Abstract
Secretion of proteases is critical for degradation of the extracellular matrix during an inflammatory response. Cathepsin (Cat) S and L are the major elastinolytic cysteine proteases in mouse macrophages. A 65 amino acid segment of the p41 splice variant (p4165aa) of major histocompatibility complex class II–associated invariant chain (Ii) binds to the active site of CatL and permits the maintenance of a pool of mature enzyme in endosomal compartments of macro-phages and dendritic cells (DCs). Here we show that interaction of p4165aa with mature CatL allows extracellular accumulation of the active enzyme. We detected mature CatL as a complex with p4165aa in culture supernatants from antigen-presenting cells (APCs). Extracellular accumulation of mature CatL is up-regulated by inflammatory stimuli as observed in interferon (IFN)-γ–treated macrophages and lipopolysaccharide (LPS)-activated DCs. Despite the neutral pH of the extracellular milieu, released CatL associated with p4165aa is catalytically active as demonstrated by active site labeling and elastin degradation assays. We propose that p4165aa stabilizes CatL in the extracellular environment and induces a local increase in the concentration of matrix-degrading enzymes during inflammation. Through its interaction with CatL, Ii may therefore control the migratory response of APCs and/or the recruitment of effectors of the inflammatory response.
Keywords: protease, invariant chain, secretion, antigen-presenting cells, extracellular matrix degradation
Introduction
Lysosomal proteases of the cathepsin (Cat) family play a critical role in antigen processing and in the formation of peptide-receptive dimers during MHC class II–restricted antigen presentation (1, 2). These enzymes are furthermore involved in the remodeling of extracellular matrix proteins (3). Destruction of elastin-rich tissue during inflammatory responses is associated with a local accumulation of macrophages that contain high levels of a variety of elastinolytic enzymes. These cells secrete metallo-, serine-, and cysteine proteases into extracellular space to degrade matrix components. As elastin is resistant to most forms of proteolytic attack (4, 5), both the sources and the regulation of the enzymes involved in its dissolution are of major interest.
Major elastinolytic cysteine proteases of the cathepsin family are CatS, CatK, and CatL. The expression of CatK in mouse macrophages is still a matter of debate. CatS and CatL are synthesized as inactive proenzymes (3, 6), a feature shared by most lysosomal hydrolases. For the different cathepsins, the propiece is located at the NH2 terminus and can vary in size. The role of the propiece in the maturation from precursor to active protease resembles the role of invariant chain (Ii) in MHC class II maturation. Ii serves as a chaperone to facilitate the folding and assembly of class II αβ dimers, addresses them to the endocytic pathway, and blocks their antigen binding site, thus preventing their premature interaction with endogenous substrates (1, 2, 7). Similarly, the occupancy of the enzyme active site by the propiece stabilizes the protease conformation at neutral pH (3, 8). In the endocytic pathway, the drop in pH promotes removal of the propiece and proteolytic conversion to the active, mature protease. With the exception of CatS, which is so far thought to be unique in its ability to survive and maintain enzymatic activity at neutral pH (9, 10), most endosomal hydrolases are unstable under such conditions (3). While many cell types secrete cathepsins only as proforms, activated macrophages have been reported to secrete mature enzymes (11). How mature forms of proteases survive in the extracellular space is an important point that remains to be resolved.
The CatL proregion consists of 96 amino acids (12). The mature active forms of CatL are the single-chain (30 kD) and the two-chain forms, composed of a 24/25 kD heavy-chain linked to a 5 kD light-chain by disulfide bonds (3, 6). The CatL two-chain forms are prevalent in cells from the monocytic lineage. The protein and activity levels of this mature form of CatL are controlled by MHC class II–associated Ii in APCs (13).
In mice, Ii is synthesized as two major isoforms, p31 and p41, which are distinguished by alternative splicing of Ii transcripts (14, 15). Mice that express either p31 or p41 have been extensively characterized (13, 16, 17). The p41 isoform contains an extra 65 amino acid segment (p4165aa) that resembles a cysteine-rich thyroglobulin type-1 domain. Both Ii isoforms function as chaperones for class II folding and intracellular trafficking (16, 17). P31 and p41 are expressed in different ratios in the various types of APCs. Whereas p41 represents only 10% of the total pool of Ii in splenocytes, its expression level is considerably higher in macrophages, dendritic cells (DCs) and Langerhans cells (18, 19). P4165aa was found noncovalently bound to the mature form of CatL purified from human kidney (20). The x-ray crystallography analysis of the CatL/p4165aa complex revealed that the fragment occupies the active site of the enzyme in the manner of a competitive inhibitor (21). We found that the levels of mature CatL are considerably decreased in cells that lack p41. Therefore we proposed that p41 stabilizes the conformation of mature CatL by binding to its active site, thus serving as a chaperone to help maintain a pool of mature enzyme in endocytic compartments of APCs (13).
