The lymphatic vascular system plays important roles in the maintenance of tissue fluid homeostasis, in the mediation of the afferent immune response, and in the metastatic spread of malignant tumors to regional lymph nodes. It consists of a dense network of blind ending, thin-walled lymphatic capillaries and collecting lymphatics that drain extravasated protein-rich fluid from most organs and transport the lymph via the thoracic duct to the venous circulation (1). Originally discovered as “milky veins” by Gasparo Aselli in the 17th century (2), the mechanisms controlling the normal development of lymphatic vessels and the molecular regulation of their biological function have remained poorly understood in contrast to the rapid progress made in elucidating the formation and molecular control of the blood vascular system (3, 4).
100 yr ago, Florence Sabin proposed that the lymphatic system develops by the sprouting of endothelial cells from embryonic veins, leading to the formation of primitive lymph sacs from which lymphatic endothelial cells then sprout into surrounding organs to form mature lymphatic networks (5, 6). Since these pioneering studies, however, the field of lymphatic research has remained rather neglected, mainly due to the lack of molecular tools to specifically detect and functionally characterize the lymphatic endothelium. The recent identification of several new markers for lymphatic endothelial cells and of lymphatic growth factors and receptors, together with the characterization of genetic mouse models with impaired lymphatic development and/or function, has now led to a “rediscovery” of the lymphatic vascular system and has provided important new insights into the molecular mechanisms that control its development and biological function (7). Importantly, these studies have largely confirmed Sabin's original hypothesis regarding lymphatic development in the mammalian system (Fig. 1) .
Figure 1.
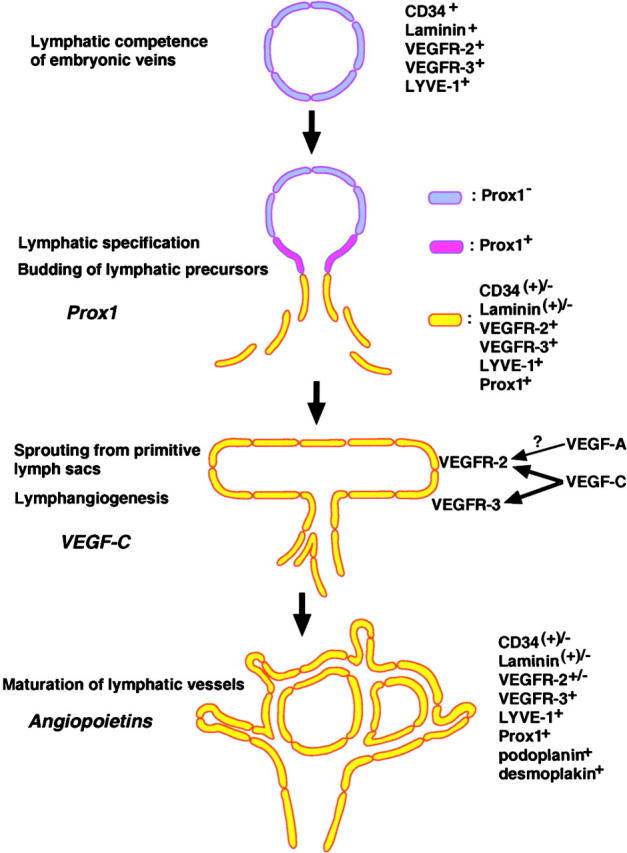
Hypothetical model of the distinct steps involved in the embryonic development of the mammalian lymphatic vasculature.
