Abstract
The biological actions of anandamide (arachidonylethanolamide), an endogenous cannabinoid lipid, are terminated by a two-step inactivation process consisting of carrier-mediated uptake and intracellular hydrolysis. Anandamide uptake in neurons and astrocytes is mediated by a high-affinity, Na+-independent transporter that is selectively inhibited by N-(4-hydroxyphenyl)-arachidonamide (AM404). In the present study, we examined the structural determinants governing recognition and translocation of substrates by the anandamide transporter constitutively expressed in a human astrocytoma cell line. Competition experiments with a select group of analogs suggest that substrate recognition by the transporter is favored by a polar nonionizable head group of defined stereochemical configuration containing a hydroxyl moiety at its distal end. The secondary carboxamide group interacts favorably with the transporter, but may be replaced with either a tertiary amide or an ester, suggesting that it may serve as hydrogen acceptor. Thus, 2-arachidonylglycerol, a putative endogenous cannabinoid ester, also may serve as a substrate for the transporter. Substrate recognition requires the presence of at least one cis double bond situated at the middle of the fatty acid carbon chain, indicating a preference for ligands whose hydrophobic tail can adopt a bent U-shaped conformation. On the other hand, uptake experiments with radioactively labeled substrates show that no fewer than four cis nonconjugated double bonds are required for optimal translocation across the cell membrane, suggesting that substrates are transported in a folded hairpin conformation. These results outline the general structural requisites for anandamide transport and may assist in the development of selective inhibitors with potential clinical applications.
When administered as a drug, the endogenous cannabinoid anandamide (1, 2) elicits a spectrum of pharmacological responses that closely resemble those of Δ9-tetrahydrocannabinol, the main psychoactive constituent of marijuana (3). Unlike Δ9-tetrahydrocannabinol, however, anandamide has a very short duration of action because of a rapid inactivation process consisting of carrier-mediated transport into cells followed by intracellular hydrolysis. Indeed, rat brain neurons and astrocytes in primary culture avidly take up radioactively labeled anandamide through a mechanism that meets four key criteria of a carrier-mediated transport: temperature dependence, high affinity, substrate selectivity, and saturation (2, 4, 5). Within cells, anandamide is hydrolyzed to arachidonic acid and ethanolamine by a membrane-bound amidohydrolase that also reacts with other fatty acid amides and esters (6–10).
There is considerable experimental and medical interest in understanding the mechanism of anandamide transport and in developing pharmacological agents that selectively interfere with it. Anandamide transport inhibitors may be used as experimental tools to reveal the possible physiological functions of this biologically active lipid. Many of these functions are still elusive despite a growing body of evidence suggesting that the endocannabinoid system is intrinsically active not only in brain and spinal cord (for review, see ref. 11), but also in peripheral tissues (12–14). Furthermore, anandamide transport inhibitors may offer a rational therapeutic approach to a variety of disease states, including pain (13, 14), psychomotor disorders (15), and multiple sclerosis (16–18), in which elevation of native anandamide levels may bring about a more favorable response and fewer side effects than direct activation of CB1 receptors by agonist drugs.
In our search for selective inhibitors of anandamide transport, we found that the compound N-(4-hydroxyphenyl)-arachidonamide (AM404) prevents [3H]anandamide uptake in vitro and enhances various effects of anandamide administration both in vitro and in vivo (4, 19). These results, together with data from initial experiments on the selectivity of [3H]anandamide uptake by rat brain astrocytes (4), suggest that the interactions of anandamide with its putative transporter protein are governed by strict structural requirements. In the present study we have used a human astrocytoma cell line to conduct a systematic investigation on the structure-activity relationship of substrates and inhibitors of anandamide transport. Our results delineate the broad molecular requisites for this process, thus providing a basis for the design of more potent and selective inhibitors with potential applications to medicine.
MATERIALS AND METHODS
Chemistry.
