Abstract
Stimulation of Flt3 receptor tyrosine kinase through its cognate ligand expands early hematopoietic progenitor and dendritic cells (DCs) in humans and mice. The exact developmental stages at which hematopoietic progenitors express Flt3, are responsive to its ligand, and subsequently develop to DCs, are not known. Here we show that common lymphoid and common myeloid progenitors, as well as steady state DCs in thymus, spleen, and epidermis, express Flt3. The receptor is down-regulated once definitive B cell, T cell, and megakaryocyte/erythrocyte commitment occurs, and Flt3 is not detectable on other steady state hematopoietic cell populations. Upon in vivo Flt3 ligand (Flt3L) administration, Flt3+ progenitor cells and their progeny DCs are expanded, whereas Flt3− downstream progenitors are not, or are only slightly increased. Transplantation of common lymphoid and common myeloid progenitors and subsequent Flt3L injection increases progeny DCs of both precursor populations. These findings provide a definitive map of Flt3 expression in the hematopoietic hierarchy and directly demonstrate that Flt3L can drive DC development along both the lymphoid and myeloid developmental pathways from Flt3+ progenitors to Flt3+ DCs.
Keywords: Flt3/Flt3L, dendritic cells, development, hematopoietic progenitors
Introduction
Flt3 (also termed Flk-2 [1] and STK-1 [2]), a receptor tyrosine kinase with homology to c-Kit (the receptor for steel factor [SLF]*/stem cell factor) and c-fms (the receptor for M-CSF), is highly expressed in hematopoietic progenitor cells (for review see 3). Important information about its function in hematopoiesis has been gained through mice with targeted gene disruption of Flt3 (4) or Flt3 ligand (Flt3L; 5), through in vivo Flt3L injection (6–8) and through overexpression of Flt3L (9, 10) or introduction of constitutively active flt3 mutations (11) in hematopoietic cells. Flt3−/− mice show normal peripheral blood counts, however, pro-B cell numbers are diminished and bone marrow progenitors of these mice display a reduced ability to competitively reconstitute lethally conditioned recipients, most pronounced in the T and myeloid lineages (4). Flt3L−/− mice show no significant changes in red blood cells and platelets but 27–45% decreased numbers of complete nucleated cells in peripheral blood, bone marrow, spleen, and lymph nodes, most marked in relative lymphocyte numbers (5). In addition, the total numbers of CFU-GM and B cell precursors are reduced (5). Flt3L−/− mice also show about fivefold lower numbers of NK cells and 4–14-fold reduced, but functional DCs in lymphoid organs (5). Injection of human Flt3L that displays a high homology and is cross-reactive to mouse Flt3L is reported to increase numbers of lymphocytes, granulocytes, and monocytes, and massively mobilize immature, myeloid colony-forming and long-term (6 mo) reconstituting cells into peripheral blood (6). Also, it dramatically increases NK cells (12) and DCs in bone marrow and lymphoid organs (4–27-fold), with up to 30% of spleen cells expressing CD11c and MHC class II (8, 13). Mice with Flt3L overexpression or constitutive active Flt3 signaling develop myeloproliferative disease and are prone to develop leukemias with both lymphoid and myeloid marker expression (9–11). Finally, Flt3L as a single cytokine is able to induce all major DC populations in vitro from whole bone marrow cells (14, 15). Based on these data, Flt3 signaling has its most pronounced effect on the expansion of early hematopoietic progenitor cells and on the generation of mature, steady state NK cells and DCs.
We have recently isolated clonal common lymphoid and myeloid progenitors in mouse bone marrow (16, 17) and we and others demonstrated that both lymphoid and myeloid progenitors give rise to both CD8α+ and CD8α− DCs in vivo (18–20). Also, we and others have shown that short-term but not long-term hematopoietic stem cells (HSCs) express Flt3 (21, 22). Given the relevance of Flt3/Flt3L in early hematopoiesis and DC generation, here we wanted to directly test (a) Flt3 receptor expression on defined successive lymphoid and myeloid progenitors and on mature, steady state hematopoietic cell populations, (b) in vivo progenitor and mature hematopoietic cell expansion in response to Flt3L, and (c) if Flt3L acts on the generation of DCs along both the lymphoid and myeloid developmental pathway.
Materials and Methods
Mice.
C57BL/Ka-Thy1.1 mice, homozygous for either CD45.1 or CD45.2, and F1 C57BL/Ka-Thy1.1 (CD45.1 × CD45.2) mice were used as previously described (18, 19). All mice were maintained in Stanford University's Research Animal Facility in accordance with Stanford guidelines.
Antibodies and Flow Cytometry.
The following antibodies were used: M1/70 (anti–Mac-1/CD11b), 8C5 (anti–Gr-1), 6B2 (anti-B220), KT-31 (anti-CD3), GK1.5 (anti-CD4), 53-6.7 (anti-CD8α), anti-TER119, A7R34 (anti–IL-7Rα/CD127), 2B8 (anti–c-Kit/CD117), 3C11 (anti–c-Kit/CD117), E13-161-7 (anti–Sca-1), 19XE5 (anti–Thy-1.1), A20.1.7 (anti-CD45.1), AL1-4A2 (anti-CD45.2), 6C3 (anti–BP-1; own laboratory hybridoma cell lines), and goat anti–rat IgG (PE or Cy5-PE conjugated; Caltag). Antibodies purchased from BD Biosciences: 2.4G2 (anti-FcγRII/III), RAM34 (anti-CD34), NK1.1, S7 (anti-CD43), 7D4 (anti–IL-2Rα/CD25), 1D3 (anti-CD19), AF6-120.1 (anti-IAb), HL3 (anti-CD11c), and rat IgG2a as isotype control. Antibodies purchased from eBioscience: 30-F1 (anti-HSA/CD24) and A2F10.1 (anti-Flt3/CD135). For visualization of biotinylated antibodies, streptavidin-conjugated PE, Cy5-PE, Cy7-PE, and Texas Red (Invitrogen) were used.
