Abstract
Neurotrophins (nerve growth factor [NGF], brain-derived neurotrophic factor [BDNF], neurotrophin [NT]-3, and NT-4) have been observed in elevated concentrations in allergic diseases. Neurotrophin levels are up-regulated endobronchially after allergen challenge. This coincides with an influx of activated eosinophils into the bronchial lumen. These eosinophils have an increased viability and CD69 expression 18 h after segmental allergen provocation (SAP) which is not present in peripheral blood. To investigate whether these observations are related we studied the influence of neurotrophins on eosinophil function in allergic asthma.
Incubation with NGF, BDNF, NT-3, or NT-4 caused a significant increase in the viability and CD69 expression of isolated eosinophils from bronchoalveolar lavage fluid (BALF) but not from peripheral blood, suggesting a unique sensitivity of endobronchial eosinophils to neurotrophins. To elucidate the underlying mechanisms expression of the neurotrophin receptors p75NTR, trkA, trkB, and trkC on eosinophils was analyzed by RT-PCR and immunocytology. After SAP expression of all neurotrophin receptors was markedly elevated on eosinophils from BALF. Our findings suggest that neurotrophin-mediated activation of bronchial eosinophils might play a role in the regulation of eosinophilic inflammation in allergic asthma.
Keywords: allergic asthma, eosinophils, neurotrophins, neurotrophin receptors, segmental allergen provocation
Introduction
Bronchial asthma is a chronic inflammatory disorder with an increased responsiveness of the airways to nonspecific stimuli. Inhalation of allergen causes a biphasic, reversible airflow obstruction referred to as the early and late phase response. During the late phase response an infiltration of the airways with activated T lymphocytes and eosinophils has been recognized and this has been associated with an increase in bronchial hyperreactivity (BHR)* (1–4).
Neurotrophins such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, and NT-4 have recently gained widespread attention in allergic diseases. Initial evidence came from investigations in humans where NGF concentrations in the peripheral blood correlated with the severity of allergic manifestations and highest levels of NGF were measured in patients with asthma. They were also correlated with other atopy-related parameters such as eosinophil cationic protein (ECP) and IgE Ab levels (5). Further investigations found increased concentrations of NGF and NT-3 in bronchoalveolar lavage fluid (BALF) in patients with asthma compared with nonatopic normal controls (6). Recently, we were able to report that segmental allergen provocation (SAP) induces an increase in the concentrations of NGF, BDNF, and NT-3 in BALF suggesting that neurotrophins are produced locally following allergen provocation (7). Possible sources of neurotrophins in allergic inflammation are neurons, neuron-associated cells (8–10) and immune cells such as macrophages, mast cells, T cells, B cells, epithelial cells, and eosinophils (11–18).
It has been speculated that the release of neurotrophins in atopic diseases after allergen challenge might link inflammatory changes observed in asthma with the increase in bronchial hyperresponsiveness. In an animal model of asthma NGF can mimic several key features of asthma by augmenting BHR and allergic inflammation (15), probably due to neurotrophin-induced up-regulation of neuropeptide production in sensory neurons (19, 20). Neurotrophins not only affect nerve cells but can also interfere with allergy related immune cell functions such as mast-cell degranulation, Th2 cytokine synthesis, Ab production from B cells, and eosinophil survival (15, 21–23).
The neurotrophin effects are mediated by the low affinity (Kd 10−9) pan-neurotrophin receptor p75NTR to which all members of the neurotrophin family bind with similar affinity (24, 25), and/or the tyrosine protein kinase receptors of the tyrosine kinase, high affinity neurotrophin receptor (trk) family to which neurotrophins bind with high affinity (Kd 10−11). TrkA has been identified as the preferred receptor for NGF (26, 27) and trkB has been reported to exert the effects of both BDNF and NT-4 (28, 29). For NT-3, trkC plays a central role in cellular signaling (30). Neurotrophin receptors are expressed on several immune cells including mast cells, T cells, B cells, and macrophages (14, 21, 31–37). Previous investigations about neurotrophin receptor expression in the bone marrow indicated that expression on eosinophils depended on the stage of differentiation. Polymorphnuclear eosinophils in human bone marrow preparations showed immunoreactivity for trkB kinase, the truncated trkC and the trkC kinase (38). In contrast, peripheral blood granulocytes do not express mRNA for neurotrophin receptors (39).
Based on the pleiotrophic actions of neurotrophins on other cells of the inflammatory cascade in allergic asthma we hypothesized that neurotrophins might have direct effects on eosinophil function in human allergic inflammation. To address this question neurotrophin receptor expression on eosinophils was investigated before and after segmental allergen challenge in peripheral blood and BALF cells from patients with allergic asthma and eosinophil function and survival in response to neurotrophins was tested in vitro.
Materials and Methods
Subjects.
Eight nonsmoking, allergic subjects (3 female and 5 male) with mild asthma (mean age 27.4 ± 1.1 yr, range 23–33) and a mean FEV1 (forced expiratory volume in one second) of 99.5 ± 3.9% (84.2–117.0%) of predicted (40) were included in the study. Mean total IgE levels were 318.4 ± 106.1 kg units per liter (kU/l; range 71.8–986.0 kU/l). All patients had positive skin prick test reactions (Allergopharma) and elevated specific IgE concentrations (36.5 ± 12.7 kU/l; Kabi, Pharmacia CAP System®) to at least one common aeroallergen. All subjects had a history of intermittent wheeze, chest tightness, cough, and sputum production and reversible bronchoconstriction after inhalation of allergens (Table I). There was no evidence suggesting respiratory tract infections before or at the time of the segmental allergen challenge. All subjects received inhaled β2-agonist therapy as needed. Inhaled corticosteroids were withdrawn at least 7 d before the study. All subjects gave informed consent. The study protocol was approved by the Ethics Committee of the University of Freiburg.
Table I.
Patient Characteristics
| Sex | Age | FEV1 baseline (liter) |
FEV1% pred | IgE (kU/l) |
Allergen | Specific IgE (kU/l) |
Allergen Dose (AU) |
Medication |
|---|---|---|---|---|---|---|---|---|
| f | 33 | 3.1 | 98.7 | 72 | birch | 6.2 | 160 | β |
| f | 27 | 2.9 | 84.2 | 83 | birch | 16.0 | 3 | β, ICS |
| m | 29 | 4.1 | 91.2 | 214 | D. pt. | 35.0 | 6 | β |
| m | 29 | 4.5 | 96.5 | 242 | birch | 93.5 | 88 | β, ICS |
| m | 26 | 3.7 | 97.6 | 161 | birch | 59.2 | 526 | β |
| m | 27 | 5.7 | 114.4 | 986 | rye | >100 | 98 | β |
| m | 23 | 5.8 | 117.0 | 306 | D. pt. | 33.9 | 4,700 | β |
| f | 25 | 3.1 | 87.0 | 483 | D. pt. | 99.5 | 35 | β |
Birch, birch pollen; D. pt., Dermatophagoides pteronyssinus; rye, rye pollen; β, β2-agonists; ICS, inhaled corticosteroids.
The healthy donors had no history of atopic diseases, normal pulmonary function tests, IgE levels within the normal range, and had not been taking any medication 4 wk before the study. All patients and normal controls were nonsmokers.
Inhaled Allergen Provocation.
The inhaled allergen provocation was performed as described previously (41). The individual provocative dose of allergen (rye pollen, birch pollen, or house dust mite allergen) that caused a 20% fall in FEV1 (PD20) was extrapolated for each patient according to the cumulative dose of allergen inhaled until a drop of the FEV1 of >20% was recorded. A 10-fold higher dose of allergen was then used for the subsequent segmental allergen provocation. Inhaled and segmental allergen provocations were at least 3 wk apart.
Segmental Allergen Provocation.
Bronchoscopy and SAP were performed as described previously (41) using an Olympus BF 1T30 (Olympus Optical Co.) following local anesthesia (Novesine®; Wander). Saline (2.5 ml) was instilled into one segment of the lower left lobe (usually B8) and into the inferior lingular bronchus (B5 left; control). 10 min later, the lower lobe segment was lavaged with 100 ml prewarmed saline. Two aliquots of allergen extract were diluted in 2.5 ml of saline and instilled into two segments of the right lung (B8 and B5). After another 10 min after endoscopic allergen deposition, the lower lobe segment was lavaged with 100 ml of normal, prewarmed saline in aliquots of 20 ml. Patients were rebronchoscoped after 18 h at which time the lingular and middle lobe bronchus were again lavaged with 100 ml of saline as described above.
Analysis of Bronchoalveolar Lavage Leukocytes.
BALF samples were filtered through a two layer sterile gauze into sterile plastic vials, centrifuged at 4°C and 500 g for 10 min. After removing the supernatants, cytospins were made and cells stained with May Grünwald Giemsa solution. Differential cell counts were performed on all nucleated cells and results expressed as total number of cells per ml of recovered fluid.
Purification of Eosinophils.