All amides were synthesized by the reaction of the fatty acid or fatty acid chloride with the appropriate amine or aminoalcohol as described (20). 1- and 2-Arachidonylglycerols were prepared by a modification of the procedure established by Serdarevich and Carroll (21) for the synthesis of fatty acid monoglycerides. Briefly, 1,3-O-benzylidine-sn-glycerol, prepared by the reaction of glycerol with benzaldehyde in the presence of p-toluenesulfonic acid, was allowed to react with arachidonyl chloride in the presence of pyridine. Subsequent treatment with boric acid in triethylborate to remove the benzylidine moiety gave 2-arachidonylglycerol. For the preparation of 1(3)-arachidonylglycerol, commercially available 1,2-O-isopropylidene-sn-glycerol was esterified with arachidonic acid in a similar manner, followed by removal of the isopropylidene group by treatment with bromodimethylborane (22). The synthesis of compounds 32–34 will be described in detail elsewhere. Radioactively labeled fatty acid ethanolamides were prepared by the reaction of acid chlorides (Nu-Check Prep, Elysian, MN) with [3H]ethanolamine (10–30 Ci/mmol; American Radiolabeled Chemicals, St. Louis) as described (6). All compounds were purified by HPLC or flash column chromatography, and their identities were established by NMR and/or gas chromatography-mass spectrometry. Additional compounds were purchased from Avanti Polar Lipids, Cayman Chemicals (Ann Arbor, MI), Nu-Check Prep, Research Biochemicals, or Sigma.
[3H]Anandamide Competition Assay.
Human CCF-STTG1 astrocytoma cells (American Type Culture Collection) were grown in RPMI 1640 culture medium containing 10% FBS and 1 mM glutamine. For standard competition assays, confluent cells grown in 24-well plates were rinsed and preincubated for 10 min at 37°C in Tris-Krebs’ buffer (136 mM NaCl/5 mM KCl/1.2 mM MgCl2-6H20/2.5 mM CaCl2-2H20/10 mM glucose/20 mM Trizma base) containing 0.1–0.3% DMSO or 0.1–0.3% DMSO plus test compounds at their final concentrations (0.1–100 μM). After having discarded the preincubation media, the cells were incubated for 4 min in 0.4 ml of Tris-Krebs’ buffer containing 30 nM [3H]anandamide (220 Ci/mmol, New England Nuclear) and 0.1–0.3% DMSO, or 0.1–0.3% DMSO plus test compounds. Reactions were stopped by removing the incubation media and rinsing the cells three times with 0.4 ml of ice-cold Tris-Krebs’ buffer containing 0.1% fatty acid-free BSA (Sigma). Radioactive material in Triton X-100 cell extracts was measured by liquid scintillation counting. Preliminary analyses carried out by TLC demonstrated that >95% of this radioactive material was nonmetabolized [3H]anandamide, suggesting that our astrocytoma cell preparation contains no significant anandamide amidohydrolase activity.
[3H]Anandamide Transport Assay.
For standard transport assays, confluent astrocytoma cells grown in 90-mm plates were incubated at 37°C in 10 ml of Tris-Krebs’ buffer containing 10–50 × 106 dpm/ml of one the following radioactive tracers (unless indicated otherwise, specific radioactivity was 0.31–0.69 mCi/mmol): [3H]anandamide (220 Ci/mmol, New England Nuclear), [3H]oleylethanolamide (18:1Δ9), [3H]eicosaenoylethanolamide (20:1Δ11), [3H]eicosadienoylethanolamide (20:2Δ8,11), [3H]eicosatrienoylethanolamide (20:3Δ8,11,14), [3H]2-arachidonylglycerol (100 mCi/mmol; New England Nuclear, custom-synthesized), [3H]N-(4-hydroxyphenyl) arachidonamide (200 Ci/mmol; American Radiolabeled Chemicals). At various times after the addition of tracer (0–20 min), 1-ml samples of the incubation media were collected for liquid scintillation counting. Under these conditions, clearance of radioactive material from the incubating medium provides an accurate estimate of transport into cells, as indicated both by previous work with rat brain neurons (2) and by preliminary experiments with astrocytoma cells (data not shown).
Transport Kinetics.
