Abstract
Mice lacking the p110δ catalytic subunit of phosphatidylinositol 3-kinase have reduced numbers of B1 and marginal zone B cells, reduced levels of serum immunoglobulins, respond poorly to immunization with type II thymus-independent antigen, and are defective in their primary and secondary responses to thymus-dependent antigen. p110δ−/− B cells proliferate poorly in response to B cell receptor (BCR) or CD40 signals in vitro, fail to activate protein kinase B, and are prone to apoptosis. p110δ function is required for BCR-mediated calcium flux, activation of phosphlipaseCγ2, and Bruton's tyrosine kinase. Thus, p110δ plays a critical role in B cell homeostasis and function.
Keywords: Akt, Btk, calcium, gene targeting, p110δ
Introduction
B lymphocyte development, selection, and activation are critically dependent on signal transduction events mediated by the B cell antigen receptor (BCR)* (1). The BCR is tightly associated with nonpolymorphic subunits, CD79a and b, which contain immunoreceptor tyrosine–based activation motifs. Immunoreceptor tyrosine–based activation motifs serve to recruit tyrosine kinases of the Src and Syk families that initiate the signal transduction cascade by phosphorylation of multiple substrate proteins (2).
A number of studies have provided evidence for a role for phosphatidylinositol 3-kinase (PI3-K) and the second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP-3) in BCR signal transduction. PI3-K is activated downstream of the BCR for antigen (3, 4) and the CD19 coreceptor (5, 6). The class IA PI3-K's consist of three catalytic subunits p110α, β, and δ that are encoded by distinct genes and interact with a family of adaptor proteins that regulate location and enzyme activity (7). Mice deficient in the p85α or p85/p55/p50α adaptor proteins (8, 9) of PI3-K display an immune defect similar to xid mice in which the Pleckstrin homology domain of Bruton's tyrosine kinase (Btk) can no longer bind PIP-3 (10). Btk, a member of the TEC family of tyrosine kinases, is thought to be a major effector of PI3-K in B cells. Btk acts, in part, to regulate calcium flux through the phosphorylation and activation of phospholipaseCγ2 (PLCγ2; reference 11). The regulation of Btk membrane association and activation by phosphoinositides plays an important role in regulating B cell responses (12).
B cells from mice deficient in the breakdown of PIP-3, such as those lacking the SH2 domain-containing inositol polyphosphate 5′-phosphatase 1, display accelerated development and are hyperresponsive to BCR stimulation (13–15). Furthermore, mice deficient in the phosphatase and tensin homolog gene that encodes a PIP-3 3′ phosphatase develop B cell hyperplasia, lymphoma, and hypergammaglobulinaemia (16).
Each of the three class IA catalytic subunits of PI3-K are expressed in B cells, however their relative roles in B cell development and function are unknown. Mutant mice lacking p110α (17) and p110β (18) have previously been reported to have lethal phenotypes that precluded analysis of immune cell development and function. p110δ is the most recently identified PI3-K catalytic subunit with expression reported to be highest in hematopoietic cells (19, 20). We have used gene-targeting to generate mice that lack p110δ function. Our analysis of B cell development in these mice revealed an essential role for p110δ in the development of B1 and marginal zone (MZ) B cells. Furthermore, B cell responses to thymus-dependent and -independent antigens required p110δ function. Analysis of BCR signal transduction revealed an important role for p110δ in the regulation of proliferation and calcium flux. These defects can be attributed to a failure to activate protein kinase B (PKB) and Btk.
Materials and Methods
Generation of p110δ Knockout Mice.
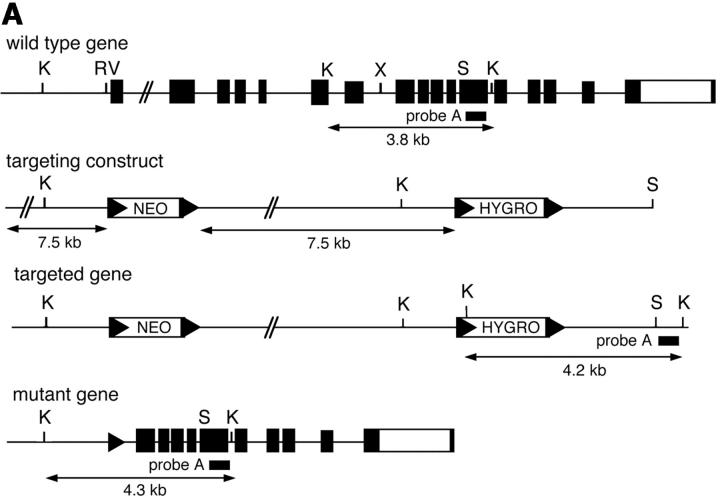
The structure of murine pik3cd genomic clones isolated from a 129/Sv genomic library has been described previously (21). The targeting vector consists of LoxP flanked neomycin and hygromycin-resistance cassettes cloned 7.5 kb apart into the EcoRV and XhoI sites respectively of the pik3cd genomic clone. This strategy was adopted in an attempt to generate a conditional allele of pik3cd. The targeting vector was transfected into PC3 mouse embryonic stem cells (22) and analyzed for the targeting event using Southern blotting of KpnI-digested DNA with probe A (a 700-bp EcoRI fragment). Correctly targeted clones were injected into blastocysts to produce chimaeric mice. The resulting chimaeras, which express the Cre enzyme in the male germline (22), were bred to obtain lines of mice harboring a pik3cd gene which had undergone Cre-mediated recombination and thus deleted exons 1–9 encoding the first 490 amino acids of p110δ. These were detected by Southern blot analysis using KpnI-digested DNA and by PCR. All mice were bred at the Babraham Institute Small Animal Barrier Unit (SABU) and housed according to UK Home Office guidelines under project licence 80/1263. p110δ-deficient mice were born in normal Mendelian ratios from heterozygous intercrosses and were fertile and healthy under SPF conditions.
Measurement of PIP-3 Levels.
Lipid extracts were assayed from stimulated B cells after precipitation with 0.5 M trichloroacetic acid. A time resolved fluorescence resonance energy transfer ligand displacement assay was performed using the general receptor for phosphoinositides-1 Pleckstrin homology domain as a PIP-3–specific binding protein (23) (unpublished data).
Immunoprecipitation and Western Blot Analysis.
Purification of splenic B cells, immunoprecipitation and Western blotting were performed using previously described methods (24). B cell purity was around 95% as assessed by flow cytometry of lymphocytes (unpublished data). Antibodies to Btk provided by V. Tybulewicz (National Institute for Medical Research, London, UK), antisera against, amino acids 74–89 of murine p110δ and against the COOH-terminal 20 amino acids of murine PLCγ2 were from (Babraham Technix), anti–Vav-1 (24), phospho-Btk (25), have been described previously. Phospho-IκBα, phospho-PKB, and pan-PKB were from Cell Signaling Technology, p110α (H-201), p110β (S-19) IkBα (C-21) were from Santa Cruz Biotechnology, Inc., anti–Bcl-XL was from BD Transduction Laboratories, anti-p85α was from Upstate Biotechnology, anti-PLCγ2 PY 759 antibody will be described previously (unpublished data).
Immunofluorescence Staining of Tissue Sections.
Spleens were harvested and immediately frozen by dipping in liquid nitrogen. Spleens were mounted in OCT and 8-μm sections were cut, air-dried, and stored at –20°C until use. Sections were fixed in ice-cold acetone for 15 min, air-dried briefly, rehydrated in PBS and then blocked with 5% normal rat serum for 15 min. Sections were stained with FITC-conjugated MOMA-1 antibody (rat IgG2a; Serotec), and biotinylated anti-IgM (clone R6–60.2, rat IgG2a; BD BioSciences), or control FITC-conjugated rat IgG2a (BD BioSciences) for 1 h at room temperature. Sections were washed in PBS and stained with a 1:200 dilution of streptavidin-TRITC (Jackson ImmunoResearch Laboratories) for 1 h. Sections were washed and mounted in Aqua PolyMount (Polysciences, Inc.) and viewed with an Olympus BX-40 epifluorescence microscope using appropriate filters. Images were digitally captured using a high-resolution CCD camera (F-View) using analySIS® software (SIS, GmbH) and processed using Adobe Photoshop® v.7.0.
Antibody Responses.
Staining of cells with fluorescent antibodies was performed as described previously (24). All antibodies were purchased from BD PharMingen except anti–IgM-Cy5 and anti-IgD-PE (Jackson ImmunoResearch Laboratories). Serum Igs in naive mice were determined by ELISA using antibodies purchased from BD PharMingen. For immune responses, 8-wk-old mice were injected intraperitoneally with either 5 μg DNP-Ficoll in a solution of PBS or 50 μg DNP conjugated to KLH in a solution of PBS. Serum antibody levels were determined by ELISA as described previously (24). The relative units of DNP-specific antibodies are shown as optical density values. A dilution series of the serum samples was measured and for each isotype a single dilution factor which fell in the linear part of the curve is represented for all time points. Serum dilution factors were as follows: thymus-independent responses 1:800 for IgM and 1:800 for IgG3. For thymus-dependent responses, the dilutions were 1:800 for IgM, IgG2b, and IgG3, 1:1,600 for IgG1, and 1:200 for IgG2a.
B Cell Proliferation and Apoptosis Assays.
Purified B cells were cultured for 72 h at an initial concentration of 106 cells per milliliter with the indicated doses of polyclonal anti-IgM or monoclonal anti-IgM (clone B7.6), monoclonal anti-CD40 (clone 3/23) and recombinant murine IL-4 in RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 × 10−5 M 2-mercaptoethanol. Proliferation was measured by incorporation of [3H]thymidine following a 16-h pulse. For analysis of apoptosis, B cells were cultured in the above media but without antibodies or IL-4 and apoptosis determined by flow cytometry of permeabilized cells stained with propidium iodide as described previously (26).
Calcium Flux Analysis.