Recently, Wigle and Oliver (8) and Wigle et al. (9) have identified the first gene that is essential for early lymphatic development. Beginning at E9.5 of mouse development, the homeobox gene Prox1 starts to become specifically expressed in a subpopulation of endothelial cells located on one side of the anterior cardinal vein (8). At this stage, the venous endothelium also expresses the hyaluronan receptor LYVE-1, a CD44 homologue (10), and vascular endothelial cell growth factor receptor (VEGFR)-3, a receptor for the lymphangiogenesis factors vascular endothelial growth factor (VEGF)-C and VEGF-D (11). The expression of both of these receptors later becomes restricted to lymphatic endothelium (Fig. 1; reference 12). This is followed by the polarized budding and migration of Prox1+ lymphatic endothelial progenitor cells (8) that progressively down-regulate the expression of blood vascular genes, such as CD34 and laminin (9), and express increasing levels of lymphatic markers such as VEGFR-3 and secondary lymphoid chemokine (CCL21), a ligand for the chemokine receptor CCR7 (13). Importantly, in Prox1 null mice, the budding and sprouting of lymphatic endothelial cells from the veins is arrested around E11.5-E12.0 and they completely lack a lymphatic vascular system (8). With the reported haploinsufficiency effect of Prox1 during the development of the enteric lymphatic system, these findings reveal an essential role of Prox1 during early lymphatic specification and development (8, 9). The exact mechanisms of action of Prox1 during and after the switch from the blood vascular to the lymphatic phenotype remain to be identified. However, recent studies revealed that ectopical expression of Prox1 in primary human blood vascular endothelial cells was sufficient to up-regulate the expression of the lymphatic endothelial cell markers podoplanin and VEGFR-3, and repress the expression of several genes that have been associated with the blood vascular endothelial cell phenotype (14). These results identify Prox1 as a master control gene in the program specifying lymphatic endothelial cell fate (14).
Recent studies in angiopoietin-2–deficient mice suggest an important role of the angiopoietins and their receptor Tie2 for the final developmental steps of lymphatic network patterning (Fig. 1) and lymphatic vessel maturation (14). However, the molecular mechanisms controlling the sprouting of lymphatic endothelial cells from primitive lymph sacs and their migration into adjacent organs and tissues (lymphangiogenesis) have remained unclear. In this issue, Saaristo et al. (15) identify VEGF-C as a potent inducer of lymphatic sprouting and provide experimental evidence that in addition to VEGFR-3, VEGFR-2 may also be required for this process. Previously, the authors had shown that signaling via VEGFR-3 was sufficient to induce hyperplasia of cutaneous lymphatic vessels because transgenic mice with skin-specific overexpression of a mutated VEGF-C (K14-VEGF-C156S) that selectively activates VEGFR-3 developed lymphatic vessel enlargement in the skin (17). In contrast, wild-type VEGF-C activates both VEGFR-3 and, after proteolytic processing, VEGFR-2.
In the study by Saaristo et al., K14-VEGF-C or K14-VEGF-C156S transgenic mice were crossed with VEGFR-3+/LacZ mice in which one allele of VEGFR-3 had been replaced by the LacZ gene, thereby enabling the visualization of lymphatic vessels by X-gal staining. Importantly, whereas VEGF-C156S overexpression mainly caused the enlargement of preexisting lymphatic capillaries, wild-type VEGF-C induced lymphatic vessel sprouting during embryogenesis (16). Similarly, an increased number of cutaneous lymphatic vessels was detected in adult VEGF-C transgenic mice and in adult mice that were intradermally injected with an adenovirus encoding VEGF-C, whereas chronic transgenic delivery of VEGF-C156S or intradermal injection of a VEGF-C156S–encoding adenovirus predominantly induced lymphatic enlargement. Moreover, only VEGF-C but not VEGF-C156S also induced angiogenesis and vascular hyperpermeability in these studies, most likely via interaction with VEGFR-2 on blood vascular endothelium. These results indicate that VEGF-C, through interaction with both VEGFR-3 and VEGFR-2, plays an important role in lymphangiogenesis, i.e., the sprouting of lymphatics from preexisting vessels. This is similar to the effects of VEGF-A in angiogenesis where it induces sprouting of new blood vessels (18, 19). Future studies in mice deficient for VEGF-C or VEGF-D, a related lymphangiogenesis factor with comparable VEGFR binding properties, should reveal whether the activation of VEGFR-3 and VEGFR-2 is only sufficient, as shown here, or also necessary for the induction of lymphangiogenesis during normal embryonic development. Moreover, additional studies are needed to investigate whether or not mesenchymal lymphatic progenitor cells might contribute to embryonic (or postnatal) lymphangiogenesis, as has been recently proposed for the early wing bud development in birds (20).
