Abstract
Formation of a mature thymic epithelial microenvironment is an essential prerequisite for the generation of a functionally competent T cell pool. It is likely that recently identified thymic epithelial precursors undergo phases of proliferation and differentiation to generate mature cortical and medullary thymic microenvironments. The mechanisms regulating development of immature thymic epithelial cells are unknown. Here we provide evidence that expansion of embryonic thymic epithelium is regulated by the continued presence of mesenchyme. In particular, mesenchymal cells are shown to mediate thymic epithelial cell proliferation through their provision of fibroblast growth factors 7 and 10. In contrast, differentiation of immature thymic epithelial cells, including acquisition of markers of mature cortical and medullary epithelium, occurs in the absence of ongoing mesenchymal support. Collectively, our data define a role for mesenchymal cells in thymus development, and indicate distinct mechanisms regulate proliferation and differentiation of immature thymic epithelial cells. In addition, our findings may aid in studies aimed at developing strategies to enhance thymus reconstitution and functioning in clinical certain contexts where thymic epithelial cell function is perturbed.
Keywords: fibroblast growth factors, stromal cells, thymus gland, fibroblasts, cell division
Introduction
Initiation of thymus development in the mouse occurs around embryonic day 10–11 (E10–11), and involves formation of an epithelial bud that is at least in part derived from endoderm of the third pharyngeal pouch (1, 2). By E12, this epithelial rudiment is surrounded by a layer of mesenchyme (3, 4), and is beginning to be colonized by cells of haemopoietic origin (4–6). Perithymic mesenchyme migrates into the epithelial rudiment at E13, where it establishes a network that interacts with immature thymocytes as well as immature epithelial cells. Establishment of an intrathymic mesenchymal network is accompanied by proliferation and differentiation of immature thymic epithelium. How immature thymic epithelial cells, such as the recently identified Mts24+ precursors (7, 8), are triggered to generate defined cortical and medullary microenvironments capable of supporting T cell maturation, is unclear (9).
Several lines of evidence suggest that mesenchyme plays a functional role during thymus development. For example, extirpation of neural crest mesenchyme in birds results in abnormal thymus development (10), while murine fetal thymus lobes devoid of mesenchyme fail to support T cell development under organ culture conditions (3, 5, 11, 12). There are at least two distinct mechanisms by which mesenchyme may influence thymic function. First, mesenchyme may be able to directly influence development of immature CD4−8− T cell precursors, by presentation of soluble growth factors on extracellular matrix (ECM) components (11, 13, 14). Second, mesenchyme may also influence the development of immature thymic epithelial cells, and so play a direct role in the establishment of a functional thymic microenvironment. Interestingly, mesenchymal cells are known to directly influence development of epithelial cells in other embryonic rudiments such as the limb bud (15). Moreover, bone morphogenic proteins (BMPs) and fibroblast growth factors (FGFs)* have been identified as molecular mediators of mesenchymal function in these systems (15). In contrast, while a role for epithelio-mesenchymal interactions in thymus development is long established (3), the mechanisms involved remain unclear.
Here, we have analyzed the role of mesenchyme during thymus development by separately analyzing the effects of mesenchyme on the proliferation and differentiation of immature epithelial cells in the fetal thymus. We provide evidence that after E12 of gestation, the continued differentiation of immature thymic epithelial cells into cortical and medullary phenotypes is independent of sustained interactions with mesenchyme. In contrast, the continued presence of mesenchymal cells, via their production of FGF7 and FGF10, is necessary to support the proliferation of thymic epithelial cells leading to thymus growth. Our data thus define a key role for mesenchyme in direct regulation of thymus development, and identify some of the molecular mediators involved.
Materials and Methods
Mice
Thymic lobes were obtained from Balb/c and CD3ɛtg26 (reference 16; The Jackson Laboratory) mouse embryos, where E0 was the day of vaginal plug detection. Mice were maintained under specific pathogen free conditions in the Birmingham University Animal Unit.
Antibodies
The following antibodies were used for flow cytometry: anti-BrdU-FITC (BD Biosciences), anti-pan cytokeratin (clones: C-11, PCK-26, CY-90, Ks-1A3, M20, and A53-B/A2; Sigma-Aldrich), goat anti–mouse Ig PE (Caltag), anti-IAd-FITC (clone AMS-32.1; BD Biosciences). Antibodies used for immunohistology: anti-keratin 8 (clone LE41, a gift from Dr. B. Lane, University of Dundee, Dundee, UK), anti-keratin 5 (Covance), which were detected with goat anti–mouse Ig FITC (Caltag) and goat anti–rabbit Ig Alexa Fluor 350 (Molecular Probes), respectively. For immunomagnetic selection, anti-EpCAM-1 (clone G8.8, a gift of Dr. Andy Farr, University of Washington, Seattle, WA) and anti-PDGFRα (BD Biosciences), were coated onto anti-rat Ig Dynabeads (Dynal).
Removal of Perithymic Mesenchyme and Organ Culture of E12 Thymic Epithelial Rudiments
Thymic lobes were dissected and washed in PBS. Lobes were incubated in 2.5% Collagenase D (Boehringer/Roche Ltd.) in RPMI with 5% FCS for 30 min, and thymic mesenchyme was removed by drawing lobes into finely drawn glass pipettes. Lobes were submerged in 200 μl DMEM in V-bottomed 96-well plates and incubated in 60% oxygen at 37°C. For analysis of thymic epithelial differentiation by cytospin detection of keratin phenotype and PCR analysis, lobes were cultured for 2 d, while cultures were left for 6 d for analysis of MHC class II expression.
