Abstract
Vaccination with leishmanial Ag and CpG oligodeoxynucleotides (ODN) confers sustained cellular immunity and protection to infectious challenge up to 6 mo after immunization. To define the cellular mechanism by which CpG ODN mediate their adjuvant effects in vivo, the functional capacity of distinct dendritic cell (DC) subsets was assessed in the lymph nodes (LNs) of BALB/c mice, 36 h after immunization with the leishmanial antigen (LACK) and CpG ODN. After this immunization, there was a striking decrease in the frequency of the CD11c+B220+ plasmacytoid DCs with a proportionate increase in CD11c+CD8−B220− cells. CD11c+CD8+B220− cells were the most potent producers of interleukin (IL)-12 p70 and interferon (IFN)-γ, while plasmacytoid DCs were the only subset capable of secreting IFN-α. In terms of antigen presenting capacity, plasmacytoid DCs were far less efficient compared with the other DC subsets. To certify that DCs were responsible for effective vaccination, we isolated CD11c+ and CD11c− cells 36 h after immunization and used such cells to elicit protective immunity after adoptive transfer in naive, Leishmania major susceptible BALB/c mice. CD11c+ cells but not 10-fold higher numbers of CD11c− cells from such immunized mice mediated protection. Therefore, the combination of LACK antigen and CpG ODN adjuvant leads to the presence of CD11c+ DCs in the draining LN that are capable of vaccinating naive mice in the absence of further antigen or adjuvant.
Keywords: plasmacytoid dendritic cell, Toll-like receptor, Leishmania major, vaccine, Th1 cells
Introduction
Currently there are no uniformly effective vaccines for infections such as Mycobacterium tuberculosis, malaria, and HIV. For such infections, the cellular immune response comprising primarily Th1 and CD8+ effector T cells has been shown to be critical in mediating protection against infection and/or development of disease (1). Potential difficulties in generating potent and durable cellular immune responses in vivo with current vaccines may be due to multiple factors. First, induction of Th1 cells requires a differentiation process that involves both Ag stimulation and a Th1-inducing cytokine (2, 3). Second, generation of CD8+ effector T cells requires intracellular processing of Ag, which is most readily elicited by either live-attenuated or DNA vaccines; however, live-attenuated vaccines for diseases such as HIV or M. tuberculosis might be precluded for safety and/or manufacturing considerations. In addition, DNA vaccines are far less potent for inducing immune responses in humans than in rodents. One recent approach that has shown promise for generating broad cellular immune responses in vivo is use of Toll-like receptor (TLR)* ligands as vaccine adjuvants (4).
At present, there are 10 TLR receptors that respond to different pathogen-associated molecular patterns (5). One of the most potent inducers of both innate and adaptive immunity is mediated through the interaction of cytosine phosphate guanosine oligodeoxynucleotide (CpG ODN) with TLR 9 (4, 6). At the initiation of an immune response, this interaction leads to increased expression of costimulatory markers such as CD40, CD80, and CD86 and production of proinflammatory cytokines such as TNF-α, IL-12, and IL-6 from DCs (7–9). In turn, this facilitates induction of both Th1 and CD8+ T cell responses (10–13). The physiologic relevance for the immune effects of CpG ODN in vivo was demonstrated in a mouse vaccine model of Leishmania major infection in which mice immunized with heat-killed or recombinant leishmanial Ag and CpG ODN had sustained cellular immune responses and were protected up to 6 mo after immunization (14). Similarly, in other studies, vaccination with plasmid DNA encoding various leishmanial Ag also conferred long-term protection in a Th1 and CD8+ T cell–dependent manner (15). Thus, while the aforementioned vaccine studies elucidated the immune correlates of protection, it remained to be proven how these different types of vaccine formulations were initiating such responses at the level of the APC. In this regard, a common feature of plasmid DNA and CpG ODN + protein vaccines is the ability to transfect (16) and activate (17) DCs, respectively.
CD11c+ DCs can direct the differentiation of CD4+ T cells into Th1- or Th2-type cells (18) as well as CD8+ T cell responses either directly or through cross-priming (19). The ability of DCs to induce such responses depends on the specific subset (20), the maturation state (21), and the conditions of stimulation (22). The great majority of prior studies analyzing the function of DCs have used either in vitro–generated or in vitro–cultured bone marrow or splenic DCs. In addition, characterization of ex vivo-derived DCs has been limited to nonpathogenic Ag. Thus, there has not been a systematic study using an entirely in vivo model in which subsets of DCs from peripheral LNs were characterized after immunization with a protein derived from a specific pathogen plus CpG ODN for their ability to present the Ag, secrete cytokines, and mediate protection to an infectious challenge after adoptive transfer into naive mice.
The aim of the studies presented here was to determine the cellular mechanism by which CpG ODN were mediating protective immunity in vivo when used as a vaccine adjuvant with a leishmanial Ag. For the leishmanial Ag, we used the Leishmania homologue of the receptor for activated C kinase (LACK) protein. LACK is a highly conserved, immunodominant Ag with a single I-Ad–restricted epitope that has been shown to regulate the natural course of infection in BALB/c mice and been useful in vaccine studies for determining the requirements for Th1 and CD8+ T cells in maintaining long-term memory responses sufficient to mediate protection (15, 23–25). We hypothesized that DCs from mice immunized with LACK + CpG ODN mediate T cell immunity and protection following transfer into in naive, Leishmania major–susceptible BALB/c mice. The results of this study provide in vivo evidence for the cellular mechanism by which CpG ODN, which previously has been shown to have demonstrable adjuvant activity, mediates protective immunity in vivo.
Materials and Methods
Mice.
Female BALB/c mice were purchased from the Division of Cancer Treatment (National Cancer Institute, National Institutes of Health). Mice were maintained in the Vaccine Research Center Animal Care Facility (Bethesda, MD) under pathogen-free conditions.
ODN.
For all in vivo immunizations, phosphorothioate-modified ODN sequence 1826 containing two CpG motifs (underlined: TCATGACGTTCCTGACGTT) was provided by Coley Pharmaceutical Group. To assess production of IFN-α under in vitro stimulation, ODN sequence 1585 containing one CpG motif (underlined: GGGGTCAACGTTGAGGGGGG) was used. The ODN contained endotoxin levels < 0.1 EU/mg using the limulus amebocyte lysis assay (Associates of Cape Cod, Inc.).
Immunization.
To generate CD11c+ DCs in vivo, naive BALB/c mice (20–25 mice/group) were injected s.c. in both hind footpads and flanks with sterile PBS, CpG ODN (25 μg), or recombinant leishmanial LACK protein (50 μg; Paragon Bioservices, Inc.) with or without CpG ODN. This protein contained < 5.0 EU/dose. Each injection was suspended in sterile PBS and given in a volume of 50 μl. For some experiments, mice were vaccinated s.c. in the footpad with 50 μg of LACK protein with either 25 μg CpG ODN or 1 μg IL-12 protein and boosted 2 wk later as described previously (26).
DC Isolation.