The cells were incubated for 4 min at 37°C in the presence of 10–500 nM anandamide containing 0.05–2.5 nM [3H]anandamide. Nonspecific accumulation (measured at 0–4°C) was subtracted before determining kinetic constants by Lineweaver–Burke analysis.
Data Analysis.
A minimum of three independent experiments conducted in triplicate was used to define the concentration needed to produce half-maximal inhibition (IC50) for each compound. IC50 values were obtained by nonlinear least-squares fitting of the data, using the prizm software package. All other experiments were carried out in triplicate and repeated at least twice with identical results. Data are expressed as mean ± SEM.
Molecular Modeling.
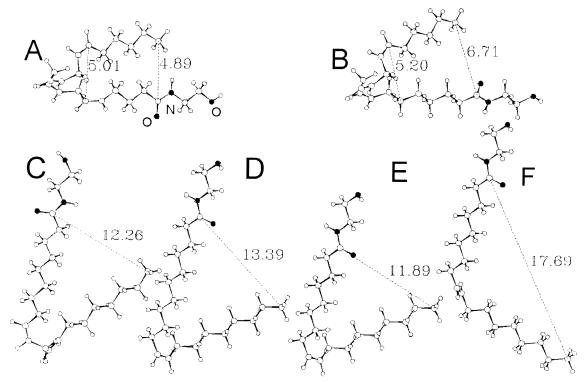
Molecular modeling was conducted on a SGI Octane R10000 workstation (Silicon Graphics, Mountain View, CA) with the Tripos Sybyl 6.4 and Tripos empirical force field molecular modeling package. The initial structures were generated by using standard bond lengths and angles from the Sybyl package. Charges were calculated for all molecules by the semiempirical method (MOPA/AM1), and energies were minimized by using the Tripos force field in two stages. First, the steepest descent method was applied for the first 200 steps, followed by the conjugate gradient method until the maximum derivative was less than 0.001 kcal/mole per Å. The conformation of anandamide was refined by using the Tripos random search module on the six rotatable single bonds within the group of four nonconjugated cis double bonds. Conformer A depicted in Fig. 4 has a hairpin conformation similar to that described by others for anandamide (23) and arachidonic acid (24). The preferred conformers of all anandamide analogs were generated from A by appropriate structural modifications followed by minimization.
Figure 4.
Low-energy conformers of various fatty acid ethanolamides with hydrophobic carbon chains differing in their degree of unsaturation. The numbers indicate calculated interatomic distances in Å. (A) Anandamide (1); (B) cis-eicosatrienoylethanolamide (2, 20:3Δ8,11,14); (C) cis-eicosadienoylethanolamide (3, 20:2Δ8,11); (D) cis-eicosaenoylethanolamide (4, 20:1Δ11); (E) oleylethanolamide (6, 18:1Δ9); and (F) trans-octadecenoylethanolamide (7, 18:1Δ9).
RESULTS
[3H]Anandamide Transport in Astrocytoma Cells.
As expected of a carrier-mediated process, [3H]anandamide accumulation in human astrocytoma cells is rapid (t1/2 = 3 min), temperature dependent, and saturable, displaying an apparent Michaelis constant (Km) of 0.6 ± 0.1 μM and a maximal accumulation rate (Vmax) of 14.7 ± 01.5 pmol/min per mg of protein (n = 5). The accumulation is not affected by replacement of Na+ with choline, suggesting that it is mediated by a Na+–independent mechanism (data not shown). In addition, [3H]anandamide accumulation is prevented by the anandamide transport inhibitor N-(4-hydroxyphenyl)-arachidonamide (AM404, 22) with a IC50 value of 2.2 ± 0.2 μM, whereas its positional isomer N-(3-hydroxyphenyl)-arachidonamide (23) is 10 times less effective (IC50 = 21.3 ± 3.4). Finally, a variety of compounds that are substrates or inhibitors of membrane transporters have no effect on [3H]anandamide accumulation when tested at concentrations ranging from 10 to 100 μM. These compounds include: prostaglandins (PGE2, PGF2α, PGD2, and PGJ2), hydroxyeicosatetraenoic acids (5-HETE, 12-HETE, and 15-HETE), epoxyeicosatrienoic acids (5,6-EET and 8,9-EET), leukotrienes (LTC4 and LTB4), organic anions (taurocholate, p-aminohippurate, sulfobromophthalein, and taurine), ceramide, verapamil, digoxin, urea, amino acids (glutamate, γ-aminobutyrate, glycine, and proline) and biogenic amines (norepinephrine, epinephrine, dopamine, dihydroxyphenylalanine, histamine, 5-hydroxytryptamine, and choline) (data not shown). Together, these results indicate that [3H]anandamide accumulation in human astrocytoma cells is mediated by a high-affinity, Na+–independent transporter functionally analogous to that described in rat brain neurons and astrocytes (4).