Eosinophils were purified as described (42). Peripheral blood eosinophils were obtained from 27 ml EDTA blood of asthmatics 10 min before and 18 h after SAP or from 54 ml EDTA blood of healthy donors. Blood was diluted 1:1 with PBS and 20 ml aliquots were overlayered onto 20 ml of isotonic Percoll® solution (density 1.8 g/ml; Amersham Biosciences) and centrifuged for 30 min at 1,000 g at 4°C. After centrifugation, the supernatant was removed and the mononuclear cells at the interface were aspirated. Erythrocytes and platelets were removed by hypotonic lysis (0.2% NaCl for 30 s). Eosinophils were separated by negative selection of neutrophils, using immunomagnetic beads. The pellet was resuspended in 1 ml PBS (Dulbecco) containing 2% heat inactivated FCS (PBS/2% FCS; GIBCO BRL) and 5 μl of CD16 microbeads (Miltenyi Biotech) per 107 neutrophils were added for 30 min at 4°C with occasional mixing. The cell suspension was added on top of the separation column which was placed in a magnetic field (VarioMACS®; Miltenyi Biotech). Eosinophils were eluted with ice cold PBS/2% FCS. After separation, cells were washed in PBS/2% FCS and then diluted in culture medium (RPMI 1640 with 10% heat inactivated FCS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin [Seromed; Biochrom]). The purity of eosinophils was consistently >98%, as assessed by Kimura staining.
The procedure of isolating eosinophils from BALF was performed in a similar fashion. BALF cells were diluted in 5 ml culture medium and overlayered onto 5 ml Percoll® solution. After washing the cells twice, differential cell counts were performed and eosinophils were separated from neutrophils as described above. The purity of BALF eosinophils was also consistently >98%, as assessed by Kimura staining.
Cell Culture.
Eosinophils (0.5 × 105 cells/ml) were cultured at 37°C in a humidified atmosphere with 5% CO2 either in the culture medium alone or in the presence of 0.05, 0.5, 5, or 50 ng/ml rhuman β-NGF (BioConcept), BDNF (Biosource International) rhuman NT-3, rhuman NT-4 (both BioConcept), or 10 ng/ml rhuman IL-5 (Genzyme). All neurotrophins and the IL-5 contained less than 0.1 ng endotoxin per μg. Before immunofluorescence labeling, cells were washed twice in PBS/2% FCS.
Flow Cytometric Analysis of CD69 Cell Surface Expression.
Flow cytometric analysis of CD69 cell surface expression was performed as described previously (42). 20 μl of cell suspensions (5 × 104 cells) were incubated in the presence of saturating concentrations of PE conjugated mouse anti–human CD69 mAbs (Becton Dickinson) or PE-conjugated IgG1 (DakoCytomation), respectively, for 30 min on ice. Cells were then washed once in PBS/2% FCS and resuspended in 100 μl of a propidium-iodide solution (PI; 0.5 μg/ml dissolved in PBS; Sigma-Aldrich). Flow cytometry was performed on at least 5,000 cells from each sample with a FACScan™ Flow Cytometer (Becton Dickinson). To include only viable cells in the analysis, PI positive, nonviable cells were excluded by appropriate gating in a separate fluorescence channel. Nonspecific fluorescence was determined by incubating cells with mouse IgG of the same isotype but with irrelevant Ag specificity (IgG1). The specific mean fluorescence (SMF) for each population was determined by subtracting the nonspecific mean fluorescence (NMF) of the isotype control. To correct for variations in cell autofluorescence between patients and different cell populations, the relative SMF (rSMF) was calculated, which is the ratio of the SMF emitted by the bound specific mAbs and that of the NMF of the corresponding isotype control (43).
Survival Assay.
Survival of cultured eosinophils was assessed in a PI assay. 5 × 104 cells were washed once in PBS and resuspended in 100 μl of a PI solution. Flow cytometry was performed on at least 5,000 cells from each sample. The data were aquired and analyzed by the CELLQuest™ software (Becton Dickinson).
Neutralization of Neurotrophin-induced Survival and CD69 Surface Expression
To verify that the observed effects were mediated by the different neurotrophins, BALF eosinophils were incubated with either 50 ng/ml NGF, BDNF, NT-3, or NT-4 alone or in combination with either a monoclonal mouse IgG1 (20 μg/ml) or a mouse NGF, BDNF, NT-3, or NT-4 mAb (each 20 μg/ml, IgG1; R&D Systems). Viability and CD69 expression of eosinophils were measured after 10 d or after 4 h, respectively, as described above.
RT-PCR.
Neurotrophin receptor mRNA expression of purified eosinophils obtained 18 h after SAP from peripheral blood or BALF fluid of patients with asthma, respectively, was examined. mRNA was extracted from total cellular RNA using commercially available (m)RNA-isolation kits (RNeasy Mini Kit and Oligotex mRNA Isolation Kit, both QIAGEN). Reverse transcription was performed with 7.5 ng of mRNA from each sample in a 20 μl reaction for 60 min at 37°C followed by 5 min at 95°C according to the manufacturer's recommendation (Sensiscript RT-Kit; QIAGEN). As control, 7.5 ng mRNA of each sample was treated according to the same protocol with addition of water instead of the Reverse Transcriptase. Real time PCR was performed on the Light Cycler Instrument (Roche Molecular Biochemicals) using the DNA-binding dye SYBR green (Light Cycler - Fast Start DNA Master SYBR Green I; Roche Molecular Biochemicals). PCR reaction mix contained 2 μl PCR master mix supplemented with 2.5 mM MgCl2 (magnesium chloride), custom synthesized primers (MWG Biotech), and 2 μl of cDNA or control RNA, respectively, in a final volume of 20 μl. The specific primers used for PCR were β-actin (sense: 5′-TCCCTGGAGAAGAGCTACGA-3′, antisense: 5′-AGCACTGTGTTGGCGTACAG-3′; GenBank/EMBL/DDBJ accession no. BC013380; expected product length 194 bp), p75NTR (sense: 5′-CCTACGGCTACTACCAGGATG-3′; antisense: 5′-TGGCCTCGTCGGAATACG-3′; GenBank/EMBL/DDBJ accession no. M14764; calculated product length: 147 bp) (44), trkA (sense: 5′-AATGCCTCGGTGGATGTG-3′; antisense: 5′-AGCGTGTAGTTGCCGTTGTT-3′; GenBank/EMBL/DDBJ accession no. NM_002529; expected product length: 479 bp), trkB (sense: 5′-CCCACTCACATGAACAATGG-3′; antisense: 5′-TCAGTGACGTCTGTGGAAGG-3′; GenBank/EMBL/DDBJ accession no. NM_006180; calculated product length: 221 bp), and trkC (sense: 5′-AAGCGAGAACTGGGTGAGG-3′; antisense: 5′-ATGTGGAGCATTTGGGAGAG-3′, GenBank/EMBL/DDBJ accession no. S76475; expected product length: 356 bp). After an initial denaturation step at 95°C for 10 min the PCR reaction was performed with an annealing temperature of 60°C for 10 s, followed by an extension phase at 72°C for 9 s, and a denaturation cycle at 95°C for 1 s. At the end of each extension phase, fluorescence was observed at 72°C. The PCR reaction was completed after 45 cycles. The melting point analysis was performed by heating the amplicon from 65 to 95°C and revealed the characteristic melting point for each product. After cooling down to 40°C the product was extracted from the capillary. 10 μl of each reaction were run onto a 1.5% agarose gel and visualized by staining with ethidium bromide. PCR products were commercially sequenced (GATC Biotech).
Immunocytochemistry.
To examine neurotrophin receptor protein expression on eosinophils, immunocytochemistry was performed on acetone fixed cytospin preparations from BALF and purified blood eosinophils after SAP with the EnVision™ Alkaline Phosphatase System for mouse and rabbit Abs/Fast Red (DakoCytomation) according to the manufacturer's instructions. After washing in aqua bidest and in TBS (Tris-phosphate buffered saline; 0.05 M Tris, 0.9% NaCl, pH 7.6), cytospin preparations were blocked for 2 h with 2% goat serum (DakoCytomation) in TBS. Primary Abs diluted in TBS were added in optimized concentrations (up to 10 μg/ml) and incubated overnight at 4°C. Irrelevant isotypes from the same species served as negative controls. In one experiment, the 10-fold concentration of each isotype control antibody was used to ascertain the specificity of the binding of the antibodies used. After washing in TBS (3 × 5 min), EnVision™ anti–rabbit/mouse was added for 30 min. Three washes with TBS were followed by counterstaining with the Fast Red solution (one tablet and three drops levamisole dissolved in 3 ml of buffer). All cytospins were microscopically examined and photodocumented with the help of the photomicrographic system SC 35 (Olympus).
Antibodies.
A monoclonal anti–human nerve growth factor receptor (p75NTR) antibody, clone NGFR5 (NeoMarkers) directed against the extracellular domain of the receptor, was used to study surface and cytoplasmatically localized p75NTR immunoreactivity. The polyclonal rabbit antibodies TrkA (763), TrkB (794) (all Santa Cruz Biotechnology, Inc.), TrkB23–36 (Chemicon), TrkB (H-181), and TrkC (798) (both from Santa Cruz Biotechnology, Inc.) and the monoclonal mouse antibodies TrkA, clone H-10 (Cymbus Biotechnology LTD, distributed by Chemicon) and TrkC, clone 75219 (R&D Systems), were used to study Trk family receptor expression. TrkA (763) reacts with a carboxy terminal epitope of human TrkA (amino acids 763–777). TrkB (794) and TrkC (798) with carboxy terminal peptides (794–808 and 798–812 residues, respectively) of gp145TrkB or gp145TrkC proteins. TrkB23–26 is raised against a synthetic peptide from TrkB (amino acids 23–36) and TrkB (H-181) against a recombinant protein corresponding to amino acids 160–340 mapping within the extracellular domain of TrkB of human origin. Mouse IgG1 was used as isotype-control for the anti-p75NTR antibody, mouse IgG2a for the monoclonal TrkA antibody, mouse IgG2b for the monoclonal trkC antibody (both from DakoCytomation), and rabbit IgG (R&D Systems) for the polyclonal antibodies.
