Abstract
To gain insight into the biological role of mast cell chymase we have generated a mouse strain with a targeted deletion in the gene for mast cell protease 4 (mMCP-4), the mouse chymase that has the closest relationship to the human chymase in terms of tissue localization and functional properties. The inactivation of mMCP-4 did not affect the storage of other mast cell proteases and did not affect the number of mast cells or the mast cell morphology. However, mMCP-4 inactivation resulted in complete loss of chymotryptic activity in the peritoneum and in ear tissue, indicating that mMCP-4 is the main source of stored chymotrypsin-like protease activity at these sites. The mMCP-4 null cells showed markedly impaired ability to perform inactivating cleavages of thrombin, indicating a role for mMCP-4 in regulating the extravascular coagulation system. Further, a role for mMCP-4 in connective tissue remodeling was suggested by the inability of mMCP-4 null peritoneal cells to process endogenous fibronectin.
Keywords: chymase, mast cell, fibronectin, thrombin, mouse mast cell protease 4
Introduction
Mast cells (MCs)* are mainly known for their harmful effects during allergic inflammation (1). However, recent investigations using MC-deficient mice have shown that MCs are essential for the manifestation of various other types of disease, e.g., experimental allergic encephalomyelitis (rodent analogue to multiple sclerosis; reference 2), antibody-induced rheumatoid arthritis (3), tumor angiogenesis (4), and experimental bullous pemphigoid (5). When MCs are activated, e.g., by cross-linking of IgE bound to their high affinity receptor FcɛRI by specific antigen, they respond by degranulation with the concomitant release of a panel of preformed inflammatory mediators such as histamine, cytokines, proteoglycans, and various neutral proteases. In addition, MC activation leads to de novo synthesis and release of several lipid-derived mediators, e.g., PGD2, LTC4, and platelet activating factor (for reviews, see references 6–8).
The neutral proteases fall into three classes: tryptases, chymases, and carboxypeptidase A (CPA; for reviews, see references 9–11). The chymases are chymotrypsin-like serine proteases, and are further divided, based on structural comparisons, into α-chymases and β-chymases. In humans only one chymase gene, belonging to the α-family, has been identified. In contrast, rodents have been shown to express a number of β-chymase genes, designated mouse MC protease 1 (mMCP-1), mMCP-2, and mMCP-4 as well as one α-chymase, mMCP-5. The different chymases differ significantly as regards tissue distribution. Connective tissue type MCs (CTMCs; located in various tissues such as skin, tongue, peritoneum) show predominant expression of mMCP-4 and –5 whereas the mucosal subtype of MCs (MMCs; located in the intestinal tract) instead express mMCP-1 and –2. In addition to mMCP-4 and –5, CTMCs express the tryptases mMCP-6 and –7 as well as CPA, all of which are lacking in MMCs. The CTMC chymases are stored in tight complexes with heparin proteoglycan in the secretory granules. In contrast, the MMC chymases are of lower affinity for the negatively charged glycosaminoglycans present in the secretory granule and may, accordingly, be constitutively secreted by the MCs (12).
The fact that the only human chymase gene known belongs to the α-family suggests that mMCP-5, the only α-chymase in mouse, may be the murine counterpart of human chymase. However, recent data have shown that mMCP-5, due to an amino acid difference in position 216 (Val instead of the Gly that is present in human chymase), does not have chymotrypsin-like activity but in fact has elastase-like specificity (13). Thus, it is likely that the functional counterpart to human chymase may be found in the rodent β-chymase family. Of the known mouse β-chymases, mMCP-4 has the most similar tissue distribution as human chymase, e.g., skin, and has also similar heparin-binding (14, 15) and angiotensin I-converting (16) properties. It may therefore be suggested that mMCP-4 is the functional homologue to human chymase. While the other CTMC-specific chymase, mMCP-5, as well as CPA and the tryptases mMCP-6, and –7 are expressed early during MC differentiation induced by IL-3, mMCP-4 is only expressed after inducing terminal MC differentiation with stem cell factor (17).
Previous investigations have indicated a role for chymase in various inflammatory conditions. Injection of human chymase into the skin of guinea pigs was shown to cause marked neutrophil influx and was also shown to cause increased vascular permeability (18, 19). More recently it has been shown that specific chymase inhibitors may ameliorate a variety of pathological conditions, including fibrosis in NC/Nga mice (20), eosinophil accumulation in mice infected with Nippostrongylus brasiliensis (21), allergen-induced skin reactions (22), and angiogenesis in hamster sponge granulomas (23). It is important to stress that although the reports described above provide evidence for an involvement of chymases in various pathological conditions, the exact mode of action for chymase has not been determined, i.e., the in vivo substrates for chymases have not been identified. Further, limited knowledge is available as regards the individual contribution of the various MC chymases. To gain further insight into the biological role of MC chymase we have here inactivated the gene for mMCP-4.
Materials and Methods
Reagents.
The chromogenic peptide substrates S-2586, S-2238 and S-2288 were from Chromogenix. The CPA substrate M-2245 (N-(4-Methoxyphenylazoformyl)-Phe-OH was from Bachem). Bovine α-thrombin was a gift from Ingemar Björk (Swedish University of Agricultural Sciences, Dept. of Veterinary Medical Chemistry, Uppsala, Sweden). The calcium ionophore A23187 was purchased from Calbiochem. Purified anti–mouse IgE (R35–72) was from BD Biosciences, donkey anti–rabbit Ig conjugated to horseradish peroxidase was purchased from Amersham Biosciences, and goat anti–rat Ig conjugated to horseradish peroxidase was purchased from Sigma-Aldrich. Antisera toward mMCP-4, -5, -6, and CPA were as described (24). The antisera to mMCP-4 and –5 were gifts from Lars Hellman (Uppsala University, Uppsala, Sweden). Antisera against mMCP-4, mMCP-6, CPA, and fibronectin were raised in rabbits whereas the antiserum toward mMCP-5 was of rat origin. The anti-fibronectin antiserum was a kind gift from Staffan Johansson (Uppsala University).
Knockout Techniques.
