Abstract
The marginal zone of the spleen is a precisely ordered region that contains specialized subsets of B lymphocytes and macrophages. Disruption of the negative signaling inositol phosphatase, SH2-containing inositol-5-phosphatase 1 (SHIP), results in the loss of marginal zone B cells (MZBs) with reorganization of marginal zone macrophages (MZMOs) to the red pulp of the spleen. This primary macrophage defect, as revealed by selectively depleting SHIP in myeloid cells shows that MZMOs are specifically required for the retention of MZBs. The MZMO phenotype was reverted in SHIP/Bruton's tyrosine kinase (Btk) double knockout mice, thus identifying the Btk activating pathway as an essential component being regulated by SHIP. Furthermore, we identified a direct interaction between the MARCO scavenger receptor on MZMOs and MZBs. Activation or disruption of this interaction results in MZB migration to the follicle. The migration of the MZMOs was further studied after the response to Staphylococcus aureus, which induced MZMOs to move into the red pulp while MZBs migrated into the follicular zone. The marginal zone is therefore a dynamic structure in which retention and trafficking of B cells requires specific macrophage–B cell interactions.
Keywords: SHIP, Btk, MARCO, migration, Staphylococcus aureus
Introduction
Blood-borne pathogens first encounter the adaptive immune system in the marginal zone region of the spleen where the convergence of innate and adaptive immune mechanisms insures an early and effective response to pathogen antigens (1, 2). Both thymic-independent and -dependent responses are elicited in response to infection (1, 3). The thymic-independent response involves the targeting and activation of marginal zone B cells (MZBs)*through their interaction with the repetitive antigenic determinants of pathogens with complement and B cell antigen receptors (4, 5). In contrast, the thymic-dependent Ab response is driven by the interaction and reciprocal stimulation of APCs, T lymphocytes, and B cells. The organization of the splenic white pulp nodule into discrete zones enriched for either B cells, T cells, or APCs provide a spatial microenvironment that facilitates an efficient interaction of pathogens with the various cellular populations required for insuring an efficient immune response (6–8). Antigen presentation and stimulation of T and B cells ultimately results in the formation of germinal centers, high affinity neutralizing Abs, and memory cells. Recent reports have begun to define the cellular components and molecular signals that are necessary to establish the marginal zone. B cell intrinsic pathways have been described involving specific chemokines and their receptors, molecules involved in B cell activation, as well as adhesion molecules and their ligands (9, 10). Apart from the MZB, the other predominant cell of the marginal zone is the marginal zone macrophage (MZMO), which is distinct from the metallophilic macrophage, defined by the marker MOMA-1, located at the border of the marginal and follicular zone (11). The MZMO is defined by its location, interspersed in several layers within the marginal zone, and by its expression of the markers MARCO and ER-TR9 (12, 13). The former molecule is a scavenger receptor belonging structurally to the class A receptor family whereas the latter is identical to the C-type lectin SIGN-RI (14–17). MARCO has been shown to bind a range of microbial Ags including Staphylococcus aureus and Escherichia coli whereas SIGN-RI is the predominant receptor for uptake of polysaccharide dextran by MZMOs. Even though both MZBs and MZMOs are implicated in both thymus-dependent and -independent immune responses, the exact roles of the two cell types in initiation of the response to blood-borne pathogens is not known. We now define a unique role for the MZMO in regulation of MZB retention and activation and show that movement of this subset of macrophages to the red pulp of the spleen involves signaling via SH2-containing inositol-5-phosphatase 1 (SHIP) and Bruton's tyrosine kinase (Btk). In addition, we show a direct interaction between MZMOs and MZBs via the MARCO receptor on MZMOs and a ligand on MZBs.
Materials and Methods
Mice.
C57BL/6 mice obtained from The Jackson Laboratory were used as WT mice and controls unless otherwise stated. Founders of SHIP-deficient mice were provided by G. Krystal (Terry Fox Laboratory, BC Cancer Agency, Vancouver, Canada; reference 18) and Btk-deficient mice were purchased from The Jackson Laboratory. Op/op mice were provided by J. Pollard (Albert Einstein College of Medicine, New York, NY) and LysMCre transgenic mice (19) were provided by I. Forster (Technical University of Munich, Germany). Abs and bacteria was injected i.v. in the tail vein and all experiments involving mice were performed in accordance with National Institutes of Health (NIH) guidelines. All mice were maintained under specific pathogen-free conditions at The Rockefeller University.
Antibodies and Reagents.
For histological examination 6-μM frozen sections were stained, and for FACS® analysis erythrocyte-depleted spleen cells were used. Macrophages were detected using MOMA-1, MARCO Abs from Serotec, and ER-TR9 from Accurate Chemical & Scientific Corp. Abs to CD1d, B220, CD19, CD21/CD35 (CRI/II), CD23, MAC-1, anti–rat alkaline phosphatase, and anti–rabbit horseradish peroxidase were from BD Biosciences. Secondary Abs for immunohistochemistry, anti-biotin, anti-FITC F(ab′) horseradish peroxidase, or alkaline phosphatase were from DakoCytomation and rabbit anti–SHIP used for Western blot was from Upstate Biotechnology. Vector Blue Alkaline Phosphatase Substrate from Vector Laboratories and DAB peroxidase substrate from Sigma-Aldrich were used for development of immunohistochemistry stains. Soluble MARCO receptor was provided by T. Pikkarainen (The Karolinska Institute, Stockholm, Sweden; reference 20) and was biotinylated using the EZ-Link™ kit from Pierce Chemical Co. The biotinylated soluble MARCO was detected using Streptavidin-CyChrome™ from BD Biosciences. S. aureus fluorescent bioparticles were purchased from Molecular Probes, Inc. and MACS anti-FITC and anti-biotin beads were from Miltenyi Biotec. Cl2MDP (or clodronate) and PBS liposomes were provided by Roche Diagnostics.
