Abstract
Immune responses are regulated by opposing positive and negative signals triggered by the interaction of activating and inhibitory cell surface receptors with their ligands. Here, we describe novel paired activating and inhibitory immunoglobulin-like receptors, designated myeloid-associated immunoglobulin-like receptor (MAIR) I and MAIR-II, whose extracellular domains are highly conserved by each other. MAIR-I, expressed on the majority of myeloid cells, including macrophages, granulocytes, mast cells, and dendritic cells, contains the tyrosine-based sorting motif and the immunoreceptor tyrosine-based inhibitory motif-like sequences in the cytoplasmic domain and mediates endocytosis of the receptor and inhibition of IgE-mediated degranulation from mast cells. On the other hand, MAIR-II, expressed on subsets of peritoneal macrophages and B cells, associates with the immunoreceptor tyrosine-based activation motif-bearing adaptor DAP12 and stimulates proinflammatory cytokines and chemokine secretions from macrophages. Thus, MAIR-I and MAIR-II play important regulatory roles in cell signaling and immune responses.
Keywords: ITAM, ITIM, innate immunity, DAP12, myeloid cells
Introduction
The activating and inhibitory cell surface receptors play important regulatory roles in immune responses (1, 2). The immune inhibitory receptors are characterized by a consensus amino acid (aa)* sequence, immunoreceptor tyrosine-based inhibitory motif (ITIM), present in their cytoplasmic domains. The prototype six-aa sequence for ITIM is (I/V/L/S)-x-Y-x-x-(L/V), where x denotes any aa. Upon ligand binding, immune inhibitory receptors result in tyrosine phosphorylation, which provides a docking site for the recruitment of Src homology 2 (SH2)–containing cytoplasmic phosphatases (3, 4), shutting down activation signals by dephosphorylation of intracellular substrates at the earliest steps of the activation response. An expanding family of immune inhibitory receptors has been identified on a variety of immune cell types (1).
Recent progress has further demonstrated that many of these immune inhibitory receptors pair with activating, as well as inhibitory, isoforms (2, 5), both of whose genes are located in small clusters on a chromosome (6). For example, the NK inhibitory receptor families KIR, CD94/NKG2, and Ly49, which recognize MHC class I ligands, contain both activating and inhibitory receptors within the families (7). The paired activating and inhibitory receptor families have also been identified on other cell types. The CD28 and CTLA4 expressed on T cells bind the same ligands (i.e., CD80 and CD86) yet deliver opposing positive and negative signals, respectively (8). Similarly, B and/or myeloid cells express paired receptor families, including human and murine Fcγ receptors (9, 10), murine paired Ig-like receptors (PIRs; references 11, 12) and possibly its human homologue, Ig-like transcripts (ILT; CD85; references 13–15), and signal regulatory proteins (16–18). In contrast to the inhibitory receptors, the activating receptors have a short cytoplasmic domain lacking ITIM but containing a charged aa residue in the transmembrane region, which is involved in association with immunoreceptor tyrosine-based activation motif (ITAM)–bearing adaptor transmembrane proteins, such as FcɛRIγ or DAP12, or with DAP10 adaptors containing PI3 kinase binding motif (19, 20). Among these activating and inhibitory receptors, KIR, PIR, ILT, and signal regulatory proteins belong to the Ig supergene family, which is characterized by the extracellular portion containing Ig-like domain, in some cases binding the same ligands that are highly homologous to each other (2, 6). The genes encoding most of these receptors are located on human chromosome 19q13.4 or its syntenic region on murine chromosome 7 (6).
In the present investigation, we have identified novel paired activating and inhibitory Ig-like receptors, designated myeloid-associated immunoglobulin-like receptor (MAIR) I and MAIR-II, expressed on the majority of macrophages, granulocytes, dendritic cells, mast cells, and a subset of B cells. Here, we describe the molecular and functional characteristics of the MAIR.
Materials and Methods
Antibodies.
Control rat and mouse IgGs, anti–mouse TCRβ, CD45R/B220, CD11b (Mac1), CD11c, Ly6G (Gr-1), CD21, CD23, and CD32/16 mAbs and DX-5 mAb were purchased from BD Biosciences; antiphosphotyrosine (4G10) and anti-FcɛRIγ mAbs were purchased from Upstate Biotechnology; anti-Flag mAb was purchased from Sigma-Aldrich; anti–SHP-1, anti–SHP-2, and anti–SH 2 domain–containing inositol-5–phosphatase (SHIP) antibodies were purchased from Santa Cruz Biotechnology, Inc.; and rat IgE anti-DNP mAb was purchased from Zymed Laboratories. Anti-DAP12 polyclonal antibody was a gift from M. Ono (Ehime University School of Medicine, Ehime, Japan). TX-8 (anti–MAIR-I), TX-10 (anti–MAIR-I and MAIR-II), and TX-13 (anti–MAIR-II) mAbs were generated in our laboratory by fusing the Sp2/0 myeloma cell line with popliteal lymph node cells from rats that had been immunized by injection of Ba/F3 transfectants expressing the MAIR-I or MAIR-II, and each extracellular protein was fused with the Fc portion of human IgG into footpads, as described previously (21). F(ab′)2 fragments were prepared by digesting TX-8 and TX-13 mAbs with immobilized pepsin (10 mg IgG in 10 ml of 0.2 M sodium citrate, 0.15 M NaCl buffer, pH 3.5, with 2.5 ml of immobilized pepsin for 1 h at 37°C; Pierce Chemical Co.), and afterwards, by removing residual intact mAb by protein A affinity chromatography. Purity of the F(ab′)2 fragments were determined by SDS-PAGE.
Representational Difference Analysis (RDA) and cDNA Cloning.
Total RNA was isolated using Isogen LS solution (Nippon Gene), and oligo(dT)-primed double-stranded cDNA was synthesized using a cDNA synthesis system (GIBCO BRL) according to the manufacturer's instructions. RDA was performed, as described previously (22), using day 14 fetal livers from wild-type and PU.1−/− mice. Full-length MAIR-Ia cDNA was cloned by screening of a macrophage cDNA library, using a cDNA fragment generated by RDA as a probe. Full-length MAIR-Ib, MAIR-IIa, and MAIR-IIb cDNAs were also isolated by screening the same cDNA library, using the extracellular domain of MAIR-Ia cDNA as a probe.
Northern Blot Analysis.
MAIR-I cDNA was labeled with [32P]dCTP using the Ready to Go DNA Labeling Beads (Amersham Biosciences). Membranes containing ∼10 μg of total RNA in each lane from different mouse tissues were hybridized with the [32P]dCTP–labeled cDNA probes at 68°C in modified Church's hybridization buffer (0.5 M Church's phosphate buffer, 7% SDS, and 1% BSA). The membranes were washed at 68°C for 1 h in Church's washing buffer (40 mM Church's phosphate buffer and 1% SDS) and developed by autoradiography.
Reverse Transcription(RT)–PCR.
RNA was subjected to RT and PCR using MAIR-I–specific primers (5′-GCTGATAGGCATCCAGAGC-3′ and 5′-AAGGAGTCACAGGTAAAGGTC-3′), MAIR-II–specific primers (5′-TCGAGCCTTGAGAGTGGTAGAC-3′ and 5′-AGGAGCTGTGTTTAGGGA-CAG-3′), DAP12-specific primers (5′-GGCTCTGGAGCCC-TCCTGGT-3′ and 5′-CTGTGTGTTGAGGTCACTG-3′), or HPRT-specific primers (5′-GCTGGTGAAAAGGACCTCT-3′ and 5′-CACAGGACTAGAACACCTGC-3′). The amount of cDNA was normalized by the quantitative PCR using TaqMan rodent GAPDH control reagent (Applied Biosystems; reference 23). Conditions used for PCR were as follows: 30 or 35 cycles of 20-s denaturation (94°C), 20-s annealing (55°C), and 30-s extension (72°C).
Fluorescence in Situ Hybridization (FISH).
The direct R-banding FISH method was used for chromosomal assignment of the MAIR-I gene in mouse and rat. Preparation of R-banded chromosomes and FISH were performed, as described by Matsuda et al. (24) and Matsuda and Chapman (25).
Establishment of Transfectants Expressing MAIR-I or MAIR-II.
The MAIR-I and MAIR-II cDNA tagged with the flag cDNA at the NH2 terminus were subcloned into the pMX retroviral vectors (26). To generate site-directed MAIR-I mutant at residues Y233, PCR primers, which contained a codon for F233 (TTT) instead of Y233 (TAT), were designed. BW5147, Ba/F3, and RAW cells stably expressing Flag-tagged MAIR-I and MAIR-II were established as described previously (27).
Serotonin-release Assay.
Bone marrow cells obtained from the femur and tibia of adult Balb/c mice were cultured at a concentration of 2 × 105 cells/ml in RPMI 1640 medium containing 10% FBS, 4 ng/ml mouse rIL-3 (BD Biosciences), and 10 ng/ml mouse rSCF (provided by KIRIN). The bone marrow–derived nonadherent cells were transferred weekly into new culture dishes over a 4–10-wk culture interval. Ig-E–mediated serotonin release assay was performed as described previously (28). In brief, bone marrow–derived mast cells were incubated with 5 μCi/ml [3H]serotonin (5-[1,2-3H(N)]-hydroxytryptamine creatinine sulfate; NEN Life Science Products) for 5 h at 37°C, washed, and reincubated for an additional 1 h to reduce background radioactivity. [3H]Serotonin-loaded mast cells were plated in 96-well plates that contained 10 μl of rat IgE, 25 μg/ml anti-DNP mAb, and various concentrations of F(ab′)2 fragments of rat anti–MAIR-I or control IgG, incubated for 30 min at 4°C, washed, and resuspended in 25 μl of culture medium. Antibody-primed mast cells were challenged with 25 μl of F(ab′)2 fragments of 40 μg/ml rabbit anti–rat Ig antibody for 30–60 min at 37°C. The reactions were terminated by adding 50 μl of cold PBS. [3H]Serotonin in the supernatants were measured by a liquid scintillation counter.