In addition to providing new insights into the mechanisms directing lymphatic development, this study by Saaristo et al. raises new questions regarding the molecular control of angiogenesis versus lymphangiogenesis. In this study, VEGFR-2 was implicated in the induction of lymphatic sprouting and strong expression of VEGFR-2 was detected on collecting lymphatic vessels. Therefore, one might expect that VEGF, thus far thought to specifically induce blood vascular angiogenesis (21), might also be able to activate lymphatic vessel sprouting via the activation of VEGFR-2. Indeed, VEGFR-2 is expressed by cultured lymphatic endothelial cells (22, 23) and VEGF equally stimulates lymphatic and blood vascular endothelial growth in vitro (23 and unpublished data). Moreover, intradermal injection of a VEGF165-encoding adenovirus into mouse ears resulting in high levels of VEGF expression, potently induced the formation of new lymphatic vessels that persisted for up to 1 yr (Dvorak, H.F., personal communication). In contrast, cutaneous wound healing is associated with up-regulated expression of VEGF (24) and the formation of a richly vascularized granulation tissue that initially contains no or only a few lymphatic vessels (unpublished data). Is the formation of VEGFR-3 and VEGFR-2 heterodimers needed for the efficient formation of lymphatic vessel sprouts, as suggested by Saaristo et al. (16)? Does VEGF need simultaneous activation/binding of VEGFR-1, most likely not expressed by lymphatic endothelium in vivo (unpublished data) but by cultured lymphatic endothelial cells (22), and of VEGFR-2 to exert its angiogenic effects under pathological conditions, as suggested by recent findings in placenta growth factor–deficient mice (21)? Does VEGF, via its vascular permeability–inducing activity, create a tissue environment that is permissive for blood vascular endothelial proliferation and sprouting, but not for lymphangiogenesis despite the activation of VEGFR-2, possibly due to the differential expression of extracellular matrix receptors by lymphatic endothelium? Do the observed effects of adenoviral VEGF expression on lymphangiogenesis represent a physiological response of lymphatic endothelium to increased tissue fluid accumulation, or are they caused by the induction of VEGF-C expression in vascular endothelium as has been reported (25)? Future in vivo and in vitro studies, including gene expression profiling, are needed to address this unresolved discrepancy.
Impaired formation of lymphatic vessels results in insufficient fluid drainage from tissues, leading to chronic lymphedema that is characterized by edematous swelling of the skin, epithelial hyperplasia, dermal fibrosis, delayed tissue repair, and impaired immune response (1). Recently, missense mutations in the VEGFR-3 gene have been detected in some cases of primary congenital lymphedema (Milroy disease), indicating an important role of VEGF-C and/or VEGF-D in the normal development of the human lymphatic system (26). Consequently, a heterozygous inactivating VEGFR-3 mutation was identified in Chy mutant mice that develop cutaneous lymphedema and chylous ascites after birth and may serve as a convenient mouse model for primary lymphedema (27). Importantly, virus-mediated VEGF-C gene therapy stimulated the growth of functional lymphatics in this model (27), indicating the potential applicability of growth factor gene therapy to at least some cases of human lymphedema. However, adenoviral VEGF-C gene therapy also induced blood vascular enlargement and increased vascular permeability via interaction with VEGFR-2, unwanted side effects in the context of clinical antilymphedema therapy (28). Saaristo et al. (16) now provide evidence that these blood vascular side effects were avoided by viral gene transfer of a VEGFR-3–specific mutant form of VEGF-C (VEGF-C156S) to wild-type and Chy lymphedema mice. Remarkably, the authors detected functional cutaneous lymphatic vessels as confirmed by their ability to transport intradermally injected FITC-dextran even 8 mo after the injection of the VEGF-C156S-adeno–associated virus into the ear skin of Chy mutant mice, whereas no changes of blood vascularity were observed.
These findings have potential implications for the development of novel therapies for human lymphedema, and it will be of interest to see whether the intradermal injection of naked VEGF-C156S plasmid cDNA, as previously described for VEGF treatment of peripheral artery disease (29), or of recombinant VEGF-C156S protein will also be able to specifically induce the formation of functional lymphatics, avoiding potential side effects associated with the in vivo application of adenoviral constructs. However, one has to keep in mind that thus far missense mutations of VEGFR-3 have only been detected in a minority of all patients with congenital lymphedema and additional gene mutations are likely responsible for the majority of these cases. The recent identification of inactivating mutations of the FOXC2 gene in the autosomal-dominant disorder lymphedema-distichiasis (30), together with the detection of lymphedema, chylous ascites, or chylothorax in an increasing number of mutant mouse models such as α9 integrin and angiopoietin-2–deficient mice (15, 31), and the identification of novel lymphatic-specific markers such as Prox1, LYVE-1, and podoplanin (32), suggests the presence of additional disease-specific targets for the future treatment of primary lymphedemas.
