Abstract
Interferon (IFN)-γ is necessary for tumor immunity, however, its initial cellular source is unknown. Because γδ T cells primarily produce this cytokine upon activation, we hypothesized that they would provide an important early source of IFN-γ in tumor immunosurveillance. To address this hypothesis, we first demonstrated that γδ T cell–deficient mice had a significantly higher incidence of tumor development after challenge with a chemical carcinogen methylcholanthrene (MCA) or inoculation with the melanoma cell line B16. In wild-type mice, γδ T cells were recruited to the site of tumor as early as day 3 after inoculation, followed by αβ T cells at day 5. We then used bone marrow chimeras and fetal liver reconstitutions to create mice with an intact γδ T cell repertoire but one that was specifically deficient in the capacity to produce IFN-γ. Such mice had a higher incidence of tumor development, induced either with MCA or by inoculation of B16 melanoma cells, compared with mice with IFN-γ–competent γδ T cells. Moreover, genetic deficiency of γδ T cells resulted in impaired IFN-γ production by tumor antigen-triggered αβ T cell upon immunization with tumor lysate. These results demonstrate that γδ T cells can play a necessary role in tumor immunity through provision of an early source of IFN-γ that in turn may regulate the function of tumor-triggered αβ T cells.
Keywords: cytokines, immunosurveillance, immune regulation, tumor immunosurveillance
Introduction
IFN-γ is a necessary cytokine in the innate and adaptive immune responses that protect against tumor development (1, 2). Endogenously produced IFN-γ forms the basis of a tumor surveillance system against the development of primary chemically induced sarcomas (3, 4). For example, mice deficient in the IFN-γ receptor or in Stat1, required for IFN-γ signaling, develop tumors more rapidly and with greater frequency in response to the chemical carcinogen methylcholanthrene (MCA)*when compared with wild-type mice. Lymphocytes also are necessary for immunity to spontaneously induced tumors, with their role dependent upon IFN-γ synthesis (5). Tumor cells themselves are the major target of the action of IFN-γ, as determined using IFN-γ–insensitive tumor cell lines and a transplant tumor model (6).
Several immunological and nonimmunological mechanisms have been proposed to explain the effect of IFN-γ in tumor immunity (1). Perhaps most importantly, IFN-γ is critical for the development of an effective adaptive immune response, primarily directing the balance of Th1/Th2 cytokines toward protection. Notably, the primary cellular source for the initial production of IFN-γ has not yet been defined. It has been proposed that innate cells or innate-like lymphocytes, such as NK, NK T, and γδ T cells, might provide the early source of this cytokine (2).
γδ T cells have many features that distinguish them from αβ T cells (7–9). Most of the former have a phenotype of spontaneous activation with a fast turnover rate in vivo (10), and they quickly expand upon pathogen challenge in the first few days after infection (11–14). γδ T cells also regulate CD4+ and CD8+ T cell function. For example, in a Coxsackie virus infection model, Vγ1 cells direct CD4+ T cells toward a dominant Th2 response, whereas Vγ4 cells bias them toward a dominant Th1 response (15). In a similar manner, γδ T cells are responsible for the establishment of a protective CD8+ memory response during Listeria monocytogenes infection (16), whereas γδ T cell–deficient mice have an inadequate CD8+ T cell immune response against Encephalitozoon cuniculi infection (17).
γδ T cells provide the host with several potential advantages over conventional lymphocytes in host protection against tumors. CD8+ T cells recognize processed peptide antigens in the context of self-MHC class I molecules that may have impaired expression on tumor cells, thereby rendering the tumor immunologically invisible to αβ T cells. By contrast, γδ T cells recognize antigens directly, with no requirement for antigen processing or presentation (8, 9). More recently, γδ T cells have been shown to recognize the MHC class I–related molecules MICA and MICB, which are induced by stress resulting from infection or injury, or by cellular transformation (18, 19). These molecules have been shown to be stimulatory ligands for the NK cell receptor NKG2D that is also found on γδ T cells (20, 21). Moreover, skin-associated γδ T cells play an important role in preventing tumor development through NKG2D-mediated cytolytic mechanisms (22).
To precisely define the role of γδ T cells in the protective immune responses against tumor development, we analyzed tumor formation and growth in B6 TCRδ-deficient (TCRδ−/−) mice compared with wild-type mice, as well as in bone marrow chimeras or fetal liver–reconstituted mice in which γδ T cells selectively lack the ability to produce IFN-γ. We demonstrate that γδ T cells play a critical role in the protective immune response against tumor development by providing an important early source of IFN-γ that in turn regulates αβ T cell effector function.
Materials and Methods
Mice.
C57BL/6J (B6), C57BL/6J-Tcrbtm1Mom (B6 TCRβ-deficient mice [TCRβ−/−]), C57BL/6J-Tcrdtm1Mom (B6 TCRδ−/−), and C57BL/6-Ifngtm1Ts (B6 IFN-γ–deficient [IFN-γ−/−]) mice were purchased from The Jackson Laboratory. B6 IFN-γ−/− mice and B6 TCRβ−/− mice were bred to create B6 TCRβ−/− IFN-γ−/− double deficient mice. For bone marrow chimeras, the donors and recipients are listed in Table I. All animals were maintained under specific pathogen-free conditions and used at 6–8 wk of age unless specifically mentioned.
Table I.
Generation of Bone Marrow Chimeras
| Group | Donors 1 | Donors 2 | Recipients | Expected outcome |
|---|---|---|---|---|
| Experimental | TCRβ2/2 IFN-γ2/2 | TCRδ2/2 | IFN-γ2/2 | γδ T cells cannot produce IFN-γ |
| Control | TCRβ2/2 IFN-γ1/1 | TCRδ2/2 | IFN-γ2/2 | γδ T cells can produce IFN-γ |
Bone marrow chimeras were prepared as described in Materials and Methods. Bone marrow cells from TCRδ−/− mice (normal αβ T cells without γδ T cells) together with bone marrow cells either from TCRβ−/− IFN-γ−/− mice (γδ T cells lack the ability to produce IFN-γ) or from TCRβ−/− IFN-γ+/+ mice (γδ T cells can produce IFN-γ) in a 1:1 ratio were injected intravenously into irradiated IFN-γ−/− mice (to eliminate any radio-resistant source of endogenous IFN-γ). All mice were on the B6 background and were age and sex matched. 12 wk after reconstitution, mice were injected with MCA or with B16 F0 melanoma cells.
Reagents.