Competition with [3H]Anandamide Transport.
To explore the structural features involved in the interaction of substrates and inhibitors with the anandamide transporter, we synthesized a series of anandamide analogs and examined their abilities to interfere with [3H]anandamide uptake. The anandamide structure reveals three potential pharmacophores that lend themselves to structural modification (Scheme S1): (A) the highly hydrophobic cis-tetraene carbon chain, (B) the polar carboxamido group, and (C) the hydroxyethyl head group.
Scheme 1.
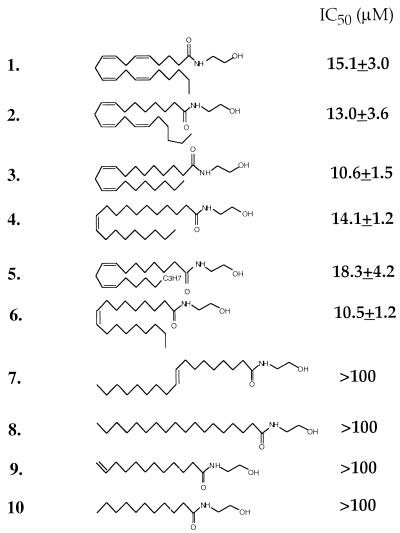
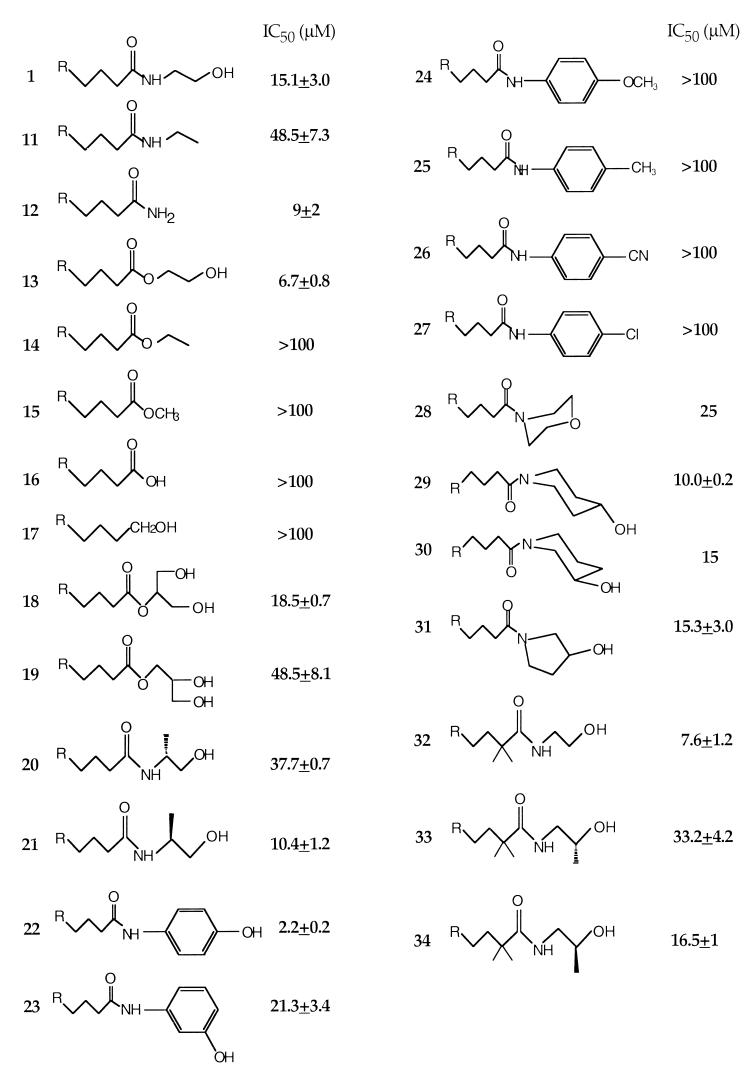
We explored the correlation between ligand structure and function by varying the structures of these three components. The results indicate that analogs incorporating either a C-18 or a C-20 hydrophobic tail with one, two, or three nonconjugated cis double bonds in the middle part of the chain (Fig. 1; 2–6) compete successfully with [3H]anandamide for transport. By contrast, analogs with fully saturated chains or with a trans or terminal double bond fail to do so (7–10). Exploration of the B pharmacophore suggests that compounds containing primary (Fig. 2; 12), secondary (11), and tertiary carboxamido groups (e.g., 28–31) as well as hydroxyethyl ester (13) or glycerol ester moieties (18 and 19) are capable of competing with [3H]anandamide, but exhibit a wide range of potencies. Conversely, compounds containing a free carboxylic acid (16), carboxyethyl and carboxymethyl groups (14 and 15), or a primary alcohol (17) are inactive. Structural variations of the hydroxyethyl head group (C) also lead to compounds with diverse selectivities for the anandamide transporter. Thus substitution of the terminal hydroxyl with a hydrogen (11) causes a substantial decrease in potency, whereas elimination of the hydroxyethyl moiety yields compounds that are as potent as anandamide, as illustrated by arachidonamide (12) or oleamide (Ki = 11.1 ± 2.6 μM; not shown). Introduction of a methyl group alpha to the amido nitrogen also leads to active compounds (20 and 21). These chiral molecules display considerable enantioselective inhibition of [3H]anandamide transport. As illustrated in Fig. 2, the (S) enantiomer 21 is approximately four times more potent than