Abstract
Human hematopoietic tissue contains rare stem cells with multilineage reconstituting ability demonstrable in receptive xenogeneic hosts. We now show that within 3 wk nonobese diabetic severe combined immunodeficiency (NOD/SCID) mice transplanted with human fetal liver cells regenerate near maximum levels of daughter human hematopoietic stem cells (HSCs) able to repopulate secondary NOD/SCID mice. At this time, most of the human HSCs (and other primitive progenitors) are actively proliferating as shown by their sensitivity to treatments that kill cycling cells selectively (e.g., exposure to high specific-activity [3H]thymidine in vitro or 5-fluorouracil in vivo). Interestingly, the proliferating human HSCs were rapidly forced into quiescence by in vivo administration of stromal-derived factor-1 (SDF-1) and this was accompanied by a marked increase in the numbers of human HSCs detectable. A similar result was obtained when transforming growth factor-β was injected, consistent with a reversible change in HSCs engrafting potential linked to changes in their cell cycle status. By 12 wk after transplant, most of the human HSCs had already entered Go and treatment with SDF-1 had no effect on their engrafting activity. These findings point to the existence of novel mechanisms by which inhibitors of HSC cycling can regulate the engrafting ability of human HSCs executing self-renewal divisions in vivo.
Keywords: TGF-β, SDF-1, cell cycle, stem cells, engraftment
Introduction
In the last decade, major advances have been made in the functional characterization of human hematopoietic stem cells (HSCs).* Key to this progress has been the discovery that these cells (even at limiting dilutions) can generate multiple types of differentiating progeny for prolonged periods of time, both in stromal-based culture systems (1–5) and in immunodeficient xenogeneic recipients (6–10). Sublethally irradiated nonobese diabetic (NOD)/SCID mice are particularly useful hosts for in vivo studies of human HSCs because of the efficiency (11), selectivity (12), and speed (7, 13) with which these mice are repopulated with the lymphoid and myeloid progeny of a very primitive subset of transplantable human cells. It has thus become practical to measure and resolve differences in human HSC frequencies in variously manipulated populations using relatively short term endpoints of human multilineage repopulation of NOD/SCID mice (8, 14). During the initial 4–6 wk after transplantation of human HSCs into primary NOD/SCID mice, the number of human cells able to produce both lymphoid and myeloid progeny in secondary NOD/SCID mice also rapidly increases (14–16). This finding suggests that at least some of the human HSCs that produce differentiating lymphoid and myeloid cells after injection into NOD/SCID mice also generate daughter HSCs that can be detected by injection into secondary NOD/SCID mice. More recent analyses of secondary recipients of retrovirally marked human HSCs provide further support for this view (17). However, little is known about the factors that control human HSC cycling in vivo or whether these may be active in engrafted NOD/SCID mice.
The present studies were designed to address these questions in NOD/SCID recipients of human fetal liver cells. This source of human HSCs was chosen because of the high HSC content in fetal liver and the ability of fetal liver HSCs to readily amplify their numbers in this model. Two complementary approaches were used to assess the proliferative activity of the regenerated human HSCs. The first relied on the ability of high specific activity [3H]thymidine to deliver a lethal dose of radiation exclusively to cells passing through S-phase (18) and the use of a sufficiently prolonged exposure time to allow all HSCs in G1, G2, or M to also enter S-phase (19). As a result, only HSCs in Go would not be killed by this treatment. The second approach exploited the ability of in vivo administered 5-fluorouracil (5-FU) to kill proliferating hematopoietic cells selectively but by a different mechanism (20). Because it had been shown that HSCs undergo marked but reversible fluctuations in their engraftment activity as they transit the cell cycle (21–23) we hypothesized that treatment of primary NOD/SCID hosts with agents able to arrest human HSC cycling would cause an apparent increase in the number of human HSCs detected in secondary recipients if, and only if, the HSCs in the primary hosts were proliferating. Both TGF-β and stromal-derived factor 1 (SDF-1) have been found to inhibit the cycling of primitive human progenitors detectable in vitro, including cells able to sustain hematopoiesis for at least 6 wk in stroma-containing cultures (24–26). Evidence that TGF-β can block the cell cycle entry of transplantable human HSCs has also been reported (27). Accordingly, 3 wk after transplant, we injected primary recipients of human fetal liver cells with either TGF-β or SDF-1 and then looked for effects on the subsequent repopulating activity detected upon transfer of the treated cells to secondary NOD/SCID recipients.
Materials and Methods
Human Cell Preparation.
Fetal livers obtained from 12 to 16 wk aborted fetuses according to approved institutional procedures were suspended using dispase (Dispase II; Sigma-Aldrich) and DNase (DNase I; Sigma-Aldrich) as described previously (28). Low-density (<1.077 g/ml) cells were isolated using Ficoll/Hypaque (Amersham Biosciences) and then cryopreserved in DMSO plus 90% FCS (StemCell Technologies Inc.).
Animals.
NOD/LtSz-scid/scid mice were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC, Canada) under microisolators and were provided with sterilized food and water. After being irradiated, mice were given acidified water containing ciprofloxacin (100 mg/l; Bayer AG). Mice were irradiated with 350 cGy 137Cs γ-rays at 6 to 8 wk of age and then injected intravenously with 107 freshly thawed low-density human fetal liver cells. Primary mice were killed and analyzed for the presence of human cells and progenitors either 2 1/2 to 3, or 12 wk later, as indicated. In some experiments, groups of mice were injected intravenously with either 150 mg/kg 5-FU (Faulding) or PBS 2 d before being killed. In others, groups of primary mice were injected intraperitoneally twice, 24 h apart, with PBS containing either 1 μg TGF-β1 (R&D Systems), or 10 μg SDF-1 (synthesized and purified as described [29]) or no other agent, 1 or in some cases 3 d before being killed. Secondary recipients were irradiated in the same way as primary mice and injected intravenously with equal proportions of marrow cells harvested from primary mice. All secondary recipients were killed 6 to 8 wk after transplant and their marrow cells were then analyzed for the presence of human cells by FACS® as described below.