At various times after immunization, mice from each group were killed and their draining popliteal and inguinal LN passed through a 100-μm cell strainer and digested for 30 min at 37°C by 1 mg/ml collagenase D and 15 μg/ml DnaseI (Roche). All subsequent procedures were performed on ice in HBSS. The cells were blocked using 1 mg/ml purified rat IgG or 1:100 2.4G2 Ab (BD Biosciences). CD11c+ cells were positively selected using magnetized Ab for CD11c (N418; Miltenyi Biotec). After this step, there were ∼60% CD11c+ cells. CD11c+ DCs were further enriched by staining with FITC-labeled anti-CD3 and APC-labeled anti-CD19 and sorted for to remove such cells. After cell sorting, there were > 95% CD11c+CD3−CD19− cells. To isolate specific DC subsets, CD11c+ cells enriched following magnetic bead separation were stained with FITC-labeled anti-CD3 and CD19, PE-labeled anti-CD11c (HL3), PerCP-labeled anti-B220, or APC-labeled anti-CD8 (Ly-2) and sorted on a FACSVantage™ cell sorter. The resulting population was consistently >99% CD11c+, with fewer than 0.5% T cells. CD11c− cells were further depleted of CD3+ cells by cell sorting resulting in a population that was >90% CD19+ cells.
T Cell Proliferation Assay.
CD4+ T cell hybridoma LMR 7.5, detects LACK in the context of MHC class II expressing I-Ad (27). To detect the antigen presenting capacity of LACK from APC immediately ex vivo, 105 LMR 7.5 hybridoma cells were cultured with varying numbers of CD11c+ or CD11c− cells from mice immunized with PBS, LACK protein, CpG ODN, or LACK + CpG ODN or IL-12 protein. Supernatants were harvested 24 h later and assayed for IL-2 content by ELISA using OptEIA kits (BD Biosciences). As a positive control for the ability of APC to present LACK independent of processing of the LACK protein in vivo, 105 LMR 7.5 hybridoma cells were cultured with varying numbers of CD11c+ or CD11c− cells from naive or immunized mice with 10 μg/ml LACK peptide (amino acids 156–173; FSPSLEHPIVVSSW). The detection limit for IL-2 production was 3 pg/ml.
Adoptive Transfer of CD11c+ and CD11c−Cells.
Varying numbers of CD11c+ DC (104–5 × 104) or CD11c− cells (5 × 104–5 × 105) from mice immunized with PBS, CpG ODN, LACK ± CpG ODN, or IL-12 protein were sorted, resuspended in sterile HBSS (50 μl), and transferred s.c. into the footpad of naive BALB/c mice. Mice were boosted 2 wk later in an identical manner.
LACK-specific Production of IFN-γ from BALB/c Mice After Adoptive Transfer of CD11c+ Cells from Immunized Mice.
After adoptive transfer of CD11c+ DCs into naive BALB/c mice, LACK-specific production of IFN-γ was assessed from LN before infection or spleens after infection by culturing 2 × 105 total LN or spleen cells/200 μl in flat-bottom 96-well plates with or without LACK protein (10 μg/ml). Supernatants were harvested 48 or 72 h later and analyzed for IFN-γ content by ELISA using OptEIA kits. The detection limit for IFN-γ was 62.5 pg/ml.
Assessment of Cytokine Production from DCs Isolated After Immunization.
2 d after immunization, total CD11c+ or sorted subsets of DCs (104/ml) were isolated from draining LNs, added to 96-well tissue culture plates, and stimulated in media alone, Staphylococcus aureus Cowens (SAC; 1:1,000) and/or anti-CD40 Ab (1 μg/ml), CpG ODN (5 μg/ml), or CpG ODN + anti-CD40 Ab. Supernatants were collected at 24–48 h for IL-12 p40, p70, and IL-10 assays. IL-12 p40, p70, and IL-10 were detected using OptEIA kits. The limit of detection for IL-12 p40 was 125 pg/ml; for IL-10, 62.5 pg/ml; and for IL-12 p70, 7.8 pg/ml. For detection of IFN-α, cells were stimulated with CpG ODN and poly I:C (25 μg/ml). IFN-α was detected using kits purchased from R&D Systems. The limit of detection was 16 pg/ml.
Characterization of Cell Surface Markers on DCs.
After enrichment of CD11c+ DCs from mice immunized with LACK, CpG ODN, both, or neither, cells were stained with the following Ab: FITC-labeled anti-I-Ad (AMS32.1), anti-CD40 (3/23), anti-CD80 (16–10A1), anti-CD86 (GL1), APC-labeled CD11b (M1/70), or Ly-6G/C (RB6–8C5). The relative expression of these markers was assessed using a FACSCalibur™ flow cytometer (Becton Dickinson) and analyzed using Flow Jo software.
Infectious Challenge.
L. major clone V1 (MHOM/IL/80/Friedlin) promastigotes were grown as described previously (26). Briefly, infective-stage metacyclic promastigotes of L. major were isolated from stationary cultures (4–5 d old) by negative selection using peanut agglutinin (Vector Laboratories). BALB/c mice were infected 2 wk after boost with 105 metacyclic promastigotes. Parasites were injected into the footpad s.c. contralateral from the site in which they had received CD11c+ or CD11c− cells from immunized mice. Weekly footpad swelling measurements using a metric caliper were recorded.
Preparation of Bone Marrow–derived DCs for Detection of LACK-Specific CD4+ and CD8+ IFN-γ Responses.
To measure LACK-specific T cell immune responses after adoptive transfer, we used normal bone marrow–derived DCs from BALB/c mice (1.2 × 106) alone or pulsed with LACK protein anti-CD40, and CpG ODN. These were cocultured for 2 h with total splenocytes (6 × 106) from BALB/c mice that had received CD11c+ or CD11c− cells from mice immunized with LACK ± CpG ODN. BFA (10 μg/ml) was then added and 4 h later cells were fixed, permeabilized, and stained with FITC-labeled anti-CD3ɛ (145–2C11), PE-labeled anti-CD8α (Ly-2), PerCP-labeled anti-CD4 (L3T4), or APC-labeled anti-IFN-γ (XMG1.2). The frequency of CD4+ and CD8+ T cells producing IFN-γ was assessed after collecting 300,000 total events in each group.
Results
CD11c+ Cells From Mice Immunized With LACK ± CpG ODN Present Ag to LACK-Specific CD4+ T Cells.
