Abstract
By transgenic expression of ovalbumin (OVA) as a model self antigen in the β cells of the pancreas, we have shown that self tolerance can be maintained by the cross-presentation of this antigen on dendritic cells in the draining lymph nodes. Such cross-presentation causes initial activation of OVA-specific CD8 T cells, which proliferate but are ultimately deleted; a process referred to as cross-tolerance. Here, we investigated the molecular basis of cross-tolerance. Deletion of CD8 T cells was prevented by overexpression of Bcl-2, indicating that cross-tolerance was mediated by a Bcl-2 inhibitable pathway. Recently, Bim, a pro-apoptotic Bcl-2 family member whose function can be inhibited by Bcl-2, was found to play a critical role in the deletion of autoreactive thymocytes, leading us to examine its role in cross-tolerance. Bim-deficient T cells were not deleted in response to cross-presented self-antigen, strongly implicating Bim as the pro-apoptotic mediator of cross-tolerance.
Keywords: CD8-positive T lymphocytes, antigen presentation, T cell tolerance, Bcl-2, apoptosis
Introduction
CD8 T cells recognize antigen presented by MHC class I molecules. Access of antigen to the MHC class I presentation pathway was originally thought to be restricted to endogenous proteins, i.e., those expressed within the cytoplasm of the cell presenting the antigen. It has subsequently been shown, however, that under certain circumstances professional APCs, such as dendritic cells (DCs),* can present exogenous antigens in the class I pathway (1, 2); a process referred to as cross-presentation (3). In an immunogenic response, the advantage of cross-presentation is that proteins from pathogens that do not infect DCs can still be processed in the MHC class I pathway of such professional APCs, allowing them to prime naive CD8 T cells, a process termed cross-priming (4).
Tissue-associated self-antigens can also be cross-presented by DCs (2). This creates the potential for autoimmunity when antigens whose expression is limited to peripheral tissues are cross-presented to autoreactive CD8 T cells. However, we have shown that cross-presentation of self antigen leads to deletion of naive, autoreactive CD8 T cells. This process, termed cross-tolerance, is preceded by the activation and limited proliferation of autoreactive CD8 T cells (5, 6). Our study seeks to further understand the molecular mechanisms underlying the deletion of CD8 T cells by cross-tolerance.
Apoptosis is involved in many aspects of the control of peripheral T cell numbers including: normal cell turnover in naive (unimmunized) animals, reduction of the pool of activated, antigen-specific T cells at the termination of an immune response, and maintenance of peripheral tolerance to tissue antigens (7, 8). Apoptosis of activated T cells can occur either passively or actively. Active apoptosis, or activation-induced cell death (AICD), is mediated by certain members of the TNF receptor (TNF-R) superfamily, including CD95 (Fas/APO-1) and TNF-R1 (9, 10). Passive apoptosis, or death by neglect, arises from a lack of survival signals such as those provided by cytokines or costimulatory molecules. A number of studies have shown that death by neglect can be inhibited by the pro-survival molecule Bcl-2, both in vitro and in vivo (11). Bcl-2 is a cytoplasmic membrane protein that belongs to a family containing both pro- and anti-apoptotic members that act in concert to control cell survival (12). Mice lacking bcl-2 have decreased numbers of lymphocytes (13–15) and conversely, bcl-2 transgenic mice have enhanced survival of antigen stimulated lymphocytes and prolonged immune responses (16–19).
Among the family of Bcl-2–related proteins are anti-apoptotic members, such as Bcl-2 itself and Bcl-xL, as well as pro-apoptotic members, which include Bad, Bax, Bim, and many others (7). Of the pro-apoptotic members, Bim has recently been reported to play a critical role in T cell development, being essential for the negative selection of autoreactive thymocytes (20). Here, we provide evidence that Bim is required for peripheral deletion mediated by cross-tolerance, and Bcl-2 overexpression can inhibit this process.
Materials and Methods
Mice.
All mice were bred and maintained at the Walter and Eliza Hall Institute for Medical Research. Transgenic, OT-I (founder C57BL/6 × C57BL/6-H-2bm1; reference 21), rat insulin promoter (RIP)-membrane-bound OVA (mOVA; founder C57BL/6-H-2bm1; reference 22), and RIP-OVAhi (founder C57BL/6-H-2bm1; reference 23) mice have been described previously. These mice were all crossed to C57BL/6 mice to achieve an H-2b MHC haplotype. RIP-mOVA mice were also crossed to C57BL/6-H-2bm1 mice to achieve an H-2bm1 MHC haplotype. Background gene expression for all these transgenic mice was pure C57BL/6. It should be noted that RIP-mOVA mice develop diabetes when large numbers (>5 × 106) of naive OT-I cells are transferred, whereas RIP-OVAhi mice only develop diabetes when activated, but not naive, OT-I cells are adoptively transferred (23).
Two distinct bcl-2 transgenic lines were used: Eμ-bcl-2–25 (Eμ-bcl-2) mice (founder C57BL/6JWehi × SJL/JWehi and backcrossed for 18 generations to C57BL/6), which express Bcl-2 under the control of the 5′ IgH enhancer (Eμ) in T cells (16), and vav-bcl-2–69 (vav-bcl-2) transgenic mice (founder mouse C57BL/6), which express bcl-2 in all hematopoietic cells under the control of the vav promoter (24). OT-I.Eμ-Bcl-2 mice were generated by crossing OT-I with the Eμ-bcl-2–25 line and OT-I.vav-Bcl-2 were generated by crossing OT-I with vav-bcl-2–69 mice, provided by Drs. Jerry Adams, Suzanne Cory, and Alan Harris (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Victoria, Australia). OT-I.Bim mice were generated by crossing OT-I mice for two generations with bim-deficient mice (line 266; founder C57BL/6 × 129/Sv, backcrossed for 10 generations to C57BL/6; reference 25).
B6.lpr (backcrossed 11 generations to C57BL/6J from MRL/MpJ donor strain) were obtained from The Jackson Laboratory and crossed to OT-I mice to generate OT-I.lpr mice
Adoptive Transfer and FACS® Analysis.
