Abstract
Protective immunity against pathogens such as Leishmania major is mediated by interleukin (IL)-12–dependent Th1-immunity. We have shown previously that skin-dendritic cells (DCs) from both resistant C57BL/6 and susceptible BALB/c mice release IL-12 when infected with L. major, and infected BALB/c DCs effectively vaccinate against leishmaniasis. To determine if cytokines other than IL-12 might influence disease outcome, we surveyed DCs from both strains for production of a variety of cytokines. Skin-DCs produced significantly less IL-1α in response to lipopolysaccharide/interferon γ or L. major when expanded from BALB/c as compared with C57BL/6 mice. In addition, IL-1α mRNA accumulation in lymph nodes of L. major–infected BALB/c mice was ∼3-fold lower than that in C57BL/6 mice. Local injections of IL-1α during the first 3 d after infection led to dramatic, persistent reductions in lesion sizes. In L. major–infected BALB/c mice, IL-1α administration resulted in increased Th1- and strikingly decreased Th2-cytokine production. IL-1α and IL-12 treatments were similarly effective, and IL-1α efficacy was strictly IL-12 dependent. These data indicate that transient local administration of IL-1α acts in conjunction with IL-12 to influence Th-development in cutaneous leishmaniasis and prevents disease progression in susceptible BALB/c mice, perhaps by enhancing DC-induced Th1-education. Differential production of IL-1 by C57BL/6 and BALB/c mice may provide a partial explanation for the disparate outcomes of infection in these mouse strains.
Keywords: dendritic cell, IL-1, Leishmania major, infection, T helper cell type 1/T helper cell type 2 immune response
Introduction
Leishmania infections represent a significant health problem worldwide. Current therapeutic and vaccination strategies are inadequate. Control of disease and long lasting immunity in humans and mice is associated with Th1-predominant immunity, whereas development of Th2-responses leads to progressive disease (for a review, see reference 1). Recent data suggests that IFN-γ release by CD8+ Leishmania major–specific T cells (Tc1) may also promote the development of protective immunity (2, 3).
In cutaneous leishmaniasis, inoculated promastigotes are rapidly taken up by resident dermal MΦ. Several laboratories, including our own, have suggested that epidermal Langerhans cells (LCs)* or dermal DCs rather than MΦ, are responsible for the initiation of anti-parasite immunity in cutaneous leishmaniasis (4–6). We have suggested that LCs are ultimately recruited to L. major inoculation sites where they ingest parasites (presumably released from lysed MΦ, fibroblasts, or apoptotic neutrophils). This leads to LC/DC activation (5) and translocation of LC/DC from skin to draining LN eventuating in T cell priming (7).
Development of protective Th1-responses requires IL-12 during priming of naive T cells. Although MΦ comprise the major parasite reservoir in vivo, they fail to produce IL-12 after infection with Leishmania (8). In contrast, L. major–infected skin DCs do release IL-12 (5). Thus, DCs may be an important early source of IL-12 and they are likely to be involved in the initiation of protective immunity. Interestingly, infection of both C57BL/6 and BALB/c LC-like DCs with L. major stimulated IL-12 production and injection of infected BALB/c LCs or DCs loaded with antigen into naive susceptible animals induced Th1-immunity that prevented disease (4, 9).
In the present study, we surveyed DCs from both Leishmania-susceptible and -resistant mice for production of other cytokines that might influence Th1-differentiation and the outcome of infection. We found that skin-DCs from BALB/c mice produced several-fold less IL-1 in response to various stimuli than their C57BL/6 counterparts. We also determined that treatment of infected mice with IL-1 during T cell priming enhanced Th1-development and attenuated lesion development and disease progression in both susceptible BALB/c and resistant C57BL/6 mice. We propose that differences in the production of IL-1 by skin-derived DCs in BALB/c and C57BL/6 mice may influence the outcome of infection in these mouse strains.
Materials and Methods
Animals.
6 to 8-wk-old BALB/c and C57BL/6 mice were purchased from the NCI Animal Production Program. BALB/c mice with targeted mutations of the IL-12 p40 gene (10) were kindly provided by Dr. E. Schmitt (Institute of Immunology, University of Mainz, Germany). All animals were housed and used in experiments in accordance with institutional guidelines.
Propagation of Cells and Parasites.
Immature LC-like fetal skin–derived DCs (FSDDCs) were generated as described previously (5, 11). In brief, fetal skin cells from day 16 mice were cultured in GM-CSF– and M-CSF–supplemented media and, after ∼2 wk, DC aggregates were isolated. Inflammatory skin-derived MΦ (skin-MΦ) were elicited by subcutaneous injection of polyacrylamide beads and enriched to homogeneity as described (5, 12–14). Bone marrow–derived DCs (BMDCs) were generated in GM-CSF and IL-4–containing media (15) and harvested as immature DCs on day 6. Bone marrow–derived MΦ and thioglycollate-elicited peritoneal MΦ (P-MΦ) were generated for comparison (16).
Metacyclic promastigotes or amastigotes of L. major clone VI (MHOM/IL/80/Friedlin) were prepared as described previously (5, 13). Isolated parasites were opsonized with 5% BALB/c normal mouse serum and washed before in vitro infections.
Cytokine Analyses.
DC and MΦ populations were subcultured in their basal media (RPMI 1640/5% FBS with 10 ng/ml GM-CSF and M-CSF [FSDDC], RPMI/5% FCS with 10 ng/ml GM-CSF and IL-4 [BMDC] and DMEM/10% FBS [MΦ], respectively) in 24-well plates at 2 × 105 cells/ml/well. L. major amastigotes (3 organisms/cell) or E. coli LPS (100 ng/ml; kindly provided by Dr. Stephanie Vogel, Uniformed Services University of the Health Sciences, Bethesda, MD), and IFN-γ (1,000 U/ml; Genzyme) were added as indicated.
Total RNA was isolated from FSDDCs using RNAzol B (Tel-Test) after incubation for 18 h in the presence or absence of agonists. Cytokine mRNA was quantitated using a multiprobe RNase protection assay system (RiboQuant; BD Biosciences) and template set mCK2. 3 μg of total RNA and 3 × 105 cpm of the radiolabeled probe were used per lane. Spontaneous and agonist-induced cytokine production was measured in DC and MΦ supernatants using ELISA kits specific for murine IL-1α and IL-1β (Endogen). Cell-associated IL-1 was determined as described previously (11).