Secondary lymphedema is frequently induced by the surgical removal or radiation of lymph nodes in cancer patients, whereas filariasis, a chronic infection with the parasitic worms Brugia malayi or Wuchereria bancrofti, is the leading cause in the developing world. Secondary lymphedema after surgery is associated with the interruption of the normal lymphatic drainage system. Recent studies in an experimental postsurgery lymphedema model, involving the removal of lymphatic vessels from rabbit ears, showed that the injection of VEGF-C protein into the wounded area induced the growth of functional lymphatics along with normalization of the tissue structure (33). Therefore, postsurgical lymphedemas might constitute additional targets for VEGF-C– or VEGF-C165S–based protein or gene therapies. The recent discovery of a direct correlation between experimental tumor-associated lymphangiogenesis and enhanced lymph node metastasis (34–37), however, suggests that future studies are warranted to evaluate whether therapeutic regeneration of lymphatic vessels after lymph node removal might increase the risk for enhanced spread of tumor metastases.
Tumor metastasis to regional lymph nodes represents the first step of tumor dissemination in many common human cancers and serves as a major prognostic indicator for the progression of the disease. In contrast to the extensive molecular and functional characterization of tumor angiogenesis (38), i.e., the induction of new blood vessel growth, little is known about the mechanisms through which tumor cells gain entry into the lymphatic system. A widely held view has suggested that lymphatic endothelium only plays a passive role during this process (38) and lymphatic invasion only occurs once stroma-infiltrating tumor cells happen upon preexisting peritumoral lymphatic vessels (Fig. 2 A). However, the recent identification of lymphatic growth factors and receptors, together with the discovery of lymphatic-specific markers and the development of orthotopic cancer metastasis models, have provided important new insights into the formation of tumor-associated lymphatic vessels (7) and their active contribution to lymphatic tumor spread (Fig. 2 B). An increasing number of clinicopathological studies have shown a direct correlation between tumor expression of the lymphangiogenesis factors VEGF-C or VEGF-D and metastatic tumor spread in many human cancers, including cancers of the breast, lung, prostate, cervix, and colon (for review see reference 39), providing circumstantial evidence for the involvement of lymphangiogenesis in tumor progression.
Figure 2.
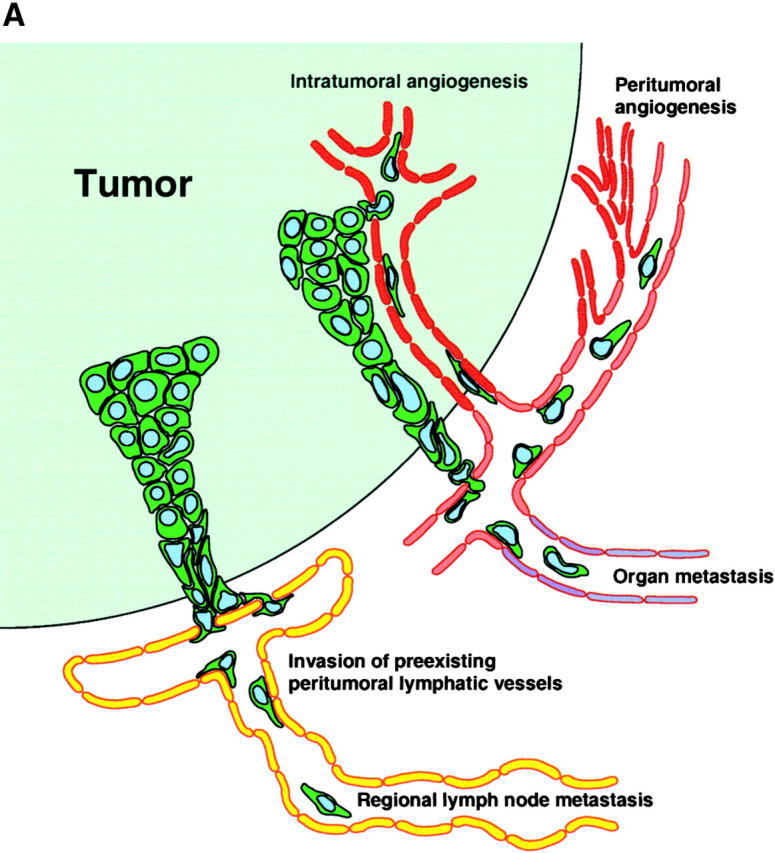
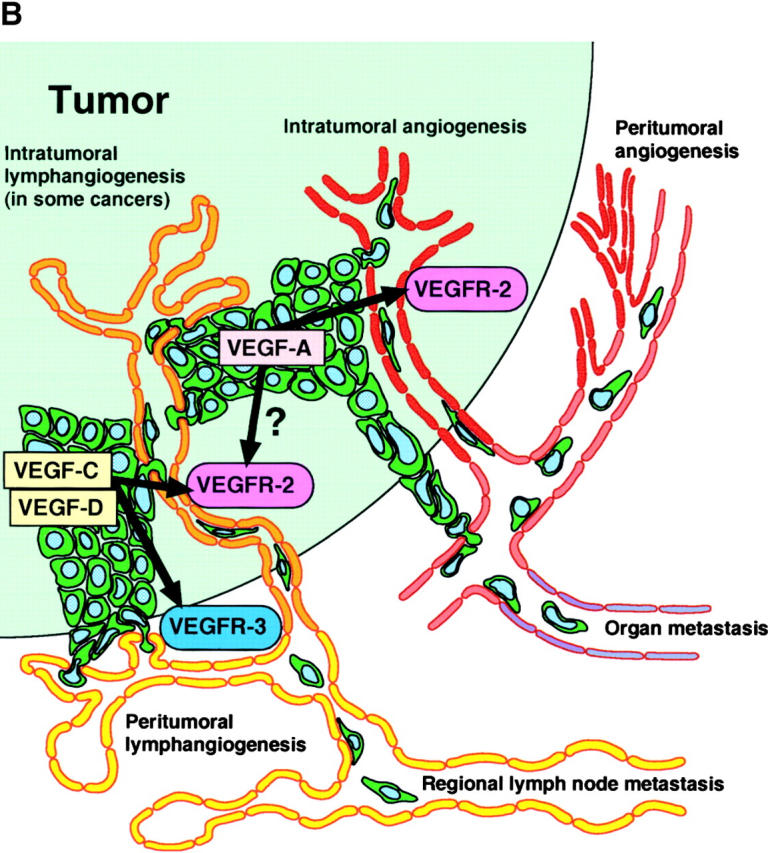