Preparation of OT-I cells, carboxy fluorescein diacetate succinimidyl ester (CFSE)-labeling and analysis on a FACScan™ (Becton Dickinson) were performed as described previously (5). For adoptive transfer experiments, 5 × 106 OT-I T cells were intravenously injected into recipient mice and after 6–8 wk, spleen and lymph nodes were removed and assessed for the number of OT-I T cells present by staining with anti-TCR antibodies or tetramers. For antibody staining anti-Vβ5-FITC (MR9–4), anti-CD8-PE (Caltag Labs), and anti-Vα2-biotin (B20.1) revealed with Streptavidin Tricolor (Caltag Labs) were used. For tetramers, OT-I T cells were revealed by staining with Kb-OVA257–264 tetramer-PE and anti-CD8-FITC (Caltag Labs). The total number of OT-I T cells was determined from the formula: (% OT-I in the CD8+ cells of adoptively transferred mice − background) × (% CD8+ T cells in total live cells) × total cell number/10,000. Background was the % TCRVβ5+Vα2+ or tetramer+ cells in uninjected mice, on average this was 1.4% for mAb and 0.1% for tetramer stained cells. Total cell numbers were determined either by hemocytometer cell count with trypan blue for viability or by flow cytometry. For flow cytometry cell number determination, a known number of small nonfluorescent Sphero™ beads (BD Biosciences) were added to a known volume of the cell sample, therefore cells/ml = number cells collected × (numbers beads in sample/number beads collected)/sample volume.
Bone Marrow Chimeras.
RIP-mOVA mice expressing the MHC class I molecule H-2Kb on bone marrow–derived cells and H-2Kbm1 on nonbone marrow–derived tissue (B6 → RIP-mOVA.bm1) were generated as follows. Adult RIP-mOVA.bm1 mice were lethally irradiated, with either one dose of 900 cGy or two doses of 550 cGy 3 h apart, and reconstituted with 5 × 106 T cell–depleted C57BL/6 bone marrow cells. T cell depletion was performed by incubating with anti-CD4 (RL172), anti-CD8 (3.168), and anti-Thy1 (J1j) mAbs and treatment with rabbit complement. On day 1 after reconstitution, all mice were injected intraperitoneally with 100 μg anti-Thy1 mAb (T24) to eliminate radioresistant host T cells. The mice were left for 8–10 wk before use.
Proliferation Assays.
RIP-OVAhi mice or nontransgenic littermate mice were adoptively transferred with OT-I.Bim cells and 4 wk later, lymph node cells were collected. Cells were analyzed by flow cytometry after staining with anti-CD8 and Kb-OVA257–264 tetramer to determine the proportion of OT-I.Bim cells present. Lymph node cells from these mice or mice that received no OT-I.Bim cells were titrated in twofold dilutions in 100 μl with 5 × 105 OVA257–264-coated irradiated (15 Gray) B6 spleen cells in 96 well round-bottom plates. Cultures were pulse with [3H]thymidine for 8 h on day 3. To determine OT-I.Bim–specific proliferation, [3H]thymidine incorporation of cultures from mice that received no OT-I.Bim cells was subtracted from that of the same lymph node cell concentration for mice containing OT-I.Bim cells.
Results
Previously, we have shown that tissue antigens can be cross-presented on MHC class I molecules of bone marrow–derived DCs and that this leads to an initial phase of proliferation followed by deletion of naive autoreactive CD8 T cells (2, 5). This has been referred to as cross-tolerance (6). Our model involves the adoptive transfer of OVA-specific CD8 T cells from the OT-I transgenic line (OT-I cells [21]) into RIP-mOVA mice, which express mOVA under the control of the RIP in the pancreatic islets and the proximal tubule cells of the kidneys (22). Because we must transfer large numbers of OT-I cells to measure deletion by flow cytometry, these studies must utilize B6 → RIP-mOVA.bm1 bone marrow chimeras. This prevents damage to islet cells and the induction of diabetes that would otherwise occur (26). In such chimeras, the MHC class I molecule Kb is expressed on the bone marrow stem cell–derived cells and Kbm1 on all other tissues. Kbm1 is unable to present the MHC class I–restricted OVA257–264 peptide SIINFEKL to the OT-I TCR, and this precludes OT-I cells from attacking RIP-mOVA islet β cells. In this report, we have initially used this model to examine the role of Bcl-2 in cross-tolerance.
Bcl-2 Overexpressing CD8 T Cells Proliferate in Response to Stimulation by Cross-Presented Self-Antigen.
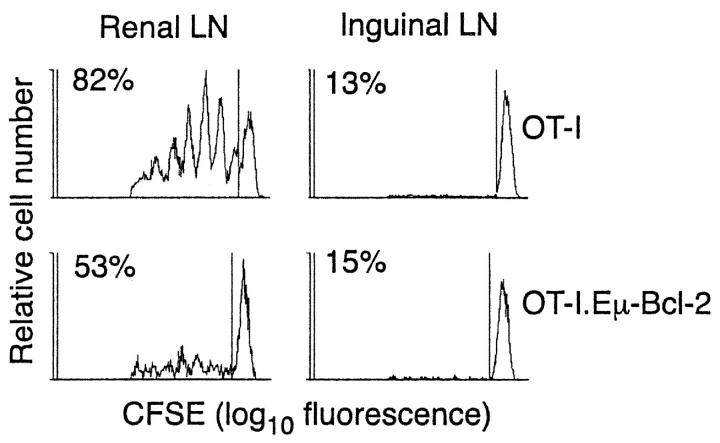
To analyze the effect of Bcl-2 on deletion by cross-tolerance, OT-I.Eμ-Bcl-2 mice were generated by crossing OT-I mice to transgenic mice overexpressing Bcl-2 in T cells under the control of the IgH enhancer (16). Before examining the effect of overexpression of Bcl-2 on T cell deletion, it was important to show that OT-I.Eμ-Bcl-2 cells could respond to cross-presented self-antigen. To test this, OT-I or OT-I.Eμ-Bcl-2 cells were labeled with CFSE and adoptively transferred into RIP-mOVA mice. After 3 d, cells were recovered from the renal (draining) and inguinal (nondraining) lymph nodes and assessed for proliferation on the basis of dilution of CFSE fluorescence (Fig. 1) . Like OT-I cells, OT-I.Eμ-Bcl-2 cells proliferated in the draining lymph nodes of OVA-expressing mice. The proportion of proliferating OT-I.Eμ-Bcl-2 cells was slightly decreased compared with control OT-I cells, which is consistent with previous observations that Bcl-2 overexpression can slow cell cycle entry (27–29). Nevertheless, OT-I.Eμ-Bcl-2 cells were clearly capable of detecting and responding to self-antigen.
Figure 1.
In vivo proliferation of OT-I and OT-I.Eμ-Bcl-2 CD8 T cells in response to stimulation by cross-presented self-antigen. 2 × 106 CFSE-labeled OT-I or OT-I.Eμ-Bcl-2 cells were adoptively transferred into RIP-mOVA mice. After 52 h, lymphocytes from the draining renal (left panel) and nondraining inguinal LNs (right panel) were analyzed by flow cytometry. Profiles were gated on CFSE+CD8+PI− cells. The numbers indicate the percentage of OT-I cells that have undergone division. These results are representative of two independent experiments.