Internalization Assay.
Peritoneal macrophages were incubated with biotin-labeled anti–MAIR-I mAb (TX-8), followed by allophycocyanin-labeled streptavidin at 4° or 37°C for 30 min. Cells were treated or not with 2.5% trypsin and analyzed by flow cytometry. Ba/F3 transfectants expressing Flag-tagged MAIR-I at the NH2 terminus were incubated with anti-Flag mAb, followed by cross-linking with FITC-labeled anti–mouse IgG secondary antibody at 4°C. Cells were resuspended in RPMI 1640 containing 10% FBS, plated onto polylysine-precoated chamber slide (Lab-Tek II; Nunc) and incubated at 37°C for 30 min. Cells were centrifuged at 1,500 rpm for 5 min at 4°C, washed with PBS twice, and fixed with 3% paraformaldehyde. Cells were mounted with VECTASHIELD including DAPI (Vector) and analyzed under a confocal scanning laser microscope.
Biochemistry.
BW5147 transfectants expressing Flag-tagged MAIR-I or -II were lysed in Tris-buffered saline (50 mM Tris and 15 mM NaCl, pH 8.0) containing 1% NP-40, protease inhibitors (1 mM PMSF and 20 Kallikrein inhibitor U/ml aprotinin), and phosphatase inhibitors (1 mM EGTA, 10 mM NaF, 1 mM Na4P2O7, 0.1 mM β-glycerophosphate, and 1 mM Na3VO4). To examine N-glycosylation of MAIR-I, lysates of BW5147 transfectants expressing Flag-tagged MAIR-I were immunoprecipitated with anti-Flag mAb or control Ig and treated or not with N-glycosidase F (Boehringer) using the conditions recommended by the manufacturers. To examine tyrosine phosphorylation of MAIR-I or association of MAIR-I with phosphatases, mast cells were stimulated or not with 100 mM sodium pervanadate for 10 min at 37°C. Cells were lysed in 1% digitonin (Sigma-Aldrich) buffer (0.12% Triton X-100; Katayama Pure Chemical), 150 mM NaCl, 20 mM triethanolamine (Katayama Pure Chemical), or 1% NP-40 buffer, containing the protease and phosphatase inhibitors. For analysis of association of MAIR-II with DAP12 or FcɛRIγ, 293T or RAW transfectants expressing MAIR-II or B cells separated from spleen cells were lysed in 1% digitonin buffer, and cell lysates were immunoprecipitated with control Ig, anti-DAP12, or anti-FcɛRIγ.
Lysates or immunoprecipitates were separated by SDS-PAGE, transferred (100 V, 1 h in 25 mM Tris, 195 mM glycine, and 20% methanol) to PVDF membranes (Immobilon-P; Millipore). Membranes were incubated overnight in Tris-buffered saline containing 0.5% Tween 20, MgCl2, and 3% BSA at 4°C, and incubated with primary antibodies (1 µg/ml in Tris-buffered saline with 3% BSA) for 2 h at room temperature. Proteins were detected by using HRP-conjugated goat anti–mouse, anti–rat, or anti–rabbit Igs (Amersham Biosciences), and were developed with SuperSignal CL-HRP substrate (Pierce Chemical Co.). Chemiluminescences were detected by autoradiograph or image analyzer (LAS model 1000 mini; Fuji Film).
ELISA.
RAW cells expressing Flag-tagged MAIR-II or peritoneal macrophages derived from C57BL/6 mice were pretreated with anti–CD32/16 (FcγR) to block Fcγ receptors, stimulated with plastic-coated control Ig, anti-Flag, or anti–MAIR-II mAb, and cultured for 24 or 48 h. TNF-α, IL-6, and monocyte chemoatractant protein 1 (MCP-1) concentrations in culture supernatants were measured using an ELISA kit (eBioscience) according to the manufacturer's instructions.
Results
Cloning and Molecular Characteristics of MAIR-I and MAIR-II.
To identify novel genes involved in immune responses by myeloid cells, we performed representational differential analysis (RDA), which is a PCR-based subtractive hybridization, using day 14 fetal livers from PU.1−/− mice lacking myeloid cells and control littermates. We identified several cDNA clones unique to myeloid cells. Full-length cDNAs were cloned by screening a macrophage cDNA library. The predicted aa sequence demonstrates that the protein encoded by one of these cDNAs is a type-1 transmembrane protein with a 27-aa leader sequence, a 152-aa extracellular domain, a 23-aa transmembrane domain, and a 112-aa cytoplasmic region (Fig. 1 A). A pair of cysteine residues in the extracellular domain is flanked by consensus sequences for one Ig-like domain, indicating that the protein, designated MAIR-Ia, is a member of the Ig superfamily. The extracellular domain has one potential site for N-linked glycosylation, suggesting that MAIR-Ia is a glycoprotein. The cytoplasmic domain contains the five tyrosines at residues 233, 258, 270, and 299. Among these tyrosines, the residues at 258 and 270 may consist of ITIM-like sequences (VEY258STL and LHY270SSV, respectively) based on the consensus sequence for ITIMs (I/V/L/SxYxxL/V), suggesting that MAIR-I may recruit protein tyrosine phosphatases and mediate inhibitory signals. We found that the motif Y233VNL is also a consensus sequence involved in receptor internalization (29, 30).
Figure 1.
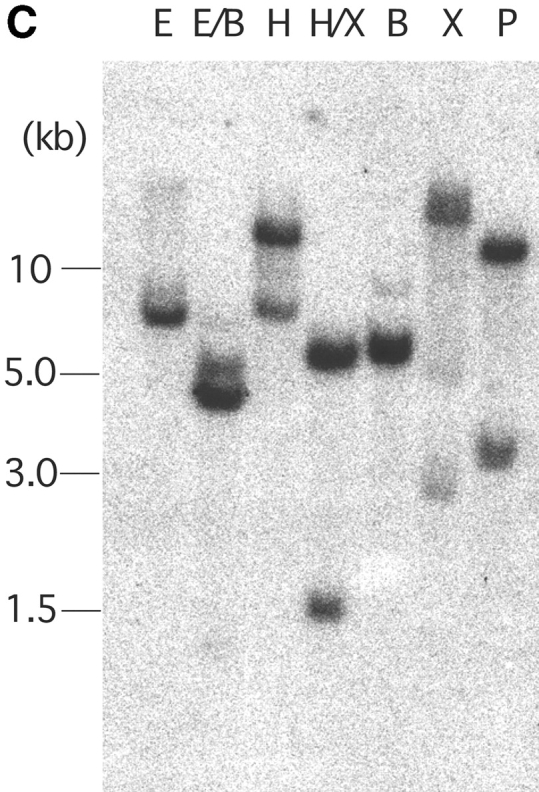
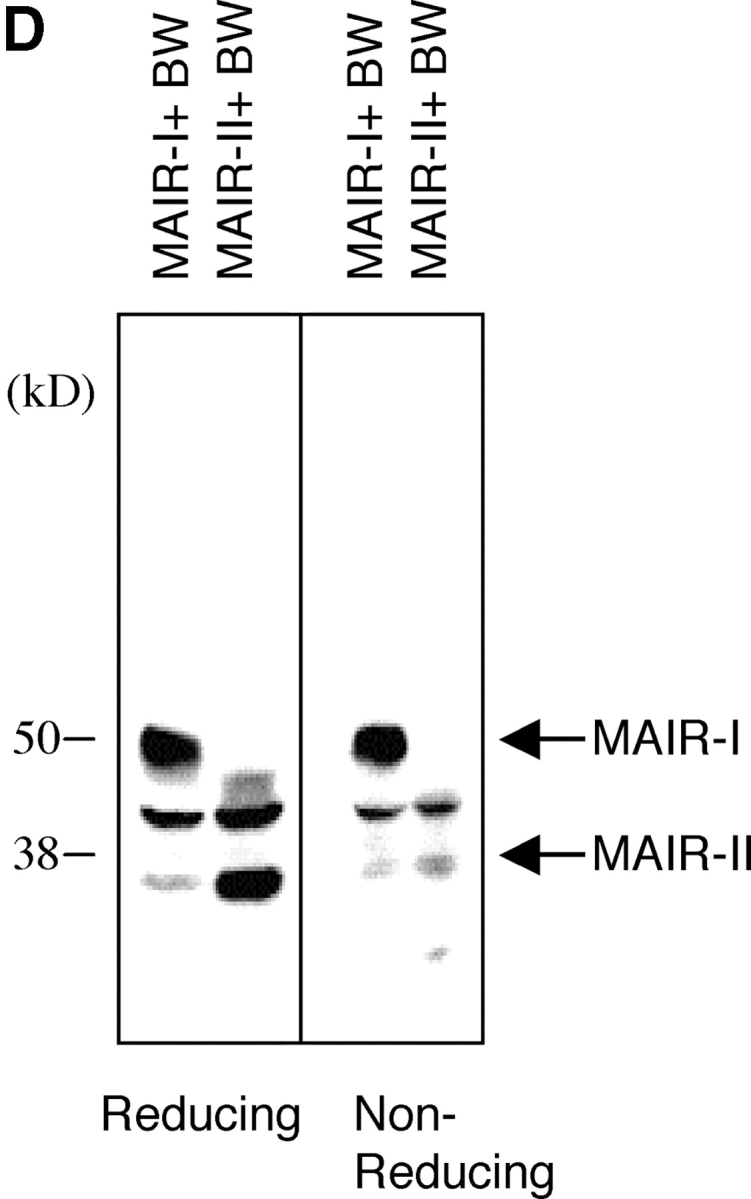
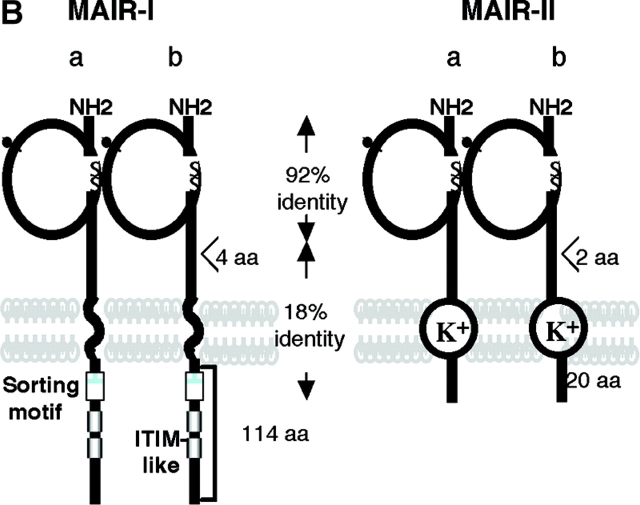
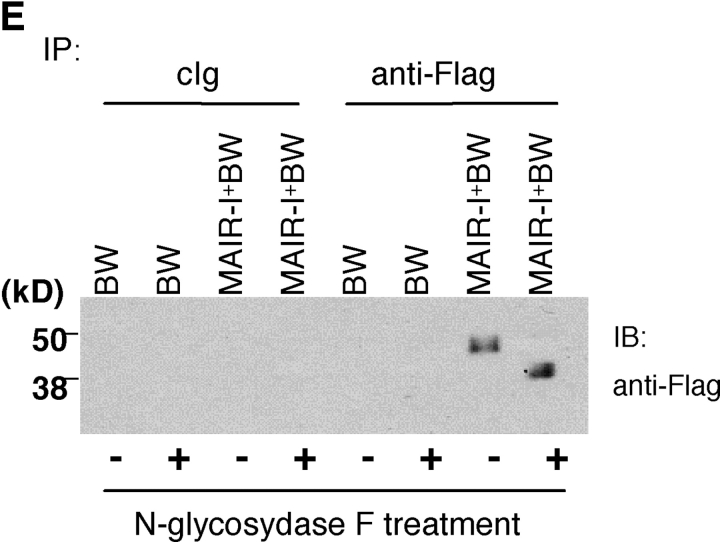