A ∼16 kb fragment of the mMCP-4 gene was cloned from a 129SVJ mouse genomic lambda FIX II library (Stratagene) using a full-length mMCP-4 cDNA as probe (a kind gift from Lars Hellman [Uppsala University, Sweden]). The 16 kb genomic fragment was released from the library vector with NotI enzyme and subcloned into the pZero vector (Invitrogen). The subcloning allowed further characterization of the structure of the mMCP-4 gene and identification of suitable restriction sites for construction of the target vector to be used for homologous recombination in ES cells. A BamH1 site was identified between exons 1 and 2, and this site together with the NotI site of the pZero/lamda vector was used for cloning of a ∼4.5 kb downstream arm of the mMCP-4 gene into the target vector (Fig. 1 B). A suitable ∼3 kb fragment with EcoRI and BglII sites was found upstream of the mMCP-4 gene, covering the promotor region (Fig. 1 B). The blunted EcoRI and BglII sites were used for cloning of the upstream arm into a blunted XhoI site of the target vector containing the neo cassette (indicated by solid lines connecting the upper and lower parts of Fig. 1 B). This strategy thus deletes exon 1 of the mMCP-4 gene. The targeting vector was electroporated into ES cells using 1.5 μg of DNA/106 ES cells. The ES cell work was performed using a standard protocol but without negative selection, i.e., omitting the selection against thymidine kinase expression. Cell cultures derived from the different ES cell clones obtained were divided into two portions. One of the portions was frozen (−80°C) whereas the remaining cells were cultured further and used for preparation of genomic DNA.
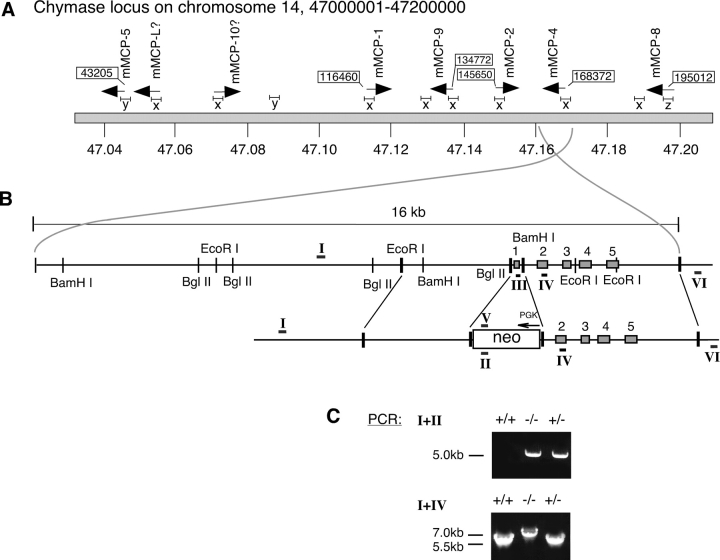
Figure 1.
(A) Genomic organization of the mouse MC chymase locus. The positions of the different chymase genes and their transcriptional orientations are indicated by arrows. mMCP-5 and mMCP-8, which are clearly different from the other chymases in terms of structure or tissue specificity (see Results), are positioned at the flanks of the locus. The more closely related chymases mMCP-1, -2, -4, and –9 are clustered in the middle of the locus. (B) Construct used for targeting of the mMCP-4 gene. Detailed organization of a 16 kb genomic fragment covering the mMCP-4 gene and 12 kb of upstream regions. EcoRI, BglII, and BamHI indicate restriction sites found. Depicted is also the final targeting construct used for homologous recombination. Neo corresponds to the neomycin resistant cassette used in the target vector, with promotor (PGK) and transcriptional orientation (arrow) indicated. Roman numerals indicate the positions of primers used in genomic PCR (primer I is upstream outside construct, II is inside Neo, III is in exon 1, IV is in exon 2, V is reverse complementary to II, VI is downstream outside construct). (C) PCR screening of targeted mice. Genomic DNA was prepared from WT (+/+), heterozygous (+/−) or homozygous (−/−) littermates and was subjected to PCR analysis to trace the mMCP-4 mutation. PCR products were separated on 1% agarose gels. The top panel shows a standard result using primers I+II, yielding the 5 kb product characteristic of the mutant mMCP-4 gene. The bottom panel shows the result obtained using primers I+IV, where the insertion of the mutant mMCP-4 gene results in a 7 kb PCR product.
A PCR based strategy was designed and used for the screening of possible positive clones: one oligonucleotide primer, specific for the neomycin cassette (primer II; see Fig. 1 B), was used together with a primer located outside the construct, upstream from the mMCP-4 gene (primer I; see Fig. 1 B). In clones where homologous recombination had occurred, these primers will give rise to a 5 kb fragment upon PCR amplification. Amplification was performed with shuttle PCR using TaKaRa enzyme with annealing and melting temperatures of 68°C and 98°C, respectively. Approximately 400 ES cell clones were screened and out of these we identified 6 ES cell clones where homologous recombination had occurred, i.e., a 5 kb PCR product was obtained using primers I and II. To verify that the homologous recombination was correct a second PCR was performed. We designed one primer (primer VI; see Fig. 1 B) for specific targeting downstream of the construct that, in combination with primer V (see Fig. 1 B), should yield a 7 kb PCR product. All six clones gave the expected size in the second PCR. Clone number 338 was chosen for injection into blastocysts. Injected blastocysts were implanted into pseudo-pregnant C57/CBA F1 mice, and a total of four chimeras were generated. One chimera gave germline transmission of the targeting construct (the other three chimeras were not further tested). Genotyping of F1 offspring (using tail DNA) by the described PCR approach (see above, using primer I and II) identified the 5 kb PCR product in the expected 50% ratio. Heterozygous animals were backcrossed to the C57BL/6J strain. F1 and F2 heterozygote animals were then crossed to obtain animals homozygous for the mutation. The mMCP-4 null genotype was found in the expected 25% ratio among the offspring. The mMCP-4−/− animals do not show any difference from their wild-type and heterozygote littermates when studied for phenotypic or behavioral signs using a simplified version of the SHIRPA protocol (25). Homozygous null animals were mated and were found to be fertile. For the purpose of this study, we used null mice obtained from crossings of heterozygote animals of either F1 or F2 generations. In all cases, +/+ and +/− littermates were used as controls.
Cell Culture.