Purified splenic B cells were loaded for 30 min at RT in the dark with 3 μM Fluo-4 a.m. (Molecular Probes) at a density of 6 × 106 cells per milliliter in 0.5% BSA/PBS. The cells were washed in indicator free medium and then resuspended at 3 × 106 cells per milliliter in 0.5% BSA/PBS containing 1 mM CaCl2. After a further incubation of 30 min to allow complete deesterification of intracellular Fluo-4 a.m. ester, the variations in absorbance were measured using a Perkin-Elmer LS55 Luminescence Spectrometer. [Ca2+]i was calculated as described previously (27).
Results
Impaired BCR Stimulated PIP-3 Production in the Absence of p110δ.
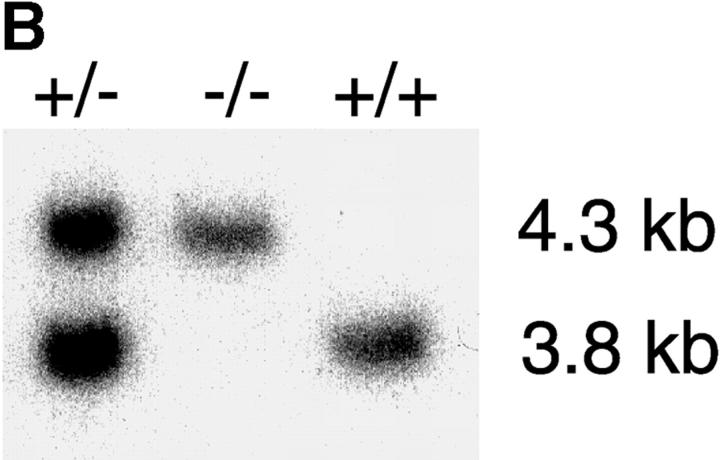
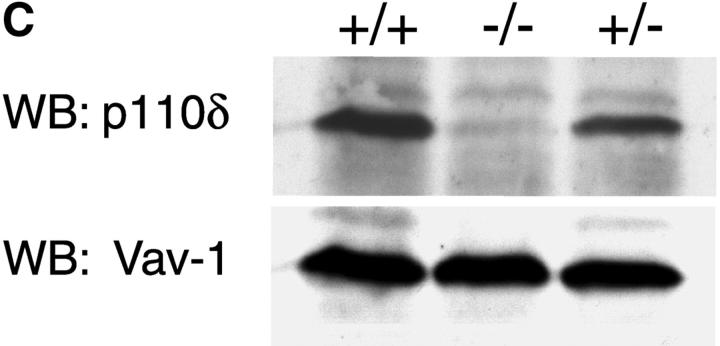
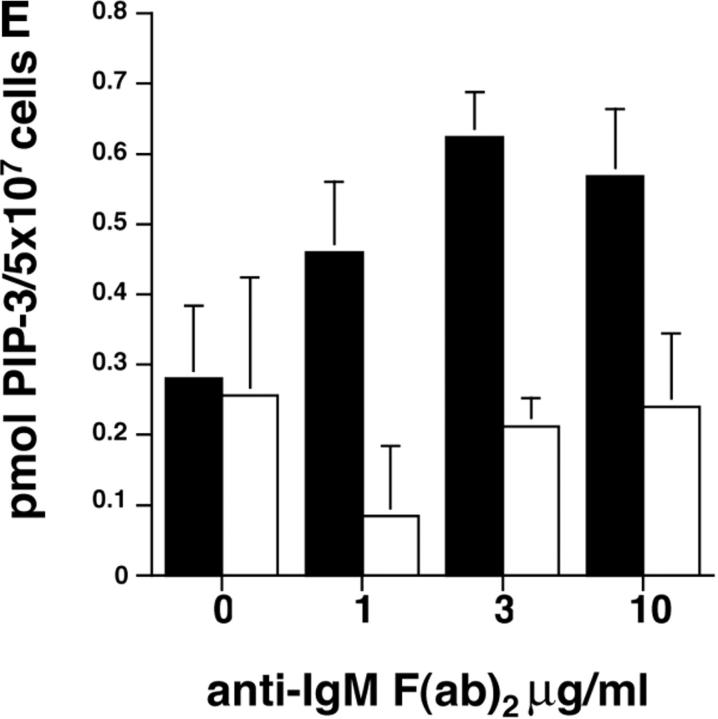
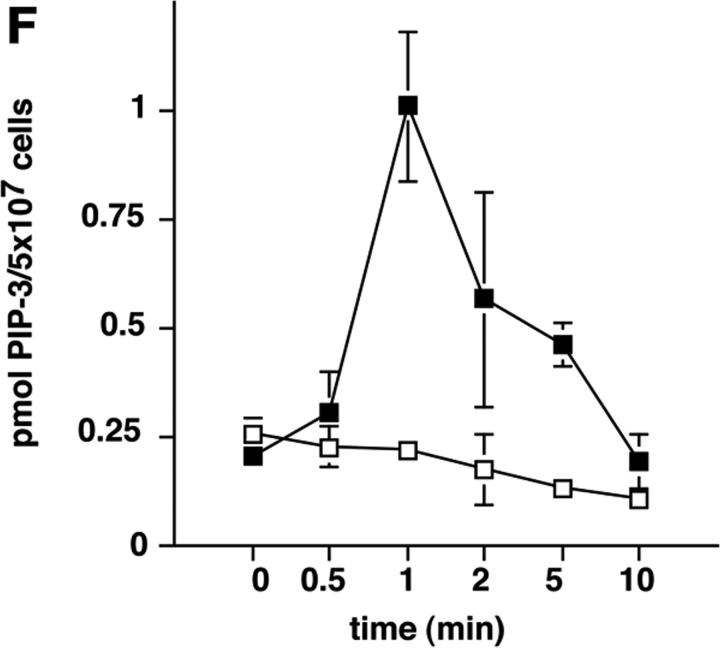
To address the function of p110δ in B cells, we used gene targeting to produce p110δ null mice (Fig. 1, A and B) . Thymocytes and B cells from these mice lacked p110δ protein (Fig. 1 C and unpublished data). B cells from control and mutant mice expressed similar levels of p110α and p110β catalytic subunits, but showed a small reduction in the levels of the p85α and P55/p50α adaptor subunits (Fig. 1 D). To determine the impact of this mutation on BCR-induced PIP-3 production we employed a novel assay that permits determination of PIP-3 levels in primary cells, without the need for biosynthetic labeling. We observed that BCR-stimulated B cells from p110δ-deficient mice produced little PIP-3 (Fig. 1, E and F). In control B cells, PIP-3 levels peaked after 1 min of BCR stimulation and returned to baseline by 10 min, in mutant mice no increases in PIP-3 levels were observed within 10 min of stimulation (Fig. 1 F). These data indicate p110δ was responsible for most of the BCR-induced PIP-3 production.
Figure 1.
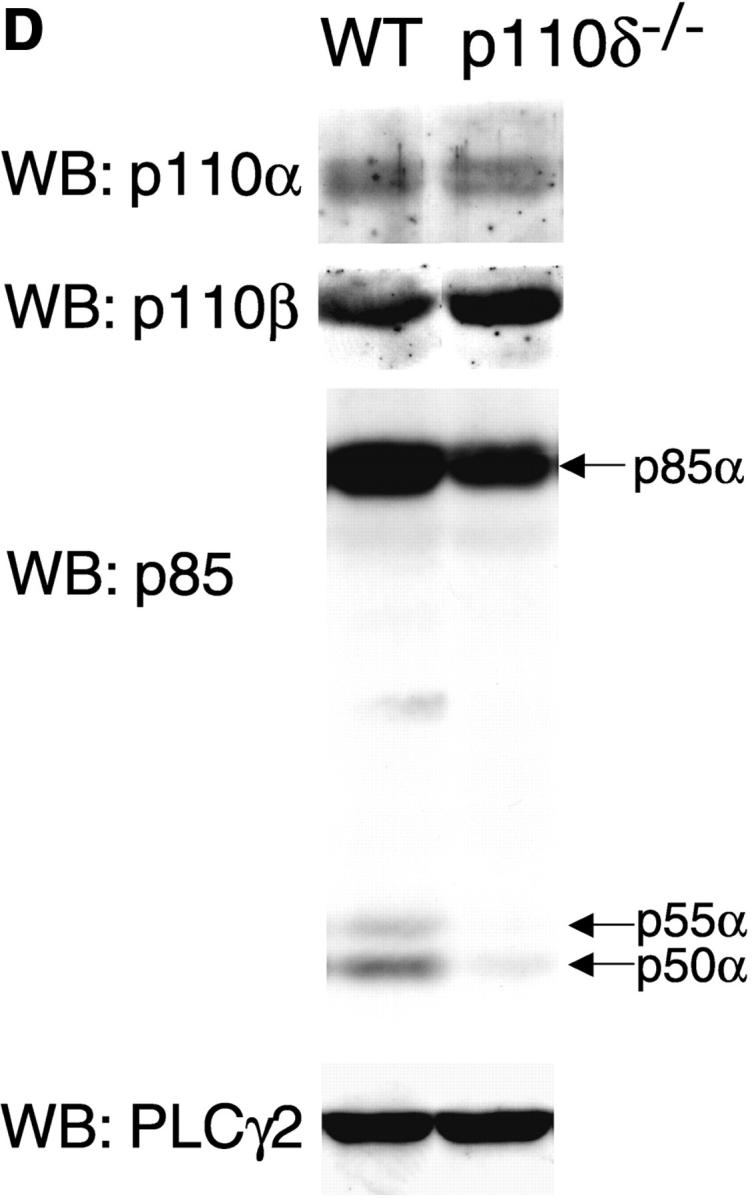
p110δ mutant B cells fail to produce PIP-3. (A) Gene targeting strategy. (B) Southern blot analysis of tail DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mutant mice using probe A. (C) Western blot analysis of wild-type, heterozygous, and homozygous mutant thymocytes using antibody against p110δ. The blot was reprobed with anti–Vav-1 to demonstrate equal protein loading. (D) Western blot analysis of wild-type and homozygous mutant B-lymphocytes for levels of p110α, p110β, the p85/55/50α subunits of PI3-K, and PLCγ2. (E) BCR stimulated PIP-3 production in wild-type and mutant B cells. Wild-type is represented by black bars, mutant by white bars. (F) Time course of PIP-3 production in wild-type and mutant B cells stimulated with 10 μg/ml anti-IgM F(ab)2. In E and F, error bars represent the variance of triplicate determinations.