Molecular and biochemical characteristics of MAIR-I and MAIR-II. (A) Predicted aa sequences of MAIR-I and MAIR-II. The putative leader and transmembrane domain are underlined. Potential N-linked glycosylation sites in the extracellular domain and the charged aa residue in the transmembrane region are circled. Potential cysteine residues involved in disulfide bonding of the Ig-like domains are indicated in bold. ITIM-like or sorting motif in the cytoplasmic domain are boxed. The cDNA sequence data are available from GenBank/EMBL//DDBJ under accession nos. AB091765, AB091766, AB091767, and AB091768 (MAIR-Ia, MAIR-Ib, MAIR-IIa, and MAIR-IIb, respectively). (B) Schematic diagram of MAIR-I and MAIR-II proteins. A pair of cysteine residues in the extracellular portion potentially able to participate in intrachain disulfide bonding for the formation of Ig-like domains are indicated. (C) Southern blot analysis of genomic MAIR-I and MAIR-II. Mouse genomic DNA was digested with the indicated enzymes (E, EcoRI; B, BamHI; H, HindIII; X, XbaI; and P, PstI) and probed with the cDNA encoding the extracellular portion of MAIR-I. (D) BW5147 cells expressing the Flag-tagged MAIR-I or MAIR-II were lysed and proteins were analyzed by immunoblotting with anti-Flag mAb under reducing and nonreducing conditions. (E) BW5147 cells expressing the Flag-tagged MAIR-I or parental BW5147 cells were lysed and proteins were immunoprecipitated with anti-Flag. Precipitates were treated or not with N-glycosidase F and immunoblotted with anti-Flag mAb under nonreducing conditions.
To examine whether MAIR-Ia is a member of paired activating and inhibitory receptors, we further screened the macrophage cDNA library using the cDNA encoding extracellular domain of MAIR-Ia as a probe. We identified a clone encoding the protein, designated MAIR-IIa, which contains one Ig-like domain with 92% aa identity to that of MAIR-Ia in extracellular domain, a transmembrane region with a charged (Lys aa) and a short (20 aa) cytoplasmic tail (Fig. 1, A and B). In addition, we found the isoforms of MAIR-Ia and MAIR-IIa, designated MAIR-Ib and MAIR-IIb, which contain additional four and two aas, respectively, in the extracellular domain. These are alternate splicing variants of MAIR-Ia and MAIR-IIa, as suggested by the genomic DNA sequence of MAIR-I and MAIR-II (unpublished data). Southern blot analysis of murine genomic DNA, using the cDNA encoding the conserved Ig-like domain in the extracellular domain of MAIR-I and MAIR-II, demonstrated a simple hybridization pattern, suggesting that each MAIR-I and MAIR-II is encoded by a single gene (Fig. 1 C). The MAIR-I and MAIR-II genes are located to the proximal region of the E2 band of mouse chromosome 11 and consist of six and four exons, respectively, as determined by FISH and a genomic DNA sequence database (unpublished data). Database search demonstrated that MAIR-I and MAIR-II are most similar to human CMRF-35-H9 (31) with 44% homology and to human CMRF-35 (31) with 49% homology, respectively. Moreover, both CMRF-35-H9 and CMRF-35 genes are located near to human chromosome 17, syntenic region of mouse chromosome 11 (32), suggesting that these are the human homologues to murine MAIR-I and MAIR-II.
Mouse T cell leukemia BW5147 cells were transfected with the MAIR-Ia and MAIR-IIa cDNAs tagged with Flag peptide at the NH2 terminus, which resulted in surface expression of the receptor as detected by immunofluorescence using an anti-Flag mAb (unpublished data). Immunoprecipitation of the Flag-tagged MAIR-Ia and MAIR-IIa receptors on the BW5147 transfectants with an anti-Flag mAb revealed ∼50 and ∼35 kD proteins, respectively, when analyzed under both reducing and nonreducing conditions (Fig. 1 D). The mobility of MAIR-Ia decreased from ∼50 to ∼45 kD after treatment with N-glycosidase F, which is consistent with the size of the polypeptide predicted from the MAIR-I cDNA and the presence of one potential N-linked glycosylation site in the extracellular domain (Fig. 1 E).
Expression of MAIR-I and MAIR-II.
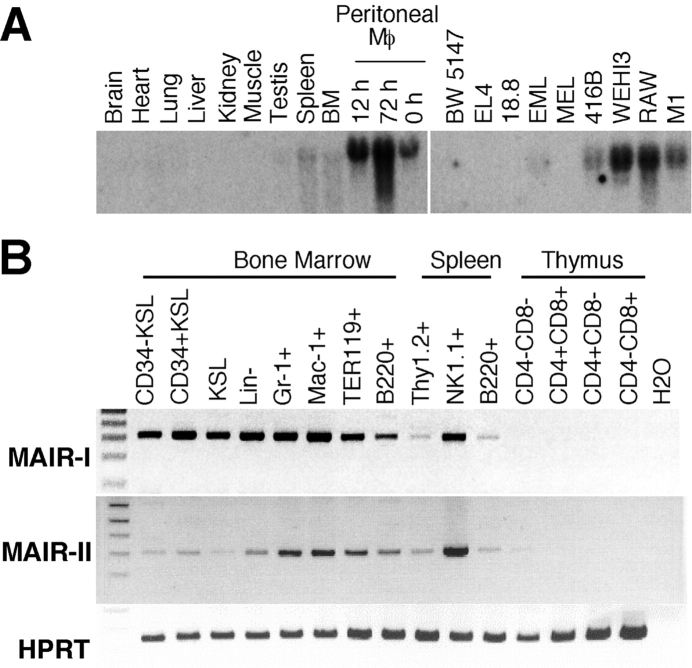
Northern blot analysis using the full-length MAIR-Ia cDNA, containing the conserved cDNA sequence of the MAIR-I and MAIR-II, as a probe demonstrated that MAIR-I and/or MAIR-II transcripts were detected in lymphohematopoietic tissues, including spleen and bone marrow, but not in thymus and nonhematopoietic organs (Fig. 2 A). We also observed that peritoneal macrophages express significant amount of MAIR transcripts, which were up-regulated in these cells after in vivo stimulation with thioglycollate. The preferential expression of MAIR transcript was also observed in myeloid cell lines, including 416B, WEHI3, RAW, and M1 cells (Fig. 2 A).
Figure 2.
Expression of MAIR-I and MAIR-II transcripts. (A) Expression of MAIR-I and/or MAIR-II transcripts was analyzed by Northern blot analysis using cDNA encoding MAIR-I extracellular portion as a probe. The comparable amount of β-actin transcript expression in each lane using β-actin cDNA was confirmed (not depicted). (B) cDNA, adjusted to comparable quantities using a HPRT control, was prepared from purified cells by cell sorter, as indicated. These cDNA and water (used as a negative control) were used as templates for RT-PCR. PCR products were amplified using specific primers for MAIR-I and MAIR-II.