Organ Culture of E14 Thymus Lobes and Thymic Epithelial Cells
Fetal thymus organ cultures (11) were used to compare proliferation of epithelium in E14 wild-type and CD3ɛtg26 thymus lobes. In some experiments, reaggregate thymus organ cultures (11) were used to analyze epithelial cell proliferation. Isolated thymus lobes were washed in Ca2+/Mg2+ free PBS and incubated in 0.25% trypsin/0.02% EDTA for 20 min at 37°C to give a single cell suspension. For some experiments such suspensions were reaggregated without further manipulation by depositing cells onto the surface of a 0.8-μm pore size Nucleopore filters under organ culture conditions. In other experiments, reaggregate cultures were made from purified thymic epithelial cells isolated from freshly trypsinized E14 lobes by sequential incubations in anti-EpCAM-1 and anti–rat Ig MicroBeads (Miltenyi Biotech) followed by positive isolation using a MiniMacs separation system (Miltenyi Biotech). Any contaminating CD45+ and PDGFRα+ cells were removed using Dynabeads (Dynal). Where necessary, recombinant FGF7 and FGF10 (R&D Systems) were added to cultures at a final concentration of 100 ng/ml at the outset of a 2 d culture period.
Cytospin Analysis of Thymic Epithelial Cell Subpopulations
Unmanipulated or mesenchymal free E12 fetal thymus lobes, cultured in the presence of 60% oxygen for 2 d, were trypsinized as described above. Cell suspensions were depleted of mesenchyme and residual haemopoietic cells using anti-CD45 and anti-PDGFRα coated Dynabeads. Approximately 4 × 104 cells were resuspended in 200 μl PBS with 10% FCS and cytospun onto multispot slides (C.A. Hendley-Essex Ltd.) at 400 rpm for 5 min. Slides were air-dried and fixed in methanol at 4°C for 40 min. Cytospins were stained with antibodies to keratin 5 and keratin 8, followed by anti–rabbit Ig-Alexa Fluor 350 (Molecular Probes) and anti-mouse Ig-FITC (Caltag).
Functional Analysis of Thymic Epithelium Cultured in the Absence of Mesechyme
E12 fetal thymus lobes, after removal of perithymic mesenchyme, were cultured in V-bottomed 96-well plates in the presence of 60% oxygen for 7 d. After culture, lobes were recovered, disaggregated with 0.25% trypsin in 0.02% EDTA, and reassociated as reaggregate organ cultures with equal numbers of preselection CD4+8+ thymocytes (11). As a comparison, CD4+8+ thymocytes were also reaggregated with equal numbers of thymic stromal cells obtained from 15 d thymus lobes pretreated for 6 d in 1.35 mM 2-deoxyguanosine (11). After 6 d, viable thymocytes were recovered and analyzed for expression of CD4 and CD8 by flow cytometry.
Flow Cytometric Analysis of Thymic Epithelium
Analysis of Cell Division in Thymic Epithelium.
Fetal thymuses and reaggregate thymic organ cultures were pulsed for 18 h with 10 μM BrdU (Sigma-Aldrich). Lobes were washed in Ca2+/Mg2+ free PBS, trypsinized as detailed above, and suspensions permeabilized using OrthoPermeafix (Ortho Diagnostic Systems) for 40 min at room temperature. Cells were DNAase I treated for 10 min at 37°C and incubated sequentially with anti-pan cytokeratin and goat anti–mouse Ig-PE. After incubation with 10% normal mouse serum for 30 min, cells were stained with anti-BrdU-FITC for 30 min at 4°C. Cells were then resuspended in 1% paraformaldehyde and analyzed using a BD LSR flow cytometer, with forward and side scatter gates set to exclude nonviable cells.
Phenotypic Analysis of Thymic Epithelium.
MHC class II expression was analyzed in fresh E12 thymus lobes, and in unmanipulated or mesenchymal free E12 fetal thymus lobes, cultured in the presence of 60% oxygen for 6 d. Lobes were trypsinized and cell suspensions labeled with FITC-conjugated anti-mouse I-Ad. Cells were resuspended in 1% paraformaldehyde and analyzed using a BD LSR flow cytometer, with forward and side scatter gates set to exclude nonviable cells.
RT-PCR
Semiquantitative RT-PCR was performed as described (17). For some experiments (see Fig. 2), stromal cell cDNAs of thymic epithelium and mesenchymal cells were purified by immunomagnetic selection from disaggregated E14 thymus lobes. Epithelial cells were selected using anti-EpCAM1 coated Dynabeads, and mesenchymal cells were isolated using anti-PDGFRα coated Dynabeads. Purities obtained were >95% (unpublishe data). cDNA was also prepared from total E14 CD4−8− thymocytes, which were obtained at >98% purity (unpublished data). Analysis of thymic epithelial cell differentiation was also performed in either freshly isolated E12 thymus lobes, or in E12 thymus lobes organ cultured for 2 d after mesenchyme removal (see Fig. 4). The following primer pairs were used: β-actin: forward GTTACCAACTGGGACGACA, reverse TGGCCATCTCCTGCTCGAA; Aire: forward CAGACCATGGCAGCTTCTGTCCAG, reverse GAGGCAGCAGGAGCATCTCCAGAG; Plunc: forward ACTGCCTTCAAATCCCACAG, reverse CCTCCCCTGATTGTCTTTCA; FGF7: forward CCGACTCCGCTCTACAGACC, reverse GTTGCAATCCTCATTGCATTC; FGF10: forward ACATTGTGCCTCAGCCTTTC, reverse TTCCATTCAATGCCACATACAT; FGFR2IIIb: forward CCCATCCTCCAAGCTGGACTGCCT, reverse ATCTGGGGAAGCCGTGATCTCCTT.