Interaction of active, endocytic proteases with competitive inhibitors has been proposed as a mechanism to allow their survival in extracellular space. Such a scenario was invoked for CatK for which a stable, secretory complex with Cystatin C (CysC) was described (22). Unlike free mature CatL, the enzyme complexed to p41 can survive in a neutral pH environment (20). We therefore investigated the possibility that p4165aa promotes extracellular accumulation of active mature CatL released from APCs.
Materials and Methods
Mice.
C57BL/6 and Ii−/− mice were purchased from the The Jackson Laboratory. CatL−/−, p31, and p41 mice have been described (10, 13, 16, 17, 23).
Preparation of Bone Marrow–derived APCs.
Bone marrow from 2–4 mo mice were cultured in RPMI 1640/10% FCS/10 ng/ml rmGM-CSF (PeproTech). Macrophages were harvested at day 5 (13) and activated with 100 U/ml rmIFN-γ for 2 d. To isolate pure DCs, magnetic cell sorting with anti-CD11c beads was performed (MACS; Miltenyi Biotec). To control proper separation, anti-CD11c or anti-I-Ab (BD Biosciences) FACScan™ analysis (Becton Dickinson) was performed. DCs were activated with LPS (1 μg/ml) overnight.
Preparation of Cell Culture Supernatants.
Macrophages and DCs were incubated overnight in FCS-free RPMI/GM-CSF. Supernatants were harvested, centrifuged (5 min, 200 g) and analyzed. The pH was monitored throughout the experiments and was close to pH7, unless modified with citric acid.
Gel Filtration.
Experiments were performed to preserve CatL/p41 interaction (20). Ammonium sulfate precipitation was performed from supernatant of ∼107 cells. Precipitate was resuspended in 200 μl 50 mM sodium acetate, pH 5.0/1 mM EDTA. Sample were fractionated on a Superdex 75 column HR 10/30 (Amersham Biosciences). Immunoblots with anti-CatL and anti-p41 sera were performed on each fraction (60 μl).
Antibodies and Immunoblotting.
Experiments were performed as described (13). Reagents were from A. Erickson (anti-CatL) and E. Weber (anti-CatD and S). The JV7 serum is directed against a peptide in the p4165aa fragment (13).
Active Site Labeling.
DCG-04 was synthesized as described (24, 25). PH was adjusted with citric acid (pH 3.0) to a range from 4.5–6.5. 50 μl supernatant was incubated with DCG-04 (final concentration 10 μM) for 1 h at 37°C. Reactivity of DCG-04 with active cysteine proteases was detected as described (13).
Elastin Degradation Assays.
Elastinolytic activity in supernatants was analyzed with Elastin-Remazol microspheres (Elastin Products Co.) according to the manufacturers' protocol. 50 μl of an Elastin-Remazol suspension (10 mg/ml in PBS/EDTA) was reacted with 100 μl of fivefold concentrated supernatants for 8 h at 37°C. After centrifugation, 100 μl was reacted with 50 μl of 0.2 M Tris, pH 8.8. Optical density (OD) was measured at 590 nm. Incubation of the Elastin-Remazol suspension with medium alone was used to define the background. All experiments were performed in the presence of EDTA and PMSF (100 μM). In selected experiments, DCG-04 (10 μM) was added.
Results
P41-Ii Promotes Accumulation of Extracellular Mature CatL in Macrophage Supernatants.