To further analyze the expression of the MAIR-I and MAIR-II on hematopoietic cells, RNA was obtained from lymphohematopoietic progenitors and lineage-committed cells purified by flow cytometry from the bone marrow, thymus, and spleen. RT-PCR analysis using the specific primers for MAIR-I and MAIR-II demonstrated that both the transcripts were expressed in multilineage cells, including lymphohematopoietic progenitors, granulocytes, macrophages, erythroid cells, and B cells in bone marrow, and in T, B, and NK1.1+ cells in the spleen. However, neither of the MAIR-I and MAIR-II transcripts was detected in immature and mature T cells in the thymus (Fig. 2 B).
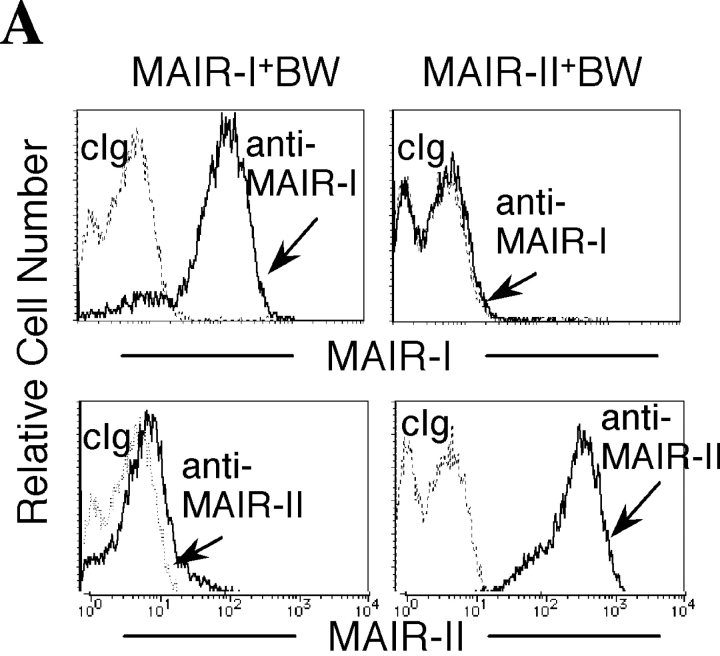
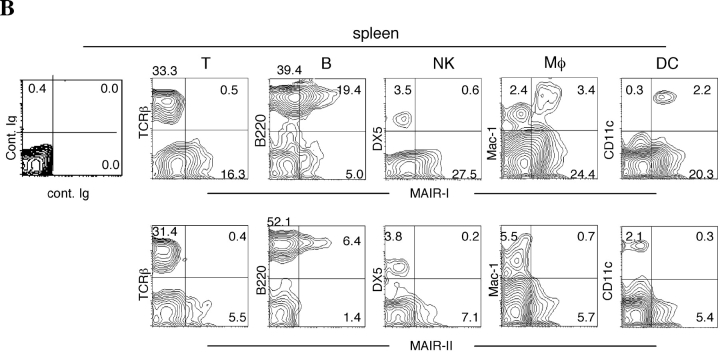
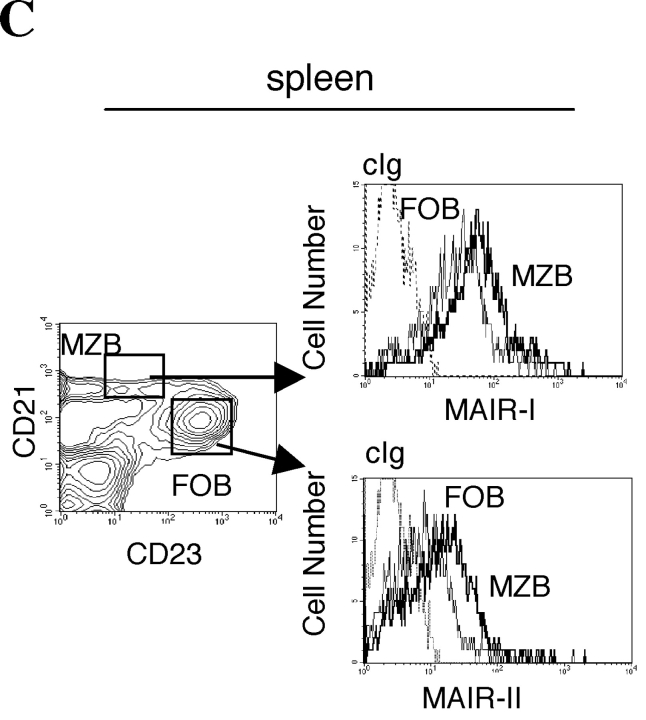
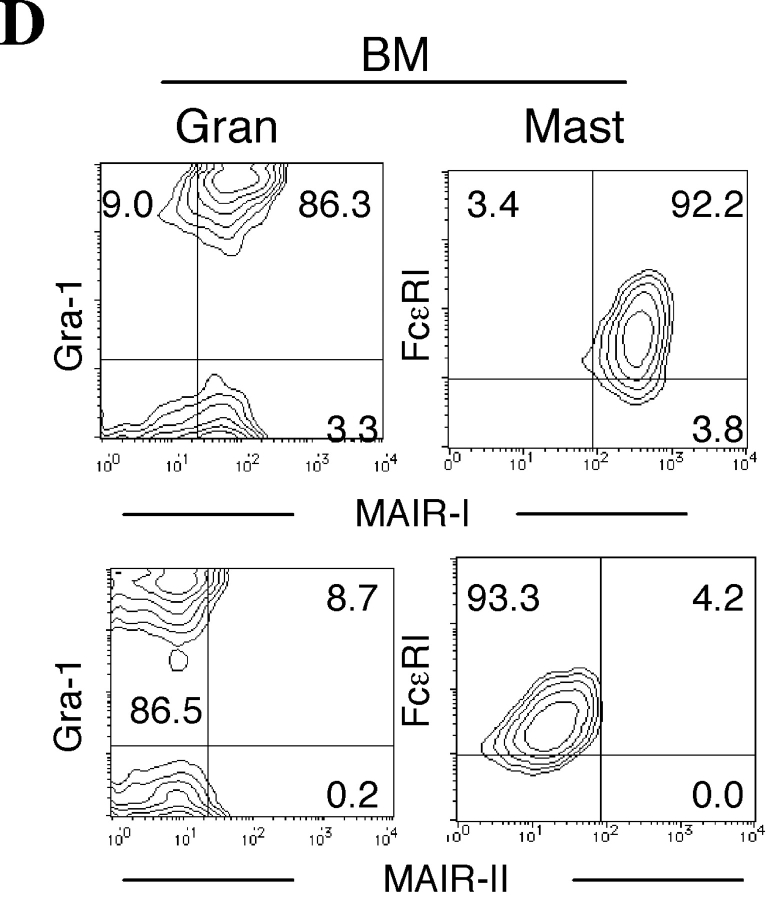
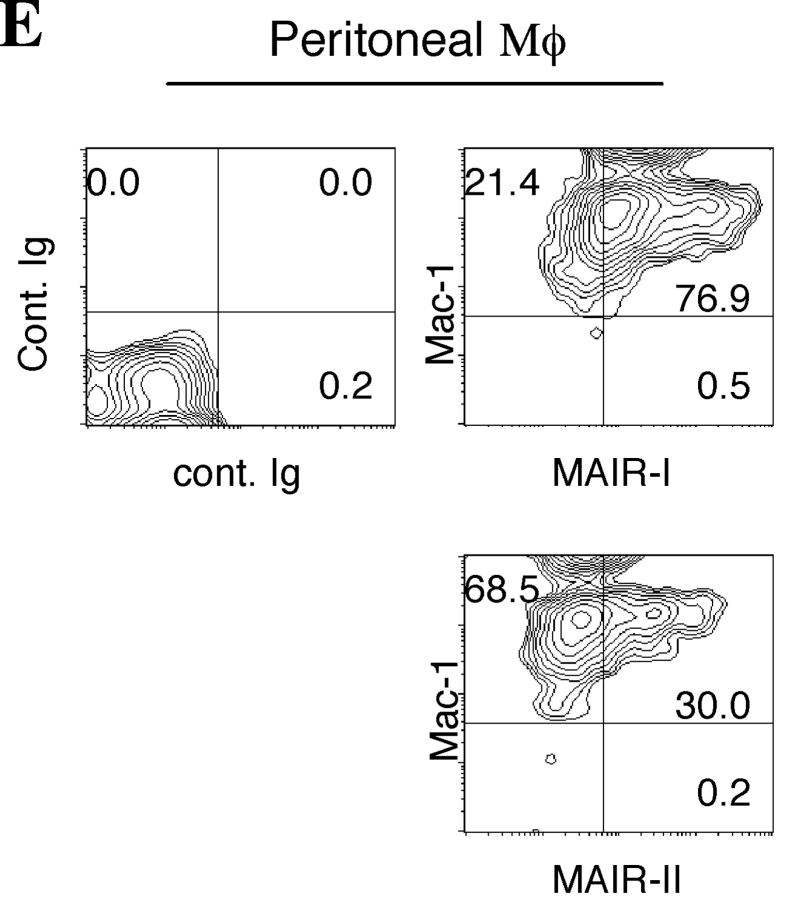
To examine the cell surface expression of the MAIR-I and MAIR-II proteins, we generated monoclonal antibodies TX-8 and TX-13, which preferentially react to MAIR-I and MAIR-II, respectively (Fig. 3 A). Analysis of spleen and bone marrow cells by flow cytometry showed that MAIR-I is expressed on the majority of myeloid cells, including macrophages, dendritic cells, granulocytes, and bone marrow–derived cultured mast cells, and MAIR-1 is expressed on a subset of B cells, but neither on T nor NK cells (Fig. 3, B and D). In contrast, MAIR-II protein is detected only on cell surface of subsets of B cells and peritoneal macrophages (Fig. 3, B and E). These results indicated that the transcript's expressions of MAIR-I and MAIR-II do not always result in cell surface expression of the proteins. To further characterize the B cell subsets expressing MAIR-I or MAIR-II, spleen cells were simultaneously stained with anti-CD21 and anti-CD23 mAbs in combination with either anti–MAIR-I or anti–MAIR-II. The marginal zone B cells that are characterized by cell surface phenotype of CD21hiCD23lo express higher amount of MAIR-I and MAIR-II than the CD21intCD23hi follicular B cells (Fig. 3 C; reference 33). These results suggest that marginal zone B cells consist of the major population of MAIR-II expressing B cell subset in spleen.