Peritoneal cells from mMCP-4+/+ mice and corresponding mMCP-4+/− or −/− littermates (8–12 wk old) were collected by peritoneal washing with 10 ml of cold PBS, pH 7.4. Cells were centrifuged (300 g, 4°C, 10 min) and cultured in serum-free medium, HybridoMed DIF (Biochrom KG). The serum-free medium was supplemented with 50 μg/ml of gentamycin (GIBCO BRL). The cells were distributed in 24-well plates (Nunc; ∼0.5 × 106 cells in 0.4 ml/well). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. For activation of MCs, either the calcium ionophore A23187 (final concentration 2 μM), or anti–mouse IgE (2 μg) were added. To study the ability of the peritoneal cells to inactivate thrombin, 1 μg of thrombin (in 10 μl PBS) was added to the cell cultures. Samples from the conditioned media (50 μl) were taken at various time points and were frozen at –20°C. For analysis of residual thrombin activities, 20 μl of the samples were added to individual wells of 96-well microtiter plates, followed by the addition of 200 μl PBS and 20 μl of a 1.8 mM (in H2O) solution of the chromogenic thrombin substrate S-2238. The absorbance at 405 nm was monitored with a Titertek Multiscan spectrophotometer (Flow Laboratories) and initial reaction velocities were determined with the DeltaSoft3 software.
Analysis of Protease Activities in Tissues.
Ear extracts were obtained by the addition of 1 ml of PBS/2 M NaCl per ear, followed by homogenization using a PT1200 Polytron device (Kinematica AG). After homogenization, Triton X-100 was added to give a final concentration of 0.5%. Extracts were centrifuged (10,000 g), the supernatants were recovered and samples from the supernatants were used for activity measurements. Peritoneal cells (2 × 106 cells) were solubilized in 200 μl lysis buffer (PBS/2 M NaCl, 0.5% Triton X-100). 10 μl of the peritoneal cell or ear extracts were mixed with 90 μl H2O, followed by the addition of 20 μl of 1.8 mM solutions (in H2O) of chromogenic substrates for either chymotrypsin-like proteases (S-2586), trypsin-like proteases (S-2288), or CPA (M-2245). Changes in absorbance at 405 nM were measured as above.
Western Blot Analysis.
Samples of ear tissue extracts, solubilized peritoneal cells or cell culture media were mixed with 3× SDS-PAGE sample buffer containing 5% β-mercaptoethanol. Samples (40 μl) of these mixtures were subjected to SDS-PAGE on 12% gels. Proteins were subsequently blotted onto nitrocellulose membranes, followed by blocking with 5% milk powder in PBS (overnight, 20°C). Next, the membranes were incubated with antisera (1:250) in TBS/5% milk powder/0.1% Tween 20, at 20°C for 1 h. After washing the membranes extensively with TBS/0.1% Tween 20, the membranes were incubated with secondary antibody (anti–rat or anti–rabbit, respectively) conjugated to horseradish peroxidase (Amersham Biosciences; 1:3,000 dilution in TBS/0.1% Tween 20). After 45 min of incubation at 20°C, the membranes were washed extensively with TBS/0.1% Tween 20, followed by washing with TBS without detergent. The membranes were developed with the ECL system (Amersham Biosciences) according to the protocol provided by the manufacturer.
Results
Genomic Organization of MC Chymase Genes.
A genomic contig spanning the entire mouse chymase locus on chromosome 14 was extracted from the Sanger Institute's database, Ensembl Mouse Blast view (in April 2003). The organization of the chymase genes over a total of 160 kb of DNA starts with the mMCP-5 gene which is followed downstream by the mMCP-1 (at 75 kb; in opposite orientation), mMCP-9 (at 85 kb), mMCP-2 (at 105 kb; in opposite orientation), mMCP-4 (at 125 kb), and finally, at 155 kb, the mMCP-8 gene (Fig. 1 A). In addition we also identified the possible location of the mMCP-L (at 13 kb) and mMCP-10 (at 30 kb; in opposite orientation) genes. Interestingly, the transcriptional orientations of the CTMC chymases (mMCP-4 and mMCP-5) are opposite to those of the MMC protease(s) (mMCP-1 and mMCP-2). Possibly, the tissue-specific transcription of the CTMC and MMC chymase genes, respectively, may be related to their genomic orientation. The mMCP-9 gene, considered to be expressed in uterine MCs only (26), and the mMCP-8 gene, which has been suggested to be a basophilic cell marker (27), have the same transcriptional orientations as the CTMC chymases.
Previous work has identified highly conserved promotor regions in the mMCP-1, -2, -4, and –L genes (28). In addition, Kitamura and coworkers have shown in several studies that most chymase genes are regulated by the transcription factor MITF through specific E-boxes located 300 to 1,200 bases upstream of respective gene (29, and references therein). A comparison of the promotor region of mMCP-9 (26), bases +1 to –1,200, against the chymase locus extracted from the Sanger database reveals a number of homologous DNA segments throughout the entire locus (Fig. 1 A). The promotor regions in the mMCP-1, -2, -4, -L, and –10 genes all share homology with the promotor region of mMCP-9 (“x” in Fig. 1 A). Note also that “x” promotor regions are present at positions without any connection to a known chymase gene. In contrast, the promotor region of mMCP-5 (“y” in Fig. 1 A), bases −5 to –905, reveals only a partial homology with one segment of the chymase locus (located at 40 kb; see Fig. 1 A). The promotor region of mMCP-8 (“z” in Fig. 1 A), bases +1 to –825, does not show any homology with other parts of the chymase locus, in agreement with a previous study (30).
Construction of Targeting Vector, Electroporation, Production of Knockout Mice.
After successful screening of the 129SVJ genomic library and subcloning of the mMCP-4 gene fragment we cloned two arms (Fig. 1 B) into the pNeo plasmid and used the target construct for electroporation into ES cells (GSI-I). 400 clones were screened for the mutation using a PCR-based strategy. A total of six positive clones were identified and one of these (clone 338) was used for blastocyst injection. Implantation of injected blastocysts into pseudo-pregnant C57/CBA F1 females resulted in chimeric animals and subsequent breeding of these revealed germ line transmission of the targeting construct. The agouti F1 animals were genotyped by PCR using the primer pair I+II (see Fig. 1 B), which should give a 5 kb product. As expected, ∼50% of the animals were heterozygous for the mMCP-4 mutation. Heterozygous F1 animals were then crossed to obtain homozygous mMCP-4 null animals. The offspring from these crossings arrived at the expected Mendelian frequencies: 25% wt, 50% heterozygous, and 25% homozygous for the mMCP-4 mutation. To identify animals that were homozygous for the mMCP-4 mutation, the offspring was genotyped by PCR using the primer pair I+IV (see Fig. 1 B), that should yield a 7 kb product. Indeed, heterozygous animals gave two products of expected sizes, 5.5 kb for the wt- and 7 kb for the targeted allele. However, the 7 kb PCR product was barely detectable on gel (see Fig. 1 C) but was easily detected by southern hybridization using the neo-specific primers (II or V, Fig. 1 B) as probes (unpublished data). The homozygous null animals were identified by the presence of the 7 kb PCR product only (Fig. 1 C).
