Abstract
Access to the splenic white pulp is restricted to lymphocytes and dendritic cells. Here we show that movement of molecules from the blood into these confined areas is also limited. Large molecules, such as bovine serum albumin (68 kD), immunoglobulin G (150 kD), and 500 kD dextran are unable to enter the white pulp, whereas smaller blood-borne molecules can directly permeate this compartment. The distribution is restricted to a stromal network that we refer to as the splenic conduit system. The small lumen of the conduit contains collagen fibers and is surrounded in the T cell areas by reticular fibroblasts that express ER-TR7. It also contains the chemokine CCL21. Conversely, in B cell follicles the B cell–attracting chemokine CXCL13 was found to be associated with the conduit and absence of ER-TR7+ fibroblasts. These results show heterogeneity of reticular fibroblasts that enfold the conduit system and suggest that locally produced chemokines are transported through and presented on this reticular network. Therefore, the conduit plays a role in distribution of both blood-borne and locally produced molecules and provides a framework for directing lymphocyte migration and organization of the splenic white pulp.
Keywords: spleen, mouse, reticular network, antigen, chemokine
Introduction
Due to its strategic position in the blood stream and the abundance of macrophage-laden cords within the red pulp, the spleen is an effective filter for particles, such as dead blood cells and bacteria. The splenic white pulp is structurally similar to a lymph node (1), allowing for the generation of antigen-specific immune responses.
One major difference between the white pulp and lymph nodes is the mode of antigen transport. Lymph nodes collect antigens from the periphery via afferent lymphatics, whereas the spleen has no afferent lymphatic vessels and receives its antigen directly from the blood. Lymph-borne antigens initially end up in the subcapsular sinus of the lymph node, from where they are transported either directly into the medullary sinuses or into a reticular conduit system (2). The reticular meshwork that defines this conduit system has been described as a network of collagen fibers ensheathed by fibroblastic reticular cells, which extend from the subcapsular sinus to the deeper cortex and medullary regions and even connect with high endothelial venules (3, 4). These fibers comprise an extracellular space through which fluid drainage can occur, thus bringing antigens from afferent lymphatics into the parenchyma of the lymph node. Additionally, this conduit has been shown to drain peripherally produced chemokines, which can then be presented on the luminal side of high endothelial venules affecting leukocyte entry (5, 6).
In the spleen the vasculature is designed to allow most of the blood to flow directly into the red pulp, while a minor part reaches the marginal zone, the area between the white and red pulp (7, 8). Because the white pulp is restricted to lymphocytes, blood-borne particles and nonlymphoid cells leave the fenestrated marginal sinus at the red pulp side, thereby traversing the marginal zone (9). This region is densely packed with dendritic cells, macrophages, and marginal zone B cells, which are able to take up passing antigens and subsequently enter the white pulp to present them to T and B cells (7). However, little is known about the permeability of the white pulp for soluble molecules. If small antigens or cytokines could simply distribute through the white pulp, they could also be taken up by APCs or directly affect leukocytes, respectively. Therefore, in this study we analyzed the distribution of i.v. injected tracer molecules in the spleen, which revealed that the white pulp also contains a conduit system.
Materials and Methods
Mice.
C57BL/6 and lymphotoxin-α (LTα)−/− mice were maintained in the animal facilities at the Vrije Universiteit Faculty of Medicine, Amsterdam, Netherlands, under conventional conditions.
Reagents.
Fluorescein-conjugated, lysine-fixable dextran (Molecular Probes) used: 10 kD (5 mg/ml), 70 kD (5 mg/ml), and 500 kD (2 mg/ml). FITC-conjugated OVA (OVA-FITC; 5 mg/ml) was obtained from Molecular Probes and FITC-conjugated BSA (BSA-FITC; 5 mg/ml) and horseradish peroxidase (HRP; 100 mg/ml; activity 100 U/mg) were obtained from Sigma-Aldrich. mAb RA3-6B2 (IgG2a) was affinity purified from hybridoma culture supernatant and injected at 1 mg/ml.
Injection of Tracer Molecules and Tissue Handling.
