Abstract
Using human autoimmune sera as molecular probes, we previously described the association of phosphorylated serine/arginine splicing factors (SR splicing factors) with the U1-small nuclear ribonucleoprotein (U1-snRNP) and U3-small nucleolar RNP (snoRNP) in apoptotic cells. SR proteins are highly conserved autoantigens whose activity is tightly regulated by reversible phosphorylation of serine residues by at least eight different SR protein kinase kinases (SRPKs), including SRPK1, SRPK2, and the scleroderma autoantigen topoisomerase I. In this report, we demonstrate that only one of the known SRPKs, SRPK1, is associated with the U1-snRNP autoantigen complex in healthy and apoptotic cells. SRPK1 is activated early during apoptosis, followed by caspase-mediated proteolytic inactivation at later time points. SRPKs are cleaved in vivo after multiple apoptotic stimuli, and cleavage can be inhibited by overexpression of bcl-2 and bcl-xL, and by exposure to soluble peptide caspase inhibitors. Incubation of recombinant caspases with in vitro–translated SRPKs demonstrates that SRPK1 and SRPK2 are in vitro substrates for caspases-8 and -9, respectively. In contrast, topoisomerase I is cleaved by downstream caspases (-3 and -6). Since each of these SRPKs sits at a distinct checkpoint in the caspase cascade, SRPKs may serve an important role in signaling pathways governing apoptosis, alternative mRNA splicing, SR protein trafficking, RNA stability, and possibly the generation of autoantibodies directed against splicing factors.
Keywords: apoptosis, autoantibodies, autoimmunity, kinase, RNA splicing
Introduction
The breakdown of tolerance to self-antigens is a common feature of autoimmune diseases including systemic lupus erythematosus (SLE)*, systemic sclerosis, Sjögren's syndrome, and mixed connective tissue disease (MCTD), resulting in the production of autoantibodies (1). Accumulating data suggests that such a breakdown of tolerance may be attributed to posttranslational modifications of autoantigens during apoptosis or cellular stress, resulting in the creation of neoepitopes to which the immune system has never been exposed or has been tolerized in the periphery (2). A variety of apoptosis-specific posttranslational modifications has been described, including proteolysis by caspases and granzyme B (3–6), and reversible phosphorylation/dephosphorylation of a subset of autoantigens (7, 8).
Autoimmune sera have proven to be very useful as molecular probes to study important biochemical processes such as mRNA splicing, endoplasmic reticulum transport, and apoptosis signaling. In addition to caspase cascades, other signaling pathways are activated during apoptosis, including the stress-activated protein kinase pathway (9). Previously, we demonstrated that, in response to multiple different apoptotic stimuli, serine/arginine splicing factors (SR splicing factors) are reversibly phosphorylated during apoptosis, and they physically associate with the U1-small nuclear ribonucleoprotein (U1-snRNP) and U3-small nucleolar ribonucleoprotein (U3-snoRNP) autoantigen complexes (8, 10). Moreover, an SR protein kinase (SRPK) activity was found to coprecipitate with the U1-snRNP using human sera derived from patients with SLE or MCTD, although the SRPK was not identified in our initial reports (7, 8).
In this study, we demonstrate that one of the major known SRPKs, SRPK1, is physically associated with the U1-snRNP autoantigen complex in cells and can be immunoprecipitated using mAbs directed against U1-snRNP components or SR proteins, as well as human serum derived from patients with SLE or MCTD. Moreover, three different SRPKs (SRPK1, SRPK2, and topoisomerase I) are differentially cleaved by recombinant caspases in vitro, and in vivo in several different cells lines. SRPK1 is activated and phosphorylates the SR protein ASF/SF2 at an early stage of apoptosis, before cleavage by caspases, while the SRPK1 and SRPK2 cleavage products lack kinase activity. These results suggest that SRPK1 and perhaps other SRPKs are activated during cell stress and that the fidelity of the apoptosis program is assured through caspase-mediated cleavage of SRPKs that sit at “apoptosis checkpoints.” These results have important implications for understanding molecular mechanisms underlying alternative splicing regulation, apoptosis signaling, and the generation of autoantibodies in SLE and MCTD.
Materials and Methods
Cell Culture.
Jurkat cells or human MCF7 breast carcinoma cells were grown in 5% CO2 at 37°C using RPMI 1640 (Bio Whittaker) supplemented with 9% heat-inactivated fetal calf serum (HI-FCS; Bio Whittaker) and penicillin and streptomycin (GIBCO BRL). Cells were grown and harvested at mid-log phase. Adherent cells were plated the night before each experiment such that cells were at 50–80% confluence on the day of the experiment. Jurkat cells engineered to stably overexpress bcl-2 (or empty vector), a gift from J.C. Reed (La Jolla Cancer Research Foundation, La Jolla, CA), or MCF7 cells engineered to stably overexpress bcl-xL (or empty vector), a gift from N. Kedersha (Brigham & Women's Hospital, Boston, MA) were grown in RPMI 1640 medium as described above, supplemented with G418 (GIBCO BRL) at a final concentration of 500 μg/ml. Overexpression was confirmed by Western blot analysis.
Cell Lysis.
Jurkat cells or MCF7 cells were solubilized in Nonidet P40 (NP40) lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, pH 7.8, 1 mM EDTA, and a protease inhibitor cocktail described previously; reference 11) or detergent lysis buffer (50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 0.1% vol/vol NP-40, 5 mM EDTA, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, 10 mM benzamidine, 100 μg/ml PMSF, 10 μg/ml N-tosyl-phenylalanine chloromethyl ketone, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml aprotinin, 1 μg/ml leupeptin prepared as described previously; reference 16). All chemicals were purchased from Sigma-Aldrich. After addition of lysis buffer, cells were incubated on ice for 30 min, centrifuged in a refrigerated microfuge (Biofuge Fresco, Kendro Laboratory, Hanau, Germany) at 13,000 rpm for 15 min, and the supernatant was used immediately for each experiment. MCF7 cells were trypsinized, washed with PBS, and lysed as described above. Identical results were obtained in other experiments performed by scraping cells from plates in the absence of trypsin.
UV Irradiation.
Jurkat cells were plated in 100 × 15 mm polystyrene petri dishes (Nunc) at a concentration of 2 × 106 cells/ml and irradiated in a Stratalinker 2400 (Stratagene) at a distance of 9 cm for 30 s (6). After irradiation, cells were incubated at 37°C for the indicated times before harvesting.
Cellular Activation.
Jurkat cells were treated with anti-Fas antibody 7C11 (IgM; provided by M. Robertson, Indiana University, Bloomington, IN) from hybridoma supernatants at a final dilution of 1:500. Cells were incubated at 37°C for the indicated times before harvesting. For experiments using caspase inhibitors, Jurkat cells were pretreated for 1 h at 37°C with either 10 μM ZVAD-fmk or 10 μM DEVD-fmk (Kamiya Biomedical Co.; reference 6). After 1 h, apoptosis was induced by the addition of 7C11, and the cells were processed as described above. Jurkat cells or MCF7 cells were treated with 10 μg/ml anisomycin or 2 μM staurosporine (Sigma-Aldrich) for varying times before harvesting, and the cells were processed as described previously.
Plasmid Construction and Preparation of Recombinant ASF/SF2.
cDNA clones encoding human SRPK1 and SRPK2 (SRPKs) were provided by X.D. Fu (UCSD School of Medicine, La Jolla, CA). Mouse SRPKs were provided by M. Hagiwara (Tokyo Medical and Dental University, Tokyo, Japan). Human Clk/Sty 1–4 have been described previously and were provided by O. Nayler, Max-Planck-Institute for Biochemistry, Martinsried, Germany (12). Epitope-tagged ASF/SF2 was constructed by PCR amplification using a human ASF/SF2 construct as a template, provided by A.R. Krainer (Cold Spring Laboratory, Cold Spring Harbor, NY). Primer sequences were as follows: sense primer, 5′-GAGAGAGGATCCGTCGGGAGGTGGTGTGA-TT-3′; and anti-sense primer, 5′-GAGAGAAAGCTTTGTACGAGAGCGAGATCT-3′. The PCR product was then cloned in-frame into pTriEx-4 (Novagen) using the restriction enzymes BamHI and HindIII, and the constructs were sequence-verified for both strands. His-tagged ASF/SF2 was expressed in Escherichia coli Origami (DE3) pLacI (Novagen) with IPTG induction, extracted using a Ni-NTA Spin kit (QIAGEN), analyzed by BCA protein assay (Pierce Chemical Co.), Coomassie staining, and Western blotting using antibodies specific for both His-C tag (Invitrogen), and HSV tag (Novagen), respectively.
In Vitro Transcription/Translation.
[35S] methionine-labeled p35, IL-1β, Ich-1, mouse SRPKs, human SRPKs, or Clk/Sty kinases 1–4 were in vitro transcribed and translated using the TNT rabbit reticulocyte lysate kit (Promega), according to the manufacturer's instructions. Reactions were performed using 0.25 μg plasmid in a 10 μl transcription/translation reaction mixture containing 0.5 μl of translation grade [35S] methionine (7.9 μCi/ml; NEN Life Science Products, Inc.).
In Vitro Caspase Cleavage Assays.
In vitro–translated proteins synthesized as described previously were incubated in caspase cleavage buffer with recombinant caspases (caspases 1, 2, 3, 8, 9, or a control bacterial lysate) for 90 min at 30°C as described previously (6). cDNAs encoding individual caspases were a gift of H. Li and J. Yuan (Harvard Medical School, Boston, MA). The data for caspase-8 cleavage of SRPK1 was confirmed using recombinant, purified His-tagged caspase-8 (Sigma-Aldrich). Recombinant caspases were prepared as described and frozen at −80°C until used (6). In a separate reaction, the mixture was then separated by SDS-PAGE, transferred to nitrocellulose (OSMONICS) and exposed for autoradiography. In separate experiments, each caspase was incubated with in vitro–translated proteins including p35 (a gift of V. Shifrin, Scriptgen, Inc., Medford, MA), pro-caspase 2, or IL-1β (gifts of H. Li and J. Yuan, Harvard Medical School) to confirm their activity (unpublished data).