(A) Traditional model of tumor metastasis via lymphatic and blood vessels. (B) Active lymphangiogenesis model of tumor metastasis.
Several studies in animal tumor models have now provided direct experimental evidence that increased levels of VEGF-C or VEGF-D promote tumor lymphangiogenesis and lymphatic tumor spread to regional lymph nodes and that these effects can be suppressed by blocking VEGFR-3 signaling (34–37, 40–42). Most of these studies used an antibody against the hyaluronan receptor LYVE-1, a lymphatic-specific CD44 homologue (10, 43), to identify and quantify tumor-associated lymphatic vessels. Although some LYVE-1 expression has been detected in liver sinusoidal endothelial cells that are involved in hyaluronan uptake (44), recent studies applying the combined immunostains of a variety of experimental tumors with antibodies to LYVE-1 and the lymphatic-specific transcription factor Prox1 found that all LYVE-1+ tumor-associated lymphatic vessels also expressed Prox1 (7, 9, 45), confirming the specificity of LYVE-1 expression for lymphatic endothelium.
Despite the accumulated evidence for an active role of VEGF-C– or VEGF-D–induced tumor lymphangiogenesis in cancer metastasis to regional lymph nodes, the existence and biological function of lymphatics within experimental and human tumors has remained controversial. High interstitial pressure within tumors has been proposed to prevent intratumoral lymphatic vessel growth and function as assessed by the lack of lymphatic uptake of tracers that were injected in the vicinity of experimental tumors (46, 47). However, the mechanisms controlling metastatic tumor cell invasion and transport within lymphatic vessels are most likely distinct from those involved in fluid uptake and transport. Indeed, proliferating intratumoral lymphatic vessels have been detected in rapidly progressing tumor xenotransplants and in slowly growing, chemically induced orthotopic squamous cell carcinomas in mice and is associated with lymphatic metastasis (7, 9, 34, 45). Proliferating intratumoral lymphatics have also been found in human head and neck squamous cell carcinomas that were characterized by the correlation of the density of LYVE-1+ tumor-associated lymphatic vessels with the presence of regional lymph node metastasis (48). In contrast, no evidence for tumor lymphangiogenesis was found in invasive breast cancer by the same group of investigators (49). Taken together, these results indicate that active tumor-associated lymphangiogenesis induced by VEGF-C, VEGF-D, or other not yet identified growth factors leads to the proliferation and enlargement of peritumoral and, in some cancers, intratumoral lymphatic vessels, likely enhancing the metastatic spread of many different types of human tumors (Fig. 2 B).
