Abstract
Control of infection with virulent Mycobacterium tuberculosis (Mtb) in mice is dependent on the generation of T helper (Th)1-mediated immunity that serves, via secretion of interferon (IFN)-γ and other cytokines, to upregulate the antimycobacterial function of macrophages of which the synthesis of inducible nitric oxide synthase (NOS)2 is an essential event. As a means to understanding the basis of Mtb virulence, the ability of gene-deleted mice incapable of making NOS2 (NOS2−/−), gp91Phox subunit of the respiratory burst NADPH-oxidase complex (Phox−/−), or either enzyme (NOS2/Phox−/−), to control airborne infection with the avirulent R1Rv and H37Ra strains of Mtb was compared with their ability control infection with the virulent H37Rv strain. NOS2−/−, Phox−/−, and NOS2/Phox−/− mice showed no deficiency in ability to control infection with either strain of avirulent Mtb. By contrast, NOS2−/− mice, but not Phox−/− mice, were incapable of controlling H37Rv infection and died early from neutrophil-dominated lung pathology. Control of infection with avirulent, as well as virulent Mtb, depended on the synthesis of IFN-γ, and was associated with a substantial increase in the synthesis in the lungs of mRNA for IFN-γ and NOS2, and with production of NOS2 by macrophages at sites of infection. The results indicate that virulent, but not avirulent, Mtb can overcome the growth inhibitory action of a Th1–dependent, NOS2-independent mechanism of defense.
Keywords: M. tuberculosis, virulence, NOS2 synthase, NADPH-oxidase, Th1 immunity
Introduction
The onset of expression of acquired, T cell–mediated immunity to airborne infection with virulent strains of Mycobacterium tuberculosis (Mtb)* in genetically resistant mice is evidenced by inhibition of Mtb growth in the lungs starting at ∼20 d of infection, after which infection is held at an approximately stationary level for a protracted period of time (discussed in reference 1). It is generally agreed that anti-Mtb immunity is Th1 mediated predominantly by CD4 and CD8 T cells via the secretion of IFN-γ and other Th1 cytokines (2–5) that function to upregulate the antimicrobial function of macrophages in which Mtb bacilli reside at sites of infection. The ability of activated macrophages to inhibit growth of virulent Mtb depends on the synthesis by these cells of the inducible isoform of nitric oxide synthase (NOS)2 that catalyzes high output generation of nitric oxide (NO) from l-arginine. Mice functionally deleted of the gene for NOS2 fail to control Mtb growth and die much earlier than wild-type (WT) mice of progressive, fulminating granulocyte-dominated lung pathology (6, 7). Mice treated with inhibitors of NOS2 are likewise incapable of controlling infection with virulent strains of Mtb (6, 8).
Published studies of antituberculosis immunity in mice deal almost exclusively with immunity to infection with virulent strains of Mtb that are considered virulent because of their ability to persist in stationary number and cause progressive lung disease, in spite of the acquisition by the host of Th1-mediated immunity. The role of Th1-mediated immunity in the control of infection with avirulent strains of Mtb, on the other hand, has received little attention. It was considered important for a better understanding of virulence, therefore, to know whether the requirements for control of infection with avirulent strains of Mtb are the same as those for the control of infection with virulent strains. To this end the study presented here used targeted gene-deleted mice to determine whether control of infection with avirulent Mtb, like control of infection with virulent Mtb, depends on the generation of Th1 immunity and an ability of host macrophages to generate NO and reactive oxygen. The results show that control of infection with either of two avirulent strains of Mtb depended on the generation of Th1 immunity, but did not depend on the synthesis of NOS2 by macrophages. They also show that the ability to generate reactive oxygen and its metabolites was not required to control infection with virulent or avirulent Mtb. Thus virulence is a property that enables Mtb to overcome the growth inhibitory action of a Th1-dependent, NOS2-independent mechanism capable of inhibiting the growth of avirulent Mtb.
Materials and Methods
Mice.
WT mice and NOS2−/− mice, and mice deleted of the gene for the gp91Phox subunit (9) of the respiratory burst gp91Phox subunit of the respiratory burst NADPH-oxidase complex (Phox−/ − mice), as well as double mutant mice (NOS2/Phox−/− mice), all on a C57BL/6 background were purchased from the Trudeau Institute Animal Breeding Facility. The mutant mice were from breeding stock supplied by C. Nathan (Cornell University Medical College, New York, NY). Phox−/− mice were originally supplied by M. Dinauer (Indiana University Medical Center, Indianapolis, IN). Mice deleted of the gene for the TCR-α chain (TCR-α/β−/− mice) and IFN-γ (IFN-γ−/− mice) on a C57BL/6 background were purchased from The Jackson Laboratory. All mice were used when they were 10 wk old.
Bacteria and Infection.
The H37Rv (TMC# 102), H37Ra (TMC# 201), and R1Rv (TMC# 205) strains of Mtb were originally obtained from the Trudeau Mycobacterial Culture Collection (TMC), Trudeau Institute. The avirulent H37Ra and virulent H37Rv strains were derived from the virulent H37strain as smooth and rough colony in 1934, respectively (10). The H37 parental strain was isolated from a human with pulmonary tuberculosis in 1905 (11). The R1Rv strain was derived in 1946 from the virulent R1 strain that was isolated from a human with lung disease in 1891 (12). All strains are currently available from the American Type Culture Collection. They were grown in suspension culture in Proskauer and Beck medium containing 0.01% Tween 80. The cultures were harvested after reaching ∼108 CFU per ml, dispersed by ultrasound, and stored in vials in 1 ml lots at –70°C until needed. To infect mice via the respiratory route, a vial was thawed, diluted appropriately in PBS containing 0.01% Tween 80, dispersed further by two 5 s bursts of ultrasound, and 10 ml of the diluted culture used to load the nebulizer reservoir of an aerosol infection apparatus (Tri Instruments). Exposure to the aerosol for 30 min resulted in each mouse being infected with ∼102 CFU. This was determined by enumerating CFU in the lungs 1 d after aerosol exposure. Changes in the numbers of CFU in lungs, livers, and spleens against time of infection was determined by plating 10-fold serial dilutions of whole organ homogenates on enriched Middlebrook 7H11 agar, and counting colonies after 3 wk incubation at 37°C as described previously (13).