Flow Cytometry and Calculation of Human HSC Numbers.
The procedure used to detect human cells in suspensions harvested from transplanted mice has been outlined in detail previously (14, 25). Briefly, red cells were lysed with 8.3% ammonium chloride, the nucleated cells then counted, washed in Hanks Balanced Salt Solution with 2% FCS, and incubated with human serum and 3 μg/ml of an anti–mouse IgG receptor antibody to minimize nonspecific staining. One aliquot was then stained with anti–human CD34-FITC and anti–human CD19-PE and CD20-PE (Becton Dickinson) with propidium iodide (PI; Sigma-Aldrich) added to the last wash to permit the identification of viable (PI−) human progenitor (CD34+) and exclusively B-lineage (CD34− CD19/20+) populations. A second aliquot was stained with anti-human CD45-PE (Hlel; Becton Dickinson), CD71-PE (OKT-9), CD15-FITC, and CD66b-FITC and PI to quantify the total viable human hematopoietic (CD45/71+) cell population present as well as a subset of exclusively granulopoietic (CD15/66b+) cells within this population. Additional aliquots were stained with irrelevant antibodies labeled with PE or FITC to set gates for positive staining based on exclusion of 99.99% of events seen in these controls. LYSIS II software (Becton Dickinson) was used for acquisition and analysis of FACS® data. For each analysis a minimum of 2 × 104 viable (PI−) cells were assessed and only when >5 positive events were recorded (for each gate setting) was the value used to quantitate a specific human cell phenotype. The frequency of human HSCs present in a given test cell suspension was calculated using the L-calc software (StemCell Technologies, Inc.) as described for cells referred to as competitive repopulating units (CRUs) from the proportions of negative mice detected in groups given different doses of test cells, where the definition of a negative mouse was one that was not positive for both human myeloid and human B-lineage cells (14). To calculate the total number of human HSCs present in the marrow of primary mice, it was assumed that two femurs and two tibias contained 25% of all the marrow cells in a mouse (30).
In Vitro Progenitor Assays.
Human colony-forming cells (CFCs) were enumerated in marrow cell suspensions harvested from mice by plating the cells in methylcellulose cultures that incorporated conditions that minimize the simultaneous detection of murine CFCs (7). Human long-term culture-initiating cells (LTC-ICs) were measured by coculturing aliquots of the same cell suspensions with murine feeders engineered to produce human Steel factor (SF), IL-3, and G-CSF for 6 wk at 37°C as described previously (3) and then counting the number of colonies obtained when the cells harvested from these cultures were plated in CFC assays that are selective for human cells. Erythroid CFCs (burst forming erythroids [BFU-Es]) and granulopoietic CFCs (CFU-GMs) were subdivided into primitive and mature categories based on their ability to generate large and small colonies using well established criteria (31).
[3H]Thymidine Suicide Experiments.
Marrow cells harvested from primary mice were depleted of red cells by ammonium chloride lysis, washed in PBS, resuspended in Iscove's medium containing a serum substitute (BIT™; StemCell Technologies, Inc.) supplemented with 10−4 2-mercaptoethanol (Sigma-Aldrich), 40 μg/ml high density lipoproteins (Sigma-Aldrich) and recombinant human SF (100 ng/ml, prepared and purified in the Terry Fox Laboratory), IL-3 (20 ng/ml; Novartis), and G-CSF (20 ng/ml; StemCell Technologies), and incubated at 2 × 106 cells/ml for 16 h with or without high specific activity [3H]thymidine (20 μCi/ml, 25 Ci/mmol; Amersham Biosciences) as described previously (19). The cells were then washed and assayed by plating suitable aliquots in CFC or LTC-IC assay cultures or by injecting suitable aliquots into irradiated NOD/SCID mice.
Results
Most of the Human HSCs Present in NOD/SCID Mice Injected 3 wk previously with Human Fetal Liver Cells Are Cycling.
Previous experiments have described the rapid regeneration of human hematopoietic cells, including CFCs and LTC-ICs that occurs in the marrow of sublethally irradiated NOD/SCID mice transplanted with 107 low-density human fetal liver cells (13). During the first 2 wk after transplant, the number of all of these cell types increases 100 to 1,000-fold followed in the next 2 wk by a slower pace of expansion with peak values being achieved at the end of that time. Secondary transplant studies have demonstrated that by 4 wk after transplant, the marrow of such primary mice contains many more transplantable human HSCs than were initially injected (11, 14). Therefore, we chose the 3 wk posttransplant time to look for evidence of human HSC proliferation in this model.