HSCs, lymphoid progenitors, myeloid progenitors, DC precursor–containing populations, and DCs were sorted as reported elsewhere (16–19, 21, 23–25). Mature cells were sorted from spleen or blood cells as follows: B cells: CD19+ B220+; T cells: CD3+ TCRβ+ NK1.1−; NK cells: NK1.1+ CD3−; NKT cells: NK1.1+ CD3+; granulocytes: Gr-1hi Mac-1+ CD11c−; monocytes/macrophages: Gr-1−/int Mac-1+ CD11c− B220−. Langerhans cells were isolated and sorted as previously described (26). For all experiments, dead cells were excluded by propidium iodide staining and appropriate isotype-matched, irrelevant control monoclonal antibodies were used to determine the level of background staining.
Cells were sorted and analyzed using a modified dual laser FACS Vantage™ (Becton Dickinson) and a dual laser MoFlo® cytometer (DakoCytomation), both equipped with a 488-nm argon laser and a 599-nm dye laser. All transplanted progenitors as well as all cells for PCR analysis were purified by sorting and then resorting, and were >99% pure for the indicated surface marker phenotype.
RT-PCR Analysis.
RNA was extracted from double sorted populations, reverse transcribed to cDNA, and analyzed for the presence of flt3 sequences as previously described (flt3 sense: 5′ ACC ATG GAT TCG GGC TCA CCT 3′; flt3 antisense: 5′ CTG GGC GTC ATC ATT TTC TGC 3′, 445-bp PCR product; reference 27). β actin was amplified as a control. PCR amplification consisted of an initial denaturation step at 94°C for 2 min, followed by 30–38 cycles at 94°C for 30 s, annealing at 65°C for 30 s, and extension at 72°C for 60 s in each cycle. PCR products were electrophoresed on an ethidium bromide–stained 1.5% agarose gel. For each PCR, RNA equivalents from 125 cells (progenitors) or 500 cells (splenocytes) were used. PCR amplification was repeated at least twice for at least three separately prepared cDNA samples for each experiment.
In Vitro DC Differentiation Assay.
Common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs) were differentiated in vitro into DCs as previously described (19). In brief, CLPs were cultured over 5 d in Iscove's modified Dulbecco medium (Invitrogen) supplemented with 10% FCS, 10−4 M 2-mercaptoethanol, sodium pyruvate, antibiotics, and 10 ng/ml recombinant mouse IL-1β, 20 ng/ml IL-3, 10 ng/ml IL-4, 10 ng/ml IL-7, 10 ng/ml TNFα, 10 ng/ml SLF, and 10 ng/ml Flt3L. CMPs were cultured over 8 d in 20 ng/ml IL-3, 10 ng/ml IL-4, 10 ng/ml TNFα, 10 ng/ml SLF, 10 ng/ml Flt3L, and 10 ng/ml GM-CSF. Peripheral spleen monocytes were cultured over 5 d in the media described above supplemented with 10 ng/ml IL-4, 10 ng/ml GM-CSF, and where indicated, 10 ng/ml Flt3L. In additional experiments sorted Flt3+ and Flt3− CLPs or CMPs were cultured on a confluent layer of irradiated (25 Gy) murine AC6 stromal cells as previously described (28) with the addition of 10 ng/ml IL-7, 10 ng/ml SLF, and 10 ng/ml Flt3L for CLPs or 10 ng/ml SLF and 10 ng/ml Flt3L for CMPs to provide cells with an optimized support for in vitro development.
In Vivo Reconstitution Assays and Flt3L Injection.
For in vivo reconstitution assays, purified progenitors or whole bone marrow cells were injected i.v. into 8–12-wk-old sublethally (4.75 Gy) or lethally irradiated (9.5 Gy) congenic mice, which differed only at the CD45 allele. Donor-derived DCs were evaluated at different time points after transplantation as previously described (18, 19).
Flt3L (10 μg in 100 μl PBS; provided by Immunex) or control PBS was injected daily s.c. as previously described (8) over the time indicated in the results section.
Results
Flt3 Is Expressed on CLPs and CMPs and Is Subsequently Down-regulated in B Cell, T Cell, and Megakaryocyte/Erythrocyte Commitment.
Flt3 receptor expression was tested by nonquantitative RT-PCR and for quantitative assessment, by FACS®. As reported before (21, 22), the Lin− c-Kit+ Thy-1.1+ Sca-1+ population contains Flt3-expressing cells, which are short-term HSCs (Fig. 1, A and E) . Flt3 is highly up-regulated in a major fraction of CLP (60–70%) and is consecutively reduced in pro-B and pro-T cells. Although on both pro-B (A) and pro-T1 cells, Flt3 is detectable as surface protein by FACS®, only RT-PCR reveals Flt3 expression in pro-B (BC) cells and neither method shows it in pro-T2 cells (Fig. 1, A and E, and not depicted). During myeloid commitment, Flt3 is highly expressed on more than half of CMPs (50–65%) and is reduced to lower levels on granulocyte/macrophage progenitors (GMPs), whereas it is not detectable on cells committed to the megakaryocyte-erythrocyte lineage (megakaryocyte/erythrocyte progenitors [MEPs]; Fig. 1, A and E). Therefore, Flt3 is transiently up-regulated during both lymphoid and myeloid commitment, but consecutively down-regulated in definitive B cell, T cell, and megakaryocyte/erythrocyte lineage development.
Figure 1.