Conditional Targeting of SHIP.
Floxed SHIP mice were created by insertion of loxP sites flanking the 10th and 11th exons (see Fig. 2 a) of the SHIP gene. The targeting vector was introduced into embryonic stem (ES) cells by electroporation and clones were selected with neomycin and ganciclovir and verified by Southern blot and PCR. Properly integrated ES clones were transiently transfected with a Cre-expressing plasmid. Clones were subsequently selected for a conditional floxed allele (SHIPflox) or null allele (SHIPnull) using Southern blot and PCR. Appropriate ES clones were then injected into blastocysts to generate chimeric mice. The chimeric mice were then bred with C57BL/6 mice to achieve germline transmission. These mice were subsequently crossed with mice expressing Cre in the myeloid compartment (LysMcre; reference 19) to generate Cre+/null/flox mice. Mice were screened for respective genotype by PCR and SHIP protein expression using Western blot (21) on equal numbers of spleen cells purified by MACS (Miltenyi Biotec) sorting according to protocol from the manufacturer. Relative expression of SHIP in macrophage and B cell populations (comparing wt/null with flox/null/cre) were estimated using Alpha imager software from Alpha Innotech Corp.
Figure 2.
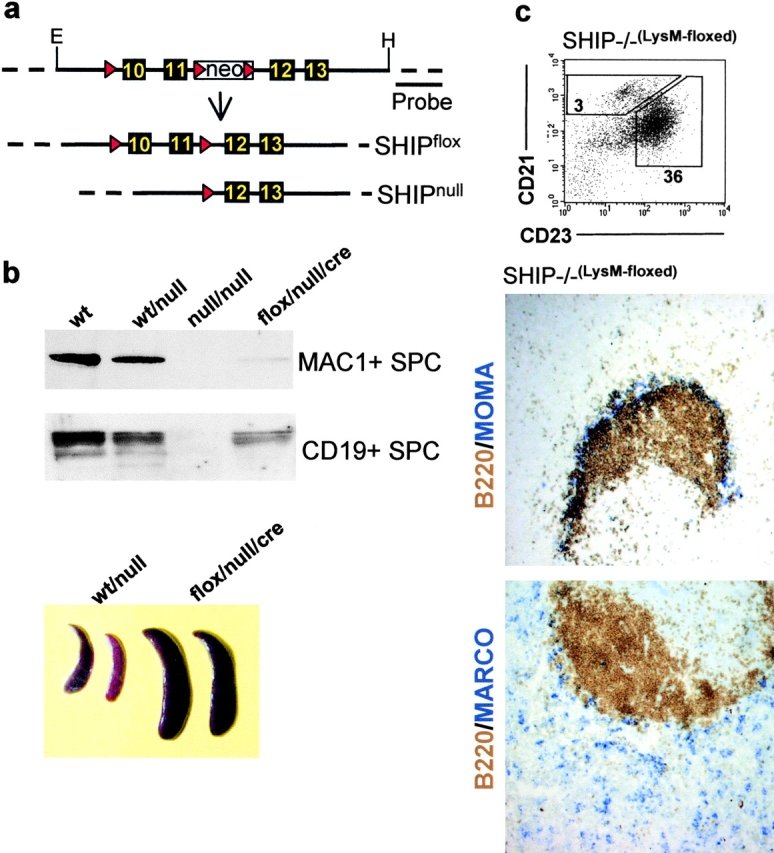
Conditional targeting of SHIP in macrophages results in MZMO displacement and reduced numbers of MZBs. (a) A targeting construct covering exons 10 to 13 of SHIP, from EcoRI (E) to HindIII (H), was made. Boxes represent exons and triangles represent loxP sites flanking exons 10 to 11 and a neomycin resistance gene (neo). Properly integrated ES cell clones were transiently transfected with Cre recombinase to create conditional floxed (SHIPflox) or null (SHIPnull) clones. These cells were subsequently used to create floxed (flox) and null mice, which were crossed to mice expressing Cre from a macrophage-specific lysosomal promoter (cre). (b) Western blot analysis of MAC1+ and CD19+ spleen cells (SPC) from WT, WT/null, null/null, LysM floxed (flox/null/cre), and relative spleen size of 6-wk-old WT/null and flox/null/cre SHIP mice. (c) FACS® and histological profiles of single cell suspensions from the spleen of the conditionally targeted SHIP KO mice. MZBs were measured as the CD19+, CRIhigh, and CD23low population. The numbers shown represent percent of CD19+ cells for the depicted gates as an average of five mice and the numbers for the follicular B cells are shown for comparison. For representative immunohistochemical analysis, at least four serial sections were stained for MOMA-1+ (blue, top) metallophilic macrophages or MARCO+ MZMOs (blue, bottom). Sections were also stained for B220 (brown) to show the positioning of the follicle. Refer to Fig. 1 for SHIP+/− and SHIP−/− profiles. ×10.