Statistical Analysis.
Results are expressed as arithmetic mean ± SEM. Statistical evaluation was performed with SigmaStat® for Windows. Differences between two normal variable groups were analyzed using the unpaired Student t test. Differences before and after SAP in normally distributed groups were analyzed using the paired t test or the signed rank test for nonparametric samples, respectively. Differences with P values <0.05 were considered statistically significant.
Results
Eosinophils in Peripheral Blood and BALF after Segmental Allergen Provocation.
A marked increase in the number of eosinophils was observed in the allergen challenged segment 18 h after allergen provocation which was significantly higher (P < 0.05) compared with the segment lavaged 10 min after allergen challenge or 10 min and 18 h after saline challenge (Table II).
Table II.
Cellular Composition in BALF after SAP (Cells × 103/ml; Mean ± SEM)
| Recovery | Macrophages(×103/ml) | Lymphocytes(×103/ml) | Eosinophils(×103/ml) | Neutrophils(×103/ ml) | |
|---|---|---|---|---|---|
| 10 min saline | 55 ± 4 ml | 297.8 ± 137.2 | 28.2 ± 8.2 | 1.4 ± 0.6 | 0.3 ± 0.2 |
| 10 min allergen | 52 ± 5 ml | 286.7 ± 130.9 | 14.2 ± 2.5 | 1.5 ± 0.4 | 1.1 ± 0.5 |
| 18 h saline | 63 ± 5 ml | 433.1 ± 171.3 | 34.1 ± 3.8 | 1.3 ± 0.3 | 5.1 ± 1.5 |
| 18 h allergen | 54 ± 3 ml | 357.9 ± 124.2 | 87.6 ± 18.6* | 129.6 ± 35.0* | 109.1 ± 41.8* |
10 min saline: control segment lavaged 10 min after instillation of 2.5 ml normal saline; 10 min allergen: allergen challenged segment lavaged 10 min after instillation of allergen with 10× of the inhaled provocation dose causing a 20% fall in forced expiratory volume in one second (PD20); 18 h saline: control segment lavaged 18 h after instillation of 2.5 ml normal saline, 18 h allergen: allergen challenged segment lavaged 18 h after instillation of allergen 10× PD20. Cell numbers are given in absolute numbers; n = 8; *P < 0.05 compared to 18 h saline.
In addition, there was a significant increase in the number of peripheral blood eosinophils 18 h after allergen instillation compared with the number of eosinophils 10 min before SAP (P < 0.05; Table III).
Table III.
Cellular Composition in Venous Blood 10 min before and 18 h after SAP (Cells × 103/ml; Mean ± SEM)
| Cells × 103/ml | Monocytes | Lymphocytes | Eosinophils | Neutrophils | Basophils |
|---|---|---|---|---|---|
| 10 min before SAP | 488.0 ± 108.4 | 1,945.9 ± 112.4 | 248.4 ± 62.0 | 3,949.2 ± 552.7 | 41.7 ± 11.5 |
| 18 h after SAP | 549.5 ± 76.7 | 1,958.3 ± 242.7 | 378.6 ± 82.0* | 5,064.2 ± 742.4 | 46.7 ± 11.3 |
10 min before SAP: venous blood taken 10 min before SAP, 18 h after SAP: venous blood taken 18 h after SAP; n = 8; *P < 0.05 compared to 10 min before SAP.
Due to the limited total number of cells recovered, it was possible to separate sufficient quantities of eosinophils from both peripheral blood 10 min before and 18 h after SAP as well as from allergen challenged lung segments 18 h after allergen treatment in only four patients for further in vitro experiments. In contrast, the purification of eosinophils from the BALF of the saline challenged lung segments after 10 min and 18 h as well as those of the allergen treated segment after 10 min did not yield sufficient numbers of eosinophils to allow cell culture and further analysis.
CD69 Expression on Eosinophils after SAP.
To determine the activation state of eosinophils, the expression of the activation marker CD69 was measured. CD69 on peripheral blood eosinophils of healthy donors (n = 8) was 0.1 ± 0.0 rSMF and showed no difference to that of patients with asthma 10 min before SAP. There was a slight increase 18 h after allergen challenge (0.3 ± 0.1 rSMF) but this difference did not reach statistical significance. In contrast, eosinophils obtained from BALF 18 h after SAP showed a markedly increased CD69 surface expression (4.9 ± 0.3 rSMF; P < 0.05 to blood eosinophils taken 18 h after SAP).
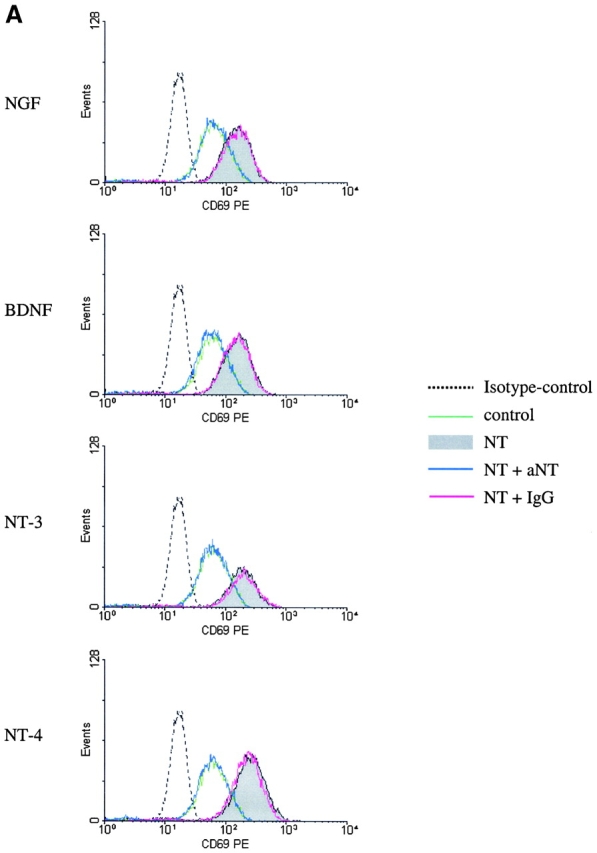
CD69 Expression of BALF and Peripheral Blood Eosinophils after Stimulation with Neurotrophins.
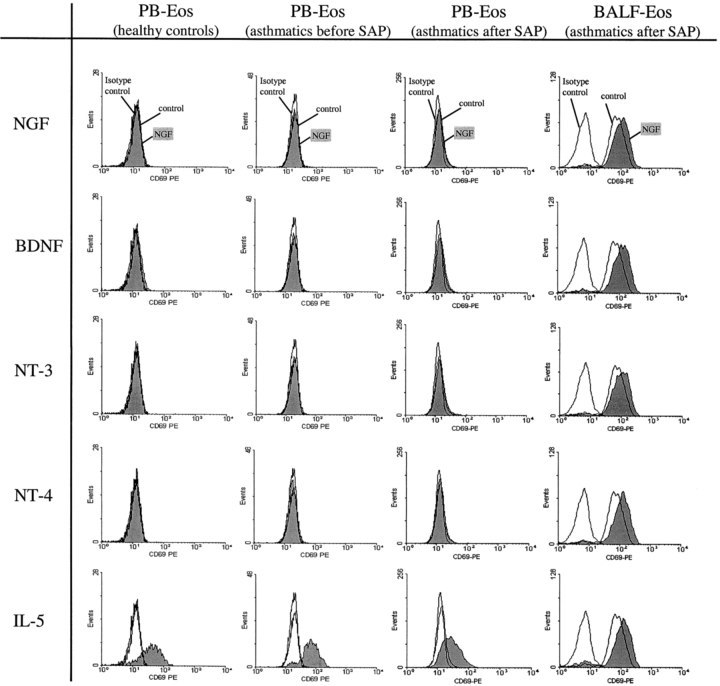
To assess whether neurotrophins can induce CD69 expression, purified eosinophils obtained from peripheral blood of healthy donors and from asthmatics 10 min before and 18 h after SAP were cultured with NGF, BDNF, NT-3, or NT-4 (50 ng/ml each) for 4 h. No significant effect on the activation of the cells as determined by CD69 expression was detected (n = 8 [healthy controls] or n = 4 [asthmatics], respectively; Fig. 1) , whereas stimulation with IL-5 caused a significant increase (P < 0.05) in CD69 (healthy donors: 2.1 ± 0.3 rSMF, asthmatics 10 min before SAP: 2.3 ± 0.4 rSMF, asthmatics 18 h after SAP: 1.9 ± 0.3 rSMF).
Figure 1.
Comparison of the CD69 expression of peripheral blood eosinophils with BALF eosinophils after SAP. Purified eosinophils (0.5 × 105/ml) of peripheral blood obtained from healthy donors or from asthmatics 10 min before and 18 h after SAP and BALF eosinophils from asthmatics obtained 18 h after SAP were incubated with 50 ng/ml NGF, BDNF, NT-3, or NT-4, respectively, or with cell culture medium alone (control). After 4 h, the CD69 expression was measured by flow cytometry. The figures show representative histograms of at least four independent experiments.
Stimulation of BALF eosinophils both with NGF, BDNF, NT-3 or NT-4, respectively, caused a significant increase in CD69 surface expression (NGF: 5.8 ± 0.3 rSMF, BDNF: 6.4 ± 0.5 rSMF, NT-3: 5.7 ± 0.2 rSMF, NT-4: 6.8 ± 0.7 rSMF; n = 4, each P < 0.05 compared with control [4.9 ± 0.3 rSMF]; Fig. 1). In BALF eosinophils 50 ng/ml of neurotrophins caused a similar degree of eosinophil activation (as measured by CD69 expression) as did 10 ng/ml IL-5 (5.8 ± 0.3 rSMF).