The p41 isoform of Ii maintains a pool of mature enzyme in endocytic compartments of bone marrow-derived macrophages by means of a direct interaction (13, 20; Fig. 1 A). What functional role does this interaction between CatL and p41 play in APCs? One possibility is that CatL bound to p41 is packaged into vesicles of endosomal origin and released into extracellular space since, unlike free mature CatL, this complex can survive at neutral pH. We analyzed supernatants from IFN-γ–treated macrophages of WT, CatL (CatL−/−), and Ii knockout (Ii−/−) mice for the presence of CatL. Supernatants from cells that contain only the p31 or only the p41 splice variant of Ii were investigated (referred to as p31 and p41). Expression of proCatL is independent of p41, and anti-CatL immunoblotting experiments indeed showed presence of equal amounts of CatL zymogen in supernatants from all strains (Fig. 1 B). In contrast, the secreted 24 kD mature form of CatL was detected only in supernatants from WT and p41 macrophages, but not in Ii−/− or p31-derived samples (Fig. 1 B). As all supernatants contain large amounts of proCatL, extracellular activation of proforms can be ruled out as a mechanism for the generation of mature CatL. We performed an anti-CatS blot on the same supernatants and detected equal amounts of pro- and mature CatS in all samples (Fig. 1 B). Thus, accumulation of mature extracellular CatL is selectively regulated by the presence of p41. A comparison of supernatants prepared from untreated and stimulated macrophages shows the same pattern of accumulation, with considerably lower levels of mature CatL but larger amounts of proCatL in supernatants from untreated cells (Fig. 1 C).
Figure 1.
Intra- and extracellular CatL. (A) Intracellular expression of CatL and CatS. Exposure time was adjusted to visualize mature, but not proCatL. The mature-25 kD enzyme is present in all samples but CatL−/−. Mature-24 kD CatL is coexpressed with p41. CatS is evenly detected in all samples. (B) Analysis of macrophage supernatants. ProCatL is detected in supernatants from all mouse strains. Mature CatL is detected only in wt and p41supernatants, but is absent from Ii−/− or p31 samples. Pro- and mature CatS are detected evenly in all samples irrespective of Ii expression. (C) Comparison of supernatants from resting and activated macrophages. Accumulation of extracellular mature CatL is up-regulated by IFN-γ.
The possibility that mature CatL in supernatants from wt or p41 mice is simply derived from cell leakage is ruled out by the comparative analysis for intracellular protease content. We detect intracellular proforms of CatL and the mature-25 kD CatL in all macrophages but the CatL−/− (Fig. 1 A). As expected, the expression of the 24 kD form of CatL was restricted to cells that express p41 (13). If cell leakage had occurred, it should have resulted in the detection of mature-25 kD CatL in supernatants from li−/− and p31 mice, as sufficient amounts are present inside the cells. We therefore conclude that p41 is required for detection of extracellular mature CatL.
Extracellular Accumulation of Mature CatL Is Up-regulated during Activation of DCs.
Do these observations extend to bone marrow–derived DCs? We show that DCs also require the presence of intracellular p41 for expression of mature-24 kD CatL (13; Fig. 2 A). In line with this result, we find that extracellular accumulation of mature CatL is impaired in DCs that lack p41 (Fig. 2 B). In addition, significantly larger amounts of mature-24 kD CatL are detected in supernatants collected from LPS-activated DCs (Fig. 2 B).
Figure 2.
Expression and release of CatL from resting and activated DCs. (A) Levels of intracellular CatL are slightly downregulated by DC activation. (B) Significantly larger amounts of mature CatL accumulate in supernatants from activated DCs. Reprobing of the membrane shows modest upregulation of proCatD secretion during activation.
Analysis of intracellular protease content of DCs shows that the pool of intracellular CatL is slightly downregulated by LPS (Fig. 2 A) and that more of the mature enzyme is found in the supernatant (Fig. 2 B). This suggests that the mechanism of release of mature CatL is specifically up-regulated during DC activation, consistent with a role for CatL secretion in the course of inflammation.
Mature CatL Accumulates in the Extracellular Space in Association with p4165aa.
To demonstrate that extracellular mature CatL is indeed associated with the p41 isoform of Ii, we analyzed supernatants from wt, CatL−/−, Ii−/−, p31, and p41 macrophages for the presence of p41. An anti-p4165aa serum-reactive protein fragment of ∼10 kD, corresponding to the size of the previously described p4165aa, is detected only in supernatants that contain mature CatL (Fig. 3 A). No full-length p41 is found extracellularly (data not depicted). Reprobing of the membrane with an anti-CatD serum shows the presence of equal amounts of proCatD in all samples (Fig. 3 A, top). The fact that p4165aa is detected only in 24 kD CatL-containing supernatants and vice versa strongly suggests that these two proteins were secreted together.