Real-Time RT-PCR Quantitation of mRNA Synthesis for IFN-γ and NOS2.
Lungs were harvested at the times indicated in the Results section, and snap frozen in liquid nitrogen. Total RNA was extracted from lungs homogenized in Trizol (Life Sciences), according to the manufacturer's instructions. The RNA pellet was dissolved in DEPC-treated distilled water. To remove contaminated genomic DNA, RNA samples were treated with RNase-free DNase I (Ambion) for 1 h at 37°C. Aliquots of RNA samples were then passed through RNeasy minicolumns (QIAGEN), treated with DNA-free kit (Ambion), and stored at –70°C. RiboGreen™ Quantitation kit (Molecular Probes) was used to quantify RNA for real-time RT-PCR analysis. The assay was repeated three times using different dilutions of the samples.
Primers and probes were designed with Primer Express Software (PE Biosystems), and purchased from Integrated DNA Technologies. Probes contained a fluorescent dye (6-carboxy-fluorescein; FAM) and a quencher (Black Hole Quencher 1; BHQ1). The melting temperature of the hybridized probe (∼70°C) was always 10°C higher than the PCR primers (57–60°C).
To make RNA standards, each amplicon of IFN-γ and NOS2 was generated by PCR from WT mouse lung mRNA using the same primers as those used for real-time RT-PCR. The amplicons were cloned behind the T7 RNA polymerase promoter in the pGEM-T Easy Vector System (Promega). The sequence of each cloned amplicon was determined by thermocycler sequencing. After linearization of plasmid DNA, amplicons were transcribed using T7 RNA polymerase (Promega). Template DNA was removed by digestion with DNase I, and RNA was purified by using RNeasy minicolumns and quantified with the RiboGreen assay. It was determined that 1 μg of an average 1,000 bp mRNA contained 1.8 × 1012 molecules. To obtain a standard curve, serial dilutions of each transcript was performed in triplicate to give dilutions ranging from 109 to 101 molecules. The dilutions were then subjected to real-time RT-PCR analysis as described below.
For real-time RT-PCR, 2 μg of RNA was transcribed by using a random hexamer and a TaqMan Gold RT-PCR kit (PE Biosystems), according to the manufacturer's instructions. Real-time PCR to enumerate IFN-γ and NOS2 amplicons was performed in the ABI-prism 7700 Sequence Detector. Reaction conditions were programmed on a dedicated Power Macintosh 7200 computer. PCR amplification was performed in a total of 25 μl containing 10 μl of cDNA sample, 2.5 μl of 10× Taqman Buffer A, 5.5 mM MgCl2, 200 μM each of dATP, dCTP, and dGTP, 400 μM dUTP, 0.25 μM of each primer, 0.625 U of AmpliTaq Gold, and 0.25 U of AmpErase Uracil N-glycosylase (Perkin Elmer/Applied Biosystems). The reaction also contained 0.2 μM of detection probe. Amplification was performed in triplicate wells under the following conditions: 2 min at 50°C and 10 min at 94°C followed by a total of 40 two-temperature cycles (15 s at 94°C and 1 min at 60°C). The copy number in each sample was calculated according to the formula N = (Ct-b)/m, where N is copy number, Ct is the threshold cycle, b is the y-intercept, and m is the slope of the standard curve line.
Histology and Immunocytochemistry.
Lungs were fixed in 10% neutral buffered formaldehyde for 18 h. They were then washed for several hours in distilled water, dehydrated in 70 and then 100% ethanol, and embedded in paraffin according to standard procedures. Paraffin sections were cut at a thickness of 5 or 10 μm with a rotary microtome, stained for acid-fast bacteria with a modified basic fuchsin stain (14), and counter stained with methylene blue. Immunocytochemical staining for NOS2 involved reacting 10 μm-thick section with 0.1 μg/ml of affinity-purified monospecific rabbit Ig anti–mouse NOS2 (Transduction Laboratories) as the primary reagent. After washing, the sections were reacted with biotinylated goat Ig anti–rabbit Ig as the second reagent. The sections were then reacted with avidin-coupled horseradish peroxidase and with diaminobenzidine as substrate to produce a brown reaction product in cells containing NOS2. The secondary and tertiary reagents were supplied in kit form (Vectastain ABC kit; Vector Laboratories) and used according to the supplier's instructions. Photomicrographs were taken with a Nikon Microphot-Fx microscope.
Results
Susceptibility of NOS2−/− and Phox−/− Mice to Infection with Virulent Versus Avirulent Mtb.
To investigate whether NOS2 and NADPH-oxidase are required for resistance to infection with virulent versus avirulent Mtb strains, WT, NOS2−/−, Phox−/−, and NOS2/Phox−/− mice were infected via the respiratory route with ∼102 CFU of H37Rv, R1Rv, or H37Ra, and bacterial growth monitored in lungs, livers, and spleens over time. As shown in Fig. 1 , WT mice were capable of controlling growth of H37Rv in their lungs and other organs starting on day 20 of infection, and of maintaining infection at an approximately stationary level from day 20 until day 60 when the experiment was terminated. In NOS2−/− mice, in contrast, H37Rv grew progressively beyond day 20, resulting in death before day 50. In Phox−/− mice, on the other hand, the course of H37Rv infection was essentially the same as in WT mice, whereas in NOS2/Phox−/− mice infection was essentially the same as in NOS2−/− mice.