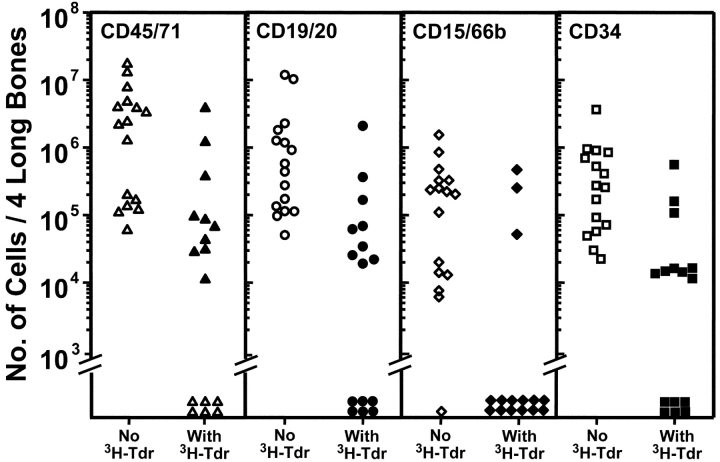
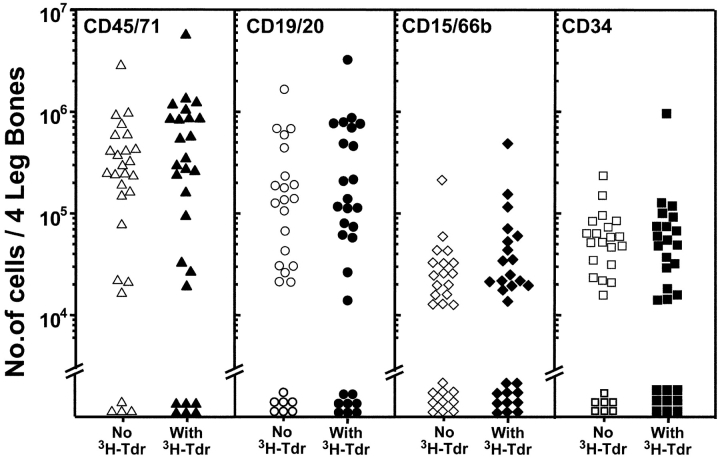
Fig. 1 shows the combined results of a first series of three experiments in which cells harvested from the marrow of primary NOD/SCID mice injected with 107 low-density human fetal liver cells 3 wk previously were incubated overnight in the presence or absence of high specific activity [3H]thymidine before being assayed for their ability to repopulate secondary NOD/SCID mice. It can be seen that 15 of the 16 secondary recipients of control cells showed multilineage engraftment (≥5 CD34−CD19/20+ and ≥5 CD45+CD15/66b+ cells per 2 × 104 viable cells analyzed [14]). In contrast, this was true for only 3 of the 15 secondary recipients of identical aliquots of initial cells that had been exposed to [3H]thymidine. When Poisson statistics were used to calculate the frequency of transplantable human multilineage HSCs detectable in each group as described in Materials and Methods, a 13-fold lower value was obtained for the [3H]thymidine-treated HSCs. Thus, a majority of the human HSCs from the primary mice must have entered S-phase at some point during the 16 h period of exposure to [3H]thymidine in vitro resulting in a 93% kill value. This indicates that >90% of the human HSCs present in the 3-wk engrafted mice were proliferating, and conversely, that <10% were in Go. Similar results were obtained for the human CFCs and LTC-ICs present in the same cell suspensions (unpublished data, but see also controls in Fig. 4, discussed below and previous studies [7, 25]).
Figure 1.
[3H]Thymidine cell suicide assays show most of the human HSCs present in NOD/SCID mice transplanted 3 wk before with human fetal liver cells are cycling. Marrow cells were removed from both femurs and tibias of primary NOD/SCID recipients of 107 low-density human fetal liver cells transplanted 18 to 21 d previously (12 to 20 mice per experiment, three experiments), pooled, and exposed to [3H]thymidine (or not) for 16 h in vitro before being injected into secondary NOD/SCID mice (equal aliquots of 0.56–0.88 primary mouse marrow equivalents per secondary mouse). Each symbol indicates the number of human cells of the phenotype indicated measured in the same four hind leg bones of individual secondary recipients of control (open symbols) or [3H]thymidine-treated primary bone marrow cells (solid symbols) assessed 6 to 8 wk later.
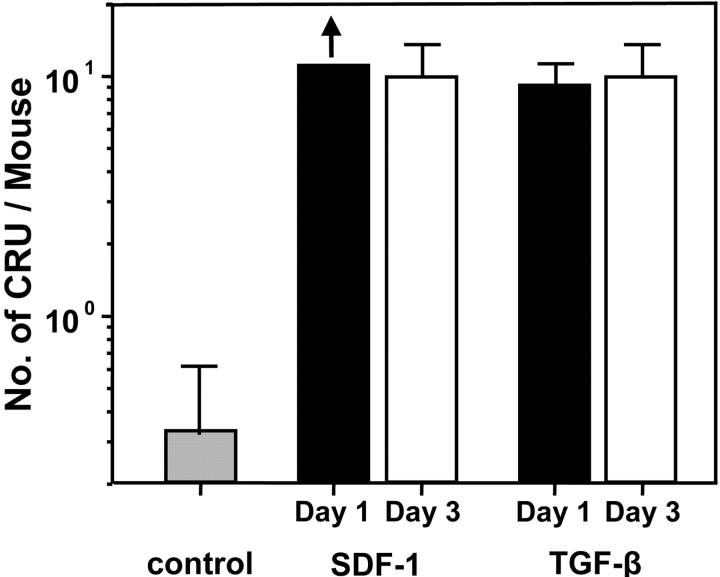
Figure 4.
In vivo administration of TGF-β1 or SDF-1 enhances the ability of human HSCs in regenerating primary NOD/SCID mice to engraft secondary mice. Equal aliquots of primary mouse marrow cells were transplanted into secondary mice (.25 primary mouse marrow equivalents per secondary mouse) and 6 to 8 wk later the number of different types of human cells in the four hind leg bones of each mouse was determined. These were used to identify mice as positive or negative for human multilineage engraftment and thence to provide data for HSC calculations. Each bar indicates the mean ± SEM of the total number of HSCs determined to be present in the control (gray bar) primary mice or in the primary mice infected with SDF-1 or TGF-β1 and assessed wither 1 d (black bars) or 3 d (white bars) after the last infection.
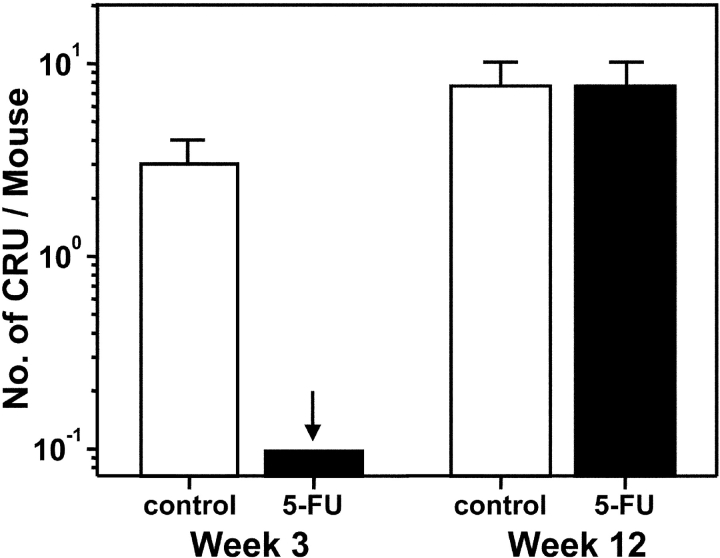
In a second series of eight experiments, the cycling activity of the human HSCs present in such 3-wk posttransplant mice was assessed by measuring their numbers 2 d after being injected (or not) with 150 mg/kg 5-FU. The numbers of human HSCs detectable in the untreated and 5-FU–treated primary mice were calculated from the frequencies of negative secondary recipients of equal aliquots of marrow from the two types of primary mice and the values obtained are shown in the left part of Fig. 2. It can be seen that at 3 wk after transplant, 5-FU treatment reduced the human HSC population in the primary mice by a factor of >30 (29 negative secondary mice of 29 injected with cells from 5-FU–treated primary mice versus 11 negative secondary mice of 29 injected with control cells). 5-FU treatment also decreased significantly (P < .05) the numbers of other types of human progenitors assayed in the same experiments (Table I).