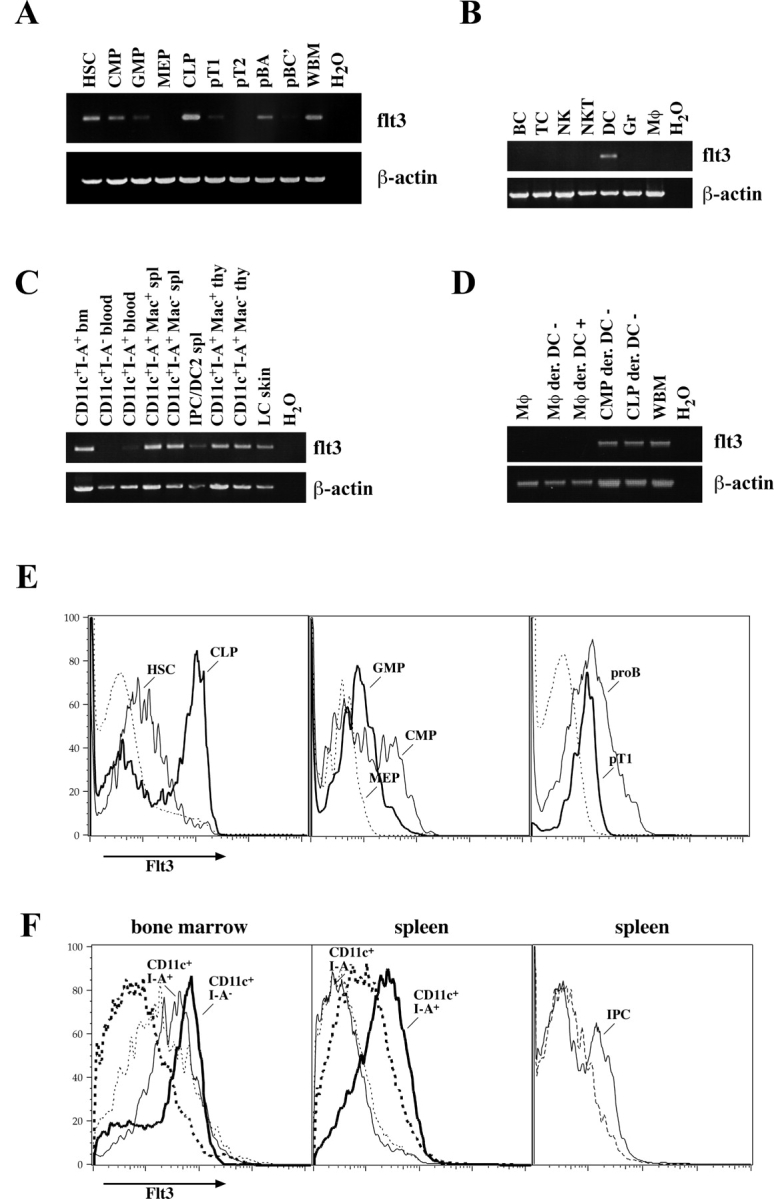
Flt3 expression on defined hematopoietic progenitor cells, steady state DCs, and other mature hematopoietic cells. Expression of Flt3 was examined by nonquantitative RT-PCR on (A) hematopoietic progenitor populations from bone marrow and thymus, (B) on mature hematopoietic cells from spleen (BC, B cells; TC, T cells; NK, NK cells; NKT, NKT cells; DC, dendritic cells; Gr, granulocytes; Mφ, monocytes/macrophages), and (C) on steady state DC populations as indicated from bone marrow (bm), blood, spleen (spl), thymus (thy), and Langerhans cells (LC) from the epidermis. (D) In vitro–derived DCs (CD11c+ I-Ab+) from either splenic monocytes or common myeloid and lymphoid progenitors were sorted, and Flt3 expression was analyzed by RT-PCR. (E) FACS® analysis of Flt3 surface expression is shown on the left panel. thin line, HSCs; bold line, CLPs; dashed line, whole bone marrow for comparison. In the middle panel: thin line, CMPs; bold line, GMPs; dashed line, MEPs. On the right panel: thin line, pro-B cells; bold line, pro-T1 cells from thymus; dashed line, whole thymus. (F) Flt3 expression on CD11c+ I-Ab+ cells (thin line) and CD11c+ I-Ab− cells (bold line) from bone marrow (left) and spleen (middle). Right panel shows FACS® analysis of IPCs in the spleen. Dashed lines in F always show staining with the according isotype control antibody.
Flt3 Is Expressed on Steady State DCs, but Not on Other Mature Steady State Hematopoietic Cells.
To test whether Flt3 is expressed on mature cells of hematopoietic origin under steady state conditions, we evaluated all major nucleated cell lineages isolated from spleen. Only CD11c+ MHC class II+ DCs but not B cells, T cells, NK cells, NKT cells, granulocytes, and monocytes/macrophages expressed flt3 mRNA or Flt3 surface protein (Fig. 1 B and not depicted). Further analysis of DC subpopulations showed that Flt3 is expressed in CD8α− CD11b+ and CD8α+ CD11b− DCs in spleen and thymus as well as in Langerhans cells of the epidermis and, at slightly lower levels, on CD11clo B220+ Gr-1+ interferon α–producing cells (IPCs; Fig. 1, C and F, and not depicted). Therefore, with the exception of follicular DCs that were not analyzed, all major steady state DC populations in primary and secondary lymphoid tissues and in the epidermis express Flt3 whereas other mature cell populations do not.
Because early lymphoid and myeloid progenitors as well as steady state DCs expressed Flt3, we were interested to test whether Flt3 is continuously expressed during DC development. CD11c+ MHC class II− cells in blood were recently described as immediate DC-committed progenitors (25). As the residence time of tested nucleated cells (T cells and B cells, granulocytes, monocytes, LT-HSCs, ST-HSCs, and multipotent progenitors/MPP) can be measured in minutes (29), it can also be assumed that blood CD11c+ MHC class II− cells are brief transients from bone marrow to tissues where both they should be contained in cell populations that display a similar phenotype. Although bone marrow CD11c+ MHC class II− and CD11c+ MHC class II+ cells expressed high levels of Flt3, we were not able to detect Flt3 in blood and spleen CD11c+ MHC class II− cells and only low levels in blood CD11c+ MHC class II+ DCs (Fig. 1, C and F). Therefore, if CD11c+ MHC class II− cells in blood and spleen are indeed the major immediate DC progenitor populations of lymphoid organ DCs, Flt3 would be transiently down-regulated in development and consecutively up-regulated on DCs again.
Flt3 Is Expressed on Lymphoid and Myeloid Progenitor–derived, but Not on Monocyte-derived, DCs In Vitro.
To test whether DCs derived from different precursors express Flt3, CLPs, CMPs, and spleen monocytes/macrophages were cultured, and differentiated CD11c+ MHC class II+ cells were sorted for RT-PCR analysis. DCs derived from both CLPs and CMPs expressed Flt3, whereas no Flt3 signal could be detected on DCs derived from Flt3− monocytes (Fig. 1 D). Therefore, although in vitro–derived DCs from early Flt3+ progenitors are Flt3+, Flt3 expression seems not to be an essential prerequisite for DC development and is not expressed on monocyte-derived DCs in vitro.