Production of IL-1α mRNA in vivo was quantitated by semi-quantitative RT-PCR. RNA was extracted from draining lymph nodes using the RNeasy Kit (QIAGEN) and reverse transcribed using Stratascript reverse transcriptase (Stratagene). Primers for murine GAPDH and IL-1α were designed using Primer Express software (Applied Biosystems). Sequences were as follows: GAPDH 5′ CCA TCA ACG ACC CCT TCA TTG ACC, 3′ TGG TTC ACA CCC ATC ACA AAC ATG; IL-1α 5′ CAC CTT ACA CCT ACC AGA GTG ATT TG, 3′ TGT TGC AGG TCA TTT AAC CAA GTG. PCR amplification was performed with ∼150 pg cDNA in 20 μl 1× reaction buffer with 0.5 U Taq polymerase (Applied Biosystems), 200 μM dNTP, 0.4 μM of each primer (5 min 94°C, followed by 20 (GAPDH) or 32 cycles (IL-1α) of 94°C 15 s, 64°C 25 s, 72°C 20 s, and 7 min at 72°C). Control reactions were stopped at lower and higher cycle numbers to document amplification in the linear range of the PCR. PCR products were resolved by 3% agarose gel electrophoresis and quantitated using a LumiQuant densitometer (Boehringer). Strain differences in IL-1α expression in vivo were confirmed by real time RT-PCR. PCR amplifications were performed in duplicates with 5 μl of dilutions (1:40 to 1:640) of the RT-reaction mix using the QuantiTectTM SYBR® Green PCR Kit (QIAGEN). PCR reactions were performed with an ABI Prism®7900 HT Cycler (Applied Biosystems). PCR-results were analyzed using SDS 2.0 software (Applied Biosystems). Relative abundances of IL-1α transcripts were normalized to GAPDH.
Infections.
Groups of five mice were infected with high dose (2 × 105) or low dose (103) inocula of infectious stage metacyclic promastigotes (17) by injection of ∼10 μl of PBS containing parasites into the dorsal dermis of the left ears. For treatment purposes, murine IL-1α, IL-1β, IL-6, IL-12 p70, or IL-18 (all from R&D Systems) were diluted in serum-free PBS and injected intradermally as indicated in doses between 0.1 and 100 ng/10 μl. Lesion volumes were measured weekly in 3 dimensions using a caliper, and are reported (in mm3) as ellipsoids [(a/2× b/2× c/2) × 4/3 × π]. Organisms present in lesional tissue were enumerated at the indicated times (≥3 animals/group) using a limiting dilution assay in Schneider's Drosophila media (BioWhittaker), 2% human urine, 10% FCS, 2% glutamine, 1% penicillin/streptomycin, and 0.5% HEPES as described (17).
For measurement of in vitro cytokine production, retroauricular LNs were recovered and single-cell suspensions were prepared. In some experiments, CD4+ and CD8+ T cells were enriched from LN suspensions by positive selection using antibody-coated magnetic beads (Miltenyi Biotec). Cells were added to 96-well plates without antigen, with BMDCs (APC/T cell ratio: 1:5) and with L. major-lysate (17) (at 106 LN cells or 5 × 105 T cells/ 200 μl) of complete RPMI containing β-mercaptoethanol (5 × 10−5 M). Culture supernatants were assayed for cytokine production after 48 h using ELISAs specific for murine IFN-γ, IL-12 p40 (R&D Systems), and IL-4 (BD Biosciences).
Statistics.
Statistical analysis was performed using the unpaired Student's t test.
Results
Skin-derived APCs from Leishmania-susceptible Mice Produce Less IL-1α.
We have previously shown that FSDDC from both Leishmania-resistant C57BL/6 and susceptible BALB/c mice release comparable amounts of IL-12 p70 when infected with L. major amastigotes in vitro, and that infected BALB/c DC can vaccinate against disease (9). To determine if strain-dependent differences in factors other than Th1-promoting IL-12 might be responsible for genetic determination of disease outcome, we surveyed DCs from both strains for production of other cytokines. We observed up-regulation of mRNA levels for IL-12 p35 and p40, IL-1α/β, IL-1RA as well as MIF in stimulated FSDDC compared with untreated control cells (unpublished data). Quantitation of mRNA expression in three independent experiments revealed that LPS/IFN-γ–stimulated or L. major–infected FSDDCs from BALB/c mice contained 2.9 and 1.7-fold less IL-1α mRNA (Fig. 1 A), respectively, whereas no strain differences in expression of IL-12 p40, IL-1RA, or IL-1β mRNA were found (unpublished data). Thus, the level of bioactive IL-1, as determined by the ratio of IL-1/IL-1RA, is reduced in activated BALB/c DCs compared with DCs from C57BL/6 mice.
Figure 1.
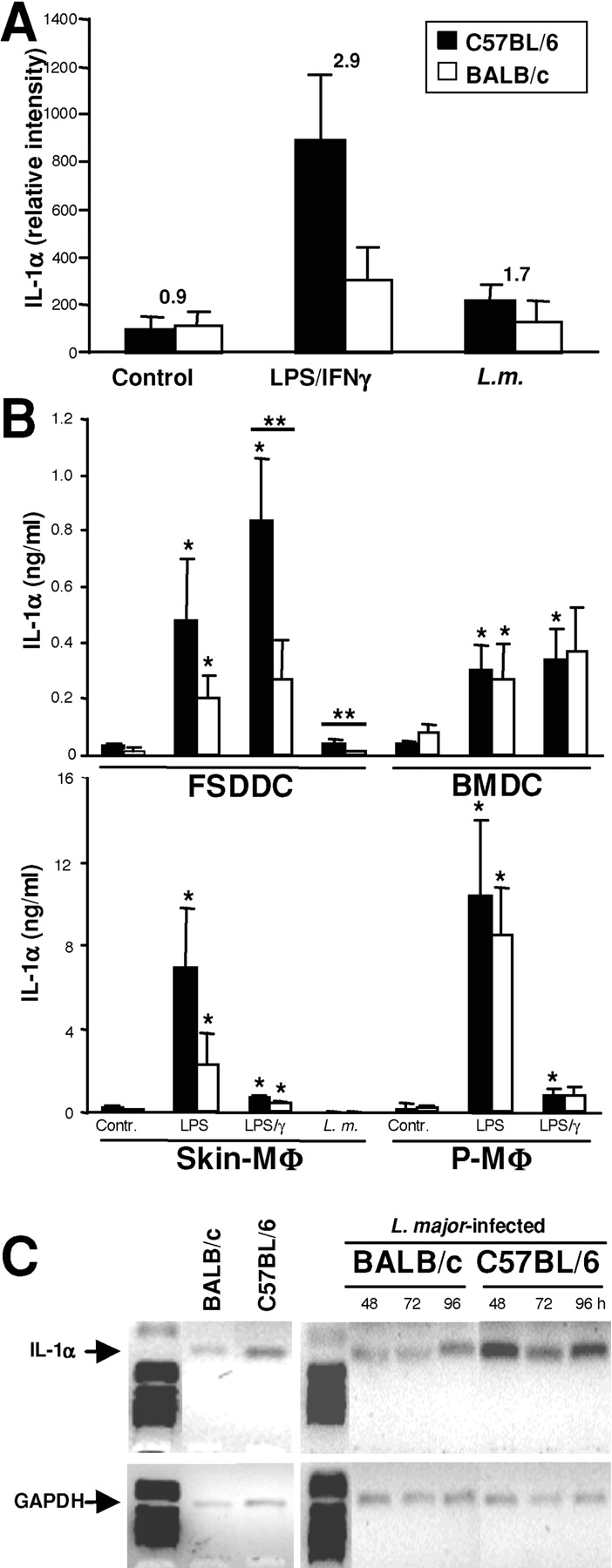
Langerhans cell-like dendritic cells of Leishmania-susceptible BALB/c mice produce less IL-1α than dendritic cells of resistant C57BL/6 mice. (A) FSDDCs were stimulated with LPS/IFN-γ (100 ng/1,000 U/ml) or L. major amastigotes (3:1 organisms/cell) for 18 h and expression of various cytokines was determined using RNase protection assays. Relative intensities of bands corresponding to IL-1α mRNA and mean fold differences between C57BL/6 and BALB/c DC of three independent experiments are shown. (B) FSDDCs (n ≥ 5), BMDCs (n = 6), peritoneal MΦ (P-MΦ, n = 5), and skin-MΦ (n ≥ 4) were stimulated as indicated. After 18 h, protein levels in cell lysates were determined by ELISA (ng/ 106 cells, mean ± SEM). *P < 0.05 compared with untreated control, differences between mouse strains are marked with bars above columns (**P < 0.05). (C) IL-1α mRNA accumulation in lymph nodes of uninfected mice or, in a separate experiment, mice infected with 2 × 105 L. major was assessed by semi-quantitative RT-PCR at the time points indicated. Results shown are representative of two independent experiments.
