Abstract
Bacillus subtilis contains three Fur homologs: Fur, PerR, and Zur. Despite significant sequence similarities, they respond to different stimuli and regulate different sets of genes. DNA target site comparisons indicate that all three paralogs recognize operators with a core 7-1-7 inverted repeat. The corresponding consensus sequences are identical at five or more of the seven defined positions. Using site-directed mutagenesis, the Per box at the mrgA promoter was altered to mimic the core 7-1-7 motif of the Fur and Zur boxes. In vitro, the mrgA promoter containing a Zur box was only recognized by Zur, as demonstrated by DNase I footprinting assays. In contrast, both Fur and PerR bound to the mrgA promoter region containing a consensus Fur box. Expression analysis of these promoters is consistent with the in vitro data demonstrating as few as 1 or 2 base changes per half-site are sufficient to alter regulation. Similarly, the Fur box at the feuA promoter can be converted into a Per or a Zur box by appropriate mutations. While both Fur and PerR could recognize some of the same synthetic operator sequences, no naturally occurring sites are known that are subject to dual regulation. However, the PerR-regulated zosA gene is controlled from a regulatory region that contains both Per and Fur boxes. Although purified Fur protein bound to the candidate Fur boxes, Fur has little effect on zosA expression—possibly due to the location of the Fur boxes relative to the zosA promoter. Together, our results identify two nucleotide positions that are important for the ability of PerR, Fur, and Zur to distinguish among the many closely related operator sites present in the B. subtilis genome.
The ferric uptake regulator (Fur) protein mediates iron-responsive gene regulation in a variety of gram-negative and low-GC gram-positive bacteria. Fur represses transcription by binding to target genes when the intracellular concentration of Fe(II) exceeds a threshold level. In addition to iron acquisition and storage, Fur regulates the production of some pathogenicity factors, the acid shock response, chemotaxis, and oxidative stress defenses (27, 48). In Bacillus subtilis, three Fur paralogs regulate different sets of genes in response to distinct stimuli. Fur represses a regulon of ∼40 genes in response to iron sufficiency (3), PerR controls ∼12 genes involved in an oxidative stress response (7, 31, 32), and Zur represses two operons encoding zinc transporters (20, 21). There does not appear to be any overlap between these three regulons, indicating that each of these three proteins has distinct DNA recognition properties.
Fur proteins bind with high affinity to a DNA sequence known as a Fur box. The classical Fur box is a 19-bp (9-1-9) inverted repeat, GATAATGATAATCATTATC (13). Originally, it was envisioned that this 19-bp sequence might represent the binding site for a single dimer of Escherichia coli Fur. However, subsequent studies using synthetic oligonucleotides led to a model in which Fur recognizes repeated arrays of a hexamer motif, GATAAT (16). Our analysis of the Fur regulon in B. subtilis suggested a new interpretation. A multiple sequence alignment of all identified Fur-regulated genes led to a consensus Fur box containing a heptameric inverted repeat (7-1-7 motif) of TGATAATNATTATCA (3). Two such motifs, offset by 6 bp, generate a 21-bp sequence containing the classical 19-bp Fur box. We therefore proposed that a single Fur dimer recognizes each 7-1-7 operator and that the binding of two Fur dimers to opposite faces of the DNA duplex would occur at a 19-bp Fur box (2). Support for this model for B. subtilis Fur was provided by using electrophoretic mobility shift assays (EMSAs) with synthetic oligonucleotides (2). In addition, modeling of the recently determined structure of Pseudomonas aeruginosa Fur onto a DNA duplex also suggests that two dimers bind per 19-bp Fur box (41). A similar conclusion has been reached from mutational analysis of E. coli Fur binding to the fepD-entS regulatory region (33, 34). Taken together, these results suggest that the primary DNA determinants for Fur binding are likely to be similar in all three organisms and include a 7-1-7 (or closely related) consensus (2, 34).
The B. subtilis Fur box, defined as the minimal site needed for high-affinity binding (TGATAATNATTATCA) (2) is nearly identical to the consensus Per box, TTATAATNATTATAA (5, 9, 11, 18, 32). In addition, recent DNA sequence alignments indicate that Zur binds to DNA targets with a similar 7-1-7 core element, but with a 3-bp extension (lowercase letters) on both sides, aaaTCGTAATNATTACGAttt (21). We have demonstrated previously that Fur binds to a model oligonucleotide substrate containing a Fur box, while it does not bind the same substrate containing a Per box, which differs at only 2 nucleotides (2). Thus, it is likely that only small differences in the binding sites are sufficient to distinguish among Fur, Per, and Zur boxes. In this study, we identify those bases that are critical for each B. subtilis Fur homolog to distinguish among the closely related operator sites present in the B. subtilis genome.
MATERIALS AND METHODS
Bacterial strains, phage, and plasmids.
The selected B. subtilis strains, phage, E. coli strains, and plasmids used in this study are listed in Table 1. B. subtilis strains were grown at 37°C in Luria broth (LB). SPβ phage are derivatives of SPβc2Δ2 (40) and were constructed by integration of a promoter region-cat-lacZ operon fusion constructed in pJPM122 into strain ZB307A as described previously (36).
TABLE 1.