Various tracers were injected i.v. in 200 μl. To prevent aspecific binding of OVA-FITC and BSA-FITC, mice received 100 μl unconjugated protein 10 min before injection of an equal amount of the conjugated protein. Before HRP injections, 25 μg promethazine was injected i.m. to prevent anaphylactic shock. Mice were killed 20 s, 1, 6, or 10 min after injection of the injected tracer by immediate CO2 suffocation or decapitation in case of analysis after 20 s. Spleens were rapidly removed and either immersion fixed overnight at 4°C in freshly made 4% paraformaldehyde/0.1% glutaraldehyde in PBS or for 3 h in 3% formaline in PBS. Tissues were then washed in PBS and saturated in 30% sucrose/PBS before freezing in Tissue-Tek (Sakura Finetek).
Macrophage Depletion.
Systemic depletion of macrophages was performed by treatment with clodronate-filled liposomes as previously described (10). Clodronate was provided by Roche Diagnostics. 2 d after liposome injection, efficiency of macrophage depletion in the spleen was assessed by immunohistochemical methods.
Immunohistochemistry.
10-μm cryostat sections were incubated with the following mAbs: ER-TR9 (detecting MZM), ER-TR7 (detecting stromal cells; provided by P. Leenen, Erasmus Medical Center, Rotterdam, Netherlands), 8.1.1 (hamster anti–gp38; provided by A. Farr, University of Washington, Seattle, WA), N418 (detecting CD11c+ DC), and RA3-6B2 (detecting B cells), and visualized with Alexa 488– or Alexa 594–conjugated anti–rat or anti–hamster Ig (Molecular Probes). Injected HRP was detected by 10-min incubation in 0.5 mg/ml diaminobenzidine in 0.1 M Tris-HCl, containing 0.01% H2O2. For visualization of CCL21 or CXCL13, sections were incubated overnight at 4°C with specific biotin-labeled polyclonal goat IgG (R&D Systems). Specificity of the chemokine staining was tested by adding recombinant chemokine to a concentration of 0.5 μg/ml. The Alexa Fluor 488 or Alexa 594 tyramide amplification system was then used to enhance the signal according to the manufacturer's protocol (Molecular Probes). Three-dimensional reconstructions were generated by performing x-y-z scans in 120- or 10-μm thick fixed splenic sections, using a confocal microscope (Leica).
Transmission Electron Microscopy.
∼100-μm slices of HRP-injected and fixed spleens were rinsed in 0.1 M Tris-HCl buffer, pH 7.6, preincubated in 0.1 mg/ml diaminobenzidine/0.1 M Tris-HCl solution (for 1 h at room temperature), and incubated in the same solution freshly made containing 0.01% H2O2 (for 1 h at room temperature). The tissue was prepared according to standard procedures and examined with a Philips CM100 electron microscope.
Flow Cytometry.
Isolated spleens were digested for 20 min with collagenase (1). Splenocytes were incubated with ER-TR7 mAb for 30 min in the presence or absence of 0.5 μg/ml CCL21 (R&D Systems). Preincubation of 0.5 μg/ml CCL21 with 50 μg/ml full-size heparin (Leo Pharmaceutical Products BV) was performed for 30 min. ER-TR7 was detected with Alexa Fluor 488–labeled goat anti–rat IgG (Molecular Probes), and then cells were blocked with 20% goat serum and stained with anti–CCL21-biotin (R&D Systems), followed by streptavidin-APC (BD Biosciences). Stainings were performed in 0.1% BSA/PBS and dead cells were excluded using 7-aminoactinomycin D (Molecular Probes).
Online Supplemental Material.
Three videos show three-dimensional reconstructions of the splenic conduit, visualized by the presence of 10 kD dextran 10 min after i.v. injection. The videos were created by combining x-y confocal images at successive z-planes. They are available at http://www.jem.org/cgi/content/full/jem.20021801/DC1.
Results
Intrasplenic Distribution of Blood-borne Tracer Molecules.
To study the permeability of the splenic white pulp for molecules present in blood, mice were injected i.v. with fluorescently labeled dextran of different molecular weights (10, 70, and 500 kD). The distribution of these tracers at 10 min after injection revealed that access to the white pulp was size dependent: 10 and 70 kD dextran were clearly present within this lymphoid compartment, whereas the 500-kD form could not be detected within the white pulp and remained in the marginal zone and the red pulp (Fig. 1 A). For all three sizes of dextran, the marginal zone was discernible as a clear rim surrounding the white pulp and contained large fluorescent cells, which were identified as ER-TR9+ marginal zone macrophages (MZM; not depicted).
