Abstract
Altered peptide ligands (APLs) and their antagonistic or partial agonistic character–influencing T cell activation have mainly been studied in vitro Some studies have shown APLs as a viral escape mechanism from cytotoxic CD8+ T cell responses in vivo. However, whether infection or superinfection with a virus displaying an antagonistic T cell epitope can alter virus–host relationships via inhibiting T cell–mediated immunopathology is unclear. Here, we evaluated a recently described CD4+ T cell escape lymphocytic choriomeningitis virus (LCMV) variant that in vitro displayed antagonistic characteristics for the major histocompatibility complex class II–restricted mutated epitope. Mice transgenic for the immunodominant LCMV-specific T helper epitope that usually succumb to wild-type LCMV-induced immunopathology, survived if they were simultaneously coinfected with antagonistic variant but not with control virus. The results illustrate that a coinfecting APL-expressing virus can shift an immunopathological virus–host relationships in favor of host survival. This may play a role in poorly cytopathic long-lasting virus carrier states in humans.
Keywords: APL, LCMV, antagonistic peptide, CD4+ T cells, protection
Introduction
Altered peptide ligands (APLs)* were originally described as modified MHC class II peptides with a single amino acid change, which in vitro induced cytokine secretion without proliferation of the CD4+ T cells. This contrasted with activation and proliferation after stimulation with the WT peptide (1, 2). Subsequently, complete antagonistic (3, 4) or partial agonistic features (5, 6) of APLs have been described. Most of these data were collected with CD4+ T cells in vitro (1) or by peptide immunization in vivo (7–9). The general hope is that such APL might be used to interfere with, or prevent the induction of, autoimmunity. Two clinical therapeutic trials have used CD4+ T helper cell–specific APL peptides in multiple sclerosis patients (10, 11) without finding any clear effect on disease progression. Mutations of CD8+ cytotoxic (CTL) epitopes with APL features have been described as a possible viral escape mechanism from protective cytotoxic T cell responses. During HIV (12, 13), hepatitis B (14), hepatitis C (15), and lymphocytic choriomeningitis virus (LCMV; 16–18) infections mutated MHC class I epitopes have been identified in the viral genome of infected patients or mice. The possibility that the presence of both WT and APL virus in vivo can influence CD4+ or CD8+ T cell immune responses and the outcome of disease caused by non or poorly cytopathic viruses, however, has not been studied so far.
We have recently analyzed a CD4+ T cell escape mutant of the RNA virus LCMV-WE, a noncytopathic mouse pathogen (19). This escape mutant arose in LCMV-WE–infected mice treated with TCR transgenic (tg) CD4+ T cells specific for the major MHC class II LCMV glycoprotein peptide gp61 (19). The deleted single point mutation in the viral RNA leads to an amino acid substitution at position 75 of the LCMV glycoprotein I from K to R. When analyzed in vitro, the peptide gp61-75R was able to antagonize proliferation and cytokine production of the WT peptide, thereby revealing activities as shown for APLs. Because the assessment of T cell–mediated immunoprotection is usually sensitive to low T cell numbers over a narrow range, whereas susceptibility to immunopathological disease is dependent upon large numbers of effector T cells over a rather broad range, the latter situation should offer the possibility to evaluate the effects of APL in LCMV-induced CD4+ T cell–dependent immunopathology.
Materials and Methods
Mice.
Smarta1/2, TNFR1−/−, and Fas−/− mice were purchased from the Institute für Labortierkunde (University of Zurich, Zurich, Switzerland) and kept under specific pathogen-free conditions. TNFR1−/− knockout mice were backcrossed to Smarta2 tg mice. Genotype was confirmed by PCR (TNFR1 knockout) and FACS® (Smarta TCR).
Virus.
LCMV strain WE, Docile (provided by F. Lehmann-Grube, Heinrich Pette Institute, Hamburg, Germany), and Armstrong (provided by R. Ahmed, Emory University School of Medicine, Atlanta, GA) were propagated on L929 cells. Mice were infected intravenously with the indicated dose.
Peptides.
The following peptides were used: gp61 (aa61-80): GLNGPDIYKGVYQFKSVEFD; gp61-75R (aa61-80): GLNGPDIYKGVYQFRSVEFD; and p8 (aa415-433): SSKHQVFEHPHIQDAASQL.
FACS®.
Peripheral blood was collected by retroorbital bleeding and the lymphocytes were stained with rat anti–mouse CD4 tri-color conjugated antibody (Caltag), anti–mouse CD69 FITC-conjugated antibody (BD Biosciences), and anti–mouse CD62L-PE–conjugated antibody (BD Biosciences), and then analyzed with the Becton Dickinson FACScan™. The determination of virus titer by plaque assay was performed as previously described (20).
Immunohistochemistry.
This was performed on cryosection and as previously described (21).
CD8 Depletion.
CD8 depletion was performed with monoclonal rat antibodies as previously described (19).
Statistical Analysis.
Statistical analysis was performed with the one-tailed Mann-Whitney U test using GraphPad Prism3.00 for Windows (GraphPad Software). P < 0.05 was interpreted as statistically significant.