B1 and MZ B Cells Require p110δ..
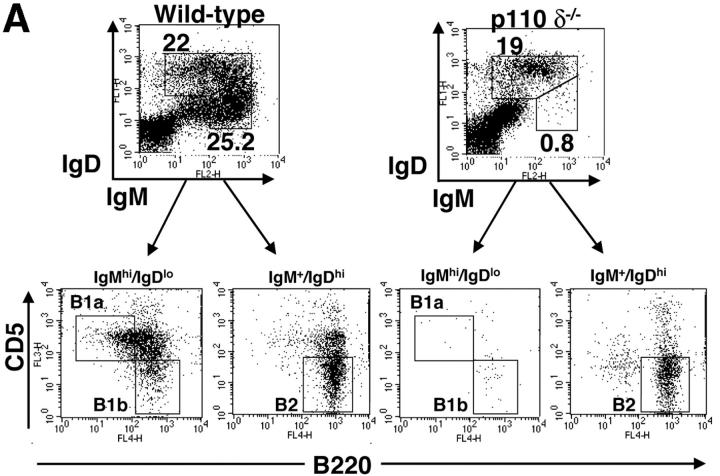
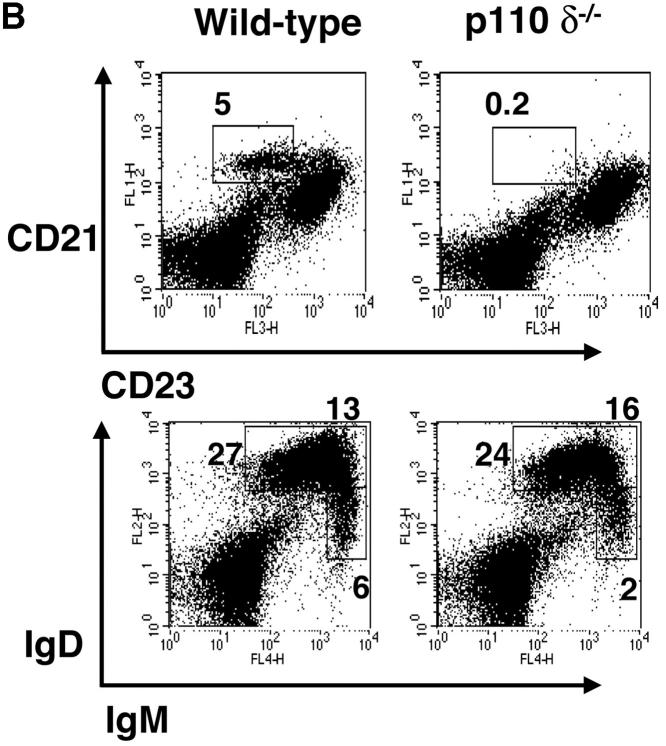
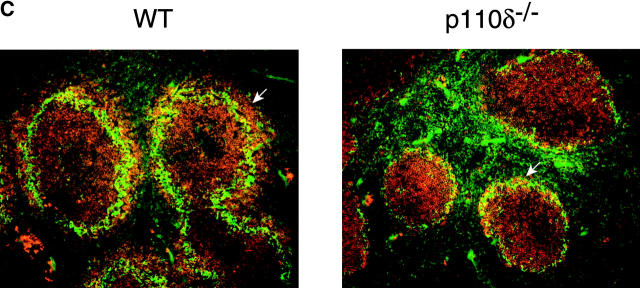
Flow cytometric analysis of the bone marrow of p110δ−/− mice did not reveal any major blocks in B cell development (Table I). However, we observed a marked reduction in the B1 subset resident in the peritoneal cavity (Fig. 2 A and Table I). Furthermore, CD21hi CD23lo MZ B cells of the spleen were also significantly reduced in number (Fig. 2 B and Table I). This conclusion was further supported by noting the absence of the splenic B220+ CD1high population in the mutants (unpublished data). Examination of frozen splenic tissue sections stained with anti-IgM and anti–MOMA-1 (which stains MZ metallophilic-macrophages), revealed that in the p110δ mutant there were very few B cells beyond the marginal sinus (delineated by MOMA-1 staining), supporting the conclusion that the MZ B cell population was severely reduced. The number of conventional B2 B cells was significantly reduced in young p110δ−/− mice (Table I). By contrast, older p110δ−/− mice had only marginally reduced numbers of B2 cells (Table I). Subdivision of splenic B cells using IgM and IgD staining revealed that p110δ−/− mice had near normal numbers of IgMlo IgDhi B cells, which are mature recirculating follicular (RF) B cells (Fig. 2 B and Table I). The level of surface IgM staining on follicular B cells was not different between control and mutants mice (Fig. 2 B). Thymocyte development, and the number of CD4+, CD8+ T cells, and Mac1+ macrophages in the spleens of P110δ−/− mice were not different from littermate controls (Table I and unpublished data).
Table I.
Lymphocyte Populations in p110δ−/− Mice
| Tissue/cell type | Control | p110δ−/− |
|---|---|---|
| Bone Marrowa | ||
| Fraction A–C | 8.6 × 105 (2.7) | 7.5 × 105 (1.5) |
| Fraction D | 4.1 × 106 (1.3) | 3.1 × 106 (0.8) |
| Fraction E | 9.6 × 105 (4.0) | 7.2 × 105 (1.4) |
| Fraction F | 6.6 × 105 (2.4) | 9.6 × 105 (4.2) |
| Spleen (8–10 wk old) | ||
| Large Mac1+ | 1.5 × 106 (0.2) | 1.2 × 106 (0.3) |
| CD4+ | 2.5 × 107 (0.5) | 2.5 × 107 (0.9) |
| CD8+ | 1.3 × 107 (0.3) | 1.1 × 107 (0.4) |
| CD21hi CD23lo | 3.7 × 106 (0.8) | 0.6 × 106 (0.05) b |
| B220+ | 4.4 × 107 (1.3) | 3.1 × 107 (0.7) |
| IgMhi IgDlo | 3.6 × 106 (1.1) | 2.6 × 106 (0.8) |
| IgMhi IgDhi | 1.1 × 107 (0.3) | 1.1 × 107 (0.2) |
| IgMlo IgDhi | 2.6 × 107 (0.8) | 1.6 × 107 (0.4) |
| Spleen (21-d old) | ||
| IgMhi IgDlo | 2.7 × 106 (0.8) | 106 (0.1) c |
| IgMhi IgDhi | 9 × 106 (1.4) | 2.2 × 106 (0.4) c |
| IgMlo IgDhi | 3.2 × 106 (0.2) | 0.8 × 106 (0.2) d |
| Peritoneum | ||
| B1 total | 106 (0.2) | 0.08 × 106 (0.02) c |
| B1a | 2.4 × 105 (0.2) | 0.1 × 105 (0.02) c |
| B1b | 1.9 × 105 (0.3) | 0.07 × 105 (0.01) c |
| B2 | 5.7 × 105 (2.9) | 4.9 × 105 (3.6) |
Values are given as mean with SD in parenthesis. n = 9 for determination of B cell numbers in the spleen of adult mice; n = 4 for all other determinations, except the mutant peritoneum where n = 3.
Fractions assessed using the criteria of Hardy. Significant differences are highlighted in bold, significance assessed by Student's t test.
P < 0.02.
P < 0.001.
P = 0.02.
Figure 2.
Lack of B1 and MZ B cells. (A) FACS® plot of scatter gated peritoneal cells stained with the indicated combinations of monoclonal antibodies. The gates in the top panel were used to generate the plots in the bottom panels which reveal the B1a, B1b, and B2 subsets. A proportion of B cells in the wild–type are B220high CD5+, which are also missing in the mutant. (B) Splenic cells were stained with the indicated combinations of mAbs to reveal MZ and follicular B cell profiles. Numbers indicate the percentage of lymphocytes falling within the gate. These gates were used to calculate the numbers of cells in Table I. (C) Immunofluorescence performed on splenic cryosections using MOMA-1 (green) and anti-IgM (red) revealed a marked reduction in MZ B cells in p110δ−/− mice (right). The position of MZ B cells is arrowed. The results shown are representative of four mutant spleens examined. Original magnification: 200×.
Impaired Antibody Responses in p110δ−/− Mice.