Figure 1.
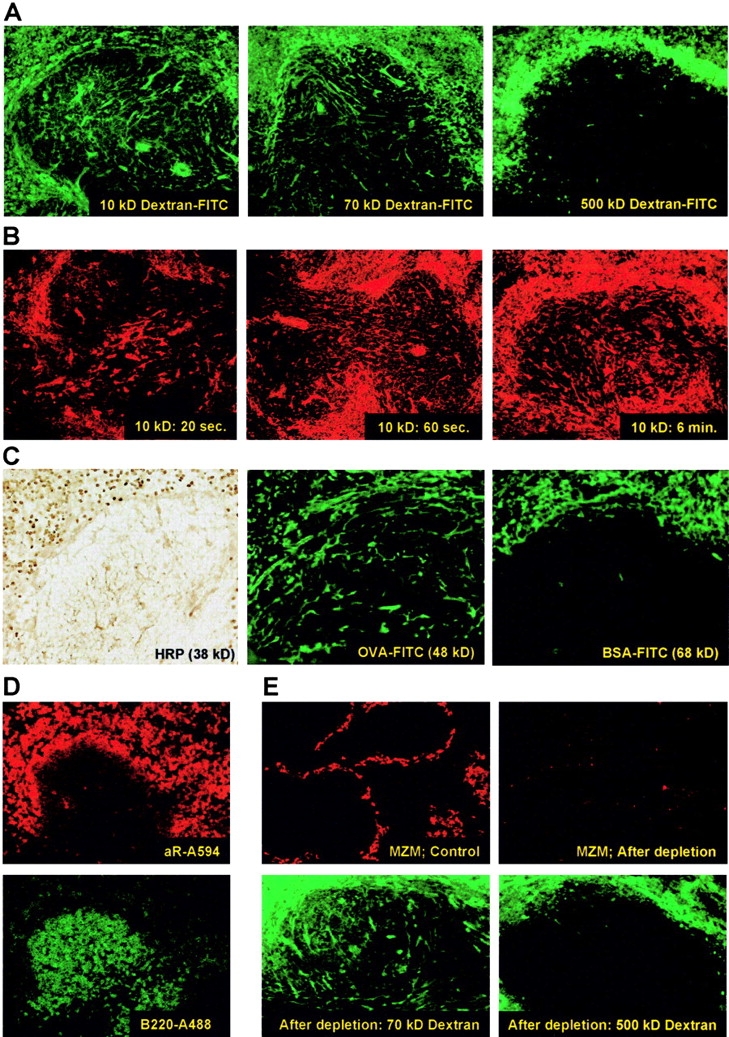
(A) Localization of 10, 70, and 500 kD FITC-labeled dextran 10 min after i.v. injection. (B) Presence of 10 kD TR-labeled dextran at 20 s, 60 s, or 6 min after injection. Exposure times were adjusted to obtain comparable fluorescence intensity. (C) Localization of protein tracers HRP, OVA-FITC, and BSA-FITC 10 min after i.v. injection. (D) Detection of i.v. injected, B cell–specific IgG mAb RA3-6B2 on B cells in the marginal zone and red pulp (using an anti rat–Ig conjugate; top), but not in the follicles (visualized by topical application of Alexa 488–labeled RA3-6B2; bottom). (E) i.v. injection with a single dose of clodronate-filled liposomes effectively depleted macrophages from the marginal zone within 2 d (stained for ER-TR9+ MZM; top), but did not affect the distribution of 70 and 500 kD dextran-FITC in the spleen (bottom). Original objective magnification for all panels is ×20, except the top panels of E, which are ×10.