Results and Discussion
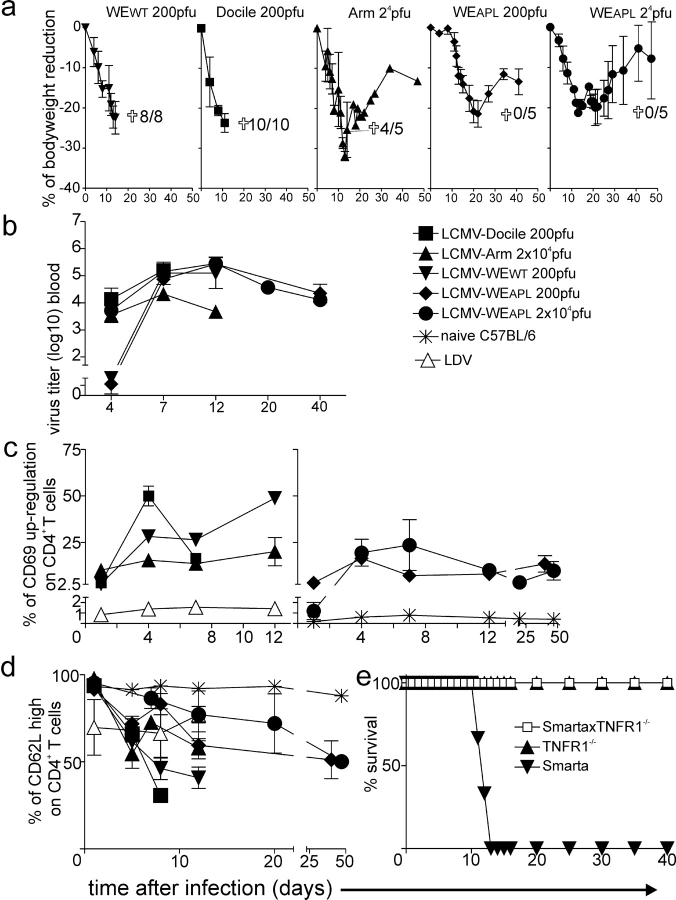
As an in vivo readout of the APL activities of the mutated virus we used CD4+ TCR tg mice (Smarta1 mice in which the CD41 T cells are specific for the MHC class II LCMV glycoprotein peptide gp61; 22), which express the tg TCR in >98% of the CD4+ T cells and have only low numbers of CD8+ T cells. Although this tg CD4+ T cell condition displaying a limited T cell repertoire represents a model situation, it reduces complexities and may allow for the determination of the key parameter change. Smarta mice were infected with different LCMV strains. In order of increasing replication kinetics and hepatotropism, they are: Armstrong (LCMV-Armstrong), WE WT (LCMV-WEWT), Docile (LCMV-Docile), or the mutated LCMV-WE APL virus (LCMV-WEAPL). Mice infected with different LCMV strains show strain-dependent clinical courses and clearance characteristics (23). Although LCMV-Docile and LCMV-Armstrong differ at several other sites, they differ only at position 63 from the gp61-80 helper epitope of LCMV-WEWT. However, this change is irrelevant for the recognition and activation of the tg Smarta CD4+ T cells in vitro (unpublished data) and in vivo. Upon infection with 200 plaque-forming units (pfu) LCMV-WEWT, Smarta1 animals showed rapid signs of systemic disease, lost 22% of their body weight, and died within 11 to 14 d (Fig. 1 a). Similarly, infection with 200 pfu LCMV-Docile was lethal within 8 to 10 d and the mice showed an accelerated reduction in bodyweight when compared with LCMV-WEWT (Fig. 1 a). Mice infected with a higher dose (2 × 104 pfu) of the less rapidly replicating LCMV-Armstrong lost up to 35% of bodyweight within 12 to 15 d. The mortality was 80% (Fig. 1 a). In contrast, after infection with the CD4+ T cell escape virus (LCMV-WEAPL) all animals survived independently of the applied dose (Fig. 1 a). After infection with 200 pfu LCMV-WEAPL, the bodyweight reduction was delayed by 8 to 10 d compared to LCMV-WEWT and maximal weight loss was around 20% on day 20. The mice completely recovered thereafter (Fig. 1 a). Similarly, after infection with 2 × 104 pfu LCMV-WEAPL, the bodyweight reduction was delayed by 2 to 4 d compared to LCMV-WEWT, LCMV-Docile, or LCMV-Armstrong infection. Again, the maximal bodyweight reduction was ∼20% and all mice survived (Fig. 1 a). The amount of replicating virus in the blood correlated well with the infecting virus dose except for LCMV-Docile, which is known to replicate faster in vivo than the others. All surviving mice became chronic LCMV carriers (Fig. 1 b). The tg CD4+ T cells were activated upon infection with all LCMV strains, as shown by the up-regulation of the early T cell activation marker CD69 (Fig. 1 c) and the down-regulation of the late T cell activation marker CD62L (Fig. 1 d). The intensity of CD69 up-regulation at day 4 correlated well with the reduction in bodyweight. Surprisingly and in contrast to the stimulation results obtained in vitro, APL virus–infected mice also showed CD69 up-regulation and CD62L down-regulation, although less extensively than that observed in LCMV-Docile– or