Immunoprecipitation and Western Blot Analysis.
Lysates were precleared once with 100 μl of a 50% solution of protein A-Sepharose (Amersham Pharmacia Biotech) in detergent lysis buffer and 5 μg rabbit anti–mouse IgG (Jackson ImmunoResearch Laboratories) for 1–2 h. Mouse mAbs (2–5 μg monoclonal and 5 μg rabbit anti–mouse IgG) were used as follows: anti-SRPK1 and anti-SRPK2 (Transduction Laboratories); anti-cdc2 (cyclin-dependent kinase [CDK]1; Santa Cruz Biotechnology, Inc.); anti-SC35 (Sigma-Aldrich); and anti-U2B″ (4G3, a gift of W.J. van Venrooij, University of Nijmegan, Nijmegen, The Netherlands) (13). Human autoimmune serum samples were employed as follows: 3 μl human polyclonal anti–Scl-70 or anti–U1-snRNP (Immunovision). U1-snRNP-specific sera that were previously shown to coprecipitate SR proteins and control sera have been described previously (7, 8). 2 μl anti–DNA-dependent protein kinase (DNA-PKCS) (Serotec) was used for IP kinase and Western blotting experiments. Immunoprecipitations were performed after addition of detergent lysis buffer to a total volume of 500 μl, and rotation in a 4°C cold room for 2–4 h. Precipitates were harvested by centrifuging for 20 s at 12,000 rpm in a refrigerated Heraeus microfuge, washing three times with detergent lysis buffer, resuspending in SDS loading buffer with 9% 2-mercaptoethanol, boiling for 5 min, and separating by SDS-PAGE as described previously (6). Proteins were transferred to nitrocellulose for Western blotting experiments. Antibodies and dilutions used were as follows: anti-cdc2 (CDK1) (1:100; Santa Cruz Biotechnology, Inc.); anti–DNA-PKCS (1:3,000; Serotec); anti-SRPKs (1:1,000; Transduction Laboratories); anti-topoisomerase I (1:100; Arthritis Foundation/CDC Reference Sera); anti–bcl-2 (1:100; BD PharMingen); anti–bcl-xL (1:400; Santa Cruz Biotechnology, Inc.); anti–phospho-cdc2 (CDK1)/Tyr-15 (1:500; New England BioLabs); anti-PARP (1:500; Transduction Laboratories); mAb104 (1:5 dilution of hybridoma supernatants, a gift of R. Reed, Harvard University School of Medicine); and anti-Smith complex (Sm) (1:50; Immunovision). Nitrocellulose filters were blocked with 5% Blotto (Bio-Rad Laboratories) in PBS overnight at 4°C. Bands were visualized using species-specific antibody conjugated to HRP (Amersham Pharmacia Biotech) at a dilution of 1:7,500 in 5% Blotto in PBS, and developed using ECL chemiluminescence performed according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Immunoprecipitation Kinase Assays.
Immunoprecipitation kinase assays have been described previously (7, 14–16). For cdc2 (CDK1) kinase assays, individual precipitates were washed three times in detergent lysis buffer and once in wash buffer A: (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2), resuspended in 20 μl reaction buffer A: (50 mM Tris, pH 7.4, 10 mM MgCl2, 2.0 μM ATP) containing 5 μCi [γ-32P] ATP, 125 μg/ml Histone H1 for cdc2 (CDK1)/Histone H1; (kinase/substrate) or 10 μCi [γ-32P] ATP, 25 pmoles bacterial ASF/SF2 for cdc2 (CDK1)/ASF/SF2 (kinase/substrate), then incubated at 30°C for 10 min, or at 30°C for 30 min, respectively (16). For DNA-PKCS kinase assays, individual precipitates were washed three times in detergent lysis buffer and once in wash buffer B (50 mM Tris, pH 7.4, 5 mM MnCl2), resuspended in 20 μl of reaction buffer B (50 mM Tris, pH 7.4, 5 mM MnCl2, 5 mM DTT, 5 mM NaF, 40 μM MgCl2, 1 mM sodium orthovanadate, 4 μM ATP, 50 μg/ml salmon sperm DNA) containing 10 μCi [γ-32P] ATP, 20 μg α-casein, then incubated at 30°C for 15 min (14). For SRPK1, SRPK2, and topoisomerase I kinase assays, individual precipitates were washed three times in detergent lysis buffer and once in wash buffer C (40 mM Hepes, pH 7.5, 120 mM NaCl), resuspended in 20 μl of reaction buffer C (40 mM Hepes, pH 7.5, 120 mM NaCl, 2 mM DTT, 10 mM MgCl2, 2 mM benzamidine, 20 μM ATP) containing 10 μCi [γ-32P] ATP, 25 pmoles bacterial ASF/SF2, then incubated at 30°C for 30 min (15). All kinase reactions were terminated by boiling in equal volume of 2× SDS loading buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. The extent of phosphorylation of individual substrates was detected by autoradiography, then quantified using Molecular Imager® FX (Bio-Rad Laboratories). The extent of phosphorylation was determined by subtracting background counts (CPM/mm2) from total counts (CPM/mm2). The results depict the means of three representative experiments. In some experiments, individual immunoprecipitates were washed three times in detergent lysis buffer and once in wash buffer C, then incubated in 20 μl of reaction buffer C containing 20 μCi [γ-32P] ATP at 30°C for 30 min (15).
Dissociation of SRPK1 from Immunoprecipitate by 1 M MgCl2.
The effect of antibody binding on the dissociation of SRPK1 from the immunoprecipitate was investigated by including 1 M MgCl2 in the buffers, either before or after the binding of antibodies to proteins in the lysate had occurred (17).
For one set of immunoprecipitations, 10–13 million Jurkat cells were solubilized in NET/NP40 buffer (150 mM NaCl, 2 mM EDTA, and 50 mM Tris-HCl, pH 7.5 [NET], and 0.3% NP-40) containing 0.5 mM PMSF and 1 μg/ml aprotinin. Lysates were then precleared, and immunoprecipitations were performed as described previously. Immunoprecipitates were washed three times with 0.5 M NaCl NET (standard immunoprecipitation), or washed twice with 1 M MgCl2 NET/NP40, and once with NET. The other set of immunoprecipitations was performed in an identical manner using extracts prepared from cells lysed in 1 M MgCl2 NET/NP40.
Results
Autoimmune Sera Derived from MCTD and SLE Patients Coprecipitate SRPK1 and SR Splicing Factors.
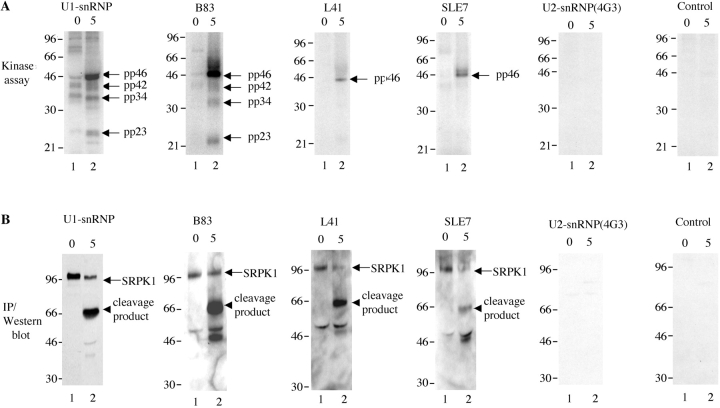
Thus far, eight kinases have been reported to have the capacity to phosphorylate SR proteins, including SRPK1 and SRPK2, Clk/Sty kinases 1–4, topoisomerase I (Scl-70), and cdc2 (CDK1) (12, 15, 18–20). SRPK1 is the best characterized of these kinases and is known to phosphorylate several different SR proteins including SRp55 (pp54), SC35, ASF/SF2, SRp20 (pp23), and U2AF65 (21). Previously, we demonstrated that autoimmune sera derived from patients with SLE, overlap syndromes, and MCTD precipitate at least seven distinct phosphoproteins (designated pp200, pp54, pp46, pp42, pp34, pp23, and pp17) from apoptotic Jurkat cell lysates, as well as an unidentified kinase activity (7, 8). We went on to demonstrate that pp54, pp42, pp34, and pp23 are SR proteins that associate directly with the U1-snRNP and U3-snoRNP during apoptotic stress (8, 10). We hypothesized that one or more of the eight known SRPKs was a component of the spliceosome and was responsible for the SRPK activity we observed in vitro. To test this hypothesis, we repeated the in vitro kinase assays reported previously, using human serum specific for the U1-snRNP complex (7, 8). The immunoprecipitates were then analyzed by Western blot assays using antibodies specific for individual SRPKs. As shown in Fig. 1 , Jurkat cells were incubated for the time indicated above the panels in the presence of anti-Fas, which engages and activates the Fas death receptor, then solubilized in detergent lysis buffer. Proteins were immunoprecipitated using the indicated sera or antibodies, and subjected to in vitro phosphorylation kinase assays (Fig. 1 A). As expected, U1-snRNP specific human sera, but not a mAb specific for a U2-snRNP component, U2B, precipitate SR proteins (pp54, pp46, pp42, pp34, and pp23) from apoptotic Jurkat cell lysates (Fig. 1 A). Over 40 other autoimmune sera specific for autoantigens other than the U1-snRNP failed to coprecipitate SR proteins, suggesting that members of the SR splicing factor family uniquely associate with the U1-snRNP during apoptosis (reference 8, and unpublished data). We next performed Western blotting of the membranes using antibodies specific for individual SRPKs. Surprisingly, all of the U1-snRNP-specific serum samples precipitated SRPK1 (lanes numbered 1, Fig. 1 B). SRPK1 was not precipitated using control sera, using sera from patients with other autoimmune diseases, or using a U2-snRNP specific mAb (Fig. 1 B and unpublished data). SRPK2, topoisomerase I, and cdc2 (CDK1) were not present in the immunoprecipitates (unpublished data), suggesting that SRPK1 is the major SRPK associated with the U1-snRNP autoantigen complex, the major target antigen in patients with MCTD. When lysates were prepared from apoptotic Jurkat cells (lanes numbered 2), SRPK1 was less abundant (e.g., U1-snRNP and B83, left panels of Fig. 1 B) or nearly completely absent (e.g., L41 and SLE 7, middle panels of Fig. 1 B) from the immunoprecipitates, and a 66-kD protein was recognized by SRPK1-specific antibodies. This result suggests that SRPK1 is cleaved in vivo during apoptosis, and that at least one of the cleavage fragments remains associated with the U1-snRNP autoantigen complex.