Flt3+, but Not Flt3−, Lymphoid- and Myeloid-committed Progenitors Generate DCs.
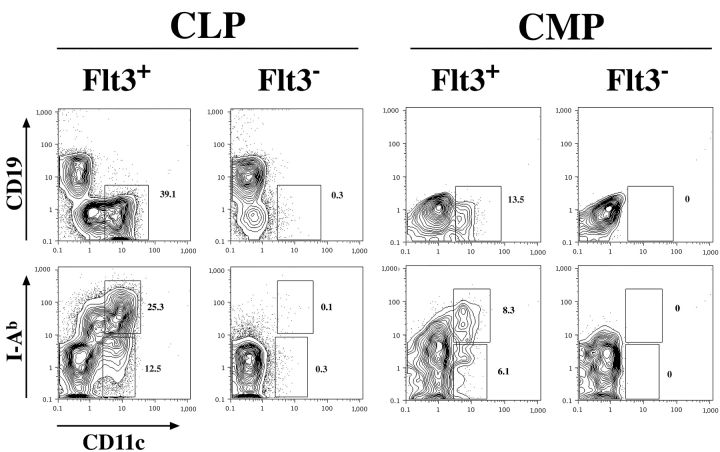
As shown above, both CLPs and CMPs are heterogeneous in Flt3 expression. Therefore, we wanted to investigate if Flt3+ and/or Flt3− CLPs and CMPs, respectively, would generate DCs (Fig. 2) . To do so we isolated subpopulations of CLP and CMP according to their expression of Flt3 and tested their developmental potential. 2,000 highly purified cells of each population were cultured per well in the presence of AC6 stroma cells and cytokines that provided an optimized environment for DC development in vitro. After 6 d cultures were analyzed by FACS® for the generation of lymphoid and/or myeloid cells as well as DCs. The data in Fig. 2 show that CLPs, whether they express Flt3 or not, give rise to B cells identified by their expression of CD19. But only the Flt3+ subpopulation also give rise to CD11c+ DCs that are mainly positive for MHC II. In contrast, the majority of CMP-derived cells were CD11bhi macrophages (∼80% in average) characterized by their high autofluorescence properties. The FACS® plots showing CMP progeny in Fig. 2 are therefore gated on SSClow/intermediate cells to exclude cells with a high autofluorescence. No CD19+ B cells were detected. But comparable to the result with CLPs, only the Flt3+ subpopulation of CMPs was able to generate CD11c+ MHC II+ DCs. Therefore, Flt3+ CLPs have lymphoid and DC potential and Flt3+ CMPs have myeloid and DC potential. Thus, Flt3 expression on CLPs or CMPs does not define a Flt3+ common DC progenitor. Rather, DC developmental potential segregates with both the lymphoid and myeloid lineage.
Figure 2.
Only Flt3+ CLPs and CMPs generate DCs. Flt3+ and Flt3− subpopulations of CLPs and CMPs were sorted and 2,000 cells each were cultured in vitro on AC6 stroma cells in presence of IL-7/SLF/Flt3L for CLPs or SLF/Flt3L for CMPs. After 6 d these cultures were analyzed by FACS® and the percentages of the DC populations are given. Plots showing CMP-derived cells are gated on SSClow/intermediate cells to exclude macrophages with characteristically high autofluorescence. Data shown are representative for three independent experiments.
Flt3L Expands Flt3-expressing Lymphoid- and Myeloid-committed Progenitor Cells but No or Little of Their Immediate Downstream Flt3− Progeny In Vivo.
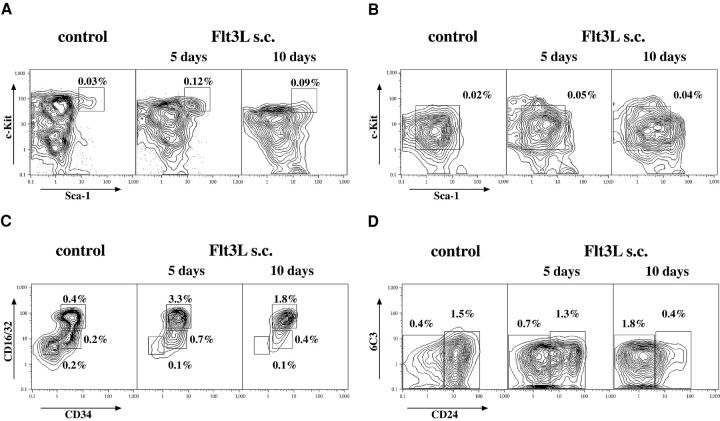
To directly test the effect of Flt3 stimulation on successive early hematopoietic progenitors, mice were injected with human Flt3L and relative and absolute numbers of progenitors were evaluated by FACS® after 5 and 10 d of injection, respectively (Fig. 3 and Table I). All lymphoid and myeloid Flt3+ progenitors expanded and, with the exception of pro-B (Fig. 3 A) cells, showed higher expansion levels after 5 d compared with 10 d. Remarkably, GMPs showed the highest, near 10-fold, overall expansion. Thus, although Flt3-expressing progenitors were expanded through cognate ligand stimulation, immediate downstream Flt3− progeny populations were not or only slightly, possibly due to the lack of concurrent up-regulation of additional cytokines necessary for further differentiation as IL-7 for lymphoid or thrombopoietin/erythropoietin for megakaryocyte/erythrocyte development.
Figure 3.
Flt3L expands hematopoietic progenitors. FACS® analysis of hematopoietic progenitor populations in the bone marrow after 5 and 10 d of s.c. Flt3L injections. Mice injected for 5 and 10 d (not depicted) with PBS were used as a control. (A) HSCs (electronically gated on Lin−/lo Thy1.1lo cells). (B) CLPs (gated on Lin−/lo Thy1.1− IL-7Rα+). (C) CMPs (CD34+ CD16/32lo), GMPs (CD34+ CD16/32hi), and MEPs (CD34− CD16/32lo; gated on Lin−/lo Sca-1− c-Kit+ cells). (D) Pro-B cells (gated on IgM− NK1.1− B220+ CD43+ cells): pro B (A) are CD24−/lo 6C3− and pro-B (BC) are CD24+. Numbers delineate relative percentages of the according population in total bone marrow.