LCMV-WEWT–infected mice (Fig. 1, c and d). Interestingly, upon infection of tg mice with lactate dehydrogenase elevating virus (LDV), we also observed a slight CD69 up-regulation (Fig. 1 c) and a comparable CD62L down-regulation (Fig. 1 d) after infection with LCMV-WEAPL. This suggests a general rather than a specific effect, indicating that the observed CD4+ T cell activation after LCMV-WEAPL and LDV could partially be due to CD4+ T cell bystander activation in a tg, almost monoclonal, situation (24). In addition, viral revertants or minimal contamination of quasispecies in the viral stocks could be responsible for the observed CD4+ T cell activation after LCMV-WEAPL infection. We also performed intracellular IFNγ staining 8 d after infection with 200 pfu LCMV-WEWT or LCMV-WEAPL. However, we were not able to observe any relevant IFNγ production in the spleen compared to the situation in nontransgenic C57BL/6 mice (unpublished data). This correlates with the down-regulated TCR, which is usually paralleled by CD69 up-regulation, and a dilution by nonprimed TCR tg CD4+ T cells in this almost monoclonal situation. However, activation and proliferation of the Smarta TCR tg CD4+ T cells after adoptive transfer experiments has been described in several prior publications (19, 22, 25–27).
Figure 1.
Virus-induced immunopathology. (a) Smarta1 mice were infected intravenously with 200 pfu LCMV-WEWT (▾), LCMV-Docile (▪), or LCMV-APL (♦), or with 2 × 104 pfu LCMV-Armstrong (▴), LCMV-WEAPL (•), or LDV (▵). Bodyweight (n = 3 or 4 mice per group) and mortality rate (cross represents all mice used) are indicated. (b) Virus titers were measured in the blood (n = 3 or 4 per group). The activation of blood CD4+ T cells was measured by (c) CD69 up-regulation (n = 3 or 4 per group) and (d) CD62L down-regulation (n = 3 or 4 per group). (e) Smarta2 mice backcrossed to TNFR1−/− (□, n = 5), TNFR1−/− (▴, n = 5), or Smarta2 (▾, n = 3) were CD8 depleted on days 3 and 1 and infected with 200 pfu LCMV-WEWT on day 0, respectively. The mortality rate was measured.
To further analyze the mechanism of LCMV-induced cachexia and mortality in Smarta mice, we backcrossed Smarta2 mice with TNFR1−/− mice. Due to logistical reasons, Smarta2 mice, which have near normal CD8+ T cell levels, were used. After CD8 depletion by monoclonal anti-CD8 antibodies, Smarta2 mice behave identically to Smarta1 mice, not only in regard to mortality but also in regard to protection by the LCMV-WEAPL (unpublished data). In the absence of CD8+ T cells, Smarta × TNFR1−/− double tg mice were protected from death when infected with LCMV-WEWT at a dose of 200 pfu (Fig. 1 e). Similar results were obtained with 2 × 106 LCMV-Docile (unpublished data). In contrast, the backcrossing of Smarta2 mice with Fas knockout mice could not prevent death when mice were infected with LCMV-Docile (unpublished data). This indicates that LCMV-induced disease in Smarta mice is mainly mediated by CD4+ T cells mediated via the action of TNFα. This is similar to the situation in perforin knockout mice, in which LCMV-induced cachexia, anemia, and mortality is also mediated by TNFα (28).