Bcl-2 Overexpression Prevents Deletion of T Cells by Cross-Tolerance.
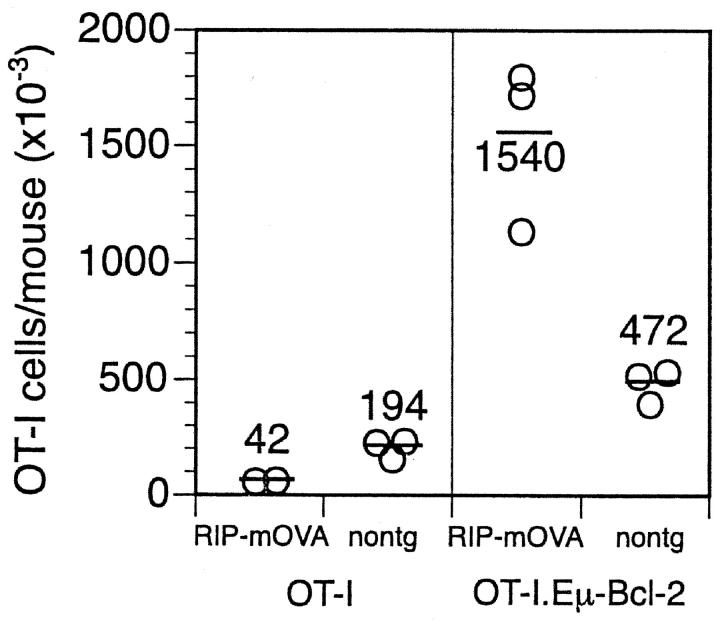
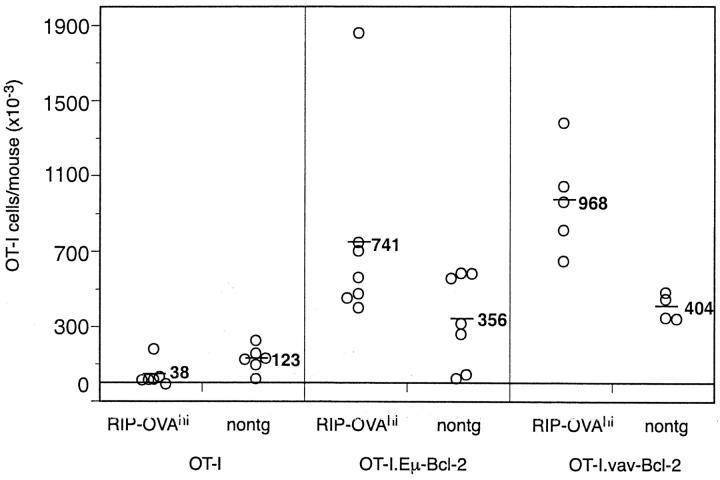
To examine the role of Bcl-2 in the deletion of naive, self-reactive CD8 T cells, OT-I or OT-I.Eμ-Bcl-2 cells were adoptively transferred into B6 → RIP-mOVA.bm1 chimeras or into control B6 → bm1 littermate chimeras. After 6–8 wk, the number of surviving T cells was quantitated by counting total cellularity in pooled spleen and lymph nodes and by immunofluorescent staining for OT-I TCR expressing T cells. Fig. 2 shows that although most control OT-I cells were deleted in RIP-mOVA mice (an average of 42 × 103 cells in RIP-mOVA mice versus 194 × 103 cells in nontransgenic mice), OT-I.Eμ-Bcl-2 cells increased in number (an average of 1,540 × 103 cells in RIP-mOVA mice versus 472 × 103 in nontransgenic mice). This 3–4-fold increase in OT-I.Eμ-Bcl-2 cells in RIP-mOVA mice compared with nontransgenic controls would be expected if the deletion process was inhibited, as proliferation in the pancreatic and renal nodes would generate more OT-I.Eμ-Bcl-2 cells with time. These results demonstrate that transgenic overexpression of Bcl-2 prevented the deletion of self-reactive CD8 T cells by the cross-tolerance mechanism.
Figure 2.
The deletion of OT-I cells in response to cross-presentation of self-antigen is prevented by overexpression of Bcl-2. Bone marrow from C57BL/6 mice was grafted into irradiated RIP-mOVA.bm1 mice and nontransgenic (nontg) littermates and the mice were left for 8–12 wk for reconstitution. 5 × 106 OT-I or OT-I.Eμ-Bcl-2 cells were then adoptively transferred, and after a further 6 wk the number of remaining OT-I cells in the LNs and spleen was determined. Open circles represent individual mice and the bar and number corresponds to the average number of OT-I cells per group. These results are representative of five independent experiments.
Bcl-2 Overexpression Does Not Enhance the Seeding of Adoptively Transferred CD8 T Cells, but Enhances Their Survival.
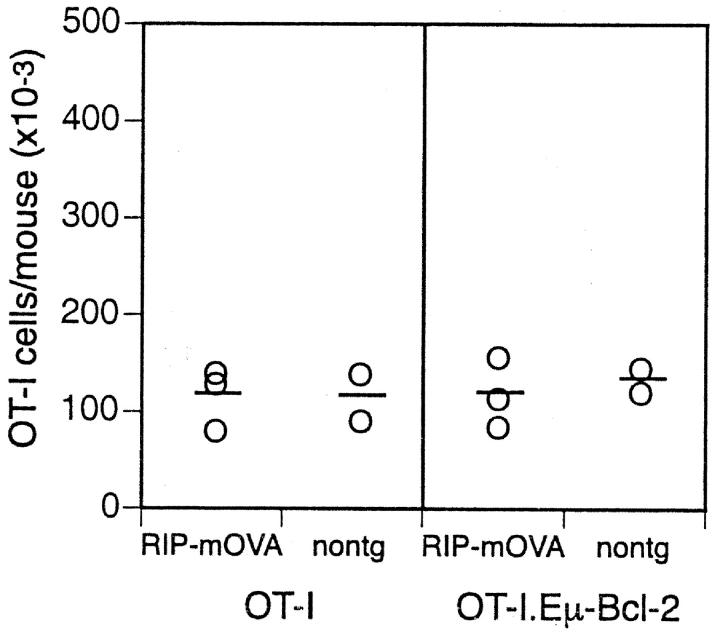
In addition to preventing deletion by cross-tolerance, Bcl-2 overexpression increased the number of OT-I cells surviving long term in hosts lacking the OVA antigen (nontransgenic mice; Fig. 2). Thus, while ∼2 × 105 OT-I cells could be found in nontransgenic mice after 6 wk, 5 × 105 OT-I.Eμ-Bcl-2 cells were evident. Two explanations for this observation were possible: (a) Bcl-2 expression might increase the survival of T cells from OT-I mice or (b) it might allow more efficient initial seeding after adoptive transfer. To distinguish between these possibilities, RIP-mOVA and nontransgenic mice were adoptively transferred with T cells from either OT-I or OT-I.Eμ-Bcl-2 mice, and 3 d later their spleen and lymph nodes were harvested and transgenic T cell numbers quantitated (Fig. 3) . This showed that similar numbers of OT-I and OT-I.Eμ-Bcl-2 cells were found in each group, indicating that the initial seeding of T cells was not affected by Bcl-2 overexpression. This indicated that Bcl-2 promotes long-term survival of T cells in recipients lacking OVA antigen, a finding that is consistent with the increased T cell numbers in unmanipulated bcl-2 transgenic mice (24, 30).