LPS/IFN-γ–stimulated or L. major–infected FSDDC also produced significantly less IL-1α protein (Fig. 1 B) when isolated from BALB/c rather than C57BL/6 mice (266 ± 142 vs. 839 ± 217 and 12 ± 3 vs. 41 ± 11 pg/106 cells/ml, P < 0.05). Similar to skin-derived FSDDCs, LPS-stimulated inflammatory skin-MΦ derived from BALB/c mice also contained 2.5-fold less IL-1α than skin-MΦ from C57BL/6 mice (Fig. 1 B). Previously, L. major was shown to selectively increase IL-1α mRNA accumulation in MΦ, although levels were lower than those induced by LPS (18). In our hands, L. major–infection of skin-MΦ did not induce production of detectable amounts of IL-1α. In contrast to skin DCs, standard APC populations from noncutaneous sources (BMDC, P-MΦ) did not exhibit strain-dependent differences in IL-1α synthesis (Fig. 1 B).
To test the in vivo relevance of our in vitro findings, we performed additional studies to quantify IL-1α expression in vivo after L. major infection of BALB/c or C57BL/6 mouse ear skin (Fig. 1 C). Using semi-quantitative RT-PCR, we determined that IL-1α mRNA levels in draining LN were ∼3-fold lower in BALB/c as compared with C57BL/6 mice (e.g., 48 h: 1.9- and 4,3-, 96 h: 2.8- and 3.1-fold difference in two independent experiments). Preinfection controls and earlier time points did not show striking differences (0.8- to 1.2-fold less IL-1α RNA in BALB/c compared with C57BL/6 LN). Strain differences in IL-1α mRNA expression was confirmed using real time PCR (48 h: 3.2- and 2.3-fold difference between BALB/c and C57BL/6 LN preparations in two independent experiments).
Administration of IL-1α During T Cell Priming Improves Disease Outcome in both Leishmania-susceptible and -resistant Mice.
To determine if skin-DC-derived IL-1α might influence disease outcome, we injected IL-1α into sites of standard high dose inoculation (2 × 105 metacyclic promastigotes; Fig. 2, A and B) . In both BALB/c and C57BL/6 mice, administration of IL-1α during the first 3 d dramatically reduced ear lesion volumes when followed over the course of 3 mo. BALB/c mice treated with 50 ng IL-1α on days 1–3 after infection were protected from progressive disease for more than 12 wk, whereas PBS-treated controls had to be killed after 6 weeks due to uncontrolled lesion evolution with tissue ulcerations (Fig. 2 A). Daily treatment of Leishmania-susceptible BALB/c mice with IL-1α for up to 2 wk after parasite inoculation did not additionally improve disease outcome (unpublished data). Lesion development in Leishmania-resistant C57BL/6 mice was also significantly attenuated by treatment with IL-1α (Fig. 2 B).
Figure 2.
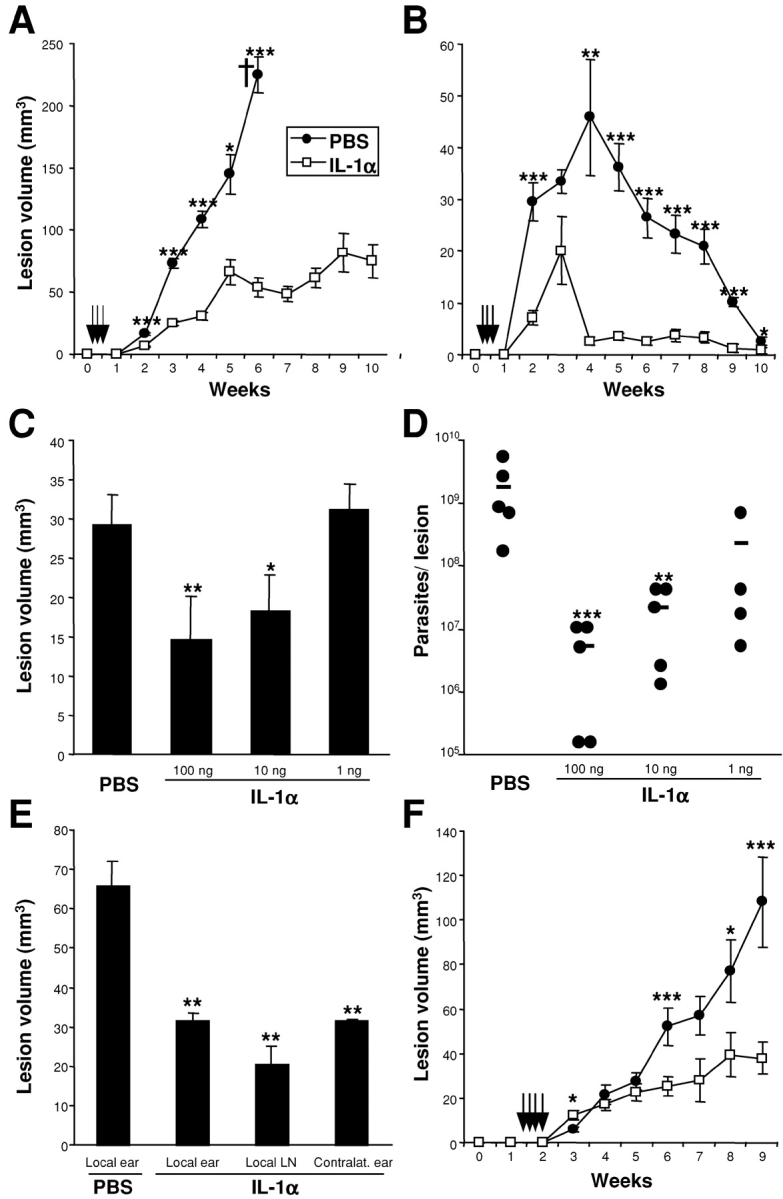
Improvement of disease outcome in Leishmania-susceptible and -resistant mice after systemic treatment with IL-1α. Groups of ≥5 BALB/c (A) or C57BL/6 (B) mice were infected with 2 × 105 L. major and treated locally with 50 ng IL-1α or PBS on days 1, 2, and 3 after infection. Lesion development was assessed weekly (mean ± SEM). Results are representative of ≥3 experiments. (C and D) BALB/c mice were infected as described above and treated with different doses of IL-1α as indicated. Lesion volumes were measured after 3 wk and the number of parasites/lesion was determined in limiting dilution assays. Dots represent number of parasites in one ear, bars show the arithmetic mean of all mice/group. (E) Groups of three BALB/c mice were infected with L. major promastigotes and treated with 50 ng IL-1α or PBS on days 1–3 after infection. Treatment was delivered locally, into the contralateral ear or intradermally into the retroauricular region of the infected side (local LN). 3 wk later, lesion sizes were assessed (mean ± SEM). (F) IL-1α treatment prevents progressive disease in low dose cutaneous leishmaniasis. Groups of >5 BALB/c mice were infected with 1,000 L. major promastigotes and treated locally with 50 ng IL-1α or PBS on days 11–14 after infection. Development of lesions was expressed as mean ± SEM. (A–F) Statistical differences between treatment groups at different time points are indicated as *P < 0.05, **P < 0.005, and ***P < 0.002 compared with PBS-treated control mice.