Figure 3.
Extracellular mature CatL is detected as a complex with p4165aa. (A) Mature CatL is detected only in supernatants that contain p4165aa and vice versa. (B) Gel filtration of the CatL/p4165aa complex. Mature CatL coelutes with p4165aa. A double exposure of a blot that was probed consecutively with anti-CatL and anti-p4165aa sera is shown. An unknown band of ∼16 kD reactive with anti-CatL serum was detected (*, 17–21).
To assess the presence of stable CatL/p4165aa complexes, supernatants from p41 cells were subjected to gel filtration. Subsequently, fractions were analyzed for the presence of CatL and p41. We observe perfect coelution of mature-24 kD CatL and p4165aa from the column, according to its Stokes' radius (Fig. 3 B, fractions 13–17). A protein fragment whose size (∼16 kD) exceeds that of p4165aa elutes later than the CatL complex (Fig. 3 B, asterisk), showing that p4165aa must be associated with CatL to elute at this position. These results show that mature CatL detected in supernatants from APC cultures is associated with p4165aa. Virtually the same results are obtained when gel filtration is performed with supernatants from WT cells (unpublished data).
Mature CatL is Accumulated in its Active Form.
Detection of secreted, mature CatL requires expression of p41 (Fig. 1, B and C, and Figs. 2 B and 3, A and B). Unlike the free mature enzyme, CatL complexed to p41 can survive at neutral pH (20). Thus, the instability of extracellular mature CatL might be overcome by its interaction with p4165aa. One would therefore predict that CatL bound to p4165aa maintains its enzymatic activity at neutral pH. To test this hypothesis, we conducted active site labeling experiments using DCG-04, a peptide epoxide derivative that recognizes cysteine proteases (24, 25), and covalently and irreversibly modifies their active site. The pH of the supernatants was modified with citric acid (pH 3.0) to a range from pH 6.5 to pH 4.5 before labeling (Fig. 4 A). Only supernatants from p41 mice show considerable amounts of active CatL at neutral pH (pH 7.5, Fig. 4 A). No such activity is detected in supernatants from p31 mice (Fig. 4 A). We detected active CatB and active CatS in all samples, according to their expected pH activity ranges and with their estimated MW (Fig. 4 A). As expected, CatL was more active at low pH in contrast to CatS, whose activity decreased under acidic conditions (Fig. 4 A). We conclude that p4165aa allows survival and activity of CatL at neutral pH, a condition so far considered as suboptimal for this enzyme.
Figure 4.
Extracellular mature CatL is active at neutral pH. (A) Active site labeling of macrophage supernatants with DCG-04 at different pH. Supernatants from p41 but not p31 mice contain active CatL. Active CatB and CatS were detected in all samples. (B) Mature active CatL is elastinolytic. Elastin-Remazol was incubated with macrophage supernatants at neutral pH. Enhanced elastinolytic activity is detected in supernatants from wt and p41 macrophages only. DCG-04 blocks elastinolytic activity. Experiments (n = 4) were performed in duplicates and are given as mean ± SEM.
Active CatL Is the Major Elastin-degrading Enzyme at Neutral pH.
We analyzed the ability of secreted active CatL to degrade elastin as a physiologically relevant substrate. Elastin microparticles coupled to remazol were incubated with macrophage supernatants at neutral pH. Degradation of this modified elastin results in release of remazol from the matrix, allowing a quantitative estimate of enzyme activity. Significant elastinolysis at neutral pH is observed only in supernatants from wt and p41 macrophages (Fig. 4 B). All experiments were performed in the presence of EDTA and PMSF which should inhibit matrix metallo- and serine proteases (amongst which is the major elastase, CatG), and therefore allows an assessment of the contribution of cysteine proteases to elastin degradation. Inclusion of DCG-04 in the experiment decreases elastinolytic activity significantly (Fig. 4 B), showing that we indeed measure the activity of a cysteine protease. Significantly lower elastinolytic activity of CatL−/− samples implies that neither matrix metalloproteases nor CatB and CatS, despite their elastin-destructive potential, contribute significantly in this assay (Fig. 4 B). This stresses the notion that CatL is a major active cysteine protease capable of degrading elastin in our experimental setting. Auto-activation of proCatL and conversion to the mature active enzyme could be expected over the 8-h interval of the assay. However, the moderate levels of elastinolytic activity observed in p31 samples indicate that this mechanism is of lesser importance, if it occurs at all. In summary, our data show that the CatL/p4165aa complex is a potentially active, complexed version of the enzyme that can process physiologic substrates at neutral pH, i.e., under conditions otherwise unfavorable for the free mature enzyme.