Effect of mMCP-4 Inactivation on MC Phenotype.
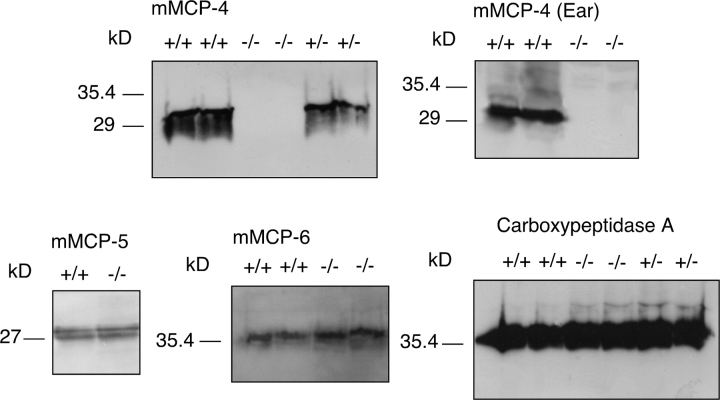
Two different MC-rich tissues, the peritoneum and ears, were used to study the consequence of the mMCP-4 inactivation on MC phenotype. Immunoblot analysis using a specific antibody to mMCP-4 (24) confirmed that the mutant mice lacked immunoreactive mMCP-4 protein, whereas mMCP-4 antigen was readily detected in wt cells both from peritoneum and ear tissue (Fig. 2) . Previous investigations have shown that the lack of one MC mediator may affect the storage of other MC mediators (31, 32). Therefore, experiments were performed to assess whether the lack of mMCP-4 influenced the presence of the other CTMC proteases, mMCP-5, -6, and CPA. However, immunoblot analysis revealed that the mMCP-4 inactivation did not lead to any marked effect on the concentration of any of these proteases in peritoneal cells (Fig. 2).
Figure 2.
Effect of mMCP-4 inactivation on the storage of CTMC proteases. Extracts were prepared from peritoneal cells and from ears of mMCP-4+/+, mMCP-4+/−, or mMCP-4−/− littermates. Samples of the extracts were subjected to Western blot analysis using antisera toward mMCP-4, -5, -6, or CPA as described in Materials and Methods.
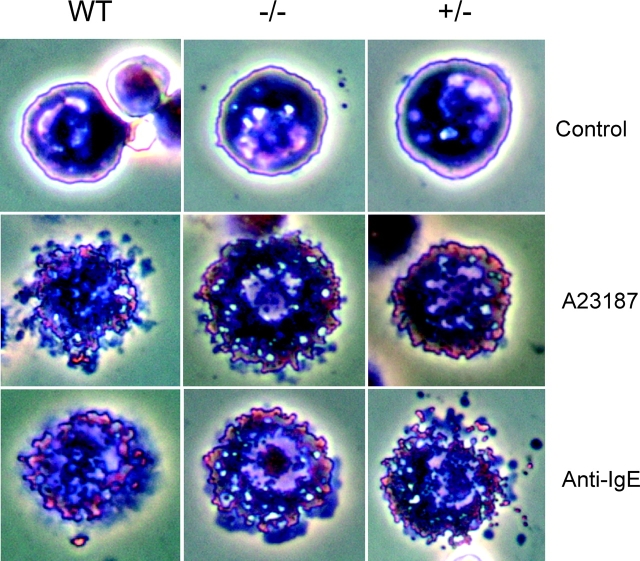
The inactivation of the mMCP-4 gene did not affect the number of MCs being present in the peritoneum, with ∼2–3% of the cells in both wt and mMCP-4−/− animals being identified as MCs by staining with May-Grünwald/Giemsa. Moreover, the mMCP-4 inactivation did not lead to any noticeable effects on MC size or apparent morphology (Fig. 3) . We also investigated if the mMCP-4−/− MCs responded normally to MC-degranulating agents. Results displayed in Fig. 3 show that treatment of both wt and mMCP-4−/− MCs with either the calcium ionophore A23187 or anti-IgE antibody resulted in MC degranulation.
Figure 3.
Effect of the mMCP-4 targeting on MC morphology and ability to degranulate. Peritoneal cells were recovered from mMCP-4+/+ mice and from −/− or +/− littermates. Cytospin slides were prepared from unstimulated cells or after the cells had been subjected to stimulation with either calcium ionophore A23187 (2 μM) or anti-IgE antibody (4 μg/ml) for 30 min. Slides were stained May-Grünwald/Giemsa.
Contribution of mMCP-4 to Total Amount of Stored Chymotrypsin-like Activities in Tissues.
Studies were performed to study the contribution of mMCP-4 to the total amount of stored chymotrypsin-like activities in the peritoneal cell population and in ear tissue. Further, the possibility that the lack of mMCP-4 could influence the levels of other protease activities was investigated. Results displayed in Tables I and II show that chymotrypsin-like activities were clearly detected in both peritoneal cells and in ear tissue extracts from mMCP-4+/+ animals. However, the targeting of mMCP-4 completely abolished the chymotrypsin-like activities in these tissues, demonstrating that mMCP-4 is the major source for chymotrypsin-like activity in peritoneal cells and in ear tissue. The inactivation of mMCP-4 did not lead to any marked effects on the levels of trypsin-like or CPA activities in peritoneal cells or ear tissue, indicating that mMCP-4 does not influence the activity of the other CTMC proteases.
Table I.