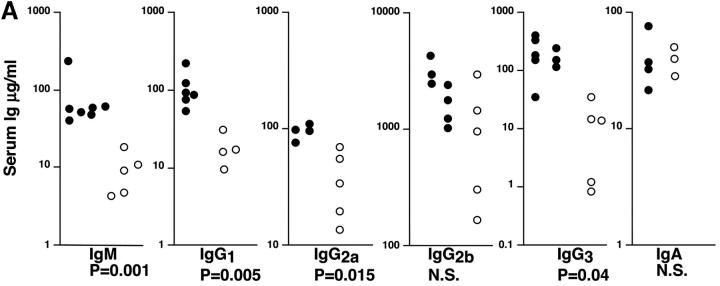
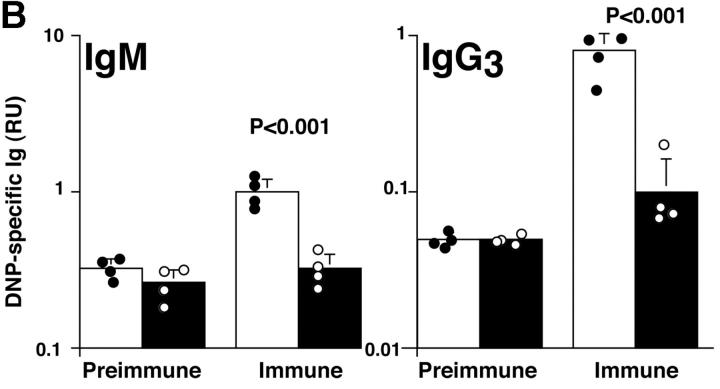
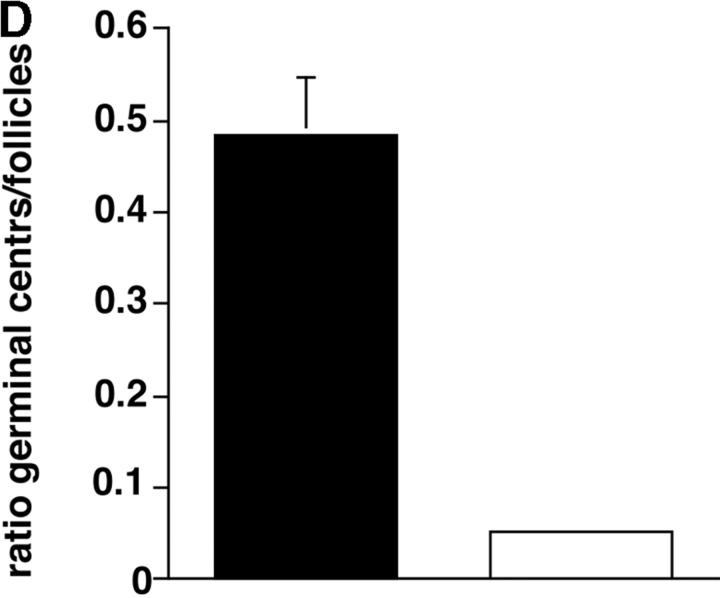
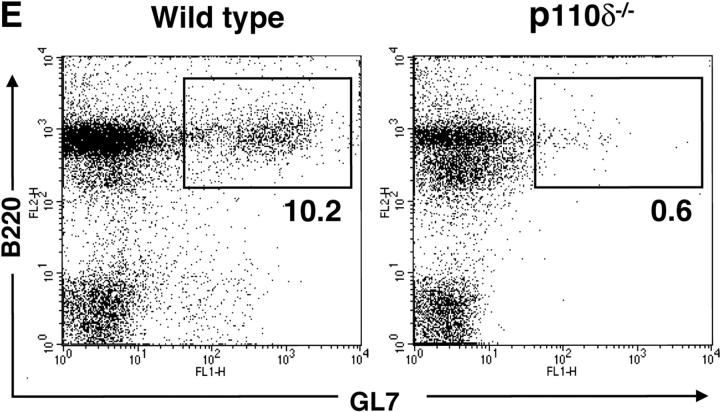
In nonimmunized P110δ−/− mice the levels of serum IgM, IgG1, IgG2a, and IgG3 and were significantly reduced, whereas IgG2b and IgA were in the normal range (Fig. 3 A). To determine the ability of p110δ−/− mice to mount humoral responses, we immunized p110δ−/− mice with the T cell–independent type II antigen, DNP-Ficoll. The hapten-specific IgM response of p110δ−/− mice after immunization was significantly reduced when compared with that of control mice, similarly, the levels of DNP-specific IgG3 produced were significantly lower in p110δ−/− mice (Fig. 3 B). The primary response to thymus-dependent (TD) antigens was measured 7 d after administration of DNP-KLH. Wild-type littermate mice produced IgM, IgG1 IgG2a, IgG2b, and IgG3 antibodies against DNP (Fig. 3 C). By contrast, p110δ−/− mice produced significantly less anti-DNP antibody of these subclasses. By 21 d after the primary immunization antigen specific Ig levels had generally fallen and reimmunization of control animals with DNP-KLH led to a secondary response. Although mice lacking p110δ produced antigen-specific Ig, there was a significant defect in the production of antigen-specific Ig of all subclasses tested (Fig. 3 C). Analysis of germinal center formation in the spleen after immunization revealed a reduction in the p110δ−/− mice (Fig. 3 D). Those germinal centers that were found were often atypical in size or location (unpublished data). This defect was underscored by a reduction in the number of Peyer's patches on the small intestine from 7.75 ± 1.7 in control mice (n = 4) to 4 ± 1.2 in p110δ−/− mice (n = 4). Furthermore, the Peyer's patches were smaller in the mutant mice and staining of B lymphocytes with GL7, an activation antigen expressed on germinal center B cells, also revealed a defect in p110δ−/− mice (Fig. 3 E).
Figure 3.
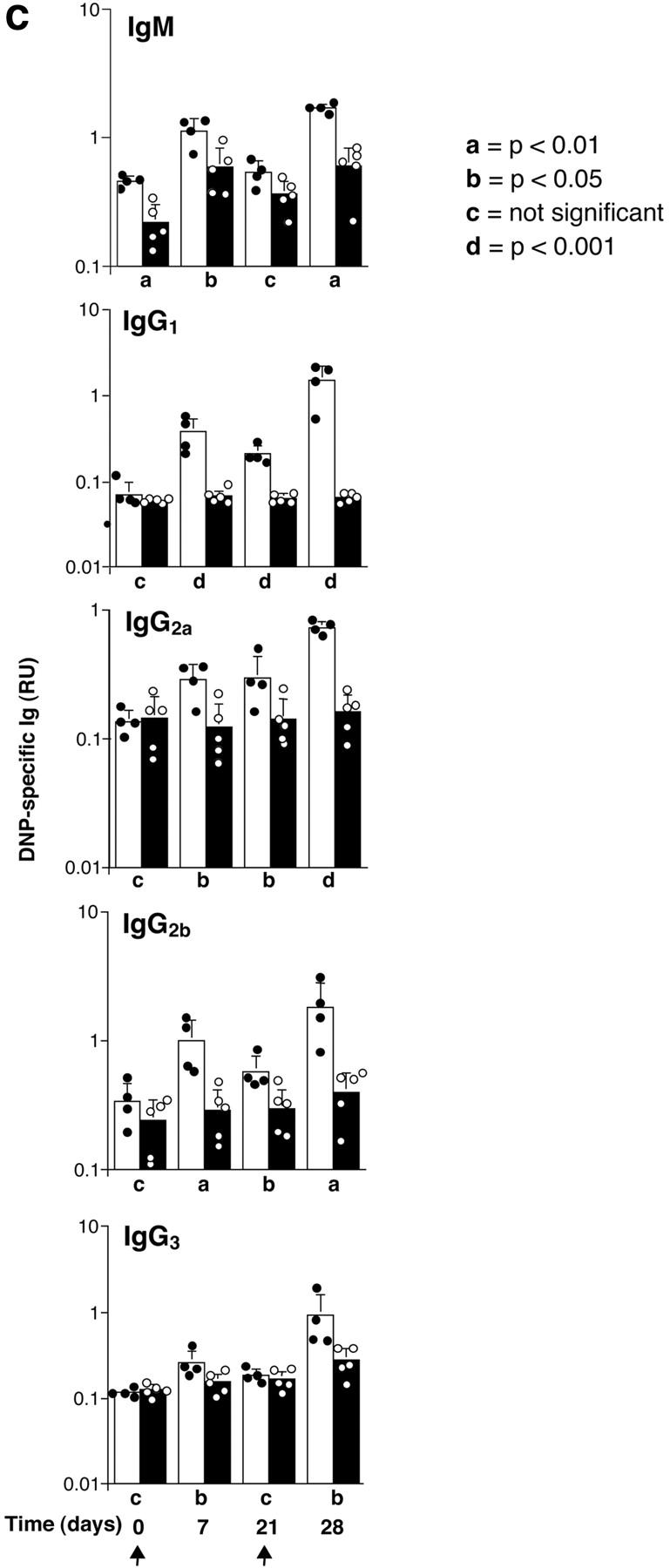
Immune function in p110δ mutant mice. (A) Serum Ig levels in naive mice were measured by ELISA. (B) DNP-specific Ig of the indicated isotypes were determined by ELISA of “preimmune” collected before immunization, and “immune” sera collected 7 d after immunization with DNP-Ficoll. (C) DNP-specific Ig was measured in preimmune serum, and in serum taken 7 and 21 d after immunization with DNP-KLH. Mice were then reimmunized at 21 d and bled 7 d later to measure the secondary response. In each set of graphs, the relative unit (absorbance) value for individual control animals is represented by a filled circle, individual p110δ-deficient mice are represented by open circles. Bars represent the average and SD for the group. In the TD responses, arrows represent the points at which mice were immunized (days 1 and 21). P values denote the levels of significance between sera of control and p110δ−/− mice as determined by the Student's t test. a, P < 0.01; b, P < 0.05; c, not significant; d, P < 0.001. (D) Quantitation of germinal center formation in spleens taken 10 d after immunization. The results are expressed as the ratio of PNA+ germinal centers/B220+ follicles. Black bar, wild type; white bar, mutant. Data was compiled from two control mice and four mutant mice. (E) Flow cytometric analysis of lymphocytes from Peyer's patches. Activated B cells are gated as B220+ GL7+, the numbers refer to the percentage of lymphocytes falling within the indicated gate.
Impaired In Vitro Responses of p110δ−/− B Cells.