Results and Discussion
Mice deficient in the inhibitory signaling molecule SHIP display pleiotropic defects in macrophages, NK cells, and lymphocytes (18, 22). A prominent feature of these mice is their splenomegaly resulting from dysregulation of myeloid proliferation. As seen in Fig. 1 , SHIP-deficient mice also display a specific defect in the organization of the splenic follicle with the loss of MZBs measured as the CD21high/CD23low population in FACS® and in sections as the B220+ cells localizing peripherally to the MOMA-1+ cells (Fig. 1, a and b). In the SHIP-deficient mice the MARCO+ MZMO cells are no longer organized within the marginal zone and adjacent to the MOMA-1 macrophages but are redistributed to the red pulp, whereas MOMA-1+ metallophils remain unaffected (Fig. 1 b). Because SHIP is expressed in most hematopoietic cells, including lymphoid and myeloid subsets, we determined if this marginal zone phenotype in SHIP-deficient mice was the result of primary macrophage dysregulation. A conditional disruption of SHIP was generated in which macrophages displayed an approximate >90% reduction in SHIP expression whereas B cell expression was reduced by <10% (Fig. 2 , a and b). This is consistent with the expression patterns of Cre recombinase, driven by the lysosyme promoter used (19). The mice developed a splenomegaly at ∼5 wk of age (Fig. 2 b), similar to that of complete SHIP deletion, thus implicating a primary macrophage defect as the cause for splenomegaly in SHIP−/− mice (18). In addition, the mice displayed essentially the same marginal zone phenotype with significantly reduced MZBs as defined by flow cytometry and reorganization of the MZMOs as observed by histological staining (Fig. 2 c). To confirm that the SHIP phenotype is B cell nonautonomous and that SHIP-deficient B cells can give rise to MZB populations when WT MZMOs are available, we produced BM chimeras using SHIP-deficient BM combined with WT BM and injected these cells into irradiated WT recipients. In the resulting chimeric mice the SHIP-deficient and WT BMs contributed equally to the MZB population (unpublished data).
Figure 1.
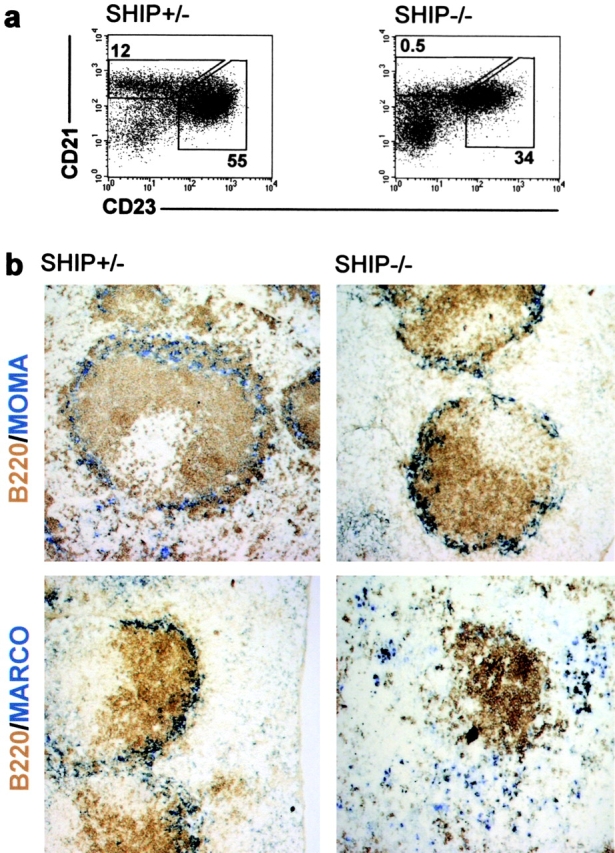
SHIP-deficient mice lack MZBs and MZMOs are displaced to the red pulp. (a) FACS® profiles of single cell suspensions from the spleen of SHIP-heterozygous (SHIP+/−) and -deficient (SHIP−/−) mice. MZBs were measured as the CD19+, CRIhigh, and CD23low population. The numbers shown represent percent of CD19+ cells for the depicted gates as an average of five mice. Numbers for the follicular B cells are shown for comparison. (b) Representative immunohistochemical analysis of above listed mice. At least four serial sections from each mouse were stained for MOMA-1+ (blue, top) metallophilic macrophages or MARCO+ MZMOs (blue, bottom). Sections were also stained for B220 (brown) to show the positioning of the follicle. ×10.
In B cell lines it has been shown that SHIP functions as a negative regulator of cellular activation by regulating the association of the positive signaling kinase Btk with the membrane, thus raising the threshold required for stimulation (23). It does so by hydrolyzing PIP3, the substrate for Btk association with the membrane, thereby reducing the ability of Btk to become membrane associated and activated (24). Because both SHIP and Btk are expressed in macrophages and a link between these molecules had been suggested, we reasoned that the myeloid proliferation and MZMO phenotype leading to the loss of MZBs might be the result of inappropriate activation of Btk in macrophages of SHIP-deficient animals (25, 26). Disruption of Btk in macrophages may thus be sufficient to restore normal signaling thresholds in SHIP-deficient mice. Combining the SHIP deficiency with a Btk deficiency resulted in the restoration of both the normal marginal zone structure (Fig. 3 a) and spleen size (Fig. 3 b) indicating that Btk is an important target of SHIP in myeloid cells in vivo. Similarly, Btk deficiency counteracted the over responsiveness of myeloid progenitors to GM-CSF and M-CSF in SHIP-deficient mice (unpublished data). Both the dysregulation of myeloid proliferation and follicular architecture likely result from enhanced signaling through the Btk pathway in myeloid cells. Reversion of the MZB and myeloid phenotypes in SHIP−/− mice by deletion of Btk suggests that Btk is the dominant Tec family member regulated by SHIP in these cells. The observation that other members of the family are expressed in macrophages and have been shown to be able to substitute for Btk both in vivo and in KO mice indicates a surprising degree of specificity to the SHIP inhibitory pathway (27–29).
