Abstract
Granulocyte/macrophage colony-stimulating factor (GM-CSF)–induced monocyte-derived macrophages (GM-MΦ) are permissive to M-tropic HIV-1 entry, but inhibit viral replication at posttranscriptional and translational levels, whereas M-CSF-induced macrophages (M-MΦ) produce a large amount of HIV-1. M-MΦ express a high level of Hck and a large isoform of C/EBPβ, and HIV-1 infection increases the expression of Hck but not of C/EBPβ. GM-MΦ express a high level of C/EBPβ and a low level of Hck, and HIV-1 infection drastically increases the expression of a short isoform of C/EBPβ but decreases that of Hck.
Treatment of M-MΦ with antisense oligonucleotide for Hck (AS-Hck) not only suppresses the expression of Hck, but also stimulates the induction of the short isoform of C/EBPβ and inhibits the viral replication. Treatment of GM-MΦ with a moderate amount of AS-C/EBPβ not only inhibits the expression of the small isoform of C/EBPβ preferentially, but also stimulates the induction of Hck and stimulates the virus production at a high rate. These results suggest that CSF-induced and HIV-1–mediated distinct regulation of Hck and small isoform of C/EBPβ represent the heterogeneous susceptibility of tissue MΦ to HIV-1 infection, and the regulation of Hck and C/EBPβ are closely related and these two molecules affect one another.
Keywords: macrophages, HIV-1, Hck, C/EBPβ, CSF
Introduction
Monocytes/macrophages (Mo/MΦ)* are a major target of HIV type I (HIV-1) infection and serve as a reservoir for viral persistence in vivo (1, 2). Most tissue MΦ are permissive to macrophage (M)-tropic virus entry and release a small amount of virus particles in the asymptomatic carrier (3, 4), but they occasionally produce a large amount of viral particles in the AIDS patients or HIV-1 patients complicated with opportunistic infection (5, 6). Recent studies suggest that anti–HIV-1 therapy (highly active antiretroviral therapy) strongly inhibits HIV-1 replication at levels of RT activity and viral DNA replication in aggressively divided T cells, and reduces viral antigenemia (7, 8). HIV-1–infected Mo/MΦ in some lymphoreticular tissue, however, cannot be removed by this therapy, and the residual cells can generate and spread virus particles in the human body (9, 10). In contrast to the advanced and profound studies of the T-tropic HIV-1 replication system on both the viral and host sides, the precise mechanism of M-tropic HIV-1 replication in Mo/MΦ are not fully understood, but is a key factor in the control of HIV-1 suffering.
A nonreceptor type of Src-like tyrosine kinase, human hematopoietic cell kinase (Hck), primarily expressed in neutrophils and MΦ, induces other cellular kinase activation (11, 12), and can bind to HIV-1 Nef at its SH3 domain to change it active form (13, 14). Nef is known to promote high HIV-1 and simian immunodeficiency virus (SIV) replication in vitro and progress AIDS-like disease in monkey (15, 16, 17). In addition, transfection of Hck to human 293 T cells partially helps HIV-1 entry and transactivation of HIV-1 LTR promoter region (18).
In contrast to Hck, CCAAT-enhancer binding protein (C/EBPβ) is widely expressed, and has large and small isoform generated by alternative initiation of protein translation from a single transcript (19, 20). A mutant HIV-1 that lacks C/EBPβ binding sites of the HIV-1LTR promoter region cannot replicate in macrophage-lineage U937 cells (21), and cotransfection of the HIV-1-LTR construct and C/EBPβ expression plasmids strongly activates the HIV-1 promoter in NTera-2 cells (22). In contrast, induction of a small isoform of C/EBPβ by mycobacterium infection and IFN-β strongly inhibits HIV-1 replication in macrophage-lineage THP-1 cells and alveolar MΦ (A-MΦ; references 23 and 24). These findings suggest that both types of C/EBPβ isoforms have exactly opposite effects for HIV-1 replication; large isoform stimulates but small isoform inhibits the HIV-1 replication.
In the present study, we show that human Mo-derived MΦ induced by macrophage CSF (M-CSF) and by GM-CSF (M-MΦ and GM-MΦ, respectively) are distinct in their susceptibility to M-tropic HIV-1 infection via different basal- and HIV-1–mediated expression of Hck and small isoform of C/EBPβ.
Materials and Methods
Preparation and Culture of MΦ.
Mo were obtained from PBMCs of normal healthy volunteers using a magnetic cell separation system (MACS; Miltenyi Biotec) with anti-CD14 mAb coated microbeads as described previously (25).
CD14+ Mo were cultured in RPMI 1640 medium (Nissui Seiyaku Co., Ltd.), 3 mg/ml of a filtered glutamine (Sigma-Aldrich), 100 U/ml penicillin G potassium (Banyu Seiyaku Co., Ltd.), 100 μg/ml streptomycin (Meiji Seika Co., Ltd.), 10% of autoclaved NaHCO3, 10% heat-inactivated FCS (Z.L. Bockneck Laboratories Inc.) with the following human recombinant cytokines at optimal concentrations: 5 ng/ml GM-CSF (Schering-Plough Japan) or 50 ng/ml M-CSF (Morinaga Milk Industry Co., Ltd.) at 37°C in humidified 5% CO2 for 7 d. During the culture, Mo differentiated to MΦ (26).
Preparation and Activation of CD4+T cells.
CD4+T cells were positively isolated from CD14− PBMCs by using a MACS with anti-CD4 mAb coated microbeads. The selected cell population was >93% positive for CD3 and CD4. CD4+ T cells were stimulated for 5 d with PHA (10 μg/ml) and IL-2 (30 U/ml) (Genzyme).
HIV-1 Strains and Infection.
HIV-1BaL and HIV-1JR-FL was collected from culture supernatant of these viral HIV-1 strains-infected M-MΦ as a viral resource. MΦs were incubated for 2 h at 37°C with 100 pg/ml p24 antigen of DNase-treated viral supernatant (p24, the 50% tissue culture infective dose (TCID50) and multiplicity of infection (MOI) are 50 ng/ml, ∼3,000 and 0.05, respectively) and then cultured in RPMI 1640 containing 10% FCS and CSF. If necessary, the viral innocula was pretreated with 100 μM azidothymidine (AZT) for 2 h at 4°C. Fresh culture medium containing CSF was added every 3–4 d (20% of the volume; references 23 and 27). Heat-inactivated virus (1 h, 56°C) was used as negative control. The kinetics of viral production were followed by sequential measurement of p24 antigen in supernatants by an ELISA using a combination of two antibodies: anti-gag-p24 monoclonal antibody (Nu24) and peroxidase-labeled 10B5 (28). For detection of the intracellular p24 distribution, cells were fixed with 100% ethanol and stained with Nu24 using a commercial kit (Histofine: SAB-PO(M) kit; Nichirei Co., Ltd. Tokyo).
Detection of HIV-1 DNA by Nested PCR and Semi-Dilution PCR.
Cell lysates were prepared in 100 μl of lysis buffer containing 10 mM Tris-HCl (pH 8.3), 0.5% Tween 20, 0.5% Nonidet P-40, 0.5 mM EDTA, and 100 mg of proteinase K per ml, and then incubated at 55°C for 1 h. Crude DNA was isolated by nucleic acid extraction kit (IsoQuick; Microprobe Co., Ltd.) and 75% ethanol precipitation, and then dissolved in TE (pH 8.3). DNA samples (200 ng) were added to 0.5 μM each primer and 0.2 μM each dNTP, 1.5 mM MgCl2, and 1 U Taq DNA polymerase (Takara Biomedicals) in 50 μl final volume. After 3 min at 95°C, 30 cycles were performed in an automated DNA Thermal Cycler (PerkinElmer), consisting of 30 s at 95°C, annealing at 55°C, and extension at 72°C for 1 min. HIV LTR and gag primers were JAM 62 (5′-GCTTCAAGTAGTGTGTGCCCGTCTG-3′) and JAM65 (5′-AATCGTTCTAGCTCCCTGCTTGCCC-3′). For the nested PCR, 10 μl of amplified products were submitted to another 30-cycle amplifications under the same conditions using internal primers JAM 63 (5′-GTGTGACTCTGGTAACTAGAGATCC-3′) and JAM 64 (5′-CCGCTTAATACTGACGCTCTCGCAC-3′) (28). The amplification products were analyzed by electrophoresis on 2% agarose gel stained with ethidium bromide for UV visualization (expected size of HIV is DNA 245 bp).