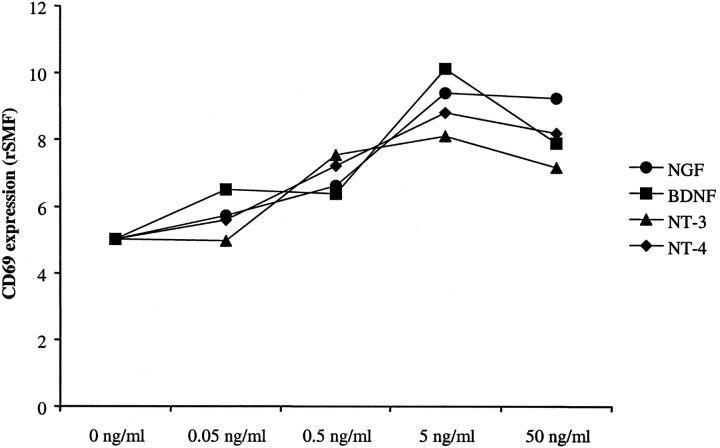
The neurotrophin-mediated increase of CD69 expression was dose-dependent and comparable results were found for NGF, BDNF, NT-3, and NT-4 (Fig. 2) .
Figure 2.
Concentration dependence of the CD69 expression in BALF eosinophils after SAP. Purified BALF eosinophils (0.5 × 105/ml) from asthmatics obtained 18 h after SAP were incubated with 0.05, 0.5, 5, or 50 ng/ml NGF, BDNF, NT-3, or NT-4, respectively, or with cell culture medium alone (control, 0 ng/ml). After 4 h, the CD69 expression was measured by flow cytometry. The figure shows representative results from one of two independent experiments.
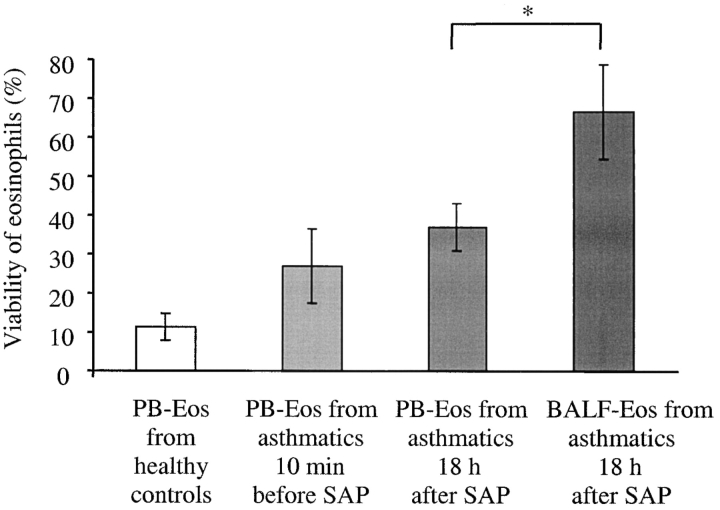
Ex Vivo Viability of Eosinophils Obtained from Peripheral Blood and BALF after SAP.
The viability of eosinophils was examined by PI exclusion. The percentage of viable eosinophils from BALF was significantly increased compared with peripheral blood cells from healthy controls and from asthmatics 10 min before and 18 h after SAP (Fig. 3) . After 3 d in culture, the viability of BALF eosinophils was significantly increased (66.4 ± 12.2%, P < 0.05, n = 4) compared with that from peripheral blood eosinophils isolated 10 min before (22.1 ± 11.1%) and 18 h (37.0 ± 6.2%) after SAP and those from blood of healthy donors (11.3 ± 3.4%, n = 6). In contrast, the differences between the percentage of viable peripheral blood eosinophils from patients with asthma 10 min before and 18 h after SAP and healthy controls did not reach statistical significance.
Figure 3.
Ex vivo viability of BALF eosinophils after 72 h is increased in comparison to peripheral blood eosinophils. Peripheral blood (PB-Eos; 0.5 × 105) isolated 10 min before and 18 h after SAP or BALF eosinophils (BALF-Eos; 0.5 × 105/ml) isolated 18 h after SAP, respectively, were cultured for 72 h in cell culture medium (each n = 4). Percentage of cell survival was determined by cytometry with a PI exclusion assay. Control blood eosinophils were isolated from a separate group of unchallenged healthy donors (n = 9); *P < 0.05.
Ex Vivo Viability of Peripheral Blood and BALF Eosinophils after Stimulation with NGF, BDNF, NT-3, or NT-4.
The effect of neurotrophins on eosinophil survival was assessed after incubation of peripheral blood eosinophils from healthy donors with 50 ng/ml NGF, BDNF, NT-3, or NT-4. Neurotrophins did not change viability which was 13.5 ± 4.8% (control) after 3 d in culture (n = 6; Fig. 4) .
Figure 4.
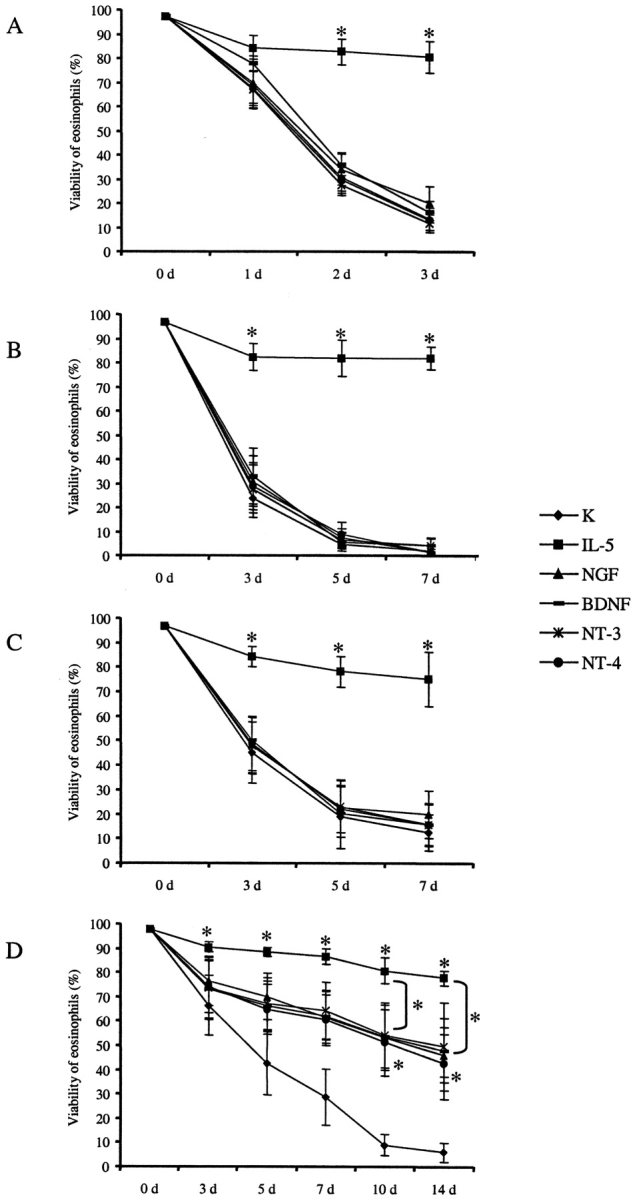
Comparison of the viability of peripheral blood eosinophils with BALF eosinophils after stimulation with neurotrophins. Isolated eosinophils (0.5 × 105/ml) of peripheral blood (PB-Eos) from healthy subjects (A, n = 6) or asthmatics 10 min before (B, n = 4) and 18 h after SAP (C, n = 4) as well as BALF eosinophils collected 18 h after SAP (BALF-Eos; D, n = 4) were incubated with 50 ng/ml NGF, BDNF, NT-3, or NT-4, respectively, or with 10 ng/ml IL-5 or cell culture medium alone (control). The viability was assessed by flow cytometry with PI immediately after isolation (0 d) and after 1, 2, and 3 d (PB-Eos from healthy subjects) or after 3, 5, and 7 d (PB-Eos from asthmatics before and after SAP) or after 0, 3, 5, 7, 10, and 14 d, respectively (BALF-Eos from asthmatics); * significant differences (P < 0.05).
Because of the observed differences in the survival of eosinophils obtained from peripheral blood of healthy subjects, and from asthmatics before and after SAP compared with BALF eosinophils from patients with asthma, viability of peripheral blood eosinophils from asthmatics was also assessed after 5 and 7 d in culture, whereas survival of BALF eosinophils was also measured after 10 and 14 d. After 7 d in culture, only 2.6 ± 0.8% of the peripheral blood eosinophils obtained from asthmatics before SAP were still viable. Neither NGF, BDNF, NT-3, nor NT-4 had any significant influence on cell viability (Fig. 4). After stimulation of peripheral blood eosinophils obtained 18 h after SAP with neurotrophins, comparable results were observed (Fig. 4). In contrast, incubation of BALF eosinophils for 14 d with either 50 ng/ml NGF, BDNF, NT-3, or NT-4 increased the percentage of viable, PI negative cells significantly (controls: 5.8 ± 3.8%, NGF: 45.9 ± 8.9%, BDNF: 47.9 ± 13.0%, NT-3: 49.3 ± 18.0%, NT-4: 42.6 ± 14.8%; n = 4; P < 0.05; Fig. 4). There were no significant differences in the percentage of viable cells after stimulation with the different neurotrophins.