Figure 3.

SHIP and Btk interact in myeloid proliferation and activation. (a) FACS® and histological profiles of single cell suspensions from the spleen of SHIP and Btk double KO mice (SHIP−/−/Btk−). MZBs were measured as the CD19+, CRIhigh, and CD23low population. The numbers shown represent percent of CD19+ cells for the depicted gates as an average of four mice and the numbers for the follicular B cells are shown for comparison. For representative immunohistochemical analysis, at least four serial sections from were stained for MOMA-1+ (blue, top) metallophilic macrophages or MARCO+ MZMOs (blue, bottom). Sections were also stained for B220 (brown) to show the positioning of the follicle. ×10. (b) Relative spleen size of 5-wk-old heterozygous KO or double KO mice.
These results suggested that MZMOs might be critical to the organization of the white pulp nodule and localization of MZBs in this structure. To test this directly we exploited the observation that MZMOs can be ablated by their preferential ingestion of macrophage-depleting liposomes (30). At a low concentration of these liposomes we could see preferential depletion of MARCO+ MZMOs as opposed to the adjacent MOMA-1 macrophages (Fig. 4) . Other phagocytic cells in the spleen, such as red pulp macrophages and dendritic cells were largely unaffected by this treatment (not depicted). When MZMOs were depleted in this fashion, we observe a specific reduction in the MZBs by both flow cytometry and histological staining. In contrast, MOMA-1 macrophages are specifically absent in the CSF-1–deficient strain op/op but these mice retain MARCO+/ER-TR9− MZMOs (31, 32). The absence of the MOMA-1+ cells and the ER-TR9 marker did not result in reduction in MZBs, but rather, an expansion of these cells is observed, indicating that the macrophage population that is required for MZB retention are the MARCO+ MZMOs.
Figure 4.

MARCO+ MZMOs are required for retention of MZBs. Representative immunohistochemical analysis and FACS® profiles of spleens from at least four WT mice treated with liposomes or untreated op/op mice. WT mice were injected i.v. with 100 μl PBS containing liposomes or with liposomes containing clodronate at a 1:24 dilution where MZMOs were preferentially depleted. 48 h later serial spleen sections were stained for MOMA-1+ (blue, top) metallophilic macrophages or MARCO+ (blue, middle) MZMOs. The sections were also stained for B220 (brown) to see the positioning of these populations in relation to the B cell follicle. ×10. Spleen cells were analyzed by FACS® analysis for detection of MZBs as measured by the CD19+, CRIhigh, and CD23low population. Numbers shown are the average percent-positive cells of four mice. Similar profiles are shown for untreated op/op mice (right). Data shown are representative of three independent experiments.
The identity of the retention signal expressed by MARCO+ MZMO cells was next determined by investigating the role of specific surface receptors on the MZMO in maintaining the marginal zone structure. The MARCO receptor, in addition to binding to bacteria (33), contains an SRCR domain that has been implicated in binding to CD19+ lymphocytes (34, 35). To determine if MARCO itself is capable of binding to MZBs, we expressed the extracellular domains of MARCO as a soluble molecule (20) and used it to stain splenic populations (Fig. 5) . Three populations of cells were distinguished by flow cytometry when stained with CD21 and CD23. Maximal binding to soluble MARCO was observed for the MZBs (CD21hi CD23low), whereas the follicular B cells (CD21low CD23hi) displayed reduced binding. None of the other splenic populations (T cells, macrophages, or dendritic cells) were capable of binding to soluble MARCO. This binding was specific for the MARCO SRCR domain, as determined by the ability of a monoclonal Ab to this domain (ED31; reference 33) to block the binding of soluble MARCO to MZBs. When the MARCO-specific Ab was injected i.v. to WT mice it resulted in disruption of the marginal zone structure in which MZBs, identified by CD1d staining, were found in the follicular region whereas MZMOs, identified by ER-TR9 staining, were retained in the marginal zone (Fig. 6) . These results suggest that a direct interaction between MZMO and MZBs is mediated by MARCO–MZB binding, through a MARCO ligand expressed on these B cells, and provides a mechanism for the retention of MZBs by MARCO-expressing MZMO cells. Perturbation of this interaction either by disruption of adhesion and/or induction of macrophage activation by MARCO cross-linking results in the appearance of cells expressing a MZB surface phenotype in the follicular zone.