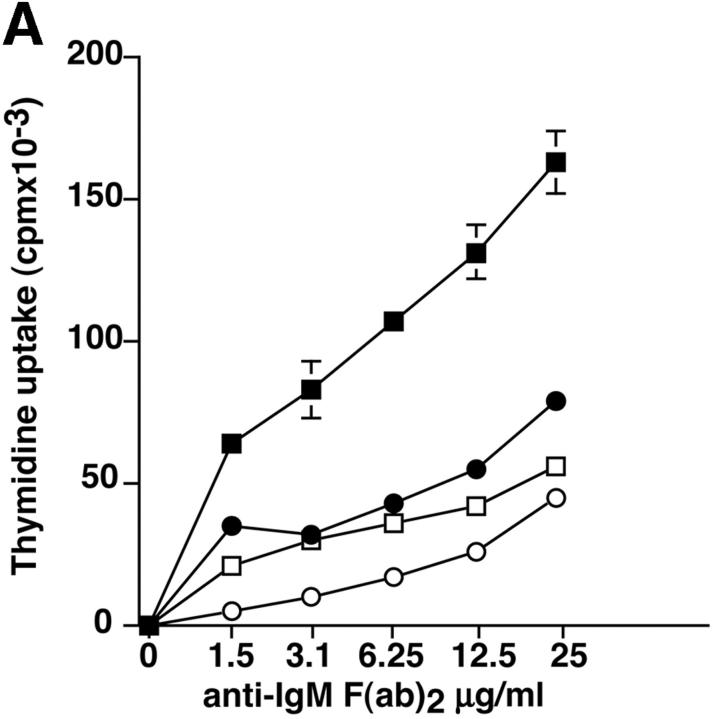
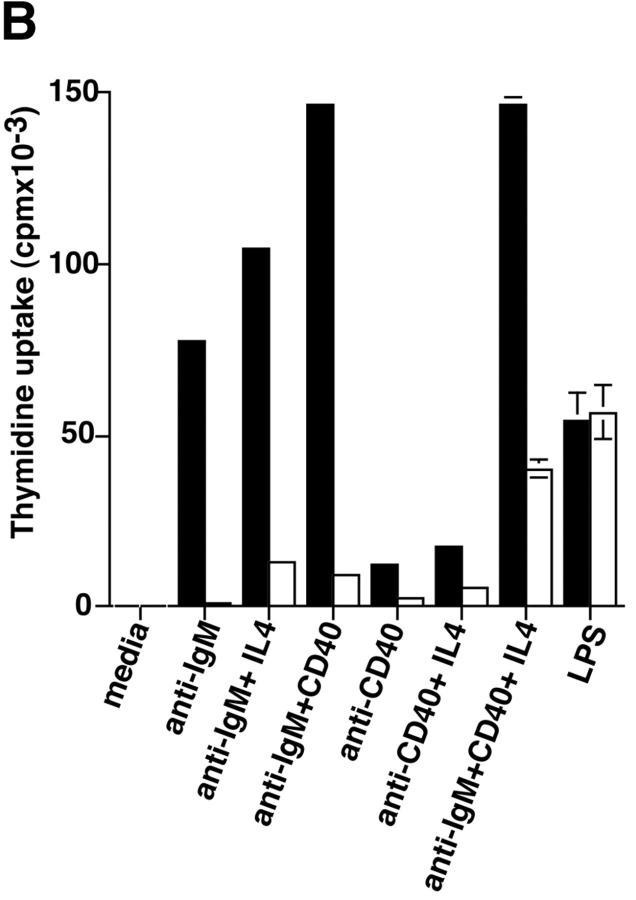
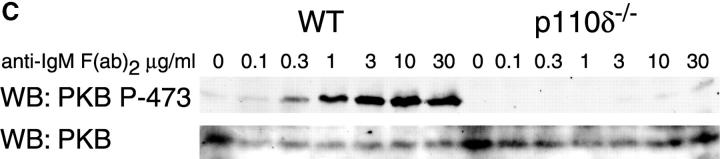
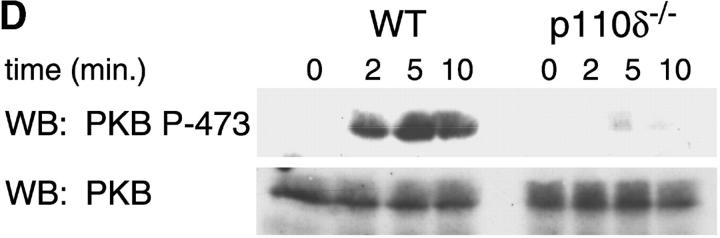
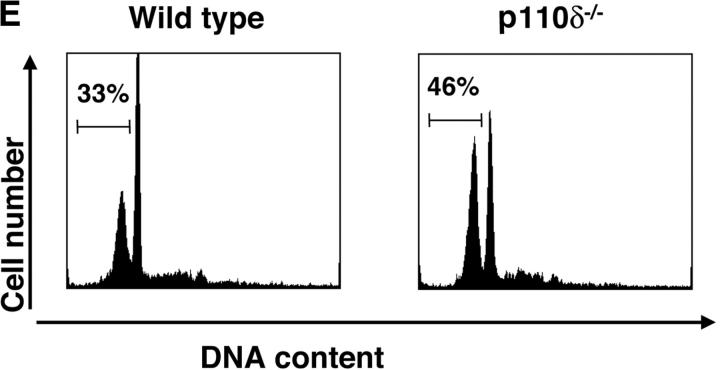
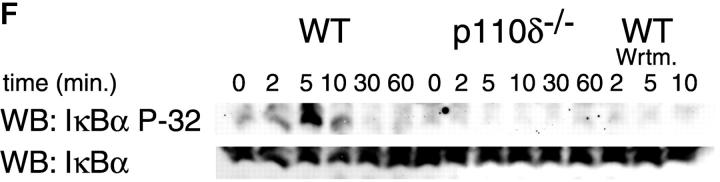
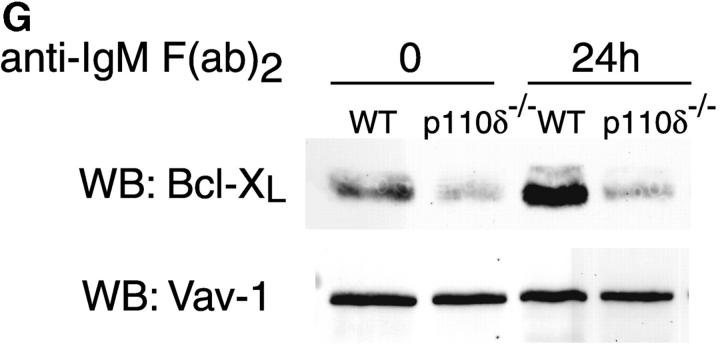
Purified splenic B cells from p110δ-deficient mice proliferated poorly in response to in vitro stimulation with polyclonal anti-IgM antibody, which is a potent B cell mitogen (Fig. 4 A). Supplementation of the media with IL-4 enhanced proliferation in both wild-type and p110δ−/− B cells. p110δ-deficient B cells were also poorly responsive to combinations of monoclonal anti-IgM, CD40, and IL-4 that elicited high levels of proliferation in control B cells (Fig. 4 B). By contrast the proliferative response of the mutant B cells to LPS was normal (Fig. 4 B). PKB is a PI3-K effector that has been shown to be involved in growth control and the suppression of apoptosis (28). The activation of PKB is an early, dose dependent, event after BCR engagement in normal B cells, however we found PKB activation was severely impaired in B cells from p110δ−/− mice (Fig. 4 C). While some PKB phosphorylation was evident in mutant B cells at the highest doses of antibody used for BCR stimulation, this phosphorylation was not sustained (Fig. 4 D), suggesting PKB was only weakly and transiently activated. When B cells from p110δ−/− mice were cultured in serum containing media, the spontaneous level of apoptosis was increased when compared with wild-type (Fig. 4 E) suggesting survival pathways were defective. Survival signaling through the BCR requires the activation of NF-κB and subsequent induction of the prosurvival bcl-2 family member Bcl-xL (29). NF-κB activation requires the phosphorylation of a cytoplasmic inhibitor (IκB) by the multiprotein (IκB-kinase) IKK complex. This targets IκB for ubiquitination and subsequent degradation and permits nuclear translocation of the transcription factor (30). We measured activation of IKK after BCR stimulation using antibodies specific to phosphorylated serine 32 of IκBα. In control B cells there was a transient induction of phosphorylation of IκBα that was blocked by the PI3-K inhibitor wortmannin (Fig. 4 F). B cells from p110δ-deficient mice did not show detectable phosphorylation of IκBα after BCR stimulation (Fig. 4 F). Bcl-xL levels in freshly isolated B cells from mutant mice were lower than control cells (Fig. 4 G). Furthermore, when cultured for 24 h in the presence of antibodies to the BCR, Bcl-xL levels increased in control but not in p110δ-deficient B cells (Fig. 4 G).
Figure 4.
Proliferation and apoptosis of p110δ−/− B cells. (A) Purified splenic B cells were cultured for 72 h with the indicated doses of polyclonal F(ab)2 goat anti–mouse IgM with (squares) and without (circles) 100 U/ml recombinant murine IL-4. (B) B cells were cultured for 72 h with media alone, or the indicated combinations of 6.25 μg/ml monoclonal anti-IgM (clone B7.6), 6.25 μg/ml monoclonal anti-CD40 (clone 3/23), and 100 U/ml recombinant murine IL-4. Control B cells are the black symbols, mutant B cells the white symbols. The numbers presented for each group represent counts per minute (cpm) plotted as mean and SD. (C) Purified B cells were stimulated with the indicated amounts of F(ab)2 goat anti–mouse IgM for 2 min and lysed in SDS-PAGE sample buffer. Western blots were developed with antibodies specific for PKB phosphorylated on serine 473 then stripped and reprobed with a pan-PKB antibody (Cell Signaling Technology). (D) Time course of PKB phosphorylation on serine 473 after stimulation with 10 μg/ml F(ab)2 goat anti–mouse IgM. (E) B cells were cultured for 24 h in RPMI 1640 plus 10% serum and apoptotic cells identified by flow cytometric analysis of DNA content using propidium iodide staining. Data are representative of B cells from three mice of each genotype. (F) Defective IκBα serine 32 phosphorylation, B cells were stimulated as in D and whole cell lysates blotted with phosphospecific antibody, top panel, the blot was then stripped and reprobed with antibody to IκBα. On the right-hand side of the panel wild-type B-lymphocytes were treated with 100 nM wortmannin (Wrtm.) before stimulation. (G) Bcl-xL levels were determined in freshly isolated B cells and in B cells that had been stimulated for 24 h with 20 μg/ml F(ab)2 goat anti–mouse IgM.
p110δ Is Required for Normal Function of Btk and PLCγ2.