Recombinant murine IL-2 and IL-12 were purchased from R&D Systems. Anti–mouse mAb used for phenotypic and cytokine analyses were all purchased from BD Biosciences.
Tumor Models.
Induction of fibrosarcomas by injection with MCA has been described (3, 22). In brief, MCA was dissolved in peanut oil overnight and sex- and age-matched groups of mice were injected intradermally in the flank (75 μg MCA/mouse). Fibrosarcoma development was recorded weekly. Alternatively, B16 F0 melanoma cells (provided by M. Mamula, Yale School of Medicine, New Haven, CT) were injected subcutaneously (5 × 104 tumor cells/ mouse) and tumor growth was observed and recorded daily for over 3 wk.
Bone Marrow Chimeras.
Bone marrow chimeras were prepared according to a previously described protocol (23). In brief, recipients were twice irradiated with 500 rads, with a 3-h interval between doses (total 1,000 rads). Donor bone marrow cells (mixed equally from two donors, see Table I for details) were injected intravenously into recipients (107 cells/ mouse). Antibiotics were added to the drinking water for the first 5 wk after reconstitution. At 12 wk after reconstitution, mice were injected intradermally in the flank with MCA or subcutaneously with B16 melanoma cells and monitored for tumor development as described above. At the time of death, splenocytes were used to examine T cell composition and determine the ability of T cell subsets (αβ vs. γδ T cells) to produce IFN-γ as described below.
Fetal Liver Reconstitution.
Fetal liver cell reconstitution was performed as previously described (24). Accordingly, donor cells were isolated from the fetal livers at 14 d of gestation, and 107 unfractioned fetal liver cells in sterile PBS were injected intraperitoneally into 3-d-old recipients. After 8 wk of reconstitution, mice were used for tumor implantation as described above. At the time of sacrifice (3 wk after tumor inoculation), splenocytes were used to examine T cell composition and determine the ability of T cell subsets (αβ and γδ T cells) to produce IFN-γ as described below.
Cell Composition and Intracellular Cytokine Staining.
To test the composition of T cell subsets from bone marrow chimeras and from mice reconstituted with fetal liver cells, splenocytes were stained with anti-CD3 (CyChrome), anti–γδ TCR (FITC), and anti–αβ TCR (PE) followed with FACS® analysis. To confirm the success of the selective depletion of the ability of γδ T cells to produce IFN-γ, 2 × 106/ml splenocytes were cultured with 10 μg/ml anti-CD3 and 1 μg/ml anti-CD28 in the presence of 5 ng/ml IL-12 as described in our previous studies (25). After 4 d of culture, cells were restimulated with anti-CD3 and anti-CD28 in the presence of brefeldin A for 6 h for intracellular IFN-γ staining as detailed below.
Intracellular Cytokine Staining.
For bone marrow chimeras and for mice reconstituted with fetal liver cells, splenocytes cultured as described above were activated with anti-CD3 and anti-CD28 for 6 h with brefeldin A added for the last 2 h. Cells were stained with CyChrome anti-αβ, and FITC anti–γδ TCR antibodies followed with fixation in 2% formaldehyde in PBS. Cells were then permeabilized and stained with PE anti–IFN-γ as previously described (25). Gating was performed on αβ and γδ T cells and the percentage of IFN-γ+ cells was reported.
Immunohistochemical Staining.
Melanoma cells (B16-F0) were inoculated into the right flank of C57BL/6 mice at the age of 7 wk. The mice were killed at various time points after injection (days 1, 3, 5, 7, 9, and 11, respectively). The injected sites, including skin and subcutaneous tissues and tumors per se, were resected and cut across the center. Tissues were then embedded in OCT compound, frozen in liquid nitrogen, and mounted for cryostat sectioning for immunohistochemical staining. 8-μm cryostat sections were fixed in cold acetone for 10 min and rinsed three times with cold PBS for 10 min to remove OCT. Endogenous peroxidase was quenched with 1.5% H2O2 for 10 min at room temperature. The sections were blocked with 10% normal goat serum for 30 min in a humidified chamber and then incubated with primary antibodies against TCR-γδ, CD4, and CD8α for 2 h at room temperature. After washing twice, a biotinylated secondary antibody was added and incubated for 30 min, followed by incubation with ABC for 30 min and the substrate 3′,3′ diaminobenzidine for 5 min, combined with hematoxylin counterstaining for 10 s. Sections of B6 mouse spleen were incubated with anti–mouse TCR-γδ, CD4, and CD8α antibodies as positive controls. Normal goat serum without primary antibodies was used as a negative control. In addition, spleen tissues of TCRδ−/− mouse were stained with anti–mouse TCR-γδ as an additional negative control. Positive cells were counted in five random high power fields under a Nikon microscope.
Preparation of Cell Suspensions from the Site of Tumor Injection.
C57BL/6 mice (n = 10) at age 7 wk were injected with 105 B16F0 melanoma cells. At day 3 after inoculation, small pieces of shaved skin were resected and digested with trypsin-GNK solution (0.29% trypsin, 0.86% NaCl, 0.041% KCl, and 0.1% glucose) at 37°C for 2 h, and then further treated with collagenase/hyaluronidase digestion solution buffer (0.27% collagenase, 0.025% hyaluronidase, 1% DNase, 0.01% Hepes, 0.01% sodium pyruvate in RPMI) for 2 h at 37°C as previously described (26). The digested skin was filtered through a 40-mM cell strainer (Becton Dickinson) and a single cell suspension was obtained containing resident cells and infiltrating cells (26). Cells were stained with anti–CD3-CyChrome and anti–γδ TCR FITC, and cells were then fixed with 2% formaldehyde and further stained for intracellular IFN-γ (anti–IFN-γ PE) as described above.
Tumor Antigen–triggered αβ T Cell IFN-γ Production.
Cultured B16 F0 cells were digested with trypsin and washed with PBS, and then sonicated and centrifuged. The protein concentration of the supernatant was determined by a Bio-Rad kit (Bio-Rad Laboratories) and adjusted to 2 mg/ml. Age- and sex-matched B6 and B6 TCRδ−/− mice were immunized with 100 μg tumor lysate emulsified in CFA in the hind footpad and rechallenged with the same concentration of tumor lysate emulsified in IFA 14 d later. On day 21, the mice were killed and draining lymph node cells were cultured with 100 μg/ml tumor lysate. Intracellular cytokine staining for the analysis of IFN-γ production by CD4+ and CD8+ T cells was performed as described above.