Figure 1.

Growth of H37Rv in the lungs, livers, and spleens of WT, Phox−/−, NOS2−/−, and NOS2/Phox−/− mice infected with 102 CFU aerogenically. Whereas in WT and Phox−/− mice growth of H37Rv was controlled from day 20 of infection on, growth of the pathogen was progressive in NOS2−/− and NOS2/Phox−/− mice. Means of five mice per time point ±SD.
In mice infected with R1Rv (Fig. 2) the results were different, in that growth of this organism was essentially the same in the lungs of NOS2−/− and WT mice over the 60 d of the experiment. In both types of mice, R1Rv infection progressed for 20 d before declining by ∼1 log by day 60. Phox−/− mice also were not deficient in an ability to control infection with R1Rv during the first 60 d of infection. On the contrary, R1Rv infection in Phox−/− mice was controlled at about a 1 log lower level than in WT mice, indicating that these mice were more resistant than WT mice, presumably because they developed compensatory mechanisms of defense. NOS2/Phox−/− mice showed the same resistance as Phox−/− mice up to day 40. A shortage of NOS2/Phox−/− mice prevented infection in these mice from being followed beyond this time.
Figure 2.

Growth of R1Rv in lungs, livers, and spleens of WT, Phox−/−, NOS2−/−, and NOS2/Phox−/− mice infected aerogenically with ∼102 CFU. Growth of R1Rv was identical in the lungs of WT and NOS2−/− mice, in that it was controlled at day 20, after which infection slowly resolved. Growth of R1Rv in Phox−/− and NOS2/Phox−/− mice was controlled at about a 1 log lower level than in WT mice after which infection slowly resolved. Dissemination of R1Rv to the liver and spleen was slower in Phox−/− and NOS2/Phox−/− mice, although by day 60 all groups showed similar CFU numbers in these organs. Means of five mice per time point ±SD.
Fig. 3 shows the results of a similar study that tested the resistance of WT, NOS2−/−, and NOS2/Phox−/− mice to infection with the avirulent H37Ra strain of Mtb. It can be seen that control of lung infection with H37Ra in NOS2−/− mice was the same as in WT mice over 100 d of infection. H37Ra infection progressed for ∼20–30 d, after which it underwent progressive resolution until the experiment was terminated. As was the case with R1Rv infection, NOS2/Phox−/− mice were somewhat more resistant to H37Ra infection than WT mice. Growth of H37Ra in the liver and spleen is not shown, because these organs did not become infected.
Figure 3.
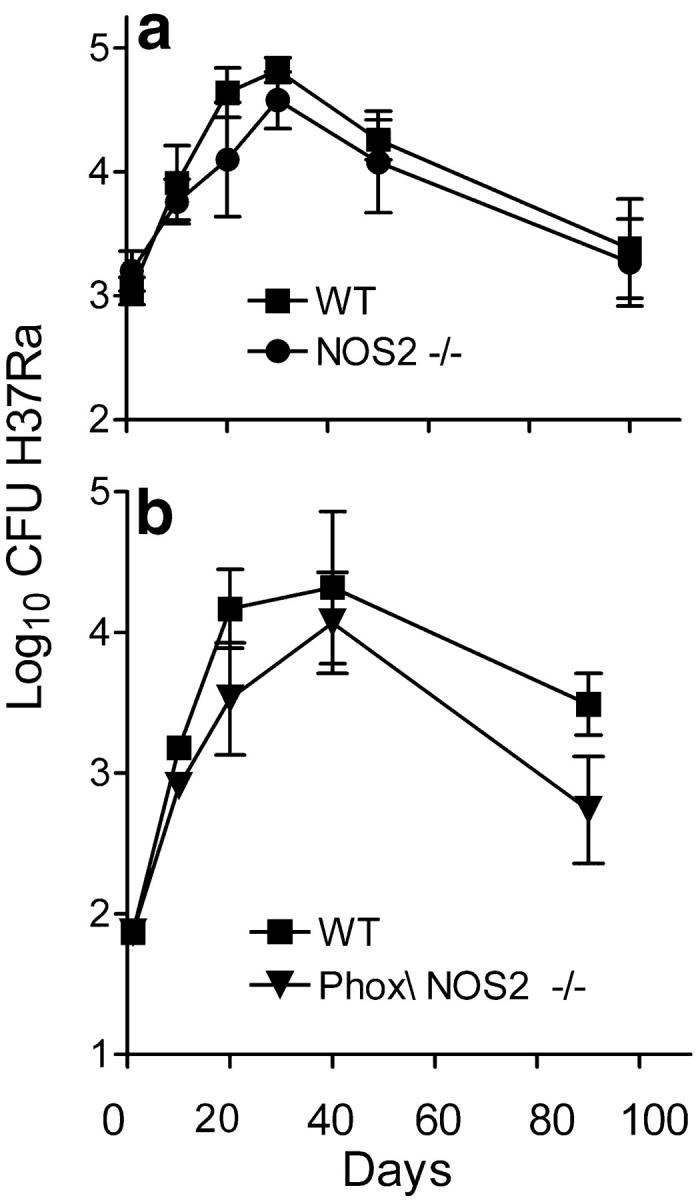
Growth of H37Ra in WT and mutant mice. Results of two experiments (a and b) showing that growth H37Ra in WT and NOS2−/− mice (a) was identical, in that in both cases H37Ra grew for 20–30 d in the lung, after which infection slowly resolved. H37Ra also grew similarly in NOS2/Phox−/− mice, except that infection was controlled at a lower level in the latter mice. Means of five mice per time point ±SD.