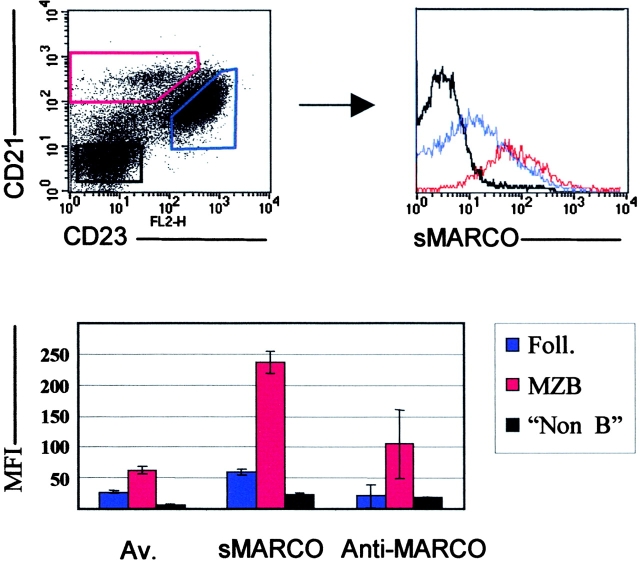
Figure 5.
Soluble MARCO receptor (sMARCO) binds preferentially to MZBs. Representative FACS® analysis of spleen cells from WT mice stained with CRI, CD23, and biotinylated sMARCO. Binding of sMARCO to different spleen cell populations was based on gates set on the CRI versus CD23 stain. red, MZBs; blue, follicular B cells; black, non-B cells. The histogram (bottom) shows the mean fluorescence index (MFI) and SD (n = 5) for the different populations as well as the avidin (Av) control and block using the MARCO-specific ED31 Ab. Data shown are representative of three independent experiments.
Figure 6.
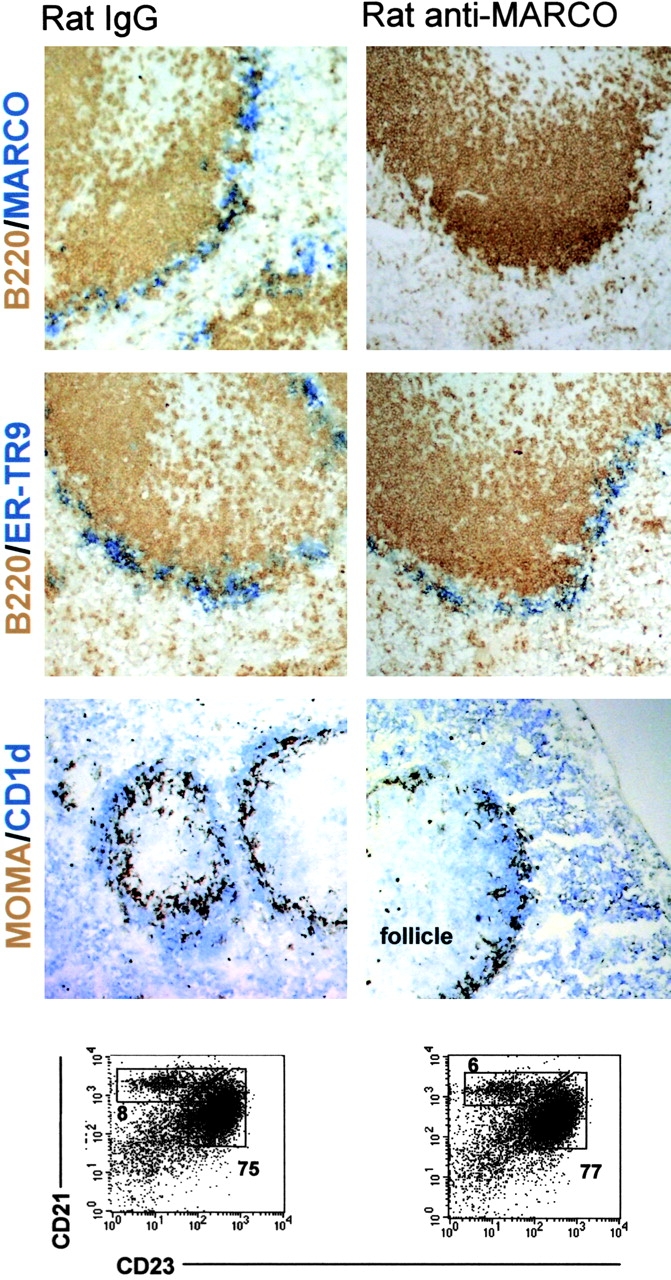
In vivo disruption of MARCO and MZB interactions leads to MZB migration to the follicle. WT mice were given 100 μg control rat IgG or anti-MARCO (ED31) IgG i.v. 3 h later the mice were killed and the spleens were stained for macrophage and B cell populations. Representative stains of serial sections from at least four different mice are shown. MZMOs were detected with anti-MARCO (blue, top) or ER-TR9 (blue, middle) antibodies whereas metallophilic macrophages were stained with MOMA-1 (brown, bottom). B220+ B cells (brown) were stained for positioning of the follicle and MZBs as the CD1high (blue, bottom) population. ×10. Part of the spleen was used for flow cytometric analysis to determine the CD19+, CRIhigh, and CD23low populations. Numbers shown are the average of four mice. The percent of CD19+ cells for either MZBs or follicular B cells is shown for comparison. Data shown are representative of two independent experiments.