In comparison, incubation with 10 ng/ml of IL-5 prolonged survival of both peripheral blood and BALF eosinophils in a similar order of magnitude. The observed differences between IL-5 stimulated eosinophils and unstimulated control cells were statistically significant (P < 0.05) in all eosinophil preparations examined irrespectively of whether cells were obtained from peripheral blood or BALF.
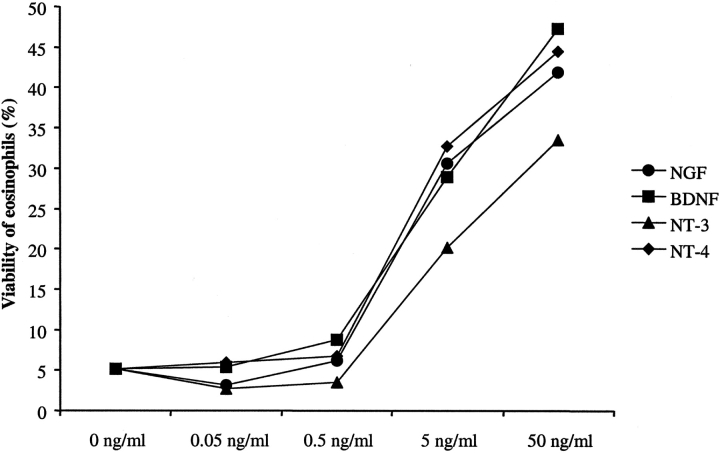
The neurotrophin-mediated increase in survival of BALF eosinophils after stimulation with NGF, BDNF, NT-3, or NT-4 was concentration-dependent. The increase in eosinophil survival after stimulation with either NGF, BDNF, NT-3, or NT-4 was of the same order of magnitude among the different neurotrophins (Fig. 5) .
Figure 5.
Concentration dependence of the viability of BALF eosinophils after stimulation with neurotrophins. Isolated BALF eosinophils (0.5 × 105/ml) collected 18 h after SAP (BALF-Eos) were incubated with 0.05, 0.5, 5, or 50 ng/ml NGF, BDNF, NT-3, or NT-4, respectively, or cell culture medium alone (control, 0 ng/ml). The viability was assessed by flow cytometry with PI after 10 d. The figure shows representative results from one of two independent experiments.
Neutralization of Neurotrophin-induced Survival and CD69 Surface Expression.
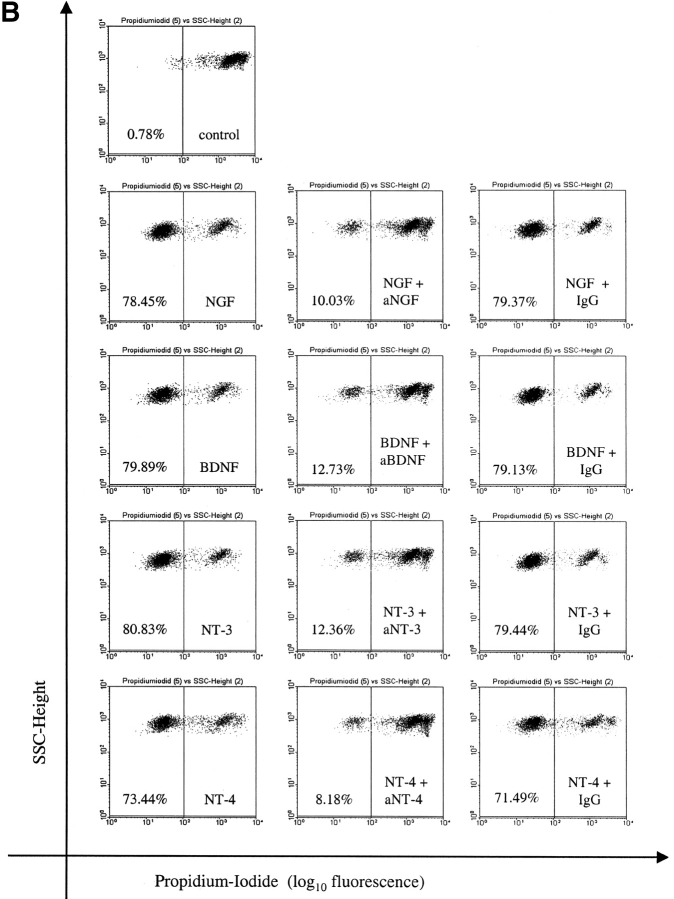
Addition of neutralizing Abs against NGF, BDNF, NT-3, or NT-4 to neurotrophin-stimulated BALF eosinophils abrogated the neurotrophin-mediated effect on CD69 expression (Fig. 6 A) and caused a significant decrease in the percentage of viable BALF eosinophils (Fig. 6 B; each n = 4, P < 0.05). In contrast, CD69 expression and eosinophil survival were not affected by coincubation with the control mAb of the same isotype (IgG1).
Figure 6.
Neutralization of the neurotrophin-induced increase in CD69 expression and viability by mAbs against NGF, BDNF, NT-3, and NT-4. The effects of NGF, BDNF, NT-3, and NT-4 on activation and viability of BALF eosinophils are inhibited by coincubation with neutralizing Abs against the neurotrophins. In contrast, the coincubation with a control Ab of the same isotype caused no change in CD69 expression measured after 4 h (A) or eosinophil survival measured after 10 d (B). Representative histograms (A) or dotplots (B) from one of four independent experiments are depicted. The depicted numbers in each graph (B) give the percentages of the viable, PI negative eosinophils.
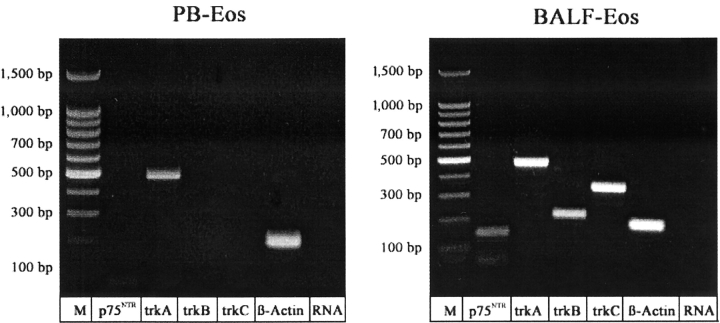
Identification of Neurotrophin Receptor mRNA Expression in Eosinophils by PCR.
To identify the mechanisms by which neurotrophins exert the observed effects on eosinophils, mRNA expression for neurotrophin receptors was investigated by RT-PCR. Fig. 7 shows the expression of mRNA for p75NTR, trkA, trkB, and trkC in BALF eosinophils of asthmatics 18 h after allergen provocation. In contrast, peripheral blood eosinophils expressed only mRNA for trkA following SAP while the levels of expression of mRNA for p75, trkB, and trkC were below the detection limit (Fig. 7).
Figure 7.
mRNA expression of neurotrophin receptors in eosinophils. Expression of mRNA for β-actin (positive control, 194 bp), p75NTR (147 bp), trkA (479 bp), trkB (221 bp), and trkC (356 bp) in peripheral blood eosinophils (PB-Eos) or BALF eosinophils, respectively, from asthmatics 18 h after SAP. M, 100 bp DNA ladder; RNA, RNA control.
Immunocytochemical Identification of Neurotrophin Receptors of Eosinophils.
To confirm the RT-PCR data, expression of neurotrophin receptors was also examined by immunocytochemistry. None of the cytospin preparations which were incubated with a negative control antibody (IgG1, IgG2a, IgG2b, or rabbit IgG, respectively) yielded positively stained cells. There was no positive staining for either extracellular or intracellular domains of the neurotrophin receptors trkA, trkB, or trkC, nor the extracellular domain of the p75NTR on peripheral blood eosinophils from healthy donors or patients with asthma before SAP (data not depicted) nor on peripheral blood eosinophils from asthmatic patients 18 h after SAP (n = 4). However, BALF eosinophils from all samples investigated (n = 4) expressed p75NTR as well as the intra- and extracellular domains of trkA, trkB, and trkC. Fig. 8 gives representative examples of the cytochemical analysis of the extracellular domains from the individual neurotrophin receptors.
Figure 8.