Selected bacterial strains and plasmids used in this studya
| Strain or plasmid | Characteristics | Reference |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| CU1065 | W168 attSPβ trpC2 | Laboratory stock |
| ZB307A | W168 SPβ c2Δ2::Tn917::pSK10Δ6 | 49 |
| HB0509 | HB1000 perR::spc | 7 |
| HB2194 | CU1065 perR::spc | This study |
| HB2078 | CU1065 perR::kan | 18 |
| HB2060 | CU1065 perR::kan amyE::cat | This study |
| HB2501 | CU1065 fur::kan | 18 |
| HB2502 | CU1065 fur::kan amyE::cat | 25 |
| HB8132 | CU1065 zur::spc | 22 |
| HB2168 | CU1065 fur::kan perR::spc | This study |
| HB2169 | CU1065 fur::kan zur::spc | This study |
| HB2170 | CU1065 perR::kan zur::spc | This study |
| HB1122 | ZB307A SPβ c2Δ2::Tn917::φ(mrgAPer box-cat-lacZ) | 11 |
| HB2144 | ZB307A SPβ c2Δ2::Tn917::φ(mrgAFur box-cat-lacZ) | This study |
| HB2145 | ZB307A SPβ c2Δ2::Tn917::φ(mrgAZur1 box-cat-lacZ) | This study |
| HB2146 | ZB307A SPβ c2Δ2::Tn917::φ(mrgAZur2 box-cat-lacZ) | This study |
| HB8206 | ZB307A SPβ c2Δ2::Tn917::φ(zosA-cat-lacZ) | 21 |
| HB0618 | ZB307A SPβ c2Δ2::Tn917::φ(feuAFur box-cat-lacZ) | This study |
| HB0619 | ZB307A SPβ c2Δ2::Tn917::φ(feuAPer box-cat-lacZ) | This study |
| HB0620 | ZB307A SPβ c2Δ2::Tn917::φ(feuAZur1 box-cat-lacZ) | This study |
| HB2218 | ZB307A SPβ c2Δ2::Tn917::φ(feuAZur2 box-cat-lacZ) | This study |
| HB8108 | CU1065 SPβ c2Δ2::Tn917::φ(zosA-cat-lacZ) | 21 |
| HB2118 | CU1065 perR::kan SPβ c2Δ2::Tn917::φ(zosA-cat-lacZ) | 18 |
| HB2111 | CU1065 fur::kan SPβ c2Δ2::Tn917::φ(zosA-cat-lacZ) | 18 |
| HB2139 | CU1065 perR::spc fur::kan SPβ c2Δ2::Tn917::φ(zosA-cat-lacZ) | This study |
| E. coli | ||
| DH5α | φ80lacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+)supE44 relA1 deoR Δ(lacZYA-argF)U169 | Laboratory stock |
| Plasmids | ||
| pJPM122 | cat-lacZ operon fusion vector for SPβ | 46 |
| pLC2260 | pJPM122 with mrgAPer box promoter | 11 |
| pMF36 | pJPM122 with mrgAFur box promoter | This study |
| pMF37 | pJPM122 with mrgAZur1 box promoter | This study |
| pMF38 | pJPM122 with mrgAZur2 box promoter | This study |
| pNB7 | pJPM122 with feuAFur box promoter | This study |
| pNB8 | pJPM122 with feuAPer box promoter | This study |
| pNB9 | pJPM122 with feuAZur1 box promoter | This study |
| pMF46 | pJPM122 with feuAZur2 box promoter | This study |
Additional strains containing reporter fusions are described in Materials and Methods.
Ampicillin (100 μg ml−1) was used for the selection of E. coli strains. Erythromycin (1 μg ml−1) and lincomycin (25 μg ml−1; for testing macrolide-lincosamide-streptogramin B [MLS] resistance), spectinomycin (100 μg ml−1), kanamycin (10 μg ml−1), and neomycin (10 μg ml−1) were used for the selection of various B. subtilis strains.
Construction of mutant strains.
Double-mutant strains were constructed by transformation of HB8132 (CU1065 zur::spc) (22) or HB2501 (CU1065 fur::kan) (18) with chromosomal DNA from HB2501 (CU1065 fur::kan), HB0509 (CU1065 perR::spc) (7), or HB2078 (CU1065 perR::kan) (18) and selection for kanamycin and spectinomycin resistance. The strains were confirmed for the presence of mutations by PCR.
Construction of reporter fusion strains.
The cat-lacZ fusions generated in strain ZB307A were moved to different backgrounds by SPβ transduction and selection for MLS and neomycin resistance. Specifically, SPβ phage from the starting strains containing the mrgA promoter region with associated operator mutations (HB1122 and HB2144 to -2146) were transduced into the CU1065 wild-type strain (generating HB0568, HB2147, HB2148, and HB2149, respectively), into the perR mutant strain (HB2060) to generate HB2150 to -2153, into the fur mutant HB2502 to generate HB2154 to -2157, and into the zur mutant HB8132 to generate HB2158 to -2161. Similarly, transduction into the fur perR double mutant HB2168 generated strains HB2171 to -2174, transduction into the perR zur double mutant HB2170 generated HB2175 to -2178, and transduction into the fur zur double mutant HB2169 generated HB2179 to -2182. The corresponding reporter phage for the feuA promoter region (Table 1; strains HB0618 to -0620 and HB2218) were transduced into CU1065 (HB0621 to -0623 and HB2211), the perR mutant (HB2188 to -2190 and HB2212), the fur mutant (HB0624 to -0626 and HB2213), the zur mutant (HB0627 to -0629 and HB2214), the fur perR double mutant (HB2191 to -2193 and HB2215), the perR zur double mutant (HB2196 to -2198 and HB2216), and the fur zur double mutant (HB2199 to -2201 and HB2217).
Primer extension mapping of mrgA and feuA promoter sites.
Total RNA from late-log-phase cells from wild-type, perR mutant, fur mutant, and perR fur mutant strains (CU1065, HB2078, HB2501, and HB2168, respectively) grown in LB was isolated with RNAWIZ reagent (Ambion). Primer extension of mrgA was performed with SuperScript II RNase H− reverse transcriptase (Invitrogen), according to the manufacturer's instructions, in a 40-μl reaction mixture containing 10 μg of total RNA and 8 pmol of end-labeled primer 522. The extension product was loaded on a 6% denaturing polyacrylamide gel alongside the DNA sequence ladder synthesized with the same labeled primer (SequiTherm Cycle sequencing kit; Epicentre Technologies). For feuA, the transcripts using in the primer extension experiment were also generated in vitro by using B. subtilis σA holoenzyme essentially as described previously (22), except [α-32P]UTP was replaced by UTP. A PCR fragment containing the feuA promoter region (generated with primers 1121 and 1123, similar to a fragment used in the DNase I footprinting assay) was used as a template for in vitro transcription.
Site-directed mutagenesis of the mrgA promoter.