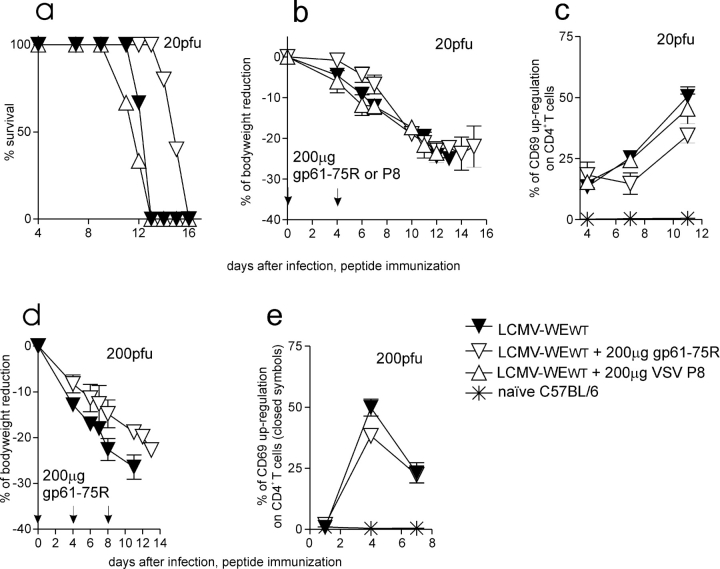
To evaluate the antagonistic capacities of the escape gp61 peptide, Smarta1 mice were immunized with 200 μg of the gp61-75R or the gp61 WT peptide in IFA s.c. Direct immunization with peptides was more efficient than immunization with peptide-loaded dendritic cells (unpublished data). No CD4+ T cell activation was observed upon immunization with 200 μg gp61-75R in IFA, but Smarta1 mice treated with the WT peptide died of generalized immunopathology within 1 to 2 d (unpublished data). Treatment on day 0 and 4 with 200 μg gp61-75R in IFA s.c. delayed lethal immunopathology by 3 to 4 d in Smarta1 mice infected intravenously with 20 pfu LCMV-WEWT (Fig. 2 a). The initial bodyweight loss was reduced and fewer CD4+ T cell were activated in comparison with 20 pfu LCMV-WEWT alone or in combination with the irrelevant H2-IAb–presented vesicular stomatitis virus helper epitope p8 (Fig. 2, a–c). This was paralleled by a reduction in CD4+ T cell activation depicted by a reduced CD69 up-regulation (Fig. 2 c). Interestingly, survival was not prolonged when mice were treated with the antagonistic gp61-75R peptide every second day (unpublished data). Upon infection with a higher dose of LCMV-WEWT (200 pfu; Fig. 2, d and e) bodyweight loss and CD4+ T cell activation (CD69 up-regulation) were slightly, but not statistically significantly reduced when LCMV was injected with 200 μg of the antagonistic gp61-75R and death was delayed by 1 d (Fig. 2 d). These results showed a dose-dependent action of the mutated peptide in vivo during a virus infection and indicated that the initial presence of the antagonistic peptide was crucial for prolonged survival.
Figure 2.
Delay of virus-induced immunopathology after immunization with APL peptide gp61-75R. (a, b, and d) Smarta1 mice were infected with 20 or 200 pfu LCMV-WEWT. 200 μg gp61-75R (▿, n = 5 in combination with 20 pfu LCMV-WEWT and n = 4 in combination with 200 pfu LCMV-WEWT) or 200 μg VSV-P8 peptide (▵, n = 3) were injected in IFA s.c. on days 0 and 4, respectively. The control mice (▾, n = 3) were left untreated. Bodyweight and mortality are indicated. (c and e) Activation of blood CD4+ T cells was examined by CD69 up-regulation by FACS® analysis. (c) Statistically significant differences were observed between the following groups (P < 0,05): ▿ versus ▾ at days 7 and 11 and (e) ▿ versus ▾ at day 4.
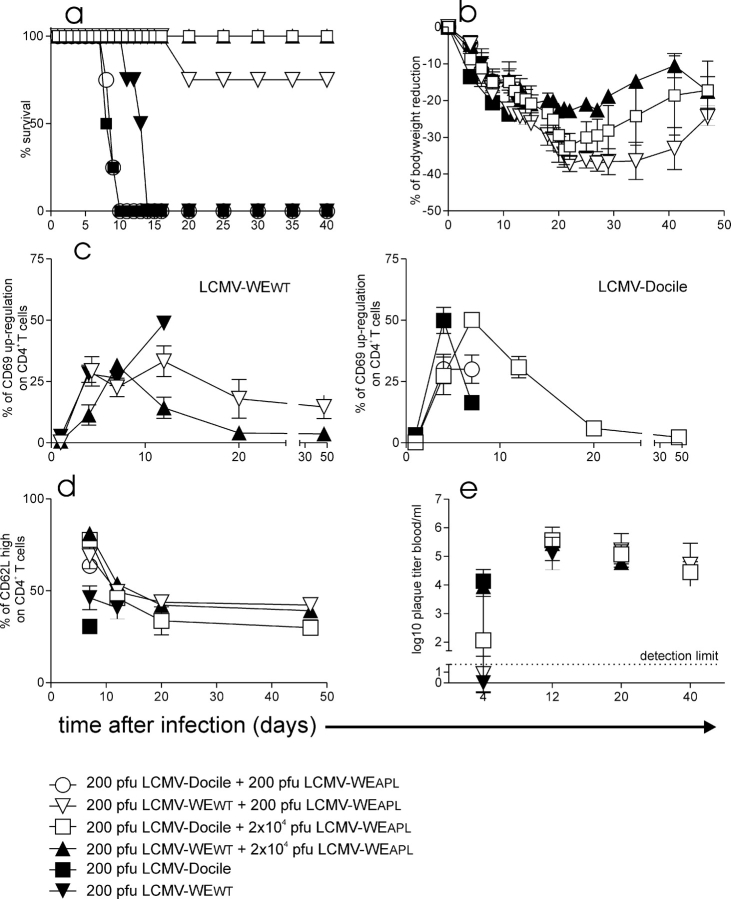
In vitro, APL effects are usually observed with specific CD4+ T cells at 10–100-fold excess of the APL peptide against a submaximal WT peptide concentration. To examine APL effects in vivo during an infection we used the gp61-75R peptide that displayed the antagonistic activity in vitro at a 100-fold excess against the WT gp61 peptide (19). The dose of WT peptide cannot be determined during a virus infection, but it is probably high. With the LCMV-WEAPL we could now test the effects of APL not only with peptide treatment but also by using the APL virus. The effects of LCMV-WEAPL were assessed in vivo by coinfecting Smarta1 mice with different doses of LCMV-WEAPL and LCMV WT strains LCMV-WEWT or LCMV-Docile at a ratio of 1:1 to 1:100, respectively. The infection of Smarta1 mice with 200 pfu LCMV-Docile and 200 pfu LCMV-WEAPL could not prevent lethal immunopathology (Fig. 3 a). The body weight reduction was comparable (Fig. 3 b), whereas CD4+ T cell activation was significantly reduced 7 d after infection in regard to CD62L down-regulation, but no statistical difference in CD69 up-regulation