Figure 3.
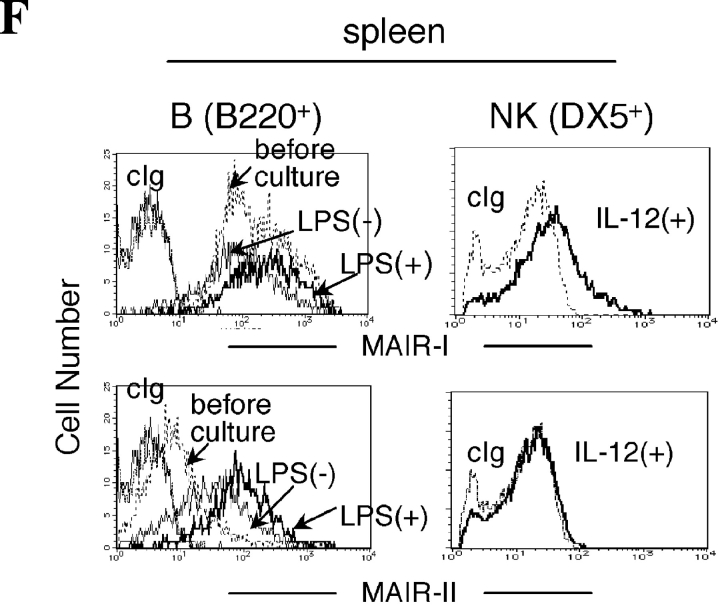
Cell surface expression of MAIR-I and MAIR-II proteins. (A) BW5147 transfectants expressing MAIR-I or MAIR-II were stained with control rat Ig or anti–MAIR-I (TX-8 mAb) or anti–MAIR-II (TX-13 mAb), followed by allophycocyanin-conjugated streptavidin. (B, D, and E) Spleen, bone marrow, or peritoneal macrophages from C57/BL6 mice were stained with either biotin-conjugated F(ab′)2 fragments of anti–MAIR-I or anti–MAIR-II and the PE-conjugated mAbs indicated, followed by allophycocyanin-conjugated streptavidin. Greater than 99% of cells stained with F(ab′)2 fragments of control Igs were present in the bottom left quadrant of the contour plots (B and D, and not depicted in C). (C) Spleen cells were stained with FITC-conjugated anti-CD21, PE-conjugated anti-CD23, and biotin-conjugated anti–MAIR-II monoclonal antibodies, followed by allophycocyanin-conjugated streptavidin. MAIR-I and MAIR-II expression on the CD21hiCD23lo (marginal zone B cells [MZB]) and CD21intCD23hi (follicular B cells [FOB]) cells were analyzed by flow cytometry. (E) B220+ B cells purified from splenocytes or total splenocytes were cultured for 48 h or not in the presence or absence of LPS or IL-12, as indicated, and stained with either biotin-conjugated F(ab′)2 fragments of control Ig, anti–MAIR-I or anti–MAIR-II and the FITC-conjugated B220 or DX5 mAb, followed by allophycocyanin-conjugated streptavidin. Cells were gated according to B220 or DX5 expressions and analyzed by flow cytometry. Data are representative in several independent experiments.
To examine regulatory mechanisms of the proteins expression on cell surface, purified B cells from spleen were cultured in medium in the presence or absence of LPS, resulting in a significant up-regulation of MAIR-II on the cell surface in both the culture condition (Fig. 3 F), although we did not observe any modulation of MAIR-I and MAIR-II proteins expressions on macrophages from spleen in the same culture condition (not depicted). Although no MAIR-I and MAIR-II proteins were detected on the NK cell surface despite the abundant presence of their transcripts, IL-12 stimulated cell surface expression of MAIR-I, but not MAIR-II, on NK cells (Fig. 3 F).
MAIR-I Mediates Endocytosis.
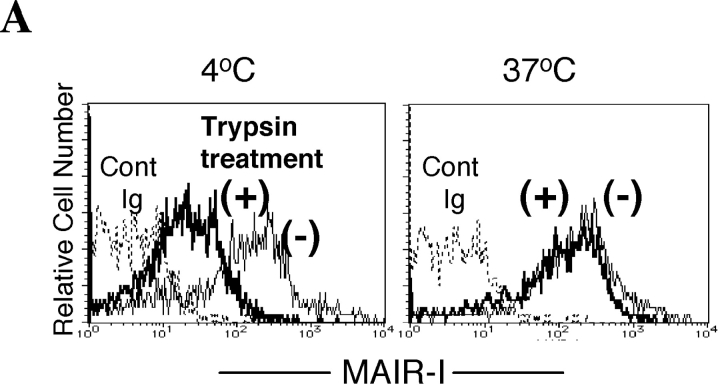
The cytoplasmic domain of the MAIR-I contains a possible tyrosine-based sorting motif (Fig. 1 A, YVNL), implicated in endosome and lysosome targeting of diverse proteins and involved in agonist-induced internalization (29, 30). To investigate whether the MAIR-I internalizes upon cross-linking, MAIR-I expressed on peritoneal macrophages were cross-linked with anti–MAIR-I mAb and incubated at 4 or 37°C for 1 h. Some of the cells were treated with trypsin to remove the cell surface MAIR-I that did not undergo internalization. Treatment with trypsin significantly decreased the fluorescence intensity of the cells, which were incubated at 4°C after cross-linking (Fig. 4 A). By contrast, the fluorescence remained constant in the cells, which were incubated at 37°C after cross-linking, despite treatment with trypsin. This suggests that cross-linking the MAIR-I induced the receptor internalization during culture at 37°C. To confirm MAIR-I internalization upon cross-linking, the Ba/F3 transfectants expressing the Flag-tagged MAIR-I at the NH2 terminus were incubated with anti-Flag mAb, cross-linked with FITC-labeled secondary antibody, and incubated at 37°C for 30 min. Although green fluorescence was detected only at the cell surface before incubation, it was abundantly observed in the cytoplasm of the transfectants 30 min after incubation at 37°C under confocal scanning laser microscope (Fig. 4 B), indicating that MAIR-I underwent internalization after cross-linking. In contrast, we did not detect internalization of the MAIR-I mutated at the tyrosine residue 233 (Y-F233) in the sorting motif (Fig. 4 B), indicating that the tyrosine at residue 233 is responsible for the MAIR-I internalization.
Figure 4.
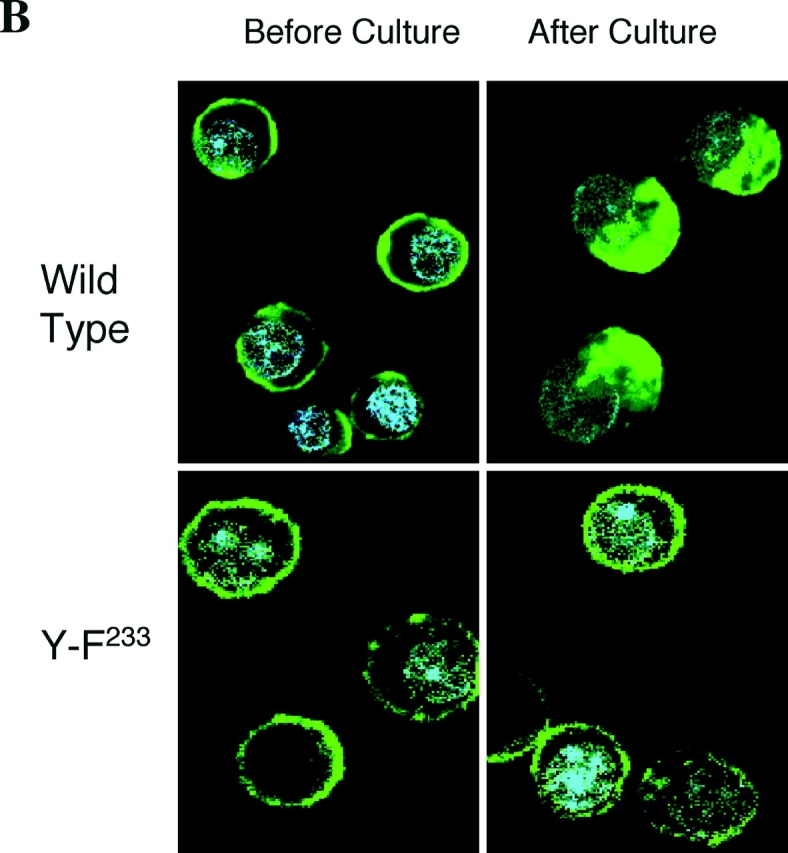
Internalization of MAIR-I. (A) Peritoneal macrophages were incubated with biotin-labeled anti–MAIR-I mAb (TX-8), followed by allophycocyanin-labeled streptavidin at 4 or 37°C for 30 min. The cells were treated (+) or not (−) with trypsin and analyzed by flow cytometry. (B) Ba/F3 transfectants expressing Flag-tagged wild-type and mutated MAIR-I were incubated with anti-Flag mAb, followed by cross-linking with FITC-labeled anti–mouse IgG secondary antibody at 4°C. Cells were incubated or not at 37°C for 30 min, fixed, mounted with VECTASHIELD including DAPI, and analyzed using a confocal scanning laser microscope. Data are representative in several independent experiments.