Statistics.
Statistical significance was evaluated by two-tailed, unpaired Student's t test or nonparametric analysis if SD were significantly different between the two compared groups using software InStat 2.03 for Macintosh (GraphPad Software). The incidence of tumor development was compared and analyzed using the log rank test, performed by GraphPad Prism Version 3.0a for Macintosh (GraphPad Software). Throughout the text, figures, and legends, the following terminology was used to denote statistical significance: *, P < 0.0001 or P < 0.001; **, P < 0.01; ***, P < 0.05.
Results
γδ T Cells Are Critical for Preventing Primary and Transplantable Tumor Development.
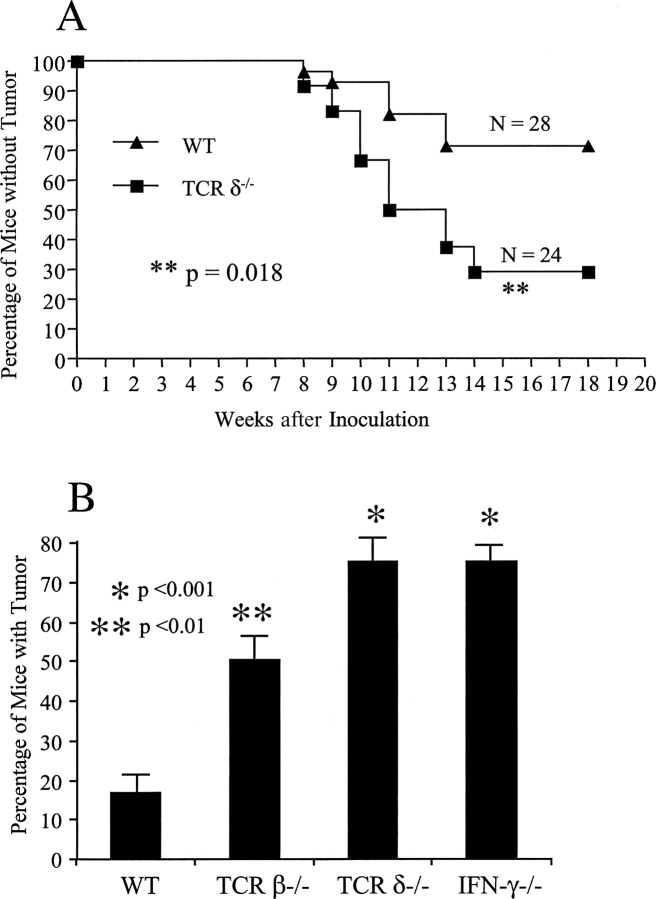
Both lymphocytes and IFN-γ are critical for tumor immunosurveillance, with overlapping effects (5). γδ T cells are critical for preventing MCA-induced fibrosarcoma development in susceptible strains of mice (22). To test the role of γδ T cells in preventing tumor development in a resistant strain, sex- and age-matched B6 wild-type (n = 28) and B6 TCRδ−/− mice (n = 24) were injected with MCA in the flank (75 μg/mouse), and tumor development was observed and measured weekly. Mice lacking γδ T cells had a significantly greater susceptibility to MCA-induced tumors, with 80% of the mice showing tumor development (tumor size larger than 4 × 4 mm) when compared with wild-type TCRδ+/+ mice (20%; Fig. 1 A, p < 0.01). Tumors between 2 × 4 mm without progression in 3 wk were excluded from analysis, as these may result from neovascularization, a IFN-γ–mediated process (2).
Figure 1.
γδ T cells are necessary to prevent tumor development. (A) Protective role of γδ T cells in the MCA-induced tumor model. Sex- and age-matched B6 wild-type (WT, n = 28) and B6 TCRδ-deficient mice (TCRδ−/−, n = 24) were injected with 75 μg MCA/mouse in the flank. Tumor development was recorded weekly after injection. Tumor size >4 × 4 mm was considered positive as previously described (reference 3). (B) Protective role of γδ T cells and IFN-γ in the B16 melanoma transfer model. Four groups of age- and sex-matched mice (B6 wild-type mice, n = 12; B6 TCRβ-deficient mice, n = 18; B6 TCRδ-deficient mice, n = 21; B6 IFN-γ–deficient mice, n = 15), all at 8 wk of age were injected subcutaneously with 5 × 104 B16 F0 melanoma cells and tumor growth was recorded daily. The percentage of mice with tumors 21 d after injection is shown. Data represent mean ± SD of three independent experiments. *, P < 0.001; **, P < 0.01.
To further explore the protective role of γδ T cells in a transplantable tumor model, T cell–intact (WT, n = 12) or T cell subset–deficient mice (TCRβ−/−, n = 18; TCRδ−/−, n = 21) and IFN-γ−/− mice (n = 15) were injected with the melanoma cell line B16 F0 subcutaneously at low doses (5 × 104 cells/mouse). In preliminary studies, titration of the number of injected tumor cells in B6 mice demonstrated that with this dose of tumor cells, only 20–30% of T cell–intact, wild-type mice showed tumor growth (unpublished data). Both αβ and γδ T cells contributed to the protective immune response against tumor development in vivo, indicating a nonredundant role for γδ T cells in transplantable melanoma growth (Fig. 1 B). Consistently, IFN-γ−/− mice also showed a higher susceptibility to tumor growth, reaffirming the critical role of IFN-γ in immunosurveillance (3, 5, 6).
γδ T Cells Are Recruited to the Tumor Injection Site Earlier Than αβ T Cells and Produce IFN-γ Ex Vivo.