Figure 2.
Changes in the number and 5-FU sensitivity of human HSCs between 3 and 12 wk after transplantation of human fetal liver cells into NOD/SCID mice. Marrow cells were removed from both femurs and tibias of primary mice 2 d after being injected with 5-FU (black bars) or not (white bars), pooled, and equal proportions transplanted into secondary NOD/SCID recipients (4–8 primary mice per group per experiment, 4 experiments, 0.25–0.5 primary mouse marrow equivalents per secondary mouse). Secondary mice were analyzed 6 to 8 wk later and human HSC numbers determined from the numbers of positive and negative secondary mice obtained. The arrow indicates a maximum value that was calculated by assuming one of the secondary mice in this group had been positive. Error bars indicate the range defined by ± SEM.
Table I.
Effect of 5-FU Administration on Human Progenitors in the Bone Marrow of NOD/SCID Mice
| Percentage kill after 5-FU
|
|||
|---|---|---|---|
| Time after transplantation |
Primitive BFU-E |
Primitive CFU-GM |
LTC-IC |
| 3 wk | 78 ± 4 | 72 ± 5 | 72 ± 6 |
| 12 wk | 77 ± 5 | 71 ± 3 | 72 ± 2 |
5-FU at 150 mg/kg. Mice killed 2 d after 5-FU administration.
The Population of Human HSCs Regenerated in Human Fetal Liver-engrafted NOD/SCID Mice Is Sustained Over Time but Becomes Quiescent by 12 wk.
Additional measurements were made in mice that had been engrafted for 12 wk to determine whether the numbers and cycling activity of the human HSCs produced by fetal liver transplants changes after plateau levels of other types of human hematopoietic cells are established. The results of limiting dilution secondary transplant assays of cells from 12-wk–engrafted primary mice are shown in the right part of Fig. 2 . It can be seen that there was only a modest (threefold) further increase in the absolute numbers of human HSCs detectable in the primary mice between 3 and 12 wk after transplant. Also, by 12 wk after transplant, an injection of 5-FU into these mice had no effect (P > .05) on human HSC detection (1 negative secondary mouse of 16 injected with either control or 5-FU–treated cells, pooled results from five experiments). In contrast, there were significantly reduced numbers of other types of primitive human cells present in the same cell suspensions from the 5-FU–treated mice (Table I). These latter findings are consistent with previous [3H]thymidine studies of the human LTC-ICs and CFCs regenerated in long-term human cord blood-engrafted mice that also showed the human LTC-ICs and CFCs to remain in cycle for at least 12 wk after transplant (25). The continuing sensitivity of these human fetal liver–derived progenitors to 5-FU also serves as an internal positive control for the lack of effect of the 5-FU treatment on the human HSCs present in the same mice.
SDF-1 or TGF-β1 Administration Markedly and Specifically Enhances the Detection of Proliferating Human HSCs in NOD/SCID Mice Transplanted with Human Fetal Liver Cells.
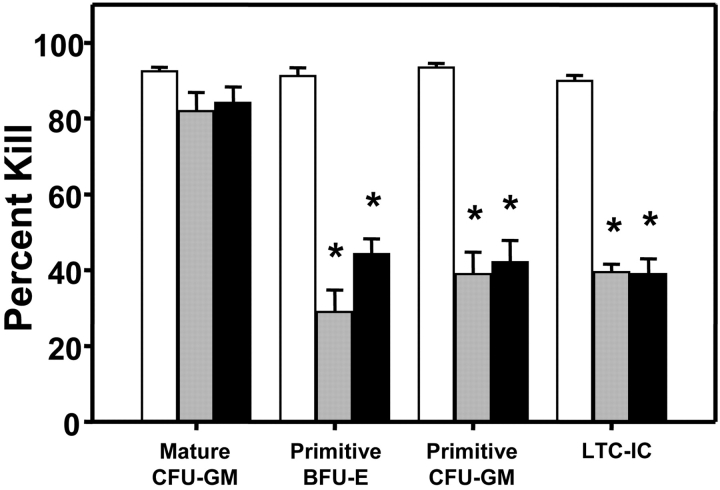
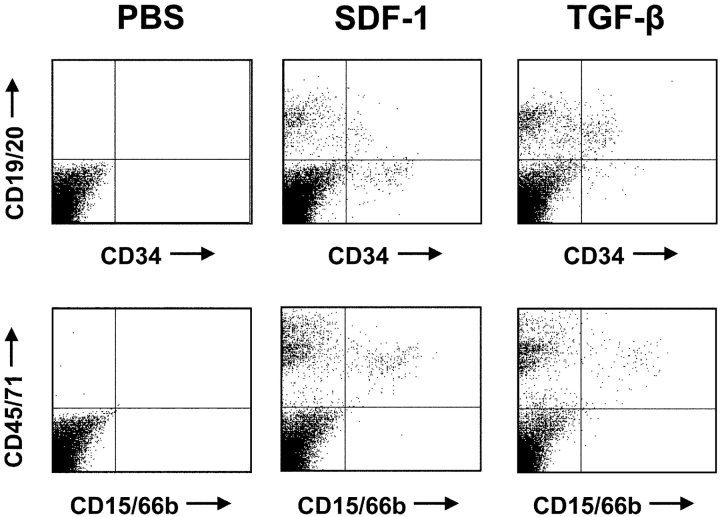
In a next series of 11 experiments, we asked whether inhibition of human HSC cycling in vivo might enhance their detection and if so, was the effect seen associated with an effect on human HSC cycling activity. As candidate inhibitors, TGF-β1 and SDF-1 were chosen. These were administered as two daily injections (TGF-β1 at 1 μg per injection per mouse and SDF-1 at 10 μg per injection per mouse) into mice that had been transplanted 3 wk previously with 107 human fetal liver cells. The doses of TGF-β1 and SDF-1 used were based on previous evidence of their effectiveness in inhibiting the proliferation (without affecting the number) of human LTC-ICs and primitive (but not mature) human CFCs in the marrow of NOD/SCID mice engrafted with human cord blood HSCs (25, 26). In seven experiments, the human HSC activity in the cells harvested from the primary mice was then assessed 1 d later by transfer into secondary NOD/SCID mice. As shown in Fig. 3 , both agents had a similar ability to significantly (P < .001) block the proliferation of the human LTC-ICs and primitive (but not mature) CFCs regenerated in human fetal liver–engrafted NOD/SCID mice. Note that there was no effect of either TGF-β1 or SDF-1 on the number of any of these progenitors detected (in the absence of [3H]thymidine, unpublished data). This indicates that the cytostatic effects seen on these target cell populations