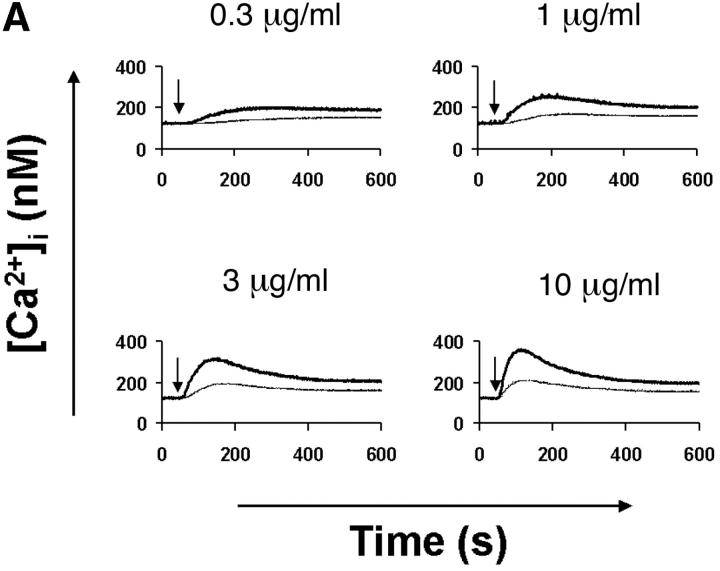
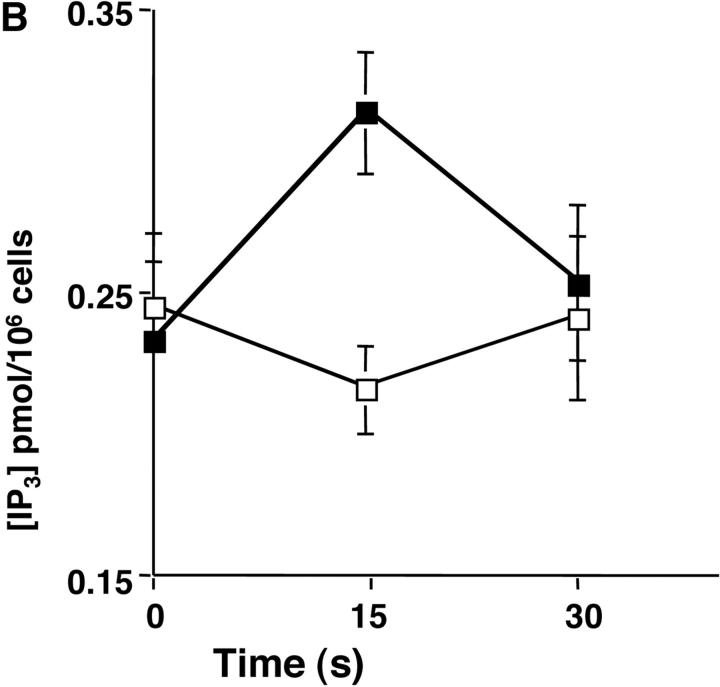
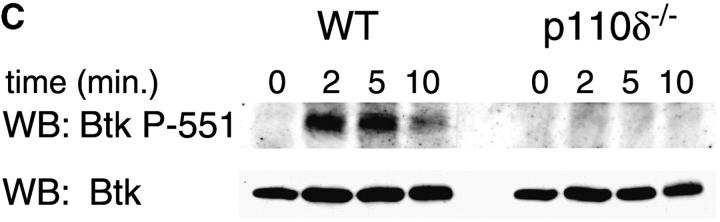
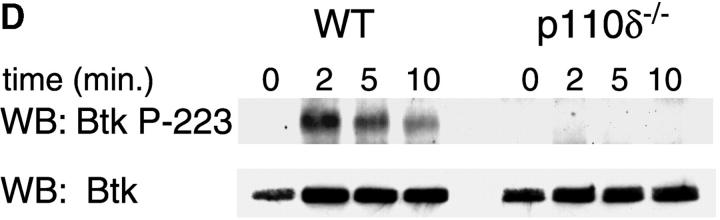
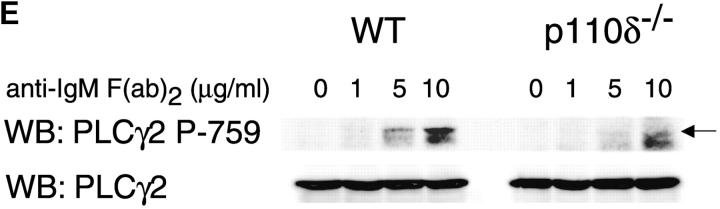
The mobilization of intracellular calcium (Ca2+) after the addition of antibodies against IgM was reduced in p110δ−/− B cells at all doses of agonist tested (Fig. 5 A). This was evident in both the initial peak response and the sustained response. To further evaluate the level at which calcium mobilization was impaired we measured BCR induced production of inositol 3,4,5 trisphosphate (IP3), the second messenger that triggers calcium release from intracellular stores. BCR stimulation of IP3 production was reduced in p110δ−/− B cells (Fig. 5 B). This observation could reflect defective activation of PLCγ2, as this lipase requires phosphorylation by Btk, a tyrosine kinase whose activity is dependent on PIP-3. Therefore, we measured the phosphorylation and activation of Btk using phosphorylation-site specific mAbs. In control mice Btk was phosphorylated on tyrosine 551 in response to BCR cross-linking (Fig. 5 C). This reflects phosphorylation in trans by Src and Syk family kinases (31–33). By contrast, phosphorylation of tyrosine 551 was not detected in p110δ-deficient B cells. Tyrosine 223 is a major autophosphorylation site of Btk, its phosphorylation can thus be used as an indicator of whether Btk has become activated. B cells from p110δ−/− mice showed no detectable phosphorylation of Btk at position 223. These results suggest that Btk activation requires the function of p110δ. Within PLCγ2 tyrosines 753 and 759 have been identified as important Btk substrates (34, 35). To establish whether PLCγ2 was phosphorylated by Btk on tyrosine 759 we employed a phosphospecific mAb. BCR stimulation of control B cells induced phosphorylation of PLCγ2 on tyrosine 759, however there was little phosphorylation of this residue in stimulated B cells from p110δ-deficient mice.
Figure 5.
Defective calcium signaling pathway in p110δ−/− B cells. (A) Intracellular calcium concentration in B cells after addition of F(ab)2 goat anti–mouse IgM, (indicated by arrow). Wild-type is thick black line and mutant thin line. (B) IP3 production was measured in control (black symbol) and mutant (white symbol) B cells after stimulation with 33 μg/ml F(ab)2 goat anti–mouse IgM using a kit purchased from New England Nuclear. The values represent the mean and SD of determinations from B cells from four individual mice. (C and D) Purified B cells were stimulated with 10 μg/ml F(ab)2 goat anti–mouse IgM for the indicated times and Btk immunoprecipitates blotted with antibodies specific for phosphorylated tyrosine 551 (C) or 223 (D). Blots were stripped and reprobed with polyclonal anti-Btk to demonstrate equal recovery of proteins. (E) Defective phosphorylation of PLCγ2. Whole cell lysates from purified B cells stimulated for 1 min with the indicated amounts of F(ab)2 goat anti–mouse IgM were blotted with antibody specific to phosphorylated tyrosine residue 759 of PLCγ2. The blot was then stripped and reprobed with antisera specific to PLCγ2 to confirm loading. The arrow indicates the position of phospho-PLCγ2.
Discussion
B cells from mice deficient in p110δ produce little PIP-3 after BCR engagement. This finding indicates that p110δ is mainly responsible for the bulk of PIP-3 production downstream of the BCR and that the p110α and β subunits, although expressed at normal levels in p110δ-deficient B cells, cannot compensate for loss of p110δ. This suggestion is further substantiated by the observations that the well characterized PI3-K effectors Btk and PKB are not, or only weakly, activated in response to BCR stimulation in p110δ-deficient B cells. Our results do not exclude roles for the p110α and β subunits in BCR signal transduction because weak phosphorylation of PKB could be detected in p110δ−/− B cells after high levels of BCR cross-linking. Furthermore, the PI3-K inhibitor wortmannin was able to mediate additional inhibition of BCR-stimulated calcium flux in p110δ−/− B cells (unpublished data). Our measurements of total cell PIP-3 do not take account of small, highly localized, concentrations of PIP-3 that may be generated (36). Methodological limitations preclude our measuring the spatio-temporal accumulation of PIP-3 in primary B cells, but such measurements will greatly increase our understanding of PI3-K function in B cell signaling. We have also noted a difference in B cell phenotype between p110δ-deficient mice and mice deficient in the p85α or p85/p55/p50α adaptor subunits of PI3-K (8, 9). Unlike p85α and p85/p55/p50α-deficient mice, the spleens of adult p110δ-deficient mice contain normal numbers and proportions of IgMlo IgDhi RF B cells. These observations presumably reflect the participation of the p110α or β subunits, coupled via the p85α adaptor, into a pathway that regulates the maturation of RF B cells. Alternatively, there may exist additional functions for the p85α subunit that are independent of the catalytic subunits. The functional interrelationships between the catalytic and regulatory subunits are poorly understood in complex systems, as exemplified by the phenotype of P85α-deficient mast cells. These cells display reduced expression of p110α but normal levels of p110β and δ (37). Intriguingly, c-kit signaling is impaired in P85α-deficient mast cells, whereas signaling through the high affinity IgE-receptor (that is also dependent on PI3-K activation) is normal (37, 38). Taken together with studies using manipulated cell lines (39), these data argue for selective roles for the individual p110 catalytic subunits.