Site-directed mutagenesis was done by the mega-primer PCR mutagenesis method as described in reference 6. PCR primers containing the desired changes within the Per box in the mrgA promoter included primer 825 (5′-CTAAATGATAATTATTATCATTTAGTATTG-3′) for mutation into the Fur box and primers 826 (5′-CTAAATCATAATTATTATGATTTAGTATTG-3′) and 827 (5′-CTAAATCGTAATTATTACGATTTAGTATTG-3′) for mutation into Zur boxes (changed bases underlined). To produce mega-primers, each oligonucleotide given above and primer 366 (5′-ACTCTCCGTCGCTATTGTAACCAG-3′, located in the cat gene) were used for the amplification reaction with pLC2260 (11), containing the mrgA-cat-lacZ operon fusion in pJPM122, as a template. The mrgA promoter in pLC2260 is an SphI-EcoRI fragment and extends from −464 to +47 relative to the start codon. The second amplification reaction contained a mega-primer and primer 535a (5′-GTACATATTGTCGTTAGAACGCGGC-3′, located in the vector and upstream of mrgA promoter). The template used in this reaction was a PCR product obtained with primers 535a and 45 (5′-GTTTGATTTGTTTTTGCG-3′, located between the mrgA Per box and the EcoRI site). These second PCR products were digested with HindIII-BamHI and cloned into pJPM122 at the same sites to generate plasmids identical to pLC2260, except for the mutations in the Per box. All constructs were verified by DNA sequencing prior to transfer into the SPβ2Δ2::Tn917::pBSK10Δ6 site of strain ZB307A (49) by double-crossover recombination.
Site-directed mutagenesis of feuA promoter.
The site-directed mutations at the Fur box of the feuA promoter were generated as described for mrgA. The mutagenic primers 765 (5′-TTATAATAGTTATAAATTGAACA-3′; changed bases underlined), 766 (5′-TCATAATAGTTATGAATTGAACA-3′), and 767 (5′-TCGTAATAGTTACGAATTGAACA-3′) were used to change the Fur box into a Per box and Zur boxes with one or two changes per half-site, respectively. The primers used to amplify the feuA promoter region (−254 to +77 relative to the start codon) from wild-type B. subtilis CU1065 are 1123 (5′-CCCAAGCTTACTACCAGCAATTAC-3′, upstream region; HindIII site is underlined) and 1121 (5′-CAGGATCCGATTCATTTTTGCTGCCG-3′, downstream region; BamHI site is underlined). All promoters were cloned into pJPM122 as BamHI-HindIII fragments, generating feuA-cat-lacZ operon fusions. All plasmids were verified by sequencing and then transferred into the SPβ2Δ2::Tn917::pBSK10Δ6 site as described above.
β-Galactosidase assays.
Cells were grown in LB overnight and collected for β-galactosidase assay by the method of Miller as described previously (10, 40). All assays were performed three times, and the values were averaged.
DNase I footprinting assays.
Purification of B. subtilis Fur, PerR, and Zur and DNase I protection assays were performed as described previously (6, 20, 32). As noted previously, Fur protein purifies in an active, metallated form (containing both iron and zinc) (26), Zur protein copurifies with activating zinc ion (20), and PerR is activated by inclusion of Mn(II) in the buffer for DNA-binding assays (32). Primer 522 (5′-GGGTATACTTGATAATTGTGTGTTCAGT-3′), located downstream of mrgA promoter, was end labeled with [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs). PCR was used to amplify templates for the footprinting experiments. The primer pair used in the PCR was made up of labeled primer 522 and primer 521 (5′-GCAAGCTTCCTGCTGTTCCGATCGCTTT-3′). The fragments were run on a 1% agarose gel and purified. The feuA promoter was prepared as an mrgA promoter, except the primers used in PCR amplification were primer 1123 and labeled primer 1121. The zosA promoter fragment was prepared by 5′ labeling on the bottom strand of a HindIII-digested PCR fragment. This PCR fragment was generated with wild-type B. subtilis chromosomal DNA and the zosA promoter amplification primers (21). Binding reaction mixtures (50-μl total volume) contained 1× binding buffer, labeled DNA fragment, and purified protein as indicated and were incubated at room temperature for 10 min. The composition of 1× buffer for each protein was as follows: for PerR, 20 mM Tris-Cl (pH 8.0), 50 mM KCl, 5% glycerol, 10-μg/ml bovine serum albumin, 1-μg/ml salmon sperm DNA, 10 μM MnCl2, and 1 mM dithiothreitol (DTT); for Fur, 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 5% glycerol, and 1 mM DTT; and for Zur, 40 mM Tris-Cl (pH 8.0), 100 mM KCl, 5% glycerol, 2-μg/ml salmon sperm DNA; and 1 mM DTT. In the binding reaction for the zosA promoter, the DTT concentration used was 5 mM. Fifty microliters of a 5 mM CaCl2-10 mM MgCl2 solution was added, and then the reactions were digested with 0.06 U of DNase I for between 1 and 3 min. Reactions were stopped by adding 700 μl of stop solution (645 μl of ethanol, 50 μl of sodium acetate, 5 μl of 1-mg/ml yeast RNA), and the nucleic acids were recovered by centrifugation for 15 min at maximum speed. The DNA was resuspended in formamide loading buffer and loaded onto a 6% sequencing gel. The G+A ladder used was generated by mixing approximately 20,000 cpm of labeled fragment with cleavage buffer (1 μl of formic acid in 1 ml of formamide loading buffer) and incubating this mixture at 104°C for 20 min (37). Gel images were obtained by exposing dried gels to a Phosphor Screen (Molecular Dynamics). The amount of protein bound to DNA was quantified with ImageQuant data analysis software.
RESULTS
Comparison of Per, Fur, and Zur boxes in B. subtilis.
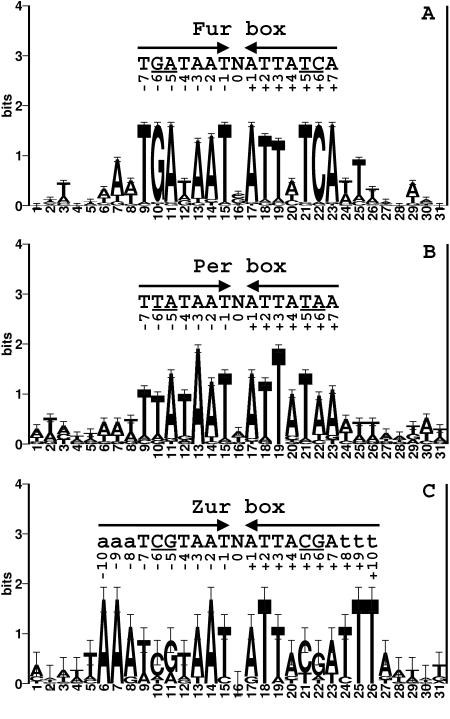
The three B. subtilis Fur homologs (Fur, PerR, and Zur) all recognize similar consensus sequences with differences apparently limited to 1 or 2 bases per half-site (Fig. 1). Each of these regulatory proteins controls multiple operons in the cell, yet we have not observed any overlap among their corresponding regulons (3, 22, 29). Thus, we hypothesized that positions 5 and 6 in each half-site are critical to allow each protein to correctly discriminate its operator sites from those recognized by other Fur homologs. It is also possible that other positions contribute to sequence discrimination. For example, Zur may require AT-rich regions flanking each 7-1-7 operator site (22).