its (R) isomer 20. A similar enantioselectivity was demonstrated in the 2,2-dimethyl arachidonate series of these chiral analogs (32–34) (Fig. 2). It is interesting to note that the enantioselectivity for anandamide transport displayed by 20 and 21 and 33 and 34 is congruent to that demonstrated for anandamide amidohydrolase, but opposite to that for the CB1 receptor (20, 25).
Figure 1.
Inhibition of [3H]anandamide uptake by various fatty acid ethanolamides. IC50 values (μM) are expressed as the mean ± SEM of three independent experiments conducted in triplicate.
Figure 2.
Inhibition of [3H]anandamide uptake by anandamide analogs containing carboxamide and polar head group modifications. IC50 values (μM) are expressed as the mean ± SEM (n = 3).
To study the effects of head group conformational preference, we synthesized a set of analogs in which the hydroxyalkyl group is partially restricted by incorporation into five- or six-member rings. The resulting 3- and 4-hydroxypiperidinyl- (29 and 30) and 3-hydroxypyrrolidinyl- (31) amides, which were tested as racemic pairs, have activities for the transporter similar to that of anandamide. Another cyclic head group analog (28) has both the amido nitrogen and the hydroxyl oxygen restricted into a morpholine ring. This analog maintains considerable activity (approximately half that of anandamide), indicating that the hydrogen in the hydroxyl head group may not be necessary for interaction with the transporter.
The most striking structure-activity correlation was observed with analogs having phenyl substitutions at the head group. Replacement of the hydroxyethyl with a hydroxyphenyl group leads to relatively potent uptake inhibitors, with the 4-hydroxyphenyl analog (AM404, 22) being distinctly the most successful. Conversely, the 4-methylphenyl analog 25 as well as other analogs with electron donating (24) or electron withdrawing (26 and 27) para substituents display no significant activity. Varying these substituents from the para to the meta or ortho position does not restore activity (data not shown). Other analogs containing multiple substituents on the phenyl ring (e.g., 3-chloro-4-hydroxyphenyl) or a bulkier aromatic moiety [e.g., 1-(4-hydroxynaphthyl)] are also less potent than 22 (data not shown).