Figure 1.
U1-snRNP specific sera derived from patients with SLE and MCTD coprecipitate SRPK1 from Jurkat cell lysates. (A) Jurkat cells were incubated in the presence of anti-Fas for the time indicated at the top of each figure, solubilized in detergent lysis buffer, and antigens precipitated using human U1-snRNP specific serum (Immunovision), anti-U2B″ (mouse monoclonal supernatant 4G3), or human serum (MCTD patient sera: B83, L41, SLE patient serum: SLE 7), and control). Individual precipitates were subjected to an in vitro kinase assay. Bands corresponding to pp46, pp42, pp34, and pp23 are shown on the right side of the panels (top panel). (B) Precipitated proteins were analyzed by IP/Western blot analysis with anti-SRPK1 (mouse monoclonal). Full-length SRPK1 and cleavage products are shown on the right sides of the panels (bottom panel).
SRPK1 Is Associated with the U1-snRNP but Is Not Directly Recognized by Serum Autoantibodies.
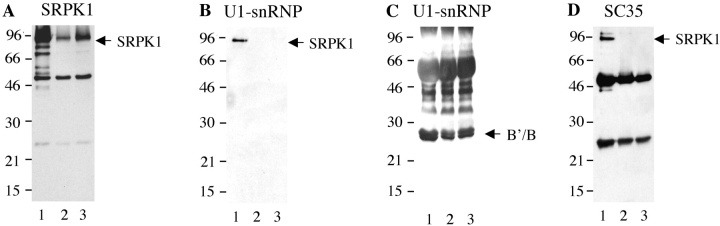
From the experiments shown in Fig. 1, it is not clear whether SRPK1 is recognized directly by serum autoantibodies, or whether the kinase is coprecipitated through noncovalent interactions with other components of the U1-snRNP (which are themselves directly recognized by serum autoantibodies). To address this question, we included 1 M MgCl2 in several of the buffers. In studies of the interaction between antibodies and snRNPs, high concentrations of MgCl2 have been shown to dissociate weak, noncovalent interactions between proteins, but not interactions between antibody and antigen(s) (17, 22). Jurkat cell lysates prepared in NET/NP40 were immunoprecipitated with autoimmune and control sera (Fig. 2) , in the presence (lanes numbered 2 and 3) or absence (lanes numbered 1) of 1 M MgCl2. Individual precipitates were analyzed by IP/Western blot as described in Materials and Methods. As expected, SRPK1 is still detected by Western blot analysis when anti-SRPK1 is used to perform the immunoprecipitation (Fig. 2 A). However, SRPK1 is not detected in MgCl2-treated immunoprecipitates prepared using a prototypic human U1-snRNP/Smith serum (Fig. 2 B) or a mAb directed against the SR splicing factor SC35 (Fig. 2 D). The Sm proteins B/B′ are retained in the immunoprecipitate (Fig. 2 C), demonstrating that treatment with MgCl2 did not disrupt direct antibody–antigen interactions. These results demonstrate that SRPK1 is coprecipitated via intermolecular binding interactions with components of the U1-snRNP complex, and is not directly recognized by serum autoantibodies. Identical results were obtained using serum samples presented in Fig. 1 (unpublished data).
Figure 2.
SRPK1 is not recognized directly by human serum autoantibodies. Jurkat cell lysates were immunoprecipitated with anti-SRPK1, anti-SC35, and human U1-snRNP specific serum (Immunovision). Immunoprecipitates were washed using standard conditions (0.5 M NaCl washing, lane 1), or with 1 M MgCl2 treatment either after (lane 2) or before (lane 3) incubation with anti-SRPK1, anti-SC35, or anti-U1-snRNP specific serum. Retention of SRPK1 or U1-snRNP components on the beads was confirmed by immunoblotting with anti-SRPK1 and human Sm specific serum (Immunovision).
SRPK1 and SRPK2 Are Differentially Cleaved by Caspases In Vitro and In Vivo.
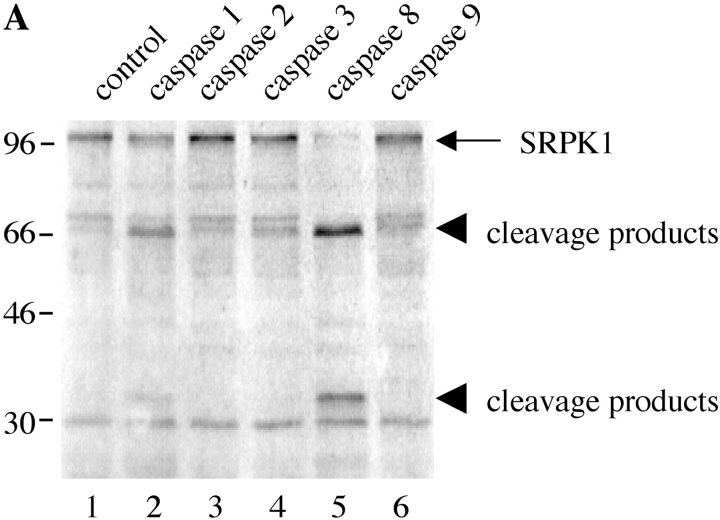
Previous studies have demonstrated that an SRPK (topoisomerase I) is cleaved by caspases-3 and -6 at unconventional sites during apoptosis (23). The results shown in Fig. 1 further suggested that another SRPK (SRPK1), and perhaps other SRPKs, may also undergo proteolysis during apoptosis. We tested this hypothesis by using a well-validated in vitro cleavage assay using recombinant caspases. In vitro–transcribed/translated SRPKs were incubated in caspase cleavage buffer in the presence of recombinant caspases or a control bacterial lysate, separated by SDS-PAGE and analyzed by autoradiography. As shown in Fig. 3, A and B , human SRPK1 and SRPK2 are differentially cleaved by recombinant caspase-8 and caspase-9, respectively, yielding signature ∼66-kD and 34-kD fragments (SRPK1) and 80-kD and 50-kD fragments (SRPK2), respectively. None of the other caspases tested had the ability to cleave SRPK1 and SRPK2, although caspase-1 appeared to partially cleave human SRPK1, generating fragments of identical size to those observed with caspase-8 (lane 2). Mouse SRPK1 and SRPK2, which are 92 and 58% conserved between mouse and man, respectively, were also differentially cleaved by caspase-8 and caspase-9, respectively, generating fragments of approximately the same molecular weight (unpublished data). In contrast, none of the Clk/Sty isoforms was cleaved by recombinant caspases (unpublished data). Taken together with the published data on topoisomerase I, these results demonstrate that at least 3 of the 8 known SRPKs are differentially cleaved by caspases.
Figure 3.
Human SRPK1 and SRPK2 are differentially cleaved by recombinant caspases in vitro. SRPK1 and SRPK2 were synthesized by in vitro transcription/translation and incubated with the recombinant caspases indicated by number at the top of each figure. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and detected by autoradiography. The relative migration of molecular size markers in kilodaltons is indicated on the left side of each panel. Lanes are numbered at the bottom of each panel.