Figure 3.
The initial take of OT-I and OT-I.Eμ-Bcl-2 cells was equivalent. Bone marrow from C57BL/6 mice was grafted into irradiated RIP-mOVA.bm1 mice and nontransgenic (nontg) littermates. 4 wk later, 5 × 106 OT-I or OT-I.Eμ-Bcl-2 cells were adoptively transferred, and after a further 3 d the number of OT-I cells in the LNs and spleen was determined. The open circle symbols represent individual mice and the bar corresponds to the average number of OT-I cells per group. These results are representative of two independent experiments.
Bcl-2 Overexpression Does Not Enhance Autoimmunity.
As Bcl-2 overexpression inhibited the deletion process and increased the number of surviving OT-I cells, we speculated that it might also enhance autoimmunity, particularly as the incidence of diabetes depends on the number of OT-I cells in this system (5). Table I shows that similar to wild-type OT-I cells (31), OT-I.Eμ-Bcl-2 cells caused diabetes in 100% of mice when 5 × 106 cells were transferred but no diabetes was evident when 50-fold fewer cells (105 cells) were transferred. This lower dose is just below the threshold maximum dose for which wild-type OT-I cells have never caused diabetes (2.5 × 105). Failure of OT-I.Eμ-Bcl-2 cells to cause diabetes at this dose indicates that Bcl-2 overexpression alone does not increase the autoimmune potential of OT-I cells.
Table I.
Diabetes Incidence After Transfer of T Cells to RIP-mOVA Mice
| Number of injected cells |
OT-I | OT-I.Eμ-Bcl-2 | OT-I.Bim |
|---|---|---|---|
| 50 × 105 | 3/3 | 5/5 | 3/3 |
| 1 × 105 | 0/5 | 0/9 | 0/4 |
Data from three independent experiments. RIP-mOVA mice were injected with semi-purified OT-I, OT-I.Eμ-Bcl-2, or OT-I.Bim cells and diabetes assessed by urine glucose analysis. Diabetes onset occurred between days 5 and 10 and nondiabetic mice were monitored for 100 d. Wild-type OT-I cells have been examined for diabetes induction in large numbers of mice in other published experiments (reference 31).
The Role of CD95 in Cross-Tolerance.
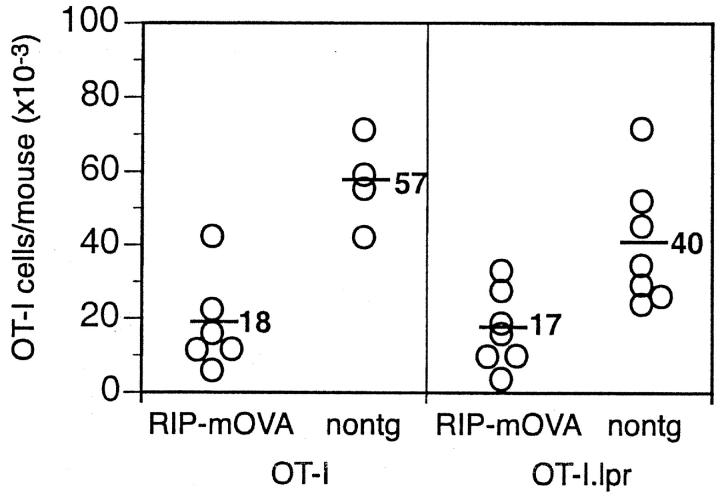
Previously, we reported that lack of CD95/Fas expression protected OT-I cells from deletion by the cross-tolerance mechanism (32). It is surprising that we now find that Bcl-2 overexpression is protective since in lymphocytes, Bcl-2 and CD95 regulate distinct apoptosis signaling pathways (17). Therefore, we reexamined our original finding with T cells from OT-I.lpr mice. Subsequent to the 2 original experiments on which the previous paper was based (32), a further 11 similar experiments were undertaken. Of the total of 13 experiments performed, 4 showed protection, 2 gave inconclusive results, and 7 showed deletion of OT-I.lpr cells. The alternative outcomes of protection or deletion were not random but could be loosely correlated with time as protection was seen with earlier experiments and only deletion seen subsequently. A basis for these variable findings emerged when the genealogy of the donor OT-I.lpr mice was examined. The mice used in the original study were derived from an OT-I to B6.lpr mating, with OT-I.lpr mice then maintained by intercrossing. Subsequently, the mice were backcrossed to B6.lpr for up to four generations. Protection from deletion was only observed for T cells from mice that had been backcrossed to C57BL/6 for 1–3 generations, whereas deletion was observed for mice backcrossed to C57BL/6 for 3–4 generations. Because of the observed changes in outcome, we then decided to reestablish the OT-I.lpr line by again mating OT-I mice to B6.lpr mice and then intercrossing. For this new OT-I.lpr line, 4/5 experiments showed deletion of OT-I.lpr cells (Fig. 4) , and 1 experiment was inconclusive. Based on the extensive studies we have performed since our original finding, we must now conclude that CD95 is not essential for deletion by cross-tolerance.
Figure 4.
The deletion of OT-I cells in response to cross-presentation of self-antigen was not prevented by the absence of CD95. Bone marrow from B6 mice was grafted into irradiated RIP-mOVA.bm1 mice and nontransgenic (nontg) littermates and mice left 8–12 wk for reconstitution. 2 × 106 OT-I or OT-I.lpr cells were adoptively transferred, and after a further 6–8 wk the number of remaining OT-I cells in the LNs and spleen was determined. Open circles represent individual mice and the bar and number corresponds to the average number of OT-I cells per group. These results are pooled from two independent experiments.
Further Support for the Ability of Bcl-2 Overexpression to Prevent Deletion by the Cross-Tolerance Mechanism.