Although the mere increase of lymphatic vessel surface area might simply increase the chance for tumor cell invasion and metastasis, lymphatic endothelial cells probably also play an active role in the chemotactic recruitment and intralymphatic transport of tumor cells. Lymphatic endothelium secretes chemokines such as CCL21 (secondary lymphoid chemokine) that binds to CCR7 (13, 22, 50), leading to chemoattraction and migration of mature dendritic cells from the skin to regional lymph nodes. CCR7 and other chemokine receptors are also expressed by some human cancer cell lines including malignant melanomas and breast cancer cells (51). Importantly, the overexpression of CCR7 in B16 malignant melanoma cells led to a >10-fold increase in the incidence of regional lymph node metastases after injection into the footpad of mice, and treatment with CCL21-blocking antibodies completely prevented metastatic tumor spread to lymph nodes (52). These findings indicate that some tumors might take advantage of preexisting molecular mechanisms designed for the physiological immune response to further their metastatic spread.
After several decades of slow progress, the study of lymphatic vessel formation and its role in malignant disease has now led to the identification of several molecular mechanisms involved in the formation and biological function of lymphatic vessels. Although much has still to be learned about the detailed steps of normal and pathological lymph vessel formation, new targets for innovative therapeutic approaches and new tools for the prognostic evaluation of human cancers are now emerging.
References
- 1.Witte, M.H., M.J. Bernas, C.P. Martin, and C.L. Witte. 2001. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 55:122–145. [DOI] [PubMed] [Google Scholar]
- 2.Asellius, G. 1627. De lactibus sive lacteis venis. Mediolani, Milan.
- 3.Gale, N.W., and G.D. Yancopoulos. 1999. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 13:1055–1066. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet, P. 2000. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6:389–395. [DOI] [PubMed] [Google Scholar]
- 5.Sabin, F.R. 1902. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1:367–391. [Google Scholar]
- 6.Sabin, F.R. 1904. On the development of the superficial lymphatics in the skin of the pig. Am. J. Anat. 3:183–195. [Google Scholar]
- 7.Oliver, G., and M. Detmar. 2002. The rediscovery of the lymphatic system. Old and new insights into the development and biological function of lymphatic vascular system. Genes Dev. 16:773–783. [DOI] [PubMed] [Google Scholar]
- 8.Wigle, J.T., and G. Oliver. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell. 98:769–778. [DOI] [PubMed] [Google Scholar]
- 9.Wigle, J.T., N. Harvey, M. Detmar, I. Lagutina, G. Grosveld, M.D. Gunn, D.G. Jackson, and G. Oliver. 2002. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerji, S., J. Ni, S.X. Wang, S. Clasper, J. Su, R. Tammi, M. Jones, and D.G. Jackson. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alitalo, K., and P. Carmeliet. 2002. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 1:219–227. [DOI] [PubMed] [Google Scholar]
- 12.Kaipainen, A., J. Korhonen, T. Mustonen, V.W. van Hinsbergh, G.H. Fang, D. Dumont, M. Breitman, and K. Alitalo. 1995. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA. 92:3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn, M.D., K. Tangemann, C. Tam, J.G. Cyster, S.D. Rosen, and L.T. Williams. 1998. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 95:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong, Y.-K., N. Harvey, Y.-H. Noh, V. Schacht, S. Hirakawa, M. Detmar, and G. Oliver. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. In press. [DOI] [PubMed] [Google Scholar]