FIG. 1.
(A) DNA sequence logo of the B. subtilis Fur DNA binding sites (3, 44). A set of 40 DNA sequences (20 sites together with their reverse complements) containing Fur box sites were aligned with CLUSTALW (47), and then a window of 31 bases around the 7-1-7 motif was used to generate a sequence logo. The height of each letter corresponds to its relative abundance at that position. The letters above the logo show the sequence of conserved bases. The underlined letters represent bases that are different among Fur, PerR, and Zur boxes. (B) Sequence logo of the B. subtilis Per box. A total of 12 Per boxes were used for alignment. (C) DNA logo of B. subtilis Zur DNA binding sites. Note that there are only four known Zur boxes found in the B. subtilis genome.
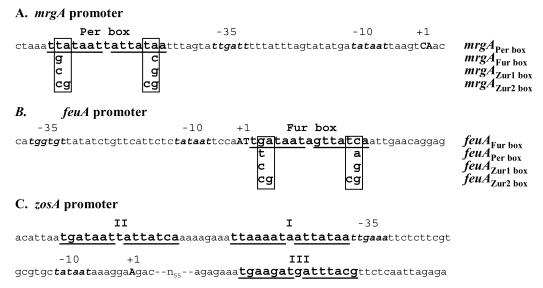
To examine the role of positions 5 and 6 in sequence discrimination, we used site-directed mutagenesis to alter the Per box of the mrgA promoter and the Fur box of the feu promoter. mrgA is a well-characterized member of the PerR regulon: expression is repressed by either manganese or iron in vivo (9-11, 18) and induced by exposure to H2O2 and other oxidants (9, 14, 35). The mrgA promoter was chosen since it contains a single Per box that matches consensus at 14 of 14 positions (Fig. 2A). In addition, it has 3-bp extensions on each side of the core sequence that are similar to the Zur box consensus sequence. The feuA promoter is regulated by the binding of Fur to a single Fur box that contains a 7-1-7 consensus motif Fig. 2B (3). In contrast, most other Fur-regulated genes contain two or more overlapping heptamer motifs generating the more common 19-bp Fur box consensus (2, 3). We have also included in our studies the zosA regulatory region (Fig. 2C), since this is a rare example of a promoter region that appears to contain both Fur and Per box regulatory sequences (21).
FIG. 2.
Promoter architecture of the mrgA, feuA, and zosA regulatory regions. (A) DNA sequence showing the Per box upstream of the mrgA promoter. The mrgA Per box mutations introduced in this study are indicated. The native mrgA promoter is designated “mrgAPer box.” The transcriptional start sites are shown in capital letters. (B) feuA regulatory region showing the Fur box overlapping the transcriptional control region. The transcriptional start sites observed in vitro (Fig. 3) are shown in capital letters. When in vivo RNA was used, transcription appeared to initiate 3 bases further upstream (data not shown). One set of candidate promoter recognition elements are indicated. The three mutant derivatives generated in this study are shown. (C) zosA regulatory region showing the promoter element and three candidate regulatory sites. Site I is a Per box, while sites II and III are Fur box-like sequences.
Identification of the mrgA and feuA promoter elements.
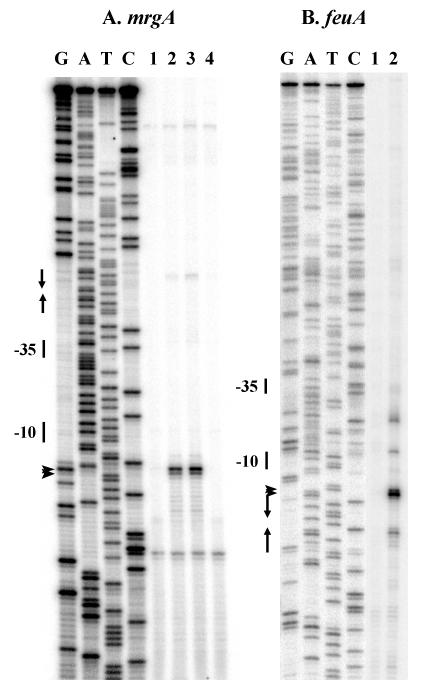
The mrgA and feuA promoter regions each contain candidate recognition elements for σA RNA polymerase (Fig. 2). To confirm the roles of these sites in transcriptional control, we have used primer extension analysis to map the 5′ ends of the corresponding transcripts (Fig. 3). Transcripts corresponding to the mrgA promoter are present in strains containing a perR null mutation, but not in the wild-type or fur mutant strains, confirming the role of PerR as the repressor of this promoter (Fig. 3A). Transcripts for the feuA region were also detected that initiated from the expected regulatory region and were modestly up-regulated in a fur mutant strain (data not shown). Localization of the feu transcription initiation site was performed with RNA generated in vitro with σA holoenzyme (Fig. 3B). Transcription initiation from this region is blocked in vitro by addition of purified Fur protein (Fig. 3B), consistent with previous studies demonstrating an ∼20-fold derepression of this gene in a fur mutant (3).
FIG. 3.
Mapping of the mrgA (A) and feuA (B) promoter sites by primer extension. (A) Total RNA was extracted from B. subtilis strains (lane 1, wild type; lane 2, perR mutant; lane 3, perR fur mutant; lane 4, fur mutant) grown in LB medium, and labeled cDNA products were prepared by reverse transcription. The arrowheads mark the corresponding major extension products; the transcription start sites are shown in Fig. 2. The Per box is indicated by arrows. The sequencing ladder was generated with the same oligonucleotide. (B) RNA was prepared from in vitro transcription reaction mixtures containing σA holoenzyme either with or without 200 nM Fur (lanes 1 and 2, respectively), and labeled cDNA products were prepared by reverse transcription. The Fur box is indicated by arrows.
Mutational analysis of the mrgA Per box: in vitro DNA-binding studies.