Because of the variable results obtained with CD95-deficient OT-I cells, we considered it critical to independently confirm the effect of Bcl-2 overexpression using a second bcl-2 expressing transgenic line. This time, a Bcl-2 overexpressing OT-I line was generated by crossing OT-I mice with vav-bcl-2 transgenic mice, which overexpress Bcl-2 under the control of the vav promoter in all hematopoietic cells, including T cells (24). In these studies, we used RIP-OVAhi mice as our recipients. These mice express secreted OVA protein in the pancreatic islets (23), and like RIP-mOVA mice, this causes deletion of OT-I cells via the cross-tolerance mechanism. They are more convenient to use than the RIP-mOVA line, however, as large numbers of naive OT-I cells fail to cause diabetes and are effectively deleted without the need to generate chimeric mice. T cells from OT-I, OT-I.Eμ-Bcl-2 or OT-I.vav-Bcl-2 mice were transferred to RIP-OVAhi or nontransgenic controls and after 7 wk the number of surviving transgenic T cells was quantitated (Fig. 5) . This revealed that like the original bcl-2 transgenic line, OT-I-vav-Bcl-2 cells were protected from cross-tolerance deletion in OVA-expressing mice.
Figure 5.
The deletion of OT-I cells is prevented by overexpression of Bcl-2. 5 × 106 OT-I, OT-I.Eμ-Bcl-2, or OT-I.vav-Bcl-2 cells were adoptively transferred into RIP-OVAhi mice and after 7 wk the number of OT-I cells in the LNs and spleen was determined. Open circles represent individual mice and the bar and number corresponds to the average number of OT-I cells per group. These results are pooled data from three independent experiments.
Bim-deficient T Cells Are Not Deleted by the Cross-Tolerance Mechanism.
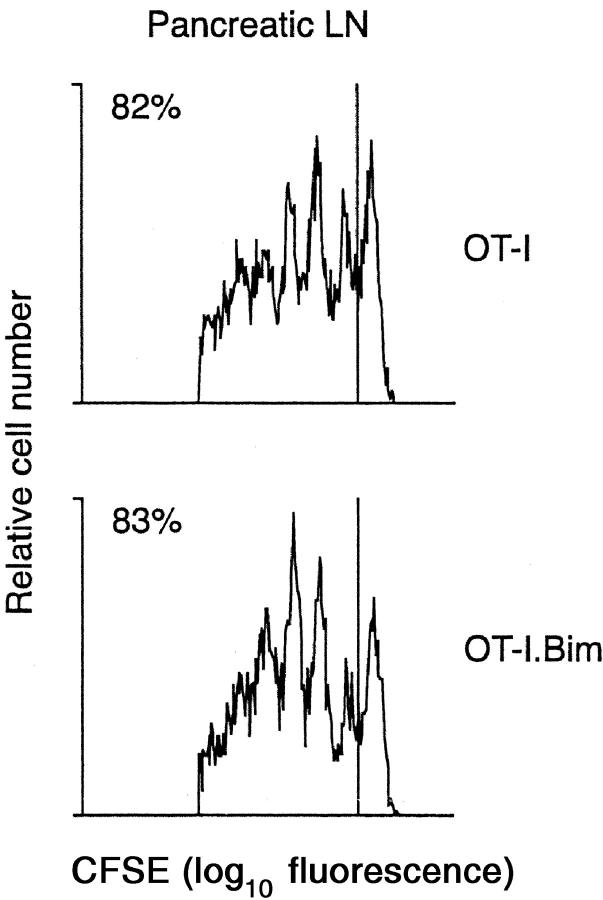
Bim is a pro-apoptotic member of the Bcl-2 protein family and plays a central role in the initiation of apoptosis signaling (33). Previous studies have shown that Bim plays a major role in T cell homeostasis (25) and the deletion of autoreactive thymocytes (20), raising the possibility that it might also play a role in the deletion of mature T cells by the cross-tolerance mechanism. To examine this idea, bim-deficient mice were crossed for two generations to the OT-I line to generate bim-deficient OT-I mice (OT-I.Bim mice). To ensure that Bim-deficient T cells could respond to cross-presented self-antigen, OT-I.Bim cells were labeled with CFSE and adoptively transferred into RIP-OVAhi mice. As with wild-type OT-I cells, OT-I.Bim cells proliferated vigorously in response to OVA cross-presented in the draining pancreatic LN (Fig. 6) .
Figure 6.
In vivo proliferation of OT-I and OT-I.Bim CD8 T cells in response to stimulation by cross-presented self-antigen. 2 × 106 CFSE-labeled OT-I or OT-I.Bim cells were adoptively transferred into RIP-mOVA mice. After 65 h, lymphocytes from the draining pancreatic LN were analyzed by flow cytometry. Profiles were gated on CFSE+CD8+PI− cells. The numbers indicate the percentage of OT-I cells that have undergone division. These results are representative of two independent experiments.
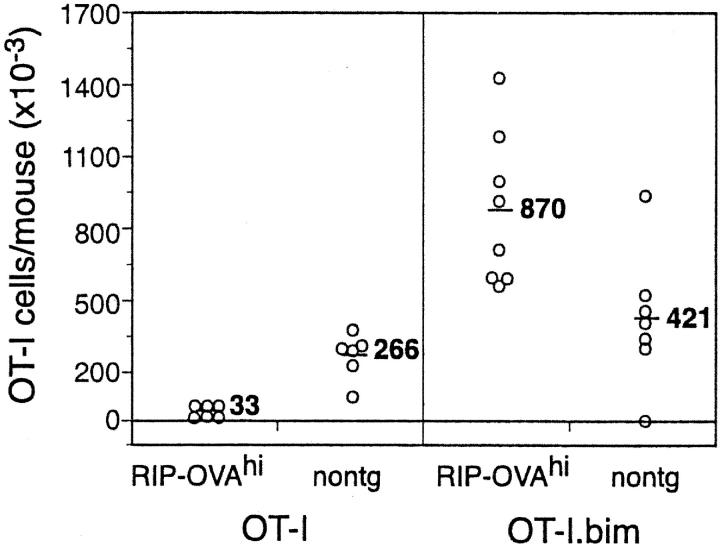
To determine whether Bim expression by T cells was essential for deletion by cross-tolerance, RIP-OVAhi mice and nontransgenic control mice were adoptively transferred with OT-I.Bim cells and 4–5 wk later examined for deletion (Fig. 7) . As observed with OT-I.Eμ-Bcl-2 and OT-I.vav.Bcl-2 cells, OT-I.Bim cells were not deleted, but increased in number during their 4–5 wk in vivo. In contrast, during this time, wild-type OT-I cells were deleted from RIP-OVAhi mice. These results indicated that the pro-apoptotic BH3 only protein Bim is essential for deletion by cross-tolerance.