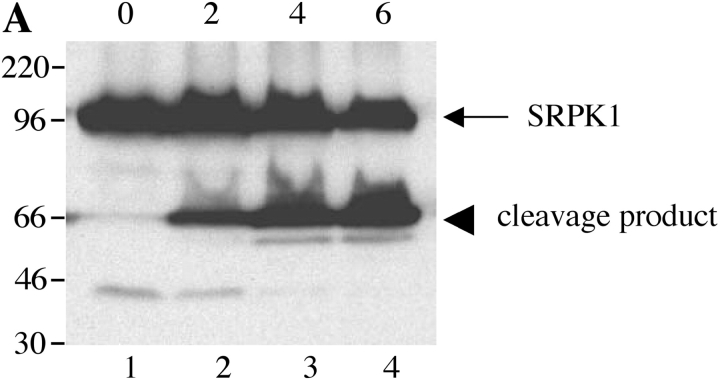
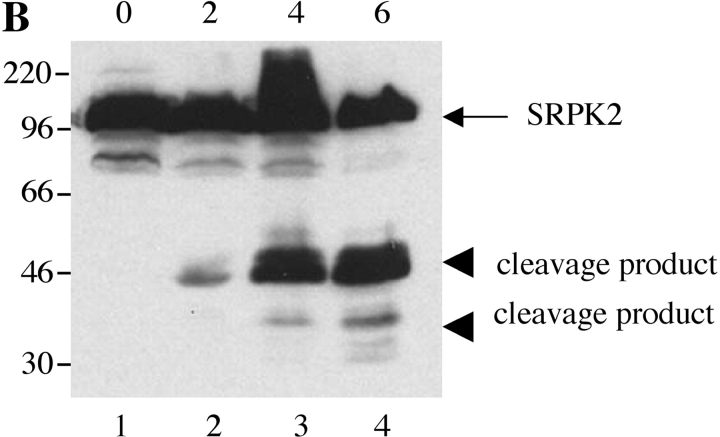
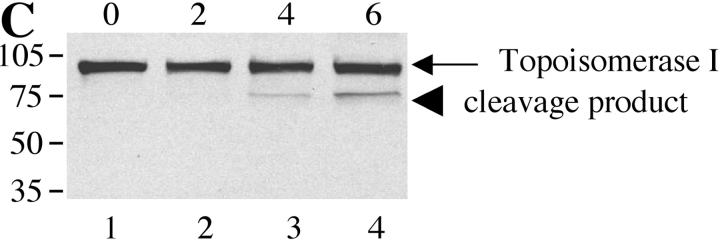
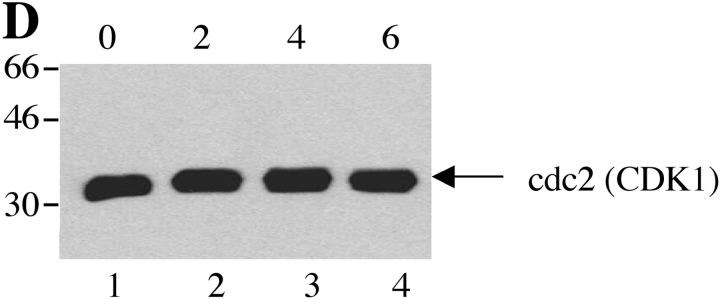
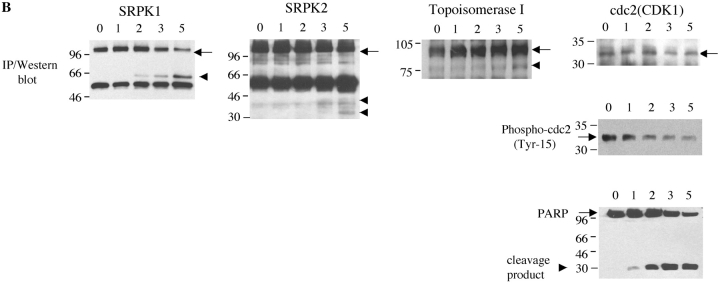
Next, we examined whether SRPKs undergo proteolysis during apoptosis in vivo. We used Jurkat cells treated with 7C11(anti-Fas) mAb, a well-established model system for apoptosis. Jurkat cells were incubated with anti-Fas for various times over a 6-h period. Western blot analysis of cell lysates was performed with anti-SRPK1, anti-SRPK2, anti-topoisomerase I, and anti-cdc2 (CDK1). As shown in Fig. 4, A and B , cleavage products of SRPK1 and SRPK2 are first detectable 2 h after anti-Fas addition. After 4 h, smaller fragments generated by further degradation were also observed for SRPK2 (Fig. 4 B). The cleavage fragments detected by Western blot analysis (Fig. 4, A and B) comigrate precisely with the fragments observed in the in vitro caspase cleavage assay using recombinant caspases (Fig. 3, A and B), suggesting that SRPK1 and SRPK2 are differentially cleaved in vivo by caspases-8 and -9, respectively. Only a partial cleavage fragment of topoisomerase I migrating at 75-kD was detected, beginning at a later time point (4 h, Fig. 4 C). Cleavage of cdc2 (CDK1) was not detected (Fig. 4 D). These results demonstrate that SRPK1, SRPK2, and topoisomerase I are cleaved in vivo during Fas-mediated apoptosis, further suggesting that (i) SRPK1, SRPK2, and topoisomerase I may play important roles in apoptosis and/or stress signaling pathways and (ii) their proteolytic cleavage during apoptosis may alter or modulate their ability to function as kinases.
Figure 4.
SRPK1 and SRPK2 are cleaved during Fas-mediated apoptosis in vivo. Jurkat cells were incubated with 7C11(anti-Fas) over a 6-h time period and harvested by NP-40 lysis at the times indicated at the top of each figure (in hours). Lysates were separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis using anti-SRPK1 (A), anti-SRPK2 (B), anti-topoisomerase I: arthritis foundation/CDC reference sera (C), and anti-cdc2 (CDK1) (mouse monoclonal) (D). The relative migration of molecular size markers in kilodaltons is indicated on the left side of each panel. SRPK1, SRPK2 and topoisomerase I cleavage products are shown on the right side of the panel. Lanes are numbered at the bottom of each panel.
SRPK1 and SRPK2 Are Cleaved after Multiple Different Apoptotic Stimuli.
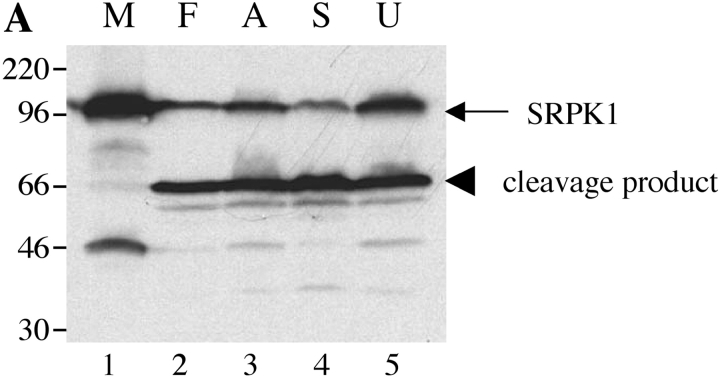
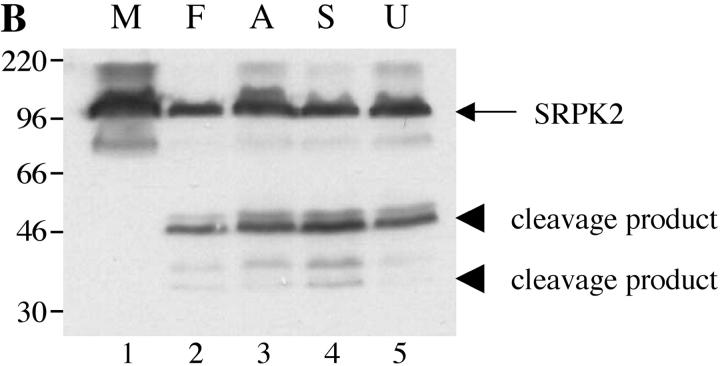
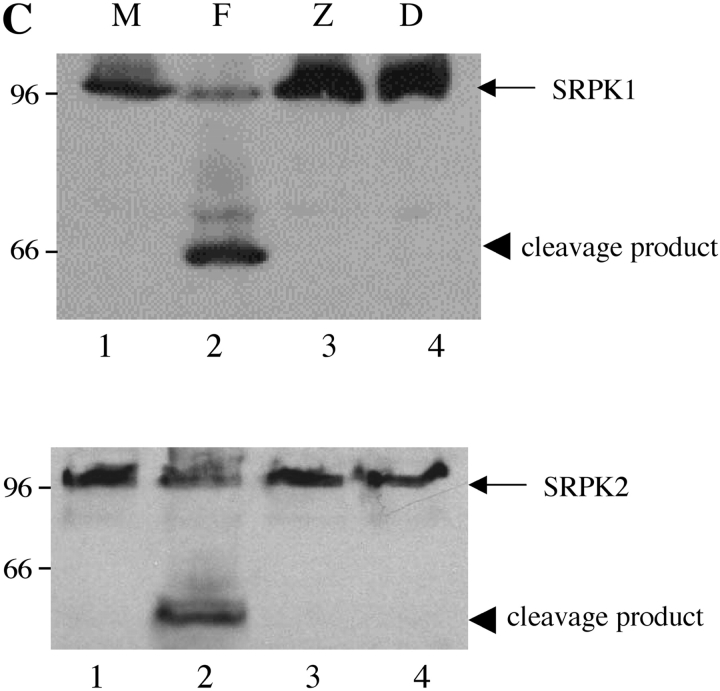
To determine whether cleavage of SRPK1 and SRPK2 was specific to Fas-induced apoptosis, Jurkat cells were treated in the absence (Fig. 5, A and B , lane 1 M) or presence of anti-Fas (lane 2 F), the antibiotic anisomycin (lane 3; Fig. 5 A), staurosporine, a broad-spectrum protein kinase inhibitor (lane 4 S, reference 24), or UV irradiation (lane 5 U). Cells were harvested after 6 h and processed as described in Materials and Methods. All four stimuli induced the cleavage of SRPK1 and SRPK2, yielding the 66-kD (SRPK1) and 50-kD (SRPK2) fragments, respectively. An additional degradation product migrating at 35 kD (for SRPK2) is also detectable (Fig. 5 B, lanes 2–5).
Figure 5.
SRPK1 and SRPK2 are cleaved in vivo after multiple different apoptotic stimuli. Jurkat cells were subjected to various apoptotic stimuli and harvested for protein analysis by NP-40 lysis after 6 h. Lysates were separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis using anti-SRPK1 (top panel) or anti-SRPK2 (bottom panel). The relative migration of molecular size markers in kilodaltons is indicated on the left side of the panel. SRPK1 and SRPK2 cleavage products are shown on the right side of each panel. The stimulus is indicated above the panel as follows: mock treatment (M), anti-Fas 7C11 (F), anisomycin (A), staurosporine (S), and ultraviolet irradiation (U). Lanes are numbered at the bottom of each panel.
Proteolytic Cleavage of SRPKs Is Prevented by Ectopic Expression of bcl-2, bcl-xL, and Preincubation with Small Peptide Caspase Inhibitors.