MAIR-I Recruits the Tyrosine Phosphatase SHIP.
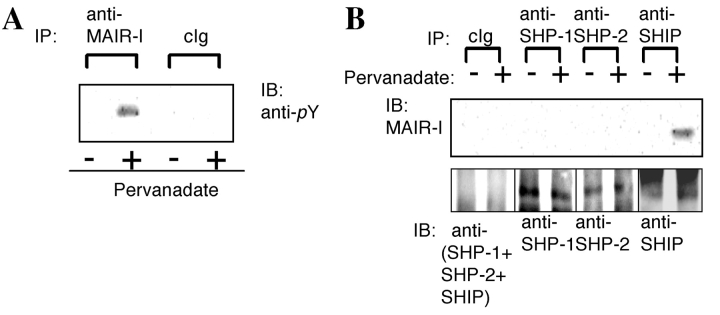
The existence of the ITIM-like sequences in the cytoplasmic region of MAIR-I suggested that MAIR-I is tyrosine phosphorylated and may recruit SH2-containing tyrosine phosphatases. Therefore, we first examined whether MAIR-I expressed on bone marrow–derived cultured mast cells were tyrosine phopshorylated by the stimulaion with pervanadate. As demonstrated in Fig. 5 A, stimulation with pervanadate significantly induced tyrosine phosphorylation of the MAIR-I (Fig. 5 A). Immunoprecipitation of the protein tyrosine phosphatases, SHP-1 and SHP-2, and SHIP demonstrated that MAIR-I associated with SHIP, but not SHP-1 and SHP-2, upon stimulation of mast cells with pervanadate (Fig. 5 B). These results suggest that MAIR-I may mediate inhibitory signal upon phosphorylation of the tyrosines in the cytoplasmic region.
Figure 5.
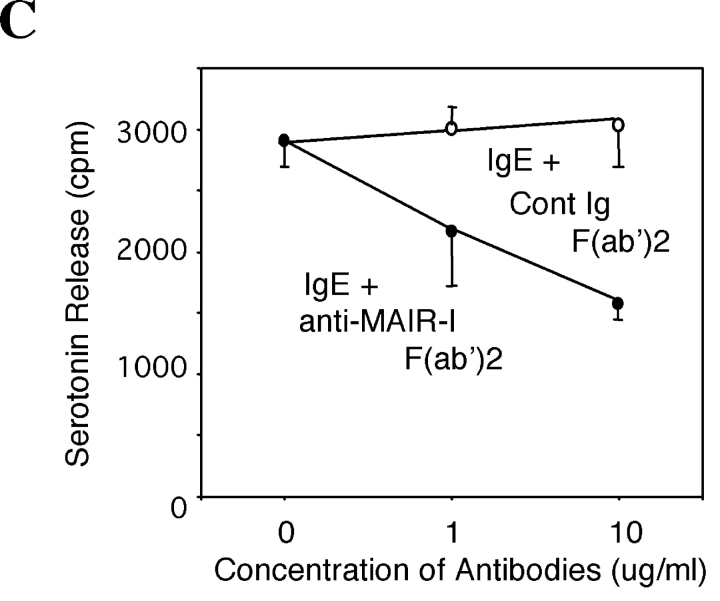
MAIR-I recruits the tyrosine phosphatase SHIP and inhibits serotonin release from mast cells. (A and B) Mast cells, derived from bone marrow cells after culture in the presence of IL-3 and stem cell factor (SCF), were stimulated or not with pervanadate for 10 min at 37°C and lysed in 1% NP-40 (A) or digitonin (B) buffer and immunoprecipitated with cIg, anti–MAIR-I, anti–SHP-I, anti–SHP-II, or anti-SHIP. The immunoprecipitates were immunoblotted with antiphosphotyrosine mAb (A) or anti–MAIR-I (B). (C) Mast cells were loaded with [3H]serotonin and stimulated with an IgE mAb plus either F(ab′)2 fragments of anti–MAIR-I or control rat IgG, followed by coligation with a common secondary reagent. [3H]Serotonin release into the supernatants was measured by a liquid scintillation counter. Data are representative in several independent experiments.
MAIR-I Inhibits IgE-mediated Serotonin Release from Mast Cells.
To address a functional role of MAIR-I in immune responses, mast cells were loaded with [3H]serotonin and stimulated with an IgE mAb plus F(ab′)2 fragments of anti–MAIR-I mAb, followed by coligation with a common secondary reagent. As demonstrated in Fig. 5 C, cross-linking MAIR-I with anti–MAIR-I mAb inhibited IgE-mediated serotonin release from bone marrow–derived mast cells in a dose-dependent manner. These results indicate that MAIR-I transduces an inhibitory signal, resulting in suppression of mast cell degranulation triggered by FcɛRI-mediated activation signals.
MAIR-II Associates with DAP12.
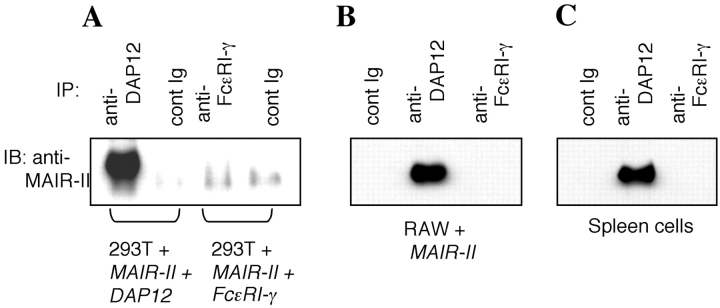
In contrast to MAIR-I, MAIR-II has a short cytoplasmic tail and a positively charged aa residue in the transmembrane domain. To test a noncovalent association of MAIR-II with an adaptor transmembrane protein, such as the FcɛRIγ or DAP12, 293T cells, which express neither FcɛRIγ nor DAP12, were transfected with MAIR-II cDNA and either FcɛRIγ or DAP12. The transfectants were lysed in 1% digitonin buffer and immunoprecipitated with anti-FcɛRIγ or anti-DAP12, and the isolated proteins were analyzed by immunoblotting with anti–MAIR-II. As demonstrated in Fig. 6 A, MAIR-II was coimmunoprecipitated with DAP12, but not with FcɛRIγ, suggesting the physical association of MAIR-II with DAP12. Similary, MAIR-II was also coimmunoprecipitated with endogenous DAP12, but not FcɛRIγ, in the lysates of mouse macrophage cells RAW after transfection with MAIR-II alone (Fig. 6 B). To confirm the physiological association of MAIR-II with DAP12 in primary cells, spleen cells were stimulated with LPS for 48 h, resulting in the up-regulation of MAIR-II expression of B cells and macrophages (unpublished data). The stimulated spleen cells were lysed in 1% digitonin buffer and immunoprecipitated with anti-FcɛRIγ or anti-DAP12. Immunoblotting of the isolated proteins with anti–MAIR-II again demonstrated the coimmunoprecipitation of MAIR-II with the DAP-12, but not with the FcɛRIγ (Fig. 6 C), indicating that DAP12 is the physiological partner for MAIR-II of stimulated B cells and/or macrophages in the spleen. These results were in agreement with the observation that DAP12 expression on cell surface of 293T cells depended on the expression of MAIR-II, although MAIR-II does not require DAP12 for cell surface expression (Fig. 6 D).
Figure 6.
Physical association of MAIR-II with DAP12. (A–C) 293T and RAW cells, which were transfected with cDNA as indicated, and spleen cells after stimulation with LPS were lysed in digitonin buffer, immunoprecipitated with control Ig, anti-DAP12, or anti-FcɛRIγ, and immunoblotted with anti–MAIR-II (TX-10). (D) 293T cells were transfected with Flag-tagged DAP12, MAIR-II, or a combination of Flag-tagged DAP12 and MAIR-II, and stained with biotin-conjugated anti–MAIR-II and FITC-conjugated anti-Flag mAbs, followed by allophycocyanin-conjugated streptavidin. Cell surface expression of MAIR-II and DAP12 was analyzed by flow cytometry.
MAIR-II Stimulates Proinflamatory Cytokines and Chemokine Secretions from Macrophages.
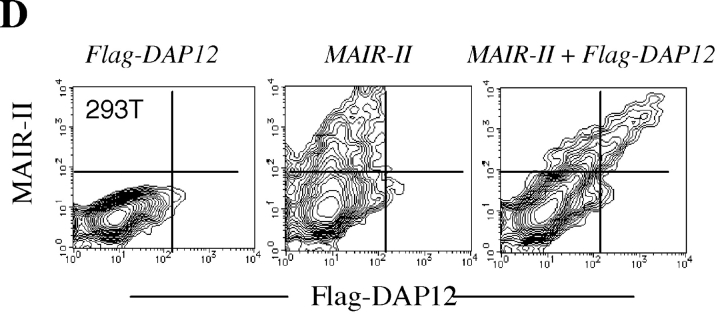
Because macrophages abundantly express DAP12 (34), we investigated a functional role of MAIR-II–mediated signaling in immune responses by macrophages. Macrophage cells, RAW, were transfected with Flag-tagged MAIR-II cDNA at the NH2 terminus and stimulated with control Ig or anti-Flag. Cross-linking MAIR-II with anti-Flag significantly stimulated the production of TNF-α from the transfectants (Fig. 7) . We further examined whether MAIR-II mediates TNF-α secretion from primary macrophages from peritoneal cavity. As shown in Fig. 7, TNF-α secretion from peritoneal macrophages was significantly increased upon cross-linking of MAIR-II. Similarly, engagement of MAIR-II stimulated IL-6 and MCP-1 secretions from peritoneal macrophages (Fig. 7). However, we did not observe a significant increase of IL-12 secretion from peritoneal macrophages after cross-linking MAIR-II (unpublished data). These results suggest that MAIR-II mediates activation signal in macrophages, leading to cytokine and chemokine secretion from macrophages.