Serially diluted DNA samples (diluted with DNA from uninfected MΦ) were added to the PCR mixture containing 5′-LTR primers, LTR5 (5′-GGCTAACTAGGGAACCCACTGCTT-3′) and LTR6 (5′-CTGCTAGAGATTTTCCACACTGAC-3′), and amplified as described above. PCR products were subjected with 2% agarose gel and capillary transferred to a nylon membrane (Pall BioSupport) with mild alkali digestion. The membrane was prehybridized, and hybridized with [γ-32P] ATP labeled JAM 62 in 20× SSC, 100× Denhart solution, 20% SDS, 5% sodium pyrophosphate, and 5 mg/ml yeast RNA for 1.5 h and overnight, respectively. The blots were washed 4 times with 2× SSC and 1% SDS, and then analyzed using a Fuji BAS 2000 bioimage analyzer (Fuji Photo Film Co., Ltd.; expected size of HIV DNA 190 bp). The threshold limitation of the PCR product was determined with serial dilutions of 8E5/LAV cells (1 copy/cell), and was 1 copy/104 cells.
Detection of C/EBPβ mRNA by RT-PCR.
Total RNA was isolated using RNA-Bee™ isolation of RNA (TEL-TEST, Inc.), and reverse-transcribed by MMLV (USB) and random primer (Takara Biomedicals). Semiquantitative RT-PCR reactions were then performed for 35 cycles using normalized cDNAs and recombinant Taq DNA polymerase. The cycling parameters were denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. PCR products were separated on agarose gel and visualized by ethidium bromide staining. The primer sequences used were: C/EBPβ sense primer 5′-ACAGCGACGAGTACAAGATCC-3′; antisense primer 5′-GCAGCTGCTTGAACAAGTTCC-3′ (29), G3PDH sense primer 5′-CCTTCATTGACCTCAACTAC-3′; antisense primer 5′-AGTG-ATGGCATGGACTGTGGT-3′ (25).
In Vitro Kinase Assay and Immunoblot Analysis.
Hck protein from MΦ lysates was immunoprecipitated with anti-p56/59hck antibody (N-30; Santa Cruz Biotechnology, Inc.) and recovered by absorption to protein A/G-PLUS Sepharose (30, 31). Kinase activity for Hck was performed with 20 μl kinase buffer containing 1 μg of the tyrosine kinase substrate p50 (GST fusion protein containing residues 331–443 of the Src substrate protein Sam 68 (51 kD); Santa Cruz Biotechnology, Inc.) and 5μCi of [γ-32P] ATP (3,000 Ci/mmol; NEN Life Science Products) for 15 min at 30°C, and then measured with liquid scintillation counting. The radiolabeled p50 were subjected with SDS-PAGE and visualized by autoradiography.
Immunoblot analysis were performed in MΦ lysates with rabbit polyclonal antibody against Hck (N-30) or C/EBPβ (C-19; Santa Cruz Biotechnology, Inc.) (32).
Antisense Treatment of Hck and C/EBPβ.
Phosphorothioate-modified antisense oligonucleotides for Hck (AS-Hck; 5′-TTCATCGACCCCATCCTGGC-3′) and C/EBPβ (AS-C/EBPβ; 5′-CAGGCGTTGCATGAACGCGG-3′), and their corresponding sense oligonucleotides (S-Hck; 5′-GCCAGGATGGGGTCGATGAA-3′ and S-C/EBPβ; 5′-CCGCGTTCATGCAACGCCTG-3′), and their unrelated nonsense oligonucleotides (NS-Hck; 5′-CCATATTTCCCGCTCGCGTG-3′ and NS- C/EBPβ; 5′-CCAGAGAGGGCCCGTGTGGA-3′) were synthesized (33, 34). A total of 2–10 μM of each oligonucleotide was incubated with serum-free RPMI 1640 medium for 15 min after the medium was preincubated with 5 μl lipofectin (Life Technologies) per ml for 30 min. Cells were incubated for 24 h in each oligonucleotide containing medium with 10% FCS and CSF, washed, and then cultured with HIV-1 strains as described above.
Statistical Analysis.
Statistical analysis of the data was performed using Student's t test. P values <0.01 were considered significant. The experiments shown are representatives of three to seven independent experiments.
Results
Different Susceptibility of Mo-derived M-MΦ and GM-MΦ to M-tropic HIV-1.
We first examined M-MΦ and GM-MΦ for their capacity to replicate M-tropic HIV-1 strains, HIV-1JR-FL and HIV-1BaL. M-MΦ produced a large amounts of HIV-1JR-FL or HIV-1BaL, whereas GM-MΦ produced no or a more limited levels of HIV-1. P24 levels of HIV-1BaL are 28–59 ng/ml versus 0–0.5 ng/ml (n = 3), and those of HIV-1JR-FL are 7.8–8.6 ng/ml versus 0–0.27 ng/ml (n = 3) on 10 d postinfection (PI), in M-MΦ versus GM-MΦ, respectively (Fig. 1 A).
Figure 1.
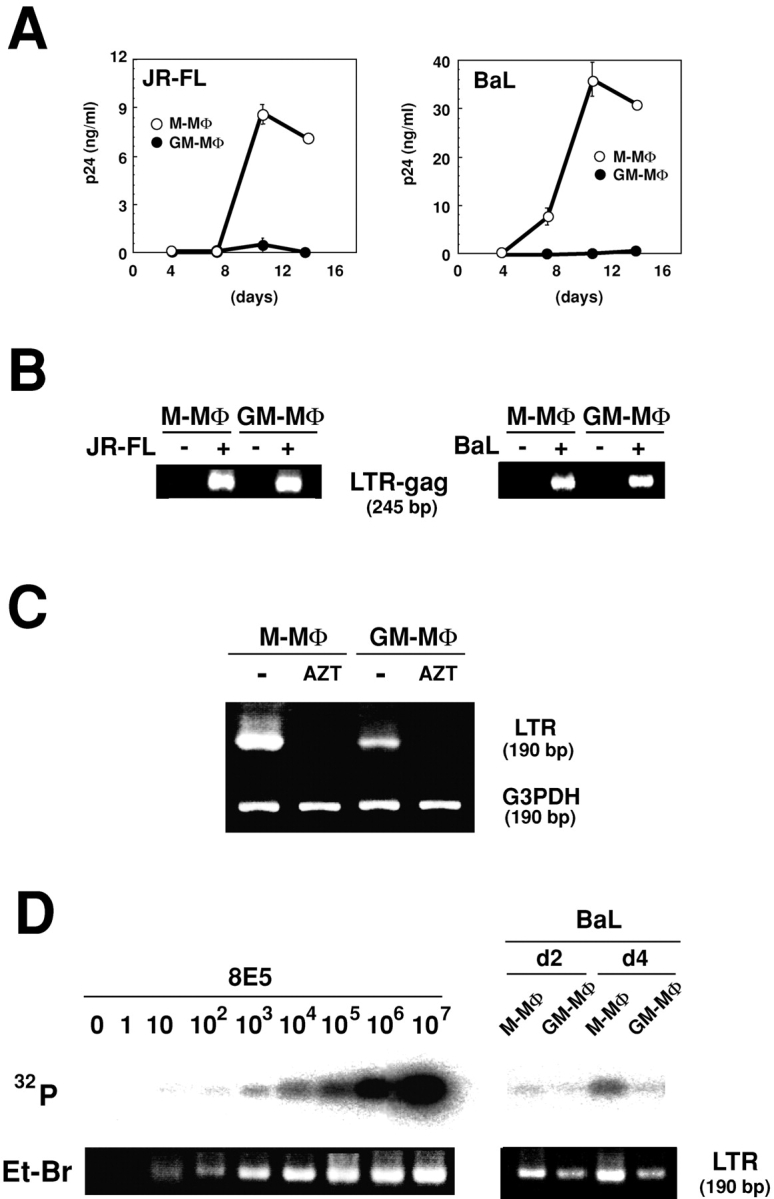
Different susceptibility of Mo-derived MΦs to M-tropic HIV-1 infection. (A) Measurement of viral production at the indicated days in the culture supernatants by p24-ELISA. The data shown are representative one of three independent experiments. (B) Detection of viral DNA in total DNA from MΦ lysates at 2 d PI by nested PCR using a specific outer- and inner-primer for HIV-1 LTR-gag region. (C) Detection of viral DNA by a single step PCR in MΦ infected by AZT-pretreated (AZT) or not treated (−) viral innocula of HIV-1BaL. The data shown here are representative one of three independent experiments. (D) Estimation of HIV-1BaL DNA levels by semi-dilution PCR using a specific primer for the HIV-1 LTR region. The levels of viral DNA in infected MΦ were compared with that of NL4–3 in 8E5 cells.
Syncitia and multinucleated giant cells appeared from d 4–7 PI in HIV-1JR-FL– or HIV-1BaL–exposed M-MΦ but not in GM-MΦ. Immunohistological study showed that p24 antigen is strongly expressed in HIV-1–infected M-MΦ, and the viral spreading is observed not only in syncitia and multinucleated giant cells but also in their surrounding cells (unpublished data).