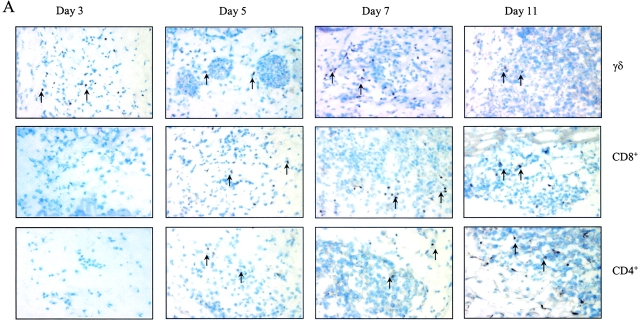
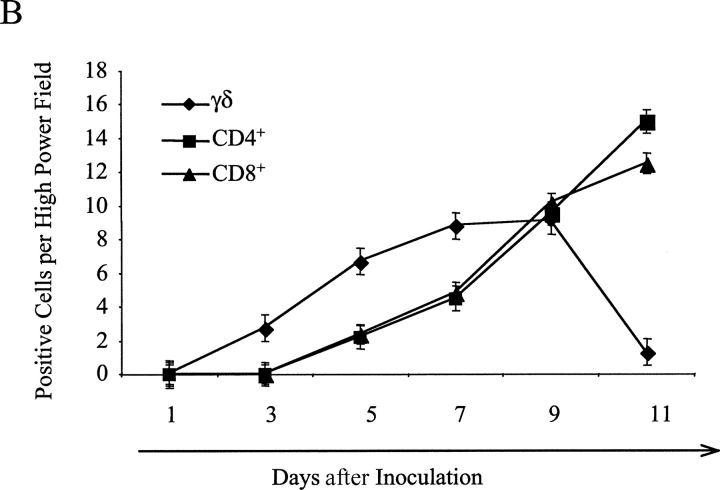
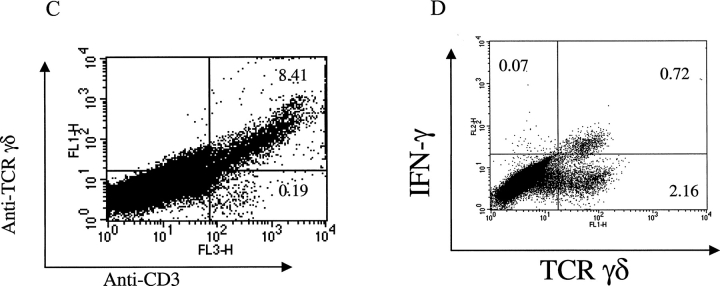
γδ T cells respond to pathogen invasion and quickly expand in the first few days of infection, and thus these T cells may contribute to early pathogen protection (11–14). To investigate whether γδ T cells are early infiltrating immune cells responding to tumor invasion, a series of tissue samples of B16 melanoma injection sites were analyzed by immunohistochemical staining using antibodies against γδ TCR or CD4+ or CD8+ T cells. TCR-γδ+ cells were observed in subcutaneous tissue as early as day 3, and the infiltration of these cells gradually increased and peaked at day 7 (Fig. 2 A, top). In contrast, CD4+ or CD8+ T cell infiltration was not observed at day 3, although these αβ T cells appeared at day 5 and gradually increased in number until reaching a peak at day 11 (Fig. 2 A, middle and bottom, and B). To test whether the infiltrating γδ T cells produce IFN-γ in situ, B6 mice were injected with B16 melanoma cells, the tissue at the tumor injection site was digested, and infiltrating cells were stained directly for anti–γδ TCR and anti-CD3 followed by intracellular IFN-γ staining. Consistent with our immunohistological staining, most of the infiltrating CD3+ lymphocytes were γδ T cells (Fig. 2 C). A minor population of CD3+ T cells (0.19%) might be NK T cells because they did not stain positive for αβ TCR (unpublished data). Strikingly, most IFN-γ–producing cells were γδ T cells and only 0.07% of IFN-γ1 cells were from another cell source (Fig. 2 D). These results imply that γδ T cells infiltrate tumor sites earlier than αβ T cells, providing IFN-γ production that may guide the function of αβ T cells.
Figure 2.
γδ T cells are recruited earlier than αβ T cells into tumor sites. B6 wild-type mice were inoculated with B16 F0 melanoma cells (5 × 104 cells/mouse) and at different time points after inoculation, the tumor injection site was frozen and stained with specific antibodies against TCR γδ, CD4, and CD8 as described in Materials and Methods. (A) One slide from each time point depicting the staining for each cell type is shown. Examples of positive cells are indicated by arrows. (B) Summary of mean ± SD of the number of positive cells per high power field is shown. (C) Cell suspensions were prepared from pools of 10 tissues collected from tumor injection sites and cells were stained with anti-CD3 and anti–γδ TCR. A representative example of a flow cytometry plot is shown. (D) Stained cells (from C) were fixed and permeabilized for intracellular IFN-γ (PE) staining. A representative plot is shown.
γδ T Cells Provide an Early Source of IFN-γ for a Protective Immune Response Against Both Primary Tumor Induction and a Transferred Tumor.
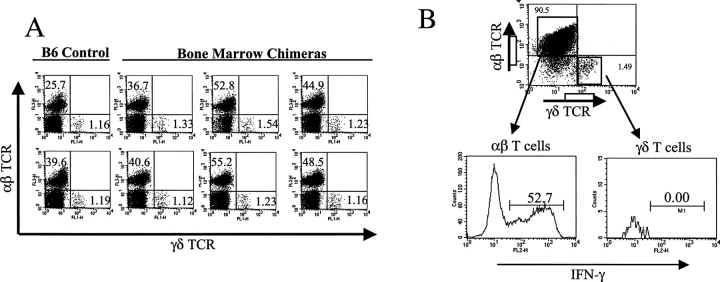
Based upon our previous findings that γδ T cells predominantly produce IFN-γ upon activation (25, 27), and on the fact that IFN-γ plays an essential role in tumor immunosurveillance (1–3), we hypothesized that the γδ T cells that were visualized at the tumor site might play an important protective role through IFN-γ production. To test this hypothesis, we first prepared bone marrow chimeras in which T cell composition is intact (both αβ and γδ T cells), except that the γδ T cells could (γδ IFN-γ+) or could not (γδ IFN-γ−) produce IFN-γ. The detailed composition of donors and recipients is shown in Table I, with γδ IFN-γ− animals labeled as the experimental group and the γδ IFN-γ+ mice as the control group. As expected, experimental chimeras (γδ IFN-γ2) were fully reconstituted with αβ and γδ T cells, with the same ratios of αβ and γδ T cells as wild-type controls (Fig. 3 A). Moreover, upon activation and restimulation in vitro, only αβ (52.7%), but not γδ (0%), T cells produced IFN-γ (Fig. 3 B), indicating the success of obtaining the expected phenotype in this group. The cell composition was very similar in the control group of chimeras (γδ IFN-γ1), although by contrast to the experimental group, their γδ T cells were fully capable of IFN-γ production as expected (unpublished data).
Figure 3.