To address the relevance of the MARCO+ MZMO and its retention of MZBs to its contribution to the development of an immune response to pathogens, we injected mice i.v. with rhodamine-conjugated S. aureus, which is a known ligand for the MARCO receptor (12). Within 30 min of injection bacteria were visualized exclusively bound to the MZMO cells, a role consistent with the phagocytic property of these scavenger receptor–expressing cells (Fig. 7) . 18 h after injection the microbes and the MZMO were found to have comigrated into the red pulp and cells with a MZB phenotype (CD1dhigh) were mostly found in the follicular region. These results are consistent with a model in which interaction of S. aureus with MARCO on MZMOs results in their migration into the red pulp and the concomitant migration of MZBs into the follicular region as has been reported for LPS and E. coli (8, 9). The deletion of the inhibitory signaling molecule SHIP results in a similar MZMO migration response, suggesting that MZMO activation can trigger migration into the red pulp. We presume that the likely explanation for the migration seen in response to S. aureus ingestion is the activation of MZMOs by their encounter with these bacteria as has been described (36, 37). A similar result was observed for E. coli suggesting a more general migratory response by MZMO cells to microbial challenge (unpublished data). The migratory response of the MZMO, carrying Ag to the red pulp, could simply be a method of clearance of particulate Ags or alternatively MZMOs could function as Ag transporters/presenters and supporters of plasmablast formation shown to take place in the red pulp (Fig. 8 ; references 38–40). This has previously been reported to be a function of dendritic cells in the T/B cell border of the follicle and by macrophages supporting B1 B cells in the peritoneum (10). Interestingly, Kang et al. (14) recently showed that phagosomes in MZMOs, after uptake of dextran polysaccarides via SIGN-RI did not stain positive for the endosomal markers LAMP-1 and transferrin. This suggests that Ags taken up by MZMOs may not necessarily take the route of normal phagosome maturation (41) resulting in destruction or Ag presentation and thus could provide a mechanism to transport intact Ag to the red pulp by MZMOs.
Figure 7.
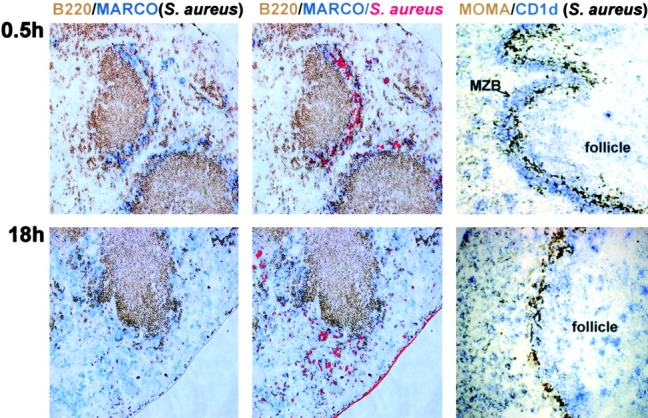
S. aureus induce MZMO movement and displacement of MZBs. WT mice were injected i.v. with 250 μg heat-killed and rhodamine-conjugated S. aureus in PBS. 0.5 or 18 h later the mice were killed and the spleens were sectioned and stained. Representative stains from at least four mice are shown. MARCO+ MZMOs (left) are stained blue and B220+ B cells are stained brown. The middle shows the same stains as in the left, merged with the fluorescent stain of S. aureus. The right shows stains for the CD1high MZB population (blue) and MOMA-1+ metallophilic macrophages (brown). ×10. The data shown are representative of two independent experiments.
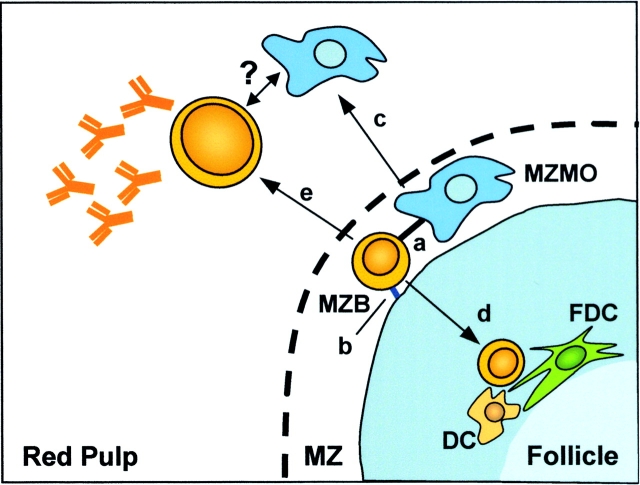
Figure 8.
Proposed model for interactions between MZMO and MZB and the response of these cells to blood-borne pathogens. In the marginal zone (MZ), MZBs interact with the MZMO via the MARCO receptor (a) and with stromal elements via the ICAM/VCAM and their respective ligands LFA-1 and α4β1 (b). Upon phagocytosis of particulate Ags, the MARCO+ MZMOs migrate to the red pulp (c) and the majority of the MZBs migrate to the follicle where they interact with cells such as dendritic and follicular dendritic (d, DC and FDC). In the early response to T cell–independent Ags, the MZB also has the capacity to migrate to the red pulp to take part in plasma cell formation (e), where a possible interaction with MZMOs and MZBs may take place.