could be observed (Fig. 3, c and d). Mice exhibited signs of systemic disease upon infection with 200 pfu LCMV-WEWT plus 200 pfu LCMV-WEAPL, but the mortality rate was reduced to 20% (Fig. 3, a–d). The infection of mice with 200 pfu LCMV-Docile plus 2 × 104 pfu LCMV-WEAPL resulted in a wasting disease but the mice recovered after 30 d (Fig. 3, a and b). Compared to infection with LCMV-Docile alone, the coinfection resulted in a statistically significant delay of the tg CD4+ T cell activation with regard to CD69 up-regulation and CD62L down-regulation (Fig. 3, c and d). After infection with 200 pfu LCMV-WEWT plus a 100-fold excess of LCMV-WEAPL (2 × 104 pfu), the bodyweight loss was considerably less severe and mice were clinically in much better condition (Fig. 3, a and b). In addition, the tg CD4+ T cells were statistically significantly less activated with regard to CD69 up-regulation at days 4 and 12 (Fig. 3, c and d). All mice became chronic LCMV carriers, although the initial virus load in the blood at 4 d after infection was reduced in LCMV-WEAPL–infected mice coinfected with LCMV-Docile compared to mice infected with LCMV-Docile alone, although not statistically significant (Fig. 3 e).
Figure 3.
LCMV-APL prevents lethal immunopathology of LCMV-WEWT and LCMV-Docile. (c) Smarta1 mice were infected with 200 pfu LCMV-WEWT (▾), LCMV-Docile (▪), LCMV-Docile plus 200 pfu LCMV-WEAPL (○), LCMV-WEWT plus 200 pfu LCMV-WEAPL (▿), LCMV-WEWT plus 2 × 104 pfu LCMV-WEAPL (□), or LCMV-Docile plus 2 × 104 LCMV-WEAPL. (a) Survival rate (n = 4 per group) and (b) bodyweight reduction are shown. (c) The activation of blood CD4+ T cells was measured by CD69 up-regulation and (d) CD62L down-regulation. (e) Virus titers were measured in blood. (b–e) 200 pfu LCMV-Docile (n = 3), LCMV-WEWT (n = 3). Coinfection of groups is n = 4 per group. Statistically significant difference between the following groups (P < 0.05): (c) ▪ versus □ at days 4 and (c and d) 7 and (d) ▪ versus ○ at day 7; (c) ▴ versus ▾ at days 4, (d) 7, and (c) 12 and ▴ versus ▿ at (c) days 4, (d) 7, and (c) 12.
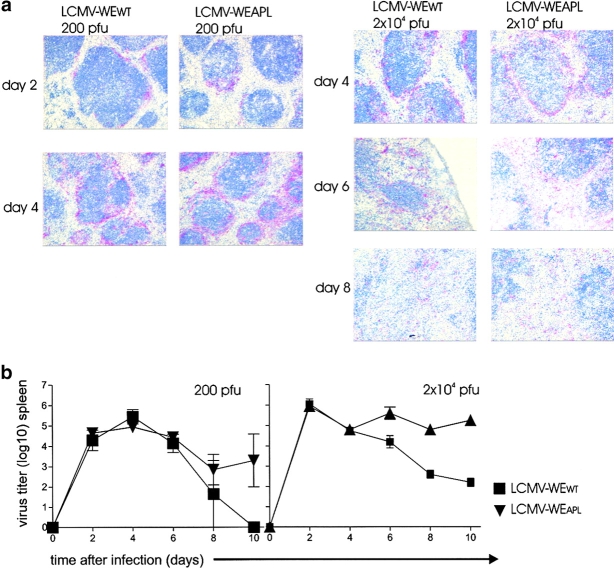
To examine whether protection by the variant virus was due to a different virus tropism in the relevant organs, we evaluated the distribution of LCMV-WEWT and LCMV-WEAPL in vivo. We used C57BL/6 WT mice because in the absence of CD8+ T cells, as is the case in Smarta1 mice, viral distribution is very rapid so that minor differences can not be detected. We could not observe any difference with regard to tropism after 200 pfu infection with LCMV-WEWT or LCMV-WEAPL. Similar virus distribution could be observed in the lung, kidney, and spleen, particularly around the marginal zone, at days 2, 4, 6, 8, and 10 (Fig 4 a, left; only the spleen on days 2 and 4 is shown). Similar results were obtained with 2 × 104 pfu infection dose. Until day 6, there was no difference in tropism and the antigen amount could be seen in the lung, kidney, and spleen. However, after 6 d a slight increase after infection with LCMV-WEAPL variant compared to LMCV-WEWT could be observed (Fig. 4 a, right; only the spleen on days 4, 6, and 8 is shown). These data were also consistent with LCMV titers in the spleen assessed with the focus-forming assay. There, similar virus titers between LCMV-WEWT and LCMV-WEAPL could be detected in the spleen until day 8 after 200 pfu (Fig. 4 b) or until day 6 after 2 × 104 pfu (Fig. 4 b) LCMV infection. Thereafter, the LCMV-WEAPL virus displayed higher titers compared to LCMV-WEWT (Fig. 4 b).