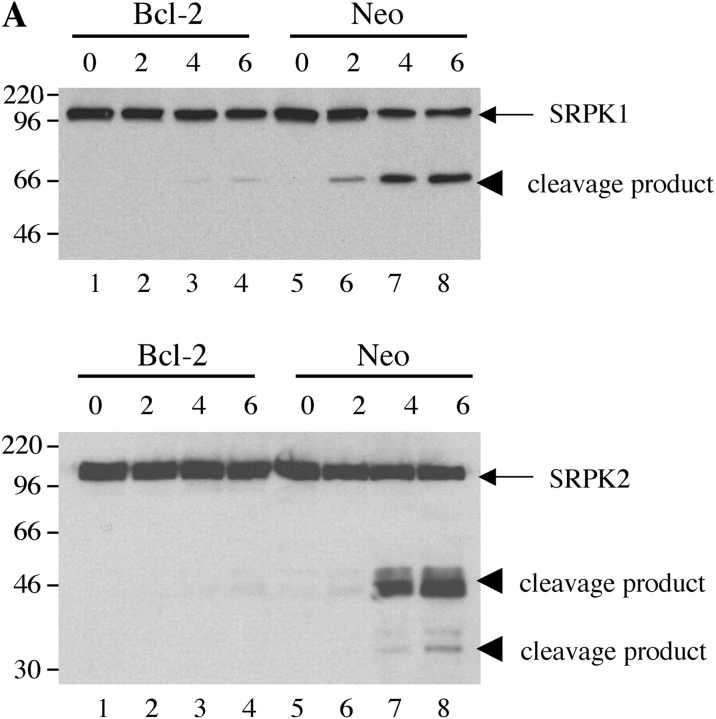
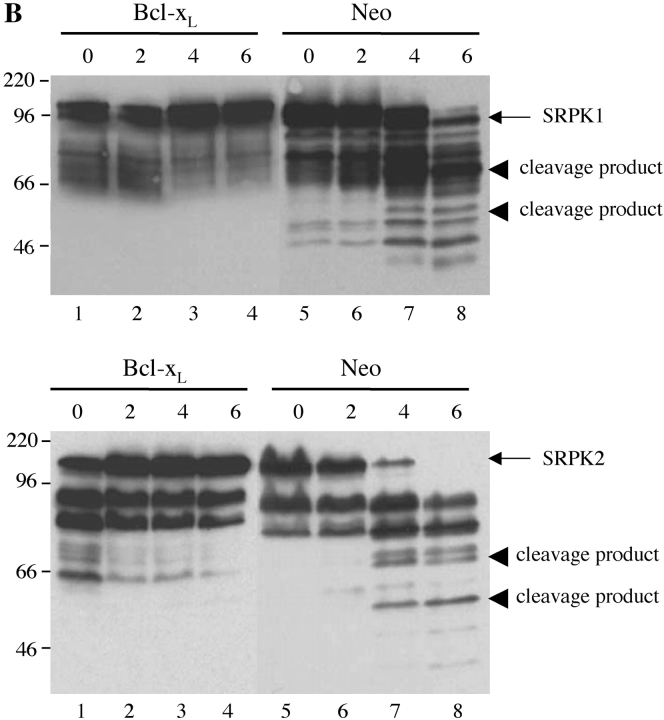
Next, we asked whether the cleavage of SRPK1 or SRPK2 could be inhibited by overexpression of bcl-2 or bcl-xL both of which prevent apoptosis induced by multiple stimuli, including γ irradiation and UV irradiation (25–28), or by the addition of small peptide caspase inhibitors. Jurkat cells stably transfected with either empty vector (Fig. 6 A, lanes 5–8) or bcl-2 (Fig. 6 A, lanes 1–4) were subjected to UV irradiation. MCF7 cells stably transfected with either empty vector (Fig. 6 B, lanes 5–8) or bcl-xL (Fig. 6 B, lanes 1–4) were incubated with anisomycin. Cells were solubilized, and lysates were separated by SDS-PAGE, transferred to nitrocellulose, and blotted with antibodies specific for SRPK1 (top panels) or SRPK2 (bottom panels). SRPK cleavage products were observed in Jurkat (neo) control cells in response to UV irradiation (Fig. 6 A, lanes 5–8) as well as MCF7 (neo) control cells in response to addition of anisomycin (Fig. 6 B, lanes 5–8), respectively. Cleavage products were absent from Jurkat (bcl-2) transformants (Fig. 6 A, lanes 1–4) as well as MCF7 (bcl-xL) transformants (Fig. 6 B, lanes 1–4), suggesting that cleavage of SRPKs is downstream of the inhibitory effects of bcl-2 and bcl-xL. Interestingly, SRPK2 in MCF-7 cells migrated at ∼130–140 kD (rather than at ∼120 kD, as observed in Jurkat cells, Fig. 4), suggesting that a novel SRPK2 isoform exists in MCF7 cells. The functional importance of this observation is under investigation.
Figure 6.
Cleavage of SRPK1 and SRPK2 is inhibited in vivo by overexpression of bcl-2 or bcl-xL, and by coincubation with specific caspase inhibitors. (A) SRPK cleavage is inhibited in Jurkat cells overexpressing bcl-2. Jurkat (bcl-2) transformants (lanes 1–4) and Jurkat (neo) control transformants (lanes 5–8) were subjected to UV irradiation, then cultured for the times indicated above each lane (in hours) and solubilized in NP-40 lysis buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was then blotted with anti-SRPK1 (top panel) or anti-SRPK2 (bottom panel). (B) SRPK cleavage is inhibited in MCF7 cells overexpressing bcl-xL. MCF7 bcl-xL transformants (lanes 1–4) and MCF7 (neo) control transformants (lanes 5–8) were treated with anisomycin, cultured for the times indicated above each lane (in hours) and solubilized in NP-40 lysis buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was then blotted with anti-SRPK1 (top panel) or anti-SRPK2 (bottom panel). (C) SRPK cleavage is inhibited by coincubation with small peptide caspase inhibitors. Jurkat cells were preincubated for 1 h with the indicated specific caspase inhibitors, cultured with anti-Fas, and harvested 5 h later for protein analysis. The membrane was blotted with anti-SRPK1 (top panel) or anti-SRPK2 (bottom panel). The stimulus is indicated above the panel as follows: mock treatment (M), anti-Fas 7C11 with no pretreatment (F), pretreatment with ZVAD-fmk (Z), and pretreatment with DEVD-fmk (D). The relative migration of molecular size markers in kilodaltons is indicated on the left side of the panel. SRPK1 and SRPK2 cleavage products are shown on the right side of the panel. Lanes are numbered at the bottom of each panel.
To determine if caspases are required for cleavage of SRPK1 and SRPK2, Jurkat cells were cocultured with ZVAD (a soluble pan-caspase inhibitor) (Fig. 6 C, lane 3), or DEVD (a soluble peptide inhibitor of CPP32 family proteases; reference 29) (Fig. 6 C, lane 4), for 1 h before the addition of anti-Fas. 5 h later, cells were solubilized, and lysates were separated by SDS-PAGE, transferred to nitrocellulose, and blotted with anti-SRPK1 or SRPK2. Both inhibitors effectively prevented the appearance of cleavage products (Fig. 6 C, lanes 3 and 4), indicating that caspases are required for cleavage of SRPK1 and SRPK2, respectively.
SRPK1 Kinase Activity Is Activated during Fas-mediated Apoptosis.
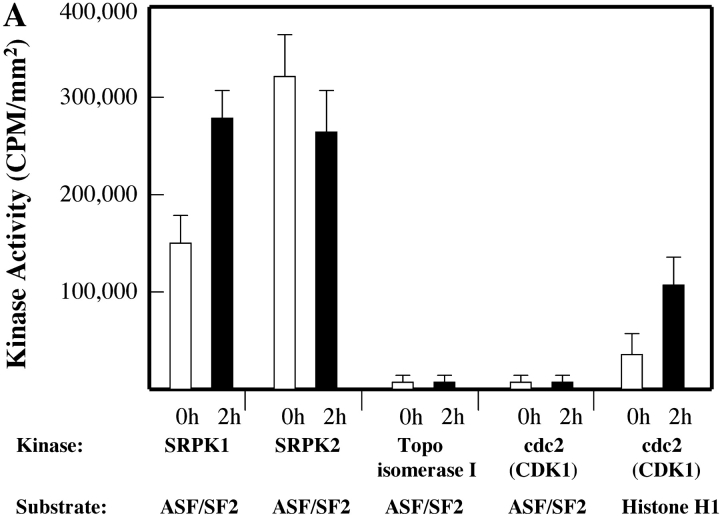
Dozens of signaling molecules are regulated during apoptosis, with varying outcomes. For example, some kinases are activated (e.g., MEKK-1 and PKC-θ), while others are inactivated (e.g., DNA-PKCS and Wee-1) or relocalized (e.g., fyn) to other cellular compartments after cleavage by caspases and other proteases (for a review, see reference 30). To determine whether any of the known SRPKs are activated during apoptosis, we performed immunoprecipitation kinase assays from lysates prepared from healthy or apoptotic Jurkat T cells, as described in detail in Materials and Methods. SRPKs were immunoprecipitated with anti-SRPK1, anti-SRPK2, anti–Scl-70 (topoisomerase I), or anti-cdc2 (CDK1), respectively. Precipitates were washed extensively, and the activity of individual kinases was determined by the ability of the kinase to phosphorylate known kinase substrates, including recombinant ASF/SF2 and purified Histone H1. As previously reported, the histone H1 kinase activity associated with cdc2 (CDK1) increases nearly threefold during Fas-mediated apoptosis (Fig. 7 A) (31). In contrast, the ability of cdc2 (CDK1) or topoisomerase I to phosphorylate ASF/SF2, either in healthy cells or dying cells, is negligible. ASF/SF2 is efficiently phosphorylated by both SRPK1 and SRPK2 when immunoprecipitations are performed using lysates prepared from healthy cells (left side of panel). However, of these two SRPKs, only SRPK1 exhibits an increase in ASF/SF2 kinase activity in response to an apoptotic stimulus (Fig. 7, left side of panel A). Analysis of the immunoprecipitates reveals that an SRPK1 cleavage product is readily detectable 2 h after anti-Fas treatment (Fig. 7 B, IP/Western blot analysis), yielding the signature 66-kD fragment. Small amounts of cleavage products (arrow heads) were detectable for SRPK2 and topoisomerase I. The level of cdc2 (CDK1) remains the same during Fas-induced apoptosis, whereas there is a significant decrease in the level of tyrosine phosphorylation at Tyr-15, an indirect result of cleavage and disruption of an upstream cdc2 kinase (Wee-1) during apoptosis (Fig. 7 B, bottom panel). Dephosphorylation of cdc2 (CDK1) activates its Histone H1 kinase activity, as shown in Fig. 7 A (right side of panel). These results demonstrate that only SRPK1 and SRPK2 efficiently utilize ASF/SF2 as an in vitro kinase substrate, and that enhanced phosphorylation of ASF/SF2 during apoptosis is attributed to direct activation of SRPK1.
Figure 7.