Control of Infection with Avirulent Mtb Depends on T Cell–mediated Immunity.
The foregoing results clearly show that, whereas NOS2 synthesis is needed for control of infection with H37Rv, it is not needed for control of infection with R1Rv or H37Ra over a 60-d period. Because synthesis of NOS2 by macrophages is induced by Th1 cytokines (4), failure to show a role for NOS2 in control of early infection with R1Rv or H37Ra could mean that resistance to infection with these avirulent strains is not dependent on Th1-mediated immunity. This possibility was investigated by determining whether infection with R1Rv or H37Ra is exacerbated in gene-deleted mice incapable of making αβ T cells or IFN-γ. As can be seen in Figs. 4 and 5 .
Figure 4.

Number of R1Rv in the lungs, livers, and spleens of WT, TCR-αβ−/−, and IFN-γ−/− mice at day 50 of an aerogenic initiated with 102 CFU. Infection was exacerbated substantially in all organs of the mutant mice. Means of five mice ±SD.
Figure 5.
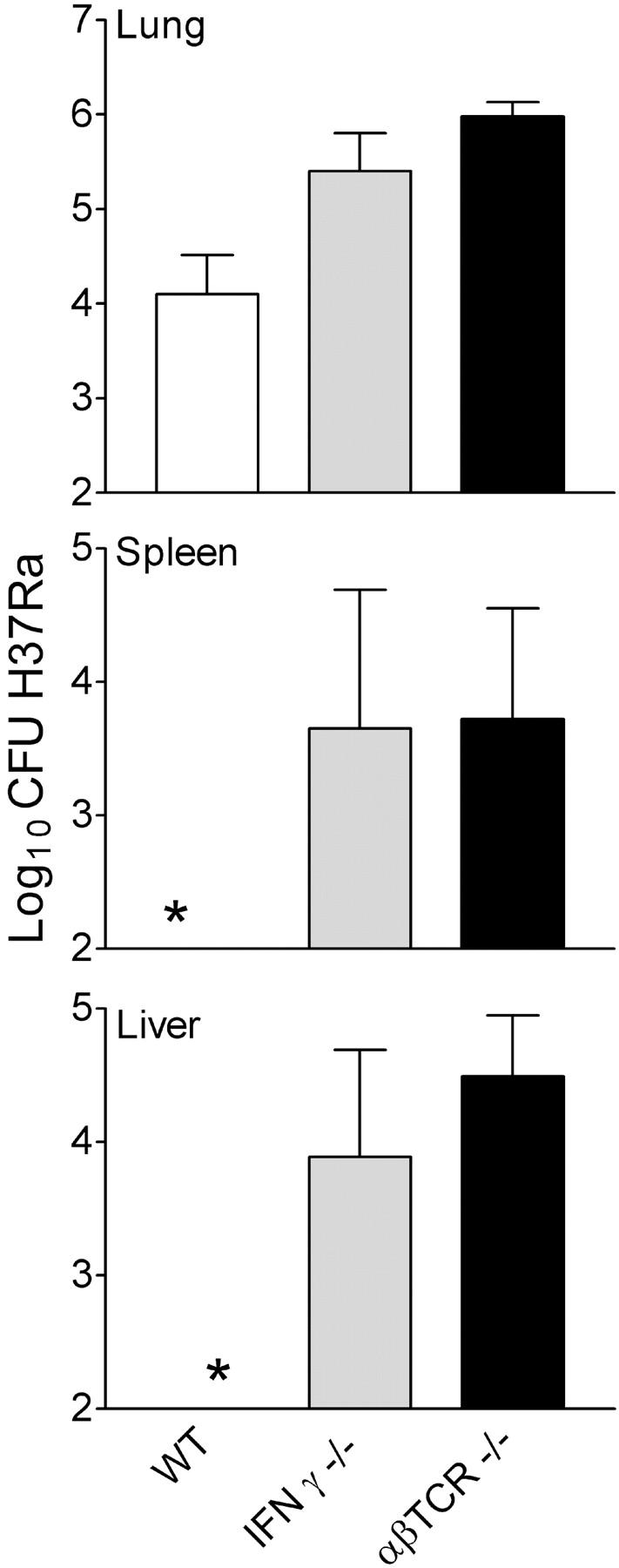
Number of H37Ra in lungs, livers, and spleens of WT, TCR-αβ−/−, and IFN-γ−/− mice at day 50 of an aerogenic infection initiated with 5 × 102 CFU. Infection in the lungs was significantly higher in mutant mice. H37Ra did not disseminate to the liver and spleen in WT mice, but did so in mutant mice incapable of expressing immunity. Means of five mice ±SD.
TCR-α/β−/− and IFN-γ−/− mice allowed significantly more growth of R1Rv and H37Ra in their lungs and other organs than WT mice over a 50-d period of infection. It will also be noted in the case of H37Ra (Fig. 5) that infection did not disseminate from the lungs to the liver and spleen in WT mice, but did so in mice devoid of αβ T cells. Thus resistance to R1Rv and H37Ra infection, like resistance to H37Rv infection, almost certainly is dependent on Th1-mediated immunity.
Increased Synthesis of mRNA for IFN-γ and NOS2 in Response to H37Rv and R1Rv Infection.