Table I.
Flt3L Expands Lymphoid and Myeloid Progenitors In Vivo
| 5 Days |
10 Days |
Fold expansion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Flt3L s.c. | Control | Flt3L s.c. | 5 Days | 10 Days | |||||
| percent | total no. ×103 | percent | total no. ×103 | percent | total no. ×103 | percent | total no. ×103 | |||
| HSCa | 0.03 | 17.5 | 0.14 | 96.6 | 0.03 | 16.2 | 0.09 | 65.3 | 5.5 | 4 |
| CLPa | 0.03 | 12.5 | 0.05 | 34.5 | 0.02 | 10.8 | 0.04 | 29 | 2.8 | 2.7 |
| CMPa | 0.22 | 110 | 0.71 | 490 | 0.22 | 119 | 0.48 | 348 | 4.4 | 2.9 |
| GMPa | 0.5 | 250 | 3.5 | 2,415 | 0.4 | 216 | 1.9 | 1,378 | 9.7 | 6.4 |
| MEPa | 0.12 | 60 | 0.1 | 69 | 0.08 | 43.2 | 0.065 | 47.1 | 1.2 | 1.1 |
| pB(A)a | 0.4 | 200 | 0.5 | 345 | 0.45 | 243 | 1.8 | 1,305 | 1.7 | 5.4 |
| pB(BC)a | 1.5 | 750 | 1.3 | 897 | 1.5 | 810 | 0.4 | 290 | 1.2 | 0.36 |
| pT1b | 0.02 | 30 | 0.08 | 104 | 0.02 | 30 | 0.05 | 60 | 3.5 | 2 |
| pT2b | 0.07 | 105 | 0.15 | 195 | 0.06 | 90 | 0.05 | 60 | 1.9 | 0.7 |
Relative percentages and total cell count of hematopoietic progenitor populations in bone marrow of two hind
legs or
thymus after 5 and 10 d of Flt3L or PBS injection, respectively. Results represent means of three to four animals per group and are representative of three independent experiments.
Flt3L Expands DCs, Natural Interferon-producing Cells, Immature Myeloid Cells, Granulocytes, and Monocytes, but Not T Cells and B Cells, in Spleen.
Next, we wanted to test how numbers of mature cell types would be affected under Flt3L injection and how this is correlated with expansion and Flt3 expression of their respective progenitor cells. Therefore, we analyzed spleen cells after 5 and 10 d of Flt3L injection (Table II). CD11c+ IAb+ DCs and CD11clo Gr-1+ B220+ IPCs (24, 30, 31) continuously expanded in relative and absolute numbers to reach on average a 21-fold and 35-fold expansion after 10 d of injection, respectively. Gr-1hi CD11b+ CD11c− immature myeloid cells and neutrophils expanded about equally (25.5-fold). Gr-1−/lo CD11b+ CD11c− B220− immature myeloid cells and monocytes expanded 4.8-fold. In contrast, B cell and T cell numbers were nearly unchanged (Table II). Therefore, beyond the expected expansion of CD11c+ IAb+ DCs (8), these data confirm that IPC numbers can be dramatically increased through Flt3L (30). Also, although CLPs, pro-B (A) cells, and pro-T1 cells are expanded by Flt3L, this did not lead to an increase in mature B cell and T cell numbers. In contrast, Flt3L-induced expansion of GMPs lead to a massive increase in immature myeloid cells and neutrophils, and a moderate increase in monocytes.
Table II.
Expansion of Spleen Cells after Flt3L Injections
| 5 Days |
10 Days |
Fold expansion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Flt3L s.c. | Control | Flt3L s.c. | 5 Days | 10 Days | |||||
| percent | total no. ×103 | percent | total no. ×103 | percent | total no. ×103 | percent | total no. ×103 | |||
| B cells | 61 | 67,100 | 54 | 72,900 | 55 | 67,000 | 26 | 68,900 | 1.1 | 1.0 |
| T cells | 35 | 38,500 | 36 | 48,600 | 36 | 44,000 | 21 | 55,600 | 1.3 | 1.3 |
| Neutrophils | 0.9 | 990 | 1.6 | 2,160 | 0.5 | 610 | 5.9 | 15,630 | 2.2 | 25.5 |
| Monocytes | 3.3 | 3,600 | 6.1 | 8,235 | 2 | 2,400 | 9.4 | 11,470 | 2.3 | 4.8 |
| DCs | 0.6 | 660 | 2.7 | 3,645 | 0.6 | 730 | 12.6 | 15,370 | 5.5 | 21 |
| IPCs | 0.04 | 44 | 0.07 | 95 | 0.05 | 61 | 1.4 | 1,708 | 2.2 | 28 |
| CLP-DCsa | 0.1 | 106 | 0.35 | 424 | 3.9 | |||||
| CMP-DCsa | 0.17 | 176 | 0.47 | 572 | 3.2 | |||||
Changes in relative percentages and total cell number of mature hematopoietic populations in the spleen after 5 and 10 d of injections with Flt3L or PBS (control group), respectively. Results represent means of three to four animals per group and are representative of three independent experiments.
Percentages and total numbers of CLP- and CMP-derived DCs in spleens of transplanted animals after 10 d of Flt3L or PBS treatment, respectively. Results represent means of three experiments each involving two to four animals in the Flt3L injection and two mice in the control group.
Flt3L Supports DC Development along Both a Lymphoid and Myeloid Developmental Pathway In Vivo.