Generation and analysis of bone marrow chimeras. (A) Composition of αβ and γδ T cells in wild-type mice versus an experimental group of bone marrow chimeras. Bone marrow chimeras were prepared as described in Materials and Methods using donors and recipients (refer to Table I). At the end of the experiments, splenocytes from the experimental group and wild-type mice were prepared and stained with FITC anti–γδ TCR (GL3), PE anti–αβ TCR (H57-597), and CyChrome anti-CD3 (145-2C11). After gating on CD3+ T cells, the composition of γδ and αβ T cells was analyzed. Two controls and six reconstituted mice are shown. (B) IFN-γ production by γδ and αβ T cells in bone marrow chimeras. Splenocytes from bone marrow chimeras (experimental group, γδ IFN-γ2) were cultured with anti-CD3 and anti-CD28 in the presence of IL-12 for 5 d. Cells were then restimulated with anti-CD3 and anti-CD28 in the presence of brefeldin A for 6 h. After staining with FITC anti–TCR γδ and CyChrome anti–TCR αβ, cells were fixed, permeabilized, and further stained with PE anti–IFN-γ. After gating on αβ and γδ T cells, the percentage of IFN-γ+ cells was detected (see representative histogram).
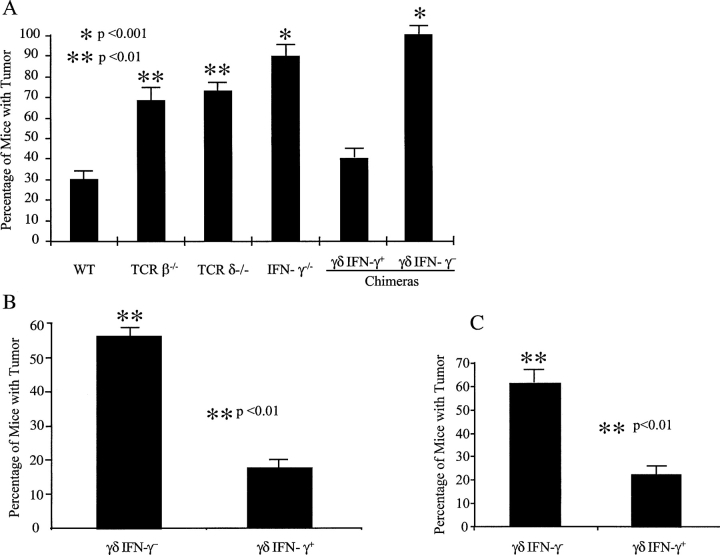
Next, we used these chimeras to explore the role in tumor protection of IFN-γ produced solely by γδ T cells. Here we compared tumor development in reconstituted animals (n = 8–9, 12 wk after reconstitution) to sex- and age-matched B6 wild-type (n = 10), B6 TCRβ−/− (n = 13), B6 TCRδ−/− (n = 11), and B6 IFN-γ−/− (n = 19) mice. At 14 wk after injection with MCA, 70% TCRβ−/− and TCRδ−/− mice showed tumor development (P < 0.05), compared with 30% of wild-type animals (Fig. 4 A, P < 0.05), confirming a nonredundant role for γδ T cells in preventing tumors (22). In addition, we observed a significantly higher percentage of tumors in the IFN-γ−/− mice (Fig. 4 A, P < 0.01), consistent with previous findings that IFN-γ is essential in tumor Immunosurveillance in the MCA-induced tumor model (3).
Figure 4.
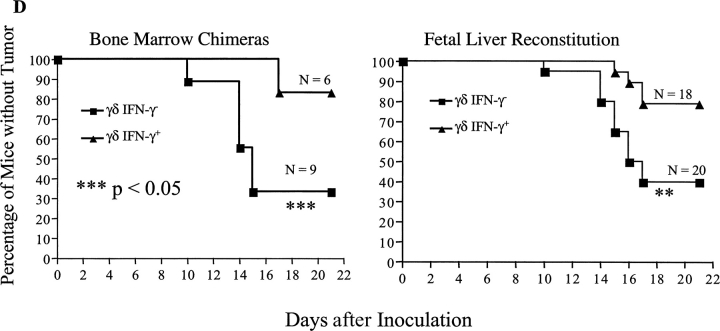
IFN-γ produced by γδ T cells is critical for protective immune responses against tumors. (A) Development of MCA-induced tumors in bone marrow chimeras and in unmanipulated mice. Four groups of age- and sex-matched mice (B6 wild-type mice, n = 10; B6 TCRβ-deficient mice, n = 13; B6 TCRδ-deficient mice, n = 11; B6 IFN-γ–deficient mice, n = 19; all at age 6 wk), and two groups of age- and sex-matched bone marrow chimeras (experimental group, n = 9; control group, n = 8; 10 wk after reconstitution) were injected with MCA (75 μg/mouse in the flank). Animals were observed weekly and tumor size was measured with a calimeter. The percentage of mice that developed tumors is shown. Data represent mean ± SD of three independent experiments. *, P < 0.001; **, P < 0.01. (B) B16 melanoma model in bone marrow chimeras. Bone marrow chimeras reconstituted as in A were injected with 5 × 104 B16 F0 cells/mouse and tumor growth was recorded. The percentage of mice that developed tumors 21 d after inoculation is shown. Data represent mean ± SD of three independent experiments. *, P < 0.001; **, P < 0.01. (C) B16 melanoma model in mice reconstituted with fetal liver cells. TCRδ−/− mice were reconstituted with fetal liver cells either from B6 TCRβ−/− mice (γδ+ IFN-γ+) or from TCRβ−/− IFN-γ−/− (γδ+ IFN-γ−). 8 wk after reconstitution, mice were inoculated with 5 × 104 B16 F0 cells/mouse and tumor growth was recorded. The percentage of mice that developed tumors 21 d after inoculation is shown. Data represent mean ± SD of three independent experiments. *, P < 0.001; **, P < 0.01. (D) Time course of B16 tumor development in bone marrow chimeras (B) and fetal liver reconstituted mice (C).
Strikingly, the experimental group of chimeras, in which the only defect was the ability of γδ T cells to produce IFN-γ (γδ IFN-γ−), had an incidence of tumor development similar to IFN-γ−/− mice (completely lacking IFN-γ), indicating the importance of IFN-γ produced by γδ T cells in preventing tumor development. Moreover, the experimental group of chimeras had a significantly higher tumor incidence than control chimeras (γδ IFN-γ+) or wild-type mice (Fig. 4 A, P < 0.01), although mice from the experimental group only differed from control chimeras or wild-type mice in the ability of their γδ T cells to produce IFN-γ. Thus, our results provide strong evidence for a necessary role of γδ T cells in providing a source of IFN-γ for the protective effect provided by this cytokine.