To investigate whether GM-MΦ are permissive to HIV-1 entry, we examined the existence of the viral DNA at 2 d PI by nested PCR using a pair primer designed from HIV-1 LTR-gag region. Both MΦs produced the viral DNA of HIV-1JR-FL and HIV-1BaL (Fig. 1 B). As viral innocula are often contaminated with viral DNA, we demonstrated that viral DNA products of HIV-1BaL were not detected in both M-MΦ and GM-MΦ when the viral innocula was preincubated with RT inhibitor AZT (Fig. 1 C). Next, levels of HIV-1BaL infectivity in these MΦs were assessed by a single step PCR using a pair primer designed from the HIV-1LTR region. Frequencies of viral DNA in 105 cells of M-MΦ and GM-MΦ at 2 d PI were almost same and were ∼10–102 copies. At 4 d PI, the frequency of viral DNA in GM-MΦ remained 10–102 copies, whereas those in M-MΦ increased to 103-104 copies (Fig. 1 D) which were similar levels to those observed in a previous study (28).
Different Expression of Hck in M-MΦ and GM-MΦ Before and After HIV-1 Infection.
MΦ express Hck that can bind to HIV-1 Nef (13, 14). Then we examined the expression of Hck in M-MΦ and GM-MΦ by immunoblots. Before HIV-1 infection, the levels of 56 and 59 kD of Hck protein in M-MΦ were ∼5 times of that in GM-MΦ (PSL of Hck in M-MΦ and GM-MΦ are 105.1 ± 1.54 and 16.7 ± 1.13, respectively; Fig. 2 A). Compared with these MΦs, PBMC and PHA-activated CD4+T cells did not express Hck and the level of Hck in Mo was significantly low (Fig. 2 A).
Figure 2.
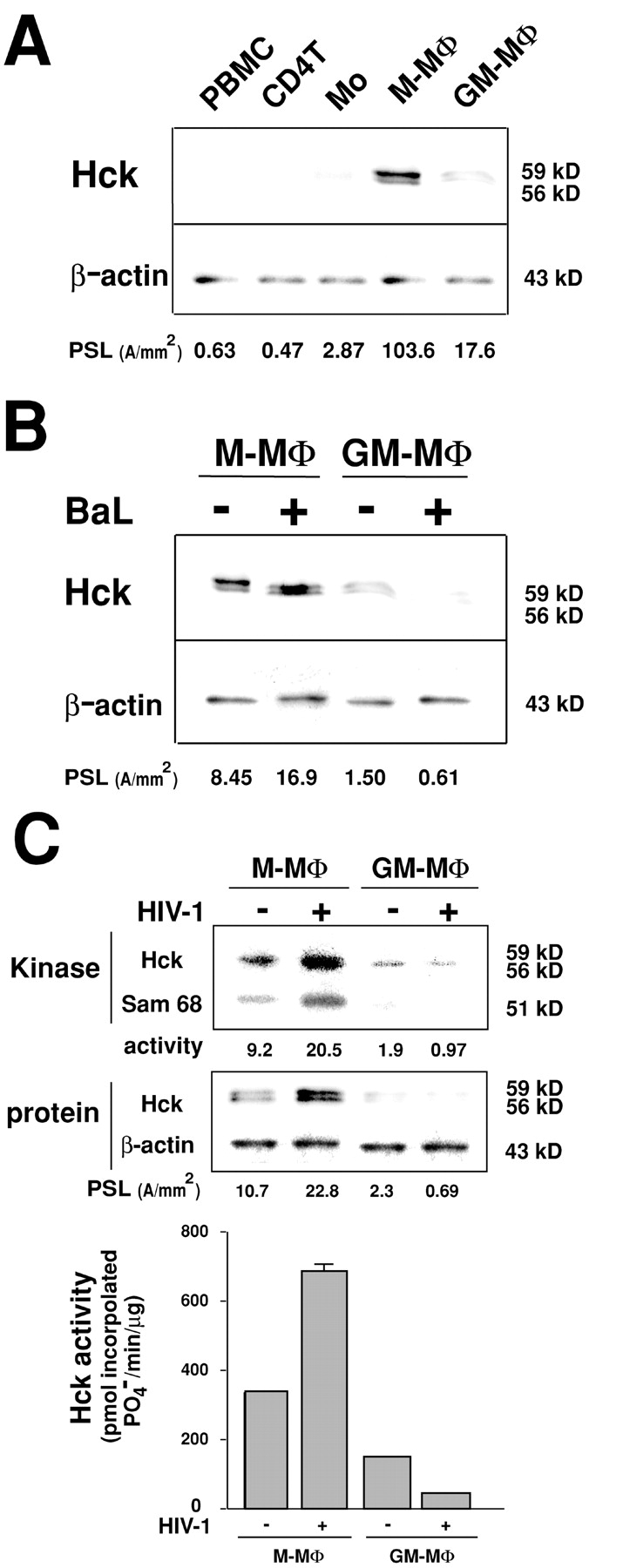
Immunoblot analysis and in vitro kinase assay of Hck in M-MΦ and GM-MΦ. (A) Immunoblots of Hck protein in PBMC, PHA-activated CD4+T cells, Mo, and Mo-derived MΦs. (B) Immuno-blots of Hck protein in HIV-1BaL–infected M-MΦ and GM-MΦ at 2 d PI. The relative amounts of the Hck in cells were measured using NIH image software (PSL; photo stimulating luminescence, A/mm2). (C) In vitro kinase assay of Hck in HIV-1BaL–infected Mo-derived MΦs. Hck protein was immunoprecipitated by a specific antibody against p56/59Hck, and reacted with a kinase buffer containing the tyrosine kinase substrate p50 (Sam 68) in the presence of [γ-32P] ATP. The samples were resolved in SDS-PAGE and autoradiography or blotted with anti-Hck antibody. The data shown here are representative one of three independent experiments.
On d 2 PI of HIV-1BaL, the expression of Hck significantly increased in M-MΦ but markedly reduced in GM-MΦ, and the difference of M-MΦ and GM-MΦ was ∼25 times (PSL of Hck in HIV-1 infected-M-MΦ and -GM-MΦ are 17.6 ± 1.0 and 0.66 ±0.14, respectively; Fig. 2 B). A similar result was observed in HIV-1JR-FL-exposed M-MΦ and GM-MΦ (unpublished data). To confirm the HIV-1 induced up- or down-regulation of Hck expression in M-MΦ and GM-MΦ, an in vitro kinase assay of Hck from these MΦs was performed using GAP-associated protein Sam 68 (51 kD) as a tyrosine kinase substrate (Fig. 2 C). In agreement with the results of immunoblot analysis, Hck activity on d 2 PI of HIV-1BaL augmented in M-MΦ but reduced in GM-MΦ, and the difference of M-MΦ and GM-MΦ was ∼25 times.
Different Expression of C/EBPβ in M-MΦ and GM-MΦ Before and After HIV-1 Infection.
Previous studies indicate that large isoform and short isoform of C/EBPβ stimulates and inhibits the HIV-1 replication in MΦ, respectively (21, 23). To investigate the possibility that the difference in the expression of the C/EBPβ isoforms correlates with the distinct susceptibility to HIV-1 replication in M-MΦ and GM-MΦ, we examined the expression of C/EBPβ isoforms in M-MΦ and GM-MΦ before and after HIV-1 infection by immunoblots. Before HIV-1 infection, the levels of C/EBPβ protein in GM-MΦ were much higher than that in M-MΦ. The small isoform (23 kD) was especially detectable in GM-MΦ but not in M-MΦ, and the relative amounts of the large band (37 kD) to the small band (23 kD; L/S ratio) of C/EBPβ in M-MΦ and GM-MΦ were 13.3 ± 0.78 and 1.32 ± 0.13, respectively (Fig. 3 A). In contrast to these MΦs, both PBMCs and Mo showed very low level expression of C/EBPβ, and the expression of C/EBPβ in PHA-activated CD4+T cells resembled that of M-MΦ.
Figure 3.