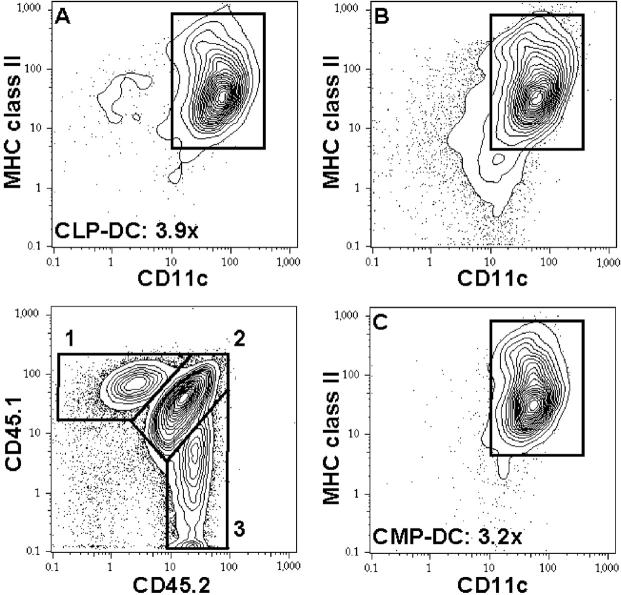
Finally, we wanted to test if Flt3L supports DC development from both CLPs and CMPs in vivo. We first evaluated whether Flt3L would expand DC numbers in irradiated animals transplanted with hematopoietic cells. Lethally (9.5 Gy) or sublethally (4.75 Gy) irradiated mice were transplanted with 2 × 105 congenic whole bone marrow cells. Flt3L was injected over 10 d, starting on day 2 after transplantation, and mice were evaluated 1 d after the last Flt3L injection. In Flt3L-injected mice, donor-derived spleen CD11c+ IAb+ DC numbers were on average 3.7-fold (9.5 Gy) and 5.4-fold (4.75 Gy) increased (two experiments each involving two mice per group; not depicted). Host spleen DCs were hardly detectable above background in lethally irradiated animals and were almost unchanged compared with controls in sublethally irradiated mice (1.2-fold expansion; not depicted). Therefore, Flt3L injection leads to an increase in DC numbers in irradiated and whole bone marrow–transplanted animals early after transplantation, although this increase is minor compared with DC expansion in Flt3L-injected healthy animals, possibly due to competing cytokine signals in irradiation-induced hematopoietic damage. Upon transplantation of 104 CLPs and 2 × 104 CMPs into 4.75 Gy irradiated animals, CLP- and CMP-derived CD11c+ IAb+ spleen DCs expanded on average 3.9-fold and 3.2-fold (Table II and Fig. 4) . These results directly demonstrate that Flt3L can drive DC development and expansion from both Flt3-expressing early lymphoid- and myeloid-committed progenitors in vivo.
Figure 4.
Flt3L expands CLP- and CMP-derived DCs in vivo. 104 CD45.1+ CLPs and 2 × 104 CD45.2+ CMPs were competitively transplanted into sublethally irradiated CD45.1/CD45.2 F1 mice. Flt3L or control PBS was injected over 10 d, starting on day 2 after transplantation, and mice were evaluated 1 d after the last Flt3L injection. Lower left contour plot shows CD11c-enriched splenic progeny of CLPs (1), host cells (2), and CMPs (3). CD11c and MHC II expression of cells in gates 1, 2, and 3 are shown in contour plots A, B, and C, respectively. Average expansions of CLP- and CMP-derived DCs are indicated. Plots show DC read out of a Flt3L-injected animal and are representative of three experiments involving two to four mice in the Flt3L-treated group and two animals as controls each. For relative and total numbers of CLP- and CMP-derived DCs see Table II.
Discussion
Immunophenotypic characterization and isolation of highly enriched hematopoietic progenitor cell populations at defined successive stages of development provides the means to study sequential changes in gene expression profiles during commitment to a lineage and the differentiation into a mature cell type. Here we mapped mRNA and cell surface expression of Flt3 on uncommitted and consecutive lineage–committed early hematopoietic progenitors and on mature steady state haematolymphoid cells, and we tested their in vivo response to Flt3L.
In early hematopoiesis, our findings show that Flt3 is transiently up-regulated from short-term HSCs (21, 22) in a substantial fraction of CLPs (60–70%) and CMPs (50–65%). Flt3 is maintained at low levels in early GMP-committed progenitor cells, and is down-regulated and lost once definitive B cell (pro-B[BC]), T cell (pro-T2), and megakaryocyte/erythrocyte (MEP) lineage commitment occurs (Fig. 1). Flt3+, but no or little of Flt3−, progenitors are expanded by in vivo Flt3 stimulation (Fig. 3 and Table I). These findings explain the observations that in vivo application of Flt3L massively increases and depletion of Flt3L decreases mixed (CFU-GEMM), CFU-GM, as well as B cell colony forming units, but no or little of CFUs or mature cells of the erythroid or megakaryocytic lineage (6), and are in agreement with previous reports evaluating Flt3 expression on nonclonally defined hematopoietic progenitor cell populations in mice and humans (1, 32–36). The dramatic increase of GMPs and downstream immature myeloid cells (Tables I and II) underlines the importance Flt3L as a myelopoietic factor, and it shall be important to determine whether this is independent of G-CSF and GM-CSF action.
In contrast to other hematopoietic lineages, developmental options for at least CD8α+ and CD8α− DCs are conserved beyond both early lymphoid and myeloid commitment (17, 19, 20), and here we directly show that Flt3L can drive DC development along both pathways (Fig. 4 and Table II). Because Flt3 is expressed on progenitors and mature, steady state DCs, and all Flt3+ cells are expanded by Flt3L, an important question is whether Flt3 expression and signaling is a prerequisite for DC development. Flt3-expressing CLPs, pro-T1 cells, CMPs, and GMPs have in vitro and in vivo DC developmental potential, whereas Flt3− fractions of CLPs, CMPs, and Flt3− MEPs do not (18–20). We were not able to detect Flt3 in pro-T2 cells. However, on a population basis, pro-T2 cells are able to develop into DCs (19, 37). Also, Flt3− monocytes generate DCs in vitro (Fig. 1) and likely in vivo (38). Conversely, pro-B (A) cells clearly express Flt3, but DC development from pro-B cells is still under debate. It was claimed that whole pro-B cells have DC potential (39), however, we were not able to detect this (19), whereas in a more specified evaluation, minor DC potential resided only in a very early pro-B (A1) fraction (with similarities to CLPs), but not within more mature pro-B cells (40). Thus, most but not all Flt3+ progenitors can develop into DCs, but Flt3 expression seems not to be essential for all DC precursor cells. Developmental outcome might be regulated by competing signals, in the case of pro-B cells possibly by the up-regulation of Pax 5 (41). It should be valuable to test whether overexpression and stimulation of Flt3 can “rescue” progenitors to the DC lineage that normally have lost DC developmental potential.