Protease Activities in Peritoneal Cells
| Source of cells | Chymotrypsin-like activity mOD/(min × 106 cells) |
Trypsin-like activity mOD/(min × 106 cells) |
CPA activity mOD/(min × 106 cells) |
|---|---|---|---|
| mMCP-4+/+ mice | 3.5 ± 2.9 | 2.0 ± 0.077 | −14.3 ± 5.0 |
| (n = 4) | (n = 4) | (n = 4) | |
| mMCP-4−/− mice | n.d. | 3.5 ± 2.0 | −14.7 ± 4.67 |
| (n = 4) | (n = 4) | (n = 4) | |
| mMCP-4+/− mice | 2.2 ± 1.58 | 1.9 ± 0.51 | −15.6 ± 3.07 |
| (n = 3) | (n = 4) | (n = 3) |
Extracts were prepared from peritoneal cells and were assayed for trypsin-like, chymotrypsin-like, and CPA activities as described in Materials and Methods. Note that hydrolysis of the CPA substrate results in decreased absorbance. n.d., none detected.
Table II.
Protease Activities in Ear Tissue Extracts
| Source of tissue | Chymotrypsin-like activity mOD/(min × mg tissue) |
Trypsin-like activity mOD/(min × mg tissue) |
CPA activity mOD/(min × mg tissue) |
|---|---|---|---|
| mMCP-4+/+ mice | 0.52 ± 0.044 | 2.3 ± 0.31 | −0.47 ± 0.11 |
| (n = 2) | (n = 2) | (n = 2) | |
| mMCP-4−/− mice | n.d. | 2.8 ± 0.87 | −0.44 ± 0.073 |
| (n = 2) | (n = 2) | (n = 2) | |
| mMCP-4+/− mice | 0.21 ± 0.015 | 3.7 ± 0.57 | −0.45 ± 0.064 |
| (n = 2) | (n = 2) | (n = 2) |
Extracts were prepared from ear tissue and were assayed for trypsin-like, chymotrypsin-like, and CPA activities as described in Materials and Methods. Note that hydrolysis of the CPA substrate results in decreased absorbance. n.d., none detected.
mMCP-4 Inactivation Results in Impaired Ability to Regulate Thrombin.
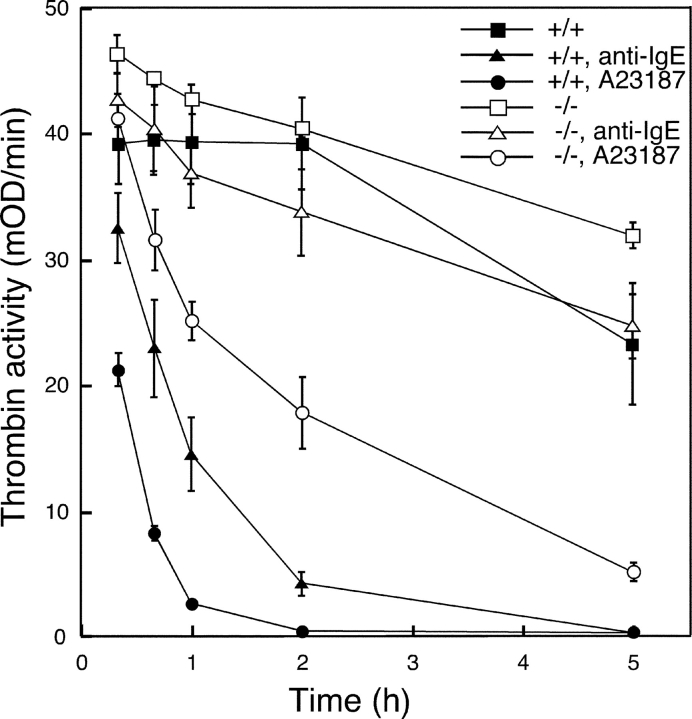
In an earlier study we showed that peritoneal cells have potent thrombin-inactivating activity, but that the thrombin-inactivating activity was severely compromised in cell cultures derived from mice lacking N-deacetylase/N-sulfotransferase 2 (NDST-2; 33 and 34). NDST-2 is an essential enzyme in the biosynthesis of MC heparin and the lack of this enzyme results in a global reduction of stored MC proteases (24, 34). Subsequent characterization of the thrombin-inactivating mechanism in wild-type cells showed that thrombin was degraded by MC serine protease activity, predominantly of chymotryptic type, although it was not possible to firmly establish which of the potential candidate proteases was the key factor in the process. Here we investigated the contribution of mMCP-4 in the thrombin-inactivating capability of peritoneal cell populations. The results displayed in Fig. 4 show that thrombin is rapidly inactivated in mMCP-4+/+ cell cultures after addition of A23187 or anti-IgE antibody to the cell cultures. In contrast, the inactivation of thrombin was markedly slower in mMCP-4−/− peritoneal cell cultures after addition of A23187 or anti-IgE, indicating that mMCP-4 is the key component of the thrombin-inactivating mechanism present in the peritoneal cell population.
Figure 4.
Targeting of mMCP-4 reduces the ability of peritoneal cells to inactivate thrombin. Peritoneal cells were prepared from mMCP-4+/+ or −/− littermates and were cultured in serum-free medium. Cells were incubated overnight and were either cultured further without any treatment or were treated with the calcium ionophore A23187 (2 μM) or with anti-IgE antibody (5 μg/ml). After 30 min, thrombin (1 μg) was added. Samples (50 μl) from the conditioned media were taken at the time points indicated and were analyzed for residual thrombin activity with the chromogenic substrate S-2238. Results are expressed as the mean of duplicate determinations ± range of variation within a single experiment. The results displayed are representative for 4 individual experiments on different mice.
mMCP-4 Inactivation Leads to Reduced Processing of Fibronectin.