Discussion
The p41 splice variant of MHC class II-associated Ii contains a 65 aa segment that binds noncovalently to the active site of CatL (20). We have previously documented the in vivo significance of this CatL/p41 interaction by showing that, in bone marrow–derived APCs, the levels of active CatL are regulated by the presence of p41. The effect is specific for the mature two-chain form of CatL. In particular, the mature-24 kD form, prevalent in activated APCs, is lacking in cells that do not express p41 (13). In the present study we are able to suggest a specific function for this CatL/p41 complex. Indeed, we show that interaction of p41 with CatL selectively regulates its accumulation as a mature, active enzyme in the extracellular space of APCs. This is inherent in the ability of p4165aa to stabilize the conformation of CatL at neutral pH (20), therefore allowing its survival in the extracellular milieu. Activated APCs (i.e., IFN-γ–treated macrophages and LPS-activated DCs) secrete significantly more mature-24 kD CatL than resting cells, suggesting that secretion of the CatL/p4165aa complex can be modulated by inflammatory stimuli. In addition, supernatants that contain CatL/p4165aa display increased elastinolytic activity at neutral pH, pointing to CatL as a major elastin-degrading protease produced by APCs. Together, these data suggest that by controlling release of mature CatL, the Ii, a component of the MHC class II antigen presentation pathway, can regulate degradation of the extracellular matrix in the course of inflammation.
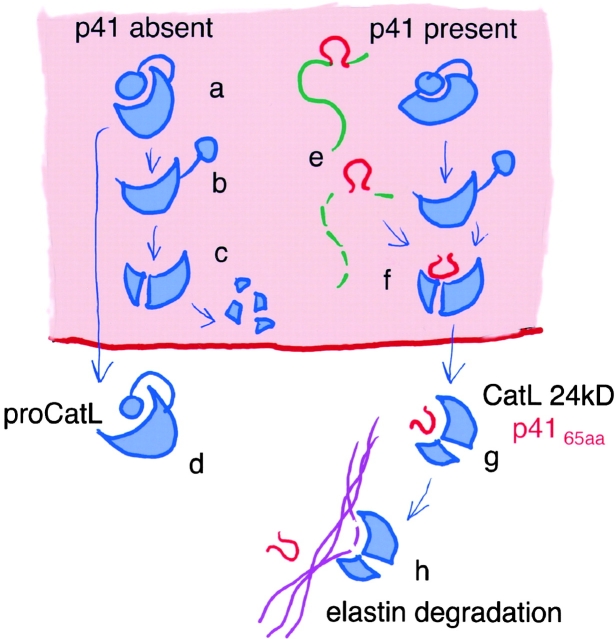
P4165aa allows the maintenance of a pool of latent active CatL in late endosomal compartments of APCs (13). This interaction stabilizes mature CatL and protects it from destruction by hydrolases. In the absence of p4165aa, the turnover of mature CatL in endosomal compartments is too fast to allow significant accumulation and subsequent secretion of the enzyme (see model; Fig. 5, a–c). This suggests that CatL binds to p4165aa. intracellularly and that both molecules are then secreted together as a stable complex (Fig. 5 g). This is supported by the fact that 24 kD CatL is detected neither inside nor outside APCs that lack p41, and that extracellular p4165aa does not accumulate in CatL-deficient cells. Once in the extracellular milieu, engagement of suitable substrates by the noncovalent CatL/p4165aa complex might displace the p4165aa fragment thus allowing substrate hydrolysis in a neutral pH environment (Fig. 5 h).
Figure 5.
Model for regulation of extracellular CatL activity by p41. (a–d) ProCatL can either be secreted or converted to mature CatL intracellularly, independent of Ii. The active, mature form CatL-24 kD is rapidly degraded when p41 is absent. (e and f) By interaction with the active site of CatL, the thyroglobulin domain of p41 protects the mature enzyme from degradation. (g and h) Protected mature CatL can then be secreted into the extracellular milieu in association with p4165aa. There, p4165aa bound to the active site of CatL can be displaced by a physiological substrate of the enzyme (e.g., elastin), which is subsequently hydrolyzed.