Figure 7.
MAIR-II stimulates cytokines and chemokines secretion from macrophages. RAW cells transfected with Flag-tagged MAIR-II cDNA at the NH2 terminus and peritoneal macrophages were pretreated with anti-CD16/32 (Fcγ receptors) to block Fc binding of mAbs, stimulated with plastic-coated control Ig, anti-Flag, or anti–MAIR-II, and cultured for 48 h. Culture supernatants were harvested, and cytokine's and chemokine's concentrations were measured by ELISA.
Expression of DAP12 in B Cells.
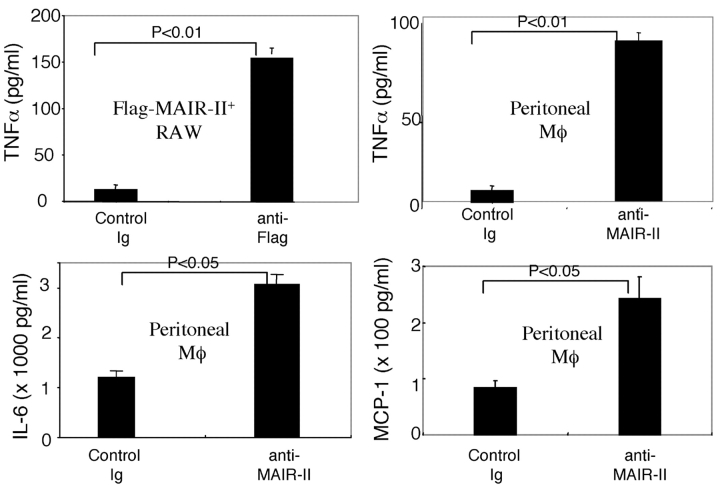
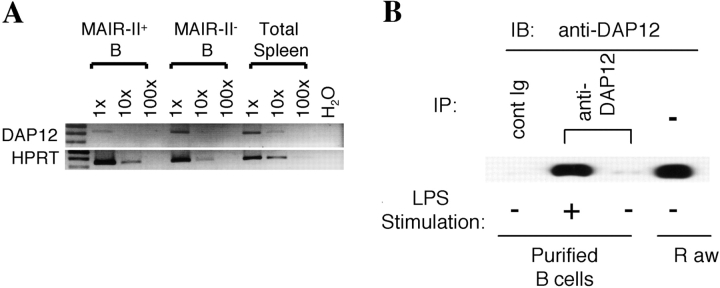
Although human primary B cells and a mouse B cell line also express DAP12 transcript (35, 36), there has been no paper reporting that primary mouse B cells express DAP12. To investigate the functional role of MAIR-II on B cells, we examined whether spleen B cells express DAP12. B cells were highly fractionated from spleen cells stimulated with LPS according to MAIR-II expression by repeating sorting on flow cytometry (>99.8% purity, as determined by reanalysis by flow cytometry) and subjected to semiquantitative RT-PCR. As demonstrated in Fig. 8 A, both fractions of B cells express DAP12 transcript. To further examine whether B cells express DAP12 protein, DAP12 was immunoprecipitated from purified B cells before or after stimulation with LPS for 24 h and immunoblotted with anti-DAP12. Although DAP12 protein was undetectable in primary resting B cells, we observed a significant up-regulation of DAP12 protein expression in stimulated B cells (Fig. 8 B). These results suggest that MAIR-II associates with DAP12 (also on B cells, at least in the activated state with LPS), resulting in MAIR-II–mediated signaling in B cells.
Figure 8.
Expression of DAP12 in B cells. (A) Spleen cells were stained with biotin-conjugated anti–MAIR-II and PE-conjugated B220, followed by allophycocyanin-conjugated streptavidin. MAIR-II+B220+ and MAIR-II−B220+ cells were purified by repeating sorting twice on flow cytometry (>99.8% purity). The RNA was extracted from the fractionated and total spleen cells and was subjected to semiquantitive RT-PCR (30 cycles) for DAP12 and HPRT, according to template dose by dilution. (B) B cells were purified from spleen cells by repeating positive selection twice with B220+ MACS-beads (>99.0% purity). 5 × 106 purified B cells were stimulated or not with 10 μg/ml LPS for 24 h, lysed in 1% NP-40 lysis buffer, and immunoprecipitated with control Ig or anti-DAP12. Immunoprecipitates or lysates of 106 raw cells were immunoblotted with anti-DAP12.
Discussion
Paired activating and inhibitory Ig-like receptors have attracted widespread interest because they are expressed on a variety of immune cell types and facilitate activation or deactivation of immune responses. Although most genes encoding these receptors are located on human chromosome 19q13.4 or its syntenic region on murine chromosome 7 (6), MAIR-I and MAIR-II genes are mapped to a unique location on murine chromosome 11E2, suggesting that the MAIR is a novel family of paired activating and inhibitory receptors.
The regulatory mechanism of paired activating and inhibitory receptor expressions on the cell surface is quite an important concept to be clarified; these receptors may play opposite roles in immune responses upon binding to their, sometimes common, ligand(s). However, their protein expression profiles on immune cells remain largely unknown, mainly because of lacking mAbs which distinguish between these paired receptors containing highly conserved extracellular domains each other. This is the case also in CMRF-35-H9 and CMRF-35 (31), possible human homologues of MAIR-I and MAIR-II, respectively.
In this paper, we have analyzed expressions of MAIR-I and MAIR-II using TX-8 and TX-13 mAbs that specifically react to MAIR-I and MAIR-II, respectively, demonstrating that MAIR-I is expressed on the majority of all the myeloid lineage cells and a subset of B cells, whereas MAIR-II is expressed only on subsets of B cells and peritoneal macrophages (Fig. 3, B, C, and D). This suggests that the MAIR-I inhibitory receptor is always dominantly expressed on these cells. These findings were not in complete agreement with the results of transcript expression, which indicated that both MAIR-I and MAIR-II are similarly expressed in the multilineage immune cells (Fig. 2 B). However, we have demonstrated that MAIR-II protein expression on B cell surface is up-regulated after culture (Fig. 3 E). Similarly, MAIR-I expression was also up-regulated on NK cells after stimulation with IL-12. We have also shown the down-regulation of MAIR-I cell surface expression by the receptor internalization upon cross-linking (Fig. 4). Although physiological significances of the up-regulation of MAIR-I and MAIR-II expression and the down-regulation of MAIR-I expression on cell surface are uncertain at present, they may play a regulatory role in cell activation when these receptors encounter their ligand(s). Further studies on the regulatory mechanisms of MAIR-I and MAIR-II expressions are required to clarify roles of these receptors in immune responses.
In the present work, we have shown that cross-linking MAIR-I and MAIR-II transduces inhibitory and activating signals, respectively. We have demonstrated the inhibition of IgE-mediated degranulation by cross-linking MAIR-I (Fig. 5). This situation is similar in murine PIR-B, an inhibitory type of paired Ig-like receptor, which, but not the activating type PIR-A, is also expressed on mast cells, where coligation of FcɛRI and PIR-B induced inhibition of IgE-mediated mast cell activation (28). MAIR-I associates with the phosphatase SHIP, which should be involved in the inhibition of mast cell activation. However, we do not conclude which ITIM-like sequence in the cytoplasmic region interacts with SHIP to mediate an inhibitory signal. Although the aa sequences VEYSTL and LHYSSV fit the consensus sequence for ITIMs (I/V/L/SxYxxL/V), we cannot exclude a possibility that the YSTL and YSSV within these sequences consist of ITAM because of the appropriate aa distance for ITAM (eight aa) between the sequences (37). In fact, SHIP also associates with ITAM in several activating adaptors, such as the β and γ subunits of FcɛRI, CD3 γ, δ, and ɛ chains as well as the T cell receptor ζ chain and Fcγ receptor via its SH2 domain (38–40), although the biological significance of these interactions has not yet been determined. Nonetheless, MAIR-I mediates an inhibitory signal and the concrete signaling pathways mediated by MAIR-I should be clarified in future papers.
On the contrary, MAIR-II associates with the ITAM-bearing adaptor DAP12 and mediates activating signals for secretion of inflammatory cytokines and chemokines, such as TNF-α and MCP-1, but not IL-12, in macrophages. TNF-α and MCP-1 are secreted from human macrophages upon initiation of DAP12-dependent pathways via TREM-1, whereas IL-6 and IL-12 are not (41). Presently, the discrepancy of IL-6 secretion mediated by mouse MAIR-II and human TREM-1 from macrophages, both of which are dependent on DAP12 pathways, is unclear. MAIR-II expression is also observed on a small subset of resting B cells and up-regulated on the majority of B cells after culture (Figs. 3, B and E). Because we have demonstrated that B cells express DAP12 (Fig. 8, A and B), MAIR-II may mediate activation signals via DAP12-dependent pathways in B cells. Future analyses are required to clarify MAIR-II–mediated signaling and the functional role of MAIR-II in B cells.