Figure 2.
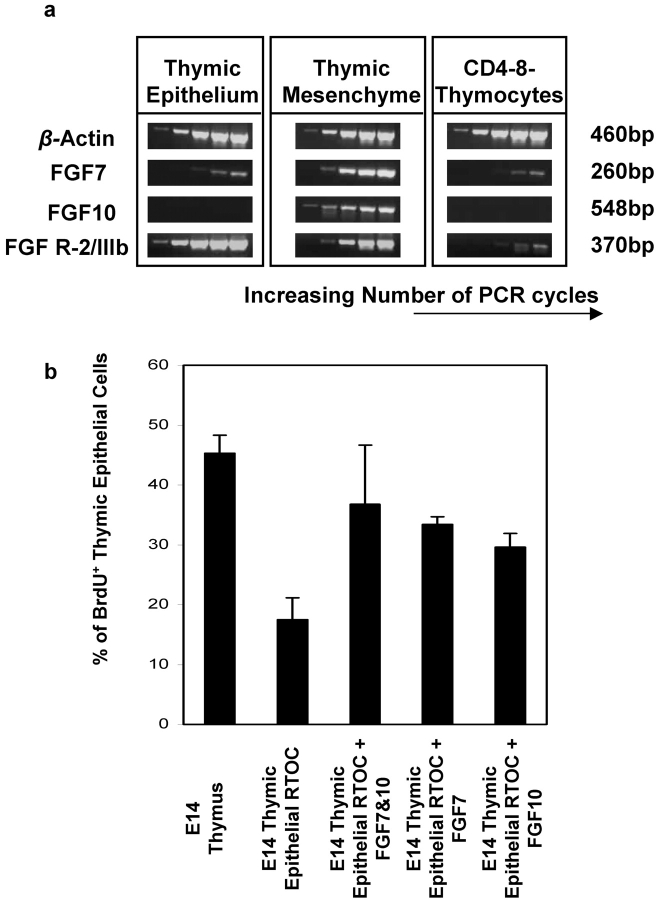
Mesenchymal production of FGF7 and FGF10 regulate proliferation of embryonic thymic epithelium. Semiquantitative PCR (a) for FGF7, FGF10, and FGFR2IIIb was performed on cDNAs isolated from thymic epithelium, thymic mesenchyme and CD4−8− thymocytes purified from E14 thymus. Equal loading of cDNA was monitored by analyzing β-actin mRNA expression. (b) Proliferation of thymic epithelium was analyzed by BrdU incorporation and Keratin expression in reaggregate cultures formed from whole E14 lobes, and from purified E14 thymic epithelium reaggregate cultures, the latter being cultured in the presence or absence of 100 ng/ml FGF7 and FGF10, either singularly or in combination. Experiments were performed three times with similar results.
Figure 4.
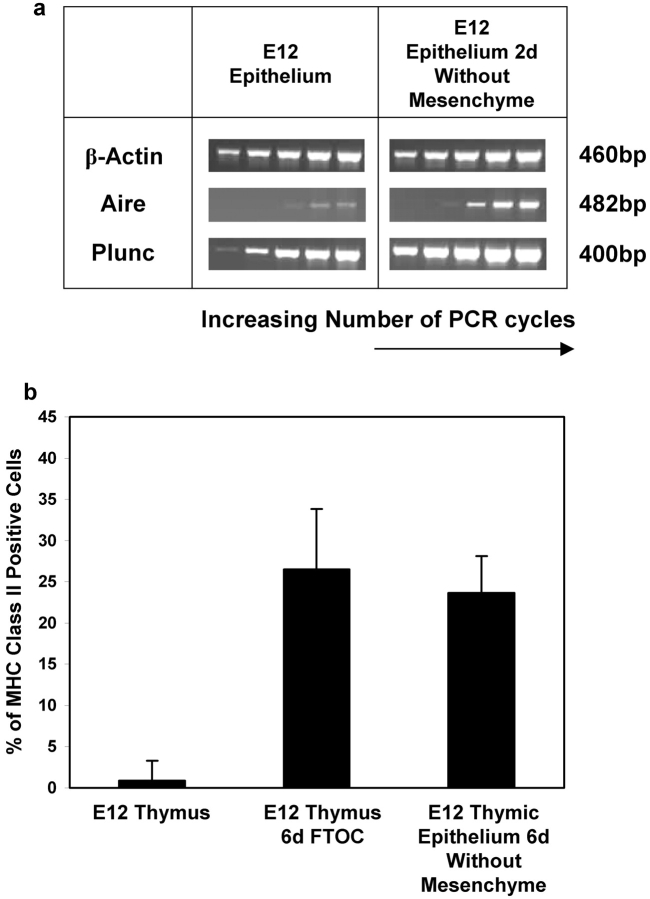
Phenotypic and genotypic differentiation of E12 thymic epithelium in the absence of mesenchyme. E12 fetal thymus lobes were organ cultured intact or after enzymatic removal of mesenchyme for either 2 d (for RT-PCR analysis) or 6 d (for flow cytometric analysis). For RT-PCR analysis (a), cDNAs were used to determine expression of Aire and Plunc, with equal cDNA loadings monitored by β-actin levels. For flow cytometry (b), 6 d cultures were disaggregated and analyzed for cell surface MHC class II expression. Data shown are representative of three separate experiments.