Our results show that p110δ is required for the development and/or survival of the B1 and MZ B cell subsets. A number of previous studies have highlighted similarities between these subsets, which appear to play important roles in immunity through the production of natural antibodies, and by being able to rapidly respond to antigenic challenge (40). Indeed, the ability to mount T cell–independent type II antigen responses has been attributed to MZ B cells (41). Our finding that the levels of IgM and IgG3 in the serum of naive mice were significantly reduced and that antibody responses to DNP-Ficoll were severely impaired is consistent with the properties of these cells. We found that p110δ was required for the generation of normal numbers of B2 B cells, particularly in young mice, but not their maturation. Taken together with the increased tendency of p110δ−/− B cells toward apoptosis this observation may reflect increased turnover of B2 cells. Confirmation of this will require further experimentation. The response to TD antigen, which is principally mediated by B2 cells, was significantly impaired in both the primary and secondary immune response. Although we observed a modest primary antigen-specific IgM response, there was no detectable class switching to IgG1 and severely impaired switching to the other IgG subtypes. Consistent with this, germinal center formation was profoundly impaired. The phenotype of p110δ−/− mice shows similarities with CD19−/− mice that also lack B1 and MZ B cells, but have apparently normal development of B2 cells which display impaired function (42–44). However, splenic B cells from CD19−/− mice are less seriously impaired in their ability to activate PKB (45, 46) and Btk (47) than are p110δ−/− B cells. It will be interesting to determine whether CD19 employs p110δ as a signal transducer. The defective proliferation of p110δ−/− B cells to anti-CD40 is also a feature shared with CD19−/− B cells (48) and suggests that the mutant B cells may be unable to respond to T cell help. CD40 ligation on B cells activates PI3-K (49), and CD40-mediated B cell proliferation is blocked by PI3K inhibitors or p85α deficiency (8, 9). Preliminary experiments suggest CD86 upregulation is also defective in p110δ−/− B cells (unpublished data), therefore cognate T–B interactions required for the normal humoral response may be defective. It will also be interesting to determine whether the ability of p110δ−/− T cells to provide help is defective. p110δ was not required for mitogenic responses to all stimuli, as 110δ−/− B cells proliferated normally in response to LPS which also activates PI3-K (50) and requires the function of p85α to exert its mitogenic effect (8, 9). Our results thus suggest that catalytic subunits other than p110δ may be mediating the mitogenic LPS signal. IL-4–mediated proliferation is also sensitive to PI3-K inhibitors and requires p85α function (8, 9). p110δ does not appear to be required for IL-4 costimulation of proliferation induced by anti-IgM, as this was generally of a similar magnitude in both wild-type and p110δ−/− B cells. Furthermore, preliminary experiments determining the ability of IL-4 to mediate survival of cultured B cells has not revealed a difference between wild-type and p110δ−/− B cells (unpublished data).
PI3-K signaling has been intimately linked with cell survival in a number of systems by virtue of its ability to regulate PKB (28). p110δ-deficient B cells were impaired in their ability to survive after in vitro culture and expressed less of the antiapoptotic protein Bcl-xL. After activation through the BCR p110δ-deficient B cells failed to appreciably activate PKB and also displayed defective phosphorylation of IκBα. In addition, the increased expression of Bcl-xL that follows BCR stimulation required the function of p110δ. Taken together, these results are consistent with the suggestion that p110δ is important for the activation of survival pathways in B cells.
A number of studies have described mice lacking components of the BCR signaling pathway that share B cell developmental phenotypes. These include xid mice as well as mice deficient in Btk, B cell linker protein, PLCγ2, Vav-1/Vav-2, and B cell adaptor for PI3-K (BCAP) (51). The similarities in phenotypes between these mice, taken together with evidence of physical association, has led to the suggestion that these molecules act as a molecular machine or “signalosome” (52, 53). One model for BCR activation of calcium flux places emphasis on Btk-mediated phosphorylation and activation of PLCγ2. Activation of Btk is dependent on PI3-K (12, 54), as PIP-3 regulates Btk function by regulating both the location (55, 56) and the catalytic activity of Btk (57). Our data are consistent with such a model as we found that Btk was not phosphorylated and activated as assessed using phosphospecific antibodies. In addition, we found PLCγ2 phosphorylation and activation was defective in p110δ−/− B cells. A recent study also implicated p110δ in the regulation of PLCγ2 function in FcεRI-stimulated RBL-2H3 cells by injecting p110δ-specific inhibitory antibodies (58). Besides activation of Btk, additional PIP-3–mediated mechanisms may contribute to PLCγ2 activation as PIP-3 has been reported to bind directly to and activate PLCγ2 (58–60). Taken together, our results suggest that p110δ is the key PI3-K catalytic subunit required for the function of the signalosome in B cells.
Acknowledgments
We thank M. Bootman, P. Kilshaw, L. Reynolds, L. Stephens, V. Tybulewicz, and L. Webb for reagents and advice, Eurof Walters for statistical analysis, S. McAdam, J. Perkins, M. George, and animal facility staff for technical assistance.
Supported by the Biotechnology and Biological Sciences Research Council (M. Turner), the ICOS Corporation, a Medical Research Council program grant to C.P. Downes, MRC studentship to E. Clayton, and the Leukemia Research Fund and Cancer Research Campaign (to M. Turner).
Footnotes
Abbreviations used in this paper: BCAP, B cell adapter for PI3-K; BCR, B cell antigen receptor; Btk, Bruton's tyrosine kinase; IP3, inositol 3,4,5 trisphosphate; MZ, marginal zone; PI3-K, phosphatidylinositol 3-kinase; PIP-3, phosphatidylinositol 3,4,5 trisphosphate; PKB, protein kinase B; PLCγ2, phospholipaseCγ2; TD, thymus-dependent antigen; RF, recirculating follicular.
References
- 1.Meffre, E., R. Casellas, and M.C. Nussenzweig. 2000. Antibody regulation of B cell development. Nat. Immunol. 1:379–385. [DOI] [PubMed] [Google Scholar]
- 2.Craxton, A., K.L. Otipoby, A. Jiang, and E.A. Clark. 1999. Signal transduction pathways that regulate the fate of B lymphocytes. Adv. Immunol. 73:79–152. [DOI] [PubMed] [Google Scholar]
- 3.Gold, M.R., V.W. Chan, C.W. Turck, and A.L. DeFranco. 1992. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J. Immunol. 148:2012–2022. [PubMed] [Google Scholar]
- 4.Yamanashi, Y., Y. Fukui, B. Wongsasant, Y. Kinoshita, Y. Ichimori, K. Toyoshima, and T. Yamamoto. 1992. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc. Natl. Acad. Sci. USA. 89:1118–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuveson, D.A., R.H. Carter, S.P. Soltoff, and D.T. Fearon. 1993. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 260:986–989. [DOI] [PubMed] [Google Scholar]
- 6.Buhl, A.M., C.M. Pleiman, R.R. Rickert, and J.C. Cambier. 1998. Qualititive regulation of B cell antigen receptor signalling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J. Exp. Med. 186:1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fruman, D.A., and L.C. Cantley. 2002. Phosphoinositide 3-kinase in immunological systems. Semin. Immunol. 14:7–18. [DOI] [PubMed] [Google Scholar]
- 8.Fruman, D.A., S.B. Snapper, C.M. Yballe, L. Davidson, J.Y. Yu, F.W. Alt, and L.C. Cantley. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science. 283:393–397. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science. 283:390–392. [DOI] [PubMed] [Google Scholar]
- 10.Salim, K., M.J. Bottomley, E. Querfurth, M.J. Zvelebil, I. Gout, R. Scaife, R.L. Margolis, R. Gigg, C.I. Smith, P.C. Driscoll, et al. 1996. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 11.Scharenberg, A.M., and J.P. Kinet. 1998. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell. 94:5–8. [DOI] [PubMed] [Google Scholar]
- 12.Bolland, S., R.N. Pearse, T. Kurosaki, and J.V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 8:509–516. [DOI] [PubMed] [Google Scholar]
- 13.Liu, Q., A.J. Oliveira Dos Santos, S. Mariathasan, D. Bouchard, J. Jones, R. Sarao, I. Kozieradzki, P.S. Ohashi, J.M. Penninger, and D.J. Dumont. 1998. The inositol polyphosphate 5-phosphatase SHIP is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 188:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helgason, C.D., C.P. Kalberer, J.E. Damen, S.M. Chappel, N. Pineault, G. Krystal, and R.K. Humphries. 2000. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of B lymphocytes in SHIP−/− mice. J. Exp. Med. 191:781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brauweiler, A., I. Tamir, J. Dal Porto, R.J. Benschop, C.D. Helgason, R.K. Humphries, J.H. Freed, and J.C. Cambier. 2000. Differential regulation of B cell development, activation, and death by the Src homology 2 domain-containing 5′ inositol phosphatase (SHIP). J. Exp. Med. 191:1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cristofano, A., P. Kotsi, Y.F. Peng, C. Cordon-Cardo, K.B. Elkon, and P.P. Pandolfi. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 285:2122–2125. [DOI] [PubMed] [Google Scholar]