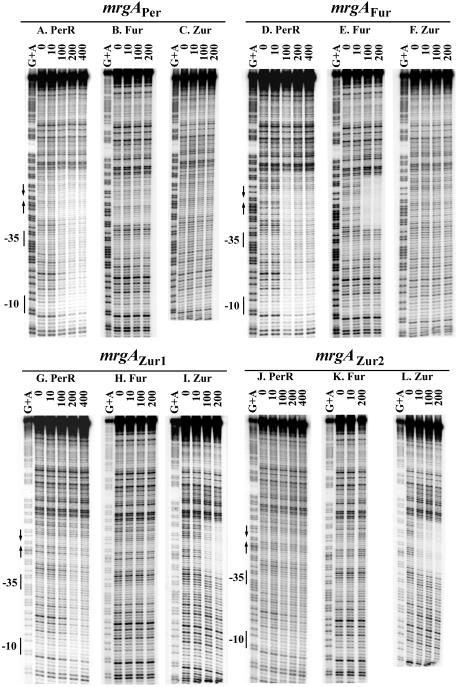
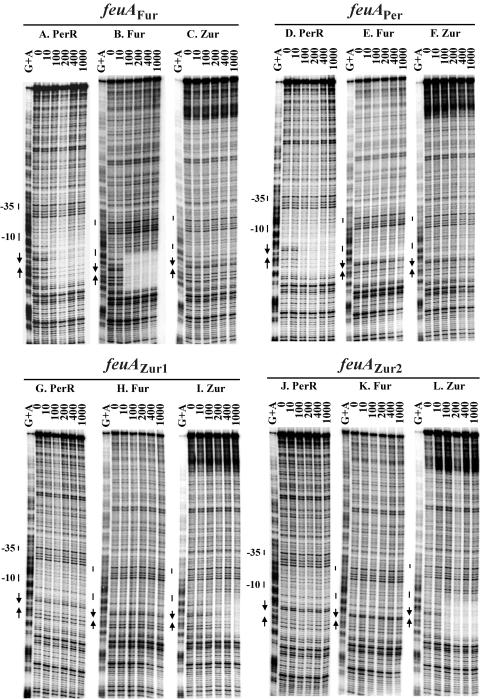
PerR binds with high affinity to the Per box-containing region of the mrgA promoter (32). So far, there has been no evidence to suggest that mrgA is under the control of either Fur or Zur. To measure the relative affinities of all three Fur homologs for this operator site in vitro, the binding of PerR, Fur, and Zur was examined by DNase I footprinting. PerR binds specifically to the mrgA promoter, but this leads to the formation of an extended DNase I footprint (Fig. 4A), consistent with previous results (32). Binding of PerR to this region of DNA initiates at the Per box region and is known to be sensitive to Per box mutations that abolish in vivo regulation (32). In contrast, no binding was detected in the reactions with Fur or Zur (Fig. 4B and C). This result confirms that among Fur homologs, PerR is the only direct regulator of mrgA.
FIG. 4.
DNase I footprinting of mrgA promoter fragments. The mrgA promoter fragments 5′ labeled on the top strand was treated with DNase I in the absence of protein and increasing concentrations of PerR (A, D, G, and J), Fur (B, E, H, and K), or Zur (C, F, I, and L). The marker lanes (G+A) are ladders generating from formamide cleavage reactions for G and A (37). The numbers on the top of footprinting lanes are the nanomolar concentrations of protein (monomer) used in the reactions. The −10 and −35 regions are shown next to the ladder. The Per box is shown with arrows. The data shown are representative of triplicate experiments.
Site-directed mutagenesis was used to change 2 bases in the mrgA Per box to generate a new promoter, mrgAFur (Fig. 2A). This change was not sufficient to abolish the binding of PerR (Fig. 4D), but did facilitate Fur binding (Fig. 4E). On the other hand, Zur did not bind to the mrgAFur promoter fragment (Fig. 4F). These results suggest that the mrgAFur promoter might be regulated by both PerR and Fur.
Like the mrgAFur box, mrgAZur1 contained 2-bp substitutions (Fig. 2A) and was bound by both PerR and Zur (Fig. 4G and I). However, the binding affinity of PerR to mrgAZur1 was lower than that to native mrgAPer (Fig. 4A and G). Note that Fur did not recognize the mrgAZur1 operator (Fig. 4H). Interestingly, when 2 additional bases were changed to better mimic the Zur box consensus (mrgAZur2, Fig. 2A), only Zur appeared to recognize the modified operator (Fig. 4J to L). In addition, Zur bound mrgAZur2 with higher affinity than mrgAZur1 (Fig. 4L and I).
Mutational analysis of the mrgA Per box: in vivo analyses.
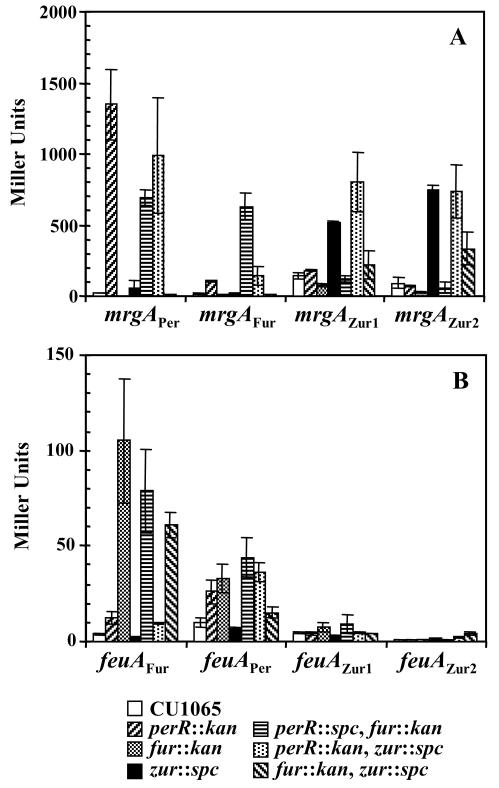
To investigate which of the three Fur homologs regulate each promoter in vivo, we measured expression in wild-type, single-, and double-mutant strains by using β-galactosidase assays. Assays were conducted with cells grown in rich medium under conditions known to provide sufficient metal ions to serve as corepressors for all three homologs. As expected, the transcription level of the mrgAPer promoter region was derepressed only in strains containing a perR mutation: the perR, perR fur, and perR zur double mutants (Fig. 5A). The expression of the mrgAFur promoter was increased in the perR and perR zur mutants, with even higher expression in the perR fur double mutant (Fig. 5A). This supports the suggestion from in vitro experiments that mrgAFur is under dual regulation of PerR and Fur. Expression from the mrgAZur1 promoter region was highest in the perR zur double mutant (Fig. 5A), indicating regulation of this promoter by both PerR and Zur. Significantly, the mrgAZur2 promoter was regulated exclusively by Zur: transcription was derepressed in all strains containing a zur mutation (Fig. 5A). Note that the absolute level of transcription from these mutant constructs is about twofold lower than that for the wild type. This may reflect a stimulatory role of the AT-rich Per box sequence, perhaps by function as an upstream promoter (UP) element (17). In sum, these in vivo results support the suggestion that the recognition of DNA sequences by each B. subtilis Fur homolog can be switched, at least in part, by changing nucleotides at position ±6 within the 7-1-7 motif.