Another critical question is whether Flt3L-expanded Flt3+ progenitors are primed to develop into DCs or whether they retain their original developmental capacities. We did not detect differences in the in vivo and in vitro lymphoid or myeloid read out, nor did we observe superior DC read out from in vivo Flt3L-expanded CLPs, CMPs, and GMPs (unpublished data). Therefore, Flt3 stimulation and consecutive expansion of early, defined progenitor populations seems not to change their biology, in agreement with previous data on Flt3-expanded, nonpurified progenitor cells (6, 7).
In contrast to preceding data, where surface Flt3 was not observed on DCs (8), we detected both mRNA and low surface protein expression on all evaluated major steady state DC populations, and on DCs generated in vitro from CLPs and CMPs, but not from monocytes (Fig. 1, C and F, and unpublished data). Flt3 on DCs might be functionally important for DC biology, e.g., in the interaction with T cells that express membrane-bound Flt3L (42), alternatively, low Flt3 surface expression on steady state DCs might be a remainder of development. Indeed, CD11c+ MHC class II− cells in bone marrow that might contain immediate DC precursors express high levels of Flt3 (Fig. 1 F), however, blood cells that are reported to be DC restricted (25) and spleen cells with the same phenotype are Flt3−. Therefore, Flt3 is either consecutively up-regulated on DCs again, or alternatively, the CD11c+ MHC class II− cell populations isolated from blood and spleen would not present an exclusive fraction of DC progenitors.
Given the importance of in vivo Flt3/Flt3L (5, 8), but not GM-CSFR/GM-CSF (8, 43), on DC numbers in lymphoid organs, how do our findings integrate Flt3L versus other DC-promoting cytokines, such as GM-CSF in a model of DC homeostasis? Likely, most of lymphoid tissue DCs derive from Flt3+ progenitors during steady state DC turnover, whereas few derive from Flt3− monocytes. This might change dramatically in inflammation, where GM-CSF becomes an abundant cytokine and tissue monocytes/macrophages likely mature to DCs that migrate to lymph nodes (38).
Acknowledgments
We thank the Immunex Corporation for providing us with recombinant human Flt3L, L. Jerabek for excellent laboratory management, S. Smith for antibody preparation, and L. Hidalgo and D. Escoto for animal care.
This work was supported in part by a fellowship from the Ernst Schering Research Foundation (to H. Karsunky), a fellowship grant from Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung für Krebsforschung (to M.G. Manz), a fellowship grant from the Swiss National Foundation (to A. Cozzio), U.S. Public Health Service grant CA86017, and the National Institutes of Health (grant AI47458 to I.L. Weissman).
Footnotes
Abbreviations used in this paper: CLP, common lymphoid progenitor; CMP, common myeloid progenitor; Flt3L, Flt3 ligand; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell; IPC, interferon α–producing cell; MEP, megakaryocyte/erythrocyte progenitor; SLF, steel factor.
References
- 1.Matthews, W., C.T. Jordan, G.W. Wiegand, D. Pardoll, and I.R. Lemischka. 1991. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 65:1143–1152. [DOI] [PubMed] [Google Scholar]
- 2.Small, D., M. Levenstein, E. Kim, C. Carow, S. Amin, P. Rockwell, L. Witte, C. Burrow, M.Z. Ratajczak, A.M. Gewirtz, et al. 1994. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc. Natl. Acad. Sci. USA. 91:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyman, S.D., and S.E. Jacobsen. 1998. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 91:1101–1134. [PubMed] [Google Scholar]
- 4.Mackarehtschian, K., J.D. Hardin, K.A. Moore, S. Boast, S.P. Goff, and I.R. Lemischka. 1995. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 3:147–161. [DOI] [PubMed] [Google Scholar]
- 5.McKenna, H.J., K.L. Stocking, R.E. Miller, K. Brasel, T. De Smedt, E. Maraskovsky, C.R. Maliszewski, D.H. Lynch, J. Smith, B. Pulendran, et al. 2000. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 95:3489–3497. [PubMed] [Google Scholar]
- 6.Brasel, K., H.J. McKenna, P.J. Morrissey, K. Charrier, A.E. Morris, C.C. Lee, D.E. Williams, and S.D. Lyman. 1996. Hematologic effects of flt3 ligand in vivo in mice. Blood. 88:2004–2012. [PubMed] [Google Scholar]
- 7.Brasel, K., H.J. McKenna, K. Charrier, P.J. Morrissey, D.E. Williams, and S.D. Lyman. 1997. Flt3 ligand synergizes with granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor to mobilize hematopoietic progenitor cells into the peripheral blood of mice. Blood. 90:3781–3788. [PubMed] [Google Scholar]
- 8.Maraskovsky, E., K. Brasel, M. Teepe, E.R. Roux, S.D. Lyman, K. Shortman, and H.J. McKenna. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand–treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan, T.S., I.K. McNiece, G. Van, D. Lacey, C. Hartley, P. McElroy, Y. Sun, J. Argento, D. Hill, X.Q. Yan, et al. 1997. Chronic expression of murine flt3 ligand in mice results in increased circulating white blood cell levels and abnormal cellular infiltrates associated with splenic fibrosis. Blood. 90:76–84. [PubMed] [Google Scholar]
- 10.Hawley, T.S., A.Z. Fong, H. Griesser, S.D. Lyman, and R.G. Hawley. 1998. Leukemic predisposition of mice transplanted with gene-modified hematopoietic precursors expressing flt3 ligand. Blood. 92:2003–2011. [PubMed] [Google Scholar]
- 11.Kelly, L.M., Q. Liu, J.L. Kutok, I.R. Williams, C.L. Boulton, and D.G. Gilliland. 2002. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 99:310–318. [DOI] [PubMed] [Google Scholar]