SRPK1 is activated and phosphorylates the SR protein autoantigen ASF/SF2 during apoptosis. Jurkat cells were incubated with anti-Fas and harvested in detergent lysis buffer at the times indicated at the top of each panel (in hours). SRPK1, SRPK2, topoisomerase I, and cdc2 (CDK1) activities were assayed by immunoprecipitation assays using purified kinase-specific substrates as described in Materials and Methods. Each kinase activity was assayed using 0.5 mg of cytosolic protein and 25 pmoles of bacterial ASF/SF2 or 5 μg of Histone H1. The extent of phosphorylation of individual substrates was detected by autoradiography, and quantified using Molecular Imager®FX (Bio-Rad Laboratories). Individual phosphorylation (CPM/mm2) is indicated as shown in A. Precipitated full-length protein (black arrow) and cleavage product (arrow head) were analyzed by IP/Western blot in the middle panels. Cleavage of PARP (bottom panel), as well as dephosphorylation of cdc2 (Tyr-15), (bottom panel) was also analyzed.
Cleavage of SRPK1 and SRPK2 Abrogates Their In Vitro Kinase Activity.
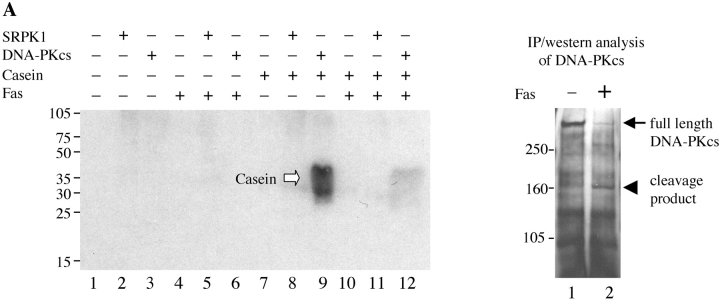
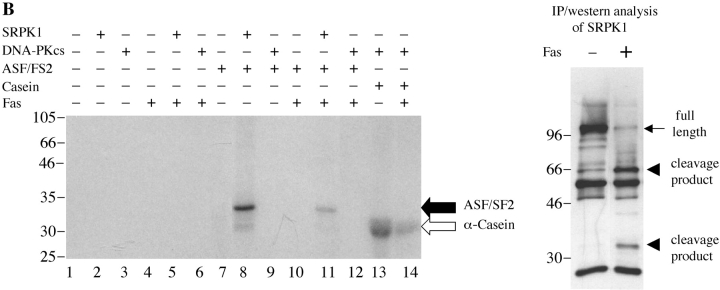
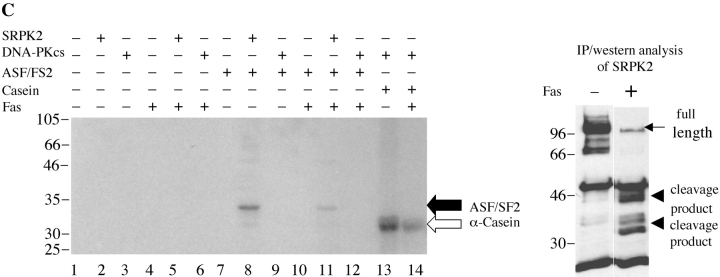
Finally, we examined whether SRPK1 and SRPK2 retained their kinase activity upon cleavage. It has been reported that several kinases are activated upon cleavage by caspases (for a review, see reference 30). We assayed the kinase activity of SRPK1 and SRPK2 before or after cleavage. Immunoprecipitation kinase assays for the autoantigen DNA-PKCS, a kinase that is disrupted after cleavage, were also performed as controls (4). Jurkat cells were incubated with anti-Fas for 24 h, and kinases were immunoprecipitated using anti-DNA-PKCS, anti-SRPK1, or anti-SRPK2, respectively. Individual precipitates were washed four times, incubated in individual reaction buffer including 20 μg of α-casein at 30°C for 15 min (DNA-PKCS) or 25 pmoles of recombinant, bacterial ASF/SF2 at 30°C for 30 min (SRPK1 and SRPK2). Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and detected by autoradiography. The kinase activities were determined by measuring the ability of the kinase to phosphorylate α-casein or ASF/SF2, as indicated. As shown in Fig. 8 A, full-length DNA-PKCS efficiently phosphorylates α-casein (lane 9), whereas the cleavage product has dramatically decreased kinase activity (lane 12). The small amount of phospho-casein (lane 12) is attributed to the remaining, uncleaved DNA-PKCS (right panel, black arrow). As shown in Fig. 8, B and C, full-length SRPK1 and SRPK2 phosphorylate ASF/SF2 (lane 8), while cleavage fragments of each kinase exhibit markedly decreased activity (lane 11). This result suggests that SRPK1 is activated during apoptosis by some mechanism other than caspase cleavage, and that SRPK1 kinase activity is disrupted following caspase-mediated proteolysis.
Figure 8.
In vivo cleavage of DNA-PKCS, SRPK1, and SRPK2 results in decreased kinase activity of the kinase fragments. Jurkat cells were incubated in the presence of anti-Fas for 24 h, solubilized in NP-40 lysis buffer, and precipitated using anti-DNA-PKCS, anti-SRPK1, or anti-SRPK2, respectively. Kinase activities were assayed by immunoprecipitation assay as described in Materials and Methods. Each kinase activity was assayed using 0.5 mg of cytosolic protein from the immunoprecipitations, and 20 μg of α-casein (DNA-PKCS) or 25 pmoles of bacterial ASF/SF2 (SRPK1 and SRPK2), respectively. Phosphorylation of substrates is demonstrated in the left panels (black arrow: ASF/SF2; white arrow: α-casein). Precipitated full-length protein (black arrow) and cleavage product (arrow head) were analyzed by IP/Western blot in the right panels. The relative migration of molecular size markers in kilodaltons is indicated on the left side of the panel. Phosphorylation of substrates is shown on the right side of the panel. Lanes are numbered at the bottom of each panel.
Discussion
SR proteins such as ASF/SF2 and SC35 are essential splicing factors in mammalian cells and are directly associated with components of the spliceosome (32). The U1-snRNP and the U3-snoRNP are the major RNP autoantigen targets in patients with SLE/MCTD and scleroderma, respectively (1), and we have previously shown that multiple different SR proteins associate with these two autoantigen complexes during cell death (7, 8). SR proteins themselves have recently been identified as novel autoantigens in connective tissue diseases (33). A striking finding in the discovery that SR proteins are unique autoantigens is that autoantibodies preferentially recognize individual SR proteins in a phosphorylation-dependent manner, with sera from some patients exclusively recognizing phosphorylated SR proteins, and others preferentially recognizing SR proteins that had been stripped of phosphate residues by preincubation with phosphatase. Few studies have directly addressed whether posttranslational modifications of antigens play a direct role in recognition by autoimmune sera. In addition to SR proteins, phosphorylated subunits of RNA polymerase I (but not isoforms lacking phosphate residues) are recognized by human serum autoantibodies (34). Also, the 70-kD component of the U1-snRNP is preferentially recognized by SLE or scleroderma sera when the protein is extracted from either apoptotic or oxidatively damaged cells, respectively (35, 36).
We had previously demonstrated that phosphorylated SR proteins reversibly associate with the U1-snRNP during a variety of different apoptotic stimuli and this could be inhibited by ectopic expression of the apoptosis inhibitory protein bcl-2. Importantly, we also demonstrated that an SR protein kinase activity was present in the coprecipitated U1-snRNP autoantigen complexes, suggesting that (i) the kinase responsible for phosphorylating SR proteins in vivo and in vitro could be identified by carefully examining immunoprecipitates prepared using serum derived from patients with SLE or MCTD and (ii) an SR protein kinase could itself be an autoantigen. With regard to the latter possibility, the results shown in Fig. 2 suggest otherwise, as incubation of the immunoprecipitates with MgCl2 disrupts binding of SRPK1 to the U1-snRNP and prevents SRPK1 coprecipitation. SRPK1 and SRPK2 are efficiently cleaved in vivo during apoptosis in a caspase-dependent manner, and this cleavage is inhibited in vivo by overexpression of either bcl-2 or bcl-xL. One of these kinases, SRPK1, is clearly coprecipitated by all serum samples derived from patients with SLE or MCTD that were tested. Analysis of available serum samples from our initial report demonstrates that SRPK1 is also present in these immunoprecipitates (Fig. 1, and unpublished data), suggesting that SRPK1 is the autoantigen kinase observed in our original reports (7, 8).
Several different subfamilies of SRPKs have been identified during the last decade, each of which were candidate kinases in our studies. Clk/Sty kinases, of which there are four main isoforms in humans, play important roles in regulation of alternative splicing (12). Since antibodies that specifically recognize human Clk/Sty kinases have not been developed, we have been unable to determine whether any members of this kinase family may be associated with either SR proteins or the U1-snRNP. However, previous experiments performed using cells labeled with [35S] methionine did not identify prominent proteins in the immunoprecipitates migrating by SDS-PAGE in the molecular weight range of Clk/Sty kinases (7, 8), arguing that these kinases are not associated in abundance with the U1-snRNP. cdc2 (CDK1) has also been reported to have SR protein kinase activity, although we have found that ASF/SF2 is a poor substrate for cdc2 (CDK1) in vitro (Fig. 7; reference 20). Moreover, CDK1 is not coprecipitated by human autoimmune sera (unpublished data). Similarly, topoisomerase I has previously been shown to be cleaved during cell death, but it is a poor kinase in in vitro assays (Fig. 7) (37), and it was not coprecipitated with the U1-snRNP (Fig. 1, and unpublished data). Our inability to demonstrate that topoisomerase I is capable of phosphorylating ASF/SF2 in vitro is in contrast to the findings of Tazi's laboratory (19), and may in part be explained by the nature of our assay, which used human autoimmune sera to precipitate topoisomerase I before performing an in vitro kinase assay directly on sepharose beads. Whether topoisomerase I, which lacks consensus kinase domains found in most protein kinases, is an important SRPK in vivo remains a controversial question. It is important to note, however, that topoisomerase I is itself a prominent autoantigen (Scl-70) in patients with the diffuse form of scleroderma (1). The mechanism(s) by which this autoantigen is targeted in such patients remain obscure.