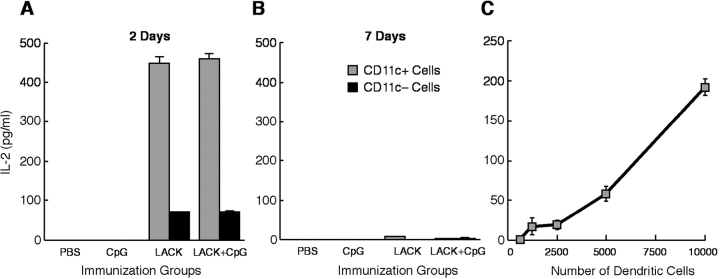
To determine the mechanism by which CpG ODN confers protection as a vaccine adjuvant with the leishmanial LACK protein, we first assessed the capacity of various populations of APCs to present LACK to a LACK-specific CD4+ T cell hybridoma (LMR 7.5) following immunization (27). Naive BALB/c mice were immunized s.c. with PBS, CpG ODN alone, or LACK ± CpG ODN. Draining LNs were then harvested at various times post immunization, and CD11c+ and CD11c− cells were isolated by cell sorting and added to LMR 7.5 cells to determine their capacity to present LACK Ag. As shown in Fig. 1 A, CD11c+ cells (104) isolated 2 d after immunization with LACK protein or LACK + CpG ODN induced substantial amounts of IL-2 from the LMR 7.5 cells. Moreover, as few as 103 CD11c+ cells from such immunized mice were sufficient to induce IL-2 from the LMR 7.5 cells (Fig. 1 C). In contrast, CD11c+ cells from mice immunized with PBS or CpG ODN alone did not induce any IL-2 production from LMR 7.5 cells. These data demonstrate that only CD11c+ cells from mice immunized with LACK ± CpG ODN have acquired the capacity in vivo to present LACK to the LMR 7.5 cells. When assessed 7 d after immunization, there was no detectable IL-2 produced by the LMR 7.5 cells from CD11c+ cells in any of the groups (Fig. 1 B). Consequently, for the remainder of the experiments shown below, CD11c+ cells were isolated from draining LN 36–48 h after immunization.
Figure 1.
LACK Ag-presenting capacity of CD11c+ and CD11c− cells derived in vivo after immunization. BALB/c mice (20 mice per group) were injected s.c. into four separate sites with PBS, CpG ODN, LACK, or LACK + CpG ODN. CD11c+ and CD11c−cells were isolated 2 d (A) or 7 d (B) later from draining LN. CD11c+ (104; A and B) or CD11c- (105; A and B) were cocultured with LMR 7.5 hybridoma cells (105) for 24 h. IL-2 content was assessed from the supernatants from triplicate wells. (C) In a separate experiment, varying numbers of CD11c+ cells from mice immunized with LACK + CpG ODN were added to LMR 7.5 cells and IL-2 content assessed. Results are expressed as geometric mean ± SEM and are representative of four individual experiments.
In comparing the efficiency of antigen presentation between CD11c+ DCs and CD11c− cells (>90% B cells), while the same number of CD11c− cells (104) from mice immunized with LACK ± CpG ODN did not induce any IL-2 from LMR 7.5 cells (unpublished data), a small amount of IL-2 was induced from LMR 7.5 cells using 105 CD11c− cells (Fig. 1 A).
CD11c+ Cells from Mice Immunized with LACK and CpG ODN Produce IL-12.
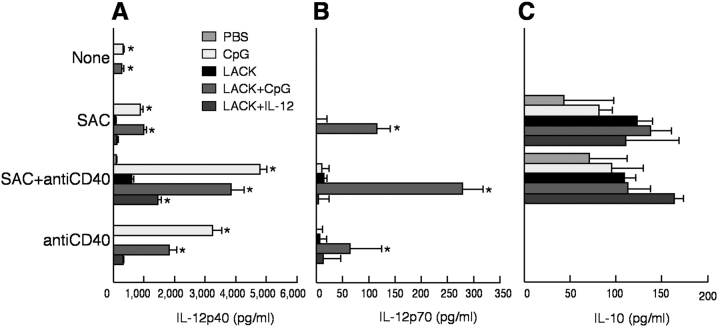
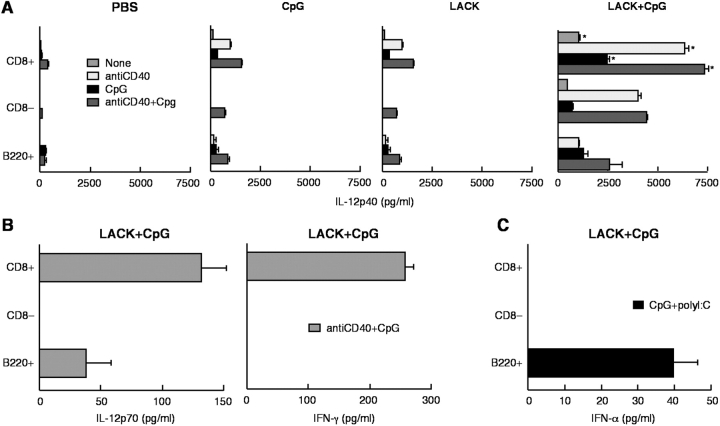
Cytokines released from CD11c+ cells have a strong influence on the CD4+ T cell response (2, 28). CD11c+ cells isolated 2 d after immunization with PBS, CpG ODN, or LACK ± CpG ODN were analyzed for production of IL-12 p40, p70, and IL-10 after restimulation in vitro. As shown in Fig. 2 A, CD11c+ cells from mice immunized with CpG ODN or LACK + CpG ODN had significant production of IL-12 p40 in response to SAC, anti-CD40, or both (P < 0.05). There was no detectable IL-12 p40 from CD11c− cells from any of the immunized groups (data not shown). IL-12 p70 was only detected from mice immunized with LACK + CpG ODN (Fig. 2 B; P < 0.05). Last, there were no significant differences in IL-10 production in response to SAC + anti-CD40 stimulation between any of the immunized groups (Fig. 2 C). Together with the previous figure, these data show that only mice immunized with both LACK protein and CpG ODN are able to present Ag and secrete IL-12 p70.
Figure 2.
IL-12 and IL-10 production from CD11c+ and CD11c− cells after immunization. CD11c+ (104) and CD11c− (104) cells from BALB/c mice immunized with PBS, CpG, or LACK + CpG ODN, or IL-12 were isolated from draining LN 2 d after immunization and cocultured with nothing, SAC, anti-CD40, or both for 24 h. IL-12 p40 (A), IL-12 p70 (B), and IL-10 (C) were measured by ELISA. Results are expressed as geometric mean ± SEM and are representative of four individual experiments. * indicates P < 0.05 for mice immunized with LACK ± CpG ODN as compared with PBS-immunized or LACK protein-immunized mice.
In this experiment, cytokine production from CD11c+ cells from mice immunized with LACK + IL-12 protein was also determined. This additional group was of interest because naive BALB/c mice vaccinated with this regimen are protected when mice are infected shortly after immunization (26, 29). Therefore, cells from such immunized mice can be used to compare whether the mechanism of protection vis a vis CD11c+ cells was similar using IL-12 protein as an adjuvant compared with CpG ODN. As shown in Fig. 2 A, production of IL-12 p40 from CD11c+ cells from LACK + IL-12–immunized mice was demonstrably less than from CD11c+ cells from LACK + CpG ODN-immunized mice.
The Frequency of DC Subsets Is Substantially Altered After Immunization.