were not complicated by any evidence of toxicity. Strikingly, as shown in Fig. 4 , there was a dramatic enhancement (∼40-fold) in the number of HSCs detected in the bone marrow of the primary mice that were injected with either TGF-β1 or SDF-1 as determined from their quantitation by limiting dilution assays in secondary mice. Examples of the markedly different levels of engraftment obtained in secondary recipients of similar aliquots of cells harvested from the bone marrow of untreated as compared with TGF-β1 or SDF-1–treated primary mice are shown in Fig. 5 . Similar results were obtained in a further series of four experiments in which the cells were not harvested from the primary mice until 3 d after the injection of either TGF-β1 or SDF-1, demonstrating that the enhancing effect was durable for at least 3 d.
Figure 3.
TGF-β1 and SDF-1 administration in vivo arrests the cycling of primitive (but not mature) human CFCs and human LTC-ICs regenerating in the marrow of primary NOD/SCID recipients of 107 low-density human fetal liver cells. 18 to 21 d after transplant, primary mice were given two intraperitoneal injections, 24 h apart, of 1 μg of TGF-β1 (gray bars) or 10 μg of SDF-1 (black bars), or an equivalent volume of PBS (white bars) and 2 d later the marrow cells from both femurs and tibias of all mice in the same group (6 to 8 per group in each experiment) were pooled and then exposed (or not) to [3H]thymidine for 16 h in vitro before plating in assays for human CFCs and LTC-ICs. Values shown are the mean ± SEM of the results obtained from a total of six experiments. Data marked with asterisks are significantly different from the corresponding control values (P < .001).
Figure 5.
FACS® profiles of the human cells regenerated in the marrow of secondary NOD/SCID recipients. The proportions of human lymphoid (top row) and myeloid (bottom row) cells are shown in the secondary recipients of marrow from primary NOD/SCID mice injected with PBS (left profiles), SDF-1 (middle profiles), or TGF-β (right profiles). All primary mice were injected with the same innoculum of human fetal liver cells from one of the experiments depicted in Fig. 4.
TGF-β is a well known proliferation inhibitor of many cell types including primitive human hematopoietic cells (24, 25, 27, 32). Human HSCs are known to be responsive to SDF-1 (33, 34), but the potential of SDF-1 to act as an inhibitor of their cycling status is less well studied, although we recently reported evidence for such a role on closely related cell types (26). Therefore it was of interest to determine whether the effect of SDF-1 on HSC detection was associated with an effect on HSC proliferation in the same model.
To examine this possibility, the same experimental design was used but all 3-wk–engrafted primary mice were injected with SDF-1 and then, before transferring the cells into secondary recipients, a [3H]thymidine suicide assay was performed. The similar levels of engraftment obtained in secondary recipients of either control or [3H]thymidine-treated cells are shown in Fig. 6 . Calculation of the HSC values derived from these results show that the percentage of HSCs killed by the exposure to [3H]thymidine was negligible (6%). These clearly establish that SDF-1 pretreatment of human HSCs proliferating in vivo caused their arrest in Go, as none entered S-phase during a subsequent period of incubation for 16 h in vitro in the presence of SF, IL-3, and G-CSF. These findings stand in marked contrast to the significant reduction in HSC activity obtained when the primary mice were not first given SDF-1 (see Fig. 1).
Figure 6.
In vivo treatment of primary mice with SDF-1 results in similar levels of engraftment in secondary recipients of either control or [3H]thymidine-treated cells. 18 to 21 d after transplant, human fetal liver–engrafted primary mice were given two intraperitoneal injections, 24 h apart, of 10 μg of SDF-1 and 2 d later the marrow cells from both femurs and tibias of all mice in each experiment (7–9 mice per experiment, four experiments) were pooled and then incubated with [3H]thymidine (filled symbols) or medium (open symbols) for 16 h in vitro. The cells were then harvested and equal aliquots of each pool injected into secondary NOD/SCID mice (25–.33 primary mouse marrow equivalents per secondary mouse). Levels of different types of human cells detected in the secondary mice were determined 6–8 wk later.