FIG. 5.
In vivo expression of mrgA (A) and feuA promoters (B). β-Galactosidase activity was determined from cells grown overnight in LB medium. The data shown represent the average ± standard deviation of three independent experiments.
Mutational analysis of the feuA Fur box.
In a reciprocal experiment, the Fur-regulated feuA gene was altered to change the associated Fur box into a Per or a Zur box. The feuA gene is the first gene in the feu operon encoding an iron transport system under Fur regulation (3, 36). The feuA Fur box contains only one 7-1-7 Fur box (Fig. 2B), which is recognized in vitro by both Fur and PerR (Fig. 6A and B). However, in vivo feuA was mainly under Fur control (Fig. 5B).
FIG. 6.
DNase I footprinting of feuA promoter fragment. The feuA fragment 5′ labeled on the bottom strand was treated with DNase I in the absence of protein and increasing concentrations of PerR (A, D, G, and J), Fur (B, E, H, and K), or Zur (C, F, I, and L). The marker lanes (G+A) are ladders generating from formamide cleavage reactions for G and A (37). The numbers on the top of the footprinting lanes represent the nanomolar concentrations of protein used in the reactions. The −10 and −35 regions are shown next to the ladder. The Per box is shown with arrows. The data shown are representative of triplicate experiments.
When the Fur box of the feuA promoter was changed to mimic a Per box (feuAper), only PerR bound to the modified operator in vitro (Fig. 6D to F). No protection was observed with purified Fur protein even at a concentration of 1 μM (Fig. 6E). Surprisingly, the feuAper operator appears to be at least partially regulated by both Fur and PerR in vivo: the activity of the feuAper promoter was derepressed in strains containing either fur or perR mutations (Fig. 5B). However, we note that the zur perR double mutant is more highly derepressed than the zur fur double mutant. This suggests that this promoter is primarily under the control of PerR, as suggested by the in vitro DNA-binding results.
Like the mrgA Per box, the feuA Fur box can be altered to resemble a Zur box by changing nucleotides at positions 5 and 6 of each repeat. When only position 6 of each repeat was changed (feuAZur1), the binding of Fur was abolished (Fig. 6H), but Zur bound only weakly (Fig. 6I). When both positions 5 and 6 in each half-site were altered, binding of Zur was significantly enhanced (Fig. 6I versus L). Note that since the promoter activity of the feuAZur1 and feuAZur2 constructs was very low, it was not possible to characterize the in vivo responsiveness (Fig. 5B). This loss of activity likely reflects the facts that the introduced mutations affect the sequence of the transcription initiation region.
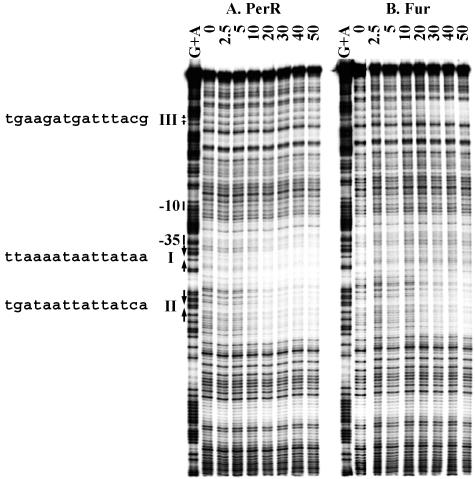
Binding of PerR and Fur to the zosA promoter.
The promoter region of zosA contains both a candidate Per box and a candidate Fur box (sites I and II in Fig. 2C and 7, respectively) (21). The Per box (TTAAAATAATTATAA) has one mismatch (underlined base) from the consensus, while the Fur box matches the 7-1-7 consensus at all positions (TGATAATTATTATCA). It has been clearly demonstrated that zosA is part of the PerR regulon: its expression is derepressed in the perR mutant and induced by H2O2 (20, 30). In footprinting experiments, PerR protein binds to both boxes (20).
FIG. 7.
DNase I footprinting of the zosA promoter. The zosA promoter fragment 5′ labeled on the bottom strand was treated with DNase I in the absence of protein and increasing concentrations of PerR (A) or Fur (B). The marker lanes (G+A) are ladders generating from formamide cleavage reactions for G and A (37). The numbers on the top of the footprinting lanes represent the nanomolar concentrations of protein (monomer) used in the reactions. The −10 and −35 regions are shown next to the ladder. DNA binding sites for PerR or Fur are shown with arrows. The sequence of each site is shown to the left. The data shown are representative of duplicate experiments.
We used DNase I footprinting to compare the binding of PerR and Fur to the zosA regulatory region. As expected, PerR binds specifically and protects both boxes (Fig. 7A) (21) with comparable affinity (Kd = 10 and 20 nM for sites I and II, respectively). Footprinting by Fur was specific to the Fur box motif (Kd = 20 nM) (Fig. 7B). The extended binding to the Per box (site I) was not detected at up to 300 nM Fur in the reaction (data not shown). Interestingly, an additional protection region by Fur (site III) was detected upstream of the ribosome binding site (Fig. 7B). This region contains a motif that has five mismatches compared to the consensus Fur box (TGAAGATGATTTACG). When the in vivo expression of zosA was investigated in various strains, transcription was derepressed >10-fold in the perR mutant and as much as 3-fold in the fur mutant, consistent with previous results (18). The significance of the derepression of zosA in the fur mutant is unclear. Most members of the PerR regulon are expressed at an elevated level in the fur mutant in medium limited for iron and manganese, the metal ion cofactors that activate PerR. Derepression is not observed in medium supplemented with iron or manganese, suggesting that it may be an indirect effect of the fur mutation (see reference 18 for discussion). Expression in the perR fur double mutant was much like that in the perR single mutant (data not shown), consistent with the conclusion that zosA is primarily regulated by PerR (18).