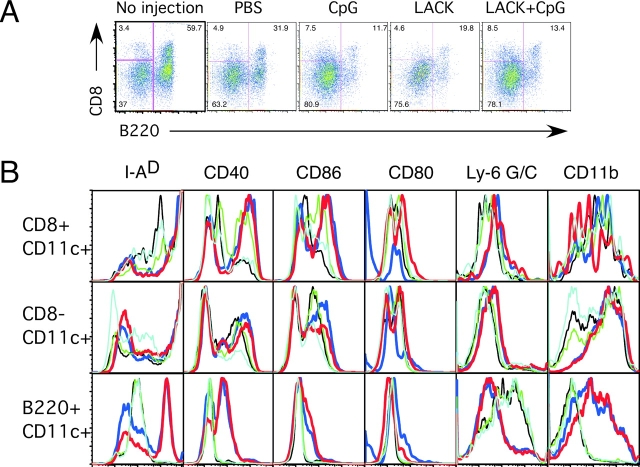
Previous studies have shown that at least five distinct subsets can be identified in LN of naive mice (22, 30). Thus, we determined the frequency and functional capacity of subsets of CD11c+ cells based on expression of CD8 and B220. These markers allowed for the historical classification of lymphoid (CD11c+CD8+ B220−), myeloid (CD11c+CD8−B220−), and plasmacytoid (CD11cintB220+) DCs. The frequency of CD11c+ cells expressing CD8 and B220 from LN of naive (no injection) and immunized mice from a representative experiment is shown in Fig. 3 A. Table I shows the percentage of these three populations of CD11c+ DCs from naive and immunized mice combined from four independent experiments. It was notable that the majority of CD11c+ cells in unimmunized naive BALB/c mice comprise B220+ (56.55% ± 4.5%) and CD8− (39.80% ± 3.9%) cells. Moreover, there is a significant decrease in the percentage of CD11c+B220+ cells after injection with PBS, LACK protein, or LACK protein ± CpG ODN (P < 0.05). This decrease is accompanied by a proportional increase in the percentage of CD11c+CD8− cells. In terms of total number of DC subsets following immunization, while mice immunized with CpG ODN ± LACK protein had a 2–3-fold increase in the number of total lymph node cells as well as CD11c+ cells at day 2 after immunization there was no appreciable change in the number of lymph node or CD11c+ cells after injection with PBS or LACK protein alone. In addition, the change in the frequency of CD11c+ DC subsets after sterile PBS immunization was a consistent finding and likely reflects inflammation association with the s.c. injection itself.
Figure 3.
Frequency of DC subsets and their expression of cell surface markers from LN after immunization. (A) CD11c+ DCs from draining LNs of naive (no injection) and or mice immunized with PBS, CpG ODN, or LACK protein ± CpG ODN mice were enriched as described in the methods and stained with antibodies against CD11c, CD8 and B220. Cells were gated on CD11c+ cells and the percentage of CD11c+CD8+B220−, CD11c+CD8−B220−, and CD11c+B220+ is shown. (B) Expression of cell surface markers was analyzed on subsets of CD11c+ DCs based on expression of CD8 and B220. Light blue, no injection; black, PBS; blue, CpG ODN; green, LACK; red, LACK + CpG ODN. Data are representative of three independent experiments.
Table I.
Frequency of DC Subsets After Immunization
| DC subset |
No injection |
PBS | CpG ODN |
LACK | LACK + CpG ODN |
|---|---|---|---|---|---|
| B220+ | 56.6 ± 4.5 | 36.6 ± 5.6 | 16.4 ± 6.6 | 26.5 ± 7.1 | 17.7 ± 9.0 |
| CD8+ | 3.7 ± 0.4 | 4.0 ± 1.8 | 4.8 ± 2.9 | 4.7 ± 2.1 | 6.7 ± 5.6 |
| CD8− | 39.8 ± 4.0 | 58.8 ± 5.0 | 78.8 ± 5.4 | 68.9 ± 8.7 | 75.7 ± 9.3 |
The frequency of CD11c+CD8+B220−, CD11c+CD8−B220−, and CD11c+B220+ cells from draining LN of naive and immunized mice was determined from four independent experiments. Results are expressed as geometric mean ± SEM: P < 0.05 when comparing any of the immunization groups to naive mice; P < 0.05 when comparing mice immunized with LACK protein, CpG ODN, or both with PBS-injected mice.
Differential Expression of Cell Surface Markers on Subsets of CD11c+ Cells After Immunization.
The expression of cell surface molecules on the DC subsets was determined from LN cells of naive or immunized mice. As shown in Fig. 3 B, both CD11c+CD8+ and CD11c+CD8− cells had increased expression of MHC class II, CD40, and CD86 compared with CD11c+B220+ cells from naive or mice immunized with CpG ODN ± LACK. In terms of other markers, Ly-6G/C, a marker used to define murine plasmacytoid DCs (31), was present on all CD11c+B220+ but not CD8+ or CD8− cells from naive mice or mice immunized with PBS or LACK protein. Of note was that expression of Ly-6G/C was decreased on CD11c+B220+ cells after immunization with CpG ODN ± LACK protein. In contrast, while expression of CD11b was low on CD11c+B220+ cells from naive mice or after PBS or LACK protein immunization, it was strongly enhanced after immunization with CpG ODN ± LACK protein.
Subsets of CD11c+ DC from Immunized Mice Have Distinct Ag-presenting Capability.
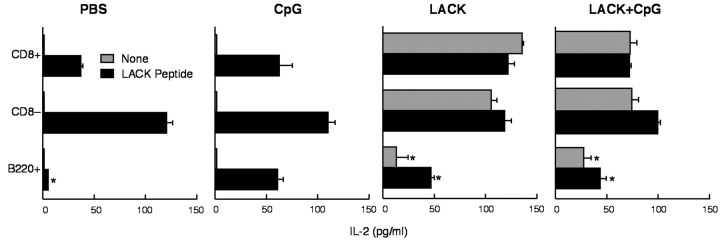
To assess whether differential expression of MHC class II and costimulatory molecules altered Ag-presenting capacity, sorted DC subsets were isolated after immunization in vivo and used to stimulate LMR 7.5 cells. As shown in Fig. 4 , all three subsets of CD11c+ cells were able to induce IL-2 secretion from LMR 7.5 cells if mice were immunized with LACK ± CpG ODN. There was a modest decrease in the production of IL-2 from LMR 7.5 cells from both CD8+ and CD8−B220− DCs in mice immunized with both LACK protein and CpG ODN compared with LACK protein alone. This was a consistent finding in other experiments and may reflect maturation of the DCs in vivo by the CpG ODN. In comparing LACK presentation among the other subsets of CD11c+ cells, CD11c+B220+ cells were far less efficient on a per-cell basis from cells derived ex vivo after immunization with LACK ± CpG ODN. Moreover, such cells were less potent at inducing IL-2 production even when LACK peptide was added in vitro compared with the other subsets of DCs (P < 0.05).
Figure 4.
LACK Ag-presenting capacity of CD11c+ DC subsets isolated directly from LNs after immunization. Sorted CD11c+CD8+, CD11c+CD8−B220−, and CD11c+CD8−B220+ (104 cells) were cultured with LMR 7.5 cells (105) for 24 h with or without LACK peptide (10 μg/ml) and IL-2 content assessed. Results are expressed as geometric mean ± SEM. * indicates P < 0.05 from CD11c+CD8+ and CD8− as compared with that from CD11c+B220+ DCs.
Subsets of CD11c+ Cells from Immunized Mice Have Distinct Cytokine-producing Capacity.