Figure 4.
No relevant difference in viral tropism and early virus titers between LCMV-WEWT and LCMV-WEAPL. (a) C57BL/6 mice were infected with 200 (left) or 2 × 104 pfu (right) LCMV-WEWT or LCMV-WEAPL. Viral distribution was analyzed by immunohistochemistry on cryosections using the anti-LCMV nucleoprotein-specific rat monoclonal antibody VL-4. Section from the spleen are shown 2 and 4 d after infection with 200 pfu and 4, 6, and 8 d after infection with 2 × 104 pfu. (b) Spleen titers after infection with 200 (left) or 2 × 104 pfu (right) LCMV-WEWT (▪, n = 3) or LCMV-WEAPL (▾, n = 3).
As an additional control experiment we examined if the slower replicating LCMV-Armstrong strain (compared to LCMV-WEWT or LCMV-Docile) could also induce protection against the WT LCMV-WE. In contrast to coinfection with the LCMV-WEAPL, infection with 100-fold excess of 2 × 104 pfu LCMV-Armstrong did not prevent or delay disease and death caused by infection with 200 pfu LCMV-WEWT (Fig. 5 , a and b) or by LCMV-Docile (unpublished data). In addition, protection was not observed if LCMV-Armstrong was given 1 d before or after infection with LCMV-WEWT or LCMV-Docile (unpublished data).
Figure 5.
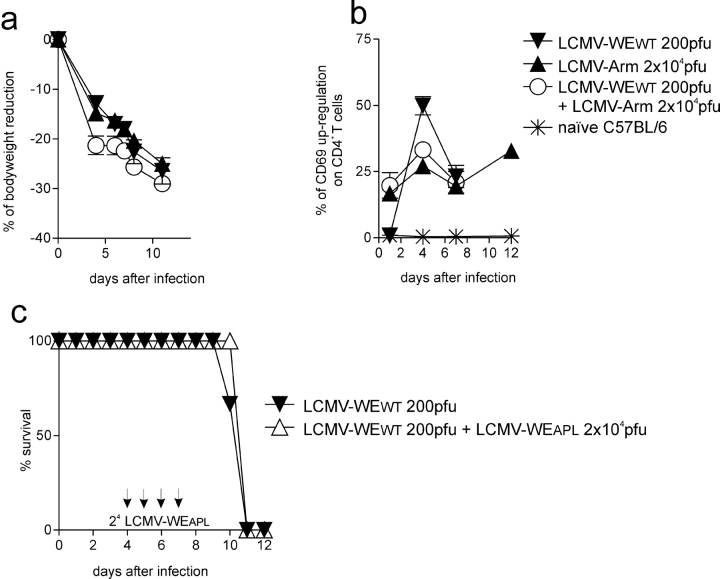
T cell antagonistic features are specific for LCMV-APL. (a and b) Smarta 1 mice were infected with 200 pfu LCMV-WEWT (▾, n = 3), LCMV-Armstrong (▴, n = 3), or LCMV-WEWT plus 2 × 104 pfu LCMV-Armstrong (○, n = 4). (a) Body weight reduction and mortality rate are shown and (b) blood CD4+ T cell activation was measured by CD69 up-regulation. (c) Limited effect of APL virus at later time points. Smarta 1 mice were infected with 200 pfu LCMV-WEWT. At days 4, 5, 6, and 7 mice were infected with 2 × 104 pfu LCMV-WEAPL (▵, n = 4). Control animals (▾, n = 3) received PBS. Survival rate is shown.
To assess the capacity of LCMV-WEAPL in its ability to prevent immunopathology if used several days after LCMV-WEWT infection, Smarta1 mice were infected intravenously with 200 pfu LCMV-WEWT on day 0 followed by 2 × 104 pfu LCMV-WEAPL on days 4–7. Under these conditions the survival kinetics (Fig. 5 c), percentage of bodyweight reduction (unpublished data), nor the clinical outcome (100% mortality) were influenced.