Subsequent studies showed that doses of 100 ng and 10 ng IL-1α administered on days 1–3 after infection were similarly efficient in reducing skin lesion sizes (Fig. 2 C). In contrast, 1 ng of IL-1α was not effective. Lesional parasite loads were also reduced upon treatment with IL-1α (Fig. 2 D). Specifically, 100 ng and 10 ng of IL-1α reduced parasite burdens of infected ear skin by >2 logs (P < 0.005), whereas 1 ng did not facilitate parasite control in vivo.
The Protective Effect of IL-1α in Cutaneous Leishmaniasis Does Not Require Local Administration.
Because local administration of IL-1α into skin is known to activate and mobilize LCs which may then acquire antigen and migrate to draining LNs (19), we sought to determine if local administration was required for the beneficial effect of IL-1α in cutaneous leishmaniasis. BALB/c mice were infected in one ear with high dose inoculae and treated with IL-1α intradermally into infected ears, opposite ears, or into the retroauricular skin of the infected ears (Fig. 2 E). Injection of IL-1α into skin sites other than the lesional tissue was as efficacious as local administration. IL-1α injection resulted in a 32 ± 5% decrease in LC density in epidermal sheets from injected, but not from contralateral ears (unpublished data). This suggests that IL-1α does not act by mobilizing LCs in lesional tissue.
IL-1α Also Protects Against Progressive Disease in Low Dose Leishmania Infections.
We also assessed the efficacy of IL-1α in infections with low dose inocula (103 infectious-stage parasites) administered intradermally (17). This experimental model is more physiologically relevant in that it more closely mimics natural parasite transmission by sand flies. In this setting, IL-1α treatment was only successful if administered for the initial 14 d after infection (unpublished data) or between days 11 and 14 after infection (Fig. 2 F). The original treatment protocol (days 1–3 after inoculation only) was without effect (unpublished data). This data suggested that IL-1α treatment was only efficacious when given coincident with the onset of T cell priming which is delayed in low dose infections (17).
Enhanced Th1-development in IL-1α–treated Leishmania-infected BALB/c Mice.
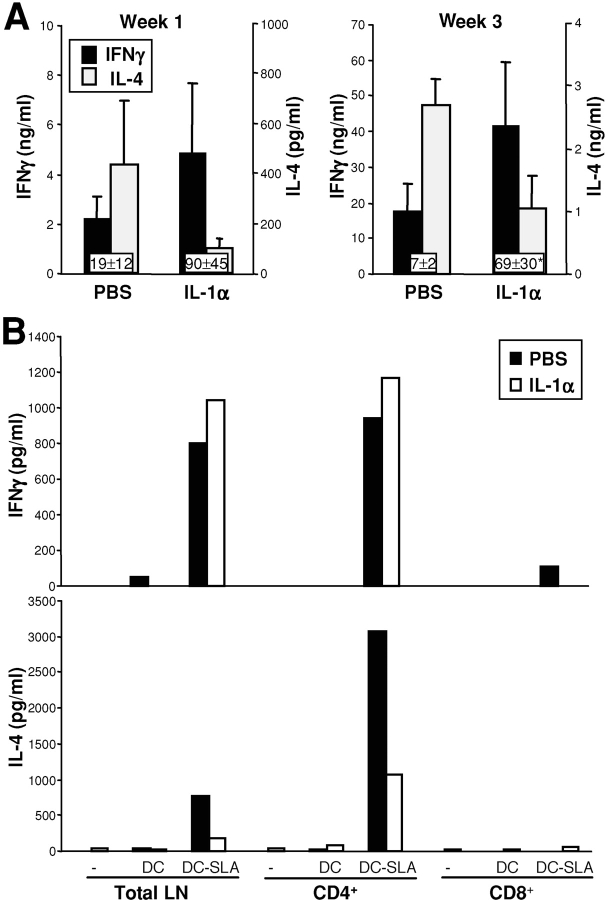
L. major specific Th1-cells are necessary for efficient elimination of Leishmania parasites from infected hosts (1). To determine if IL-1α facilitated Th1-development we assessed antigen-dependent cytokine production by lesion-draining LN cells from 1 and 3 wk Leishmania-infected BALB/c mice (Fig. 3 A). As described previously, LN cells from untreated or PBS-treated BALB/c mice infected with Leishmania promastigotes produced large amounts of IL-4 upon stimulation with Leishmania lysate (1). In contrast, LN cells of IL-1α-treated BALB/c mice showed significantly reduced IL-4 release 1 wk after infection. IFN-γ production was also >2-fold higher in IL-1α-treated mice than in PBS-treated controls at early time points. Thus, the ratio between Th/Tc1- and Th/Tc2-cytokines was significantly skewed toward a Th1-predominant immune response in IL-1α-treated Leishmania-infected mice (Fig. 3 A). This effect was still detectable after 3 wk, although treatment with IL-1α was only given on days 1, 2, and 3 after infection.
Figure 3.