Strikingly, both 10 and 70 kD dextrans were not homogeneously distributed throughout the parenchyma of the white pulp, but were only visible as long branches traversing through the lymphoid tissue (Fig. 1 A). Upon three-dimensional image reconstruction of this splenic network by confocal imaging through a 120-μm thick section, we found that these small single branches actually formed an entire network throughout the white pulp (Video 1, available at http://www.jem.org/cgi/content/full/jem.20021801/DC1). Based on the strong resemblance with the characteristics described for the conduit system in lymph nodes (5), we suggest that these fluorescent tracers expose the conduit system of the splenic white pulp. Isolation of spleens at several time points after injection of 10 kD dextran revealed that this conduit network was very rapidly filled. After 20 s the fluorescent tracer could already be seen deep in the white pulp and within 60 s the entire network was completely filled (Fig. 1 B).
Similar to the polysaccharide dextrans, we found that distribution of blood-borne proteins into the white pulp was size dependent and restricted to the conduit. Both HRP (38 kD) and OVA (48 kD) percolated into the conduit of the white pulp, whereas the larger BSA (68 kD) was only visible in the red pulp and in some blood vessels in the white pulp (Fig. 1 C).
Based on these findings it could be reasoned that large, physiologically relevant, serum-derived molecules, like immunoglobulins, would be unable to gain access to the white pulp compartment. To test this, mice were injected i.v. with mAb RA3-6B2 (150 kD), recognizing CD45R/B220 expressed on B cells. 10 min after injection the mAb could only be detected on B cells in the marginal zone and red pulp and was excluded from the white pulp (Fig. 1 D, top), whereas incubating the sections with directly labeled RA3-6B2 clearly showed the presence of B cells in the white pulp (Fig. 1 D, bottom). Besides, the injected mAb could not be detected in the conduit system. Furthermore, although MZM have high affinity for neutral polysaccharides (11), their elimination did not alter the distribution of fluorescently labeled dextran (Fig. 1 E). In conclusion, these experiments clearly demonstrate that movement of blood-borne molecules into the splenic white pulp is size dependent and restricted to a conduit system.
Tracers Present within the Reticular Fibers of the White Pulp.
To further analyze and define the conduit of the white pulp, transmission electron microscopy was performed on sections from spleens taken at 10 min after injection of HRP. We found that HRP was restricted to tubular structures in the white pulp that were enfolded by reticular cells (Fig. 2, A–C) . In certain regions where HRP had not yet fully saturated the spaces of the conduit, the collagen fibers that constitute this network could be clearly observed in both longitudinal (Fig. 2 D) and transverse sections (Fig. 2 E), as has been shown for the corresponding conduit system in lymph nodes (for review see references 3 and 4). The small, vascular-like spaces of the conduit and the presence of the collagen fibers could also be visualized in the absence of injected HRP (Fig. 2, F and G). Thus, the splenic white pulp contains a network of tubular stromal cells, a conduit system, that is accessible for small molecules from the blood.
Figure 2.
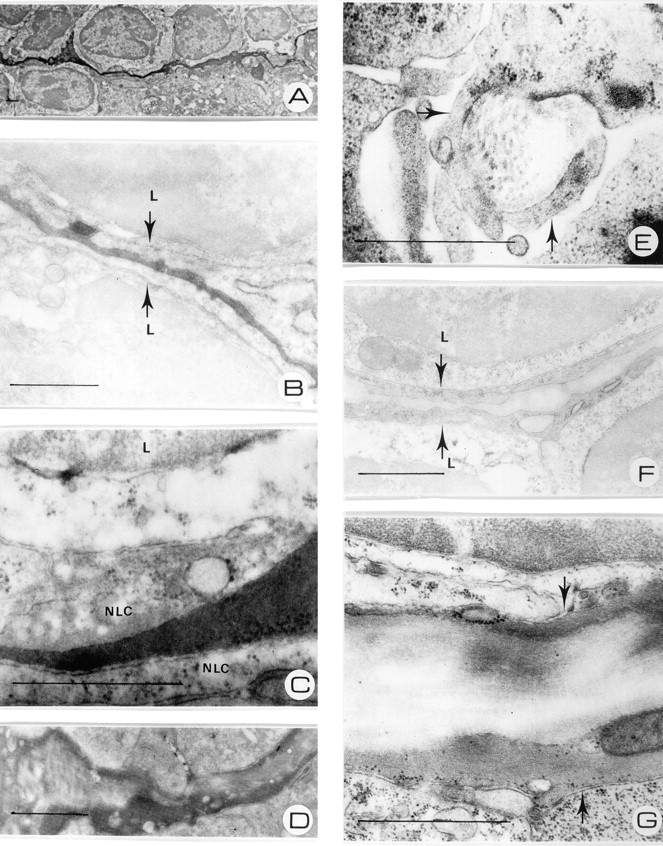
(A) 38 kD i.v. injected HRP is detected 10 min after i.v. injection in tubular structures in between lymphocytes. (B) Blood-borne HRP is located in the extracellular space enfolded by fibroblastic reticular cells (arrows), closely associated with the surrounding lymphocytes (L). (C) Detail of HRP-filled conduit, showing the lining by nonlymphoid cells (NLC). (D) Decreased density of HRP due to incomplete filling of the conduit reveals the presence of collagen fibers in the lumen. (E) Transverse section of the splenic conduit, enclosed by reticular fibroblasts (arrows), show collagen fibrils that constitute this network. (F) Control tissue, showing reticular cells (arrows) and the lumen of the conduit. (G) Detail of the conduit in control tissue, demonstrating the presence of collagen fibrils in the lumen. Bars, 1 μm.