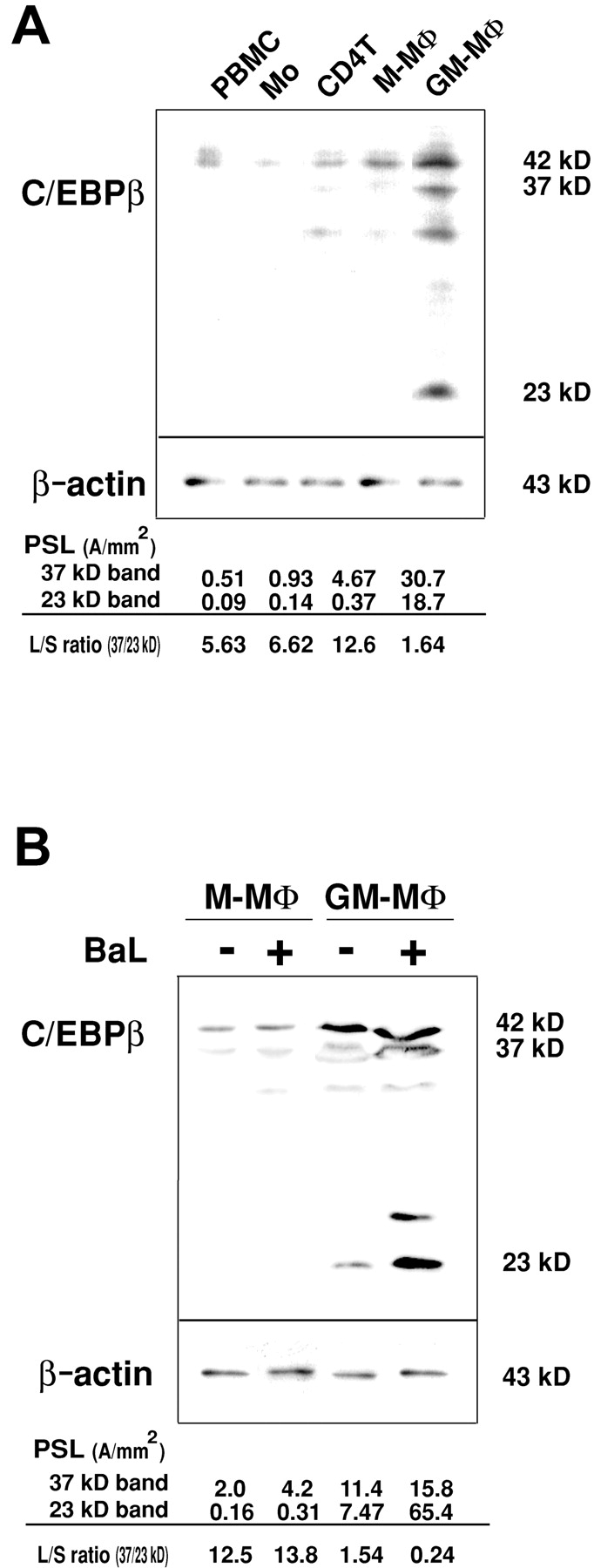
Immunoblot analysis of C/EBPβ in M-MΦ and GM-MΦ. (A) Immunoblots of C/EBPβ protein in PBMC, PHA-activated CD4+ T cells, Mo, and Mo-derived MΦs. (B) Immunoblots of C/EBPβ protein in HIV-1BaL–infected M-MΦ and GM-MΦ at 2 d PI. The relative amounts of the large band to the small band (L/S ratio) of C/EBPβ were calculated using PSL values of 37 kD and 23 kD of C/EBPβ isoforms. The data shown here are representative one of three independent experiments.
On 2 d PI of HIV-1BaL, the bands of C/EBPβ increased significantly in GM-MΦ, especially a strong induction of the small isoform of C/EBPβ was observed, and the L/S ratio of C/EBPβ markedly reduced from 1.48 ± 0.07 to 0.23 ± 0.08. In contrast to GM-MΦ, the amount of C/EBPβ and the L/S ratio of C/EBPβ were not changed significantly in M-MΦ by HIV-1BaL infection (L/S ratio of C/EBPβ in M-MΦ with and without infection were 12.3 ± 0.76 and 13.2 ± 0.56, respectively; Fig. 3 B). Similar results were also observed in HIV-1JR-FL-exposed M-MΦ and GM-MΦ (unpublished data).
Antisense Oligonucleotide for Hck Inhibits the HIV-1 Replication in M-MΦ.
The data described above suggest the possibility that highly active Hck can trigger HIV-1 replication in M-MΦ. We then examined whether down-regulation of Hck by antisense treatment reduces the ability of M-MΦ to support viral replication. Treatment with the antisense oligonucleotide probe of Hck (2 μM; AS-Hck) but not with the unrelated (NS-Hck) or sense (S-Hck) probe markedly reduced the amount of Hck protein in M-MΦ, and the level became less than one-tenth at 48 h (the level of Hck in M-MΦ treated with AS-Hck was 0.10 ± 0.004 times of that in M-MΦ treated with Lipofectin alone; Fig. 4 A). After infection with HIV-1BaL, the expression of Hck still reduced in AS-Hck-treated M-MΦ (the level of Hck in HIV-1 infected M-MΦ treated with AS-Hck was 0.11± 0.003 times of that in HIV-1 infected M-MΦ treated with lipofectin alone; Fig. 4 A), and the AS-Hck treated M-MΦ showed a markedly reduced viral production compared with NS-Hck– or S-Hck–treated M-MΦ (Fig. 4 B). However, the viral DNA was detectable in AS-Hck-treated M-MΦ (Fig. 4 C).
Figure 4.
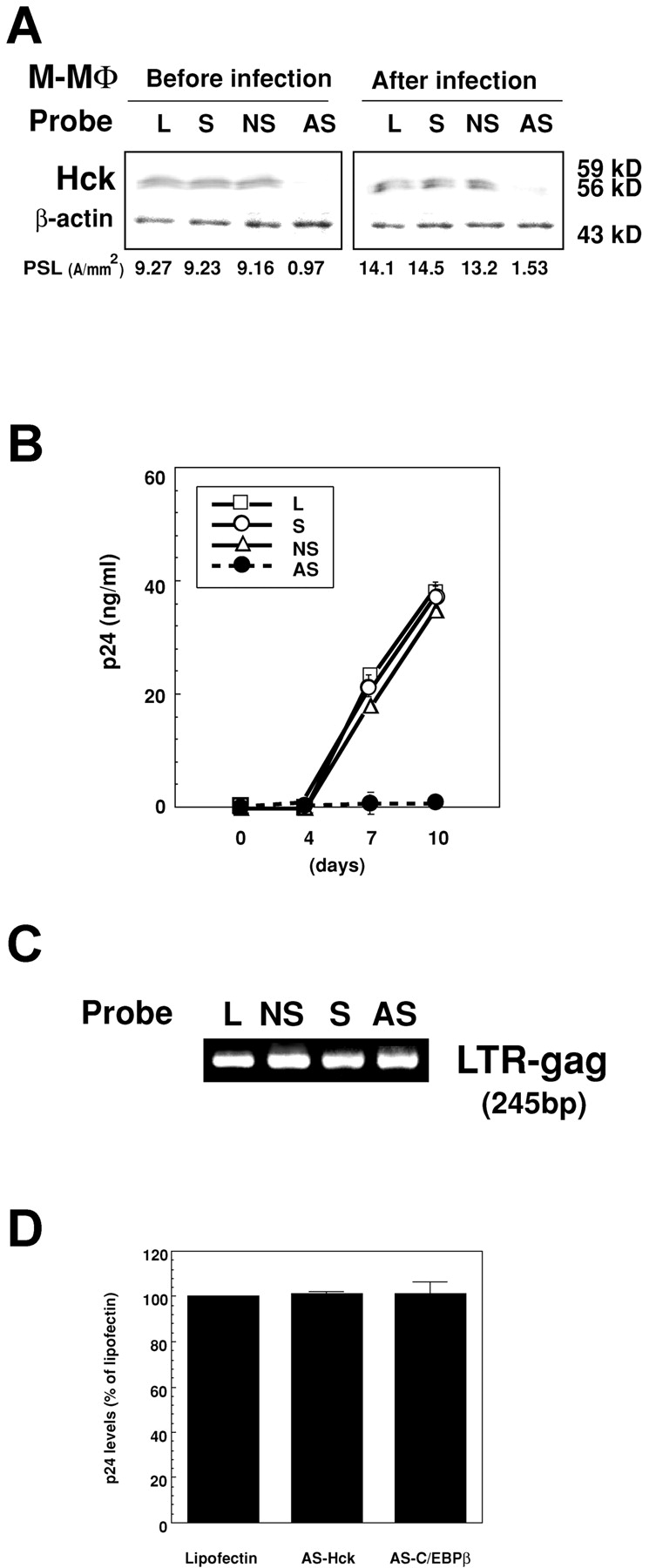
Translational suppression of Hck expression by the antisense oligonucleotide probe inhibits HIV-1BaL replication in M-MΦ. (A) Immunoblot analysis of Hck in M-MΦ treated with oligonucleotide probes for Hck (2 μM) (AS, AS-Hck; NS, NS-Hck; S, S-Hck; and L, lipofectin alone) at 24 h (left) and in HIV-1BaL–infected M-MΦ pretreated with the corresponding probes at 7 d PI, respectively (right). The data shown are representative one of three independent experiments. (B) Kinetics of the p24 levels in the culture supernatants of the oligonucleotide-pretreated HIV-1BaL–infected M-MΦ. (C) Detection of viral DNA in the oligonucleotide-pretreated HIV-1BaL–infected M-MΦ by nested PCR. The data shown are representative one of seven independent experiments. (D) p24 levels in AS-Hck or AS-C/EBPβ–treated PHA-activated CD4 T cells infected by HIV-1NL4–3 (1 ng/ml) at 7 d PI. AS-Hck or AS-C/EBPβ has no effect on T-tropic HIV-1 replication in PHA-activated CD4 T cells.