These results suggest that the interaction of MZMO cells with MZBs is required to maintain the marginal zone structure and that this association is perturbed upon MZMO binding and activation by microbial pathogens. It is likely that the MZBs migrate into the follicular zone in response to CXCL13 (9) in the absence of retention signals from the MARCO+ MZMO. This pathway is likely to be independent of the integrin pathway involving stromal VCAM/ICAM and B cell LFA-1/α4β1 because disruption of that pathway with antibodies to LFA-1 and α4β1 results in the release of MZBs to the blood stream (9), not their migration into the follicle, in contrast to the results presented here (Fig. 8). In addition, we see no effect on the localization of MZMO cells using antibodies to the stromal integrins, nor do we observe effects on their ligand expression when MZMO cells are triggered to migrate (unpublished data). These pathways are thus likely to serve different functions in the organization of the marginal zone, with the MZMO pathway specific for the antimicrobial response, leading to internalization of the organism and trafficking of B cells into the follicular zone to propagate the immune responses. MZBs have the capacity to bind polysaccharide Ags through complement-mediated pathways and transport these to the follicular area of the spleen (6, 8, 42). The events we have described appear to be another mechanism for delivery of MZBs and Ag to the T cell–rich follicular region. MZBs have mostly been implicated in the response to T cell–independent Ags, however, they are also capable of presenting Ags (43) and may thus be important both for the T cell–dependent and –independent phase of the earliest defense against a pathogen.
Acknowledgments
We would like to thank members of the Ravetch and Steinman labs at The Rockefeller University, especially Pierre Bruhns, Patrick Smith, Maggi Pack, Chae Gyu Park, and Sayori Yamazaki for technical assistance and comments on the manuscript. We also thank Dr. Jeffrey Pollard for op/op mice and Dr. Timo Pikkarainen for reagents and helpful comments.
This work was supported by the Swedish Cancer Society and the NIH.
Footnotes
Abbreviations used in this paper: Btk, Bruton's tyrosine kinase; ES, embryonic stem; MZB, marginal zone B cell; MZMO, marginal zone macrophage; SHIP, SH2-containing inositol-5-phosphatase 1.
References
- 1.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 2.Kraal, G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132:31–74. [DOI] [PubMed] [Google Scholar]
- 3.Fagarasan, S., and T. Honjo. 2000. T-Independent immune response: new aspects of B cell biology. Science. 290:89–92. [DOI] [PubMed] [Google Scholar]
- 4.Garcia de Vinuesa, C., P. O'Leary, D.M. Sze, K.M. Toellner, and I.C. MacLennan. 1999. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29:1314–1323. [DOI] [PubMed] [Google Scholar]
- 5.Guinamard, R., M. Okigaki, J. Schlessinger, and J.V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36. [DOI] [PubMed] [Google Scholar]
- 6.Pozdnyakova, O., H.K. Guttormsen, F.N. Lalani, M.C. Carroll, and D.L. Kasper. 2003. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J. Immunol. 170:84–90. [DOI] [PubMed] [Google Scholar]
- 7.Zandvoort, A., and W. Timens. 2002. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin. Exp. Immunol. 130:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray, D., D.S. Kumararatne, J. Lortan, M. Khan, and I.C. MacLennan. 1984. Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology. 52:659–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, T.T., and J.G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 297:409–412. [DOI] [PubMed] [Google Scholar]
- 10.Balazs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352. [DOI] [PubMed] [Google Scholar]
- 11.Kraal, G., and M. Janse. 1986. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 58:665–669. [PMC free article] [PubMed] [Google Scholar]
- 12.Elomaa, O., M. Kangas, C. Sahlberg, J. Tuukkanen, R. Sormunen, A. Liakka, I. Thesleff, G. Kraal, and K. Tryggvason. 1995. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 80:603–609. [DOI] [PubMed] [Google Scholar]
- 13.Dijkstra, C.D., E. Van Vliet, E.A. Dopp, A.A. van der Lelij, and G. Kraal. 1985. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 55:23–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Kang, Y.S., S. Yamazaki, T. Iyoda, M. Pack, S.A. Bruening, J.Y. Kim, K. Takahara, K. Inaba, R.M. Steinman, and C.G. Park. 2003. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15:177–186. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T.B., P.C. Groot, M.A. Nolte, S.J. van Vliet, S.T. Gangaram-Panday, G.C. van Duijnhoven, G. Kraal, A.J. van Oosterhout, and Y. van Kooyk. 2002. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 100:2908–2916. [DOI] [PubMed] [Google Scholar]
- 16.Park, C.G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B.E. Clausen, K. Inaba, and R.M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13:1283–1290. [DOI] [PubMed] [Google Scholar]
- 17.Leenen, P.J., M.F. de Bruijn, J.S. Voerman, P.A. Campbell, and W. van Ewijk. 1994. Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Methods. 174:5–19. [DOI] [PubMed] [Google Scholar]