Translocation of Substrates.
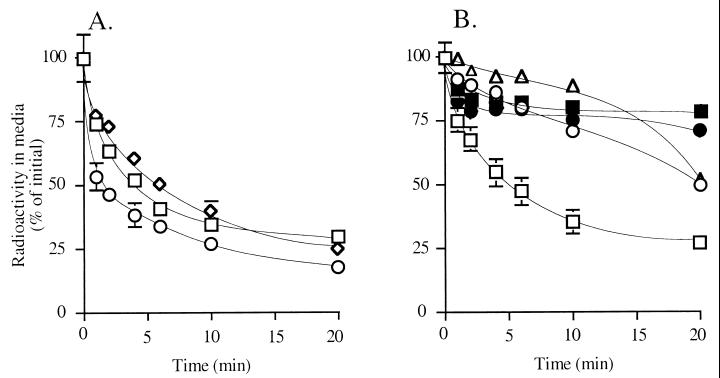
The results of the competition experiments outline the structural requirements for ligand recognition by the anandamide transporter, but do not provide information on whether the ligands also may serve as substrates for the transporter. To investigate substrate translocation we used a representative set of radioactively labeled compounds. We first tested three key analogs that compete with anandamide for uptake: [3H]arachidonamide (12), [3H]N-(4-hydroxyphenyl)arachidonamide (22), the most potent competitor in our series, and [3H]2-arachidonylglycerol (18), an endogenous cannabimimetic substance (26, 27) produced in brain hippocampus during neuronal activity (28). The three analogs are transported as rapidly and effectively as [3H]anandamide (Fig. 3A). These findings suggest that the anandamide transporter also may participate in the inactivation of 2-arachidonylglycerol, which was thought to be primarily mediated by enzymatic hydrolysis (10, 28). In agreement with this possibility, kinetic analyses indicate that [3H]2-arachidonylglycerol is accumulated in astrocytoma cells with an apparent Km of 0.7 ± 0.1 μM and a Vmax of 28 ± 6 pmol/min per mg of protein, values that are comparable to those obtained with [3H]anandamide in the same cell preparation (n = 3).
Figure 3.
Transport-mediated clearance of various radioactively labeled compounds in human astrocytoma cell cultures. (A) [3H]arachidonamide (12, ○), [3H]2-arachidonylglycerol (18, squares), [3H]N-(4-hydroxyphenyl)-arachidonamide (22, ◊). (B) [3H]anandamide (1, squares), [3H]cis-eicosatrienoylethanolamide (2, ○), [3H]cis-eicosadienoylethanolamide (3, ▵), [3H]cis-eicosaenoylethanolamide (4, ■, and [3H]oleylethanolamide (6, ●). Results are expressed as mean ± SEM (n = 3–6).
Next, we explored the effects of modifications in the hydrophobic tail. Included in this study, in addition to [3H]anandamide (1), were one cis-triene analog (2, [3H]eicosatrienoylethanolamide, 20:3Δ8,11,14), one cis-diene analog (3, [3H]eicosadienoylethanolamide, 20:2Δ8,11), and two cis monounsaturated analogs with the double bond located in the middle of the carbon chain (4, eicosaenoylethanolamide, [3H]20:1Δ11; and 6, oleylethanolamide, [3H]18:1Δ9). Although all of these fatty acid ethanolamides are able to compete with [3H]anandamide for transport (Fig. 2), only [3H]anandamide is effectively transported into cells (Fig. 3B). Of the remaining compounds, the cis-triene 2 and the cis-diene 3 are transported very slowly (t1/2 ≈20 min), whereas the two monoalkenes 4 and 6 are not transported at all. [3H]Palmitylethanolamide (16:0), a saturated acid ethanolamide that may activate an as-yet-uncharacterized peripheral CB2-like receptor (13), is not transported to any significant extent (data not shown). These findings indicate the existence of two distinct sets of structural requirements in the function of the anandamide transporter,one for substrate recognition and another for substrate translocation.
DISCUSSION
The results allow us to draw several initial conclusions on the structural requisites of substrates and inhibitors of the anandamide transporter.