To confirm that anti–HIV-1 effect of AS-Hck is due to the specific inhibition of Hck activity, and is not the nonspecific effect of the probe, we examined the effect of the AS-Hck on the replication of T-tropic HIV-1 replication in PHA-activated CD4+ T cells that do not express Hck. The AS-Hck did not inhibit T-tropic HIV-1NL4–3 replication in the CD4 T cells (Fig. 4 D). These findings suggest that active Hck protein is absolutely required for M-tropic HIV-1 replication in MΦ.
Dominant Repression of the Small Isoform of C/EBPβ by Antisense Oligonucleotide for C/EBPβ Triggers HIV-1 Replication in GM-MΦ.
As the immunoblot analysis of C/EBPβ in M-MΦ and GM-MΦ described above, and the previous studies (23, 24) provide the possibility that dominant expression of a small isoform of C/EBPβ is correlated with repression of HIV-1 replication in GM-MΦ, we investigated whether changes in the expression of C/EBPβ isoforms can account for the induction of HIV-1 replication in GM-MΦ.
The treatment of GM-MΦ with the antisense oligonucleotide probe of C/EBPβ (2 μM; AS-C/EBPβ) but not with the unrelated (NS-C/EBPβ) or sense (S-C/EBPβ) probe markedly decreased the expression of the small isoform of C/EBPβ but not the large isoform of C/EBPβ, and significantly increased the L/S ratio of C/EBPβ (L/S ratio of GM-MΦ treated with AS-C/EBPβ, S-C/EBPβ, NS-C/EBPβ, and Lipofectin alone were 2.18 ± 0.09, 1.50 ± 0.17, 1.46 ± 0.08, and 1.45 ± 0.08, respectively; Fig. 5 A). When these oligonucleotides probe-treated GM-MΦ were infected with HIV-1BaL, the L/S ratio of C/EBPβ increased in AS-C/EBPβ–treated GM-MΦ but not in NS-C/EBPβ– or S-C/EBPβ–treated GM-MΦ (L/S ratio of HIV-1 infected GM-MΦ treated with AS-C/EBPβ, S-C/EBPβ, NS-C/EBPβ, and Lipofectin alone were 3.13 ± 0.31, 0.39 ± 0.07, 0.40 ± 0.04, and 0.50 ± 0.08, respectively; Fig. 5 A). Consistent with the increase in the L/S ratio of C/EBPβ, a large amount of viral particles were produced from AS-C/EBPβ–treated GM-MΦ but not from NS-C/EBPβ– or S-C/EBPβ–treated GM-MΦ (Fig. 5 B). The viral DNA was detectable in each oligonucleotide probe-treated GM-MΦ (Fig. 5 C).
Figure 5.
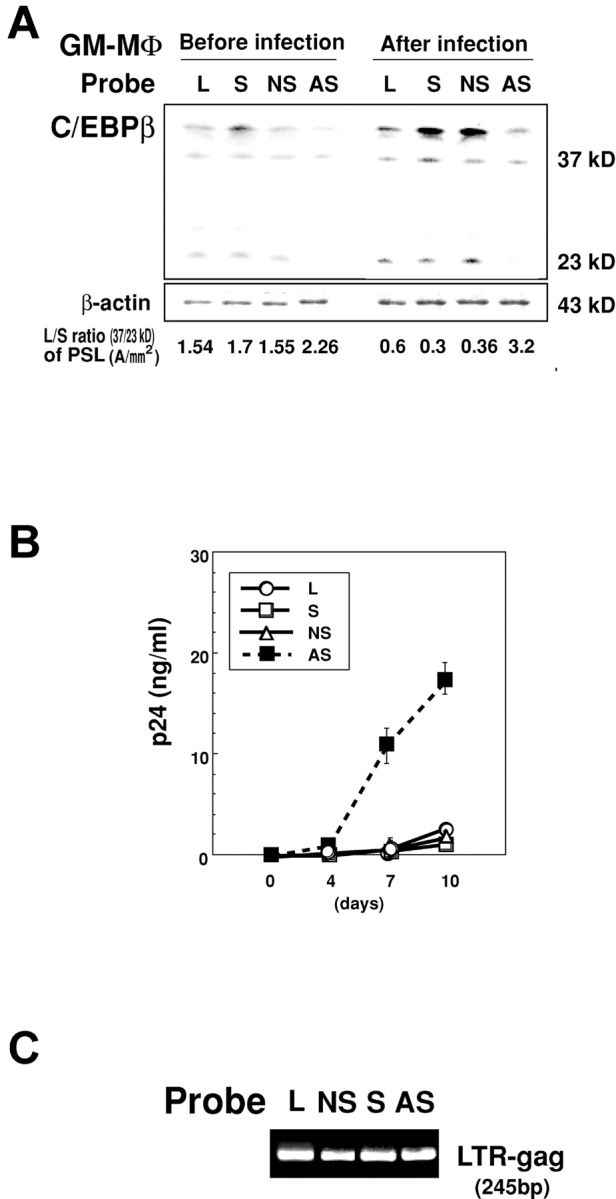
Translational inhibition of C/EBPβ expression by the antisense oligonucleotide probe triggers HIV-1BaL replication in GM-MΦ. (A) Immunoblot analysis of C/EBPβ in GM-MΦ treated with oligonucleotide probes for C/EBPβ (2 μM; AS, AS-C/EBPβ; NS, NS-C/EBPβ; S, S-C/EBPβ; and L, lipofectin alone) at 24 h (left), and HIV-1BaL–infected GM-MΦ pretreated with the corresponding probes at 7 d PI, respectively (right). The data shown are representative one of three independent experiments. (B) Kinetics of p24 levels in the culture supernatants of the oligonucleotide-pretreated HIV-1BaL–infected GM-MΦ. (C) Detection of viral DNA in the oligonucleotide-pretreated HIV-1BaL–infected GM-MΦ by nested PCR. The data shown are representative one of three independent experiments.
We next examined the dose response effect of AS-C/EBPβ on the expression of C/EBPβ and the HIV-1 replication in GM-MΦ. When GM-MΦ was treated with 1–2 μM of AS-C/EBPβ, the expression of large isoform of C/EBPβ did not change significantly, but that of small isoform of C/EBPβ decreased in a dose response manner, and L/S ratio of C/EBPβ increased in these MΦ with the maximal increase at 2 μM (L/S ratio at 2 μM were 2.2; Fig. 6 A). In consistent with the increase of L/S ratio of C/EBPβ, HIV-1 replication occurred in a dose response manner in GM-MΦ treated with 1–2 μM of AS-C/EBPβ (Fig. 6 B). When GM-MΦ were treated with higher concentration (5–10 μM) of AS-C/EBPβ, however, the reduced expression of not only a small isoform but also a large isoform of C/EBPβ was observed (Fig. 6 B). The L/S ratio of C/EBPβ in GM-MΦ treated with 5 μM was almost the same as that in GM-MΦ treated with 1 μM, and both MΦ trigger the same level of viral production (Fig. 6 B). The L/S ratio of C/EBPβ in GM-MΦ treated with 10 μM of AS-C/EBPβ was the similar level to that of nontreated GM-MΦ and no viral replication was observed in the GM-MΦ (Fig. 6 B). Blocking effect of antisense oligonucleotide is thought mainly due to the inhibition of translation. Our results also showed that AS-C/EBPβ did not significantly affect the expression of the mRNA (Fig. 6 C).
Figure 6.