Finally, because the MAIR-II+ cells coexpress MAIR-I simultaneously, a question should be raised how activation or deactivation of these cells is regulated by MAIR-I and MAIR-II if both receptors would bind the common ligand. To elucidate the regulatory roles of these receptors in cell signaling and immune responses, it is essentially required to identify and characterize the ligands for MAIR-I and MAIR-II and the receptor–ligand interactions.
Acknowledgments
We thank L. Lanier for critical reading of the manuscript, H. Kubagawa for helpful discussion, T. Takai and M. Ono for reagents, K. Yamazaki for expert assistance, flow cytometry, and DNA sequencing, T. Kameyama for technical assistance, and Y. Soeda for secretarial assistance.
This research was supported in part by the grants provided by the Ministry of Education, Science and Culture of Japan and the Special Coordination Funds of the Science and Technology Agency of the Japanese government.
The present address of M. Osawa, A. Iwama, and H. Nakauchi is the Laboratory of Stem Cell Therapy, Center for Experimental Medicine, The Institute of Medical Science, University of Tokyo, Tokyo, 108-8639 Japan.
Footnotes
Abbreviations used in this paper: aa, amino acid; FISH, fluorescence in situ hybridization; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; MAIR, myeloid-associated immunoglobulin-like receptor; MCP-1, monocyte chemoatractant protein 1; PIR, paired Ig-like receptor; RDA, representational difference analysis; RT, reverse transcription; SH2, Src homology 2; SHIP, SH2 domain–containing inositol-5–phosphatase.
References
- 1.Ravetch, J.V., and L.L. Lanier. 2000. Immune inhibitory receptors. Science. 290:84–89. [DOI] [PubMed] [Google Scholar]
- 2.Lanier, L.L. 2001. Face off–the interplay between activating and inhibitory immune receptors. Curr. Opin. Immunol. 13:326–331. [DOI] [PubMed] [Google Scholar]
- 3.Malbec, O., D.C. Fong, M. Turner, V.L. Tybulewicz, J.C. Cambier, W.H. Fridman, and M. Daeron. 1998. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J. Immunol. 160:1647–1658. [PubMed] [Google Scholar]
- 4.Smith, K.G., D.M. Tarlinton, G.M. Doody, M.L. Hibbs, and D.T. Fearon. 1998. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 187:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takai, T., and M. Ono. 2001. Activating and inhibitory nature of the murine paired immunoglobulin-like receptor family. Immunol. Rev. 181:215–222. [DOI] [PubMed] [Google Scholar]
- 6.Martin, A.M., J.K. Kulski, C. Witt, P. Pontarotti, and F.T. Christiansen. 2002. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 23:81–88. [DOI] [PubMed] [Google Scholar]
- 7.Lanier, L.L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359–393. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, C.A., and J.P. Allison. 1999. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 11:203–210. [DOI] [PubMed] [Google Scholar]
- 9.Ravetch, J.V., and R.A. Clynes. 1998. Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 16:421–432. [DOI] [PubMed] [Google Scholar]
- 10.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234. [DOI] [PubMed] [Google Scholar]
- 11.Kubagawa, H., P.D. Burrows, and M.D. Cooper. 1997. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl. Acad. Sci. USA. 94:5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayami, K., D. Fukuta, Y. Nishikawa, Y. Yamashita, M. Inui, Y. Ohyama, M. Hikida, H. Ohmori, and T. Takai. 1997. Molecular cloning of a novel murine cell-surface glycoprotein homologous to killer cell inhibitory receptors. J. Biol. Chem. 272:7320–7327. [DOI] [PubMed] [Google Scholar]
- 13.Samaridis, J., and M. Colonna. 1997. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur. J. Immunol. 27:660–665. [DOI] [PubMed] [Google Scholar]
- 14.Borges, L., M.L. Hsu, N. Fanger, M. Kubin, and D. Cosman. 1997. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 159:5192–5196. [PubMed] [Google Scholar]
- 15.Wagtmann, N., S. Rojo, E. Eichler, H. Mohrenweiser, and E.O. Long. 1997. A new human gene complex encoding the killer cell inhibitory receptors and related monocyte/macrophage receptors. Curr. Biol. 7:615–618. [DOI] [PubMed] [Google Scholar]
- 16.Kharitonenkov, A., Z. Chen, I. Sures, H. Wang, J. Schilling, and A. Ullrich. 1997. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 386:181–186. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka, Y., T. Matozaki, T. Noguchi, A. Iwamatsu, T. Yamao, N. Takahashi, M. Tsuda, T. Takada, and M. Kasuga. 1996. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16:6887–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohnishi, H., M. Kubota, A. Ohtake, K. Sato, and S. Sano. 1996. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J. Biol. Chem. 271:25569–25574. [DOI] [PubMed] [Google Scholar]
- 19.Lanier, L.L., and A.B. Bakker. 2000. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today. 21:611–614. [DOI] [PubMed] [Google Scholar]
- 20.Love, P.E., and E.W. Shores. 2000. ITAM multiplicity and thymocyte selection: how low can you go? Immunity. 12:591–597. [DOI] [PubMed] [Google Scholar]
- 21.Shibuya, A., N. Sakamoto, Y. Shimizu, K. Shibuya, M. Osawa, T. Hiroyama, H.J. Eyre, G.R. Sutherland, Y. Endo, T. Fujita, et al. 2000. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1:441–446. [DOI] [PubMed] [Google Scholar]
- 22.Iwama, A., P. Zhang, G.J. Darlington, S.R. McKercher, R. Maki, and D.G. Tenen. 1998. Use of RDA analysis of knockout mice to identify myeloid genes regulated in vivo by PU.1 and C/EBPalpha. Nucleic Acids Res. 26:3034–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto, N., K. Shibuya, Y. Shimizu, K. Yotsumoto, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, H. Nakauchi, and A. Shibuya. 2001. A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur. J. Immunol. 31:1310–1316. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda, Y., Y.N. Harada, S. Natsuume-Sakai, K. Lee, T. Shiomi, and V.M. Chapman. 1992. Location of the mouse complement factor H gene (cfh) by FISH analysis and replication R-banding. Cytogenet. Cell Genet. 61:282–285. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda, Y., and V.M. Chapman. 1995. Application of fluorescence in situ hybridization in genome analysis of the mouse. Electrophoresis. 16:261–272. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura, T., M. Onishi, S. Kinoshita, A. Shibuya, A. Miyajima, and G.P. Nolan. 1995. Efficient screening of retroviral cDNA expression libraries. Proc. Natl. Acad. Sci. USA. 92:9146–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya, K., L.L. Lanier, J.H. Phillips, H.D. Ochs, K. Shimizu, E. Nakayama, H. Nakauchi, and A. Shibuya. 1999. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 11:615–623. [DOI] [PubMed] [Google Scholar]
- 28.Uehara, T., M. Blery, D.W. Kang, C.C. Chen, L.H. Ho, G.L. Gartland, F.T. Liu, E. Vivier, M.D. Cooper, and H. Kubagawa. 2001. Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B. J. Clin. Invest. 108:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks, S.M., H. Ohno, T. Kirchhausen, and J.S. Bonifacino. 1997. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7:124–128. [DOI] [PubMed] [Google Scholar]
- 30.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L.C. Cantley, J.S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 31.Green, B.J., G.J. Clark, and D.N. Hart. 1998. The CMRF-35 mAb recognizes a second leukocyte membrane molecule with a domain similar to the poly Ig receptor. Int. Immunol. 10:891–899. [DOI] [PubMed] [Google Scholar]
- 32.Clark, G.J., B.J. Green, and D.N. Hart. 2000. The CMRF-35H gene structure predicts for an independently expressed member of an ITIM/ITAM pair of molecules localized to human chromosome 17. Tissue Antigens. 55:101–109. [DOI] [PubMed] [Google Scholar]
- 33.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 34.Bakker, A.B., E. Baker, G.R. Sutherland, J.H. Phillips, and L.L. Lanier. 1999. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl. Acad. Sci. USA. 96:9792–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldhahn N., I. Schwering, S. Lee, M. Wartenberg, F. Klein, H. Wang, G. Zhou, S.M. Wang, J.D. Rowley, J. Hescheler, et al. 2002. Silencing of B cell receptor signals in human naive B cells. J. Exp. Med. 196:1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Tomasello, E., L. Olcese, F. Vely, C. Geourgeon, M. Blery, A. Moqrich, D. Gautheret, M. Djabali, M.-G. Mattei, and E. Vivier. 1998. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP12. J. Biol. Chem. 273:34115–34119. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, A., and D.R. Littman. 1994. Signal transduction by lymphocytes antigen receptors. Cell. 76:263–274. [DOI] [PubMed] [Google Scholar]
- 38.Osborne, M.A., G. Zenner, M. Lubinus, X. Zhang, Z. Songyang, L.C. Cantley, P. Majerus, P. Burn, and J.P. Kochan. 1996. The inositol 5′-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high affinity IgE receptor aggregation. J. Biol. Chem. 273:29271–29278. [DOI] [PubMed] [Google Scholar]
- 39.March, M.E., and K. Ravichandran. 2002. Regulation of the immune response by SHIP. Semin. Immunol. 14:37–47. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura, K., A. Malykhin, and K.M. Coggeshall. 2002. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 100:3374–3378. [DOI] [PubMed] [Google Scholar]
- 41.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Inflamatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995. [DOI] [PubMed] [Google Scholar]