To further substantiate the requirement for γδ T cells in provision of an early source of IFN-γ in tumor protection, we again turned to the B16 melanoma transplantable tumor model. Sex- and age-matched bone marrow chimeras (experimental: γδ IFN-γ−, n = 9; control: γδ IFN-γ+, n = 6) were inoculated with B16 F0 melanoma cells (5 × 104 cells/mouse) and tumor growth was monitored daily. At day 21 after inoculation, >50% (6 out of 9) of the experimental (γδ IFN-γ−) group developed tumors (>4 × 4 mm), whereas only 17% of control chimeras (γδ IFN-γ+, 1 out of 6) showed sizeable tumor growth (Fig. 4 B, P < 0.01). Moreover, γδ IFN-γ2 bone marrow chimeras developed tumors much earlier when compared with γδ IFN-γ1 chimeras (Fig. 4 D, P < 0.05). This further supports the notion that γδ T cells are a critical source of IFN-γ in preventing tumor growth in vivo.
Finally, to more firmly establish the role of γδ T cells in providing the early source of IFN-γ in tumor immunosurveillance, we also reconstituted γδ T cell–deficient mice with fetal liver cells from mice with an intact γδ T cell repertoire, but with or without the capacity to produce IFN-γ. The advantage of this system is that T cell subsets distribute and repopulate similarly to wild-type mice (24). Moreover, reconstitution takes place as mice mature (during the 5–6 wk after the birth), so that experiments can be performed at ages of 6–8 wk. TCRδ−/− mice at 3 d of birth were reconstituted with fetal liver cells (14 d of gestation) either from TCRβ−/− mice (γδ T cells can produce IFN-γ; hence, mice are γδ IFN-γ+) or from TCRβ−/− IFN-γ−/− mice (γδ T cells cannot produce IFN-γ; hence, mice are γδ IFN-γ−). 8 wk after reconstitution, sex- and age-matched mice from both groups (γδ IFN-γ+, n = 20; γδ IFN-γ−, n = 18), along with unmanipulated B6 wild-type and B6 TCRδ−/− mice, were injected with B16 F0 melanoma cells (5 × 104 cells/mouse) and tumor growth was observed daily. γδ IFN-γ− mice showed a significantly higher rate of tumor growth than γδ IFN-γ+ reconstituted mice (Fig. 4 C, P < 0.01), with significantly earlier kinetics of tumor development (Fig. 4 D, P < 0.01), providing further support for the concept that γδ T cells provide a necessary source of IFN-γ in protective immunity against tumors.
γδ T Cells Regulate Tumor Antigen–triggered αβ T Cell IFN-γ Production.
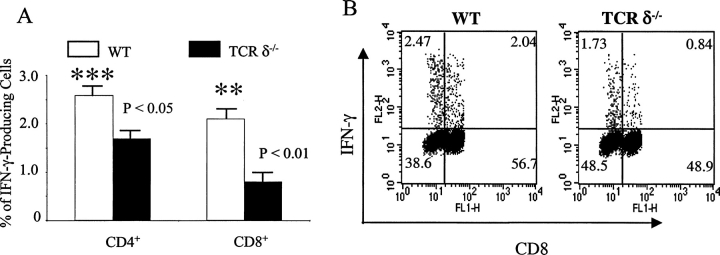
IFN-γ is a critical cytokine for directing both CD4+ and CD8+ T cell differentiation (28, 29). These T cells regulate CD4+ and CD8+ T cell effector function upon pathogen infection (16, 17). Based upon the results described above, we next hypothesized that γδ T cells might regulate tumor antigen–triggered αβ T cell IFN-γ production. To address this question, sex- and age-matched B6 wild-type and B6 TCRδ−/− mice (n = 5 for both groups) were immunized with B16 F0 cell lysates, followed by challenge with identically prepared lysates in IFA 14 d later. The production of IFN-γ by αβ T cells in draining lymph nodes was then analyzed 7 d later upon restimulation with tumor lysate in vitro. Both CD4+ and CD8+ T cells from TCRδ−/− mice produced significantly less IFN-γ upon tumor antigen restimulation in vitro compared with wild-type mice (Fig. 5 A). The finding is consistent with the previous findings that γδ T cells are required to promote IFN-γ production by both CD4+ and CD8+ T cells (15, 17).
Figure 5.
γδ T cells regulate IFN-γ production by tumor antigen–triggered αβ T cells. B6 and B6 TCRδ−/− mice (n = 5 for both strains) were immunized with tumor lysates in CFA and rechallenged in vitro with identically prepared lysates for 24 h. Brefeldin A was added for the last 4 h of culture and cells were then used for intracellular cytokine staining. After gating on CD3+ and CD8+ T cells, the percentage of IFN-γ–producing cells is shown. (A) The percentage of IFN-γ–producing cells from both CD8+ and CD4+ cells is shown (CD8−, lower left quadrant of dot plot shown in B). Data is mean ± SD of five different mice. **, P < 0.01; ***, P < 0.05. (B) One example of intra-cellular cytokine staining from wild-type (WT) and TCRδ−/− mice is shown.
Discussion
It is well established that IFN-γ is an essential mediator for the protective immune response against tumor development (1, 3, 5). However, the source of this cytokine early after tumor challenge has not been identified. NK cells and certain lymphocytes that have features of both innate and adaptive immune cells, including NK T and γδ T cells, are considered to be important for bridging the innate and adaptive immune responses and they might provide the initial source of IFN-γ in tumor protection. This in turn could facilitate the cascade of the adaptive immune response and augment later IFN-γ production (2). In this report, we demonstrate that γδ T cells play a critical role in protective immune responses against tumor development by providing such an early source of IFN-γ, which in turn regulates tumor antigen–triggered CD4+ and CD8+ T cell responses.
We first demonstrated that γδ T cells play a nonredundant role in preventing tumor formation and tumor growth in both primary and transplantable tumor models. γδ T cells have been convincingly shown in many systems to play a critical role in early pathogen protection (11, 30, 31). Recently, Girardi et al. (22) have demonstrated a necessary role for γδ T cells in cutaneous malignancy using TCRδ−/− mice, with NKG-2D–mediated cytolytic activity proposed as the antitumor mechanism. In agreement with these results, here we show that γδ T cells are able to retard tumor growth in both a MCA fibrosarcoma (Fig. 1 A) and a melanoma model (Fig. 1 B). A lack of γδ T cells rendered mice significantly more susceptible to a chemical carcinogen and to tumor cell inoculation compared with wild-type mice. Moreover, TCRδ−/− mice had a tumor incidence similar to TCRβ−/− and IFN-γ−/− mice, indicating a nonredundant role for γδ T cells in protective tumor immune responses.