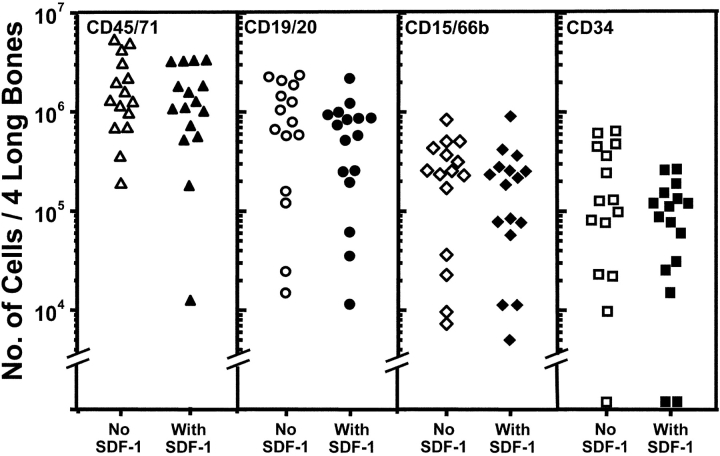
In a final series of experiments, we asked whether the in vivo SDF-1 treatment might have an independent effect on the ability of noncycling human HSCs to engraft NOD/SCID mice. Accordingly, additional primary mice that had been engrafted with human fetal liver cells for 12 wk were injected with SDF-1 (two daily doses of 10 μg/injection/mouse) or PBS (as a negative control) and the human HSC activity detectable 1 d later was then compared by transplanting equal proportions of bone marrow from the primary mice into secondary mice. All secondary mice showed regeneration of multilineage human hematopoiesis 6 to 8 wk later (i.e., 15 of 15 recipients of control cells and 16 of 16 recipients of cells from SDF-1–treated mice in a total of four experiments) and, as shown in Fig. 7 , for each type of human cell monitored, there was no difference in the level of engraftment obtained in the secondary recipients of control versus SDF-1–treated cells.
Figure 7.
In vivo administration of SDF-1 does not alter the ability of quiescent human HSCs in primary NOD/SCID mice to engraft secondary mice. Primary mice transplanted with human fetal liver cells 12 wk previously were injected on two successive days with 10 μg of SDF-1 or an equal volume of PBS and then killed 1 d later (six animals per group, four experiments). Equal aliquots from each group were transplanted into secondary mice (.38 primary mouse marrow equivalents per secondary mouse) and after a further 6 to 8 wk the number of different types of human cells in the four hind leg bones of the secondary recipients of SDF-1 (filled symbols) or PBS (open symbols) treated primary marrow cells was determined.
Discussion
The ability of intravenously injected human hematopoietic cells to home into the marrow of sublethally irradiated NOD/SCID mice and initiate multilineage hematopoiesis in this xenogeneic microenvironment has been instrumental to the development of an experimental model for characterizing the properties and in vivo behavior of transplantable human HSCs. This has included the demonstration in engrafted primary mice of human cells able to initiate multilineage hematopoiesis upon transfer to secondary recipients. Indeed, in some instances, larger numbers of such cells can be detected in the repopulated primary mice than were originally injected into them (14). These observations have suggested that human HSCs also generate progeny HSCs when they are stimulated to divide and differentiate in the marrow of the engrafted murine host. However, this type of experimental design cannot exclude the possibility that some might be cells with multilineage potential that were already present in the original innoculum but did not produce detectable progeny within the first 6–8 wk in the primary NOD/SCID hosts. Such a possibility is supported by the recent finding that CD34+ human cells with engrafting ability can be produced in vitro from CD34− cells that do not, themselves, generate mature progeny in vivo in this time frame (35, 36) and that, even in NOD/SCID mice, the human lympho-myelopoiesis seen during the first 3 to 4 wk is largely due to a more mature subset of human repopulating cells than those whose progeny appear later (17, 37, 38).
Here we provide direct evidence that the initially regenerated human HSCs (defined by a 6–8-wk multilineage repopulation read-out in secondary NOD/SCID mice) are actively dividing cells that have either acquired or retain the properties of transplantable HSCs. This was demonstrated using two different strategies that specifically target proliferating cell populations (exposure to high specific activity [3H]thymidine in vitro or 5-FU in vivo). Interestingly, we found that this proliferative activity was markedly down-regulated in the mice 9 wk later, although presumably remaining at a level just sufficient to balance the daily loss of HSCs into the pool of more differentiated progenitors since the total size of the human HSC compartment did not decrease. The lack of effect of 5-FU on human HSCs present in 12–wk–engrafted mice in contrast to the marked decrease seen in 3-wk–engrafted mice demonstrates the power of this simple strategy for future assessment of agents/conditions that can alter human HSC cycling in vivo.
Our studies also add to a growing body of evidence that the number of human HSCs detected after their transplantation into NOD/SCID mice can vary markedly according to their recent cytokine exposure history (21, 23, 39). Here we show a dramatic increase in human HSC detection can be obtained after treatment of engrafted mice with either SDF-1 or TGF-β1 under certain conditions. The increase seen is much larger than the two- to threefold effect anticipated if previously undetectable S/G2/M-phase HSCs had simply been forced to accumulate in G1 over a 2 to 3-d period. In fact, others have shown that human HSCs already begin to lose their ability to engraft upon exit from Go into G1 (21). This indicates that cells with the proliferative and differentiative potential of HSCs can be greatly underestimated by in vivo assays that depend on the integrity of their marrow homing efficiency. Conversely, if proliferating HSCs are forced to reenter Go, as indicated here by the acquired [3H]thymidine insensitivity of HSCs from mice treated with SDF-1 3 wk after transplant, then large increases in HSC detection could be obtained. Our finding that SDF-1 treatment of already quiescent HSCs (in 12-wk–engrafted mice) has no effect on their transplantability provides strong support for the concept that the effect seen on proliferating HSCs is related to the ability of SDF-1 to force their return to a Go state. More detailed characterization of the mechanism(s) responsible will clearly command attention in future studies. Involvement of various molecules implicated in HSC homing, including CXCR4, the receptor for SDF-1 and β1integrins (33, 40–43) are obvious candidates for investigation. However, regardless of the mechanisms underlying the dramatic effects of SDF-1 and TGF-β1 on proliferating human HSCs, these findings underscore the importance of cycling status on the ability of these cells to be detected or used effectively for transplantation applications. These issues will be particularly relevant to the future optimization of protocols for the clinical transplantation of HSCs to be cultured ex vivo or procured from patients that have been given treatments that activate as well as mobilize HSCs.