IL-1α treatment of Leishmania-susceptible BALB/c results in Th1-predominant immune responses. BALB/c mice were treated with 50 ng IL-1α or PBS on days 1–3 after infection with 2 × 105 L. major. (A) After 1 and 3 wk, LN cells of groups of three mice were harvested and plated at 106 cells/200 μl. Cells were stimulated with soluble Leishmania antigen (SLA) for 48 h and antigen-specific cytokine release was determined using ELISAs specific for murine IFN-γ and IL-4 (mean ± SEM). For each mouse, the ratio between IFN-γ and IL-4 was calculated (see boxes) and statistical significance was determined (*P < 0.05 compared with PBS-treated controls). (B) After 1 wk, LN cells were isolated and T cell populations isolated using magnetic beads. Total LN cells (106) or CD4+ and CD8+ T cells (5 × 105) were plated into 96-well plates (200 μl) and BMDCs and SLA added. Cytokine responses were studied after 48 h as described above. One out of two experiments with similar results is shown.
We next assessed T cell responses in IL-1α-treated BALB/c mice in more detail (Fig. 3 B). CD4+ and CD8+ T cells were isolated from IL-1α- or PBS-treated BALB/c mice 1 wk after infection and restimulated in vitro with antigen in the presence of APCs. CD8+ T cells did not release IL-4 (or IFN-γ) confirming previous results demonstrating that the source of IL-4 early in infections of BALB/c mice is CD4+ T cells (20). Those studies also indicated that the specific inability of BALB/c mice to down-regulate early IL-4 release contributed to genetic susceptibility in leishmaniasis. In IL-1α–treated mice, release of IL-4 by CD4 T cells was dramatically reduced, whereas IFN-γ levels were up-regulated. Here, the ratio between IFN-γ and IL-4 in LN cultures was altered from 0.9 in PBS-treated mice to 8.5 after IL-1α treatment. This data strongly suggests that IL-1α treatment is effective via alteration of Leishmania-specific CD4+ T cell responses.
In subsequent experiments, CD4+ T cells were depleted from BALB/c mice within the first week of infection during IL-1α treatment (unpublished data). In accordance with previous studies (21), BALB/c mice depleted of IL-4–releasing CD4+ T cells had smaller lesion volumes and decreased parasite burdens. As expected, IL-1α treatment of CD4-depleted mice was without additional benefit with respect to disease outcome.
IL-1α Treatment Is as Effective as Administration of IL-12.
The dominant role of IL-12 in development of protective immune responses against intracellular pathogens is well established (1). As we observed strain-dependent variations in the expression of IL-1α but not IL-12 by DC (9; Fig. 1 A), we tested the potency and specificity of IL-1α in leishmaniasis by comparing the effect of local administration of similar amounts of IL-12, IL-1α, IL-1β, IL-18, and IL-6 on the course of infection with L. major (Fig. 4) . BALB/c mice were infected with 2 × 105 promastigotes and recombinant cytokines were administered locally on days 1–3 after infection. As expected, mice treated transiently with IL-12 showed reduced lesion sizes after 3 wk as well as significantly reduced parasite burdens in lesional tissue. IL-1α, IL-1β, and IL-12 decreased lesion sizes and reduced parasite burdens to similar extents (Fig. 4, A and B). Importantly, both IL-12 and IL-1α increased the production of antigen-specific IFN-γ in LNs and decreased IL-4 levels (Fig. 4, C and D). In contrast, administration of IL-18 was less effective. Although we observed reduced lesion volumes and parasite loads, the differences between IL-18– and PBS-treated mice did not reach statistical significance. Treatment with IL-6 during the first 3 d of infection was ineffective.
Figure 4.
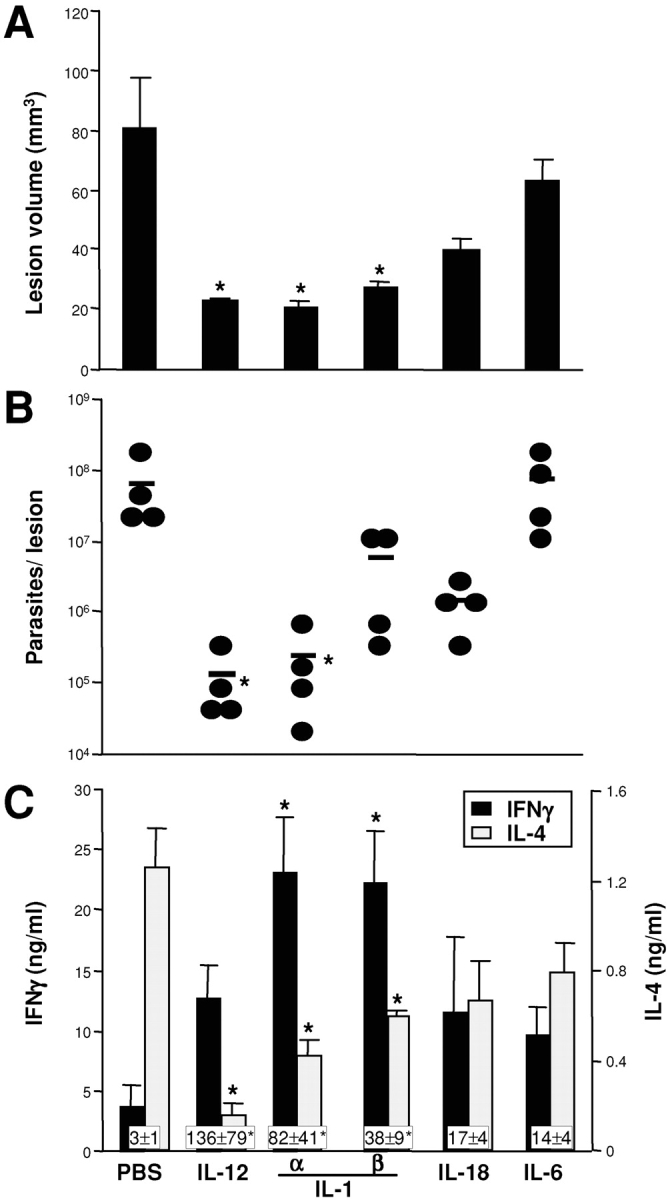
IL-1α is as efficient as IL-12 for treatment of cutaneous leishmaniasis in susceptible BALB/c mice. BALB/c mice (five/group) were inoculated intradermally in the ears with 2 × 105 metacyclic L. major promastigotes. Fifty ng of IL-12, IL-1α, IL-1β, IL-18, or IL-6 was injected locally on days 1–3. After 3 wk, lesion volumes were determined (A) and expressed as mean ± SEM. Organisms were cultured from challenge sites and enumerated as described (B). Each data point represents the number of organisms from one ear, bars indicate arithmetic means. Cytokine profiles of LN cells were determined by in vitro restimulation with soluble Leishmania antigen (SLA). Supernatants were assayed for the presence of IFN-γ (C) and IL-4 (D) after 48 h (mean ± SEM, boxes represent IFN-γ/IL-4 ratios, *P < 0.05 compared with PBS-treated controls). Data of one experiment out of three with similar results is shown.