Because Th1-mediated immunity against Mtb infection is dependent on IFN-γ synthesis by T cells, and NOS2 synthesis by macrophages (4), an additional way to determine whether acquired resistance to infection with R1Rv, like control of H37Rv infection, is associated with the expression of Th1-mediated immunity is to measure IFN-γ and NOS2 gene expression. Therefore, real-time RT-PCR was used measure changes in the copy number of mRNA for IFN-γ and NOS2 in the lungs of mice infected with either organism against time of infection. The results show (Fig. 6) that between days 11 and 20 of H37Rv infection the copy number of IFN-γ and NOS2 increased >500-fold. Moreover, increased mRNA synthesis for these two proteins was sustained until day 50 of infection when the experiment was terminated. IFN-γ and NOS2 mRNA synthesis also increased in the lungs of R1Rv-infected mice between days 11 and 20 of infection, but to a lesser extent than in the case of H37Rv-infected mice. Thus control of H37Rv at a stationary level and the slow resolution of R1Rv infection were associated with a substantial increase in mRNA synthesis of a key cytokine of Th1 cells and a key enzyme of activated macrophages.
Figure 6.
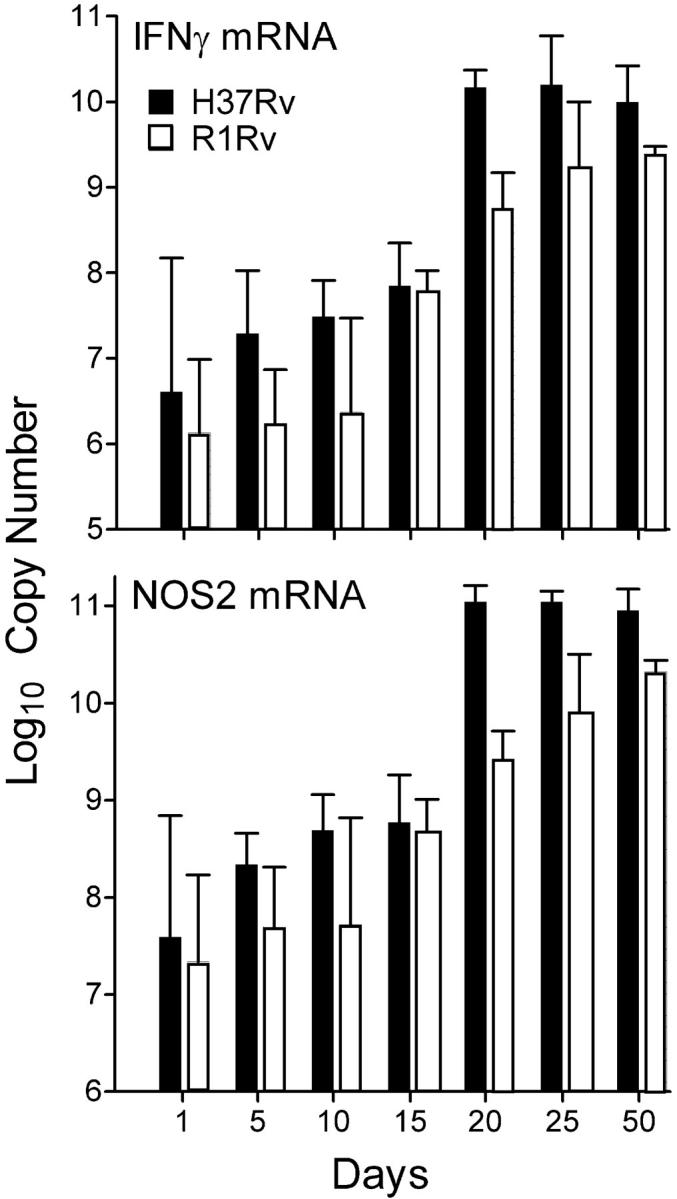
Results of a real-time RT-PCR study of IFN-γ and NOS2 gene expression in the lungs of WT mice infected aerogenically with 102 CFU of H37Rv or R1Rv. Copy numbers of mRNA for IFN-γ and NOS2 per total lung RNA increase significantly between days 11 and 20 of infection with either organism, and were sustained from day 20 to day 50 when the study was terminated. However, increased IFN-γ and NOS2 mRNA synthesis was significantly lower in the case of lungs infected with R1Rv. Means of three mice per time point ±SD.
Immunocytochemistry of NOS2 Synthesis by Macrophages in Lungs Infected with Virulent Versus Avirulent Mtb.
Lung histopatholgy induced by H37Rv in WT and NOS2−/− mice on day 40 of infection is shown in Fig. 7 . Day 40 was chosen to examine H37Rv-induced pathology because most NOS2−/− mice died before day 50. It can be seen (Fig. 7 a) that each site of lung infection in WT mice was evidenced by accumulations of macrophages in close proximity to one or more dense aggregates of lymphoid cells. Many of the macrophages in these lesions were infected with acid-fast bacilli (Fig. 7 b) and most stained positively for NOS2 by immunocytochemistry. In NOS2−/− mice on day 40, in contrast, sites of H37Rv infection were seen as large focal areas of neutrophil-dominated inflammation (Fig. 7, c and d) in which most alveoli were densely packed with degenerating neutrophils. Tissue necrosis was obviously taking place at these sites, and no cells stained positively for NOS2.
Figure 7.

Micrographs of section of lung from a WT and an NOS2−/− mouse infected 40 d earlier with 102 CFU of H37Rv aerogenically. Lesions in WT lung at low power (a) consisted of aggregates of macrophages stained for NOS2 (brown) in close proximity to aggregates to lymphoid cells (dark blue). At higher power (b) NOS2-stained macrophages were seen to contain acid-fast bacilli (red). In contrast, lesions in NOS2−/− lungs at low power (c) were seen to be composed of large numbers of neutrophils that at higher power (d) were seen to contain acid-fast bacilli and to be in the process of degeneration. No cells in NOS2−/− lungs stained for NOS2. Original magnifications: 80× for a and c; 470× for b and d.