DISCUSSION
B. subtilis contains genes that encode three Fur paralogs: Fur, PerR, and Zur. Sequence comparisons among several PerR-regulated genes led to the identification of the Per box consensus as a heptameric 7-1-7 inverted repeat (Fig. 1A) with some similarity to the longer 19-bp Fur box consensus (11, 31). The relationship between Per and Fur boxes was strengthened as a result of recent insights into the nature of the Fur box recognition element. Analysis of the B. subtilis Fur regulon by DNA microarray analysis led to the identification of 30 operons (3). When the corresponding regulatory regions were aligned, a conserved 7-1-7 motif was identified corresponding to approximately two-thirds of the classically defined 19-bp Fur box. Using synthetic operator sequences, we demonstrated that this heptamer motif binds a single dimer of Fur, whereas the longer 19-bp consensus sequence binds two Fur dimers (2). The third Fur paralog, Zur, controls a significantly smaller regulon than either PerR or Fur with a total of six Zur-regulated genes regulated by four Zur-binding sites (22). Despite this small sample size, a consensus Zur box sequence emerged that shares clear similarities with the heptameric Per and Fur boxes, albeit with additional conserved bases flanking the core elements. The significant sequence similarities between the DNA consensus sites for these three regulatory proteins suggest that regulon overlap may occur. To investigate this possibility, we have explored the effects of mutations in the mrgA Per box and feuA Fur box on DNA-protein recognition in vitro and regulation in vivo.
Our DNA-binding studies suggest that, in principle, regulon overlap could occur between these three paralogs. For example, when the mrgA Per box (which is a 14-of-14 match to the Per box consensus) was changed to a consensus Fur box, the mrgAFur regulatory region was recognized by both PerR and Fur in vitro and was under the control of both repressors in vivo (Fig. 4D and E and 5A). Note that the partial derepression of mrgAFur in the perR mutant cannot be explained by effects on fur expression. The fur gene is regulated by PerR with about two- to threefold derepression in a perR mutant grown in minimal media (18). Thus, elevated levels of Fur in a perR mutant would result in enhanced repression of mrgAFur rather than derepression. Significantly, the mrgAFur operator differs in only 1 base in each half-site relative to the native Per box. Likewise, a DNA sequence that is intermediate between the Per and the Zur box (mrgAZur1) transformed mrgA into a PerR- and Zur-regulated gene (Fig. 4G and H and 5A). In contrast, when 2 bases in each half-site were altered (mrgAZur2), the resulting operator was only recognized by Zur both in vitro and in vivo (Fig. 4J, K, and L and 5A). Similar findings resulted from the mutagenesis of the Fur box of the feuA promoter. Changing a single position of each half-site to resemble a Per box enhanced PerR binding (Fig. 5B and 6), and a complete switch to a Zur box, at least in vitro, required changes of 2 nucleotides in each half-site (Fig. 6).
While these results establish that mutationally altered target sites can be recognized by two different Fur paralogs in vitro and in vivo, we have not yet identified any naturally occurring examples of such regulon overlap. It should be noted, however, that microarray analyses of the Fur, PerR, and Zur regulons have employed only single-mutant strains (3, 22, 29), and target genes repressed by more than one paralog may have been missed. While the zosA regulatory region contains sites that interact with both Fur and PerR (Fig. 7), only PerR seems to regulate expression in vivo (18, 21).
Our mutagenesis studies indicate that two bases (positions 5 and 6 in each half-site) are key for the discrimination of DNA-binding sites by PerR, Fur, and Zur. The molecular basis for this discrimination presumably lies in differences in the recognition helices of the three Fur paralogs, although elucidation of the precise details of how these proteins have evolved to recognize distinct operator sequences will likely require high-resolution crystal structures of protein-DNA complexes.
In addition to the two key positions noted here, it is likely that other protein-DNA interactions are important in establishing the range of operator sites recognized in vivo by each paralog. For example, in B. subtilis, Per boxes frequently occur in isolation, while Fur boxes are most commonly found in overlapping arrays of two or more sites (3, 32). This suggests that cooperativity of binding may be important for establishing repression by Fur. However, it should be noted that PerR also binds to some operators in arrays, as judged by the formation of extended regions of protection in DNase I footprints (Fig. 4) (32). In the case of Zur, sequence alignments indicate that 3 additional bases are conserved in each half-site (Fig. 1C), leading to a 10-1-10 operator structure (22). Studies with model oligonucleotides support the suggestion that these bases contribute to operator recognition by Zur (F. Miyagi and J. D. Helmann, unpublished data). Finally, other regulatory factors may influence the ability of these proteins to regulate gene expression in vivo. For example, feuAPer is regulated by both Fur and PerR (Fig. 5B), although in vitro Fur binding could not be demonstrated (Fig. 6E). It has been reported that histone-like proteins, such as H-NS and integration host factor (IHF), contribute to Fur regulation in E. coli (15), perhaps by modifying the DNA conformation to facilitate contacts between regulator proteins and/or RNA polymerase (1, 12, 45).
Evolution has provided numerous examples of structurally related but functionally distinct homologs (paralogs) within bacterial cells. The three Fur paralogs of B. subtilis seem to have evolved distinctive DNA-binding selectivity and control apparently nonoverlapping regulons in response to different signals. Mutually exclusive target site recognition can result from relatively small changes in the DNA-binding domains. Well-characterized examples include the λ and 434 bacteriophage repressor proteins (28) and the E. coli CAP and FNR proteins (24). In each of these cases, structural models are available that provide a plausible mechanism by which these homologous proteins recognize related, but nonoverlapping, sets of target sites. Hybrid sites capable of interacting with both CAP and FNR have been characterized (4, 23), but there appears to be little if any regulon overlap in wild-type cells (for examples, see references 38 and 43). In contrast, other families of paralogous regulators control extensively overlapping regulons. For example, the E. coli MarA and SoxS regulators bind to a nearly identical set of target sites, but with a different hierarchy of affinities (39). Extensive regulon overlap is also apparent from the overlapping promoter selectivity of the E. coli σ70 and σRpoS holoenzymes (19). An intermediate situation is apparent from analysis of the regulons controlled by B. subtilis σ subunits of the extracytoplasmic function (ECF) subfamily. In this case, different σ factors recognize consensus promoter elements that differ by as little as a single base position (42). In the case of bcrC, for example, two different σ factors can allow transcription initiation from the same start site (8). Our results suggest that the functional divergence of the three Fur paralogs has evolved to a point at which little if any regulon overlap remains.