The Action of IL-1α Is IL-12 Dependent.
We also sought to determine if IL-1α treatment altered IL-12 levels in draining LN cells and thus contributed to Th1-predominant differentiation. Infected BALB/c mice were treated with IL-1α and LN cells were harvested on days 7 and 21 after infection (unpublished data). In all samples examined, we found slightly increased (∼30%) levels of IL-12 p40 in cultures of LN cells from mice that were treated with IL-1α compared with PBS-treated control animals. However, these differences did not reach statistical significance.
As our data suggested that the effects of IL-1α are similar to those of IL-12 in leishmaniasis, we assessed the outcome of IL-1α treatment in mice that were deficient in IL-12 p40 (Fig. 5) . Wild-type or IL-12 p40-deficient BALB/c mice were infected with 2 × 105 promastigotes and treated with IL-1α as described before. Lesion sizes as well as parasite burdens in BALB/c IL-12 p40 null mice were higher when compared with those in control animals, and treatment with IL-1α did not have an effect on disease progression (Fig. 5, A and B). As expected, we observed virtually no induction of antigen-specific IFN-γ release in IL-12 p40-deficient mice, whereas IL-4 levels were almost unchanged compared with control mice (Fig. 5, C and D). Perhaps not surprisingly, IL-1α was not able to induce Th1-differentiation in IL-12–deficient mice. The lack of effect of IL-1α on the production of IL-4 in IL-12 p40-deficient mice indicated that the IL-1α–mediated suppression of IL-4 was also dependent on the presence of IL-12.
Figure 5.
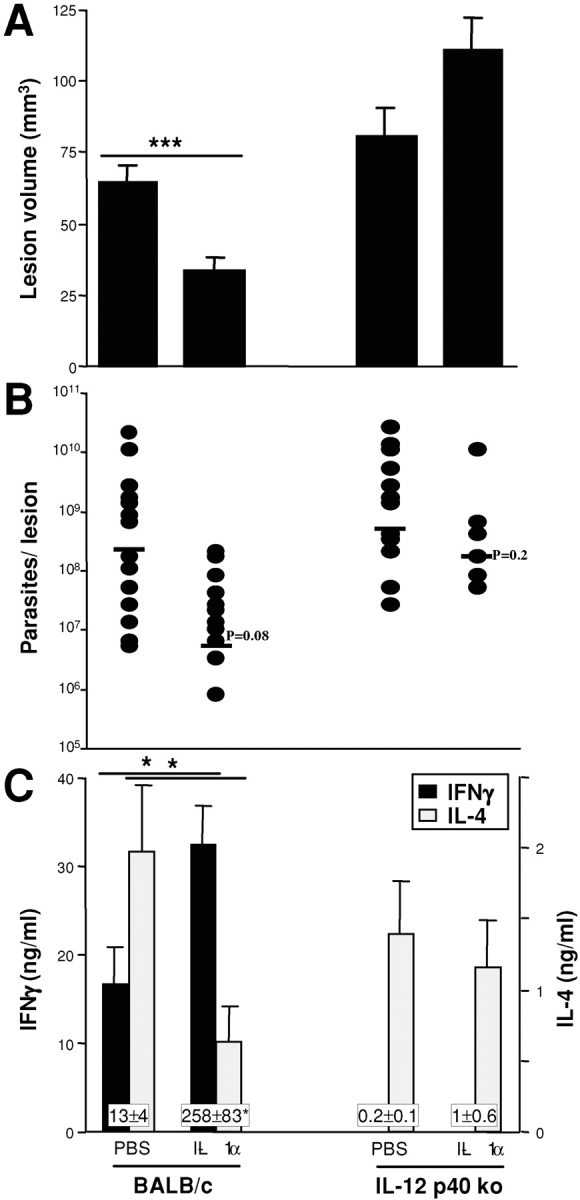
Effective treatment of cutaneous leishmaniasis in Leishmania-susceptible BALB/c mice with IL-1α requires IL-12. BALB/c or IL-12p40 ko BALB/c mice were infected with high dose inocula into ear skin. IL-1α or PBS was applied locally on days 1–3 after infection. Three weeks later, lesions sizes (A) and parasite burdens in lesional ear skin (B) were determined. Cytokine profiles of lymph node cells restimulated with antigen were assayed as described in Materials and Methods using ELISAs specific for IFN-γ (C) and IL-4 (D). All data are expressed as mean ± SEM (*P < 0.05, ***P < 0.002) and the results shown are pooled from two experiments (six mice/group).
Discussion
Susceptibility of BALB/c mice to cutaneous leishmaniasis is characterized by an inability to mount efficient anti-Leishmania Th1-immunity. DCs facilitate Th-priming in vivo by producing cytokines such as IL-12 and IFN-γ. Thus, susceptibility to infectious diseases could be influenced at the level of tissue DCs, and strain-dependent differences in Th1-promoting cytokine production by DCs might contribute to the L. major susceptibility of BALB/c mice in leishmaniasis (22). We have previously reported that L. major susceptibility does not appear to reflect a failure of BALB/c skin DCs to internalize or to be activated by parasites, or by the inability of BALB/c T cells to mount a Th1-response to DC-associated Leishmania antigens (9). We now report that, in contrast to our observations regarding IL-12 release, BALB/c DCs produce less IL-1α in response to stimulation than C57BL/6 DCs. We suggest that differences in the production of IL-1α in LN in vivo might influence the outcome of infection in these mouse strains.
The events that shape development of Th1- or Th2-predominant immunity occur during the initial phase of T cell priming. Production of cytokines (e.g., IL-12 and IL-4) that direct the subsequent development of characteristic Th-subsets are particularly relevant. Several mechanisms have been shown to contribute to L. major susceptibility of BALB/c mice (for a review, see reference 23). These include a lack of parasite containment in early lesions and higher antigen loads (24), a relative lack of IL-12 release together with increased production of IL-4 very early after infection (1), and IL-4–mediated down-regulation of the IL-12Rβ2 on Th2-cells (25, 26). In addition, IFN-γ, which could up-regulate IL-12Rβ2 expression and counteract the inhibitory effect of IL-4, is known to be produced at lower levels in L. major–infected BALB/c mice as compared with resistant animals (1).
In this study, treatment with IL-1α during T cell priming induced Th1-development that attenuated disease progression in susceptible BALB/c mice. We demonstrated that this effect was dependent on, and in the regimen employed as effective as, IL-12. Continuous administration of IL-12 protein has previously been used together with Leishmania antigen as an effective vaccine (27, 28; for reviews, see references 29 and 23). In IL-1α–treated BALB/c mice, we found slightly increased amounts of IL-12 in draining LNs suggesting that IL-1α-mediated up-regulation of IL-12 production could contribute to Th1-development. However, no obvious deficiency in IL-12 production by DCs (the principal source of IL-12 in vivo) in BALB/c mice has been demonstrated (9) and BALB/c mice expressing a transgenic IL-12Rβ2 exhibited a nonhealing phenotype to L. major infection despite maintenance of intact IL-12 signaling (30). Both studies suggest that factors other than IL-12 are relevant for development and maintenance of Th1-responses in BALB/c mice.