The production of cytokines such as IL-12 (20) and IFN-α (31–33) from DC subsets can be strongly influenced by the conditions of stimulation. As shown in Fig. 5 A, CD11c+CD8+ DCs were the most potent producers of IL-12 p40 from all immunization groups compared with the other DC subsets (P < 0.05). Immunization with both LACK protein and CpG ODN had the most dramatic effect on production of IL-12 p40 from all subsets of DCs and was especially notable for CD11c+CD8− DCs. IL-12 p70 was detected from CD11c+CD8+ and CD11c+B220+ cells but not CD11c+CD8− cells from mice immunized with LACK + CpG ODN (Fig. 5 B). These data clearly show that a TLR stimulus coupled with a signal mediated by protein immunization (i.e., CD40 ligand) is optimal for IL-12 p70 from CD11c+CD8+ (34) and plasmacytoid DCs (35, 36). Finally, as DC production of IFN-γ (37) and IFN-α (31–33) could influence Th1 and CD8+ T cell responses, the capacity of DCs to secrete such cytokines was assessed. CD11c+CD8+ cells from mice immunized with LACK + CpG ODN was induced to secrete IFN-γ, whereas production of IFN-α was only produced by CD11c+B220+ DC (Fig. 5 C).
Figure 5.
Production of IL-12, IFN-γ, and IFN-α from CD11c+ DC subsets after immunization. Sorted CD11c+CD8+B220−, CD11c+CD8−B220−, and CD11c+B220+ (5 × 103) cells were isolated from draining LN 2 d after immunization with PBS, CPG ODN, or LACK ± CpG ODN and cocultured with nothing, anti-CD40 (1 μg/ml), CpG ODN (5 μg/ml), or both. (A) IL-12 p40 was measured from all immunized groups. IL-12 p70 (B) and IFN-γ (B) were only detected from mice immunized with LACK + CpG ODN. (C) IFN-α was measured by ELISA from CD11c+B220+ cells in response to stimulation with CpG ODN 1585 and Poly I:C. Results are expressed as geometric mean ± SEM and are representative of three independent experiments.
CD11c+ Cells from Immunized Mice Induce LACK-specific Production of IFN-γ in Naive BALB/c Mice After Adoptive Transfer.
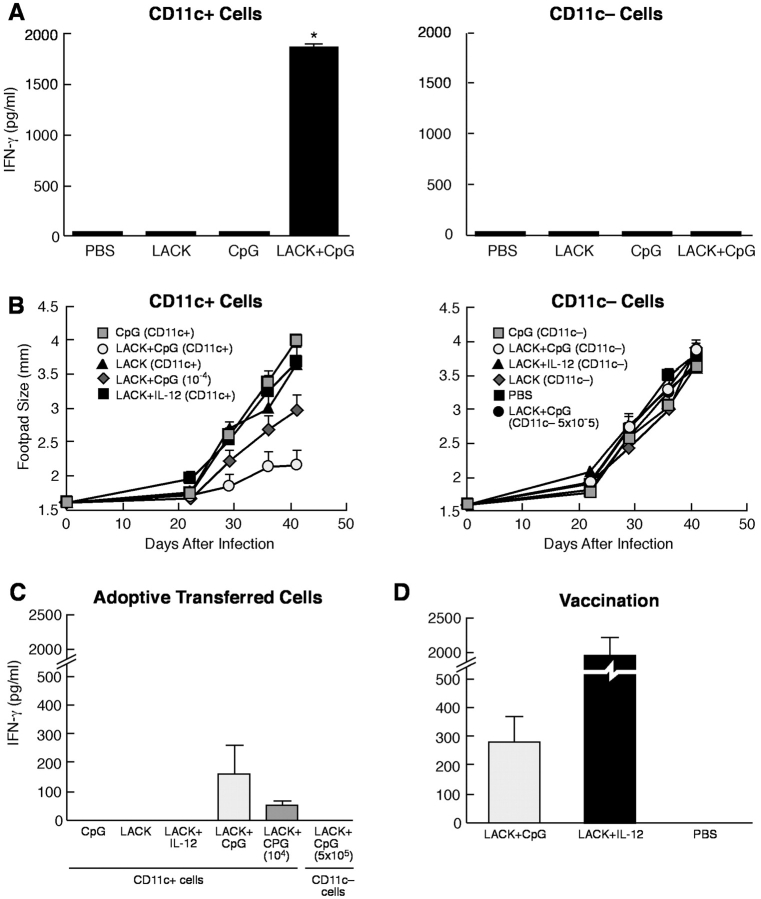
The remaining experiments focused on the ability of CD11c+ cells generated in vivo by immunization to elicit immunity and protection after adoptive transfer into naive BALB/c mice that are susceptible to L. major infection. CD11c+ cells and CD11c− cells were sorted from the various groups of immunized mice and injected into naive BALB/c mice. As shown in Fig. 6 A, only CD11c+ cells from mice immunized with LACK + CpG ODN induced production of IFN-γ from lymph node cell stimulated with LACK protein in vitro (P < 0.05). Thus, Th1 cells are induced in naive BALB/c mice after adoptive transfer of CD11c+ cells from LACK + CpG ODN immunized mice.
Figure 6.
Immunogenicity and protection induced by CD11c+ DCs from immunized mice. CD11c+ and CD11c− cells (105 cells) from immunized mice were transferred by s.c. injection into naive BALB/c mice (A). 4 d later, draining LN were removed, restimulated with LACK protein, and production of IFN-γ assessed from supernatants by ELISA. Results are expressed as geometric mean ± SEM and are representative of three independent experiments. * indicates P < 0.05 for cells of mice that received CD11c+ cells from LACK + CpG ODN-immunized mice compared with all other groups. (B) Varying numbers (5 × 104 cells for all groups except where noted) of CD11c+ or CD11c− cells from mice immunized with CpG ODN or LACK + CpG ODN or IL-12 protein were adoptively transferred s.c. into the footpad of naive BALB/c mice. Mice were boosted 2 wk later with the same regimen and then challenged with 105 L. major metacyclic promastigotes in the opposite footpad from which they received the cells from the immunized mice. Control of infection was assessed by measuring the diameter of the footpad lesions. Results are representative of three separate experiments. To assess immune responses post infection, splenocytes (2 × 105 cells/200 μl) from infected mice that received CD11c+ or CD11c− cells from immunized mice (C) or from naive BALB/c mice vaccinated with LACK + IL-12 or CpG ODN (D) were stimulated with LACK protein (10 μg/ml) for 48 h and production of IFN-γ assessed from supernatants. Results are the geometric mean of three replicate wells. Error bars represent the SEM. * indicates P < 0.05 for cells of mice that received CD11c+ cells from LACK + CpG ODN-immunized mice compared with all other groups.
CD11c+ Cells from Immunized Mice Confer Protection to Naive BALB/c Mice After Challenge with L. major.