Figure 7.
The deletion of OT-I cells is prevented by bim deficiency. 5 × 106 OT-I or OT-I.Bim cells were adoptively transferred into RIP-OVAhi mice and after 4–5 wk the number of OT-I cells in the LNs and spleen was determined. The open circle symbols represent individual mice and the bar and number corresponds to the average number of OT-I cells per group. These results are pooled data from two independent experiments and are representative of data from five experiments.
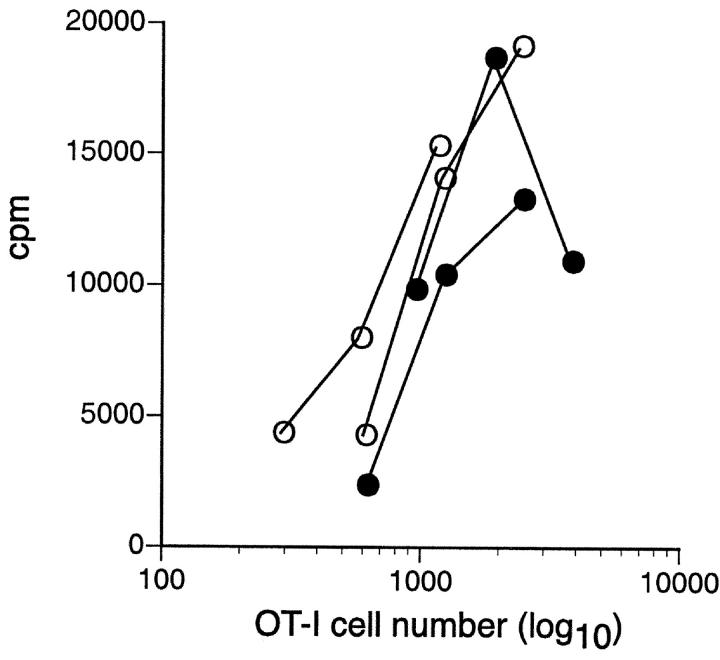
To investigate the functional status of OT-I.Bim cells that survived and expanded after transfer into RIP-OVAhi mice, cells from these mice were examined for their proliferative response to OVA in vitro. These cells were not anergic as they proliferated similarly to OT-I.Bim cells from nontransgenic recipients (Fig. 8) .
Figure 8.
OT-I.Bim CD8 T cells that fail to be deleted in RIP-OVAhi mice are not anergic. Proliferation in response to OVA257–264 peptide-coated spleen cells by OT-I.bim cells recovered from RIP-OVAhi mice (filled symbols) or nontransgenic mice (open symbols) 4 wk after adoptive transfer. These results are representative of three separate experiments.
In a single experiment to assess the autoimmune potential of OT-I.Bim cells, RIP-mOVA mice were adoptively transferred with either a high or low dose of OT-I.Bim cells (Table I). When 5 million cells were transferred OT-I.Bim cells, like wild-type OT-I cells, caused autoimmunity. When a low dose of 0.1 million OT-I.Bim cells were transferred, none of four recipient RIP-mOVA mice became diabetic. These data suggested that Bim-deficient cells were no more autoaggressive than Bim wild-type cells.
Discussion
In this work, we have shown that overexpression of Bcl-2 protects peripheral CD8 T cells from deletion in response to cross-presented self antigen. The OT-I.Eμ-Bcl-2 and OT-I.vav.Bcl-2 cells appeared similar to wild-type OT-I cells in their ability to be activated and proliferate in response to cross-presented self-antigen. If sufficiently high numbers of OT-I.Eμ-Bcl-2 T cells were transferred, they induced autoimmune diabetes (Table I). As a pro-survival molecule Bcl-2 protects lymphocytes in vitro and in vivo against apoptosis induced by growth factor deprivation, DNA damage or treatment with corticosteroids or calcium ionophores (16). For activated T cells in vivo, bcl-2 transgene expression prolongs T cell survival following injection of mice with the superantigen staphylococcus enterotoxin B (SEB; reference 16), the transfer of HY-TCR CD8 T cells into male recipients (18), the immunization of mice expressing a transgenic LCMV-TCR in CD8 T cells with LCMV peptide (19) and the immunization of mice expressing a transgenic HEL-TCR in CD4 T cells with HEL peptide (30). The most likely scenario is that Bcl-2 protects T cells against “death by neglect” caused by cytokine deprivation or lack of costimulatory ligands as the immune response is waning. This is supported by the relationship between Bcl-2 and signaling through the common γ chain (γc) cytokine receptor. Bcl-2 expression is up-regulated in human (34) or mouse (35) T cells in response to cytokines using the γc receptor, such as IL-4 and IL-7 and Bcl-2 overexpression promotes T cell development and restores T cell function in IL-7R–deficient mice (36, 37) and enhances T cell development in γc-deficient mice (38). Therefore, it is logical to speculate that Bcl-2 may protect cells from cross-tolerance deletion by blocking apoptosis caused by a cytokine deficiency or absence of costimulator molecules. Consistent with this notion, CD4 T cells are an important source of cytokines and potentially able to alter the costimulatory molecules expressed by DCs. Previously we have shown that Ag-specific CD4 T cells can impair the deletion of CD8 T cells in response to self-Ag (26).
Death receptors and members of the Bcl-2 protein family regulate distinct apoptosis signaling pathways (12, 17). Although Bcl-2 can protect against apoptosis induced by neglect, CD95 is a critical mediator for the AICD of cycling T cells that have previously been activated through their TCR (9, 10). In lymphocytes, Bcl-2 and CD95 have been shown to regulate independent pathways of apoptosis (17), although under some physiological conditions both pathways can be activated and are additive in their effects. In Eμ-bcl-2 transgenic lpr/lpr (CD95-deficient) mice, the deletion in response to superantigen SEB immunization was completely abrogated (17) and lymphadenopathy was more severe than that caused by either mutation alone (17, 39).
Although we initially described a role for CD95 in mediating cross-tolerance (32), this effect was lost when the donor OT-I.lpr mice were further backcrossed suggesting the original protective effect may have been the result of genetic drift, changes in microflora, or incomplete purity of C57BL/6 background genes. It is notable that all experiments performed here used mice on a C57BL/6 background (OT-I, B6.lpr, and RIP-mOVA or RIP-OVAhi mice), so background genes should have been identical, but it cannot be excluded that the B6.lpr line still carried other MRL background genes despite the 11 backcrosses to C57BL/6 reported by its source, The Jackson Laboratory. Interestingly, MRL mice have been reported to have a defect in B cell receptor ligation-induced AICD (40), raising the possibility that a gene(s) causing this phenotype may also impair T cell deletion. Alternatively, as we have previously shown that provision of CD4 T cell help abrogates deletion of OT-I T cells in response to cross-presentation of self-antigen (26), it is possible that minor antigens of MRL origin in the early generations of the OT-I.lpr line were able to stimulate CD4 T cell help thereby preventing deletion of the OT-I.lpr cells. From the data presented here we now conclude that the pathway for cross-tolerance involves Bcl-2 but is independent of CD95.