The Conduit Can Be Identified by the Presence of ER-TR7 Antigen and Chemokines.
To further identify the cells that enfold the collagen fibers of the conduit, dextran-injected spleens were subjected to immunohistochemical stainings for several cell-specific antigens. Staining for the B cell–specific B220 antigen revealed that the conduit system was present in both B and T cell areas of the white pulp (Fig. 3 A). CD11c+ dendritic cells were not part of the dextran-filled conduit, but were located nearby and at times closely associated with these structures (Fig. 3 B). However, analysis for stromal cells revealed that the reticular fibroblast marker ER-TR7 (12) colocalized with the conduit in the T cell area (Fig. 3 C). The stromal cell marker gp38 (13) also showed considerable overlap with the conduit in the PALS (not depicted). Extensive analysis by confocal microscopy revealed that 10 kD dextran was actually ensheathed by the ER-TR7+ fibroblasts (Fig. 3 C see Video 2, available at http://www.jem.org/cgi/content/full/jem.20021801/DC1).
Figure 3.
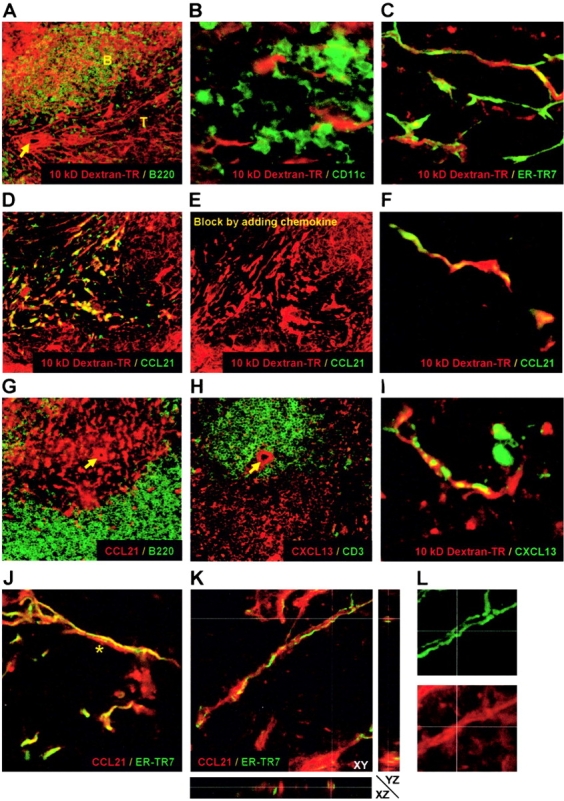
(A) The dextran-filled conduit (red) is present in both B (green) and T cell areas. (B) CD11c+ dendritic cells (green) do not overlap with the dextran-filled conduit (red). (C) ER-TR7+ reticular fibroblasts (green) in the PALS constitute the dextran-filled conduit network (red). (D) Colocalization of CCL21 (green) with dextran (red) in the PALS. (E) Inhibition of CCL21 staining by the addition of recombinant protein. (F) Close-up reveals strong overlap of dextran (red) and CCL21 (green). Restricted localization of CCL21 (G) and CXCL13 (H; red) to the conduits in the T and B cell areas, respectively. (I) CXCL13 (green) colocalizes in the follicles with i.v. injected dextran (red). (J) Colocalization of CCL21 (red) with the long ER-TR7+ fibroblasts (green) on one side of the conduit. Note the absence of ER-TR7 staining on the other side of the lumen (*). (K) X-Y-Z scan reveals that CCL21 (red) is fully enclosed by ER-TR7+ fibroblasts (green). (L) Close-up of K, revealing colocalization of ER-TR7 (green) and CCL21 (red). Arrows indicate the location of the central arteriole in the PALS. Original objective magnifications: A, D, E, G, and H, ×20; B, ×40; C, F, I, J, and K, ×63.