- 12.Shaw, S.G., A.A. Maung, R.J. Steptoe, A.W. Thomson, and N.L. Vujanovic. 1998. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J. Immunol. 161:2817–2824. [PubMed] [Google Scholar]
- 13.O'Keeffe, M., H. Hochrein, D. Vremec, J. Pooley, R. Evans, S. Woulfe, and K. Shortman. 2002. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 99:2122–2130. [DOI] [PubMed] [Google Scholar]
- 14.Brasel, K., T. De Smedt, J.L. Smith, and C.R. Maliszewski. 2000. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 96:3029–3039. [PubMed] [Google Scholar]
- 15.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X.L. Xu, G. Trinchieri, A. O'Garra, and Y.J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3 ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo, M., I.L. Weissman, and K. Akashi. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91:661–672. [DOI] [PubMed] [Google Scholar]
- 17.Akashi, K., D. Traver, T. Miyamoto, and I.L. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 404:193–197. [DOI] [PubMed] [Google Scholar]
- 18.Traver, D., K. Akashi, M. Manz, M. Merad, T. Miyamoto, E.G. Engleman, and I.L. Weissman. 2000. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 290:2152–2154. [DOI] [PubMed] [Google Scholar]
- 19.Manz, M.G., D. Traver, T. Miyamoto, I.L. Weissman, and K. Akashi. 2001. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 97:3333–3341. [DOI] [PubMed] [Google Scholar]
- 20.Wu, L., A. D'Amico, H. Hochrein, M. O'Keeffe, K. Shortman, and K. Lucas. 2001. Development of thymic and splenic dendritic cell populations from different hemopoietic precursors. Blood. 98:3376–3382. [DOI] [PubMed] [Google Scholar]
- 21.Christensen, J.L., and I.L. Weissman. 2001. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 98:14541–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adolfsson, J., O.J. Borge, D. Bryder, K. Theilgaard-Monch, I. Astrand-Grundstrom, E. Sitnicka, Y. Sasaki, and S.E. Jacobsen. 2001. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 15:659–669. [DOI] [PubMed] [Google Scholar]
- 23.Hardy, R.R., C.E. Carmack, S.A. Shinton, J.D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, et al. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 25.del Hoyo, G.M., P. Martin, H.H. Vargas, S. Ruiz, C.F. Arias, and C. Ardavin. 2002. Characterization of a common precursor population for dendritic cells. Nature. 415:1043–1047. [DOI] [PubMed] [Google Scholar]
- 26.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pharr, P.N., and A. Hofbauer. 1997. Loss of flk-2/flt3 expression during commitment of multipotent mouse hematopoietic progenitor cells to the mast cell lineage. Exp. Hematol. 25:620–628. [PubMed] [Google Scholar]
- 28.Manz, M.G., T. Miyamoto, K. Akashi, and I.L. Weissman. 2002. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. USA. 99:11872–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright, D.E., A.J. Wagers, A.P. Gulati, F.L. Johnson, and I.L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936. [DOI] [PubMed] [Google Scholar]
- 30.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 98:3520–3526. [DOI] [PubMed] [Google Scholar]
- 31.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c+ B220+ Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasko, J.E., D. Metcalf, M.T. Rossner, C.G. Begley, and N.A. Nicola. 1995. The flt3/flk-2 ligand: receptor distribution and action on murine haemopoietic cell survival and proliferation. Leukemia. 9:2058–2066. [PubMed] [Google Scholar]
- 33.Wasserman, R., Y.S. Li, and R.R. Hardy. 1995. Differential expression of the blk and ret tyrosine kinases during B lineage development is dependent on Ig rearrangement. J. Immunol. 155:644–651. [PubMed] [Google Scholar]
- 34.Rappold, I., B.L. Ziegler, I. Kohler, S. Marchetto, O. Rosnet, D. Birnbaum, P.J. Simmons, A.C. Zannettino, B. Hill, S. Neu, et al. 1997. Functional and phenotypic characterization of cord blood and bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinase. Blood. 90:111–125. [PubMed] [Google Scholar]
- 35.Tudor, K.S., K.J. Payne, Y. Yamashita, and P.W. Kincade. 2000. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 12:335–345. [DOI] [PubMed] [Google Scholar]
- 36.Gotze, K.S., M. Ramirez, K. Tabor, D. Small, W. Matthews, and C.I. Civin. 1998. Flt3high and Flt3low CD34+ progenitor cells isolated from human bone marrow are functionally distinct. Blood. 91:1947–1958. [PubMed] [Google Scholar]
- 37.Wu, L., M. Antica, G.R. Johnson, R. Scollay, and K. Shortman. 1991. Developmental potential of the earliest precursor cells from the adult mouse thymus. J. Exp. Med. 174:1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. [DOI] [PubMed] [Google Scholar]
- 39.Bjorck, P., and P.W. Kincade. 1998. CD19+ pro-B cells can give rise to dendritic cells in vitro. J. Immunol. 161:5795–5799. [PubMed] [Google Scholar]
- 40.Izon, D., K. Rudd, W. DeMuth, W.S. Pear, C. Clendenin, R.C. Lindsley, and D. Allman. 2001. A common pathway for dendritic cell and early B cell development. J. Immunol. 167:1387–1392. [DOI] [PubMed] [Google Scholar]
- 41.Nutt, S.L., B. Heavey, A.G. Rolink, and M. Busslinger. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 401:556–562. [DOI] [PubMed] [Google Scholar]
- 42.Lyman, S.D., L. James, S. Escobar, H. Downey, P. de Vries, K. Brasel, K. Stocking, M.P. Beckmann, N.G. Copeland, L.S. Cleveland, et al. 1995. Identification of soluble and membrane-bound isoforms of the murine flt3 ligand generated by alternative splicing of mRNAs. Oncogene. 10:149–157. [PubMed] [Google Scholar]
- 43.Vremec, D., G.J. Lieschke, A.R. Dunn, L. Robb, D. Metcalf, and K. Shortman. 1997. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27:40–44. [DOI] [PubMed] [Google Scholar]