Acknowledgments
The authors thank Dr. Hanno Glimm (Terry Fox laboratory, Vancouver, BC) for helpful discussions, Dr. Peter Lansdorp (Terry Fox Laboratory), Cangene (Mississauga, ON), Novartis (Basel, Switzerland), and StemCell Technologies, Inc. (Vancouver, BC) for gracious gifts of reagents, and Amy Ahamed for assistance in manuscript preparation.
This work was supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run and the Canadian Cancer Society, P01 55435 (National Heart, Lung and Blood Institute), and the Canadian Institutes of Health Research grant 36346. B. Dykstra held an Industrial Studentship from the National Science and Engineering Research Council of Canada and C.J. Eaves was a Terry Fox Cancer Research Scientist of the NCIC.
Footnotes
Abbreviations used in this paper: CFC, colony-forming cell; 5-FU, 5-fluorouracil; HSC, hematopoietic stem cell; LTC-IC, long-term culture-initiating cell; NOD, nonobese diabetic; PI, propidium iodide; SDF-1, stromal-derived factor 1.
References
- 1.Sutherland, H.J., P.M. Lansdorp, D.H. Henkelman, A.C. Eaves, and C.J. Eaves. 1990. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc. Natl. Acad. Sci. USA. 87:3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punzel, M., S.D. Wissink, J.S. Miller, K.A. Moore, I.R. Lemischka, and C.M. Verfaillie. 1999. The myeloid-lymphoid initiating cell (ML-IC) assay assesses the fate of multipotent human progenitors in vitro. Blood. 93:3750–3756. [PubMed] [Google Scholar]
- 3.Hogge, D.E., P.M. Lansdorp, D. Reid, B. Gerhard, and C.J. Eaves. 1996. Enhanced detection, maintenance and differentiation of primitive human hematopoietic cells in cultures containing murine fibroblasts engineered to produce human Steel factor, interleukin-3 and granulocyte colony-stimulating factor. Blood. 88:3765–3773. [PubMed] [Google Scholar]
- 4.Berardi, A.C., E. Meffre, F. Pflumio, A. Katz, W. Vainchenker, C. Schiff, and L. Coulombel. 1997. Individual CD34+CD38lowCD19−CD10− progenitor cells from human cord blood generate B lymphocytes and granulocytes. Blood. 89:3554–3564. [PubMed] [Google Scholar]
- 5.Miller, J.S., C. Verfaillie, and P. McGlave. 1992. The generation of human natural killer cells from CD34+DR- primitive progenitors in long-term bone marrow culture. Blood. 80:2182–2187. [PubMed] [Google Scholar]
- 6.Lapidot, T., F. Pflumio, M. Doedens, B. Murdoch, D.E. Williams, and J.E. Dick. 1992. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 255:1137–1141. [DOI] [PubMed] [Google Scholar]
- 7.Cashman, J.D., T. Lapidot, J.C.Y. Wang, M. Doedens, L.D. Shultz, P. Lansdorp, J.E. Dick, and C.J. Eaves. 1997. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood. 89:4307–4316. [PubMed] [Google Scholar]
- 8.Conneally, E., J. Cashman, A. Petzer, and C. Eaves. 1997. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc. Natl. Acad. Sci. USA. 94:9836–9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robin, C., F. Pflumio, W. Vainchenker, and L. Coulombel. 1999. Identification of lymphomyeloid primitive progenitor cells in fresh human cord blood and in marrow of nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice transplanted with human CD34+ cord blood cells. J. Exp. Med. 189:1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanjani, E.D., G. Almeida-Porada, A.G. Livingston, A.W. Flake, and M. Ogawa. 1998. Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp. Hematol. 26:353–360. [PubMed] [Google Scholar]
- 11.Cashman, J.D., and C.J. Eaves. 2000. High marrow seeding efficiency of human lymphomyeloid repopulating cells in irradiated NOD/SCID mice. Blood. 96:3979–3981. [PubMed] [Google Scholar]
- 12.Glimm, H., W. Eisterer, K. Lee, J. Cashman, T.L. Holyoake, F. Nicolini, L.D. Schultz, C. von Kalle, and C.J. Eaves. 2001. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-β2 microglobulin-null mice. J. Clin. Invest. 107:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolini, F.E., T.L. Holyoake, J.D. Cashman, P.P.Y. Chu, K. Lambie, and C.J. Eaves. 1999. Unique differentiation programs of human fetal liver stem cells revealed both in vitro and in vivo in NOD/SCID mice. Blood. 94:2686–2695. [PubMed] [Google Scholar]
- 14.Holyoake, T.L., F.E. Nicolini, and C.J. Eaves. 1999. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp. Hematol. 27:1418–1427. [DOI] [PubMed] [Google Scholar]
- 15.Hogan, C.J., E.J. Shpall, O. McNulty, I. McNiece, J.E. Dick, L.D. Shultz, and G. Keller. 1997. Engraftment and development of human CD34+-enriched cells from umbilical cord blood in NOD/LtSz-scid/scid mice. Blood. 90:85–96. [PubMed] [Google Scholar]
- 16.Cashman, J.D., and C.J. Eaves. 1999. Human growth factor-enhanced regeneration of transplantable human hematopoietic stem cells in nonobese diabetic/severe combined immunodeficient mice. Blood. 93:481–487. [PubMed] [Google Scholar]
- 17.Guenechea, G., O.I. Gan, C. Dorrell, and J.E. Dick. 2001. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat. Immunol. 2:75–82. [DOI] [PubMed] [Google Scholar]
- 18.Becker, A.J., E.A. McCulloch, L. Siminovitch, and J.E. Till. 1965. The effect of differing demands for blood cell production on DNA synthesis by hemopoietic colony-forming cells of mice. Blood. 26:296–308. [PubMed] [Google Scholar]
- 19.Ponchio, L., E. Conneally, and C. Eaves. 1995. Quantitation of the quiescent fraction of longterm culture-initiating cells (LTC-IC) in normal human blood and marrow and the kinetics of their growth factor-stimulated entry into S-phase in vitro. Blood. 86:3314–3321. [PubMed] [Google Scholar]