Because lymphoid chemokines are expressed by stromal cells in the white pulp (14), we also stained for the chemokine CCL21 (SLC) and found that expression of this chemokine overlapped to a great extent with the splenic conduit in the PALS (Fig. 3, D and E). Confocal analysis revealed that the injected dextran colocalized with CCL21 in certain parts, although we could also observe parts in which CCL21 or dextran were exclusively present (Fig. 3 F and see Video 3, available at http://www.jem.org/cgi/content/full/jem.20021801/DC1). Although CCL21 is restricted to the conduits of the T cell compartment (Fig. 3 G), we also examined the expression of the B cell chemokine CXCL13 (BLC). This chemokine, which is restricted to the follicles (Fig. 3 H), was also found to be present in association with the conduit network (Fig. 3 I). Thus, these data indicate that the splenic conduit system contains locally produced chemokines important for lymphocyte migration. Confocal analysis to further identify the precise localization of chemokines in the conduit network revealed that CCL21 is present both in the lumen of the conduit network (Fig. 3 K) as well as on the ER-TR7+ fibroblasts that enfold it (Fig. 3, J and L). However, ER-TR7 did not always fully enclose the conduit lumen because at times its expression could only be seen on one side (Fig. 3 J and see Video 2, available at http://www.jem.org/cgi/content/full/jem.20021801/DC1), indicative for either subcellular expression of the ER-TR7 antigen or presence of ER-TR7− fibroblasts.
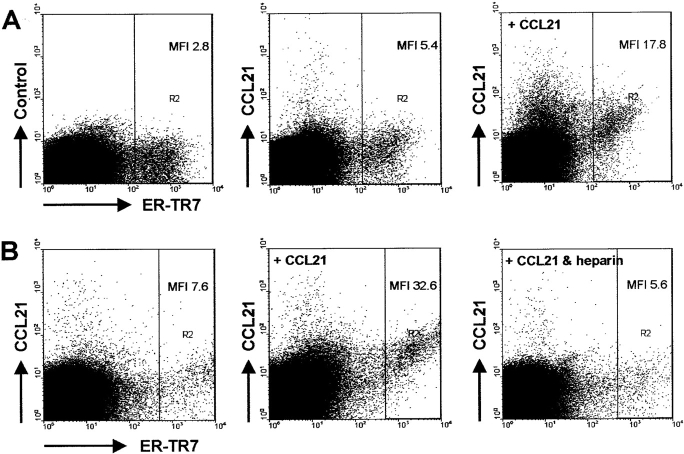
ER-TR7+ Fibroblasts Can Bind CCL21 In Vitro.
To further study the capability of ER-TR7+ stromal cells to express CCL21, we performed FACS® analysis on spleens that had been digested with collagenase. As shown in Fig. 4 A, ER-TR7+ fibroblasts could readily be detected and stained positive for CCL21. Interestingly, preincubation with recombinant chemokine strongly enhanced the staining of CCL21 on ER-TR7+ cells, but also on some ER-TR7− cells (Fig. 4 A). Because functional presentation of chemokines is generally mediated by glycosaminoglycans, we added heparin during the preincubation and found that this could prevent binding of CCL21 to the ER-TR7–expressing cells (Fig. 4 B). Thus, these data indicate that the ER-TR7+ cells that enfold the conduit are able to bind and potentially present the chemokine that is being transported through this network.
Figure 4.