PCR was performed in a PTC-200 Peltier Thermal Cycler (MJ Research). Primer annealing temperatures were: (50°C β-actin, 65°C Aire, 60°C Plunc, 55°C FGF7, 60°C FGF10 and FGFR2IIb).
Cycle points analyzed were 24 cycles and every 4 subsequent cycles up to 40 PCR cycles.
Results and Discussion
Proliferation of Embryonic Thymic Epithelial Cells Is Regulated by Mesenchyme.
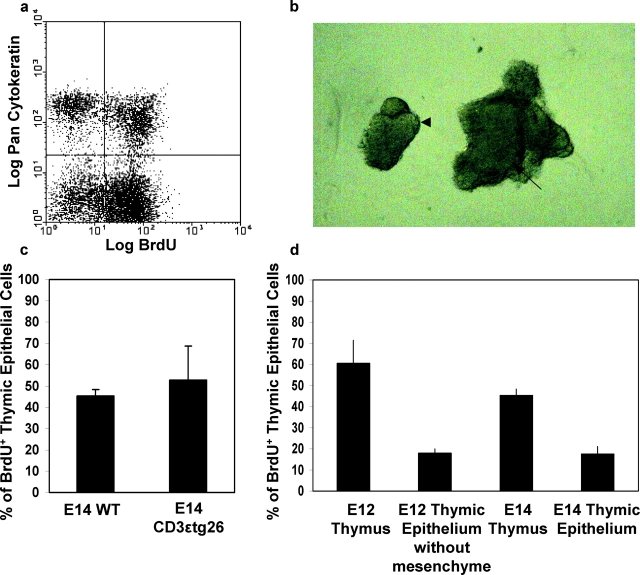
Expansion of epithelial cell numbers in the embryonic thymus is an important feature during thymus growth and the establishment of mature a thymic microenvironment. The embryonic thymus has been shown to contain a population of proliferating thymic epithelial cells, which declines with increasing gestational age (18). To investigate the mediators of epithelial cell expansion in the thymus, we analyzed cell cycle status of thymic epithelium by flow cytometric analysis of BrdU incorporation and cytokeratin (K) expression, which defines proliferating (BrdU+) Keratin (K)+ cells (Fig. 1 a). To determine whether signals derived from developing thymocytes are involved in epithelial cell expansion, we compared epithelial cell proliferation in E14 thymus lobes from WT mice with thymus lobes isolated from CD3ɛtg26 mice, which have a block in T cell development at the earliest stage (16). Isolated lobes were cultured overnight in the presence of 10 μM BrdU, then trypsinized to form a cell suspension. Analysis of BrdU incorporation into Keratin+ epithelial cells showed that, ∼50% of thymic epithelial cells are dividing in WT E14 thymus (Fig. 1 c), and analysis of epithelial proliferation in CD3ɛtg26 thymuses showed a similar proportion of Keratin+ cells incorporating BrdU (Fig. 1 c). As CD3- ɛtg26 mice show a profound block in thymocyte development from the earliest stage (16), these data suggest that the signals mediating expansion of fetal thymic epithelium is not dependent upon the presence of T cell precursors undergoing a normal program of development. Interestingly, a recent study demonstrates that the initial differentiation of immature fetal thymic epithelium is also independent of signals from thymocytes (19). Given a number of studies have demonstrated a role for thymocyte-derived signals in regulating stromal cells in the adult thymus (for a review, see reference 20), collectively these data suggest that, during the crucial phase of thymus establishment, differentiation and proliferation of thymic epithelium is not dependent upon crosstalk with thymocytes beyond the DN1 stage of maturation.
Figure 1.
Mesenchyme and not thymocytes regulate proliferation of embryonic thymic epithelium. Proliferating thymic epithelial cells were identified in fetal thymus lobes by analyzing cytokeratin expression in combination with BrdU incorporation. Panel a shows a typical example of flow cytometric analysis of E14 fetal thymus lobes, where a Keratin(K)+BrdU+ population is clearly visible. Panel c shows analysis of epithelial cell proliferation in wild-type and CD3ɛtg26 E14 thymus lobes after an 18 h BrdU pulse. The percentage of K+ epithelial cells incorporating BrdU after an 18 h pulse was also analyzed in whole E12 and E14 thymus lobes, and in E12 and E14 thymuses devoid of mesenchyme (d). Note reduction in epithelial proliferation at both developmental stages in the absence mesenchyme. In all flow cytometric analyses, a minimum of 10,000 gated events was analyzed. Panel b shows E12 thymic lobes with surrounding perithymic mesenchyme attached (arrow) and after enzymatic removal of perithymic mesenchyme to leave an epithelial thymic rudiment (arrowhead). Similar results were obtained from at least three separate experiments.