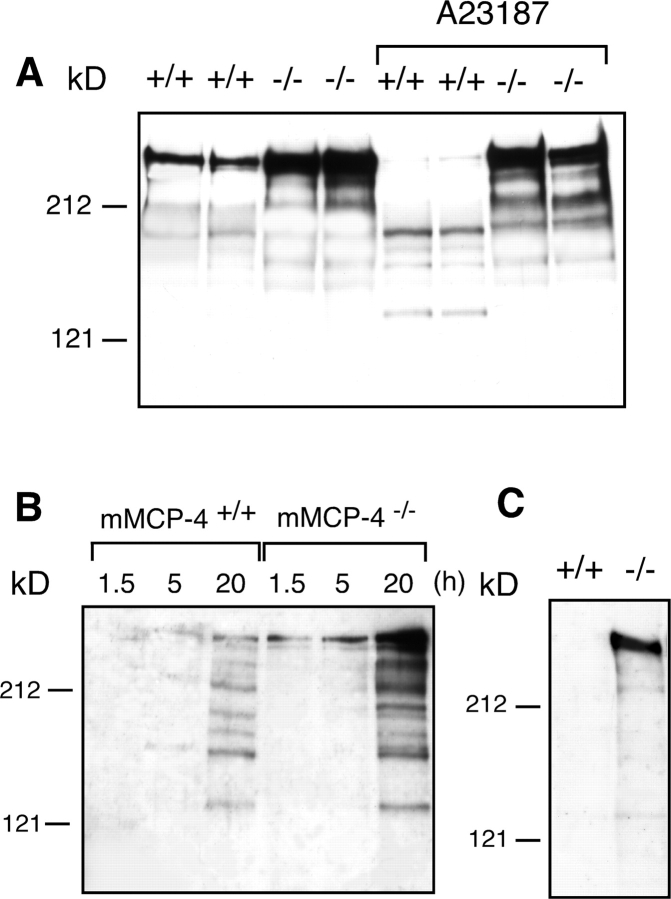
We have previously shown that NDST-2−/− peritoneal cells exhibit an impaired ability to process endogenously produced fibronectin, resulting in accumulation of intact fibronectin in the cell culture medium (35). WT cells, in contrast, continuously degrade the fibronectin and thus display markedly reduced levels of intact fibronectin in the culture medium. It was shown that the fibronectin, in nonstimulated wild-type cells, was degraded by MC chymotryptic activity that acted in concert with heparin proteoglycan (35). However, the exact identity of the fibronectin-degrading protease could not be revealed. To study the contribution of mMCP-4 in fibronectin turnover we cultured nonstimulated peritoneal cells from WT and mMCP-4 null animals and analyzed conditioned media from the cell cultures for the presence of immunoreactive fibronectin. From Fig. 5 A it is clear that intact fibronectin is present in conditioned medium from nonstimulated mMCP-4−/− cells and that the level of intact fibronectin is reduced in conditioned medium from mMCP-4+/+ peritoneal cells. Experiments were performed to investigate if stimulation of the MCs would result in degradation of the endogenous fibronectin. Indeed, addition of calcium ionophore A23187 to the peritoneal cell cultures derived from mMCP-4+/+ mice led to marked fibronectin degradation whereas very little apparent increase in fibronectin degradation was observed upon addition of A23187 to mMCP-4−/− cultures (Fig. 5 A). The results shown in Fig. 5 A were derived from relatively young (8 wk old) mice. When similar experiments were performed on peritoneal cells from older (12 wk) mice it was observed that the difference in the content of intact fibronectin in nonstimulated mMCP-4+/+ versus −/− cultures was much more pronounced (Fig. 5 B). This is in line with our observation that the level of surface-associated chymase activity increases with increasing age of the animals (unpublished data). Experiments were also performed to investigate if mMCP-4 influenced the levels of cell-associated fibronectin. Cell fractions from mMCP-4+/+ and −/− peritoneal cells were recovered and analyzed for the presence of fibronectin. Again, intact fibronectin was clearly detected in cell fractions from mMCP-4−/− cells, but was virtually undetectable in mMCP-4+/+ cells (Fig. 5 C).
Figure 5.
Effect of the mMCP-4 inactivation on fibronectin processing. (A) Peritoneal cells were obtained from mMCP-4+/+ mice and from mMCP-4−/− littermates (8 wk old). After overnight culture in serum-free medium, cells were either cultured further without treatment or treated with calcium ionophore A23187 (2 μM). Conditioned media were collected after a 5 h incubation period. (B) Peritoneal cells were prepared from mMCP-4+/+ or −/− littermates (12 wk old). Conditioned media were collected at the time points indicated. (C) Cell fractions were obtained from mMCP-4+/+ or −/− peritoneal cell cultures, by addition of SDS-PAGE sample buffer to cell cultures from which the conditioned media had been removed. (A–C) Conditioned media and cell fractions were subjected to Western blot analysis using an anti-fibronectin antiserum.
Discussion
Earlier studies in which MC granule components have been deleted have indicated that the granule content of MCs reflects a dynamic process in which one constituent may affect the storage of other components. Thus, the inactivation of the gene for NDST-2, an enzyme that is critical for the biosynthesis of MC heparin, led to drastically reduced levels of histamine and of all neutral proteases in CTMCs, as well as to a reduction in MC numbers and an altered MC morphology (24, 34). Further, the inactivation of the gene for histidine decarboxylase, the enzyme responsible for conversion of histidine to histamine, resulted in marked effects on gross MC morphology as a well as reductions in stored proteases (31). It has also been reported, so far only in abstract form, that inactivation of the gene for mMCP-5 is accompanied by reduced levels of stored CPA (32). In these cases, where the inactivation of one MC constituent thus leads to a concomitant reduction in other components, it is clear that conclusions concerning the specific function of each of the MC granule constituents are problematic, as any observed effects in a mouse strain lacking one MC granule constituent could be secondary and due to a difference in the level of other granule components. In contrast, we show here that the inactivation of the gene for mMCP-4 does not lead to any reduction in MC numbers, apparent changes in MC morphology, or to any detected effects on other MC granule constituents. Thus, the mMCP-4 null mouse strain is a relevant model for studies of the specific function of this chymase.
In earlier reports we demonstrated a reduced ability of peritoneal cells from NDST-2 null animals to process thrombin (33) and endogenous fibronectin (35) and characterization of the processing mechanisms in WT cells indicated that MC chymase in complex with heparin proteoglycan was mainly responsible. However, the NDST-2 inactivation leads to a global reduction in all MC granule components as well as to a marked reduction in MC numbers, making it difficult to firmly attribute any effects specifically to one MC product. Further, since the NDST-2 inactivation is followed by reductions in the storage (mRNA levels are not affected) of both of the CTMC specific chymases, mMCP-4 and –5, it was not possible to determine which of the two candidate chymases was mainly responsible for the fibronectin and thrombin processing. There was also the possibility that other chymotrypsin-like enzymes (e.g., cathepsin G) may have contributed. Here we show that thrombin and fibronectin degradation in the peritoneal cell population is drastically reduced in mMCP-4−/− peritoneal cells, demonstrating that mMCP-4 is the key factor in the observed processing mechanisms. Interestingly, several previous reports support the notion that fibronectin indeed is an important substrate for MC chymase. In an early study it was demonstrated that human chymase rapidly degrades fibronectin in a purified system (36) and more recently it was shown that human chymase has the ability to release fibronectin degradation products from connective tissue produced by human airway smooth muscle cells (37). Moreover, it has been suggested that the fibronectin degradation products formed by the action of chymase may be apoptotic (38). These results together with the present findings indicate that the fibronectin present in connective tissues may be one of the main physiological targets for MC chymase. Hence, we may speculate that MCs have an important role in connective tissue remodeling processes that occur in inflammatory reactions, by providing proteases that degrade the individual connective tissue components. According to the present data, chymase could thus act directly on fibronectin. On a different angle, it has been shown that chymase can promote connective tissue degradation indirectly by processing the latent form of matrix metalloproteases 9 (proMMP-9) to its active counterpart (39). Further, it has been shown that MC tryptase can contribute to connective tissue destruction indirectly by activating proMMP-3 (40) and, directly, by degrading collagen type VI (41).