The susceptibility of BALB/c mice to L. major infection is mediated by a Th2 response to the immunodominant LACK Ag (23–25). The data that CD11c+ cells from mice immunized with LACK + CpG ODN induced Th1 cells following transfer into naive BALB/c mice suggested that such cells might be sufficient to mediate protection to challenge with L. major. CD11c+ and CD11c− cells from mice immunized with LACK, CpG ODN, or LACK + CpG ODN were adoptively transferred twice at 2 wk intervals in one footpad and then challenged 2 wk later with L. major in the opposite footpad. As an assessment of disease progression, the footpad size was followed over a period of 6 wk, at which point the experiment was terminated due to ulceration and necrosis of the footpads in all groups that were not able to control infection. As shown in Fig. 6 B, naive BALB/c mice that received 5 × 104 CD11c+ cells from mice immunized with LACK + CpG ODN were able to control infection. Moreover, even 104 of such transferred cells also conferred some control of infection. In contrast, CD11c+ cells from the other immunized mice (CpG ODN or LACK alone) or CD11c− cells from all groups of immunized mice had no ability to control infection (Fig. 6 C). In addition, even 5 × 105 CD11c− cells from mice immunized with LACK + CpG ODN did not exert any control of infection. Finally, it is well established that the degree of footpad swelling is a good correlate for the parasite load in BALB/c mice after infection. In this regard, in other related experiments, we did observe a 3–10-fold decrease in parasite load in mice that received CD11c+ cells compared with CD11c− cells from leishmanial protein + CpG ODN immunized mice (unpublished data). These data clearly show that the mechanism by which CpG ODN mediates protection as an immune adjuvant in this model is through CD11c+ cells.
In Fig. 2, we showed that CD11c+ cells from mice immunized with LACK and the adjuvant IL-12 (LACK + IL-12) had relatively modest production of IL-12 after restimulation in vitro. Moreover, CD11c+ cells from mice immunized with LACK + IL-12 did not confer any protection following adoptive transfer into naive BALB/c mice (Fig. 6 B). As a positive control in the same experiment, naive BALB/c mice vaccinated with LACK + IL-12 were able to control infection following challenge (data not depicted). Thus, the mechanism that leads to development of protective immune responses appears to be different by LACK + IL-12 versus CpG ODN vaccination.
As an immune correlate of protection after infection, LACK-specific production of IFN-γ was determined 6 wk after infection in naive BALB/c mice that received CD11c+ or CD11c− cells from mice immunized with CpG ODN, LACK protein, or LACK + CpG ODN. As shown in Fig. 6 C, IFN-γ was only detected from mice that received CD11c+ cells from LACK + CpG ODN-immunized mice. Moreover, the amount of IFN-γ induced from these mice also correlated with the number of transferred cells. Production of IFN-γ was also assessed from naive BALB/c mice vaccinated with LACK + IL-12 or CpG ODN after infection (Fig. 6 D). The amount of IFN-γ from mice vaccinated with LACK + CpG ODN was comparable to that from mice receiving CD11c+ cells from mice immunized with the combination (Fig. 6 D) while mice vaccinated with LACK + IL-12 had substantial production of IFN-γ, consistent with the potent short-term induction induced by this vaccine.
CD11c+ Cells from Immunized Mice Induce Th1 and CD8+ T Cell Responses After Adoptive Transfer and Infection with L. major.
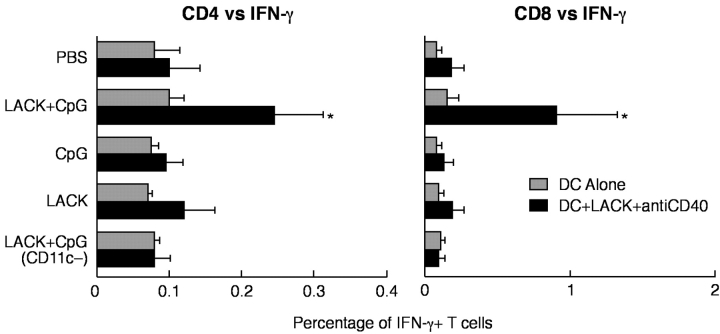
Vaccine elicited protection by leishmanial antigen plus CpG ODN against L. major requires both Th1 and CD8+ T cells (14). As shown above, CD11c+ cells from LACK+ CpG ODN immunized mice induced Th1 responses after transfer into naive BALB/c mice. Thus, we determined whether CD11c+ cells generated in vivo from LACK + CpG ODN-immunized mice could also induce LACK-specific CD8+ T cells after adoptive transfer into naive mice. To assess this, spleen cells of mice that had received CD11c+ or CD11c− cells from the indicated groups of immunized mice were harvested 3 wk after infection with L. major and stimulated in vitro with fresh bone marrow DCs pulsed with LACK protein and matured with anti-CD40 and CpG ODN. As shown in Fig. 7 , both CD4 and CD8+ IFN-γ–producing cells were only detected from mice which received CD11c+ cells from mice immunized with LACK + CPG ODN.
Figure 7.
Frequency of LACK-specific CD4+ and CD8+ IFN-γ–producing cells from BALB/c mice after transfer of CD11c+ DCs and infection. Pooled spleen cells from BALB/c mice (n = 3) that received two immunizations with sorted CD11c+ DCs from BALB/c mice immunized with LACK ± CpG ODN were harvested 3 wk after infection with L. major. Spleen cells were cultured (6 × 106) with bone marrow–derived DCs from BALB/c mice (1.2 × 106) with or without exposure to LACK protein, anti-CD40, and CpG ODN stimulation. Cells were processed and stained as described to enumerate the frequency of LACK-specific CD4+ and CD8+ T cell IFN-γ responses. Numbers represent the percentage of CD4+ or CD8+ T cells positive for IFN-γ. * indicates P < 0.05 for cells of mice that received CD11c+ cells from LACK + CpG ODN-immunized mice compared with all other groups.
Discussion
CpG ODN has potent effects in linking innate and adaptive immune responses in vivo when used as a vaccine adjuvant. In this report, to address the mechanism by which CpG ODN were responsible for vaccine protection in vivo, we performed an extensive functional analysis of distinct DC subsets from draining LN of mice immunized with LACK + CpG ODN. We show that DC from mice immunized with LACK + CpG ODN were capable of presenting antigen, secreting IL-12 p70, IFN-γ, and IFN-α and after adoptive transfer were sufficient to mediate protective immunity in naive BALB/c mice after challenge with L. major.
In characterizing the number and frequency of DC after immunization, we observed a decrease in the relatively high frequency of CD11c+B220+ cells in BALB/c (38) mice in response to injection of PBS itself, LACK protein, and CpG ODN. The change in the frequencies of DC subsets was observed in mice in which the total number of CD11c+ cells was not altered by immunization with PBS or LACK or was increased ∼2–3-fold after immunization with CpG ODN. There are several possible mechanisms to account for these changes in the respective numbers of DC subsets after immunization. First, the inflammatory responses induced by immunization could lead to the selective death and/or migration of plasmacytoid DCs in vivo compared with the other subsets. While CpG ODN can help maintain survival of plasmacytoid DCs in vitro (30, 38, 39), it is not clear whether CpG ODN have similar effects in vivo at least over the short-time period in which we evaluated this. Another possibility is that the s.c. immunization causes migration from the skin into the LN of Langerhans cells that have the effect of enriching the number of CD11c+CD8−B220− cells. A final mechanism involves alterations in lineage differentiation and commitment and whether plasmacytoid DCs develop a phenotype consistent with a different subset of DCs after immunization. At present there is no evidence from our data or previous studies to support such flexibility of plasmacytoid DCs in developing into other types of DCs (39–41). Whether one or a combination of these mechanisms explains the change in the frequency of DC subsets after immunization with CpG ODN remains an open question.