Contrary to the results here, it has been found that loss of CD95 but not Bcl-2 overexpression could protect CD4 T cells from apoptosis induced by stimulation with self-antigen (30). This disparity may reflect a differential role for CD95 in CD4 versus CD8 T cells. In vitro CD8 T cells show greater sensitivity to TNFα than FasL induced apoptosis (41), whereas in vivo models of virus infection have shown that CD8 T cell responses are unchanged in the absence of CD95 and TNF-R (42–45). Alternatively, the involvement of CD95 versus Bcl-2 regulated apoptosis signaling pathways may result from differences in TCR/MHC affinity or the quantity, nature, or manner of presentation of the self antigen. For example, it has been shown that Bcl-2 expression can be altered by the affinity of TCR/MHC interactions (46, 47). With respect to the nature of the self-ligand, it is worth pointing out that when CD4 T cells were protected from deletion by CD95 deficiency, the self-antigen was ubiquitously expressed, whereas in our case the antigen is expressed locally and is cross-presented. Hence, whether death receptors or the Bcl-2–regulated apoptosis signaling pathway is activated may be determined by the site of antigen expression or the type of cells presenting the antigen.
In this study, Bcl-2 overexpression protected CD8 T cells from deletion in response to cross-presented self-antigens. This indicates that Bcl-2 itself or a functional homologue (e.g. Bcl-x, Bcl-w, A1, or Mcl-1) is downregulated, or the function of a pro-apoptotic family member is upregulated, to cause T cell death during cross-tolerance induction. Interestingly, thymocytes lacking the pro-apoptotic Bcl-2 family member Bim, were shown to be resistant to negative selection in the thymus (20), leading us to examine a role for Bim in deletion by cross-tolerance. As shown by the use of Bim-deficient T cells in adoptive transfer experiments, the absence of Bim prevented their deletion, leading to a dramatic accumulation of autoreactive T cells. The amount of T cell accumulation caused by Bim deficiency was similar to that caused by Bcl-2 overexpression indicating that Bim is the critical initiator of apoptosis during the cross-tolerance deletion process. A role for Bim in peripheral T cells has also been recently reported in the deletion of activated T cells after their response to SEB in vivo (48). Bim is normally retained in an inactive form in the microtubular dynein motor complex by binding to dynein light chain DLC1/LC8 (49). How it is released from this complex during cross-tolerance induction is as yet unknown.
Interestingly, despite a failure to delete Bim-deficient OT-I cells, there was no apparent enhancement of autoimmunity (Table I) even though the surviving OT-I.Bim cells were not anergic (Fig. 8). The simplest explanation for these findings is that activation by cross-presented self-antigen is insufficient to generate full effector function in autoreactive T cells. Sherman and colleagues provide support for this view, showing that CD8 T cells activated by cross-presented self antigens are functionally different from those primed by virus (50). This suggests that, in this situation, the major potential problem with failing to delete autoreactive Bim-deficient CD8 T cells might relate to their continued availability for activation into fully functional autoreactive effectors if further stimulated by antigens in the context of infection.
In conclusion, we have demonstrated that Bim is essential for the deletion of autoreactive CD8 T cells induced by cross-presentation of self-antigens, thereby providing a link between the mechanisms responsible for central and peripheral tolerance and illustrating the importance of the Bcl-2 family of proteins in self-tolerance.
Acknowledgments
We thank Drs. J.M. Adams, S. Cory, and A. Harris for generously providing vav-Bcl-2 mice and Jane Langley, Tatiana Liew, and Loretta Coverdale for their technical assistance.
This work was supported by grants and fellowships from National Institutes of Health (grant AI43347), the National Health and Medical Research Council of Australia, the Leukemia and Lymphoma Society of America, and the Dr. Josef Steiner Cancer Research Foundation. W.R. Heath is a Howard Hughes International Fellow.
Footnotes
Abbreviations used in this paper: AICD, activation-induced cell death; CFSE, carboxy fluorescein diacetate succinimidyl ester; DC, dendritic cell; γc, common γ chain cytokine receptor; mOVA, membrane-bound ovalbumin; RIP, rat insulin promoter; SEB, staphylococcus enterotoxin B.
References
- 1.den Haan, J.M., S.M. Lehar, and M.J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurts, C., M. Cannarile, I. Klebba, and T. Brocker. 2001. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166:1439–1442. [DOI] [PubMed] [Google Scholar]
- 3.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. [DOI] [PubMed] [Google Scholar]
- 4.Bevan, M.J. 1987. Antigen recognition. Class discrimination in the world of immunology. Nature. 325:192–194. [DOI] [PubMed] [Google Scholar]
- 5.Kurts, C., H. Kosaka, F.R. Carbone, J.F. Miller, and W.R. Heath. 1997. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath, W.R., C. Kurts, J.F. Miller, and F.R. Carbone. 1998. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 187:1549–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton, K., and A. Strasser. 2000. Cell death control in lymphocytes. Adv. Immunol. 76:179–226. [DOI] [PubMed] [Google Scholar]
- 8.Van Parijs, L., and A.K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 280:243–248. [DOI] [PubMed] [Google Scholar]
- 9.Van Parijs, L., A. Ibraghimov, and A.K. Abbas. 1996. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 4:321–328. [DOI] [PubMed] [Google Scholar]
- 10.Nagata, S., and T. Suda. 1995. Fas and Fas ligand: lpr and gld mutations. Immunol. Today. 16:39–43. [DOI] [PubMed] [Google Scholar]
- 11.Cory, S. 1995. Regulation of lymphocyte survival by the bcl-2 gene family. Annu. Rev. Immunol. 13:513–543. [DOI] [PubMed] [Google Scholar]
- 12.Strasser, A., L. O'Connor, and V.M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217–245. [DOI] [PubMed] [Google Scholar]
- 13.Kamada, S., A. Shimono, Y. Shinto, T. Tsujimura, T. Takahashi, T. Noda, Y. Kitamura, H. Kondoh, and Y. Tsujimoto. 1995. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 55:354–359. [PubMed] [Google Scholar]
- 14.Veis, D.J., C.M. Sorenson, J.R. Shutter, and S.J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 75:229–240. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama, K., I. Negishi, K. Kuida, H. Sawa, and D.Y. Loh. 1994. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl. Acad. Sci. USA. 91:3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strasser, A., A.W. Harris, and S. Cory. 1991. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 67:889–899. [DOI] [PubMed] [Google Scholar]