- 15.Gale, N.W., G. Thurston, S.F. Hackett, R. Renard, Q. Wang, C. McClain, C. Martin, C. Witte, M.H. Witte, D. Jackson, et al. 2002. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell. In press. [DOI] [PubMed] [Google Scholar]
- 16.Saaristo, A., T. Veikkola, T. Tammela, B. Enholm, M.J. Karkkainen, K. Pajusola, H. Bueler, S. Yla-Herttuala, and K. Alitalo. 2002. Lymphangiogenic gene therapy with minimal blood vascular side-effects. J. Exp. Med. 196:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veikkola, T., L. Jussila, T. Makinen, T. Karpanen, M. Jeltsch, T.V. Petrova, H. Kubo, G. Thurston, D.M. McDonald, M.G. Achen, et al. 2001. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara, N. 1999. Vascular endothelial growth factor: molecular and biological aspects. Curr. Top. Microbiol. Immunol. 237:1–30. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak, H.F., J.A. Nagy, D. Feng, L.F. Brown, and A.M. Dvorak. 1999. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 237:97–132. [DOI] [PubMed] [Google Scholar]
- 20.Schneider, M., K. Othman-Hassan, B. Christ, and J. Wilting. 1999. Lymphangioblasts in the avian wing bud. Dev. Dyn. 216:311–319. [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet, P., L. Moons, A. Luttun, V. Vincenti, V. Compernolle, M. De Mol, Y. Wu, F. Bono, L. Devy, H. Beck, et al. 2001. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7:575–583. [DOI] [PubMed] [Google Scholar]
- 22.Kriehuber, E., G.S. Breiteneder, M. Groeger, A. Soleiman, S.F. Schoppmann, G. Stingl, D. Kerjaschki, and D. Maurer. 2001. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 194:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maekinen, T., T. Veikkola, S. Mustjoki, T. Karpanen, B. Catimel, E.C. Nice, L. Wise, A. Mercer, H. Kowalski, D. Kerjaschki, et al. 2001. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20:4762–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown, L.F., K.T. Yeo, B. Berse, T.K. Yeo, D.R. Senger, H.F. Dvorak, and L. Van De Water. 1992. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 176:1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skobe, M., and M. Detmar. 2000. Structure, function and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 5:14–19. [DOI] [PubMed] [Google Scholar]
- 26.Karkkainen, M.J., R.E. Ferrell, E.C. Lawrence, M.A. Kimak, K.L. Levinson, M.A. McTigue, K. Alitalo, and D.N. Finegold. 2000. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25:153–159. [DOI] [PubMed] [Google Scholar]
- 27.Karkkainen, M.J., A. Saaristo, L. Jussila, K.A. Karila, E.C. Lawrence, K. Pajusola, H. Bueler, A. Eichmann, R. Kauppinen, M.I. Kettunen, et al. 2001. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA. 98:12677–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saaristo, A., T. Veikkola, B. Enholm, M. Hytonen, J. Arola, K. Pajusola, P. Turunen, M. Jeltsch, M.J. Karkkainen, D. Kerjaschki, et al. 2002. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J. 16:1041–1049. [DOI] [PubMed] [Google Scholar]
- 29.Isner, J.M., K. Walsh, J. Symes, A. Pieczek, S. Takeshita, J. Lowry, K. Rosenfield, L. Weir, E. Brogi, and D. Jurayj. 1996. Arterial gene transfer for therapeutic angiogenesis in patients with peripheral artery disease. Hum. Gene Ther. 7:959–988. [DOI] [PubMed] [Google Scholar]
- 30.Fang, J., S.L. Dagenais, R.P. Erickson, M.F. Arlt, M.W. Glynn, J.L. Gorski, L.H. Seaver, and T.W. Glover. 2000. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 67:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, X.Z., J.F. Wu, R. Ferrando, J.H. Lee, Y.L. Wang, R.V. Farese, Jr., and D. Sheppard. 2000. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol. Cell. Biol. 20:5208–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleeman, J.P., J. Krishnan, V. Kirkin, and P. Baumann. 2001. Markers for the lymphatic endothelium: in search of the Holy Grail? Microsc. Res. Tech. 55:61–69. [DOI] [PubMed] [Google Scholar]