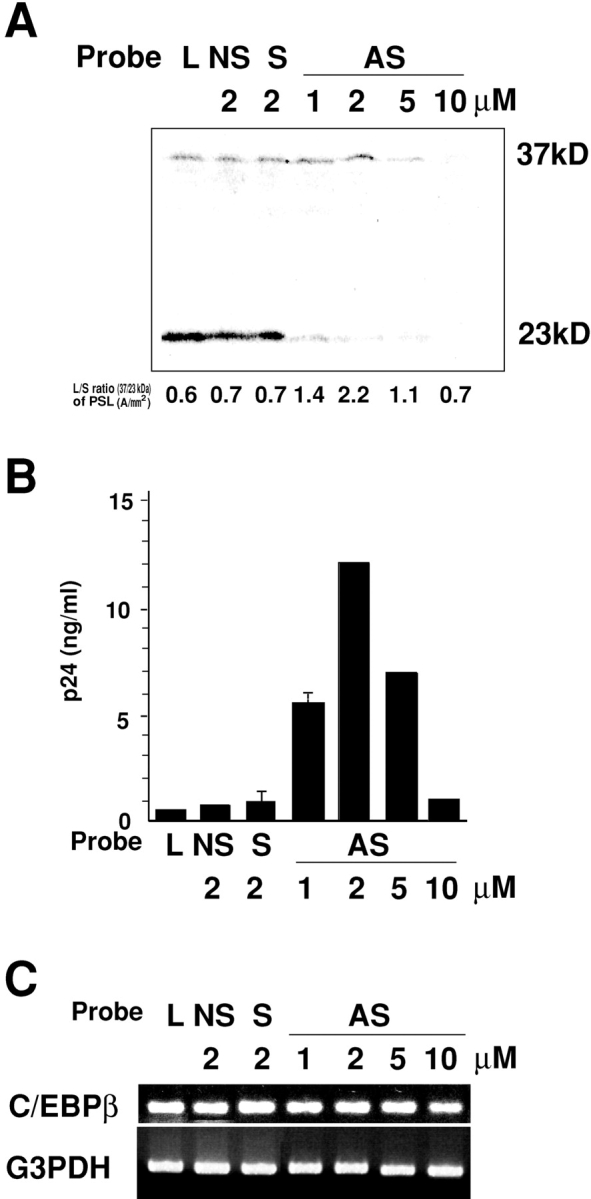
Effects of different concentrations of AS-C/EBPβ on the expression of protein and mRNA of C/EBPβ and HIV-1BaL replication in GM-MΦ. (A) Effect of different concentrations of AS-C/EBPβ on the expression of C/EBPβ protein. (B) Dependence of HIV-1BaL replication in GM-MΦ on the alternative L/S ratio of C/EBPβ expression. (C) Effect of different concentrations of AS-C/EBPβ on the expression of C/EBPβ mRNA. GM-MΦ were treated with various concentrations (1–10 μM) of AS-C/EBPβ for 24 h, and then infected with HIV-1BaL. Cell lysates for immunoblot and mRNA were obtained 2 d and 1 d after infection, respectively. p24 levels of the culture supernatants obtained 7 d after infection much correspond with the alternative L/S ratio of C/EBPβ expression (AS, AS-C/EBPβ; NS, NS-C/EBPβ; S, S-C/EBPβ; and L, lipofectin alone). The data shown are representative one of two experiments.
As AS-C/EBPβ did not affect HIV-1NL4–3 replication in PHA-activated CD4 T cells, the effect of AS-C/EBPβ on HIV-1 replication by the modulation of the L/S ratio of C/EBPβ was specifically active in GM-MΦ (Fig. 4 D). These findings suggest that the large isoform of C/EBPβ is necessary for M-tropic HIV-1 replication in MΦ and the small isoform of C/EBPβ has a deep association with the repression of M-tropic HIV-1 in GM-MΦ.
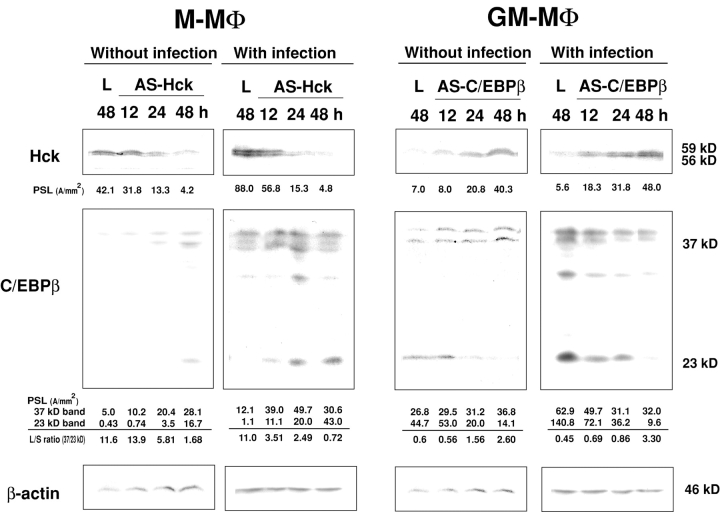
Treatment of M-MΦ with AS-Hck and of GM-MΦ with AS-C/EBPβ Modulates the Expression of C/EBPβ in M-MΦ and Hck in GM-MΦ, Respectively.
As already shown, a high level of Hck and repression of the small isoform of C/EBPβ are observed in HIV-1 susceptible M-MΦ. In contrast, a low level of Hck and dominant expression of the small isoform of C/EBPβ are observed in HIV-1 resistant GM-MΦ. We also showed that treatment of M-MΦ with AS-Hck inhibits the viral replication, and treatment of GM-MΦ with AS-C/EBPβ stimulates the viral replication. These results indicate that the phenotype of AS-Hck–treated M-MΦ and AS-C/EBPβ–treated GM-MΦ are similar to that of GM-MΦ and M-MΦ in their susceptibility for HIV-1 replication, respectively. Then we examined whether treatment of M-MΦ with AS-Hck and of GM-MΦ with AS-C/EBPβ changes the expression of C/EBPβ in M-MΦ and Hck in GM-MΦ, respectively. Treatment of M-MΦ with AS-Hck (2 μM) suppressed the expression of Hck but stimulated the expression of the short isoform of C/EBPβ and decreased the L/S ratio (Fig. 7) . When AS-Hck–treated M-MΦ were infected with HIV-1BaL, similar results were obtained (Fig. 7). Treatment of GM-MΦ with AS-C/EBPβ (2 μM) not only reduced the expression of a short isoform of C/EBPβ but also stimulated the expression of Hck (Fig. 7). Similar results were obtained when AS-C/EBPβ–treated GM-MΦ was infected with HIV-1BaL (Fig. 7). As well as the data shown in Figs. 4, 5, and 6, viral replication was inhibited in AS-Hck–treated M-MΦ and a large amount of viral particles were produced from AS-C/EBPβ–treated GM-MΦ.
Figure 7.
Immunoblot analysis of C/EBPβ in M-MΦ treated with AS-Hck and of Hck in GM-MΦ treated with AS-C/EBPβ with or without HIV-1BaL infection M-MΦ and GM-MΦ were treated with AS-Hck (2 μM), AS-C/EBPβ (2 μM) and lipofectin alone (L) for 24 h. These MΦs were infected with or without HIV-1BaL, and cultured for 48 h with M-CSF and GM-CSF, respectively. Cell lysates were obtained at indicated time points after antisense oligonucleotide treatment. The data shown are representative one of two experiments.
These results strongly suggest that Hck and C/EBPβ are closely related and these two molecules affect one another, and the regulation of Hck and small isoform of C/EBPβ contribute to determine distinct susceptibility of MΦ to HIV-1 infection.
Discussion
In the present study, we found an evidence that Mo-derived M-MΦ and GM-MΦ are distinct in their susceptibility to M-tropic HIV-1 infection; M-MΦ releases a large amount of HIV-1 particles whereas GM-MΦ does not produce or produce a very low level of the viral replication, but the viral DNA was detectable at similar levels in both MΦs. These findings suggest that the inhibition of HIV-1 replication in GM-MΦ occurs at posttranscriptional and translational levels but not at the viral entry. In fact, HIV-1 coreceptors, CD4 and CCR5, are equally expressed in both MΦs (27, and our unpublished data), and CCR5 and CXCR4 are fully expressed in CSF-induced Mo-derived MΦs and A-MΦ (35, 36, 37).
Previously, we reported that viral DNA of M-tropic HIV-1PAR was detected in both M-MΦ and GM-MΦ, but the viral replication was mainly observed in M-MΦ (27). Thus, M-MΦ is ubiquitously susceptible to many strains of M-tropic HIV-1, but GM-MΦ inhibits the viral replication at the transcriptional and translational levels. In relation to this point, it is reported that MΦ generated from Mo by human serum produce a large amount of M-CSF by HIV-1 infection, and the addition of a specific antibody against M-CSF markedly reduces HIV-1 production in these MΦ (38).
In some studies, GM-CSF augments M-tropic HIV-1 replication in human Mo-derived MΦ (39, 40). Mo-derived MΦ used in that study, however, is different from that used in our present study, because they generated MΦ from Mo by human serum and examined the effect of GM-CSF on the MΦ, but we examined the MΦ generated directly from Mo by GM-CSF or M-CSF. Thus, the difference in the effect of GM-CSF on HIV-1 replication may depend on the difference in MΦ origin.
In the present study, we showed that the CSF-induced basal- and HIV-1–induced difference in the expression of Hck and C/EBPβ isoforms deeply contributes to determine the tissue- or cell stage–specific differences of viral replication in MΦ. M-MΦ possess high levels of both the protein and kinase activity of Hck, and HIV-1 infection triggers to augment them, whereas GM-MΦ express about fivefold lower levels of both the protein and kinase activity of Hck, and HIV-1 infection reduces the levels to an undetectable one. Final difference of the expression of Hck between M-MΦ and GM-MΦ was ∼25-fold. Furthermore we provide an evidence that translational inhibition of Hck by AS-Hck can fully inhibit HIV-1 replication in M-MΦ although viral DNA is detected in the treated MΦ. Thus, the present observations suggest that the high basal level and HIV-1–mediated activation of Hck activity greatly contributes to produce HIV-1 replication in MΦ, and the repression and reduction of Hck activity maintains nonproductive (latent) infection of HIV-1 in MΦ. However, the underlying mechanism that induces such a distinct modulation of the expression of Hck is presently unclear.