- 17.Strasser, A., A.W. Harris, D.C. Huang, P.H. Krammer, and S. Cory. 1995. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14:6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teh, S.J., J.P. Dutz, B. Motyka, and H.S. Teh. 1996. Fas (CD95)-independent regulation of immune responses by antigen-specific CD4−CD8+ T cells. Int. Immunol. 8:675–681. [DOI] [PubMed] [Google Scholar]
- 19.Petschner, F., C. Zimmerman, A. Strasser, D. Grillot, G. Nunez, and H. Pircher. 1998. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur. J. Immunol. 28:560–569. [DOI] [PubMed] [Google Scholar]
- 20.Bouillet, P., J.F. Purton, D.I. Godfrey, L.-C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J.M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 relative Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. [DOI] [PubMed] [Google Scholar]
- 21.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 22.Kurts, C., W.R. Heath, F.R. Carbone, J. Allison, J.F. Miller, and H. Kosaka. 1996. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurts, C., J.F. Miller, R.M. Subramaniam, F.R. Carbone, and W.R. Heath. 1998. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J. Exp. Med. 188:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogilvy, S., D. Metcalf, C.G. Print, M.L. Bath, A.W. Harris, and J.M. Adams. 1999. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc. Natl. Acad. Sci. USA. 96:14943–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouillet, P., D. Metcalf, D.C. Huang, D.M. Tarlinton, T.W. Kay, F. Kontgen, J.M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 26.Kurts, C., F.R. Carbone, M. Barnden, E. Blanas, J. Allison, W.R. Heath, and J.F. Miller. 1997. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186:2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linette, G.P., Y. Li, K. Roth, and S.J. Korsmeyer. 1996. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc. Natl. Acad. Sci. USA. 93:9545–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Reilly, L.A., D.C. Huang, and A. Strasser. 1996. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 29.Mazel, S., D. Burtrum, and H.T. Petrie. 1996. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J. Exp. Med. 183:2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Parijs, L., D.A. Peterson, and A.K. Abbas. 1998. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity. 8:265–274. [DOI] [PubMed] [Google Scholar]
- 31.Kurts, C., F.R. Carbone, M.F. Krummel, K.M. Koch, J.F. Miller, and W.R. Heath. 1999. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 398:341–344. [DOI] [PubMed] [Google Scholar]
- 32.Kurts, C., W.R. Heath, H. Kosaka, J.F. Miller, and F.R. Carbone. 1998. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1). J. Exp. Med. 188:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor, L., A. Strasser, L.A. O'Reilly, G. Hausmann, J.M. Adams, S. Cory, and D.C. Huang. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbar, A.N., N.J. Borthwick, R.G. Wickremasinghe, P. Panayoitidis, D. Pilling, M. Bofill, S. Krajewski, J.C. Reed, and M. Salmon. 1996. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294–299. [DOI] [PubMed] [Google Scholar]
- 35.Vella, A.T., S. Dow, T.A. Potter, J. Kappler, and P. Marrack. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 95:3810–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maraskovsky, E., L.A. O'Reilly, M. Teepe, L.M. Corcoran, J.J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 89:1011–1019. [DOI] [PubMed] [Google Scholar]
- 37.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- 38.Kondo, M., K. Akashi, J. Domen, K. Sugamura, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 7:155–162. [DOI] [PubMed] [Google Scholar]
- 39.Tamura, A., M. Katsumata, M.I. Greene, and K. Yui. 1996. Inhibition of apoptosis and augmentation of lymphoproliferation in bcl-2 transgenic Fas/Fas ligand-defective mice. Cell. Immunol. 168:220–228. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida, T., T. Higuchi, H. Hagiyama, A. Strasser, K. Nishioka, and T. Tsubata. 2000. Rapid B cell apoptosis induced by antigen receptor ligation does not require Fas (CD95/APO-1), the adaptor protein FADD/MORT1 or CrmA- sensitive caspases but is defective in both MRL-+/+ and MRL-lpr/lpr mice. Int. Immunol. 12:517–526. [DOI] [PubMed] [Google Scholar]
- 41.Zheng, L., G. Fisher, R.E. Miller, J. Peschon, D.H. Lynch, and M.J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 377:348–351. [DOI] [PubMed] [Google Scholar]
- 42.Ehl, S., U. Hoffmann-Rohrer, S. Nagata, H. Hengartner, and R. Zinkernagel. 1996. Different susceptibility of cytotoxic T cells to CD95 (Fas/Apo-1) ligand-mediated cell death after activation in vitro versus in vivo. J. Immunol. 156:2357–2360. [PubMed] [Google Scholar]
- 43.Zimmermann, C., M. Rawiel, C. Blaser, M. Kaufmann, and H. Pircher. 1996. Homeostatic regulation of CD8+ T cells after antigen challenge in the absence of Fas (CD95). Eur. J. Immunol. 26:2903–2910. [DOI] [PubMed] [Google Scholar]
- 44.Reich, A., H. Korner, J.D. Sedgwick, and H. Pircher. 2000. Immune down-regulation and peripheral deletion of CD8 T cells does not require TNF receptor-ligand interactions nor CD95 (Fas, APO-1). Eur. J. Immunol. 30:678–682. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen, L.T., K. McKall-Faienza, A. Zakarian, D.E. Speiser, T.W. Mak, and P.S. Ohashi. 2000. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur. J. Immunol. 30:683–688. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima, H., and W.J. Leonard. 1999. Role of Bcl-2 in alpha beta T cell development in mice deficient in the common cytokine receptor gamma-chain: the requirement for Bcl-2 differs depending on the TCR/MHC affinity. J. Immunol. 162:782–790. [PubMed] [Google Scholar]
- 47.Auphan-Anezin, N., and A.M. Schmitt-Verhulst. 2001. Differential survival of transferred CD8 T cells and host reconstitution depending on TCR avidity for host-expressed alloantigen. J. Immunol. 166:7200–7207. [DOI] [PubMed] [Google Scholar]
- 48.Hildeman, D.A., Y. Zhu, T.C. Mitchell, J. Kappler, and P. Marrack. 2002. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 14:354–359. [DOI] [PubMed] [Google Scholar]
- 49.Puthalakath, H., D.C. Huang, L.A. O'Reilly, S.M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3:287–296. [DOI] [PubMed] [Google Scholar]
- 50.Hernandez, J., S. Aung, W.L. Redmond, and L.A. Sherman. 2001. Phenotypic and functional analysis of CD8+ T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J. Exp. Med. 194:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]