SRPK1 was first identified as an SR protein kinase that copurified biochemically with components of the spliceosome (21, 38). A closely related homologue of SRPK1, termed SRPK2, was soon identified (39). SRPK2 is 58% homologous to SRPK1, differing only by the inclusion of an additional domain of unknown function in the amino terminus. Closely related SRPK1 and SRPK2 homologues have been cloned and characterized in mice (40), and two functional SRPK1 homologues have been identified in budding yeast and fission yeast. The Saccharomyces cerevisiae SRPK1 homologue, Sky1p, regulates nuclear import of the yeast SR-like protein Np13p (41–43). Another yeast SRPK1 homologue, Dsk1, has been identified and characterized in Schizosaccharomyces pombe (44). Interestingly, a unique mammalian isoform of SRPK1 has recently been identified (45). This SRPK1 isoform is produced by alternative splicing, encoding a protein with an insert of 171 amino acids in the amino terminus, proximal to one of the kinase domains. Using this splice variant as bait in a yeast two hybrid assay, Nikolakaki et al. demonstrated that the novel isoform interacts specifically with Scaffold Attachment Factor B (45). Our observation that SRPK1 migrates significantly slower by SDS-PAGE in MCF7 cells may be explained by such a mechanism, suggesting that a novel SRPK1 isoform may exist in this cell line. Experiments designed to address this possibility are in progress.
Detailed structural analysis of SRPK1 and SRPK2 has not been reported. Their structure, as predicted from their deduced amino acid sequence, suggests that cleavage of either protein should disrupt kinase activity by preventing the amino terminal and COOH terminal kinase domains from interacting. Experiments shown in Fig. 8 support this concept, since the kinase activity of a caspase cleavage fragment of SRPK1 is markedly decreased. Cleavage of signaling molecules by caspases or other proteases that are activated in response to cellular stressors has been suggested to play an important role in many aspects of the apoptotic phenotype (30). Kinases and other signaling molecules are well positioned as caspase substrates to regulate the process of cell death. Cleavage of signaling molecules can have a variety of outcomes. For example, some kinases such as SLK, PAK2, and P27Kip1 are activated or dysregulated upon cleavage (46–48), whereas kinases such as the catalytic subunit of DNA-PKCS and CaMK IV are permanently inactivated upon cleavage (4, 49). Other kinases are relocalized to new intracellular compartments, e.g., relocalization of fyn to the cytosol through removal of a palmitoylation and myristylation site by caspase-3, allowing the kinase to interact with a novel substrate, Tctex-1 (50).
We provide data that the SR protein kinase activity associated with SRPK1 is inactivated on caspase-mediated proteolysis during cell death. One of the major functions proposed for SRPK1 is to phosphorylate SR proteins, which in turn regulates alternative splice site selection of a subset of mRNA molecules, including the apoptosis regulatory protein Ich-1 (51–53). Overexpression of SR proteins such as ASF/SF2 alters splice site selection of several RNAs, including Ich-1, and this correlates with resetting of the apoptotic threshold of transfected cells (52). Over 30 different mRNAs have been identified that exist as two or more different splice variants, each with opposing apoptotic functions (53). For example, alternative splicing of the mRNAs encoding at least three different apoptosis regulatory molecules has been recently shown to occur in vivo in dying cells. Wotawa et al. demonstrated that procaspase 2L, a proapoptotic isoform of caspase-2, is differentially downregulated in response to the apoptosis-inducing drug etoposide in tumor cells (54). Chalfant et al. showed that mRNAs encoding caspase-9 and bcl-x are differentially spliced in response to ceramide in A549 lung carcinoma cells and that alternative splicing correlated with the phosphorylation state of SR proteins (55). Although they did not address whether SRPKs might directly influence splice site selection, their results suggested a role for the protein phosphatase-1 in the dephosphorylation of SR proteins (55), which we have also observed at later time points (8, 9). Therefore, it is highly likely that enzymes such as protein phosphatase-1, SRPK1, and perhaps other SRPKs play a critical role in determining whether an individual cell will live or die in response to stressful stimuli by regulating relative levels of splice variants of such apoptosis regulatory molecules. Other functions that could be indirectly attributed to SRPK1 through its kinase activity include regulation of SR protein localization (41), shuttling of splicing factors between the nucleus and cytoplasm (56), and mRNA message stabilization (57). Experiments are in progress to determine which of these functions might be regulated by SRPKs during cell stress and apoptosis.
Caspases are the main executioners of the apoptotic program. At the pinnacle of the caspase cascade are caspases-8 and -9, which upon activation feed into the main executioners, caspase-3 and -6. Caspase-8 is the major caspase that is activated in response to death receptor–mediated signals, while caspase-9 is the initiating caspase for nonreceptor-mediated stimuli such as irradiation, growth factor withdrawal, and chemotherapy, all of which are regulated by mitochondrial-associated factors such as APAF-1, cytochrome c, and bcl-2 (58). Our in vitro caspase cleavage data (Fig. 3) and data published by others demonstrates that three SRPKs (SRPK1, SRPK2, and topoisomerase I) are differentially cleaved by each of these caspases (caspase-8, caspase-9, and caspases-3 and -6, respectively) (19, 23). Because each of these SR protein kinases are cleaved by key caspases in the cascade, our results suggest that alternative splicing may play a critical role in the sensing phase of cellular stress. mRNA splicing may represent a largely unrecognized and unexplored posttranscriptional modification that determines cell fate in response to environmental stimuli. Our results suggest that SRPKs such as SRPK1 are activated during sublethal cell stress (leading to alternative splicing of mRNAs such that antiapoptotic isoforms such as bcl-xL and Ich1-α are produced). Caspase-mediated cleavage of SRPKs after lethal stressors would then ensure that the apoptotic program is faithfully executed in condemned cells.
Acknowledgments
The authors would like to thank J. Reed, N. Kedersha, and M. Robertson for cell lines; X.D. Fu, M. Hagiwara, A. Krainer, O. Nayler, V. Shifrin, H. Li, and J. Yuan for cDNA constructs; and W.J. van Venrooij, X.D. Fu, and R. Reed for antibodies. The authors would like to thank members of the Utz and Anderson labs for insights, encouragement, and critical review of the manuscript.
This work was supported by a Stanford University Dean's Fellowship (to M. Kamachi), a Howard Hughes Medical Institute Medical Student Research Fellowship (to T.M. Le), grants from the Lupus Foundation of America and the American Association of Allergy, Asthma, and Immunology (to T.M. Le), NIH grants K08AI01521 and U19-DK61934 (to P.J. Utz), and an Arthritis Foundation Investigator Award, Bio-X grant, Baxter Foundation Career Development Award, and Northern California Arthritis Foundation Chapter Grant (to P.J. Utz).
Footnotes
Abbreviations used in this paper: CDK, cyclin-dependent kinase; DNA-PK, DNA-dependent protein kinase; MCTD, mixed connective tissue disease; NP40, nonidet P40; RNP, ribonucleoprotein; snRNP, small nuclear RNP; SLE, systemic lupus erythematosus; Sm, Smith complex; SR splicing factor, serine/arginine splicing factor; SRPK, SR protein kinase.
References
- 1.von Mühlen, C.A., and E.M. Tan. 1995. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthr. Rheum. 24:323–358. [DOI] [PubMed] [Google Scholar]
- 2.Utz, P.J., T.J. Gensler, and P. Anderson. 2000. Death, autoantigen modifications, and tolerance. Arthr. Res. 2:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casciola-Rosen, L.A., D.K. Miller, G.J. Anhalt, and A. Rosen. 1994. Specific cleavage of the 70 kDa protein component of the U1 small nuclear riboprotein is a characteristic biochemical feature of apoptotic cell death. J. Biol. Chem. 269:30757–30760. [PubMed] [Google Scholar]
- 4.Casciola-Rosen, L.A., G.J. Anhalt, and A. Rosen. 1995. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J. Exp. Med. 182:1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casciola-Rosen, L., F. Andrade, D. Ulanet, W.B. Wong, and A. Rosen. 1999. Cleavage by granzyme B is strongly predictive of autoantigen status. Implications for initiation of autoimmunity. J. Exp. Med. 190:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utz, P.J., M. Hottelet, W.J. van Venrooij, and P. Anderson. 1998. The 72 kDa component of the signal recognition particle is cleaved during apoptosis. J. Biol. Chem. 273:35299–35361. [DOI] [PubMed] [Google Scholar]
- 7.Utz, P.J., M. Hottelet, P.H. Schur, and P. Anderson. 1997. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J. Exp. Med. 185:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utz, P.J., M. Hottelet, W. van Venrooij, and P. Anderson. 1998. Association of phosphorylated SR proteins and the U1-small nuclear ribonuclear protein autoantigen complex accompanies apoptotic cell death. J. Exp. Med. 187:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson, P. 1997. Kinase cascades regulating entry into apoptosis. Microbiol. Mol. Biol. Rev. 61:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overzet, K., T.J. Gensler, S.J. Kim, M.E. Geiger, W.J. van Venrooij, K.M. Pollard, P. Anderson, and P.J. Utz. 2000. Small nucleolar RNP scleroderma autoantigens associate with phosphorylated serine/arginine splicing factors during apoptosis. Arthr. Rheum. 43:1327–1336. [DOI] [PubMed] [Google Scholar]
- 11.Karwan, R., J.L. Bennett, and D.A. Clayton. 1991. Nuclear RNase MRP processes RNA at multiple discrete sites: interaction with an upstream G box is required for subsequent downstream cleavages. Genes Dev. 5:1264–1276. [DOI] [PubMed] [Google Scholar]
- 12.Nayler, O., S. Stamm, and A. Ullrich. 1997. Characterization and comparison of four serine and arginine rich (SR) protein kinases. Biochem. J. 326:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habets, W.J., M.H. Hoet, B.A. de Jong, A. van der Kemp, and W.J. van Venrooij. 1989. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J. Immunol. 143:2560–2566. [PubMed] [Google Scholar]
- 14.Liu, L., Y.T. Kwak, F. Bex, L.F. Garcia-Martinez, X.H. Li, K. Meek, W.S. Lane, and R.B. Gaynor. 1998. DNA-dependent protein kinase phosphorylation of IκBα and IκBβ regulates NF-κB DNA binding properties. Mol. Cell. Biol. 18:4221–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colwill, K., L.L. Feng, J.M. Yeakley, G.D. Gish, J.F. Cáceres, T. Pawson, and X.D. Fu. 1996. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J. Biol. Chem. 271:24569–24575. [DOI] [PubMed] [Google Scholar]
- 16.Brooks, G. 2000. Cycline-dependent kinases and cycline-dependent kinase inhibitors. Protein kinase protocols 124. Twana Press Inc., Twana. pp. 1–161.