The most important finding presented in this report is that IFN-γ produced by γδ T cells is critical for a protective immune response against tumor development. Several of our findings support this notion. First, γδ T cells were the first group of T cells recruited into tumor injection site (Fig. 2). It has been demonstrated in many infection systems that γδ T cells are among the first group of cells to expand and infiltrate at inflammatory sites (32, 33). Our report finds for the first time that γδ T cells respond to tumor cell insults earlier than αβ T cells. Moreover, these early infiltrating γδ T cells (at day 3 after inoculation) produce IFN-γ ex vivo in the absence of extra stimulation (Fig. 2 D), strongly suggesting that the early source of IFN-γ is from γδ T cells. However, staining of tumor sites for IFN-γ was negative (unpublished data), likely a consequence of the small amounts of locally produced cytokine or due to the low avidity of anti–IFN-γ antibodies. Nevertheless, our results, together in the setting of the ability of γδ T cells to recognize antigens without the need for engagement of conventional antigen processing pathways and their default production of IFN-γ, led us to believe that the early interaction between γδ T cells and tumor cells might provide the early source of IFN-γ for subsequent αβ T cell activation and differentiation.
To directly address this issue, we constructed mice with an intact αβ and γδ T cell composition, except that the latter lacked the ability to produce IFN-γ. The advantage of these animals is that we could directly assess our hypothesis that the IFN-γ produced by γδ T cells is a critical early source for tumor immunity, in a physiological setting. Our bone marrow chimeras had a composition of αβ and γδ T cells similar to wild-type mice (Fig. 3 A), although we produced animals in which γδ T cells could or could not produce IFN-γ (Fig. 3 B). A similar phenotype was observed in mice selectively reconstituted with fetal liver cells (unpublished data). Using these mice, we demonstrated that IFN-γ made by γδ T cells resulted in susceptibility to both MCA-induced tumors (Fig. 4 A) and B16 melanoma cell inoculation (Fig. 4, B–D). This finding strongly supports our hypothesis that γδ T cells are indeed the early source of IFN-γ for protective immune responses against tumor formation and tumor growth.
Finally, IFN-γ produced by γδ T cells appeared to modulate CD4+ and CD8+ IFN-γ production. Depletion of γδ T cells resulted in significantly reduced IFN-γ production by both CD4+ and CD8+ T cells upon challenge with tumor antigen (Fig. 5), indicating that early production of IFN-γ by γδ T cells enhanced subsequent IFN-γ production by αβ T cells. Without γδ T cell synthesis of IFN-γ, the adaptive immune response was impaired, leading to enhanced tumor susceptibility.
NK and NK T cells have also been shown to play an important role in tumor immunosurveillance, in part dependent on IFN-γ production (34–36). Although NK and NK T cells are intact in our TCRδ−/− mice, the fact that these mice remain highly susceptible to MCA or B16 melanoma inoculation may be explained by the following possibilities. First, NK and NK T cells might be the down-stream targets of γδ T cells and γδ T cells might be required for the competent function of these cells, through direct interaction or provision of IFN-γ. Second, γδ T cells might be the target effector cells of NK and NK T cells. Without γδ T cells the effector function of NK and NKT cells might be impaired. Further analysis of the functional states of NK or NK T cells in TCRδ−/− mice or in our mice with selective depletion of IFN-γ produced by γδ T cells should further our understanding of the interplay between these cell populations.
It should be emphasized that γδ T cells might have other antitumor mechanisms other than provision of IFN-γ for protective immune responses. γδ T cells from synovial fluid of patients with Lyme arthritis are cytolytic toward a wide array of target cells by a Fas–FasL interaction (37), and human intestinal Vδ1 γδ T cells directly recognize tumor cells of epithelial origin (38). Recently, it has been demonstrated that skin γδ T cells directly kill skin carcinoma cells by an NKG-2D-Rae1/H60 interaction (22). However, it is unclear at the present time whether γδ T cell cytolytic function is correlated with, or requires, IFN-γ production. Further studies are needed to clarify whether these two effector arms overlap or if they are in parallel.
In summary, we have presented evidence that IFN-γ produced by γδ T cells is an essential early source of IFN-γ for tumor immunosurveillance, which in turn regulates αβ T cell IFN-γ production. These findings will not only shed light on the molecular mechanisms of immunosurveillance, but may also help in the design of novel therapeutic approaches via manipulation of the function of γδ T cells.
Acknowledgments
We thank Ping Zhu and Christie Hawley for animal care. We thank Dr. Richard Bucala, Dr. Elisabeth Ramsburg, Dr. Daniel H. Kaplan, and Dr. Mark Mamula for critical review the manuscript. We greatly appreciate the kind help provided by Dr. R.E. Tigelaar and Dr. Julia Lewis for preparation of skin cells.
This work was supported in part by an Arthritis Foundation Investigator Award, a National Institutes of Health (NIH; NIAMS) K01 AR 02188 grant, and a Yale Skin Disease Research Center PF grant (to Z. Yin). It was also supported by grants AR40072 and AR44076 from the NIH, by the Arthritis Foundation, the Lupus Foundation of America and their Connecticut chapters, the S.L.E. Foundation, Inc., and a Kirkland Scholars Award (to J. Craft).
Footnotes
Abbreviation used in this paper: MCA, methylcholanthrene.