The inactivation of mMCP-4 resulted in essentially complete abolishment of chymotryptic activity recovered in the peritoneal cell population as well as in ear tissue. This establishes firmly that mMCP-4 constitutes the main chymotrypsin-like enzyme at these sites, and rules out that other chymases or chymotrypsin-like enzymes, e.g., mMCP-5 or cathepsin G, significantly contribute to this type of activity in these tissues. The implication of this finding is that during MC activation, mMCP-4 represents by far the dominating chymotrypsin-like activity that is released into the surrounding tissue. Thus, it is likely that mMCP-4 will contribute significantly to any physiological process performed by chymotrypsin-like proteases in tissues that contain CTMCs.
The exact biological function(s) of the MC chymases is an intriguing issue. As noted in the Introduction, many lines if evidence suggest a harmful role of chymase in various inflammatory conditions. However, it is likely that MC chymases also carry beneficial functions. Indeed, it has been shown that inactivation of the MMC-specific chymase, mMCP-1, results in an impaired ability to combat infection by the nematode Trichinella spiralis (42). Further, MCs have been shown to be involved in defense against bacterial infection (43, 44), although the specific contribution of MC proteases have not been addressed so far. We believe that the mMCP-4 null mouse strain will provide a model in which the specific function of this chymase in a number of pathological conditions may be addressed. Further, as mMCP-4 may be the functional counterpart of the human chymase, we believe that the findings presented here are relevant in the context of the biological function of human chymase.
Acknowledgments
We thank the transgenic facility at Uppsala University for technical assistance throughout this work.
This work was supported by grants from Polysackaridforskning AB, AgriFunGen, the Swedish Medical Research Council, Magnus Bergvalls stiftelse, Vårdalstiftelsen, and from King Gustaf V's 80th Anniversary Fund.
Footnotes
Abbreviations used in this paper: CPA, carboxypeptidase A; CTMC, connective tissue type mast cell; MC, mast cell; MMC, mucosal mast cell; mMCP, mouse mast cell protease; NDST, N-deacetylase/N-sulfotransferase.
References
- 1.Galli, S.J. 1997. Complexity and redundancy in the pathogenesis of asthma: reassessing the roles of mast cells and T cells. J. Exp. Med. 186:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Secor, V.H., W.E. Secor, C.A. Gutekunst, and M.A. Brown. 2000. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 191:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, D.M., D.S. Friend, M.F. Gurish, C. Benoist, D. Mathis, and M.B. Brenner. 2002. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 297:1689–1692. [DOI] [PubMed] [Google Scholar]
- 4.Coussens, L.M., W.W. Raymond, G. Bergers, M. Laig-Webster, O. Behrendtsen, Z. Werb, G.H. Caughey, and D. Hanahan. 1999. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13:1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, R., G. Ning, M.L. Zhao, M.G. Fleming, L.A. Diaz, Z. Werb, and Z. Liu. 2001. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J. Clin. Invest. 108:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metcalfe, D.D., D. Baram, and Y.A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033–1079. [DOI] [PubMed] [Google Scholar]
- 7.Wedemeyer, J., M. Tsai, and S.J. Galli. 2000. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 12:624–631. [DOI] [PubMed] [Google Scholar]
- 8.Stevens, R.L., and K.F. Austen. 1989. Recent advances in the cellular and molecular biology of mast cells. Immunol. Today. 10:381–386. [DOI] [PubMed] [Google Scholar]
- 9.Miller, H.R., and A.D. Pemberton. 2002. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 105:375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caughey, G. 2002. New developments in the genetics and activation of mast cell proteases. Mol. Immunol. 38:1353–1357. [DOI] [PubMed] [Google Scholar]
- 11.Huang, C., A. Sali, and R.L. Stevens. 1998. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 18:169–183. [DOI] [PubMed] [Google Scholar]
- 12.Brown, J.K., P.A. Knight, S.H. Wright, E.M. Thornton, and H.R. Miller. 2003. Constitutive secretion of the granule chymase mouse mast cell protease-1 and the chemokine, CCL2, by mucosal mast cell homologues. Clin. Exp. Allergy. 33:132–146. [DOI] [PubMed] [Google Scholar]
- 13.Kunori, Y., M. Koizumi, T. Masegi, H. Kasai, H. Kawabata, Y. Yamazaki, and A. Fukamizu. 2002. Rodent alpha-chymases are elastase-like proteases. Eur. J. Biochem. 269:5921–5930. [DOI] [PubMed] [Google Scholar]
- 14.Sayama, S., R.V. Iozzo, G.S. Lazarus, and N.M. Schechter. 1987. Human skin chymotrypsin-like proteinase chymase. Subcellular localization to mast cell granules and interaction with heparin and other glycosaminoglycans. J. Biol. Chem. 262:6808–6815. [PubMed] [Google Scholar]
- 15.Pejler, G., and M. Maccarana. 1994. Interaction of heparin with rat mast cell protease 1. J. Biol. Chem. 269:14451–14456. [PubMed] [Google Scholar]
- 16.Caughey, G.H., W.W. Raymond, and P.J. Wolters. 2000. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim. Biophys. Acta. 1480:245–257. [DOI] [PubMed] [Google Scholar]