- 17.Satoh, M., H.B. Richards, K.J. Hamilton, and W.H. Reeves. 1997. Human anti-ribonuclear ribonucleoprotein antigen autoimmune sera contain a novel subset of autoantibodies that stabilizes the molecular interaction of U1RNP-C protein with the Smith core complex. J. Immunol. 158:5017–5026. [PubMed] [Google Scholar]
- 18.Koizumi, J., Y. Okamoto, H. Onogi, A. Mayeda, A.R. Krainer, and M. Hagiwara. 1999. The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs). J. Biol. Chem. 274:11125–11131. [DOI] [PubMed] [Google Scholar]
- 19.Rossi, F., E. Labourier, T. Forne, G. Divita, J. Derancourt, J.F. Riou, E. Antoine, G. Cathala, C. Brunel, and J. Tazi. 1996. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 381:80–82. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto, Y., H. Onogi, R. Honda, H. Yasuda, T. Wakabayashi, Y. Nimura, and M. Hagiwara. 1998. cdc2 kinase-mediated phosphorylation of splicing factor SF2/ASF. Biochem. Biophys. Res. Commun. 249:872–878. [DOI] [PubMed] [Google Scholar]
- 21.Gui, J.F., W.S. Lane, and X.D. Fu. 1994. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 369:678–682. [DOI] [PubMed] [Google Scholar]
- 22.Satoh, M., J.J. Langdon, K.J. Hamilton, H.B. Richards, D. Panka, R.A. Eisenberg, and W.H. Reeves. 1996. Distinctive immune response patterns of human and murine autoimmune sera to U1 small nuclear ribonucleoprotein C protein. J. Clin. Invest. 97:2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samejima, K., P.A. Svingen, G.S. Basi, T. Kottke, P.W. Mesner, L. Stewart, F. Durrieu, G.G. Poirier, E.S. Alnemri, J.J. Champoux, et al. 1999. Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis. J. Biol. Chem. 274:4335–4340. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson, M., J. Burne, and M. Raff. 1994. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 13:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boise, L.H., A.R. Gottschallk, J. Quintans, and C.B. Thompson. 1995. Bcl-2 and Bcl-2-related proteins in apoptosis regulation. Curr. Top. Microbiol. Immunol. 200:107–121. [DOI] [PubMed] [Google Scholar]
- 26.Itoh, N., Y. Tsujimoto, and S. Nagata. 1993. Effect of bcl-2 on Fas antigen-mediated cell death. J. Immunol. 151:621–627. [PubMed] [Google Scholar]
- 27.Reed, J.C. 1994. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 124:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sentman, C.L., J.R. Shutter, D. Hockenberry, O. Kanagawa, and S.J. Korsmeyer. 1991. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 67:879–888. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson, D.W., and N.A. Thornberry. 1997. Caspases: killer proteases. TIBS. 22:299–306. [DOI] [PubMed] [Google Scholar]
- 30.Utz, P.J., and P. Anderson. 2000. Life and death decisions: regulation of apoptosis by proteolysis of signaling molecules. Cell Death Differ. 7:589–602. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, B., H. Li, J. Yuan, and M.W. Kirschner. 1998. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA. 95:6785–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu, X.D. 1995. The superfamily of arginine/serine-rich splicing factors. RNA. 1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 33.Neugebauer, K.M., J.T. Merrill, M.H. Wener, R.G. Lahita, and M.B. Roth. 2000. SR proteins are autoantigens in patients with systemic lupus erythematosus. Importance of phosphoepitopes. Arthr. Rheum. 43:1768–1778. [DOI] [PubMed] [Google Scholar]
- 34.Stetler, D., and S. Jacob. 1984. Phosphorylation of RNA polymerase I augments its interaction with autoantibodies of systemic lupus erythematosus patients. J. Biol. Chem. 259:13629–13632. [PubMed] [Google Scholar]
- 35.Greidinger, E.L., L. Casciola-Rosen, S.M. Morris, R.W. Hoffman, and A. Rosen. 2000. Autoantibody recognition of distinctly modified forms of the U1-70-kd antigen is associated with different clinical disease manifestations. Arthr. Rheum. 43:881–888. [DOI] [PubMed] [Google Scholar]
- 36.Greidinger, E.L., M.F. Foecking, S. Ranatunga, and R.W. Hoffman. 2002. Apoptotic U1-70 kd is antigenically distinct from the intact form of the U1-70-kd molecule. Arthr. Rheum. 46:1264–1269. [DOI] [PubMed] [Google Scholar]
- 37.Casiano, C.A., S.J. Martin, D.R. Green, and E.M. Tan. 1996. Selective cleavage of nuclear autoantigens during CD95 (Fas/Apo-1)-mediated T cell apoptosis. J. Exp. Med. 184:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gui, J.F., H. Tronchere, S.D. Chandler, and X.D. Fu. 1994. Purification and characterization of a serine kinase specific for the serine- and arginine-rich pre-mRNA splicing factors. Proc. Natl. Acad. Sci. USA. 91:10824–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, H.Y., W. Lin, J.A. Dyck, J.M. Yeakley, Z. Songyang, L.C. Cantley, and X.D. Fu. 1998. SRPK2: A differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J. Cell Biol. 140:737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroyanagi, N., H. Onogi, T. Wakabayashi, and M. Hagiwara. 1998. Novel SR-protein-specific kinase, SRPK2, disassembles nuclear speckles. Biochem. Biophys. Res. Commun. 242:357–364. [DOI] [PubMed] [Google Scholar]
- 41.Yun, C.Y., and X.D. Fu. 2000. Conserved SR protein kinase functions in nuclear import and its action is counteracted by arginine methylation in Saccharomyces cerevisiae. J. Cell Biol. 150:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schenk, P.W., A.W. Boersma, J.A. Brandsma, H. den Dulk, H. Burger, G. Stoter, J. Brouwer, and K. Nooter. 2001. SKY1 is involved in cisplatin-induced cell kill in Saccharomyces cerevisiae, and inactivation of its human homologue, SRPK1, induces cisplatin resistance in a human ovarian carcinoma cell line. Cancer Res. 61:6982–6986. [PubMed] [Google Scholar]
- 43.Siebel, C.W., L. Feng, C. Guthrie, and X.D. Fu. 1999. Conservation in budding yeast of a kinase specific for SR splicing factors. Proc. Natl. Acad. Sci. USA. 96:5440–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang, Z., T. Kuo, J. Shen, and R.J. Lin. 2000. Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1. Mol. Cell. Biol. 20:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolakaki, E., R. Kohen, A.M. Hartmann, S. Stamm, E. Georgatsou, and T. Giannakouros. 2001. Cloning and characterization of an alternatively spliced form of SR protein kinase 1 that interacts specifically with scaffold attachment factor-B. J. Biol. Chem. 276:40175–40182. [DOI] [PubMed] [Google Scholar]
- 46.Sabourin, L.A., K. Tamai, P. Seale, J. Wagner, and M.A. Rudnicki. 2000. Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol. Cell. Biol. 20:684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudel, T., and G.M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 276:1571–1574. [DOI] [PubMed] [Google Scholar]
- 48.Levkau, B., H. Koyama, E.W. Raines, B.E. Clurman, B. Herren, K. Orth, J.M. Roberts, and R. Ross. 1998. Cleavage of p21/Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell. 1:553–563. [DOI] [PubMed] [Google Scholar]
- 49.McGinnis, K.M., M.M. Whitton, M.E. Gnegy, and K.K. Wang. 1998. Calcium/calmodulin-dependent protein kinase IV is cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. J. Biol. Chem. 273:19993–20000. [DOI] [PubMed] [Google Scholar]
- 50.Mou, T., J.R. Kraas, E.T. Fung, and S.L. Swope. 1998. Identification of a dynein molecular motor component in Torpedo electroplax; binding and phosphorylation of Tctex-1 by Fyn. FEBS Lett. 435:275–281. [DOI] [PubMed] [Google Scholar]
- 51.Cáceres, J.F., F. Stamm, D.M. Helfman, and A.R. Krainer. 1994. Regulation of alternative splicing by overexpression of antagonistic splicing factors. Science. 265:1706–1709. [DOI] [PubMed] [Google Scholar]
- 52.Jiang, Z.H., W.J. Zhang, Y. Rao, and J.Y. Wu. 1998. Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc. Natl. Acad. Sci. USA. 95:9155–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang, Z.H., and J.Y. Wu. 1999. Alternative splicing and apoptosis. Proc. Soc. Exp. Biol. Med. 220:64–72. [DOI] [PubMed] [Google Scholar]
- 54.Wotawa, A., S. Solier, E. Logette, E. Solary, and L. Corcos. 2002. Differential influence of etoposide on two caspase-2 mRNA isoforms in leukemic cells. Cancer Lett. 185:181–189. [DOI] [PubMed] [Google Scholar]
- 55.Chalfant, C.E., K. Rathman, R.L. Pinkerman, R.E. Wood, L.M. Obeid, B. Ogretmen and Y.A. Hannun. 2002. Denovo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J. Biol. Chem. 277:12587–12595. [DOI] [PubMed] [Google Scholar]
- 56.Cáceres, J.F., G.R. Screaton, and A.R. Krainer. 1998. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 12:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemaire, R., J. Prasad, T. Kashima, J. Gustafson, J.L. Manley, and R. Lafyatis. 2002. Stability of a PKCI-1-related mRNA is controlled by the splicing factor ASF/SF2: a novel function for SR proteins. Genes Dev. 16:594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green, D.R., and J.C. Reed. 1998. Mitochondria and apoptosis. Science. 281:1309–1312. [DOI] [PubMed] [Google Scholar]