As several studies have implicated mesenchymal cells in thymus growth and function (3, 10–12), we analyzed the role of mesenchyme in the regulation of epithelial cell proliferation. In these experiments, we analyzed the thymus at both E12 and E14 of gestation, to study any possible developmental stage–specific regulation of epithelial proliferation. To investigate the role of mesenchyme at E12 of gestation, perithymic mesenchyme was removed from freshly dissected lobes by brief incubation in 2.5% collagenase, leaving an intact epithelial core (Fig. 1 b) which was then organ cultured in the presence of BrdU. By E14, mesenchymal cells have invaginated into the inner epithelial core, and so enzymatic removal of surrounding mesenchyme cannot be used at this stage to generate mesenchymal-free thymus lobes. We therefore isolated thymic epithelium from E14 lobes on the basis of EpCAM-1 expression, and purified cells (greater than 99% purity, unpublished data) were then used to establish reaggregate thymus lobes. After overnight culture in the presence of BrdU, flow cytometric analysis showed the presence of proliferating epithelial cells in whole embryonic thymus lobes at both developmental time points analyzed (Fig. 1 d). Removal of perithymic mesenchyme at E12, or culture of purified E14 EpCAM-1+ epithelial cells alone resulted in a threefold reduction in the proportion of Keratin+ cells incorporating BrdU (Fig. 1 d). Importantly, this diminution of epithelial cell proliferation was not due to either the enzymatic treatment of E12 lobes, nor the immunomagnetic selection of E14 epithelium, as the addition of mesenchyme from fetal lung was able to induce the proliferation of these epithelial cell preparations (from ∼20% without mesenchyme to ∼50% with mesenchyme, unpublished data). Thus, in agreement with a previous report (21) proliferation of thymic epithelium is the early embryo thymus is regulated by signals derived from mesenchyme. Whether mesenchymal cells play a role in regulation of thymic epithelial cells during subsequent neonatal or adult periods is not clear. Interestingly, a recent paper (22) has suggested that mesenchymal cells present in the neonatal thymus, in contrast to fetal thymus, may not be neural crest-derived, perhaps indicating differing roles for mesenchyme at different developmental stages. However, while possible functional differences between fetal and neonatal mesenchyme in the thymus have yet to be addressed, the data shown here suggest that during the time of thymus formation, mesenchyme plays an important role in the regulation of epithelial cell growth.
FGFs Are the Molecular Mediators of Mesenchymal Support of Epithelial Proliferation in the Embryonic Thymus.
FGFs have mitogenic activity for epithelial cells in other organ systems, and so would appear to be candidates for regulating proliferation of thymic epithelium. Both FGFs and their receptors are present in thymus (2, 23), although the precise cell types responsible for their production is unclear. Moreover, thymus growth is retarded in mice lacking FGF-R2IIIb, a receptor for FGF7 and FGF10 (24), while FGF7 administration to mice following induction of graft-versus-host disease (GVHD) has a protective effect on thymic epithelium (25). Moreover, FGF10-deficient mice display a hypoplastic thymus (24). We therefore investigated these molecules further as regulators of proliferation of thymic epithelium. First, expression analysis was performed using semi-quantitative RT-PCR for FGF7, FGF10, and FGFR2IIIb. cDNAs were obtained from purified CD4−8− thymocytes, E14 EpCAM-1+ thymic epithelial cells, and mesenchyme isolated from E14 thymus lobes on the basis of expression of the α chain of platelet-derived growth factor receptor (PDGFRα), a marker for mesenchymal cells (26). While FGFR2IIIb expression was found in both thymic epithelium and mesenchyme (Fig. 2 a), mRNA for FGF7 and FGF10 was readily detectable only in mesenchyme, with only a trace of FGF7 mRNA present in thymic epithelium. CD4−8− thymocytes were found to be negative for FGF10 with only a slight trace of expression of FGFR2IIIb and FGF7 (Fig. 2 a). These data thus raised the possibility that a functional role for mesenchyme in thymus development could be the provision of FGFs to induce expansion of FGFR2IIIb+ thymic epithelial cells. However, as both thymic epithelial cells and mesenchymal cells express FGFR2IIIb (Fig. 2 a), it is not clear whether the impaired thymus development seen in FGFR2IIIb deficient mice (24) reflects an effect on mesenchymal growth or function, which then indirectly effects thymic epithelial cell development, or is a direct reflection of the importance of FGFR2IIIb expression by thymic epithelial cells. To discriminate between these possibilities, experiments were performed to determine whether recombinant FGF7 and 10, both of which are ligands for FGFR2IIIb (24) could replace embryonic mesenchyme in driving epithelial proliferation. In agreement with Fig. 1, E14 thymic epithelial cells cultured alone show a reduction in proliferation as compared with whole E14 lobes containing mesenchyme (Fig. 2 b). Addition of recombinant FGF7 and FGF10, either singularly or in combination, was found to rescue proliferation of epithelial cells cultured without mesenchyme to levels comparable to that seen in whole E14 thymus. These data therefore suggest that FGF7 and FGF10 can at least partly substitute for mesenchyme in regulating thymic epithelial cell expansion. These findings fit well with recent observations in FGF10-deficient mice, where the embryonic thymus has been shown to be smaller than the wild-type thymus, and contain fewer Ki67+ cells (24). Although FGF7-deficient mice have been generated (27), there is no description of thymus size or function in this report, making it difficult to compare thymus development in the absence of FGF7 with the effects of recombinant FGF7 shown here. However, our finding that both FGF7 and FGF10 can aid in the expansion of thymic epithelial cells fits well with the observation that mice which lack FGFR2IIIb, a receptor for both FGF7 and FGF10, display a more severe thymic hypoplasia as compared with FGF10-deficient mice (24). Interestingly, a recent study (23) has shown that mature thymocytes produce FGF7, which can influence expansion of thymic medullary epithelial cells. While this may clearly be of significance to the role of FGFs in the mature thymus, the experiments performed here are based on developmental stages where the thymus does not contain any mature single positive thymocytes, with all thymocytes still being of an immature CD4−8− phenotype (unpublished data). Moreover, in contrast to mature thymocytes, Fig. 2 shows that immature CD4−8− thymocytes do not demonstrate detectable levels of either FGF7 or FGF10 mRNA. Thus, at the stages of thymus development analyzed here, epithelial expansion is dependent upon mesenchymal provision of FGFs. Nevertheless, taken together with our findings, the data of Erikson et al. suggest FGFs can act on mature as well as immature thymic epithelial cells, and that the cellular source of FGF in the thymus may depend upon the developmental stage of the thymus.