Our proposal for the function of p4165aa in secretion of CatL is conceptually similar to the situation described for CysC and CatK (22). CatK is found in the supernatants of human macrophages in association with its endogenous inhibitor, CysC, thereby fulfilling the stability requirements for mature CatK to survive at neutral pH. However, CatK requires an acidic pH for elastinolytic activity (22). In contrast, interaction of p4165aa with CatL does not only stabilize the protease, but extends the functional range of the enzyme to activity at neutral pH. Though unlikely, our experimental setting does not formally rule out the possibility that p4165aa interacts with CatL through an unidentified, third protein and thereby regulates CatL activity indirectly.
The physiological relevance of CatL secretion may be viewed in the context of cell migration, angiogenesis and inflammatory response (5). Of note, extracellular activity of CatL promotes tumor cell invasion, where massive secretion of the enzyme is often connected to poor prognosis (5). Angiogenesis is seen as an invasive process that requires controlled release and activity of extracellular proteases (26). In addition, CatL can generate the potent anti-angiogenic molecule endostatin and thus modulate tumor vascularization and expansion (27). Interestingly, expression of Ii has been connected to tumor progression (28). Whether this observation is linked to an increased ability of malignant cells to secrete CatL remains to be examined.
Soluble CatL could be involved in the destruction of elastin-rich tissue during the initiation of an inflammatory response. Indeed, secreted active CatL has been shown to play a role in degrading the extracellular matrix and to enhance migration of activated human macrophages (11). Increased secretion of mature CatL/p4165aa complex by activated APCs might contribute to matrix remodeling events during the inflammatory response. Macrophages can acidify their pericellular milieu to allow elastase activity in their immediate microenvironment (29). The secretion of the CatL/p4165aa complex active at neutral pH might allow more expansive destruction of elastin-rich tissue and at a greater distance. Degradation of the extracellular matrix could be important for the activated APC to migrate to lymph nodes to meet the appropriate T lymphocyte, as well as for the recruitment of effectors of inflammation, e.g., neutrophils and eosinophils. Therefore, future studies should focus on comparing the response and behavior of APCs from p31, p41, and CatL−/− mice in different models of inflammation.
Acknowledgments
The authors thank Christian Hirsch for assistance with the gel filtration experiments.
The authors are recipients of fellowships from the Austrian Academy of Sciences (Max Kade Fellowship; E. Fiebiger), Boehringer Ingelheim Fonds (R. Maehr), and the Juvenile Diabetes Foundation (A.M. Lennon-Duménil). This work was supported by grants from the Juvenile Diabetes Foundation International, through the JDF Center for Islet Transplantation at Harvard Medical School, from the National Institutes of Health (AI34893, CA14051, and AI19047), from Boehringer Ingelheim, and from the Human Frontiers Science Program to H.L. Ploegh and J. Villadangos.
References
- 1.Bryant, P.W., A.M. Lennon-Dumenil, E. Fiebiger, C. Lagaudriere-Gesbert, and H.L. Ploegh. 2002. Proteolysis and antigen presentation by MHC class II molecules. Adv. Immunol. 80:71–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennon-Dumenil, A.M., A.H. Bakker, P. Wolf-Bryant, H.L. Ploegh, and C. Lagaudriere-Gesbert. 2002. A closer look at proteolysis and MHC-class-II-restricted antigen presentation. Curr. Opin. Immunol. 14:15–21. [DOI] [PubMed] [Google Scholar]
- 3.McGrath, M.E. 1999. The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 28:181–204. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet, P., L. Moons, R. Lijnen, M. Baes, V. Lemaitre, P. Tipping, A. Drew, Y. Eeckhout, S. Shapiro, F. Lupu, and D. Collen. 1997. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 17:439–444. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, H.A., R.J. Riese, and G.P. Shi. 1997. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 59:63–88. [DOI] [PubMed] [Google Scholar]
- 6.Erickson, A.H. 1989. Biosynthesis of lysosomal endopeptidases. J. Cell. Biochem. 40:31–41. [DOI] [PubMed] [Google Scholar]