- 17.Gurish, M.F., N. Ghildyal, H.P. McNeil, K.F. Austen, S. Gillis, and R.L. Stevens. 1992. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J. Exp. Med. 175:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, S., and A.F. Walls. 1998. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br. J. Pharmacol. 125:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, S., and A.F. Walls. 1998. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur. J. Pharmacol. 352:91–98. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe, N., Y. Tomimori, K. Saito, K. Miura, A. Wada, M. Tsudzuki, and Y. Fukuda. 2002. Chymase inhibitor improves dermatitis in NC/Nga mice. Int. Arch. Allergy Immunol. 128:229–234. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe, N., K. Miura, and Y. Fukuda. 2002. Chymase inhibitor ameliorates eosinophilia in mice infected with Nippostrongylus brasiliensis. Int. Arch. Allergy Immunol. 128:235–239. [DOI] [PubMed] [Google Scholar]
- 22.Tomimori, Y., N. Tsuruoka, H. Fukami, K. Saito, C. Horikawa, M. Saito, T. Muto, N. Sugiura, K. Yamashiro, M. Sumida, et al. 2002. Role of mast cell chymase in allergen-induced biphasic skin reaction. Biochem. Pharmacol. 64:1187–1193. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu, M., M. Yamada, S. Takai, and M. Miyazaki. 2002. Suppression of basic fibroblast growth factor-induced angiogenesis by a specific chymase inhibitor, BCEAB, through the chymase-angiotensin-dependent pathway in hamster sponge granulomas. Br. J. Pharmacol. 137:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsberg, E., G. Pejler, M. Ringvall, C. Lunderius, B. Tomasini-Johansson, M. Kusche-Gullberg, I. Eriksson, J. Ledin, L. Hellman, and L. Kjellén. 1999. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 400:773–776. [DOI] [PubMed] [Google Scholar]
- 25.Rogers, D.C., E.M. Fisher, S.D. Brown, J. Peters, A.J. Hunter, and J.E. Martin. 1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome. 8:711–713. [DOI] [PubMed] [Google Scholar]
- 26.Hunt, J.E., D.S. Friend, M.F. Gurish, E. Feyfant, A. Sali, C. Huang, N. Ghildyal, S. Stechschulte, K.F. Austen, and R.L. Stevens. 1997. Mouse mast cell protease 9, a novel member of the chromosome 14 family of serine proteases that is selectively expressed in uterine mast cells. J. Biol. Chem. 272:29158–29166. [DOI] [PubMed] [Google Scholar]
- 27.Poorafshar, M., H. Helmby, M. Troye-Blomberg, and L. Hellman. 2000. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur. J. Immunol. 30:2660–2668. [DOI] [PubMed] [Google Scholar]
- 28.Huang, R., and L. Hellman. 1994. Genes for mast-cell serine protease and their molecular evolution. Immunogenetics. 40:397–414. [DOI] [PubMed] [Google Scholar]
- 29.Ge, Y., T. Jippo, Y.M. Lee, S. Adachi, and Y. Kitamura. 2001. Independent influence of strain difference and mi transcription factor on the expression of mouse mast cell chymases. Am. J. Pathol. 158:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunderius, C., and L. Hellman. 2001. Characterization of the gene encoding mouse mast cell protease 8 (mMCP-8), and a comparative analysis of hematopoietic serine protease genes. Immunogenetics. 53:225–232. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsu, H., S. Tanaka, T. Terui, Y. Hori, Y. Makabe-Kobayashi, G. Pejler, E. Tchougounova, L. Hellman, M. Gertsenstein, N. Hirasawa, et al. 2001. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 502:53–56. [DOI] [PubMed] [Google Scholar]
- 32.Stevens, R.L., D. Qui, H.P. McNeil, D.S. Friend, J.E. Hunt, K.F. Austen, and J. Zhang. 1996. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene can not store carboxypeptidase A (mMC-CPA) protein in their granules. FASEB J. 10:17772. [Google Scholar]
- 33.Tchougounova, E., and G. Pejler. 2001. Regulation of extravascular fibrinolysis and coagulation by heparin-dependent mast cell chymase. FASEB J. 15:2763–2765. [DOI] [PubMed] [Google Scholar]
- 34.Humphries, D.E., G.W. Wong, D.S. Friend, M.F. Gurish, W.T. Qiu, C. Huang, A.H. Sharpe, and R.L. Stevens. 1999. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 400:769–772. [DOI] [PubMed] [Google Scholar]
- 35.Tchougounova, E., E. Forsberg, G. Angelborg, L. Kjellén, and G. Pejler. 2001. Altered processing of fibronectin in mice lacking heparin. A role for heparin-dependent mast cell chymase in fibronectin degradation. J. Biol. Chem. 276:3772–3777. [DOI] [PubMed] [Google Scholar]
- 36.Vartio, T., H. Seppa, and A. Vaheri. 1981. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J. Biol. Chem. 256:471–477. [PubMed] [Google Scholar]
- 37.Lazaar, A.L., M.I. Plotnick, U. Kucich, I. Crichton, S. Lotfi, S.K. Das, S. Kane, J. Rosenbloom, R.A. Panettieri, Jr., N.M. Schechter, and E. Pure. 2002. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J. Immunol. 169:1014–1020. [DOI] [PubMed] [Google Scholar]
- 38.Leskinen, M.J., K.A. Lindstedt, Y. Wang, and P.T. Kovanen. 2003. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler. Thromb. Vasc. Biol. 23:238–243. [DOI] [PubMed] [Google Scholar]
- 39.Fang, K.C., W.W. Raymond, S.C. Lazarus, and G.H. Caughey. 1996. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J. Clin. Invest. 97:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber, B.L., M.J. Marchese, K. Suzuki, L.B. Schwartz, Y. Okada, H. Nagase, and N.S. Ramamurthy. 1989. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Invest. 84:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kielty, C.M., M. Lees, C.A. Shuttleworth, and D. Woolley. 1993. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem. Biophys. Res. Commun. 191:1230–1236. [DOI] [PubMed] [Google Scholar]
- 42.Knight, P.A., S.H. Wright, C.E. Lawrence, Y.Y. Paterson, and H.R. Miller. 2000. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192:1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Echtenacher, B., D.N. Mannel, and L. Hultner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 381:75–77. [DOI] [PubMed] [Google Scholar]
- 44.Malaviya, R., T. Ikeda, E. Ross, and S.N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 381:77–80. [DOI] [PubMed] [Google Scholar]