Sites of R1Rv lung infection in WT mice (Fig. 8 a) at day 50 were also seen as accumulations of macrophages in close proximity to lymphoid cell aggregates. Macrophage accumulations were somewhat less extensive than in H37Rv-induced lesions, and most macrophages stained positively for NOS2. However, few macrophages showed the presence of acid-fast bacilli, in keeping with the relatively smaller number of R1Rv bacilli in the lungs of WT mice at day 50 of infection. In NOS2−/− mice, R1Rv-induced lung lesions at day 50 (Fig. 8 b) were larger and had a different appearance to those in WT mice. Each consisted of an extensive accumulation of macrophages surrounded by a partial or complete mantle of darkly stained lymphoid cells. No cells in these lesions (Fig. 8 c) stained positively for NOS2, and neutrophils were almost completely absent. Acid-fast R1Rv bacilli were present in macrophages in the centers of the lesions, but were no more numerous than in lesions of WT mice at day 50.
Figure 8.
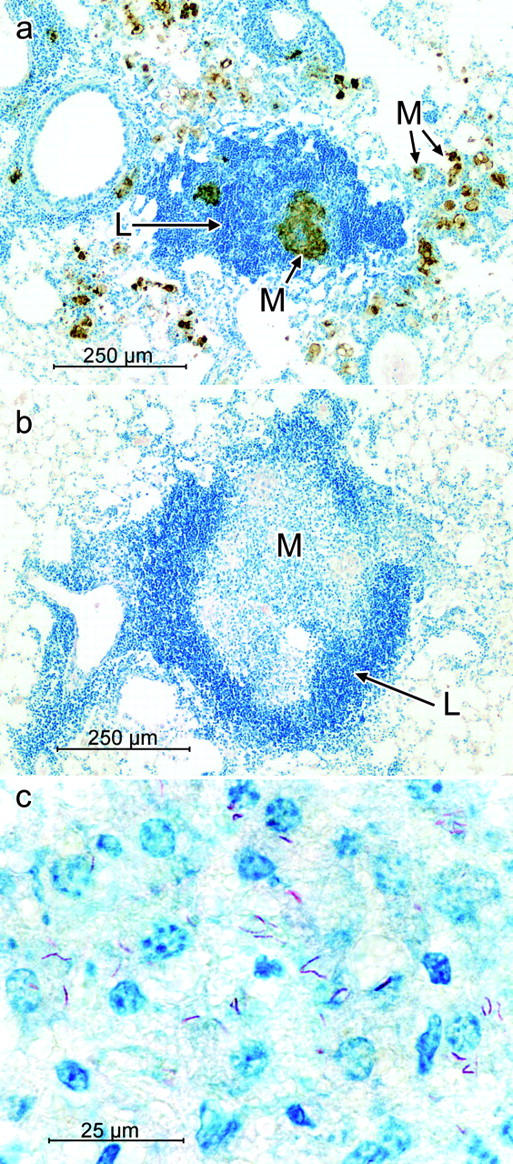
Micrographs of lung lesions in WT and NOS2−/− mice infected with 102 CFU of R1Rv aerogenically. At lower power, (a) lesions in WT lungs were composed of aggregates of lymphoid cells (blue) in close association with aggregates of macrophages containing NOS2 (brown). In the lungs of NOS2−/− mice at low power (b) lesions were seen to consist typically of a central core of macrophages surrounded by a mantle of lymphoid cells (blue). At higher power (c) none of the macrophages in the central core stained for NOS2, and some of them were infected with acid-fast bacilli (red). Original magnifications: 92× for a and b: 740× for c.
Discussion
The virulent H37Rv strain of Mtb used in this study is a widely investigated laboratory strain that was derived as a smooth colony in 1934 from the parent H37 strain isolated from a patient with active tuberculosis in 1905 (11). It is apparent from examining past and current literature that H37Rv has not lost virulence for mice since its isolation, and there is no reason to believe at present that it is less virulent for mice than most recent clinical Mtb isolates. It is shown here, in agreement with previous studies (1, 15–18) that H37Rv, like other virulent Mtb strains grows ∼4.5 logs in the lungs of mice over 20 d of infection, after which its growth is controlled and held at a stationary level by specific immunity. The avirulent H37Ra strain was derived from a parental H37 strain as a rough colony at the time of derivation of the H37Rv strain (10). It was classified as avirulent on the basis of its inability to grow progressively and cause disease in laboratory animals. Infection with this strain is progressive for ∼21 d, after which host immunity causes infection to progressively resolve, as shown here for mice, and elsewhere for guinea pigs (19). A description of differences between the IS6110 DNA fingerprint patterns of H37Rv and H37Ra has been published recently (20). The R1Rv strain is an attenuated derivative of a parental R1strain (12) that was isolated from a patient with lung disease in 1891. R1Rv is known from a previous study (21) to grow progressively in the lungs of mice for ∼3 wk, before its growth is controlled and held at stationary level for a protracted period of time. However, R1Rv, like H37Ra grows, at a much slower rate in the lungs of mice than H37Rv during the first 20 d of infection (21). It is shown here, in this connection that R1Rv grew ∼3 logs, and H37Ra ∼2 logs in the lungs of mice before infection began to resolve. According to a previous study (21), infection with R1Rv eventually stabilizes at a stationary level and causes neither progressive lung pathology nor death. This is contrast to H37Rv stationary infection that causes both progressive pathology and death. This previous finding with R1Rv led to the suggestion (21) that the ability to persist in infected tissues in the face of acquired host immunity is by itself not a measure of Mtb pathogenicity or virulence, although persistence is necessary in order for virulence to be expressed.