This study shows that CD4+ T cell antagonism by live LCMV-WEAPL is effective against fatal immunopathology if an equal dose of LCMV-WEWT or a 100-fold excess against LCMV-Docile are used. Antagonistic APLs prevent or decrease the activation of T cells by their specific agonistic peptide. How this functions in detail is not clear yet at the molecular level (1) and was not the aim of this study. The gp61-75R peptide has been shown to be an antagonistic APL in vitro (19).
In vivo in Smarta mice, rapid and strong tg CD4+ T cell activation seems to be associated with an earlier mortality and enhanced cachexia (compare LCMV-Docile vs. LCMV-WEWT and LCMV-Docile vs. LCMV-Armstrong) in Smarta mice (Fig. 1), whereas delayed activation can be protective (after a certain time point the Smarta mice are protected from death, although similar activation levels might be observed at a later time point). In contrast to a recent study analyzing wasting disease in the absence of CD8+ T cells after intracranial LCMV infection (29), the CD4+ T cell–mediated mortality in Smarta mice was associated with the presence of the TNFR1, which is similar to the situation found in perforin knockout mice (28).
Several mechanisms could be responsible for the observed nonlethal wasting disease after LCMV-WEAPL infection and the protection from LCMV-WEWT–induced immunopathology by the LCMV-WEAPL: (a) LCMV-WEAPL replicates slower than LCMV-WEWT or LCMV-Armstrong and induces a slower CD4+ T cell priming that might allow the survival of the infected Smarta mice. However, the data presented in Fig. 4, which show a comparable early replication kinetic and tropism of LCMV-WEAPL and LCMV-WEWT, argues against this. (b) LCMV-WEAPL replicates much faster than LCMV-WEWT and therefore most APC present the antagonistic peptide (gp61-75R) on their MHC class II molecule. As stated above, this is rather unlikely, because the early replication kinetic of LCMV-WEAPL and LCMV-WEWT is comparable (Fig 4; days 0–6 and day 8). However, this effect might be responsible for the observed protection when LCMV-WEAPL was used at a 100 times higher dose (Fig. 3, a and b) and this dose dependence is characteristic of antagonistic or partial agonistic APLs. (c) After the coinfection of Smarta mice with LCMV-WEAPL and LCMV-WEWT, both viruses are present in the lymphoid tissue at an equal or comparable amount when using equal infection doses and therefore the antagonistic peptide alters and delays the CD4+ T cell activation. The experiments with LCMV-Armstrong show that a delayed CD4+ T cell activation can result in reduced and delayed immunopathology with possible reduction of mortality.
According to our results the mechanism of protection is most likely mediated by the delayed, and in certain cases the reduced, activation of the gp61-specific CD4+ T cells by presenting an antagonistic APL.
It is clear that these results have model characteristics for two reasons. First, we used a CD4+ TCR tg mouse largely lacking CD8+ T cells for a defined readout and second, only combined infection but not later superinfection was effective. Nevertheless, we can hypothesize that during infection with high viral loads of HIV or during chronic hepatitis B or C such a fine-tuned mechanism may tip the virus-host-virus balances favorably away from immunopathology. During these infections, quasispecies with theoretical APL capacities have been shown to arise (14, 16) and therefore APL-expressing mutants may influence the overall balance between lethal immunopathology and host survival in these persistent viral infections in humans. Although an APL strategy may offer only limited protection against already well-activated CD4+ T cells, it may operate in chronic, persistent immunopathological viral infections where new T cells are activated cyclically.
Acknowledgments
This paper has been supported by the following grants: Swiss grants from SWF31.50900.97.2, the Kanten Zürich KTI 4235.1, and BBW1.0020 Hepatitis Therapy.
L. Hunziker and M. Recher contributed equally to this work.
Footnotes
Abbreviations used in this paper: APL, altered peptide ligand; LCMV, lymphocytic choriomeningitis virus; tg, transgenic.