In contrast to Hck, the roles of C/EBPβ in M-tropic HIV-1 replication in MΦ are very flexible and confused, because the large (37 kD) and small (23 kD) isoforms of C/EBPβ generated by alternative initiation of protein translation from a single transcript (19, 20) are reciprocal effectors; the former is required for the viral replication in Mo/MΦ but not CD4 T cells (21), whereas the latter acts as a dominant negative transcription factor (23, 24, 41). Our present data coincide with those previous study, because large isoform of C/EBPβ are dominant in HIV-1 susceptible M-MΦ, but basal- and HIV-1–mediated dominant expression of small isoform of C/EBPβ are observed in HIV-1 resistant GM-MΦ. Furthermore our data show that decrease of small isoform of C/EBPβ and increase in the L/S ratio of C/EBPβ by treatment with a low dose of AS-C/EBPβ strongly stimulates the viral replication in HIV-1–resistant GM-MΦ. These findings indicate that the large isoform of C/EBPβ is a stimulator and the small isoform is a inhibitor in the process of HIV-1 replication in Mo/MΦ and also suggest that human tissue MΦ can convert their phenotype for M-tropic HIV-1 replication from resistant to susceptible one via the modulation of the L/S ratio of C/EBPβ expression in vivo.
At present we do not know the precise mechanism of the preferential suppression of the small isoform by a low concentration of AS-C/EBPβ. One possible explanation is that the dominant expression of the small isoform of C/EBPβ usually occurs in GM-MΦ as seen in Fig. 3 B, then the small isoform is dominantly suppressed. Different protein isoforms can be produced for C/EBPβ by alternative use of translation initiation codons in the same mRNA molecule (20, 42, 43, 44). So it is also possible to consider that the AS-C/EBPβ we used in this study (corresponding to the ATG sequence of the human C/EBPβ gene [34]) preferentially affects the translation initiation site for the short isoform, and if we used another antisense oligonucleotide, then different results would be obtained.
Our present investigation using AS-Hck and AS-C/EBPβ presents the new and interesting finding that the regulation of Hck and C/EBPβ are closely related and these two molecules affect one another. Hck is a kinase, and may directly or indirectly affect the expression of the short isoform of C/EBPβ. The promoter region of Hck, however, does not contain a C/EBPβ binding site (45, 46). At present we do not know the precise mechanism of connection between Hck and C/EBPβ, and studies to clarify the mechanism are under way.
Several studies showed that A-MΦ from healthy volunteers do not stimulate the HIV-1 replication in vitro, but viral DNA formation occurs in the A-MΦ, and A-MΦ from an HIV-1 carrier contains very few copies of the HIV-1 genome and hardly produces HIV-1 particles (3, 4). Furthermore it is reported that a small isoform of C/EBPβ is dominant in A-MΦ (23, 24). Our previous report indicate that phenotype of GM-MΦ closely resembles that of human A-MΦ in their morphology, cell surface antigen expression, and several functions including resistance to H2O2-mediated cytotoxicity, H2O2 release, and catalase activity (26, 32). In this study, we demonstrated that GM-MΦ dominantly expresses small isoform of C/EBPβ, and hardly stimulates HIV-1 replication, though viral DNA is formed, indicating GM-MΦ also resembles A-MΦ in their susceptibility to HIV-1 infection. Therefore, analysis of resistance of GM-MΦ to HIV-1 replication can help to resolve the latent infection of nonactivated A-MΦ.
A-MΦ from the progressor patients or patients with opportunistic infection, however, often produces a large amount of viral particles and spreads HIV-1 infection in lung tissue (5, 6, 24). In the present study, we showed that the phenotype of GM-MΦ in HIV-1 infection can change from resistant to susceptible one after the treatment with AS-C/EBPβ. In some studies, A-MΦ from the active sites of inflammation express high levels of CD14 and c-fms gene (47, 48). Previously we reported that M-MΦ that can support HIV-1 replication also express these markers (25, 32). In addition recent study showed that addition of activated allogeneic lymphocytes reduces the expression of small isoform of C/EBPβ in A-MΦ or THP-1 cells (49). These findings suggest the possibility that a phenotypic change of resident A-MΦ from resistant to susceptible one for HIV-1 replication, and/or an influx of blood Mo-derived MΦ with high adaptability to HIV-1 replication occurs in the inflammatory lung and can account for their permissivity to M-tropic HIV-1 replication in vivo.
In conclusion, the findings of the present study strongly suggests that regulation of Hck activity and selective expression of C/EBPβ isoforms greatly contribute to determine distinct susceptibility of Mo-derived M-MΦ and GM-MΦ to M-tropic HIV-1 infection. MΦ heterogeneity induced by CSF during MΦ differentiation from precursors plays an important role for the distinct susceptibility against HIV-1. The present model of a distinct adaptability of GM-MΦ and M-MΦ to M-tropic HIV-1 through the regulation of their cellular proteins represents heterogeneous susceptibility of tissue MΦ to HIV-1 infection in vivo, and may help to develop a new anti–HIV-1 drug for prevention of persistent infection in human tissue Mo/MΦ.
Acknowledgments
This study was supported in part by grants from the Human Science Research Foundation and the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Abbreviations used in this paper: A-MΦ, alveolar macrophages; AS, antisense; AZT, azidothymidine; C/EBPβ, CCAAT enhancer binding protein; GM-MΦ, GM-CSF–induced human monocyte-derived macrophages; Hck, hematopoietic cell kinase; L/S ratio of C/EBPβ, the relative amounts of the large isoform (37 kD) to the small isoform (23 kD) of C/EBPβ; M-CSF, macrophage-CSF; MΦ, macrophages; M-MΦ, M-CSF–induced human monocyte-derived macrophages; Mo, monocytes; M-tropic, macrophage-tropic; NS, nonsense; S, sense.
References
- 1.Ghorpade, A., M.Q. Xia, B.T. Hyman, Y. Persidsky, A. Nukuna, P. Bock, M. Che, J. Limoges, H.E. Gendelman, and C.R. Mackay. 1998. Role of the beta- chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J. Virol. 72:3351–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane, B.R., D.M. Markovitz, N.L. Woodford, R. Rochford, R.M. Strieter, and M.J. Coffey. 1999. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J. Immunol. 163:3653–3661. [PubMed] [Google Scholar]
- 3.Chun, T.W., L. Carruth, D. Finzi, X. Shen, J.A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T.C. Quinn, et al. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 387:183–188. [DOI] [PubMed] [Google Scholar]
- 4.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H.M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741–9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatch, W.C., A.R. Freedman, D.M. Boldt-Houle, J.E. Groopman, and E.F. Terwilliger. 1997. Differential effects of interleukin-13 on cytomegalovirus and human immunodeficiency virus infection in human alveolar macrophages. Blood. 89:3443–3450. [PubMed] [Google Scholar]
- 6.Orenstein, J.M., C. Fox, and S.M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science. 276:1857–1861. [DOI] [PubMed] [Google Scholar]
- 7.Cavert, W., D.W. Notermans, K. Staskus, S.W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, Z.Q. Zhang, R. Mills, H. McDade, et al. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection [published erratum at 276:1321]. Science. 276:960–964. [DOI] [PubMed] [Google Scholar]
- 8.Perelson, A.S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D.D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 387:188–191. [DOI] [PubMed] [Google Scholar]
- 9.Orenstein, J.M., M. Feinberg, C. Yoder, L. Schrager, J.M. Mican, D.J. Schwartzentruber, R.T. Davey, Jr., R.E. Walker, J. Falloon, J.A. Kovacs, et al. 1999. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS. 13:2219–2229. [DOI] [PubMed] [Google Scholar]
- 10.Crowe, S.M., and S. Sonza. 2000. HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. J. Leukoc. Biol. 68:345–350. [PubMed] [Google Scholar]
- 11.Mocsai, A., Z. Jakus, T. Vantus, G. Berton, C.A. Lowell, and E. Ligeti. 2000. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J. Immunol. 164:4321–4331. [DOI] [PubMed] [Google Scholar]
- 12.Scholz, G., K. Cartledge, and A.R. Dunn. 2000. Hck enhances the adherence of lipopolysaccharide-stimulated macrophages via Cbl and phosphatidylinositol 3- kinase. J. Biol. Chem. 275:14615–14623. [DOI] [PubMed] [Google Scholar]
- 13.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moarefi, I., M. LaFevre-Bernt, F. Sicheri, M. Huse, C.H. Lee, J. Kuriyan, and W.T. Miller. 1997. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 385:650-653. [DOI] [PubMed] [Google Scholar]