- 7.Villadangos, J.A., and H.L. Ploegh. 2000. Proteolysis in MHC class II antigen presentation: who's in charge? Immunity. 12:233–239. [DOI] [PubMed] [Google Scholar]
- 8.Chapman, H.A. 1998. Endosomal proteolysis and MHC class II function. Curr. Opin. Immunol. 10:93–102. [DOI] [PubMed] [Google Scholar]
- 9.Bromme, D., A. Steinert, S. Friebe, S. Fittkau, B. Wiederanders, and H. Kirschke. 1989. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem. J. 264:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi, G.P., J.S. Munger, J.P. Meara, D.H. Rich, and H.A. Chapman. 1992. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J. Biol. Chem. 267:7258–7262. [PubMed] [Google Scholar]
- 11.Reddy, V.Y., Q.Y. Zhang, and S.J. Weiss. 1995. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA. 92:3849–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulombe, R., P. Grochulski, J. Sivaraman, R. Menard, J.S. Mort, and M. Cygler. 1996. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 15:5492–5503. [PMC free article] [PubMed] [Google Scholar]
- 13.Lennon-Dumenil, A.M., R.A. Roberts, K. Valentijn, C. Driessen, H.S. Overkleeft, A. Erickson, P.J. Peters, E. Bikoff, H.L. Ploegh, and P. Wolf Bryant. 2001. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J. 20:4055–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strubin, M., C. Berte, and B. Mach. 1986. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 5:3483–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, N. 1988. Posttranslational modifications of the Ia-associated invariant protein p41 after gene transfer. Biochemistry. 27:4097–4102. [DOI] [PubMed] [Google Scholar]
- 16.Takaesu, N.T., J.A. Lower, E.J. Robertson, and E.K. Bikoff. 1995. Major histocompatibility class II peptide occupancy, antigen presentation, and CD4+ T cell function in mice lacking the p41 isoform of invariant chain. Immunity. 3:385–396. [DOI] [PubMed] [Google Scholar]
- 17.Takaesu, N.T., J.A. Lower, D. Yelon, E.J. Robertson, and E.K. Bikoff. 1997. In vivo functions mediated by the p41 isoform of the MHC class II-associated invariant chain. J. Immunol. 158:187–199. [PubMed] [Google Scholar]
- 18.Kampgen, E., N. Koch, F. Koch, P. Stoger, C. Heufler, G. Schuler, and N. Romani. 1991. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc. Natl. Acad. Sci. USA. 88:3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierre, P., and I. Mellman. 1998. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 93:1135–1145. [DOI] [PubMed] [Google Scholar]
- 20.Ogrinc, T., I. Dolenc, A. Ritonja, and V. Turk. 1993. Purification of the complex of cathepsin L and the MHC class II-associated invariant chain fragment from human kidney. FEBS Lett. 336:555–559. [DOI] [PubMed] [Google Scholar]
- 21.Guncar, G., G. Pungercic, I. Klemencic, V. Turk, and D. Turk. 1999. Crystal structure of MHC class II-associated p41 Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J. 18:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punturieri, A., S. Filippov, E. Allen, I. Caras, R. Murray, V. Reddy, and S.J. Weiss. 2000. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 192:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa, T., W. Roth, P. Wong, A. Nelson, A. Farr, J. Deussing, J.A. Villadangos, H. Ploegh, C. Peters, and A.Y. Rudensky. 1998. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 280:450–453. [DOI] [PubMed] [Google Scholar]
- 24.Bogyo, M., S. Verhelst, V. Bellingard-Dubouchaud, S. Toba, and D. Greenbaum. 2000. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem. Biol. 7:27–38. [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum, D., K.F. Medzihradszky, A. Burlingame, and M. Bogyo. 2000. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem. Biol. 7:569–581. [DOI] [PubMed] [Google Scholar]
- 26.Liotta, L.A., P.S. Steeg, and W.G. Stetler-Stevenson. 1991. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 64:327–336. [DOI] [PubMed] [Google Scholar]
- 27.Felbor, U., L. Dreier, R.A. Bryant, H.L. Ploegh, B.R. Olsen, and W. Mothes. 2000. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 19:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishigami, S., S. Natsugoe, K. Tokuda, A. Nakajo, H. Iwashige, K. Aridome, S. Hokita, and T. Aikou. 2001. Invariant chain expression in gastric cancer. Cancer Lett. 168:87–91. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, T.H., and M. Forgac. 1997. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. 13:779–808. [DOI] [PubMed] [Google Scholar]