(i) The stereoselectivity of these requirements is illustrated by the finding that S-1′-methyl anandamide (21) is significantly more potent than its R-isomer (20) at competing with [3H]anandamide for transport. This observation is duplicated in the corresponding 2,2-dimethyl arachidonate series. The configurational preference of these chiral compounds is opposite to that found at the CB1 cannabinoid receptor and may prove useful when designing transport inhibitors devoid of direct CB1 receptor activity.
(ii) A polar nonionizable head group appears to be of primary importance for a productive interaction with the anandamide transporter. Although this interaction may be enhanced by a hydrogen-donating hydroxyl group, polar groups with hydrogen acceptors also may yield relatively potent compounds (see, for example, the ether-containing analog 28). The results obtained with phenolic amides, such as N-(4-hydroxyphenyl)-arachidonamide (22) and its meta analog 23, further indicate that a hydroxy moiety favors the interaction with the transporter and underscore the regiochemical requirements of such interaction.
(iii) Although the presence of a secondary amide moiety in the polar carboxamido group may improve affinity for the transporter, compounds that contain an ester bond are also effective, provided that an appropriately spaced hydroxyl group is also present (e.g., 13 and 14). An interesting corollary of this property is that the anandamide transporter may participate in the biological inactivation of both anandamide and 2-arachidonylglycerol, a possibility supported by the similar transport kinetics of these two substrates.
(iv) Modifications of the hydrophobic fatty acid tail reveal unexpectedly distinct requirements for recognition and translocation of substrates by the anandamide transporter. Substrate recognition requires the presence of at least one cis double bond situated at the middle of the fatty acid chain, pointing to a preference for ligands in which the hydrophobic tail can fold in the middle and adopt a bent U-shaped conformation. Indeed, analogs with fully saturated chains or those incorporating trans double bonds do not interact significantly with the transporter. By contrast, substrate translocation requires a minimum of four cis nonconjugated double bonds, as ligands containing one, two, or three olefins are transported either very slowly (2 and 3) or not at all (4 and 6). This finding suggests that for transmembrane transport to occur substrates must be capable of adopting a tightly folded conformation, one that is not energetically favorable for ligands containing an insufficient number of cis double bonds.
Molecular modeling studies of fatty acid ethanolamides differing in the degree of unsaturation of their hydrophobic carbon chains provides insight into these distinctive conformational requirements. Possible low-energy conformers of these molecules, illustrated in Fig. 4, are significantly different. The presence of one or more nonconjugated cis double bonds in the middle of the chain leads to the formation of a turn that brings in closer proximity head and tail of the molecule. The shape of this turn is determined by the number and position of the cis double bonds. Conversely, the introduction of a central trans double bond yields a more extended chain conformation and hinders the ability of the molecule to undergo folding. Thus one of the low-energy conformers of anandamide displays a folded hairpin shape with the two halves of the molecule facing each other (Fig. 4A) (23). The ω-6 cis-triene analog 2 may adopt an analogous conformation, though one that is wider than that of anandamide (Fig. 4B). The width of the turn increases considerably in the two cis-dienes 3 and 5 and the two monoalkenes 4 and 6, as illustrated by the marked increase in distance between head group and tail of the molecule, yielding a series of cognate U-shaped conformers (Fig. 4 C–E). In the corresponding trans alkene analog 7, the distance between head and tail is much greater (Fig. 4F). It is important to point out that, whereas anandamide like arachidonic acid (24) may adopt either a closed-hairpin or a U-shaped conformation depending on the properties of the surrounding milieu, the hairpin conformation may be thermodynamically unfavorable to fatty acid ethanolamides containing only one or two double bonds.
A plausible interpretation of our results is that recognition and translocation of substrates by the anandamide transporter are governed by distinct conformational preferences. Although the initial recognition step may require that substrates assume a bent U-shaped conformation of variable width, the subsequent step of translocation across the cell membrane may impose a more tightly folded hairpin conformation. Whether an analogous two-stage mechanism underlies the transmembrane transport of other lipid molecules containing multiple cis double bonds, such as polyunsaturated fatty acids (29, 30) and prostaglandins (31), is an intriguing possibility that deserves further experimentation.