(A) ER-TR7+ cells express the chemokine CCL21 directly ex vivo. Preincubation with 0.5 μg/ml CCL21 strongly enhanced the subsequent staining for this chemokine. (B) Binding of CCL21 to ER-TR7+ cells could be fully inhibited by preincubation of the chemokine with 50 μg/ml heparin. MFI, mean fluorescence intensity for CCL21 expression in gate R2.
Development of the Conduit System Does Not Require LTα Expression.
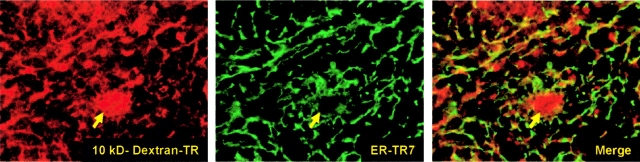
Since LTα is required for sufficient expression of lymphoid chemokines by stromal cells (14), as well as the expression of the stromal cell markers BP3 and gp38 (14, 15), we tested whether LTα is required for the development of the conduit network in the splenic white pulp. Upon i.v. injection of 10 kD dextran–Texas Red (TR) into LTα−/− mice, the fluorescent tracer displayed a network-like distribution within the splenic lymphoid compartment that was accompanied by normal ER-TR7 expression (Fig. 5) . Thus, the development of the splenic conduit network is independent of LTα.
Figure 5.
i.v. injected dextran-TR (red) into LTα−/− mice reveals a conduit network, characterized by ER-TR7+ reticular fibroblasts (green). The central arteriole is indicated with an arrow. Original objective magnification is ×20.
Discussion
Here we have described the presence of a conduit system in the splenic white pulp, consisting of a tubular, collagen-containing network ensheathed by reticular fibroblasts. Blood-borne molecules rapidly gain access to this network, which is dependent on the size of the molecule. However, the discrepancy in localization of apparently similar-sized dextran (70 kD) and BSA (68 kD) indicates that the three-dimensional configuration or electrostatic charge of these molecules, rather than their absolute molecular weight is decisive in this respect. Based on the localization of small dextran molecules early after injection (Fig. 1 B), as well as the three-dimensional reconstruction presented in Video 1 (available at http://www.jem.org/cgi/content/full/jem.20021801/DC1), it appears that the conduit in the white pulp is connected to the marginal zone, as well as to the central arteriole. From an electron microscopy study in rats it has been concluded that reticular cells in the white pulp are continuous with the connective tissue spaces of arterioles and that the components of this reticular network might therefore be the same as those in the arterioles (16). In addition to the rapid filling of the conduit, we therefore assume that small blood-borne molecules can freely move into the small lumen of the conduit, although we cannot formally exclude the involvement of vesicular transport, as suggested in lymph nodes (17).
Despite the strong resemblance of the splenic conduit system with the corresponding network in lymph nodes (for review see references 3 and 4), a marked difference is that the former is not connected to the lymphatic system, but is in contact with the blood. We also found a different molecular exclusion of the conduit in these organs because the splenic conduit was permeable for 70 kD dextran but not BSA, whereas its counterpart in lymph nodes was accessible for BSA, but not 70 kD dextran (Fig. 1; reference 5). Altogether, this suggests that the molecules transported through either network are of a different nature and that both conduits might serve a different function. It is conceivable that through the splenic conduit changes in levels of cytokines in the bloodstream may directly affect immune interactions in the lymphoid compartment of the spleen.
Another important feature of the splenic conduit system is that it contains locally produced chemokines, CCL21 in the T cell area and CXCL13 in the B cell area. These chemokines are present within the tubular system, as well as exposed on the reticular cells that enfold it (Fig. 3, J and K). Together with the ability of ER-TR7+ cells to bind CCL21 (Fig. 4), these results suggest that the splenic conduit system can transport and present locally produced chemokines through the white pulp, as it has been shown for the lymph node conduit (5, 6). This could thus provide a framework along which T and B cells can migrate to and within their respective areas of the white pulp, comparable with the presence of “corridors” in lymph nodes (for review see references 3 and 4).