Acknowledgments
We thank Noel Baichoo for construction of the feuA operator mutations and helpful discussions.
This work was supported by grants from the NSF (MCB-0235255) and NIH (GM59323).
REFERENCES
- 1.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 2.Baichoo, N., and J. D. Helmann. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baichoo, N., T. Wang, R. Ye, and J. D. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613-1629. [DOI] [PubMed] [Google Scholar]
- 4.Bell, A. I., K. L. Gaston, J. A. Cole, and S. J. Busby. 1989. Cloning of binding sequences for the Escherichia coli transcription activators, FNR and CRP: location of bases involved in discrimination between FNR and CRP. Nucleic Acids Res. 17:3865-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bsat, N., and J. D. Helmann. 1999. Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo. J. Bacteriol. 181:4299-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 8.Cao, M., and J. D. Helmann. 2002. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function σ factors. J. Bacteriol. 184:6123-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., and J. D. Helmann. 1995. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol. Microbiol. 18:295-300. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L., L. P. James, and J. D. Helmann. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J. Bacteriol. 175:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dame, R. T., and N. Goosen. 2002. HU: promoting or counteracting DNA compaction? FEBS Lett. 529:151-156. [DOI] [PubMed] [Google Scholar]
- 13.de Lorenzo, V., S. Wee, M. Herrero, and J. B. Neilands. 1987. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J. Bacteriol. 169:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowds, B. C. 1994. The oxidative stress response in Bacillus subtilis. FEMS Microbiol Lett. 124:255-263. [DOI] [PubMed] [Google Scholar]
- 15.Dubrac, S., and D. Touati. 2000. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 182:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1998. Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J. Mol. Biol. 283:537-547. [DOI] [PubMed] [Google Scholar]
- 17.Estrem, S. T., T. Gaal, W. Ross, and R. L. Gourse. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 95:9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuangthong, M., A. F. Herbig, N. Bsat, and J. D. Helmann. 2002. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J. Bacteriol. 184:3276-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaal, T., W. Ross, S. T. Estrem, L. H. Nguyen, R. R. Burgess, and R. L. Gourse. 2001. Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol. Microbiol. 42:939-954. [DOI] [PubMed] [Google Scholar]
- 20.Gaballa, A., and J. D. Helmann. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaballa, A., and J. D. Helmann. 2002. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45:997-1005. [DOI] [PubMed] [Google Scholar]
- 22.Gaballa, A., T. Wang, R. W. Ye, and J. D. Helmann. 2002. Functional analysis of the Bacillus subtilis Zur regulon. J. Bacteriol. 184:6508-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, J., A. S. Irvine, W. Meng, and J. R. Guest. 1996. FNR-DNA interactions at natural and semi-synthetic promoters. Mol. Microbiol. 19:125-137. [DOI] [PubMed] [Google Scholar]
- 24.Green, J., C. Scott, and J. R. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 25.Guedon, E., and J. D. Helmann. 2003. Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol. Microbiol. 48:495-506. [DOI] [PubMed] [Google Scholar]
- 26.Guedon, E., C. M. Moore, Q. Que, T. Wang, R. W. Ye, and J. D. Helmann. 2003. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA, and SigB Regulons. Mol. Microbiol. 49:1477-1491. [DOI] [PubMed]
- 27.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 28.Harrison, S. C. 1992. Molecular characteristics of the regulatory switch in phages 434 and lambda, p. 449-473. In S. L. McKnight and K. R. Yamamoto (ed.), Transcriptional regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Helmann, J. D., M. F. W. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmann, J. D., M. F. W. Wu, P. A. Kobel, F.-J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbig, A., and J. D. Helmann. 2002. Metal ion uptake and oxidative stress, p. 405-414. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. From genes to cells. ASM Press, Washington, D.C.
- 32.Herbig, A., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 33.Lavrrar, J. L., C. A. Christoffersen, and M. A. McIntosh. 2002. Fur-DNA interactions at the bidirectional fepDGC-entS promoter region in Escherichia coli. J. Mol. Biol. 322:983-995. [DOI] [PubMed] [Google Scholar]
- 34.Lavrrar, J. L., and M. A. McIntosh. 2003. Architecture of a Fur binding site: a comparative analysis. J. Bacteriol. 185:2194-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leichert, L. I. O., C. Scharf, and M. Hecker. 2003. Global characterization of disulfide stress in Bacillus subtilis. J. Bacteriol. 185:1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, H., K. Haga, K. Yasumoto, Y. Ohashi, H. Yoshikawa, and H. Takahashi. 1997. Sequence and analysis of a 31 kb segment of the Bacillus subtilis chromosome in the area of the rrnH and rrnG operons. Microbiology 143:2763-2767. [DOI] [PubMed] [Google Scholar]
- 37.Liu, S. T., and G. F. Hong. 1998. Three-minute G + A specific reaction for DNA sequencing. Anal. Biochem. 255:158-159. [DOI] [PubMed] [Google Scholar]
- 38.Lombardo, M.-J., A. A. Lee, T. M. Knox, and C. G. Miller. 1997. Regulation of the Salmonella typhimurium pepT gene by cyclic AMP receptor protein (CRP) and FNR acting at a hybrid CRP-FNR site. J. Bacteriol. 179:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, R. G., W. K. Gillette, and J. L. Rosner. 2000. Promoter discrimination by the related transcriptional activators MarA and SoxS: differential regulation by differential binding. Mol. Microbiol. 35:623-634. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Pohl, E., J. C. Haller, A. Mijovilovich, W. Meyer-Klaucke, E. Garman, and M. L. Vasil. 2003. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol. Microbiol. 47:903-915. [DOI] [PubMed] [Google Scholar]
- 42.Qiu, J., and J. D. Helmann. 2001. The −10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic-function σ factors σX and σW. J. Bacteriol. 183:1921-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawers, G., M. Kaiser, A. Sirko, and M. Freundlich. 1997. Transcriptional activation by FNR and CRP: reciprocity of binding-site recognition. Mol. Microbiol. 23:835-845. [DOI] [PubMed] [Google Scholar]
- 44.Schneider, T. D., and R. M. Stephens. 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 18:6097-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder, O., and R. Wagner. 2002. The bacterial regulatory protein H-NS—a versatile modulator of nucleic acid structures. Biol. Chem. 383:945-960. [DOI] [PubMed] [Google Scholar]
- 46.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 49.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]