Continued Presence of Mesenchyme Is Not Required for E12 Thymic Epithelial Cell Differentiation.
Recent evidence suggests that at least in the embryonic thymus, K5+8+ epithelial cells, which may also be Mts24+, represent precursors for both mature K5−8+ cortical and K5+8− medullary epithelial cells (7, 8). The developmental program of immature thymic epithelial cells that ultimately results in the formation of mature cortical and medullary subsets is poorly defined. As mentioned earlier, recent experiments have shown that the initial differentiation and lineage specification of fetal K5+8+ thymic epithelial cells into K5+8− medullary and K5−8+ cortical cells occurs in CD3ɛtg26 mice. As these mice demonstrate a profound block early in thymocyte development (16, 19), these data are consistent with the notion that crosstalk between developing thymocytes and epithelial cells is not required during the specification of fetal thymic epithelium, although the possible effects of small numbers of residual immature CD4−8− thymocytes in CD3ɛtg26 thymi cannot be formally excluded.
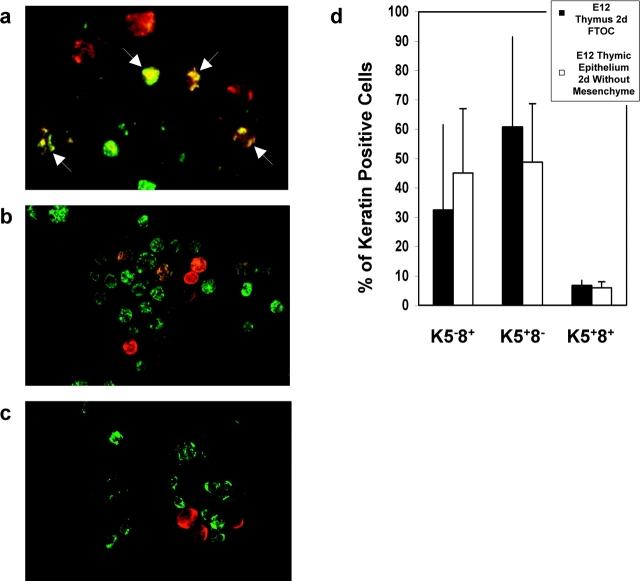
To investigate whether mesenchymal cells influence the differentiation of embryonic thymic epithelium, we analyzed the acquisition of cortical and medullary epithelial phenotypes by E12 thymic epithelium cultured in the absence of mesenchyme. To facilitate analysis of the appearance of K5+8− medullary and K5−8+ cortical cells, we performed cytospin analysis of fetal thymic epithelium, enabling quantitation of epithelial subsets on the basis of differential keratin expression. In line with previous reports (7, 8, 19), a large proportion of thymic epithelial cells at E12 of gestation, which appear as yellow cells due to overlap of green Keratin 8 and red Keratin 5, showed dual expression of K5 and K8 (Fig. 3 a). When E12 thymus lobes were cultured intact for 2 d, the proportion of K5+8+ epithelial cells reduced dramatically, with a concomitant increase in the proportion of K5+8− medullary and K5−8+ cortical epithelial phenotypes (compare Fig. 3 a with Fig. 3, b and d), consistent with epithelial cell maturation from an immature to a more mature phenotype, as seen in vivo (19). A similar differentiation progression was seen when epithelial-only rudiments, prepared by enzymatic stripping of the perithymic mesenchyme (Fig. 1 b), were cultured over a similar period (Fig. 3, c and d), suggesting that the continued presence of mesenchyme is not an essential for the differentiation of E12 thymic epithelium. Consistent with this, we also found genotypic evidence of epithelial cell differentiation in the absence of mesenchyme. RT-PCR analysis of E12 thymic epithelium cultured for 2 d in the absence of mesenchyme, in comparison to freshly isolated E12 thymic epithelium (Fig. 4 a) showed an up-regulation of Aire and Plunc expression (Fig. 4 a), genes normally expressed by medullary epithelium (28–30), together with an induction of MHC class II expression (Fig. 4 b), a characteristic of functionally mature cortical epithelium. Interestingly, our observation that MHC class II expression occurs on thymic epithelium following removal of mesenchyme at E12 of gestation contrasts with another study (31), suggesting that the precise timing of removal of mesenchyme from thymic epithelium may be an important factor.
Figure 3.
Embryonic day 12 thymic epithelial cells acquire K5+8− and K5−8+ phenotypes in the absence of mesenchyme. Cytospins were prepared from E12 thymus (a), and E12 thymus lobes cultured for 2 d with either mesenchyme attached (b) or removed (c). Cytospins were stained with antibodies to keratin 5 (red) and keratin 8 (green), with K5+8+ cells visible where fluorochromes overlap to give yellow (arrowed). Frequencies of K5+8+, K5−8+, and K5+8− subsets (d) was determined by counting at least 100 cells per experiment using a fluorescent microscope. Data are representative of three separate experiments.