In assessing the antigen presenting capacity of DCs after immunization, we found that CD11c+ cells capable of presenting LACK to the LACK-specific LMR 7.5 hybridoma could be detected from draining LN as early as 18 h after immunization, which peaked at 36–48 h and was not present at 7 d. The kinetics of the response are consistent with previous reports showing intimate contact of T cells and DCs occurring within hours after antigenic challenge (42) that begin to dissociate after 36–48 h (43). These data suggest that CD11c+ cells capable of presenting LACK died, migrated, and/or lost expression of LACK/MHC complexes on their cell surface from LN 2 d after immunization. In terms of the relative potency of Ag presentation, both CD11c+CD8+ and CD8− cells were substantially more efficient than CD11c+B220+ DCs in presenting Ag immediately ex vivo. Thus, due to the relatively high frequency of CD11c+CD8− cells in the LN; they would likely have the most prominent role in initiating T cell immune responses (44). These data are consistent with the finding that only CD11c+CD11b+CD8− DCs are capable of presenting LACK Ag after infection with L. major (44a). In contrast, the relative deficiency of B220+ cells for Ag presentation compared with the CD8+ and CD8− might involve a qualitative difference in Ag processing or presentation resulting in diminished ability to assemble peptide–MHC complexes (45). Moreover, CD11c+B220+ DCs have a demonstrable decrease in expression of MHC class II and other costimulatory markers (31, 38, 45). Finally, the high sensitivity of the LMR 7.5 hybridoma for detecting LACK Ag might have overestimated even the relatively limited Ag-presenting capacity of CD11c+B220+ cells. In this regard, recent reports showing that freshly isolated naive or virally exposed splenic plasmacytoid DCs were unable to induce proliferation of naive CD4+ T cells (45, 46) but could promote expansion of previously activated T cells (45) might be analogous to our studies showing that plasmacytoid DCs induce low but detectable IL-2 production from a T cell hybridoma. While these data do not absolutely preclude a role of LN plasmacytoid DCs in Ag presentation with protein and CpG ODN immunization in vivo, they suggest that the relatively high frequency of such cells in normal LN of BALB/c mice might be more involved in maintaining tolerance in their steady-state and upon activation provide stimulation through their production of cytokines.
In addition to Ag presentation, DCs strongly influences the qualitative and quantitative aspects of cellular immune responses. CD11c+CD8+B220− DCs were the most potent producers of IL-12 p40 and p70 (20) and the only DC subset able to secrete IFN-γ after immunization with LACK + CpG ODN. Plasmacytoid DCs also produced IL-12 p70 and IFN-α after immunization. Thus, due to the relatively low frequency of CD11c+ CD8+B220− DCs in LN of naive or immunized mice and the limited capacity of plasmacytoid DCs for Ag presentation, these particular subsets of DCs through production of IL-12 p70, IFN-α, and IFN-γ likely mediate their effects by providing a favorable milieu to activate Th1 (28, 47, 48) and CD8+ T cell (49) responses. A final point is whether the effects of CpG ODN on DC subsets are direct and/or indirect. In mice, it has recently been shown that CD8α+, CD8α− and plasmacytoid DCs derived from spleens had comparable mRNA expression for TLR-9 and all could be induced to secrete IL-12 p40 after stimulation with CpG ODN (46, 50). These data are consistent with our findings that CpG ODN directly activates all the DC subsets studied here with induction of inflammatory cytokines and up-regulation of costimulatory molecules. In addition, CpG ODN likely have substantial indirect effects for generating adaptive cellular immune responses. Such indirect effects could be through IL-12 and IFN-α inducing NK production of IFN-γ which would enhance Th1 responses. In addition, IFN-α induced from plasmacytoid DC can also enhance the maturation of other DC subsets (51). Thus, the effect of CpG ODN on DC subsets and T cell responses involves both direct and indirect mechanisms. In summary we speculate that there is a cooperative effect with potential specialization of the various DC subsets in vivo for inducing a functional cellular immune response. For the model studied here, CD11c+CD8− would be primarily involved in antigen presentation, while CD11c+CD8+B220− and plasmacytoid DCs provide a favorable cytokine milieu for enhancing adaptive immune responses.
The demonstration that in vivo-derived LN CD11c+ DCs after immunization with LACK + CpG ODN are sufficient to mediate immunity and protection against an intracellular infection provides strong evidence for how CpG ODN mediate their effects as an adjuvant in vivo. With regard to how the transferred CD11c+ DCs mediates their effects in vivo, there are two potential mechanisms. First is the importance of IL-12 from the transferred CD11c+ cells from immunized mice in priming Th1 responses in the recipient mice. It has been shown recently that wild-type but not IL-12–deficient Langerhans cells pulsed in vitro with a variety of leishmanial Ag including LACK could confer protection (52). Thus, it is likely that IL-12 would be required from the transferred CD11c+ cells. The second consideration is whether the induction of Th1 and/or CD8+ T cell responses after adoptive transfer of cells from mice immunized with LACK protein + CpG ODN into naive mice is direct and/or indirect via cross-priming from recipient cells.
To conclude, these studies focused exclusively on peripheral LN DCs. While there is heterogeneity among DC populations and function between LN, spleen, and bone marrow–derived DCs, we believe these results examining how peripheral LN DCs are altered by s.c. immunization has direct relevance for vaccine design in humans. In this regard, despite the broad distribution of TLR-9 expression amongst mouse DC subsets (50), its expression in primates and humans is limited to B cells and plasmacytoid DCs (53). Thus for humans, the antigen presenting capacity and production of cytokines of plasmacytoid DCs in vivo will largely determine whether CpG ODN alone are effective vaccine adjuvants for inducing adaptive cellular immune response in people. If indeed such cells are not optimal for antigen presentation as in the mouse, combination strategies aimed at targeting additional DC populations using other TLR ligands or combinations of TLR ligands might be necessary for efficient generation of cellular responses in humans. Nevertheless, this report showing that freshly isolated CD11c+ but not CD11c− cells from mice immunized using CpG ODN as an adjuvant are capable of transferring protective immunity should provide a more targeted and rational approach to vaccine development for diseases requiring cellular immune responses.
Acknowledgments
We thank Brenda Rae Marshall for editorial assistance.
J.A. Shah is an HHMI-NIH Research Scholar.
Footnotes
Abbreviations used in this paper: CpG ODN, cytosine phosphate guanosine oligodeoxynucleotide(s); LACK, Leishmania homolog of the receptor for activated C kinase; SAC, Staphylococcus aureus Cowens; TLR, Toll-like receptor.