- 20.Hodgson, G.S., and T.R. Bradley. 1979. Properties of hematopoietic stem cells surviving 5-fluorouracil treatment: Evidence for a pre-CFU-S cell? Nature. 281:381–382. [DOI] [PubMed] [Google Scholar]
- 21.Gothot, A., J.C.M. Van der Loo, W. Clapp, and E.F. Srour. 1998. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34+ cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 92:2641–2649. [PubMed] [Google Scholar]
- 22.Habibian, H.K., S.O. Peters, C.C. Hsieh, J. Wuu, K. Vergilis, C.I. Grimaldi, J. Reilly, J.E. Carlson, A.E. Frimberger, F.M. Stewart, and P.J. Quesenberry. 1998. The fluctuating phenotype of the lympho-hematopoietic stem cell with cell cycle transit. J. Exp. Med. 188:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glimm, H., I. Oh, and C. Eaves. 2000. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G2/M transit and do not reenter Go. Blood. 96:4185–4193. [PubMed] [Google Scholar]
- 24.Fortunel, N.O., A. Hatzfeld, and J.A. Hatzfeld. 2000. Transforming growth factor-β: pleiotropic role in the regulation of hematopoiesis. Blood. 96:2022–2036. [PubMed] [Google Scholar]
- 25.Cashman, J.D., I. Clark-Lewis, A.C. Eaves, and C.J. Eaves. 1999. Differentiation stage-specific regulation of primitive human hematopoietic progenitor cycling by exogenous and endogenous inhibitors in an in vivo model. Blood. 94:3722–3729. [PubMed] [Google Scholar]
- 26.Cashman, J., I. Clark-Lewis, A. Eaves, and C. Eaves. 2002. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 99:792–799. [DOI] [PubMed] [Google Scholar]
- 27.Dao, M.A., N. Taylor, and J.A. Nolta. 1998. Reduction in levels of the cyclin-dependent kinase inhibitor p27kip-1 coupled with transforming growth factor β neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells. Proc. Natl. Acad. Sci. USA. 95:13006–13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishikawa, S.-I., S. Nishikawa, H. Kawamoto, H. Yoshida, M. Kizumoto, H. Kataoka, and Y. Katsura. 1998. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 8:761–769. [DOI] [PubMed] [Google Scholar]
- 29.Crump, M.P., J.-H. Gong, P. Loetscher, K. Rajarathnam, A. Amara, F. Arenzana-Seisdedos, J.-L. Virelizier, M. Baggiolini, B.D. Sykes, and I. Clark-Lewis. 1998. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 16:6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggs, D.R. 1984. The total marrow mass of the mouse: a simplified method of measurement. Am. J. Hematol. 16:277–286. [DOI] [PubMed] [Google Scholar]
- 31.Cashman, J., A.C. Eaves, and C.J. Eaves. 1985. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 66:1002–1005. [PubMed] [Google Scholar]
- 32.Fan, X., G. Valdimarsdottir, J. Larsson, A. Brun, M. Magnusson, S.E. Jacobsen, P. ten Dijke, and S. Karlsson. 2002. Transient disruption of autocrine TGF-beta signaling leads to enhanced survival and proliferation potential in single primitive human hemopoietic progenitor cells. J. Immunol. 168:755–762. [DOI] [PubMed] [Google Scholar]
- 33.Peled, A., I. Petit, O. Kollet, M. Magid, T. Ponomaryov, T. Byk, A. Nagler, H. Ben-Hur, A. Many, L. Shultz, et al. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 283:845–848. [DOI] [PubMed] [Google Scholar]
- 34.Rosu-Myles, M., L. Gallacher, B. Murdoch, D.A. Hess, M. Keeney, D. Kelvin, L. Dale, S.S.G. Ferguson, D. Wu, F. Fellows, and M. Bhatia. 2000. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc. Natl. Acad. Sci. USA. 97:14626–14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia, M., D. Bonnet, B. Murdoch, O.I. Gan, and J. Dick. 1998. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat. Med. 4:1038–1045. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura, Y., K. Ando, J. Chargui, H. Kawada, T. Sato, T. Tsuji, T. Hotta, and S. Kato. 1999. Ex vivo generation of CD34+ cells from CD34− hematopoietic cells. Blood. 94:4053–4059. [PubMed] [Google Scholar]
- 37.Kerre, T.C., G. De Smet, M. De Smedt, F. Offner, J. De Bosscher, J. Plum, and B. Vandekerckhove. 2001. Both CD34+38+ and CD34+38− cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J. Immunol. 167:3692–3698. [DOI] [PubMed] [Google Scholar]
- 38.Hogan, C.J., E.J. Shpall, and G. Keller. 2002. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc. Natl. Acad. Sci. USA. 99:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebel, V.I., M. Tanaka, J.-S. Lee, S. Hartnett, M. Pulsipher, D.G. Nathan, R.C. Mulligan, and C.A. Sieff. 1999. One-day ex vivo culture allows efficient gene transfer into human nonobese diabetic/severe combined immune-deficient repopulating cells using high-titer vesicular stomatitis virus G protein pseudotyped retrovirus. Blood. 93:2217–2224. [PubMed] [Google Scholar]
- 40.Papayannopoulou, T., C. Craddock, B. Nakamoto, G.V. Priestley, and N.S. Wolf. 1995. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc. Natl. Acad. Sci. USA. 92:9647–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermeulen, M., F. Le Pesteur, M.-C. Gagnerault, J.-Y. Mary, F. Sainteny, and F. Lepault. 1998. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 92:894–900. [PubMed] [Google Scholar]
- 42.Peled, A., V. Grabovsky, L. Habler, J. Sandbank, F. Arenzana-Seisdedos, I. Petit, H. Ben-Hur, T. Lapidot, and R. Alon. 1999. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Invest. 104:1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potocnik, A.J., C. Brakebusch, and R. Fassler. 2000. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 12:653–663. [DOI] [PubMed] [Google Scholar]