- 33.Szuba, A., M. Skobe, M.J. Karkkainen, W.S. Shin, D.P. Beynet, N.B. Rockson, N. Dakhil, S. Spilman, M.L. Goris, H.W. Strauss, et al. 2002. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. In press. [DOI] [PubMed] [Google Scholar]
- 34.Skobe, M., T. Hawighorst, D.G. Jackson, R. Prevo, L. Janes, P. Velasco, L. Riccardi, K. Alitalo, K. Claffey, and M. Detmar. 2001. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7:192–198. [DOI] [PubMed] [Google Scholar]
- 35.Stacker, S.A., C. Caesar, M.E. Baldwin, G.E. Thornton, R.A. Williams, R. Prevo, D.G. Jackson, S. Nishikawa, H. Kubo, and M.G. Achen. 2001. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 7:186–191. [DOI] [PubMed] [Google Scholar]
- 36.Mandriota, S.J., L. Jussila, M. Jeltsch, A. Compagni, D. Baetens, R. Prevo, S. Banerji, J. Huarte, R. Montesano, D.G. Jackson, et al. 2001. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karpanen, T., M. Egeblad, M.J. Karkkainen, H. Kubo, S. Yla-Herttuala, M. Jaattela, and K. Alitalo. 2001. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61:1786–1790. [PubMed] [Google Scholar]
- 38.Carmeliet, P., and R.K. Jain. 2000. Angiogenesis in cancer and other diseases. Nature. 407:249–257. [DOI] [PubMed] [Google Scholar]
- 39.Stacker, S.A., M.E. Baldwin, and M.G. Achen. 2002. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 16:922–934. [DOI] [PubMed] [Google Scholar]
- 40.Skobe, M., L.M. Hamberg, T. Hawighorst, M. Schirner, G.L. Wolf, K. Alitalo, and M. Detmar. 2001. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 159:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattila, M.M., J.K. Ruohola, T. Karpanen, D.G. Jackson, K. Alitalo, and P.L. Harkonen. 2002. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int. J. Cancer. 98:946–951. [DOI] [PubMed] [Google Scholar]
- 42.He, Y., K. Kozaki, T. Karpanen, K. Koshikawa, S. Yla-Herttuala, T. Takahashi, and K. Alitalo. 2002. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 94:819–825. [DOI] [PubMed] [Google Scholar]
- 43.Prevo, R., S. Banerji, D.J. Ferguson, S. Clasper, and D.G. Jackson. 2001. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276:19420–19430. [DOI] [PubMed] [Google Scholar]
- 44.Carreira, C.M., S.M. Nasser, E. di Tomaso, T.P. Padera, Y. Boucher, S.I. Tomarev, and R.K. Jain. 2001. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 61:8079–8084. [PubMed] [Google Scholar]
- 45.Hawighorst, T., H. Oura, M. Streit, L. Janes, L. Nguyen, L.F. Brown, D.G. Jackson, and M. Detmar. 2002. Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene. In press. [DOI] [PubMed] [Google Scholar]
- 46.Leu, A.J., D.A. Berk, A. Lymboussaki, K. Alitalo, and R.K. Jain. 2000. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 60:4324–4327. [PubMed] [Google Scholar]
- 47.Padera, T.P., A. Kadambi, E. di Tomaso, C.M. Carreira, E.B. Brown, Y. Boucher, N.C. Choi, D. Mathisen, J. Wain, E.J. Mark, et al. 2002. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 296:1883–1886. [DOI] [PubMed] [Google Scholar]
- 48.Beasley, N.J.P., R. Prevo, S. Banerji, R. Leek, J. Moore, P. van Trappen, G. Cox, A.L. Harris, and D.G. Jackson. 2002. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 62:1315–1320. [PubMed] [Google Scholar]
- 49.Jackson, D.G., R. Prevo, S. Clasper, and S. Banerji. 2001. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 22:317–321. [DOI] [PubMed] [Google Scholar]
- 50.Saeki, H., A.M. Moore, M.J. Brown, and S.T. Hwang. 1999. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162:2472–2475. [PubMed] [Google Scholar]
- 51.Muller, A., B. Homey, H. Soto, N. Ge, D. Cattron, M.E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, and S.N. Wagner. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature. 410:50–56. [DOI] [PubMed] [Google Scholar]
- 52.Wiley, H.E., E.B. Gonzalez, W. Maki, M.M. Wu, and S.T. Hwang. 2001. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 93:1638–1643. [DOI] [PubMed] [Google Scholar]