Acknowledgments
We dedicate this paper to the memory of Professor Kang Tsou. We thank Drs. A. Giuffrida and N. Stella for critical reading of the manuscript. This work was supported by the Neurosciences Research Foundation, which receives major support from Novartis; National Institute of Drug Abuse Grants DA3801, DA7215, and DA9158 (to A.M.), and DA12413 and DA12447 (to D.P.); and Roche Bioscience (unrestricted grant to D.P.).
References
- 1.Devane W A, Hanuš L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 2.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J C, Piomelli D. Nature (London) 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 3.Abood M E, Martin B R. Trends Pharmacol Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- 4.Beltramo M, Stella N, Calignano A, Lin S Y, Makriyannis A, Piomelli D. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 5.Hillard C J, Edgemond W S, Jarrahian A, Campbell W B. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 6.Desarnaud F, Cadas H, Piomelli D. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 7.Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- 8.Hillard C J, Wilkinson D M, Edgemond W S, Campbell W B. Biochim Biophys Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- 9.Cravatt B F, Giang D K, Mayfield S P, Boger D L, Lerner R A, Gilula N B. Nature (London) 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 10.Goparaju S K, Ueda N, Yamaguchi H, Yamamoto S. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 11.Piomelli D, Beltramo M, Giuffrida A, Stella N. Neurobiol Dis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- 12.Wagner J A, Varga K, Ellis E F, Rzigalinski B A, Martin B R, Kunos G. Nature (London) 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 13.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature (London) 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 14.Richardson J D, Kilo S, Hargreaves K M. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 15.Mueller-Wahl, K. R., Schneider, U., Kolbe, H. & Emrich, H. M. (1999) Am. J. Psychiatry, in press. [PubMed]
- 16.Petro D J, Ellenberger C. J Clin Pharmacol. 1981;21:413S–416S. doi: 10.1002/j.1552-4604.1981.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 17.Clifford D B. Ann Neurol. 1983;13:669–671. doi: 10.1002/ana.410130616. [DOI] [PubMed] [Google Scholar]
- 18.Wirguin I, Mechoulam R, Breuer A, Schezen E, Weidenfeld J, Brenner T. Immunopharmacology. 1994;28:209–214. doi: 10.1016/0162-3109(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 19.Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Eur J Pharmacol. 1997;337:R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- 20.Abadjj V, Lin S Y, Taha G, Griffin G, Stevenson L A, Pertwee R G, Makriyannis A. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 21.Serdarevich B, Carroll K K. J Lipid Res. 1966;7:277–284. [PubMed] [Google Scholar]
- 22.Kodali D R. J Lipid Res. 1987;28:464–469. [PubMed] [Google Scholar]
- 23.Barnett-Norris J, Guarnieri F, Hurst D P, Reggio P H. J Med Chem. 1998;41:4861–4872. doi: 10.1021/jm9803471. [DOI] [PubMed] [Google Scholar]
- 24.Rich M R. Biochim Biophys Acta. 1993;1178:87–96. doi: 10.1016/0167-4889(93)90113-4. [DOI] [PubMed] [Google Scholar]
- 25.Lang, W.-S., Qin, C., Lin, S.-Y., Khalnokar, A. D., Goutopolos, A., Fan, P., Abouzid, K., Meng Z.-X., Biegel, D. & Makriyannis, A. (1999) J. Med. Chem., in press. [DOI] [PubMed]
- 26.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 27.Mechoulam R, Ben-Shabat S, Hanuš L, Lígumski M, Kaminski N E, Schatz A R, Gopher A, Almog S, Martin B R, Compton D R, et al. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 28.Stella N, Schweitzer P, Piomelli D. Nature (London) 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer J E, Lodish H F. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch D, Tahl A, Lodish H F. Proc Natl Acad Sci USA. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster V L. Annu Rev Physiol. 1998;60:221–242. doi: 10.1146/annurev.physiol.60.1.221. [DOI] [PubMed] [Google Scholar]