References
- 1.Seder, R.A., and A.V. Hill. 2000. Vaccines against intracellular infections requiring cellular immunity. Nature. 406:793–798. [DOI] [PubMed] [Google Scholar]
- 2.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283. [DOI] [PubMed] [Google Scholar]
- 3.Murphy, K.M., W. Ouyang, J.D. Farrar, J. Yang, S. Ranganath, H. Asnagli, M. Afkarian, and T.L. Murphy. 2000. Signaling and transcription in T helper development. Annu. Rev. Immunol. 18:451–494. [DOI] [PubMed] [Google Scholar]
- 4.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 5.Barton, G.M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380–383. [DOI] [PubMed] [Google Scholar]
- 6.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 7.Klinman, D.M., A.K. Yi, S.L. Beaucage, J. Conover, and A.M. Krieg. 1996. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc. Natl. Acad. Sci. USA. 93:2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparwasser, T., R.M. Vabulas, B. Villmow, G.B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 30:3591–3597. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann, G., G.J. Weiner, and A.M. Krieg. 1999. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA. 96:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu, R.S., O.S. Targoni, A.M. Krieg, P.V. Lehmann, and C.V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman, M., E. Martin-Orozco, J.S. Goodman, M.D. Nguyen, Y. Sato, A. Ronaghy, R.S. Kornbluth, D.D. Richman, D.A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849–854. [DOI] [PubMed] [Google Scholar]
- 12.Cho, H.J., K. Takabayashi, P.M. Cheng, M.D. Nguyen, M. Corr, S. Tuck, and E. Raz. 2000. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat. Biotechnol. 18:509–514. [DOI] [PubMed] [Google Scholar]
- 13.Davila, E., and E. Celis. 2000. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J. Immunol. 165:539–547. [DOI] [PubMed] [Google Scholar]
- 14.Rhee, E.G., S. Mendez, J.A. Shah, C.Y. Wu, J.R. Kirman, T.N. Turon, D.F. Davey, H. Davis, D.M. Klinman, R.N. Coler, et al. 2002. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurunathan, S., D.L. Sacks, D.R. Brown, S.L. Reiner, H. Charest, N. Glaichenhaus, and R.A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casares, S., K. Inaba, T.D. Brumeanu, R.M. Steinman, and C.A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparwasser, T., E.S. Koch, R.M. Vabulas, K. Heeg, G.B. Lipford, J.W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045–2054. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado-Lopez, R., and M. Moser. 2001. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin. Immunol. 13:275–282. [DOI] [PubMed] [Google Scholar]
- 19.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. [DOI] [PubMed] [Google Scholar]
- 22.Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. [DOI] [PubMed] [Google Scholar]
- 23.Mougneau, E., F. Altare, A.E. Wakil, S. Zheng, T. Coppola, Z.E. Wang, R. Waldmann, R.M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science. 268:563–566. [DOI] [PubMed] [Google Scholar]
- 24.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 274:421–423. [DOI] [PubMed] [Google Scholar]
- 25.Launois, P., I. Maillard, S. Pingel, K.G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R.M. Locksley, H.R. MacDonald, and J.A. Louis. 1997. IL-4 rapidly produced by Vα4 Vβ8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 6:541–549. [DOI] [PubMed] [Google Scholar]
- 26.Stobie, L., S. Gurunathan, C. Prussin, D.L. Sacks, N. Glaichenhaus, C.Y. Wu, and R.A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA. 97:8427–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malherbe, L., C. Filippi, V. Julia, G. Foucras, M. Moro, H. Appel, K. Wucherpfennig, J.C. Guery, and N. Glaichenhaus. 2000. Selective activation and expansion of high-affinity CD4+ T cells in resistant mice upon infection with Leishmania major. Immunity. 13:771–782. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251–276. [DOI] [PubMed] [Google Scholar]
- 29.Afonso, L.C., T.M. Scharton, L.Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 263:235–237. [DOI] [PubMed] [Google Scholar]
- 30.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741-748.30. [DOI] [PubMed] [Google Scholar]
- 31.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 32.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 33.Rissoan, M.C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. Malefyt, and Y.J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 34.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 35.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, R. Bals, T. Giese, H. Engelmann, S. Endres, A.M. Krieg, and G. Hartmann. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3036–3037. [DOI] [PubMed] [Google Scholar]
- 36.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 305-310. [DOI] [PubMed]
- 37.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon γ production by CD8α1 lymphoid dendritic cells. J. Exp. Med. 189:1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Keeffe, M., H. Hochrein, D. Vremec, I. Caminschi, J.L. Miller, E.M. Anders, L. Wu, M.H. Lahoud, S. Henri, B. Scott, et al. 2002. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 196:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Hoyo, G.M., P. Martin, H.H. Vargas, S. Ruiz, C.F. Arias, and C. Ardavin. 2002. Characterization of a common precursor population for dendritic cells. Nature. 415:1043–1047. [DOI] [PubMed] [Google Scholar]
- 41.Martinez del Hoyo, G., P. Martin, C.F. Arias, A.R. Marin, and D. Ardavin. 2002. CD8α+ dendritic cells originate from the CD8α− dendritic cell subset by a maturation process involving CD8α, DEC-205, and CD24 up-regulation. Blood. 99:999–1004. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins, M.K., A. Khoruts, E. Ingulli, D.L. Mueller, S.J. McSorley, R.L. Reinhardt, A. Itano, A., and K.A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23-45. [DOI] [PubMed] [Google Scholar]
- 43.Stoll, S., J. Delon, T.M. Brotz, and R.N. Germain. 2002. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 296:1873–1876. [DOI] [PubMed] [Google Scholar]
- 44.Smith, A.L., and B. Fazekas de St. Groth. 1999. Antigen-pulsed CD8a+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J. Exp. Med. 189:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.44a. Filippi, C., S. Hugues, J. Cazareth, V. Julia, N. Glaichenhaus, and S. Ugolini. 2003. CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex–independent differences within dendritic cells. J. Exp. Med. 198:201–209. [DOI] [PMC free article] [PubMed]
- 45.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E.R. Unanue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naïve T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y.J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parronchi, P., S. Mohapatra, S. Sampognaro, L. Giannarini, U. Wahn, P. Chong, S. Mohapatra, E. Maggi, H. Renz, and S. Romagnani. Effects of interferon-α on cytokine profile, T cell receptor repertoire and peptide reactivity of human allergen-specific T cells. Eur. J. Immunol. 26:697-703. [DOI] [PubMed]
- 48.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D.H. Presky, U. Gubler, and F. Sinigaglia. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185:825-831. [DOI] [PMC free article] [PubMed]
- 49.Sprent, J., and C.D. Surh. 2002. T cell memory. Annu. Rev. Immunol. 20:551–579. [DOI] [PubMed] [Google Scholar]
- 50.Edwards, A.D., S.S. Diebold, E.M.C. Slack, H. Tomizawa, H. Hemmi, T. Kaisho, S. Akira, and C. Reis e Sousa. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33:827–833. [DOI] [PubMed] [Google Scholar]
- 51.Dalod, M. T. Hamilton, R. Salomon, T.P. Salazaar-Mather, S.C. Henry, J.D. Hamilton, and C.A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J. Exp. Med. 197:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berberich, C., J.R. Ramirez-Pineda, C. Hambrecht, G. Alber, Y.A. Skeiky, and H. Moll. 2003. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J. Immunol. 170:3171–3179. [DOI] [PubMed] [Google Scholar]
- 53.Hornung, V.S., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, S., and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]