A key finding presented here is that, whereas growth of H37Rv after day 20 was progressive and quickly lethal for NOS2−/− mice, growth of R1Rv slowly resolved in these mice and did so to the same extent as in WT mice. R1Rv-infected WT and NOS2−/− mice showed no signs of morbidity over 150-d period of observation (unpublished data). Moreover, whereas H37Rv induced a fulminating, neutrophil-dominated pathology in the lungs of NOS2−/− mice, R1Rv-induced lung pathology that was essentially devoid of neutrophils, although different from that induced in WT mice. Thus, whereas R1Rv-induced pathology in WT mice, like that induced by H37Rv, was characterized by accumulations of macrophages in close association with aggregates of lymphoid cells, R1Rv-induced lesion in NOS2−/− mice were seen as a central core of macrophages surrounded by a thick mantle of lymphoid cells. Another key finding presented is that although NOS2 was not needed for resistance to R1Rv infection, NOS2 was nevertheless synthesized by R1Rv-infected macrophages in lesions in WT mice, as demonstrated by immunocytochemistry. This agrees with the additional finding that control of infection with R1Rv, like control of H37Rv infection, was preceded by a large increase in the synthesis of mRNA for IFN-γ and NOS2 in the lungs. The increase was smaller in the case of R1Rv infection, which is in keeping with a log lower level of R1Rv infection. It was not surprising to find that maintenance of stationary H37Rv infection and slow resolution of R1Rv infection were associated with a sustained high level of synthesis of IFN-γ and NOS2 mRNA. Further reason for concluding that immunity to R1Rv and H37Ra infection is Th1-mediated is the demonstration that infection with either organism was not controlled by gene-deleted mice incapable of making αβ T cells or IFN-γ. It goes without saying that the presence of IFN-γ–producing T cells at sites of R1Rv infection would be expected to cause activation of Mtb-infected macrophages at these sites, as evidence by the acquisition by macrophages of NOS2, as shown here.
The findings with H37Rv serve to confirm results published by others (6, 7) showing that NOS2−/− mice are incapable of controlling Mtb growth in their lungs and other organs after day 20 of infection and quickly succumb to infection-induced pathology. However, they are in disagreement with those in a recent publications (22) showing that NOS2−/− mice are not substantially deficient in a capacity to control early airborne infection with the virulent Erdman strain of Mtb. This contradictory evidence with Mtb Erdman needs to be considered, however, in light of other publications (6, 7) showing that NOS2 is essential for immune control of infection with Mtb Erdman, and that mice incapable of making NOS2 quickly succumb to a very small aerosol challenge with this strain (7), as do mice infected by aerosol with H37Rv (1). The reason why growth of Erdman was controlled by NOS2−/− mice in one laboratory, but not in others is not known. However, in view of the findings presented here with avirulent strains of Mtb, a possibility that needs to be considered is that an Erdman strain whose growth is fairly well controlled in the absence of NOS2 is likely to be less virulent than an Erdman strain whose growth is progressive. Indeed, it is apparent from published growth curves (22, 23) that the Erdman strain that grows less in NOS2−/− mice multiplies at a slower rate in WT mice than the Erdman strains used by others (18), and that it gives rise to a lower level of stationary infection after the expression of Th1 immunity. In fact, an examination of published growth curves of the Erdman strain in question shows that it grows at a similar rate and gives rise to about the same level of infection as the avirulent R1Rv strain used in this study.
An additional finding revealed by this study is that the generation of reactive oxygen by NADPH-oxidase is not needed for control of infection with either virulent or avirulent Mtb. On the contrary, Phox−/− mice were identical to WT mice in their ability to stabilize H37Rv infection, and were more resistant than WT mice to infection with R1Rv and H37Ra. Moreover, NOS2/Phox−/− double mutant mice were no more susceptible than NOS2−/− mice to infection with R1Rv or H37Rv. The demonstration that Phox−/− mice were not more susceptible than WT mice to H37Rv infection is in overall agreement with results published by another laboratory (24) showing only a transient, small increase in the growth of Mtb in the lungs, but not in other organs, of Phox−/− mice over WT mice. The results are in disagreement, on the other hand, with a publication (25) showing that Phox−/− mice are significantly more susceptible to lung infection with virulent Mtb than WT mice, as assessed by Mtb growth in the lungs. The reason for this discrepancy is not known and is difficult to explain.
The NOS2-independent mechanism of defense that is capable of stabilizing infection with R1Rv and H37Ra was not identified by this study. It is likely to be the same mechanism that enables NOS2-deficient mice to inhibit the growth of Mycobacterium avium in major organs (26), and enables NOS2-deficient macrophages to inhibit growth of this organism in vitro (26). It may also be the same mechanism that enables human macrophages treated with inhibitors of NOS2 to control the growth of H37Ra in vitro (27). It has been shown by in vitro studies (28) that mouse macrophages are capable of expressing both NOS2-dependent and NOS2-independent mechanisms of antimycobacterial defense.
In conclusion, by showing that NOS2−/− mice can control infection with avirulent, but not virulent Mtb, this study suggests that virulence is a property that enables Mtb to multiply in the face of the growth inhibitory action of a NOS2-independent, Th1-dependent anti-Mtb defense.
Acknowledgments
This work was supported by National Institutes of Health grants AI-37844 and HL-64565.
Footnotes
Abbreviations used in this paper: Mtb, Mycobacterium tuberculosis; NO, nitric oxide; NOS, nitric oxide synthase; phox, gp91Phox subunit of the respiratory burst NADPH-oxidase complex; WT, wild-type.