References
- 1.Sloan-Lancaster, J., and P.M. Allen. 1996. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 14:1–27. [DOI] [PubMed] [Google Scholar]
- 2.Evavold, B.D., and P.M. Allen. 1991. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 252:1308–1310. [DOI] [PubMed] [Google Scholar]
- 3.De Magistris, M.T., J. Alexander, M. Coggeshall, A. Altman, F.C. Gaeta, H.M. Grey, and A. Sette. 1992. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 68:625–634. [DOI] [PubMed] [Google Scholar]
- 4.Sloan-Lancaster, J., B.D. Evavold, and P.M. Allen. 1993. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 363:156–159. [DOI] [PubMed] [Google Scholar]
- 5.Racioppi, L., F. Ronchese, L.A. Matis, and R.N. Germain. 1993. Peptide-major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor–dependent intracellular signaling. J. Exp. Med. 177:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madrenas, J., R.L. Wange, J.L. Wang, N. Isakov, L.E. Samelson, and R.N. Germain. 1995. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 267:515–518. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson, L.B., J.M. Greer, R.A. Sobel, M.B. Lees, and V.K. Kuchroo. 1995. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 3:397–405. [DOI] [PubMed] [Google Scholar]
- 8.Karin, N., D.J. Mitchell, S. Brocke, N. Ling, and L. Steinman. 1994. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon γ and tumor necrosis factor α production. J. Exp. Med. 180:2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brocke, S., K. Gijbels, M. Allegretta, I. Ferber, C. Piercy, T. Blankenstein, R. Martin, U. Utz, N. Karin, D. Mitchell, et al. 1996. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 379:343–346. [DOI] [PubMed] [Google Scholar]
- 10.Kappos, L., G. Comi, H. Panitch, J. Oger, J. Antel, P. Conlon, and L. Steinman. 2000. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat. Med. 6:1176–1182. [DOI] [PubMed] [Google Scholar]
- 11.Bielekova, B., B. Goodwin, N. Richert, I. Cortese, T. Kondo, G. Afshar, B. Gran, J. Eaton, J. Antel, J.A. Frank, et al. 2000. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6:1167–1175. [DOI] [PubMed] [Google Scholar]
- 12.Klenerman, P., S. Rowland-Jones, S. McAdam, J. Edwards, S. Daenke, D. Lalloo, B. Koppe, W. Rosenberg, D. Boyd, A. Edwards, et al. 1994. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 369:403–407. [DOI] [PubMed] [Google Scholar]
- 13.Allen, P.M., and R.M. Zinkernagel. 1994. Promethean viruses? Nature. 369:355–356. [DOI] [PubMed] [Google Scholar]
- 14.Bertoletti, A., A. Sette, F.V. Chisari, A. Penna, M. Levrero, and M. De Carli. 1994. Natural variants of cytotoxic epitopes are T cells receptor antagonists for anti-viral cytotoxic T cells. Nature. 369:407–410. [DOI] [PubMed] [Google Scholar]
- 15.Tsai, S.L., Y.M. Chen, M.H. Chen, C.Y. Huang, I.S. Sheen, C.T. Yeh, J.H. Huang, G.C. Kuo, and Y.F. Liaw. 1998. Hepatitis C virus variants circumventing cytotoxic T lymphocyte activity as a mechanism of chronicity. Gastroenterology. 115:954–965. [DOI] [PubMed] [Google Scholar]
- 16.Klenerman, P., and R.M. Zinkernagel. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 394:482–485. [DOI] [PubMed] [Google Scholar]
- 17.Tissot, A.C., C. Ciatto, P.R. Mittl, M.G. Grutter, and A. Pluckthun. 2000. Viral escape at the molecular level explained by quantitative T-cell receptor/peptide/MHC interactions and the crystal structure of a peptide/MHC complex. J. Mol. Biol. 302:873–885. [DOI] [PubMed] [Google Scholar]
- 18.Gairin, J.E., and M.B. Oldstone. 1992. Design of high-affinity major histocompatibility complex-specific antagonist peptides that inhibit cytotoxic T-lymphocyte activity: implications for control of viral disease. J. Virol. 66:6755–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciurea, A., L. Hunziker, M.M. Martinic, A. Oxenius, H. Hengartner, and R.M. Zinkernagel. 2001. CD4+ T-cell-epitope escape mutant virus selected in vivo. Nat. Med. 7:795–800. [DOI] [PubMed] [Google Scholar]
- 20.Battegay, M., S. Cooper, A. Althage, J. Baenziger, H. Hengartner, and R.M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods. 33:191–198 (erratum published 35:115 and 38:263). [DOI] [PubMed] [Google Scholar]
- 21.Ciurea A., P. Klenerman, L. Hunziker, E. Horvath, B. Odermatt, A.F. Ochsenbein, H. Hengartner, and R.M. Zinkernagel. 1999. Persistence of lymphocytic choriomeningitis virus at very low levels in immune mice. Proc. Natl. Acad. Sci. USA. 96:11964–11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxenius, A., M.F. Bachmann, R.M. Zinkernagel, and H. Hengartner. 1998. Virus-specific MHC class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400. [DOI] [PubMed] [Google Scholar]
- 23.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for α/β and γ interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehl, S., J. Hombach, P. Aichele, H. Hengartner, and R.M. Zinkernagel. 1997. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J. Exp. Med. 185:1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxenius, A., M.F. Bachmann, D. Mathis, C. Benoist, R.M. Zinkernagel, and H. Hengartner. 1997. Functional in vivo MHC class II loading by endogenously synthesized glycoprotein during viral infection. J. Immunol. 158:5717–5726. [PubMed] [Google Scholar]
- 26.Oxenius, A., R.M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 9:449–457. [DOI] [PubMed] [Google Scholar]
- 27.Ciurea, A., L. Hunziker, P. Klenerman, H. Hengartner, and R.M. Zinkernagel. 2001. Impairment of CD4+ T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 193:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder, D., M.F. van den Broek, D. Kagi, H. Bluethmann, J. Fehr, H. Hengartner, and R.M. Zinkernagel. 1998. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J. Exp. Med. 187:1903–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamperschroer, C., and D.G. Quinn. 2002. The role of proinflammatory cytokines in wasting disease during lymphocytic choriomeningitis virus infection. J. Immunol. 169:340–349. [DOI] [PubMed] [Google Scholar]