- 17.Bi, L., I. Okabe, D.J. Bernard, A. Wynshaw-Boris, and R. Nussbaum. 1999. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274:10963–10968. [DOI] [PubMed] [Google Scholar]
- 18.Bi, L., I. Okabe, D.J. Bernard, and R.L. Nussbaum. 2002. Early embryonic lethality in mice deficient in the p110β catalytic subunit of PI 3-kinase. Mamm. Genome. 13:169–172. [DOI] [PubMed] [Google Scholar]
- 19.Chantry, D., A. Vojtek, A. Kashishian, D.A. Holtzman, C. Wood, P.W. Gray, J.A. Cooper, and M.F. Hoekstra. 1997. P110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J. Biol. Chem. 272:19236–19241. [DOI] [PubMed] [Google Scholar]
- 20.Vanhaesebroeck, B., M.J. Welham, K. Kotani, R. Stein, P.H. Warne, M.J. Zvelebil, K. Higashi, S. Volinia, J. Downward, and M.D. Waterfield. 1997. P110δ, a novel phosphoinositide 3-kinase in leukocytes. Proc. Natl. Acad. Sci. USA. 94:4330–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton, E., S. McAdam, J. Coadwell, D. Chantry, and M. Turner. 2001. Structural organization of the mouse phosphatidylinositol 3-kinase p110δ gene. Biochem. Biophys. Res. Commun. 280:1328–1332. [DOI] [PubMed] [Google Scholar]
- 22.O'Gorman, S., N.A. Dagenais, M. Qian, and Y. Marchuk. 1997. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 94:14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray, A., J. Van Der Kaay, and C.P. Downes. 1999. The pleckstrin homology domains of protein kinase B and GRP1 (general receptor for phosphoinositides-1) are sensitive and selective probes for the cellular detection of phosphatidylinositol 3,4-bisphosphate and/or phosphatidylinositol 3,4,5-trisphosphate in vivo. Biochem. J. 344:929–936. [PMC free article] [PubMed] [Google Scholar]
- 24.Doody, G.M., S.E. Bell, E. Vigorito, E. Clayton, S. McAdam, R. Tooze, C. Fernandez, I.J. Lee, and M. Turner. 2001. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2:542–547. [DOI] [PubMed] [Google Scholar]
- 25.Nisitani, S., R.M. Kato, D.J. Rawlings, O.N. Witte, and M.I. Wahl. 1999. In situ detection of activated Bruton's tyrosine kinase in the Ig signalling complex by phosphopeptide-specific monoclonal antibodies. Proc. Natl. Acad. Sci. USA. 96:2221–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoletti, I., G. Migliorati, M.C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow-cytometry. J. Immunol. Methods. 139:271–279. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, D., S.C. Tovey, T.J. Collins, M.D. Bootman, M.J. Berridge, and P. Lipp. 2000. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 28:213–223. [DOI] [PubMed] [Google Scholar]
- 28.Datta, S.R., A. Brunet, and M.E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 29.Chen, C., L.C. Edelstein, and C. Gelinas. 2000. The Rel NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol. Cell. Biol. 20:2687–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zandi, E., and M. Karin. 1999. Bridging the gap: regulation and physiological function of the IκB kinase complex. Mol. Cell. Biol. 19:4547–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawlings, D.J., A.M. Scharenberg, H. Park, M.I. Wahl, S. Lin, R.M. Kato, A.-C. Fluckinger, O.N. Witte, and J.-P. Kinet. 1996. Activation of Btk by a phosphorylation mechanism initiated by Src family kinases. Science. 271:822–825. [DOI] [PubMed] [Google Scholar]
- 32.Kurosaki, T., and M. Kurosaki. 1997. Transphosphorylation of Bruton's tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J. Biol. Chem. 272:15595–15598. [DOI] [PubMed] [Google Scholar]
- 33.Baba, Y., S. Hashimoto, M. Matsushita, D. Watanabe, T. Kishimoto, T. Kurosaki, and S. Tsukada. 2001. BLNK mediates Syk-dependent Btk activation. Proc. Natl. Acad. Sci. USA. 98:2582–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe, D., S. Hashimoto, M. Ishiai, M. Matsushita, Y. Baba, T. Kishimoto, T. Kurosaki, and S. Tsukada. 2001. Four tyrosine residues in phospholipase C-γ2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signalling. J. Biol. Chem. 276:38595–38601. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez, R., M. Matsuda, O. Perisic, J. Bravo, A. Paul, Y. Light, K. Swann, R.L. Williams, and M. Katan. 2001. Tyrosine residues in PLCγ2 essential for the enzyme function in B-cell signalling. J. Biol. Chem. 276:47982–47992. [DOI] [PubMed] [Google Scholar]
- 36.Marshall, J.G., J.W. Booth, V. Stambolic, T. Mak, T. Balla, A.D. Schreiber, T. Meyer, and S. Grinstein. 2001. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fcγ receptor-mediated phagocytosis. J. Cell Biol. 153:1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukao, T., T. Yamada, M. Tanabe, Y. Terauchi, T. Ota, T. Takayama, T. Asano, T. Takeuchi, T. Kadowaki, J. Hata, and S. Koyasu. 2002. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 3:295–304. [DOI] [PubMed] [Google Scholar]
- 38.Lu-Kuo, J.M., D.A. Fruman, D.M. Joyal, L.C. Cantley, and H.R. Katz. 2000. Impaired kit-but not Fcε RI-initiated mast cell activation in the absence of phosphoinositide 3-kinase p85 α gene products. J. Biol. Chem. 275:6022–6029. [DOI] [PubMed] [Google Scholar]
- 39.Vanhaesebroeck, B., G.E. Jones, W.E. Allen, D. Zicha, R. Hooshmand-Rad, C. Sawyer, C. Wells, M.D. Waterfield, and A.J. Ridley. 1999. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat. Cell Biol. 1:69–71. [DOI] [PubMed] [Google Scholar]
- 40.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 41.Guinamard, R., M. Okigaki, J. Schlessinger, and J.V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36. [DOI] [PubMed] [Google Scholar]
- 42.Engel, P., L.J. Zhou, D.C. Ord, S. Sato, B. Koller, and T.F. Tedder. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 3:39–50. [DOI] [PubMed] [Google Scholar]
- 43.Rickert, R.C., K. Rajewsky, and J. Roes. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 376:352–355. [DOI] [PubMed] [Google Scholar]
- 44.Makowska, A., N.N. Faizunnessa, P. Anderson, T. Midtvedt, and S. Cardell. 1999. CD1high B cells: a population of mixed origin. Eur. J. Immunol. 29:3285–3294. [DOI] [PubMed] [Google Scholar]
- 45.Otero, D.C., S.A. Omori, and R.C. Rickert. 2001. CD19-dependent activation of Akt kinase in B-lymphocytes. J. Biol. Chem. 276:1474–1478. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto, M., J.C. Poe, A.B. Satterthwaite, M.I. Wahl, O.N. Witte, and T.F. Tedder. 2002. Complementary roles for CD19 and Bruton's Tyrosine kinase in B lymphocyte signal transduction. J. Immunol. In press. [DOI] [PubMed] [Google Scholar]
- 47.Buhl, A.M., and J.C. Cambier. 1999. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton's tyrosine kinase. J. Immunol. 162:4438–4446. [PubMed] [Google Scholar]
- 48.Gardby, E., and N.Y. Lycke. 2000. CD19-deficient mice exhibit poor responsiveness to oral immunisation despite evidence of unaltered total IgA levels, germinal centre and IgA-isotype switching in peyer's patches. Eur. J. Immunol. 30:1861–1871. [DOI] [PubMed] [Google Scholar]
- 49.Ren, C.L., T. Morio, S.M. Fu, and R.S. Geha. 1994. Signal-transduction via CD40 involves activation of Lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase C-γ-2. J. Exp. Med. 179:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bone, H., and N.A. Williams. 2001. Antigen-receptor cross-linking and lipopolysaccharide trigger distinct phosphoinositide 3-kinase-dependent pathways to NF-κB activation in primary B cells. Int. Immunol. 13:807–816. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki, T., K. Takeda, K. Gotoh, H. Takeshima, S. Akira, and T. Kurosaki. 2002. Essential immunoregulatory role for BCAP in B cell development and function. J. Exp. Med. 195:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fruman, D.A., A.B. Satterthwaite, and O.N. Witte. 2000. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 13:1–3. [DOI] [PubMed] [Google Scholar]
- 53.DeFranco, L.A. 2001. Vav and the B cell signalosome. Nat. Immunol. 2:482–484. [DOI] [PubMed] [Google Scholar]
- 54.Fluckiger, A.-C., Z. Li, R.M. Kato, M.I. Wahl, H.D. Ochs, R. Longnecker, J.-P. Kinet, O.N. Witte, A.M. Scharenberg, and D.J. Rawlings. 1998. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 17:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varnai, P., K.I. Rother, and T. Balla. 1999. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 274:10983–10989. [DOI] [PubMed] [Google Scholar]
- 56.Nore, B.F., L. Vargas, A.J. Mohamed, L.J. Brandén, C.-M. Bäckesjö, T.C. Islam, P.T. Mattsson, K. Hultenby, B. Christensson, and E. Smith. 2000. Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur. J. Immunol. 30:145–154. [DOI] [PubMed] [Google Scholar]
- 57.Saito, K., A.M. Scharenberg, and J.-P. Kinet. 2001. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J. Biol. Chem. 276:16201–16206. [DOI] [PubMed] [Google Scholar]
- 58.Smith, A.J., Z. Surviladze, E.A. Gaudet, J.M. Backer, C.A. Mitchell, and B.S. Wilson. 2001. p110β and p110δ Phosphatidylinositol 3-kinases upregulate FcɛRI-activated Ca2+ influx by enhancing inositol 1,4,5-trisphosphate production. J. Biol. Chem. 276:17213–17220. [DOI] [PubMed] [Google Scholar]
- 59.Bae, Y.S., L.G. Cantley, C.S. Chen, S.R. Kim, K.S. Kwon, and S.G. Rhee. 1998. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:4465–4469. [DOI] [PubMed] [Google Scholar]
- 60.Falasca, M., S.K. Logan, V.P. Lehto, G. Baccante, M.A. Lemmon, and J. Schlessinger. 1998. Activation of phospholipase Cγ by PI3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 17:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]