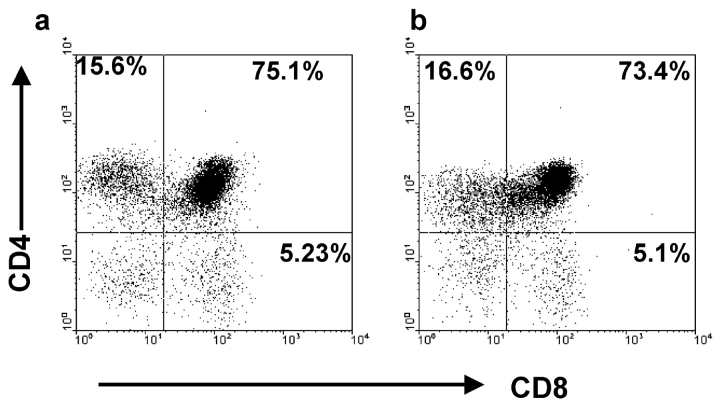
The experiments described above demonstrate that thymic epithelial cell differentiation, as indicated by phenotypic and genotypic analyses, occurs independently of interactions with mesenchyme after E12 of gestation. To provide further evidence for this, we next analyzed the functional ability of thymic epithelial cells which had undergone a period of culture in the absence of mesenchymal support. As mature thymic epithelial cells are known to be key mediators of positive selection of CD4+8+ thymocytes, we established RTOCs from 1:1 mixtures of prepositive selection CD4+8+ thymocytes and E12 thymocytes and epithelial cells previously cultured for 7 d in the absence of mesenchyme. After a further 6 d, RTOCs were harvested and recovered thymocytes were analyzed for CD4 and CD8 expression by flow cytometry. Fig. 5 shows that both CD4+8− and CD4−8+ single positive thymocytes are generated in the presence of epithelial cells that were previously cultured in the absence of mesenchyme (Fig. 5 b), in a manner similar to that seen with 2-deoxyguanosine treated thymic stroma (Fig. 5 a). Thus, this data shows that in the absence of mesenchymal support, immature thymic epithelial cells undergo functional differentiation, as demonstrated by their ability to mediate positive selection.
Figure 5.
E12 thymic epithelial cells grown in the absence of mesenchyme are functionally mature. Freshly purified CD4+8+ thymocytes, obtained from neonatal thymus lobes, were reaggregated at 1:1 mixtures with either 2-dGuo treated stroma (a), or epithelial cells from E12 thymus lobes cultured in the absence of mesenchyme for 7 d (b). After 6 d of reaggregate culture, thymocytes were harvested and stained for CD4 and CD8. Note the presence of CD4+8− and CD4−8+ cells in both cultures. In the experiment shown, 105 epithelial cells were reaggregated with 2 × 105 stromal cells, with yields of 2.5 × 104 and 2 × 104 from cultures of 2-deoxyguanosine and cultured E12 epithelium, respectively. Data shown are representative of three separate experiments.
Collectively, the phenotypic, genotypic, and functional analyses performed here demonstrate that differentiation of E12 thymic epithelium into cortical and medullary lineages occurs without the continued presence of perithymic mesenchyme. Whether or not this is indicative of cell autonomous differentiation by thymic epithelium, or whether inductive signaling by mesenchyme to thymic epithelium occurs before E12, is not known. Nevertheless, our finding that the continued presence of mesenchyme is required to sustain epithelial cell proliferation suggests that different mechanisms regulate thymic epithelial cell differentiation and expansion. Specifically, our results show that while FGFs play a role in epithelial cell proliferation in the thymus, ongoing signaling through FGF7/FGF10 binding of FGF-receptors is not required for the differentiation of immature epithelial progenitors in the E12 thymus.
Acknowledgments
We are grateful to S. Parnell, D. McLoughlin, and R. Suniara for technical support.
This work was supported by an MRC Programme Grant to E.J. Jenkinson and G. Anderson.
Footnotes
Abbreviation used in this paper: FGF, fibroblast growth factor.
References
- 1.Cordier, A., and S. Haumont. 1980. Development of thymus, parathyroids and ultimo-branchial bodies in NMR1 and nude mice. Am. J. Anat. 157:227–263. [DOI] [PubMed] [Google Scholar]
- 2.Manley, N.R. 2000. Thymus organogenesis and molecular mechanisms of thymic epithelial cell differentiation. Sems. Immunol. 12:421–428. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach, R. 1960. Morphogenetic interactions in the development of the mouse thymus gland. Dev. Biol. 2:271–284. [DOI] [PubMed] [Google Scholar]
- 4.Suniara, R.K., E.J. Jenkinson, and J.J.T. Owen. 1999. Studies on the phenotype of migrant thymic stem cells. Eur. J. Immunol. 29:75–80. [DOI] [PubMed] [Google Scholar]
- 5.Itoh, M., H. Kawamoto, Y. Katsura, and T. Amagai. 2001. Two distinct steps of immigration of hematopoietic progenitors into the early thymus anlage. Int. Immunol. 13:1203–1211. [DOI] [PubMed] [Google Scholar]
- 6.Moore, M.A.S., and J.J.T. Owen. 1967. Experimental studies on the development of the thymus. J. Exp. Med. 126:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett, A.R., A. Farley, N.F. Blair, J. Gordon, L. Sharp, and C.C. Blackburn. 2002. Identification and characterization of thymic epithelial progenitor cells. Immunity. 16:803–814. [DOI] [PubMed] [Google Scholar]
- 8.Gill, M., M. Malin, G.A. Hollander, and R.L. Boyd. 2002. Generation of a complete thymic microenvironment by MTS24+ thymic epithelial cells. Nat. Immunol. 3:635–642. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn, C.C., N.R. Manley, D.B. Palmer, R.L. Boyd, G. Anderson, and M.A. Ritter. 2002. One for all and all for one: thymic epithelial cells and regeneration. Trends Immunol. 23:391–395. [DOI] [PubMed] [Google Scholar]