- 15.Greenway, A.L., H. Dutartre, K. Allen, D.A. McPhee, D. Olive, and Y. Collette. 1999. Simian immunodeficiency virus and human immunodeficiency virus type 1 nef proteins show distinct patterns and mechanisms of Src kinase activation. J. Virol. 73:6152–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang, S.M., A.J. Iafrate, C. Stahl-Hennig, E.M. Kuhn, T. Nisslein, F.J. Kaup, M. Haupt, G. Hunsmann, J. Skowronski, and F. Kirchhoff. 1997. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat. Med. 3:860–865. [DOI] [PubMed] [Google Scholar]
- 17.Khan, I.H., E.T. Sawai, E. Antonio, C.J. Weber, C.P. Mandell, P. Montbriand, and P.A. Luciw. 1998. Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J. Virol. 72:5820–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokunaga, K., E. Kiyokawa, M. Nakaya, N. Otsuka, A. Kojima, T. Kurata, and M. Matsuda. 1998. Inhibition of human immunodeficiency virus type 1 virion entry by dominant-negative Hck. J. Virol. 72:6257–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baer, M., S.C. Williams, A. Dillner, R.C. Schwartz, and P.F. Johnson. 1998. Autocrine signals control CCAAT/enhancer binding protein beta expression, localization, and activity in macrophages. Blood. 92:4353–4365. [PubMed] [Google Scholar]
- 20.Xiong, W., C.C. Hsieh, A.J. Kurtz, J.P. Rabek, and J. Papaconstantinou. 2001. Regulation of CCAAT/enhancer-binding protein-beta isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucleic Acids Res. 29:3087–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, A.J., and K.L. Calame. 1997. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc. Natl. Acad. Sci. USA. 94:8714–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruocco, M.R., X. Chen, C. Ambrosino, E. Dragonetti, W. Liu, M. Mallardo, G. De Falco, C. Palmieri, G. Franzoso, I. Quinto, et al. 1996. Regulation of HIV-1 long terminal repeats by interaction of C/EBP(NF- IL6) and NF-kappaB/Rel transcription factors. J. Biol. Chem. 271:22479–22486. [DOI] [PubMed] [Google Scholar]
- 23.Honda, Y., L. Rogers, K. Nakata, B.Y. Zhao, R. Pine, Y. Nakai, K. Kurosu, W.N. Rom, and M. Weiden. 1998. Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J. Exp. Med. 188:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiden, M., N. Tanaka, Y. Qiao, B.Y. Zhao, Y. Honda, K. Nakata, A. Canova, D.E. Levy, W.N. Rom, and R. Pine. 2000. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J. Immunol. 165:2028–2039. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto, S., I. Komuro, M. Yamada, and K.S. Akagawa. 2001. Il-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J. Immunol. 167:3619–3625. [DOI] [PubMed] [Google Scholar]
- 26.Komuro, I., N. Keicho, A. Iwamoto, and K.S. Akagawa. 2001. Human alveolar macrophages and granulocyte-macrophage colony-stimulating factor-induced monocyte-derived macrophages are resistant to H2O2 via their high basal and inducible levels of catalase activity. J. Biol. Chem. 276:24360–24364. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda, S., K. Akagawa, M. Honda, Y. Yokota, Y. Takebe, and T. Takemori. 1995. Suppression of HIV replication in human monocyte-derived macrophages induced by granulocyte/macrophage colony-stimulating factor. AIDS Res. Hum. Retroviruses. 11:1031–1038. [DOI] [PubMed] [Google Scholar]
- 28.Tsunetsugu-Yokota, Y., K. Akagawa, H. Kimoto, K. Suzuki, M. Iwasaki, S. Yasuda, G. Hausser, C. Hultgren, A. Meyerhans, and T. Takemori. 1995. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J. Virol. 69:4544–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwama, A., M. Osawa, R. Hirasawa, N. Uchiyama, S. Kaneko, M. Onodera, K. Shibuya, A. Shibuya, C. Vinson, D.G. Tenen, and H. Nakauchi. 2002. Reciprocal roles for CCAAT/enhancer binding protein (C/EBP) and PU.1 transcription factors in Langerhans cell commitment. J. Exp. Med. 195:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briggs, S.D., and T.E. Smithgall. 1999. SH2-kinase linker mutations release Hck tyrosine kinase and transforming activities in Rat-2 fibroblasts. J. Biol. Chem. 274:26579–26583. [DOI] [PubMed] [Google Scholar]
- 31.Chiaradonna, F., L. Fontana, C. Iavarone, M.V. Carriero, G. Scholz, M.V. Barone, and M.P. Stoppelli. 1999. Urokinase receptor-dependent and - independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. EMBO J. 18:3013–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akagawa, K.S., N. Takasuka, Y. Nozaki, I. Komuro, M. Azuma, M. Ueda, M. Naito, and K. Takahashi. 1996. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 88:4029–4039. [PubMed] [Google Scholar]
- 33.Wei, S., J.H. Liu, P.K. Epling-Burnette, A.M. Gamero, D. Ussery, E.W. Pearson, M.E. Elkabani, J.I. Diaz, and J.Y. Djeu. 1996. Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J. Immunol. 157:5155–5162. [PubMed] [Google Scholar]
- 34.Adler, G. 1997. Functional NF-IL6/CCAAT enhancer-binding protein is required for tumor necrosis factor alpha-inducible expression of the granulocyte colony- stimulating factor (CSF), but not the granulocyte/macrophage CSF or interleukin 6 gene in human fibroblasts [retraction of Kiehntopf, M., F. Herrmann, and M.A. Brach. 1995. J. Exp. Med. 181:793–798]. J. Exp. Med. 186:171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Berkowitz, R.D., S. Alexander, C. Bare, V. Linquist-Stepps, M. Bogan, M.E. Moreno, L. Gibson, E.D. Wieder, J. Kosek, C.A. Stoddart, and J.M. McCune. 1998. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J. Virol. 72:10108–10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, B., M. Sharron, L.J. Montaner, D. Weissman, and R.W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA. 96:5215–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worgall, S., R. Connor, R.J. Kaner, E. Fenamore, K. Sheridan, R. Singh, and R.G. Crystal. 1999. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J. Virol. 73:5865–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutza, J., L. Crim, S. Feldman, M.P. Hayes, M. Gruber, J. Beeler, and K.A. Clouse. 2000. Macrophage colony-stimulating factor antagonists inhibit replication of HIV-1 in human macrophages. J. Immunol. 164:4955–4960. [DOI] [PubMed] [Google Scholar]
- 39.Crowe, S.M., and A. Lopez. 1997. GM-CSF and its effects on replication of HIV- 1 in cells of macrophage lineage. J. Leukoc. Biol. 62:41–48. [DOI] [PubMed] [Google Scholar]
- 40.Kedzierska, K., M.A. Rainbird, A.F. Lopez, and S.M. Crowe. 1998. Effect of GM- CSF on HIV-1 replication in monocytes/macrophages in vivo and in vitro: a review. Vet. Immunol. Immunopathol. 63:111–121. [DOI] [PubMed] [Google Scholar]
- 41.Galio, L., S. Briquet, and C. Vaquero. 1999. Real-time study of interactions between a composite DNA regulatory region (HIV-1 LTR NRE) and several transcription factors of nuclear extracts. Biochem. Biophys. Res. Commun. 264:6–13. [DOI] [PubMed] [Google Scholar]
- 42.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 67:569–579. [DOI] [PubMed] [Google Scholar]
- 43.Ossipow, V., U.K. Laemmli, and U. Schibler. 1993. A simple method to renature DNA-binding proteins separated by SDS-polyacrylamide gel electrophoresis. Nucleic Acids Res. 21:6040–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calkhoven, C.F., L. Snippe, and G. Ab. 1997. Differential stimulation by CCAAT/enhancer-binding protein alpha isoforms of the estrogen-activated promoter of the very-low-density apolipoprotein II gene. Eur. J. Biochem. 249:113–120. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenberg, U., N. Quintrell, and J.M. Bishop. 1992. Human protein-tyrosine kinase gene HCK: expression and structural analysis of the promoter region. Oncogene. 7:849–858. [PubMed] [Google Scholar]
- 46.Hauses, M., R.R. Tonjes, and M. Grez. 1998. The transcription factor Sp1 regulates the myeloid-specific expression of the human hematopoietic cell kinase (HCK) gene through binding to two adjacent GC boxes within the HCK promoter-proximal region. J. Biol. Chem. 273:31844–31852. [DOI] [PubMed] [Google Scholar]
- 47.Radzun, H.J., H. Kreipe, K. Heidorn, and M.R. Parwaresch. 1988. Modulation of c-fms proto-oncogene expression in human blood monocytes and macrophages. J. Leukoc. Biol. 44:198–204. [DOI] [PubMed] [Google Scholar]
- 48.Krombach, F., J.T. Gerlach, C. Padovan, A. Burges, J. Behr, T. Beinert, and C. Vogelmeier. 1996. Characterization and quantification of alveolar monocyte-like cells in human chronic inflammatory lung disease. Eur. Respir. J. 9:984–991. [DOI] [PubMed] [Google Scholar]
- 49.Hoshino, Y., K. Nakata, S. Hoshino, Y. Honda, D.B. Tse, T. Shioda, W.N. Rom, and M. Weiden. 2002. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 195:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]