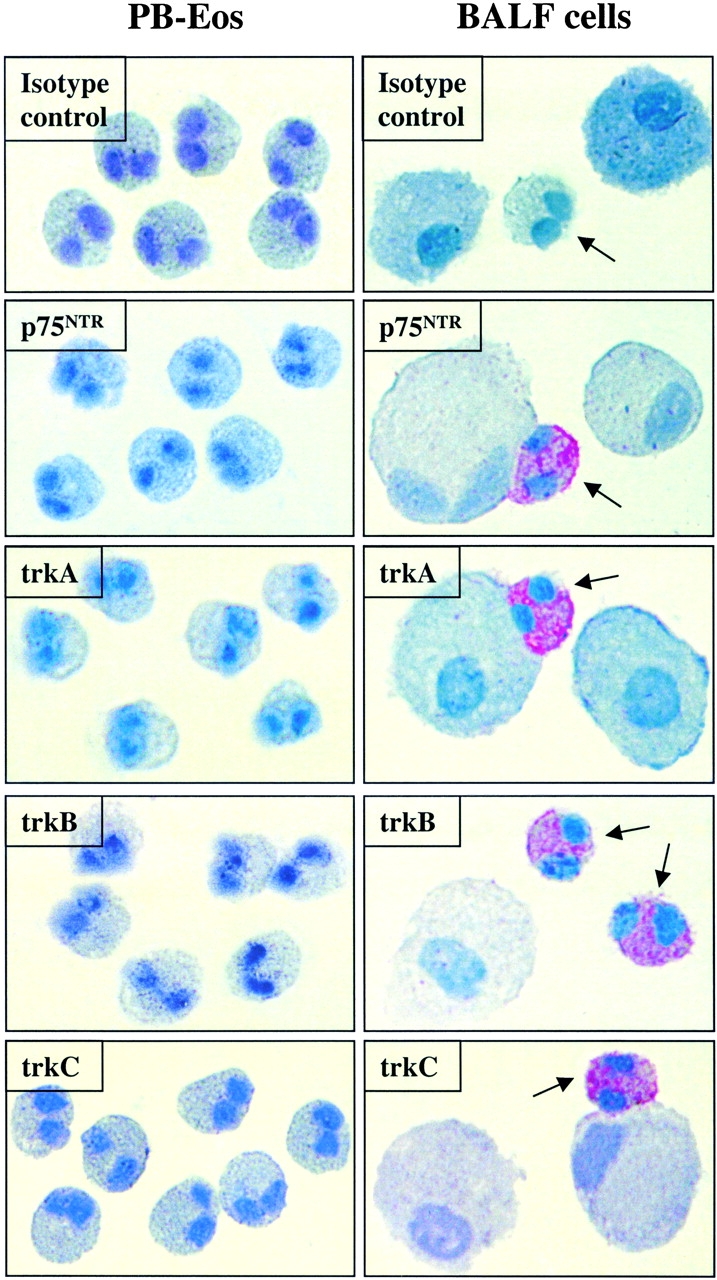
Immunocytochemical detection of neurotrophin receptor expression in cytospin preparations from purified peripheral blood eosinophils and BALF cells. In BALF cells, a strong immunoreactivity for the extracellular proportions of trkA, trkB, trkC, and the p75NTR was observed in eosinophils (arrows), whereas no immunoreactivity for any of the neurotrophin receptors was noted in eosinophils from peripheral blood (PB-Eos; trkA mAb from Cymbus Biotechnology LTD, trkB pAb from Chemicon, trkC mAb from R&D Systems, and p75NTR mAb from NeoMarkers). None of the cytospin preparations incubated with a negative control antibody (IgG1, IgG2a, IgG2b, or rabbit IgG, respectively) yielded positively stained cells (depicted are representative photographs from cells which were incubated with IgG1).
Discussion
Inflammatory processes and responses to proinflammatory mediators in vitro are potent inducers of neurotrophin release by nerve cells (45, 46) and a wide range of haematopoietic cells including mast-cells (13, 14), macrophages (12, 15, 16), T cells (11, 12, 16, 32), B cells (12, 36), and NK cells (47). Neurotrophins have been observed in elevated concentrations in several inflammatory conditions (48–50) and have recently been described in allergic diseases (5, 51–53). An up-regulation of neurotrophins was seen during the allergic late phase response in BALF following SAP (7). The late phase reaction in asthma following allergen provocation is dominated by an influx of activated eosinophils into the bronchial lumen, which correlates with the release of eosinophilic products into the airways, disease severity and the development of BHR (3, 4). The mechanisms, which regulate eosinophil activation, however, in vivo are incompletely understood. In this study, we compared the influence of the neurotrophins NGF, BDNF, NT-3, and NT-4 on survival and activation of peripheral blood eosinophils from healthy donors and from patients with mild allergic asthma before and after SAP with those of BALF eosinophils infiltrating the lung after allergen exposure. We were able to show that both viability and CD69 expression were significantly enhanced after stimulation of BALF eosinophils with NGF as well as BDNF, NT-3, and NT-4, whereas no changes in survival and induction of CD69 surface expression were observed in peripheral blood eosinophils of patients with bronchial asthma nor in those of healthy subjects. In contrast, IL-5 prolonged survival and increased CD69 expression of all investigated eosinophil preparations in a similar, nonselective manner. Addition of neutralizing anti-neurotrophin antibodies in vitro abrogated the effects of neurotrophins on BALF eosinophils. However, from our study design we cannot fully exclude that the observed changes could be due to an indirect action of neurotrophins, e.g., by triggering cytokine release from eosinophils which affect eosinophil function by autocrine or paracrine mechanisms. On the other hand such an action has not yet been reported and the neurotrophin-induced up-regulation of CD69 occurred rapidly, which would make indirect effects of neurotrophins unlikely.
NGF has previously been shown to affect the viability of peripheral blood eosinophils from healthy donors (23, 54). The results of these studies, however, gave conflicting results with respect to the influence of NGF on eosinophil survival. Both authors suggested a role for NGF in the development of allergic inflammation because NGF was shown to induce ECP release and enhance eosinophil chemotactic activity. The cytokine like functions of NGF have been highlighted by several studies recently. NGF is known to prolong the survival of murine and human neutrophils (55, 56) and to promote the growth and differentiation of myeloid and erythroid progenitors (57, 58). NGF influences the proliferation of both human B and T lymphocytes, acts as survival factor for memory B cells (59, 60), triggers several biological functions of mature myeloid cells as in monocytes/macrophages (32, 61), basophils (37, 62), and mast cells (21). Although a multitude of physiological functions for NGF have been described in the immune system, a similar role for the structurally closely related BDNF, NT-3, and NT-4 remained unclear, despite the observation that receptors for these neurotrophins are expressed on the surface of various immunocompetent cells (14, 31, 33, 34, 38).
In this study, we now provide evidence for a functional role of BDNF, NT-3, and NT-4 on eosinophil viability and activation. Interestingly, this effect of neurotrophins was only observed on cells from BALF obtained from segments that had been locally challenged by allergen but not in cells obtained from blood. As neurotrophins had no measurable effects on peripheral blood eosinophils, the effects of neurotrophin effects appear to depend on allergen-induced preactivation of eosinophils. This has not previously been demonstrated and underscores the requirement to study allergic inflammation locally.
In comparison to peripheral blood eosinophils, eosinophils of the BALF have an increased expression of the activation markers HLA-DR and CD69, an enhanced superoxide production and adhere more easily to collagen and human umbilical vein endothelial cells (HUVECs; references 63 and 64). Based on these observation we postulate that pulmonary eosinophils develop an increased expression of neurotrophin specific receptors compared with peripheral blood eosinophils.
We therefore analyzed neurotrophin receptor expression on peripheral blood eosinophils as well as on BALF eosinophils after SAP. Both immunocytochemistry and RT-PCR revealed that BALF eosinophils expressed all three tyrosine kinases as well as the p75NTR. In contrast, we observed trkA specific mRNA expression in peripheral blood eosinophils from asthmatics after SAP, but, in agreement with functional data, immunoreactivity was not observed for the trks or the p75NTR in these cells. This corresponds with our observation of comparable changes in viability and CD69 expression of BALF eosinophils to the different neurotrophins tested indicating an activation-dependent up-regulation of neurotrophin receptor expression. In contrast to our findings, neurotrophin receptor expression was recently reported in peripheral blood eosinophils from patients with mild eosinophilia and a history of allergic disease (65). The data in that study are, however, inconsistent. mRNA for these receptors was reported in about half of the allergic patients and trkA and trkC protein were detectable by Western blot only. Laurenzi et al. suggested that granulocytes in the peripheral blood of healthy donors generally lacked mRNA expression for neurotrophin receptors (39), although expression of trkB kinase, the truncated trkC, and the trkC kinase have been reported in mature eosinophils in human bone marrow (38). In conclusion, there is evidence to suggest that neurotrophin receptor expression is subject to local, allergen-dependent, compartmentalized regulation in which neurotrophin receptor expression might indicate eosinophil maturation and/or (pre) activation.
Although our findings are compatible with our hypothesis of an allergen-dependent up-regulation of all examined neurotrophin receptors in eosinophils after SAP, the detailed mechanism by which the effects of neurotrophins are mediated in eosinophils is at present unclear. Recent evidence from an animal model of allergic asthma indicates that p75NTR plays a critical role in the accumulation of eosinophils in the lung: Tokuoka et al. demonstrated that p75NTR knockout mice developed markedly diminished allergic inflammation and no airway eosinophilia (66). These data have been corroborated by Kerzel et al. who showed that blocking of p75NTR by local antibody treatment prevents eosinophilic lung inflammation in a murine asthma model (67). These findings, however, do not rule out that trks can play a critical role in eosinophil activation. Both p75NTR and trks alone or in cooperation can mediate neurotrophin signals including survival or inhibition of apoptosis. In neurons activation of p75NTR induces the expression of the transcription factor nuclear factor κB (NFκB; references 68 and 69). Activation of the tyrosine kinases induces the antiapoptotic bcl-2 and activation of phosphatidylinositol-3 kinase, the serine/threonine kinase Akt and the MAP kinase cascade (70, 71) by which the high affinity receptors as well as the p75NTR can mediate a signal for survival. Our observation that the p75NTR as well as the tyrosinkinases trkA, trkB, and trkC are expressed on the surface of BALF eosinophils is compatible with the hypothesis that both low affinity and high affinity NGF receptors play a critical role in eosinophilic inflammation in allergen induced asthma especially as p75NTR provides a positive modulatory influence on trk function as has recently been described (72, 73).