References
- 1.Mogues, T., M.E. Goodrich, L. Ryan, R. LaCourse, and R.J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boom, W.H. 1996. The role of T cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 5:73–81. [PubMed] [Google Scholar]
- 3.Cooper, A.M., B.M. Saunders, C.D. D'Souza, A.A. Frank, and I.M. Orme. 1997. Bull Inst. Pasteur. 95:85–95. [Google Scholar]
- 4.Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. [DOI] [PubMed] [Google Scholar]
- 5.Raupach, B., and S.H. Kaufmann. 2001. Immune response to intracellular bacteria. Curr. Opin. Immunol. 13:417–428. [DOI] [PubMed] [Google Scholar]
- 6.McMicking, J.D., R.J. North, R. LaCourse, J.S. Mudget, S.K. Shah, and C.F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA. 94:5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanga, C.A., V.P. Mohan, K. Tanaka, D. Alland, J.L. Flynn, and J. Chan. 2001. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis. Infect. Immun. 69:7711–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J.L., C.A. Scanga, K.E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796–1803. [PubMed] [Google Scholar]
- 9.Pollock, J.D., D.A. Williams, M.A. Gifford, L.L. Li, X. Du, J. Fisherman, S.H. Orkin, C.M. Doerchuck, and M.C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202–209. [DOI] [PubMed] [Google Scholar]
- 10.Steenken, W., W.H. Oatway, and S.A. Petroff. 1934. Biological studies of the tubercle bacillus. III. Dissociation and pathogenicity of the R and S variants of the human tubercle bacillus (H37). J. Exp. Med. 60:515–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steenken, W., and L.U. Gardner. 1946. History of H37 strain of tubercle bacillus. Am. Rev. Tuberc. 54:62–66. [DOI] [PubMed] [Google Scholar]
- 12.Steenken, W., and L.U. Gardner. 1946. R1 strain of tubercle bacillus, its dissociation and virulence of variants in normal and silicotic guinea pigs. Am. Rev. Tuberc. 54:51–60. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, P.L., and R.J. North. 1995. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology and cause mortality. Infect. Immunity. 63:3428–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis, R.C., and L.A. Zabrowarny. 1993. A safer staining method for acid fast bacilli. J. Clin. Pathol. 46:559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schell, R.F., W.F. Ealey, G.E. Harding, and D.W. Smith. 1974. The influence of vaccination on the course of experimental airborne tuberculosis in mice. J. Reticuloendoth. Soc. 16:131–138. [PubMed] [Google Scholar]
- 16.North, RJ., L. Ryan, R. LaCourse, T. Mogues and M.E. Goodrich. 1999. Growth of mycobacteria in mice as an unreliable indicator of virulence. Infect. Immun. 67:5483–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manca, C., L. Tsenova, C.E. Barry, A. Bergtold, S. Freeman, P.A. Haslett, J.M. Musser, V.H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740–6746. [PubMed] [Google Scholar]
- 18.Kelley, C.L., and F.M. Collins. 1999. Growth of a highly virulent strain of Mycobacterium tuberculosis in mice of differing susceptibility to tuberculosis. Tuberc. Lung Dis. 79:367–370. [DOI] [PubMed] [Google Scholar]
- 19.Alsaadi, A.-I., and D.W. Smith. 1973. The fate of virulent and attenuated mycobacteria in guinea pigs infected by the respiratory route. Am. Rev. Resp. Dis. 107:1041–1046. [DOI] [PubMed] [Google Scholar]
- 20.Bifani, P., S. Moghazeh, B. Shopsin, J. Driscoll, A. Ravikovitch, and B.N. Kreiswirth. 2000. Molecular characterization of Mycobacterium tuberculosis H37Rv/Ra variants. Distinguishing the mycobacterial laboratory strain. J. Clin. Microbiol. 38:3200–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn, P.L., and R.J. North. 1996. Persistent infection with virulent but not avirulent Mycobacterium tuberculosis in the lungs of mice causes progressive pathology. Med. Microbiol. 45:103–109. [DOI] [PubMed] [Google Scholar]
- 22.Cooper, A.M., J.E. Pearl, J.V. Brooks, S. Ehlers, and I.M. Orme. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immunity. 68:6879–6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valway, S.E., M.P.C. Sanchez, T.F. Shinnick, I. Orme, T. Agerton, D. Hoy, J.S. Jones, H. Westmoreland, and I.M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633–639. [DOI] [PubMed] [Google Scholar]
- 24.Cooper, A.M., B.H. Segal, A.A. Frank, S.M. Holland, and I.A. Orme. 2000. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect. Immun. 68:1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams, L.B., M.C. Dinauer, D.E. Morgenstern, and J.L. Krahenbuhl. 1997. Comparison of the roles of oxygen and nitrogen intermediates in the host response to mycobacterium tuberculosis using transgenic mice. Tuberc. Lung Dis. 78:237–246. [DOI] [PubMed] [Google Scholar]
- 26.Gomes, M.S., M. Florido, T.F. Pais, and R. Appelberg. 1999. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J. Immunol. 162:6734–6739. [PubMed] [Google Scholar]
- 27.Aston, C., W.N. Rom, A.T. Talbot, and J. Reibman. 1998. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am. J. Respr. Crit. Care Med. 157:1943–1950. [DOI] [PubMed] [Google Scholar]
- 28.Bekker, L.-G., S. Freeman, P.J. Murray, B. Ryffel, and G. Kaplan. 2001. TNF-α controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J. Immunol. 166:6728–6734. [DOI] [PubMed] [Google Scholar]