- 18.Helgason, C.D., J.E. Damen, P. Rosten, R. Grewal, P. Sorensen, S.M. Chappel, A. Borowski, F. Jirik, G. Krystal, and R.K. Humphries. 1998. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 12:1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen, B.E., C. Burkhardt, W. Reith, R. Renkawitz, and I. Forster. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277. [DOI] [PubMed] [Google Scholar]
- 20.Sankala, M., A. Brannstrom, T. Schulthess, U. Bergmann, E. Morgunova, J. Engel, K. Tryggvason, and T. Pikkarainen. 2002. Characterization of recombinant soluble macrophage scavenger receptor MARCO. J. Biol. Chem. 277:33378–33385. [DOI] [PubMed] [Google Scholar]
- 21.Ono, M., H. Okada, S. Bolland, S. Yanagi, T. Kurosaki, and J.V. Ravetch. 1997. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 90:293–301. [DOI] [PubMed] [Google Scholar]
- 22.Wang, J.W., J.M. Howson, T. Ghansah, C. Desponts, J.M. Ninos, S.L. May, K.H. Nguyen, N. Toyama-Sorimachi, and W.G. Kerr. 2002. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 295:2094–2097. [DOI] [PubMed] [Google Scholar]
- 23.Tsukada, S., D.J. Rawlings, and O.N. Witte. 1994. Role of Bruton's tyrosine kinase in immunodeficiency. Curr. Opin. Immunol. 6:623–630. [DOI] [PubMed] [Google Scholar]
- 24.Bolland, S., R.N. Pearse, T. Kurosaki, and J.V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 8:509–516. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay, S., M. Mohanty, A. Mangla, A. George, V. Bal, S. Rath, and B. Ravindran. 2002. Macrophage effector functions controlled by Bruton's tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J. Immunol. 168:2914–2921. [DOI] [PubMed] [Google Scholar]
- 26.Satterthwaite, A.B., C.A. Lowell, W.N. Khan, P. Sideras, F.W. Alt, and O.N. Witte. 1998. Independent and opposing roles for Btk and lyn in B and myeloid signaling pathways. J. Exp. Med. 188:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, C.I., T.C. Islam, P.T. Mattsson, A.J. Mohamed, B.F. Nore, and M. Vihinen. 2001. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 23:436–446. [DOI] [PubMed] [Google Scholar]
- 28.Weil, D., M.A. Power, S.I. Smith, and C.L. Li. 1997. Predominant expression of murine Bmx tyrosine kinase in the granulo-monocytic lineage. Blood. 90:4332–4340. [PubMed] [Google Scholar]
- 29.Tomlinson, M.G., T. Kurosaki, A.E. Berson, G.H. Fujii, J.A. Johnston, and J.B. Bolen. 1999. Reconstitution of Btk signaling by the atypical tec family tyrosine kinases Bmx and Txk. J. Biol. Chem. 274:13577–13585. [DOI] [PubMed] [Google Scholar]
- 30.Buiting, A.M., Z. de Rover, E. Claassen, and N. van Rooijen. 1993. In vivo distribution of particulate antigens and liposomes in murine spleen. A possible role in the humoral immune response. Immunobiology. 188:13–22. [DOI] [PubMed] [Google Scholar]
- 31.Ito, S., M. Naito, Y. Kobayashi, H. Takatsuka, S. Jiang, H. Usuda, H. Umezu, G. Hasegawa, M. Arakawa, L.D. Shultz, et al. 1999. Roles of a macrophage receptor with collagenous structure (MARCO) in host defense and heterogeneity of splenic marginal zone macrophages. Arch. Histol. Cytol. 62:83–95. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi, K., S. Umeda, L.D. Shultz, S. Hayashi, and S. Nishikawa. 1994. Effects of macrophage colony-stimulating factor (M-CSF) on the development, differentiation, and maturation of marginal metallophilic macrophages and marginal zone macrophages in the spleen of osteopetrosis (op) mutant mice lacking functional M-CSF activity. J. Leukoc. Biol. 55:581–588. [DOI] [PubMed] [Google Scholar]
- 33.Van der Laan, L.J., E.A. Dopp, R. Haworth, T. Pikkarainen, M. Kangas, O. Elomaa, C.D. Dijkstra, S. Gordon, K. Tryggvason, and G. Kraal. 1999. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 162:939–947. [PubMed] [Google Scholar]
- 34.Yokota, T., B. Ehlin-Henriksson, and G.K. Hansson. 1998. Scavenger receptors mediate adhesion of activated B lymphocytes. Exp. Cell Res. 239:16–22. [DOI] [PubMed] [Google Scholar]
- 35.Gebe, J.A., M. Llewellyn, H. Hoggatt, and A. Aruffo. 2000. Molecular cloning, genomic organization and cell-binding characteristics of mouse Spα. Immunology. 99:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotani, M., K. Matsuno, K. Miyakawa, T. Ezaki, T. Hayama, and S. Ekino. 1985. Migration of macrophages from the marginal zone to germinal centers in the spleen of mice. Anat. Rec. 212:172–178. [DOI] [PubMed] [Google Scholar]
- 37.Groeneveld, P.H., T. Erich, and G. Kraal. 1986. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology. 58:285–290. [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, K.G., T.D. Hewitson, G.J. Nossal, and D.M. Tarlinton. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448. [DOI] [PubMed] [Google Scholar]
- 39.Ho, F., J.E. Lortan, I.C. MacLennan, and M. Khan. 1986. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16:1297–1301. [DOI] [PubMed] [Google Scholar]
- 40.Sze, D.M., K.M. Toellner, C. Garcia de Vinuesa, D.R. Taylor, and I.C. MacLennan. 2000. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 192:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira, O.V., R.J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guinamard, R., N. Signoret, I. Masamichi, M. Marsh, T. Kurosaki, and J.V. Ravetch. 1999. B cell antigen receptor engagement inhibits stromal cell–derived factor (SDF)-1α chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J. Exp. Med. 189:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver, A.M., F. Martin, and J.F. Kearney. 1999. IgMhigh CD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198–7207. [PubMed] [Google Scholar]