In conclusion this is the first study to demonstrate allergen-dependent up-regulation of the neurotrophin receptors p75NTR, trkA, trkB, and trkC on eosinophils from BALF compared with peripheral blood eosinophils. This neurotrophin receptor expression translates into an increased activation and viability of BALF eosinophils in vitro after incubation with neurotrophins such as NGF, BDNF, NT-3, and NT-4. Our findings suggest that neurotrophin mediated activation of bronchial eosinophils might play a role in the regulation of eosinophilic inflammation in allergic asthma. The factors regulating neurotrophin receptor expression remain to be elucidated.
Acknowledgments
The authors thank D. Hochheim, K. Böser, and S. Mayer for excellent technical assistance.
This work was supported by a grant from the Deutsche For-schungsgemeinschaft (SFB 587, B4 and DFG Vi 193/3-1) and by the Volkswagen Stiftung.
C. Nassenstein and A. Braun contributed equally to this work.
Footnotes
Abbreviations used in this paper: BALF, bronchoalveolar lavage fluid; BDNF, brain-derived neurotrophic factor; BHR, bronchial hyperresponsiveness; FEV1, forced expiratory volume in one second; NGF, nerve growth factor; NT, neurotrophin; PI, propidium-iodide; p75NTR, low affinity pan-neurotrophin receptor; rSMF, relative SMF; SAP, segmental allergen provocation; SMF, specific mean fluorescence; trk, tyrosine kinase, high affinity neurotrophin receptor.
References
- 1.Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. [DOI] [PubMed] [Google Scholar]
- 2.Howarth, P.H. 1995. The airway inflammatory response in allergic asthma and its relationship to clinical disease. Allergy. 50:13–21. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg, M.E. 1998. Eosinophilia. N. Engl. J. Med. 338:1592–1600. [DOI] [PubMed] [Google Scholar]
- 4.De Monchy, J.G., H.F. Kauffman, P. Venge, G.H. Koeter, H.M. Jansen, H.J. Sluiter, and K. De Vries. 1985. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am. Rev. Respir. Dis. 131:373–376. [DOI] [PubMed] [Google Scholar]
- 5.Bonini, S., A. Lambiase, F. Angelucci, L. Magrini, L. Manni, and L. Aloe. 1996. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl. Acad. Sci. USA. 93:10955–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Undem, B.J., D.D. Hunter, M. Liu, M. Haak-Frendscho, A. Oakragly, and A. Fischer. 1999. Allergen-induced sensory neuroplasticity in airways. Int. Arch. Allergy Immunol. 118:150–153. [DOI] [PubMed] [Google Scholar]
- 7.Virchow, J.C., Jr., P. Julius, M. Lommatzsch, W. Luttmann, H. Renz, and A. Braun. 1998. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 158:2002–2005. [DOI] [PubMed] [Google Scholar]
- 8.Thoenen, H. 1995. Neurotrophins and neuronal plasticity. Science. 270:593–598. [DOI] [PubMed] [Google Scholar]
- 9.Leibrock, J., F. Lottspeich, A. Hohn, M. Hofer, B. Hengerer, P. Masiakowski, H. Thoenen, and Y.A. Barde. 1989. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 341:149–152. [DOI] [PubMed] [Google Scholar]
- 10.Lewin, G.R., and Y.A. Barde. 1996. Physiology of the neurotrophins. Annu. Rev. Neurosci. 19:289–317. [DOI] [PubMed] [Google Scholar]
- 11.Barouch, R., E. Appel, G. Kazimirsky, A. Braun, H. Renz, and C. Brodie. 2000. Differential regulation of neurotrophin expression by mitogens and neurotransmitters in mouse lymphocytes. J. Neuroimmunol. 103:112–121. [DOI] [PubMed] [Google Scholar]
- 12.Kerschensteiner, M., E. Gallmeier, L. Behrens, V.V. Leal, T. Misgeld, W.E. Klinkert, R. Kolbeck, E. Hoppe, R.L. Oropeza-Wekerle, I. Bartke, et al. 1999. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 189:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leon, A., A. Buriani, R. Dal Toso, M. Fabris, S. Romanello, L. Aloe, and R. Levi-Montalcini. 1994. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA. 91:3739–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam, S.Y., M. Tsai, M. Yamaguchi, K. Yano, J.H. Butterfield, and S.J. Galli. 1997. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 90:1807–1820. [PubMed] [Google Scholar]
- 15.Braun, A., E. Appel, R. Baruch, U. Herz, V. Botchkarev, R. Paus, C. Brodie, and H. Renz. 1998. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 28:3240–3251. [DOI] [PubMed] [Google Scholar]
- 16.Braun, A., M. Lommatzsch, A. Mannsfeldt, U. Neuhaus-Steinmetz, A. Fischer, N. Schnoy, G.R. Lewin, and H. Renz. 1999. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respir. Cell Mol. Biol. 21:537–546. [DOI] [PubMed] [Google Scholar]
- 17.Lommatzsch, M., A. Braun, A. Mannsfeldt, V.A. Botchkarev, N.V. Botchkareva, R. Paus, A. Fischer, G.R. Lewin, and H. Renz. 1999. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived neurotrophic functions. Am. J. Pathol. 155:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, H., G.J. Gleich, J.H. Butterfield, and H. Kita. 2002. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 99:2214–2220. [DOI] [PubMed] [Google Scholar]
- 19.de Vries, A., M.C. Dessing, F. Engels, P.A. Henricks, and F.P. Nijkamp. 1999. Nerve growth factor induces a neurokinin-1 receptor-mediated airway hyperresponsiveness in guinea pigs. Am. J. Respir. Crit. Care Med. 159:1541–1544. [DOI] [PubMed] [Google Scholar]
- 20.Hoyle, G.W., R.M. Graham, J.B. Finkelstein, K.P. Nguyen, D. Gozal, and M. Friedman. 1998. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am. J. Respir. Cell Mol. Biol. 18:149–157. [DOI] [PubMed] [Google Scholar]
- 21.Horigome, K., J.C. Pryor, E.D. Bullock, and E.M. Johnson, Jr. 1993. Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J. Biol. Chem. 268:14881–14887. [PubMed] [Google Scholar]
- 22.Kimata, H., A. Yoshida, C. Ishioka, T. Kusunoki, S. Hosoi, and H. Mikawa. 1991. Nerve growth factor specifically induces human IgG4 production. Eur. J. Immunol. 21:137–141. [DOI] [PubMed] [Google Scholar]
- 23.Hamada, A., N. Watanabe, H. Ohtomo, and H. Matsuda. 1996. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br. J. Haematol. 93:299–302. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, D., A. Lanahan, C.R. Buck, A. Sehgal, C. Morgan, E. Mercer, M. Bothwell, and M. Chao. 1986. Expression and structure of the human NGF receptor. Cell. 47:545–554. [DOI] [PubMed] [Google Scholar]
- 25.Radeke, M.J., T.P. Misko, C. Hsu, L.A. Herzenberg, and E.M. Shooter. 1987. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 325:593–597. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan, D.R., B.L. Hempstead, D. Martin-Zanca, M.V. Chao, and L.F. Parada. 1991. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 252:554–558. [DOI] [PubMed] [Google Scholar]
- 27.Klein, R., S.Q. Jing, V. Nanduri, E. O'Rourke, and M. Barbacid. 1991. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 65:189–197. [DOI] [PubMed] [Google Scholar]
- 28.Klein, R., V. Nanduri, S.A. Jing, F. Lamballe, P. Tapley, S. Bryant, C. Cordon-Cardo, K.R. Jones, L.F. Reichardt, and M. Barbacid. 1991. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 66:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ip, N.Y., C.F. Ibanez, S.H. Nye, J. McClain, P.F. Jones, D.R. Gies, L. Belluscio, M.M. Le Beau, R. Espinosa III, S.P. Squinto, et al. 1992. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA. 89:3060–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamballe, F., P. Tapley, and M. Barbacid. 1993. TrkC encodes multiple neurotrophin-3 receptors with distinct biological properties and substrate specificities. EMBO J. 12:3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibayama, E., and H. Koizumi. 1996. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am. J. Pathol. 148:1807–1818. [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrhard, P.B., U. Ganter, A. Stalder, J. Bauer, and U. Otten. 1993. Expression of functional trk protooncogene in human monocytes. Proc. Natl. Acad. Sci. USA. 90:5423–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maroder, M., D. Bellavia, D. Meco, M. Napolitano, A. Stigliano, E. Alesse, A. Vacca, G. Giannini, L. Frati, A. Gulino, and I. Screpanti. 1996. Expression of trKB neurotrophin receptor during T cell development. Role of brain derived neurotrophic factor in immature thymocyte survival. J. Immunol. 157:2864–2872. [PubMed] [Google Scholar]
- 34.Garcia-Suarez, O., J. Hannestad, I. Esteban, R. Sainz, F.J. Naves, and J.A. Vega. 1998. Expression of the TrkB neurotrophin receptor by thymic macrophages. Immunology. 94:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambiase, A., L. Bracci-Laudiero, S. Bonini, G. Starace, M.M. D'Elios, M. De Carli, and L. Aloe. 1997. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immunol. 100:408–414. [DOI] [PubMed] [Google Scholar]
- 36.Torcia, M., L. Bracci-Laudiero, M. Lucibello, L. Nencioni, D. Labardi, A. Rubartelli, F. Cozzolino, L. Aloe, and E. Garaci. 1996. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 85:345–356. [DOI] [PubMed] [Google Scholar]
- 37.Burgi, B., U.H. Otten, B. Ochensberger, S. Rihs, K. Heese, P.B. Ehrhard, C.F. Ibanez, and C.A. Dahinden. 1996. Basophil priming by neurotrophic factors. Activation through the trk receptor. J. Immunol. 157:5582–5588. [PubMed] [Google Scholar]
- 38.Labouyrie, E., P. Dubus, A. Groppi, F.X. Mahon, J. Ferrer, M. Parrens, J. Reiffers, A. de Mascarel, and J.P. Merlio. 1999. Expression of neurotrophins and their receptors in human bone marrow. Am. J. Pathol. 154:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurenzi, M.A., T. Beccari, L. Stenke, M. Sjolinder, S. Stinchi, and J.A. Lindgren. 1998. Expression of mRNA encoding neurotrophins and neurotrophin receptors in human granulocytes and bone marrow cells–enhanced neurotrophin-4 expression induced by LTB4. J. Leukoc. Biol. 64:228–234. [DOI] [PubMed] [Google Scholar]
- 40.National Heart, Lung, and Blood Institute, National Institutes of Health. 1992. International Consensus Report on Diagnosis and Treatment of Asthma, Bethesda, Publication No. 92:3091.