Analysis of spleens from LTα−/− mice revealed that formation of the splenic conduit is independent of LTα-mediated signaling because the ER-TR7–expressing reticular cells show normal colocalization with the injected tracers. Therefore, the disturbed organization of B and T cells in these mice (18) must be attributed to a lack of the stromal cells that produce the lymphoid chemokines required for compartmentalization, as previously suggested (14), rather than an absent or malfunctioning reticular network that supports transport and presentation of these chemokines.
Taken together, our findings demonstrate that the splenic white pulp is a secluded area, in which the conduit system provides restricted exchange with the blood stream. As such, this network could provide a mechanism for rapid transport of blood-borne molecules deep into the white pulp, whereas the presence of lymphoid chemokines suggests it also influences lymphocyte migration within this compartment. These findings reveal novel insight in the physiology of the spleen and will be important for further investigations of splenic homeostasis.
Acknowledgments
We thank Tom Cupedo, Jaap van den Born, and Esther van Kesteren for valuable contribution.
The online version of this article contains supplemental material.
References
- 1.Nolte, M.A., E.N. ‘t Hoen, A. Van Stijn, G. Kraal, and R.E. Mebius. 2000. Isolation of the intact white pulp. Quantitative and qualitative analysis of the cellular composition of the splenic compartments. Eur. J. Immunol. 30:626–634. [DOI] [PubMed] [Google Scholar]
- 2.Sainte-Marie, G., and F.-S. Peng. 1986. Diffusion of a lymph-carried antigen in the fiber network of the lymph node of the rat. Cell Tissue Res. 245:481–486. [DOI] [PubMed] [Google Scholar]
- 3.Ebnet, K., E.P. Kaldjian, A.O. Anderson, and S. Shaw. 1996. Orchestrated information transfer underlying leukocyte endothelial interactions. Annu. Rev. Immunol. 14:155–177. [DOI] [PubMed] [Google Scholar]
- 4.Gretz, J.E., A.O. Anderson, and S. Shaw. 1997. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156:11–24. [DOI] [PubMed] [Google Scholar]
- 5.Gretz, J.E., C.C. Norbury, A.O. Anderson, A.E.I. Proudfoot, and S. Shaw. 2000. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192:1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baekkevold, E.S., T. Yamanaka, R.T. Palframan, H.S. Carlsen, F.P. Reinholt, U.H. von Andrian, P. Brandtzaeg, and G. Haraldsen. 2001. The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraal, G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132:31–73. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt, E.E., I.C. MacDonald, and A.C. Groom. 1993. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc. 7:613–628. [PubMed] [Google Scholar]
- 9.Sasou, S., T. Madarame, and R. Satodate. 1982. Views of the endothelial surface of the marginal sinus in rat spleens using the scanning electron microscope. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 40:117–120. [DOI] [PubMed] [Google Scholar]
- 10.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 174:83–93. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey, J.H., and D. Grennan. 1981. Different macrophage populations distinguished by means of fluorescent polysaccharides. Eur. J. Immunol. 11:221–228. [DOI] [PubMed] [Google Scholar]
- 12.Van Vliet, E., M. Melis, J.M. Foidart, and W. Van Ewijk. 1986. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem. 34:883–890. [DOI] [PubMed] [Google Scholar]
- 13.Farr, A.G., M.L. Berry, A. Kim, A.J. Nelson, M.P. Welch, and A. Aruffo. 1992. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med. 176:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngo, V.N., H. Korner, M.D. Gunn, K.N. Schmidt, D.S. Riminton, M.D. Cooper, J.L. Browning, J.D. Sedgwick, and J.G. Cyster. 1999. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngo, V.N., R.J. Cornall, and J.G. Cyster. 2001. Splenic T zone development is B cell dependent. J. Exp. Med. 194:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito, H., Y. Yokoi, S. Watanabe, J. Tajima, H. Kuroda, and T. Namihisa. 1988. Reticular meshwork of the spleen in rats studied by electron microscopy. Am. J. Anat. 181:235–252. [DOI] [PubMed] [Google Scholar]
- 17.Compton, C.C., and E. Raviola. 1985. Structure of the sinus-lining cells in the popliteal lymph node of the rabbit. Anat. Rec. 212:408–423. [DOI] [PubMed] [Google Scholar]
- 18.Fu, Y.X., and D.D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433. [DOI] [PubMed] [Google Scholar]