Although IL-12 is required for IL-1 efficacy in this model, IL-1α may have effects on Th1-priming that are not directly mediated via IL-12. The importance of IL-1 in innate immune responses is well recognized (for a review, see reference 31), but its role in Th-differentiation is more controversial. IL-1 was originally reported to promote proliferation of Th2 clones (32). However, Shibuya et al. subsequently demonstrated that IL-1α (and TNF-α) are required for maximal IL-12-driven Th1-development in BALB/c, but not C57BL/6, T cells (33). In addition, recent studies indicate that IL-12 can synergize with IL-1β (or IL-18) for IFN-γ production by human T cells (34). Finally, in the absence of MyD88-signaling, mice immunized with soluble Toxoplasma-antigen (STAg) failed to develop detectable Th1-responses despite normal IL-12 release, suggesting that MyD88-dependent signals (e.g., IL-1, IL-18, and TLR) might also be critical for successful Th1-polarization (35).
We also tested IL-6 and IL-18 as potential anti-Leishmania treatments. Administration of IL-6 during T cell priming was without effect, confirming previous results using knockout mice (36). Although IL-18 and IL-1 are homologous, act via related receptors (IL-1R), and IL-18 is known to be involved in Th1-development (for a review, see reference 37), IL-18 protein was not as effective as IL-1α/β in reducing lesion development and parasite burdens in infected mice (38–40). However, IL-18 (like IL-1α) is known to potentiate IL-12–driven Th1 development of T cells from BALB/c mice (for a review, see reference 41). In the absence of sufficient amounts of IL-1α during T cell priming, IL-18 and IL-12 responsiveness by BALB/c CD4+ T cells might not be optimal and IL-4–driven Th2-development may be favored.
Previously, Hunter et al. demonstrated that IL-1β (but not IL-1α) prolonged survival of SCID mice after infection with T. gondii by inducing IFN-γ release from NK cells (42). In addition, a minor role for IL-1 in the control of parasite growth in leishmaniasis was previously noted in studies of C57BL/6 IL-1RI-deficient mice. Although lesion sizes were similar in IL-1RI knockout- and wild type-mice, parasite-specific IL-4 production in knockout animals was increased and lesional tissue contained increased numbers of parasites (43). In light of the observations by Shibuya et al. (33), a more dramatic phenotype of IL-1RI null mice on a Leishmania-resistant background cannot be expected. It has also previously been reported that delayed treatment of Leishmania-lesions with anti–IL-1RI mAb or recombinant IL-1 either did not alter disease outcome or exacerbated disease (44, 45). These findings are also not inconsistent with our results, as we observed that IL-1 was effective only when it was administered early in infections in the high dose model, or at the time of initial lesion development in the low dose setting. Our results confirm and extend these findings and highlight the role of IL-1α/β as critical mediators for successful Th1-development against an infectious organism in vivo.
IL-1 has recently been shown to contribute to the pathogenesis of several Th1-driven inflammatory and infectious diseases including arthritis and tuberculosis (46, 47). A previous report suggested that alterations in IL-1 and IL-1RA levels due to gene polymorphisms contributed to disease development (47). Specifically, IL-1RA knockout mice on the BALB/c (but not C57BL/6) background exhibited increased IL-1 bioactivity and spontaneously developed chronic inflammatory polyarthropathy (48). These findings are consistent with our findings in cutaneous leishmaniasis and highlight the role that IL-1 might play as a genetic determinant of both infectious and autoimmune disease outcome.
In summary, the present study documents an important role for IL-1 in the regulation of Th1-development and disease progression in cutaneous leishmaniasis in mice. Future studies will be directed toward additionally defining the role of DC-derived IL-1 in T cell priming and characterizing the alterations in the inflammatory cell infiltrates induced by IL-1. In conjunction with studies designed to identify key signaling components, we hope to develop a better understanding of the exact mechanism by which IL-1 regulates Th-differentiation in this important model of a human disease.
Acknowledgments
The authors thank Drs. D.L. Sacks, K. Steinbrink, and M. Maurer for helpful discussions, and M. Wilson, B. Nguyen, and E. Wiese for expert technical assistance.
Part of this work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (Ste 833/4-1, SFB548) and the MAIFOR program (University of Mainz) to E. von Stebut.
Footnotes
Abbreviations used in this paper: BMDC, bone marrow–derived dendritic cell; FSDDC, fetal skin–derived DC; LC, Langerhans cell.
References
- 1.Reiner, S.L., and R.M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151–177. [DOI] [PubMed] [Google Scholar]
- 2.Muller, I., T. Pedrazzini, P. Kropf, J. Louis, and G. Milon. 1991. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int. Immunol. 3:587–597. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., E. von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M.C. Udey, and D.L. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992–4000. [DOI] [PubMed] [Google Scholar]
- 4.Flohe, S.B., C. Bauer, S. Flohe, and H. Moll. 1998. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur. J. Immunol. 28:3800–3811. [DOI] [PubMed] [Google Scholar]
- 5.von Stebut, E., Y. Belkaid, T. Jakob, D.L. Sacks, and M.C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and IL-12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 7.Moll, H., H. Fuchs, C. Blank, and M. Rollinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595–1601. [DOI] [PubMed] [Google Scholar]
- 8.Carrera, L., R.T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D.L. Sacks. 1996. Leishmania promastigotes selectively inhibit IL-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Stebut, E., Y. Belkaid, B.V. Nguyen, M. Cushing, D.L. Sacks, and M.C. Udey. 2000. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur. J. Immunol. 30:3498–3506. [DOI] [PubMed] [Google Scholar]
- 10.Magram, J., J. Sfarra, S. Connaughton, D. Faherty, R. Warrier, D. Carvajal, C.Y. Wu, C. Stewart, U. Sarmiento, and M.K. Gately. 1996. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann. NY Acad. Sci. 795:60–70. [DOI] [PubMed] [Google Scholar]
- 11.Jakob, T., A. Saitoh, and M.C. Udey. 1997. E-cadherin-mediated adhesion involving Langerhans cell-like dendritic cells expanded from murine fetal skin. J. Immunol. 159:2693–2701. [PubMed] [Google Scholar]
- 12.Fauve, R.M., H. Jusforgues, and B. Hevin. 1983. Maintenance of granuloma macrophages in serum-free medium. J. Immunol. Methods. 64:345–351. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid, Y., B. Butcher, and D.L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389–1400. [DOI] [PubMed] [Google Scholar]
- 14.von Stebut, E., M. Metz, G. Milon, J. Knop, and M. Maurer. 2003. Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha/beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood. 101:210–215. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortier, L.A. 1999. Generation of mouse macrophage populations. Current Protocols of Immunology. John Wiley & Sons, New York. Unit 14.1.