- 41.Virchow, J.C., Jr., P. Julius, H. Matthys, C. Kroegel, and W. Luttmann. 1998. CD14 expression and soluble CD14 after segmental allergen provocation in atopic asthma. Eur. Respir. J. 11:317–323. [DOI] [PubMed] [Google Scholar]
- 42.Luttmann, W., T. Matthiesen, H. Matthys, and J.C. Virchow, Jr. 1999. Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. Am. J. Respir. Cell Mol. Biol. 20:474–480. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman, K., M. Subklewe, G. Pothoff, N. Banik, and E. Schell-Frederick. 1994. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV infection as detected by flow cytometry. Chest. 105:1324–1334. [DOI] [PubMed] [Google Scholar]
- 44.Descamps, S., V. Pawlowski, F. Revillion, L. Hornez, M. Hebbar, B. Boilly, H. Hondermarck, and J.P. Peyrat. 2001. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 61:4337–4340. [PubMed] [Google Scholar]
- 45.Cho, H.J., S.Y. Kim, M.J. Park, D.S. Kim, J.K. Kim, and M.Y. Chu. 1997. Expression of mRNA for brain-derived neurotrophic factor in the dorsal root ganglion following peripheral inflammation. Brain Res. 749:358–362. [DOI] [PubMed] [Google Scholar]
- 46.Meyer, M., I. Matsuoka, C. Wetmore, L. Olson, and H. Thoenen. 1992. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 119:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammarberg, H., O. Lidman, C. Lundberg, S.Y. Eltayeb, A.W. Gielen, S. Muhallab, A. Svenningsson, H. Linda, P.H. van Der Meide, S. Cullheim, et al. 2000. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J. Neurosci. 20:5283–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laudiero, L.B., L. Aloe, R. Levi-Montalcini, C. Buttinelli, D. Schilter, S. Gillessen, and U. Otten. 1992. Multiple sclerosis patients express increased levels of beta-nerve growth factor in cerebrospinal fluid. Neurosci. Lett. 147:9–12. [DOI] [PubMed] [Google Scholar]
- 49.Bracci-Laudiero, L., L. Aloe, R. Levi-Montalcini, M. Galeazzi, D. Schilter, J.L. Scully, and U. Otten. 1993. Increased levels of NGF in sera of systemic lupus erythematosus patients. Neuroreport. 4:563–565. [DOI] [PubMed] [Google Scholar]
- 50.Oddiah, D., P. Anand, S.B. McMahon, and M. Rattray. 1998. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport. 9:1455–1458. [DOI] [PubMed] [Google Scholar]
- 51.Lambiase, A., S. Bonini, A. Micera, L. Magrini, L. Bracci-Laudiero, and L. Aloe. 1995. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Invest. Ophthalmol. Vis. Sci. 36:2127–2132. [PubMed] [Google Scholar]
- 52.Grewe, M., K. Vogelsang, T. Ruzicka, H. Stege, and J. Krutmann. 2000. Neurotrophin-4 production by human epidermal keratinocytes: increased expression in atopic dermatitis. J. Invest. Dermatol. 114:1108–1112. [DOI] [PubMed] [Google Scholar]
- 53.Sanico, A.M., A.M. Stanisz, T.D. Gleeson, S. Bora, D. Proud, J. Bienenstock, V.E. Koliatsos, and A. Togias. 2000. Nerve growth factor expression and release in allergic inflammatory disease of the upper airways. Am. J. Respir. Crit. Care Med. 161:1631–1635. [DOI] [PubMed] [Google Scholar]
- 54.Solomon, A., L. Aloe, J. Pe'er, J. Frucht-Pery, S. Bonini, and F. Levi-Schaffer. 1998. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J. Allergy Clin. Immunol. 102:454–460. [DOI] [PubMed] [Google Scholar]
- 55.Kannan, Y., H. Ushio, H. Koyama, M. Okada, M. Oikawa, T. Yoshihara, M. Kaneko, and H. Matsuda. 1991. 2.5S nerve growth factor enhances survival, phagocytosis, and superoxide production of murine neutrophils. Blood. 77:1320–1325. [PubMed] [Google Scholar]
- 56.Kannan, Y., K. Usami, M. Okada, S. Shimizu, and H. Matsuda. 1992. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem. Biophys. Res. Commun. 186:1050–1056. [DOI] [PubMed] [Google Scholar]
- 57.Matsuda, H., M.D. Coughlin, J. Bienenstock, and J.A. Denburg. 1988. Nerve growth factor promotes human hemopoietic colony growth and differentiation. Proc. Natl. Acad. Sci. USA. 85:6508–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auffray, I., S. Chevalier, J. Froger, B. Izac, W. Vainchenker, H. Gascan, and L. Coulombel. 1996. Nerve growth factor is involved in the supportive effect by bone marrow–derived stromal cells of the factor-dependent human cell line UT-7. Blood. 88:1608–1618. [PubMed] [Google Scholar]
- 59.Ehrhard, P.B., P. Erb, U. Graumann, B. Schmutz, and U. Otten. 1994. Expression of functional trk tyrosine kinase receptors after T cell activation. J. Immunol. 152:2705–2709. [PubMed] [Google Scholar]
- 60.Otten, U., J.L. Scully, P.B. Ehrhard, and R.A. Gadient. 1994. Neurotrophins: signals between the nervous and immune systems. Prog. Brain Res. 103:293–305. [DOI] [PubMed] [Google Scholar]
- 61.Susaki, Y., S. Shimizu, K. Katakura, N. Watanabe, K. Kawamoto, M. Matsumoto, M. Tsudzuki, T. Furusaka, Y. Kitamura, and H. Matsuda. 1996. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 88:4630–4637. [PubMed] [Google Scholar]
- 62.Bischoff, S.C., and C.A. Dahinden. 1992. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 79:2662–2669. [PubMed] [Google Scholar]
- 63.Julius, P., W. Luttmann, B. Knoechel, C. Kroegel, H. Matthys, and J.C. Virchow, Jr. 1999. CD69 surface expression on human lung eosinophils after segmental allergen provocation. Eur. Respir. J. 13:1253–1259. [DOI] [PubMed] [Google Scholar]
- 64.Sedgwick, J.B., W.J. Calhoun, R.F. Vrtis, M.E. Bates, P.K. McAllister, and W.W. Busse. 1992. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J. Immunol. 149:3710–3718. [PubMed] [Google Scholar]
- 65.Noga, O., C. Englmann, G. Hanf, A. Grutzkau, S. Guhl, and G. Kunkel. 2002. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin. Exp. Allergy. 32:1348–1354. [DOI] [PubMed] [Google Scholar]
- 66.Tokuoka, S., Y. Takahashi, T. Masuda, H. Tanaka, S. Furukawa, and H. Nagai. 2001. Disruption of antigen-induced airway inflammation and airway hyper-responsiveness in low affinity neurotrophin receptor p75 gene deficient mice. Br. J. Pharmacol. 134:1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerzel, S., G. Path, W.A. Nockher, D. Quarcoo, U. Raap, D.A. Groneberg, Q.T. Dinh, A. Fischer, A. Braun, and H. Renz. 2003. Pan-neurotrophin receptor p75 contributes to neuronal hyperreactivity and airway inflammation in a murine model of experimental asthma. Am. J. Respir. Cell Mol. Biol. 28:170–178. [DOI] [PubMed] [Google Scholar]
- 68.Bothwell, M. 1996. p75NTR: a receptor after all. Science. 272:506–507. [DOI] [PubMed] [Google Scholar]
- 69.Carter, B.D., C. Kaltschmidt, B. Kaltschmidt, N. Offenhauser, R. Bohm-Matthaei, P.A. Baeuerle, and Y.A. Barde. 1996. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 272:542–545. [DOI] [PubMed] [Google Scholar]
- 70.la Sala, A., S. Corinti, M. Federici, H.U. Saragovi, and G. Girolomoni. 2000. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J. Leukoc. Biol. 68:104–110. [PubMed] [Google Scholar]
- 71.Segal, R.A., and M.E. Greenberg. 1996. Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci. 19:463–489. [DOI] [PubMed] [Google Scholar]
- 72.Barker, P.A., and E.M. Shooter. 1994. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 13:203–215. [DOI] [PubMed] [Google Scholar]
- 73.Verdi, J.M., S.J. Birren, C.F. Ibanez, H. Persson, D.R. Kaplan, M. Benedetti, M.V. Chao, and D.J. Anderson. 1994. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 12:733–745. [DOI] [PubMed] [Google Scholar]