- 10.Bockman, D.E., and M.L. Kirby. 1984. Dependence of thymus development on derivatives of the neural crest. Science. 223:498–500. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, G., E.J. Jenkinson, N.C. Moore, and J.J.T. Owen. 1993. MHC class II positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 362:70–73. [DOI] [PubMed] [Google Scholar]
- 12.Suniara, R.K., E.J. Jenkinson, and J.J.T. Owen. 2000. An essential role for thymic mesenchyme in early T cell development. J. Exp. Med. 191:1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banwell, C.M., K.M. Partington, E.J. Jenkinson, and G. Anderson. 2000. Studies on the role of IL-7 presentation by mesenchymal fibroblasts during early thymocyte development. Eur. J. Immunol. 30:2125–2129. [DOI] [PubMed] [Google Scholar]
- 14.Anderson, G., K.L. Anderson, E.Z. Tchilian, J.J.T. Owen, and E.J. Jenkinson. 1997. Fibroblast dependency during early thymocyte development maps to the CD25+CD44+ stage and involves interactions with fibroblast matrix molecules. Eur. J. Immunol. 27:1200–1206. [DOI] [PubMed] [Google Scholar]
- 15.Capadevila, J., and J.C. Izpisua Belmonte. 2001. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 17:87–132. [DOI] [PubMed] [Google Scholar]
- 16.Hollander, G.A., B. Wang, A. Nichogiannopoulou, P.P. Platenburg, W. van Ewijk, S.J. Burakoff, J.C. Gutierrez-Ramos, and C. Terhorst. 1995. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 373:350–353. [DOI] [PubMed] [Google Scholar]
- 17.Moore, N.C., G. Anderson, C.A.S. Smith, J.J.T. Owen, and E.J. Jenkinson. 1993. Analysis of cytokine gene expression in subpopulations of freshly isolated thymocytes and thymic stromal cells using semi-quantitative polymerase chain reaction. Eur. J. Immunol. 23:922–927. [DOI] [PubMed] [Google Scholar]
- 18.Anderson, K.L., N.C. Moore, D.E. McLoughlin, E.J. Jenkinson, and J.J.T. Owen. 1998. Studies on thymic epithelial cells in vitro. Dev. Comp. Immunol. 22:367–377. [DOI] [PubMed] [Google Scholar]
- 19.Klug, D.B., C. Carter, I.B. Giminez-Conti, and E. Richie. 2002. Thymocyte-dependent and thymocyte–independent phases of epithelial patterning in the fetal thymus. J. Immunol. 169:2842–2845. [DOI] [PubMed] [Google Scholar]
- 20.Ritter, M.A., and R.L. Boyd. 1993. Development in the thymus: it takes two to tango. Immunol. Today. 14:462–469. [DOI] [PubMed] [Google Scholar]
- 21.Shinohara, T., and T. Honjo. 1997. Studies in vitro on the mechanism of the epithelial/mesenchymal interaction in the early fetal thymus. Eur. J. Immunol. 27:522–529. [DOI] [PubMed] [Google Scholar]
- 22.Petrie, H.T. 2002. Role of thymic organ structure and stromal composition in steady-state postnatal T-cell production. Immunol. Rev. 189:8–20. [DOI] [PubMed] [Google Scholar]
- 23.Erikson, M., S. Morkowski, S. Lehar, G. Gillard, C. Beers, J. Dooley, J.S. Rubin, A. Rudensky, and A.G. Farr. 2002. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 100:3269–3278. [DOI] [PubMed] [Google Scholar]
- 24.Revest, J.M., R.K. Suniara, K. Kerr, J.J.T. Owen, and C. Dickson. 2001. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J. Immunol. 167:1954–1961. [DOI] [PubMed] [Google Scholar]
- 25.Rossi, S., B.R. Blazar, C.L. Farrell, D.M. Danilenko, D.L. Lacey, K.I. Weinberg, W. Krenger, and G.A. Hollander. 2002. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 100:682–691. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson, L., C. Bondjers, and C. Betsholtz. 1999. Roles of PDGF-α and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 126:2611–2621. [DOI] [PubMed] [Google Scholar]
- 27.Guo, L., L. Degenstein, and E. Fuchs. 1999. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 10:165–175. [DOI] [PubMed] [Google Scholar]
- 28.Heino, M., P. Peterson, J. Kudoh, K. Nagamine, A. Lagerstedt, V. Ovod, A. Ranki, I. Rantala, M. Nieminen, J. Tuukkanen, et al. 1999. Autoimmune regulator is expressed in the cells regulating immune tolerance in the thymus medulla. Biochem. Biophys. Res. Commun. 257:821–825. [DOI] [PubMed] [Google Scholar]
- 29.Zuklys, S., G. Balciunaite, A. Agarwal, E. Fasler-Kan, E. Palmer, and G.A. Hollander. 2000. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). J. Immunol. 165:1976–1983. [DOI] [PubMed] [Google Scholar]
- 30.LeClair, E.E., L. Nguyen, L. Bingle, A. MacGowan, V. Singleton, S.J. Ward, and C.D. Bingle. 2001. Genomic organization of the mouse plunc gene and expression in the developing airways and thymus. Biochem. Biophys. Res. Commun. 284:792–797. [DOI] [PubMed] [Google Scholar]
- 31.Itoi, M., and T. Amagai. 1998. Inductive role of fibroblastic cell lines in development of the mouse thymus anlage in organ culture. Cell. Immunol. 183:32–41. [DOI] [PubMed] [Google Scholar]