References
- 1.Ikeda, H., L.J. Old, and R.D. Schreiber. 2002. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13:95–109. [DOI] [PubMed] [Google Scholar]
- 2.Dunn, G.P., A.T. Bruce, H. Ikeda, L.J. Old, and R.D. Schreiber. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991–998. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan, D.H., V. Shankaran, A.S. Dighe, E. Stockert, M. Aguet, L.J. Old, and R.D. Schreiber. 1998. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 95:7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Street, S.E., E. Cretney, and M.J. Smyth. 2001. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 97:192–197. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. [DOI] [PubMed] [Google Scholar]
- 6.Dighe, A.S., E. Richards, L.J. Old, and R.D. Schreiber. 1994. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1:447–456. [DOI] [PubMed] [Google Scholar]
- 7.Hayday, A.C. 2000. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975–1026. [DOI] [PubMed] [Google Scholar]
- 8.Lahn, M. 2000. The role of gammadelta T cells in the airways. J. Mol. Med. 78:409–425. [DOI] [PubMed] [Google Scholar]
- 9.Carding, S.R., and P.J. Egan. 2000. The importance of gamma delta T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol. Rev. 173:98–108. [DOI] [PubMed] [Google Scholar]
- 10.Tough, D.F., and J. Sprent. 1998. Lifespan of gamma/delta T cells. J. Exp. Med. 187:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasper, L.H., T. Matsuura, S. Fonseka, J. Arruda, J.Y. Channon, and I.A. Khan. 1996. Induction of gammadelta T cells during acute murine infection with Toxoplasma gondii. J. Immunol. 157:5521–5527. [PubMed] [Google Scholar]
- 12.Tsuji, M., P. Mombaerts, L. Lefrancois, R.S. Nussenzweig, F. Zavala, and S. Tonegawa. 1994. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc. Natl. Acad. Sci. USA. 91:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S.H. Kaufmann. 1993. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 365:53–56. [DOI] [PubMed] [Google Scholar]
- 14.Moore, T.A., B.B. Moore, M.W. Newstead, and T.J. Standiford. 2000. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J. Immunol. 165:2643–2650. [DOI] [PubMed] [Google Scholar]
- 15.Huber, S.A., D. Graveline, M.K. Newell, W.K. Born, and R.L. O'Brien. 2000. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunol. 165:4174–4181. [DOI] [PubMed] [Google Scholar]
- 16.Nomura, A., G. Matsuzaki, H. Takada, K. Hiromatsu, S. Nabeshima, T. Nakamura, K. Kishihara, and K. Nomoto. 1998. The role of gammadelta T cells in induction of bacterial antigen-specific protective CD8+ cytotoxic T cells in immune response against the intracellular bacteria Listeria monocytogenes. Immunology. 95:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretto, M., B. Durell, J.D. Schwartzman, and I.A. Khan. 2001. Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection. J. Immunol. 166:7389–7397. [DOI] [PubMed] [Google Scholar]
- 18.Groh, V., R. Rhinehart, H. Secrist, S. Bauer, K.H. Grabstein, and T. Spies. 1999. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA. 96:6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groh, V., A. Steinle, S. Bauer, and T. Spies. 1998. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 279:1737–1740. [DOI] [PubMed] [Google Scholar]
- 20.Wu, J., Y. Song, A.B. Bakker, S. Bauer, T. Spies, L.L. Lanier, and J.H. Phillips. 1999. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 285:730–732. [DOI] [PubMed] [Google Scholar]
- 21.Bauer, S., V. Groh, J. Wu, A. Steinle, J.H. Phillips, L.L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729. [DOI] [PubMed] [Google Scholar]
- 22.Girardi, M., D.E. Oppenheim, C.R. Steele, J.M. Lewis, E. Glusac, R. Filler, P. Hobby, B. Sutton, R.E. Tigelaar, and A.C. Hayday. 2001. Regulation of cutaneous malignancy by gamma delta T Cells. Science. 294:605–609. [DOI] [PubMed] [Google Scholar]
- 23.Aubagnac, S., M. Brahic, and J.F. Bureau. 2002. Bone marrow chimeras reveal non-H-2 hematopoietic control of susceptibility to Theiler's virus persistent infection. J. Virol. 76:5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly, K.A., R. O'Brien, and W. Born. 1997. Reconstitution of SCID mice with haemopoietic precursors: a detailed analysis of gamma delta T-cell reconstitution. Immunology. 91:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin, Z., D.H. Zhang, T. Welte, G. Bahtiyar, S. Jung, L. Liu, X.Y. Fu, A. Ray, and J. Craft. 2000. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J. Immunol. 164:3056–3064. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Y., L.L. McCormick, S.R. Desai, C. Wu, and A.C. Gilliam. 2002. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J. Immunol. 168:3088–3098. [DOI] [PubMed] [Google Scholar]
- 27.Yin, Z., C. Chen, S.J. Szabo, L.H. Glimcher, A. Ray, and J. Craft. 2002. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J. Immunol. 168:1566–1571. [DOI] [PubMed] [Google Scholar]
- 28.Afkarian, M., J.R. Sedy, J. Yang, N.G. Jacobson, N. Cereb, S.Y. Yang, T.L. Murphy, and K.M. Murphy. 2002. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 3:549–557. [DOI] [PubMed] [Google Scholar]
- 29.O'Garra, A., and K. Murphy. 1994. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 6:458–466. [DOI] [PubMed] [Google Scholar]
- 30.Huber, S., C. Shi, and R.C. Budd. 2002. Gammadelta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J. Virol. 76:6487–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jouen-Beades, F., E. Paris, C. Dieulois, J.F. Lemeland, V. Barre-Dezelus, S. Marret, G. Humbert, J. Leroy, and F. Tron. 1997. In vivo and in vitro activation and expansion of gammadelta T cells during Listeria monocytogenes infection in humans. Infect. Immun. 65:4267–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shires, J., E. Theodoridis, and A.C. Hayday. 2001. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity. 15:419–434. [DOI] [PubMed] [Google Scholar]
- 33.Fahrer, A.M., Y. Konigshofer, E.M. Kerr, G. Ghandour, D.H. Mack, M.M. Davis, and Y.H. Chien. 2001. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl. Acad. Sci. USA. 98:10261–10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowe, N.Y., M.J. Smyth, and D.I. Godfrey. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 196:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brutkiewicz, R.R., and V. Sriram. 2002. Natural killer T (NKT) cells and their role in antitumor immunity. Crit. Rev. Oncol. Hematol. 41:287–298. [DOI] [PubMed] [Google Scholar]
- 36.Basse, P.H., T.L. Whiteside, W. Chambers, and R.B. Herberman. 2001. Therapeutic activity of NK cells against tumors. Int. Rev. Immunol. 20:439–501. [DOI] [PubMed] [Google Scholar]
- 37.Roessner, K., J. Wolfe, C. Shi, L.H. Sigal, S. Huber, and R.C. Budd. 2003. High expression of Fas ligand by synovial fluid-derived gamma delta T cells in Lyme arthritis. J. Immunol. 170:2702–2710. [DOI] [PubMed] [Google Scholar]
- 38.Maeurer, M.J., D. Martin, W. Walter, K. Liu, L. Zitvogel, K. Halusczcak, H. Rabinowich, R. Duquesnoy, W. Storkus, and M.T. Lotze. 1996. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J. Exp. Med. 183:1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]