- 17.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D.L. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977. [DOI] [PubMed] [Google Scholar]
- 18.Hawn, T.R., A. Ozinsky, D.M. Underhill, F.S. Buckner, S. Akira, and A. Aderem. 2002. Leishmania major activates IL-1 alpha expression in macrophages through a MyD88-dependent pathway. Microbes Infect. 4:763–771. [DOI] [PubMed] [Google Scholar]
- 19.Lundqvist, E.N., and O. Back. 1990. Interleukin-1 decreases the number of Ia+ epidermal dendritic cells but increases their expression of Ia antigen. Acta Derm. Venereol. 70:391–394. [PubMed] [Google Scholar]
- 20.Launois, P., I. Maillard, S. Pingel, K.G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R.M. Locksley, H.R. MacDonald, and J.A. Louis. 1997. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 6:541–549. [DOI] [PubMed] [Google Scholar]
- 21.Heinzel, F.P., and R.M. Rerko. 1999. Cure of progressive murine leishmaniasis: interleukin 4 dominance is abolished by transient CD4+ T cell depletion and T helper cell type 1-selective cytokine therapy. J. Exp. Med. 189:1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDowell, M.A., M. Marovich, R. Lira, M. Braun, and D.L. Sacks. 2002. Leishmania priming of human dendritic cells for CD40 ligand-induced IL-12p70 secretion is strain and species dependent. Infect. Immun. 70:3994–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacks, D.L., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845–858. [DOI] [PubMed] [Google Scholar]
- 24.Bretscher, P.A., G. Wei, J.N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 257:539–542. [DOI] [PubMed] [Google Scholar]
- 25.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D.H. Presky, U. Gubler, and F. Sinigaglia. 1997. Selective expression of an IL-12 receptor component by human T helper 1 cells. J. Exp. Med. 185:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Himmelrich, H., C. Parra-Lopez, F. Tacchini-Cottier, J.A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156–6163. [PubMed] [Google Scholar]
- 27.Afonso, L.C., T.M. Scharton, L.Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of IL-12 in a vaccine against Leishmania major. Science. 263:235–237. [DOI] [PubMed] [Google Scholar]
- 28.Gurunathan, S., C. Prussin, D.L. Sacks, and R.A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409–1415. [DOI] [PubMed] [Google Scholar]
- 29.Scott, P., and G. Trinchieri. 1997. IL-12 as an adjuvant for cell-mediated immunity. Semin. Immunol. 9:285–291. [DOI] [PubMed] [Google Scholar]
- 30.Nishikomori, R., S. Gurunathan, K. Nishikomori, and W. Strober. 2001. BALB/c mice bearing a transgenic IL-12 receptor beta 2 gene exhibit a nonhealing phenotype to Leishmania major infection despite intact IL-12 signaling. J. Immunol. 166:6776–6783. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello, C.A. 1996. Biologic basis for interleukin-1 in disease. Blood. 87:2095–2147. [PubMed] [Google Scholar]
- 32.Weaver, C.T., C.M. Hawrylowicz, and E.R. Unanue. 1988. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 85:8181–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibuya, K., D. Robinson, F. Zonin, S.B. Hartley, S.E. Macatonia, C. Somoza, C.A. Hunter, K.M. Murphy, and A. O'Garra. 1999. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J. Immunol. 160:1708–1716. [PubMed] [Google Scholar]
- 34.Tominaga, K., T. Yoshimoto, K. Torigoe, M. Kurimoto, K. Matsui, T. Hada, H. Okamura, and K. Nakanishi. 2000. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int. Immunol. 12:151–160. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 36.Titus, R.G., G.K. DeKrey, R.V. Morris, and M.B. Soares. 2001. Interleukin-6 deficiency influences cytokine expression in susceptible BALB mice infected with Leishmania major but does not alter the outcome of disease. Infect. Immun. 69:5189–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims, J.E. 2002. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 14:117–122. [DOI] [PubMed] [Google Scholar]
- 38.Ohkusu, K., T. Yoshimoto, K. Takeda, T. Ogura, S. Kashiwamura, Y. Iwakura, S. Akira, H. Okamura, and K. Nakanishi. 2000. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect. Immun. 68:2449–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monteforte, G.M., K. Takeda, M. Rodriguez-Sosa, S. Akira, J.R. David, and A.R. Satoskar. 2000. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J. Immunol. 164:5890–5893. [DOI] [PubMed] [Google Scholar]
- 40.Wei, X.Q., B.P. Leung, W. Niedbala, D. Piedrafita, G.J. Feng, M. Sweet, L. Dobbie, A.J. Smith, and F.Y. Liew. 1999. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J. Immunol. 163:2821–2828. [PubMed] [Google Scholar]
- 41.Heath, V.L., H. Kurata, H.J. Lee, N. Arai, and A. O'Garra. 2002. Checkpoints in the regulation of T helper 1 responses. Curr. Top. Microbiol. Immunol. 266:23–39. [DOI] [PubMed] [Google Scholar]
- 42.Hunter, C.A., R. Chizzonite, and J.S. Remington. 1995. IL-1β is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1β in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 155:4347–4354. [PubMed] [Google Scholar]
- 43.Satoskar, A.R., M. Okano, S. Connaughton, A. Raisanen-Sokolwski, J.R. David, and M. Labow. 1998. Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur. J. Immunol. 28:2066–2074. [DOI] [PubMed] [Google Scholar]
- 44.Theodos, C.M., A. Shankar, A.L. Glasebrook, W.D. Roeder, and R.G. Titus. 1994. The effect of treating with anti-IL-1 receptor antibody on the course of experimental murine cutaneous leishmaniasis. Parasite Immunol. 16:571–577. [DOI] [PubMed] [Google Scholar]
- 45.Ajdary, S., M.H. Hosseini, and M.H. Alimohammadian. 1997. Recombinant IL-1 promotes leishmaniasis in susceptible mice. Microbiol. Immunol. 41:281–283. [DOI] [PubMed] [Google Scholar]
- 46.Ji, H., A. Pettit, K. Ohmura, A. Ortiz-Lopez, V. Duchatelle, C. Degott, E. Gravallese, D. Mathis, and C. Benoist. 2002. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J. Exp. Med. 196:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attur, M.G., M.N. Dave, M.Y. Leung, C. Cipolletta, M. Meseck, S.L. Woo, and A.R. Amin. 2002. Functional genomic analysis of type II IL-1beta decoy receptor: potential for gene therapy in human arthritis and inflammation. J. Immunol. 168:2001–2010. [DOI] [PubMed] [Google Scholar]
- 48.Horai, R., S. Saijo, H. Tanioka, S. Nakae, K. Sudo, A. Okahara, T. Ikuse, M. Asano, and Y. Iwakura. 2000. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 191:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]