Abstract
Platelet-endothelial cell adhesion molecule (PECAM)-1 has been implicated in leukocyte migration through the perivascular basement membrane (PBM) though the mechanisms involved are unclear. The present results demonstrate that the ability of α6 integrins to mediate neutrophil migration through the PBM is PECAM-1 dependent, a response associated with PECAM-1–mediated increased expression of α6β1 on transmigrating neutrophils in vivo. An anti-α6 integrins mAb (GoH3) inhibited (78%, P < 0.001) neutrophil migration through interleukin (IL)-1β–stimulated cremasteric venules, primarily at the level of the PBM, as analyzed by intravital and electron microscopy. In PECAM-1–deficient mice (KO), a reduced level of neutrophil transmigration elicited by IL-1β (4-h reaction) was observed in both the cremaster muscle (55% inhibition, P < 0.05) and in the peritoneum (57% inhibition, P < 0.01) but GoH3 had no additional inhibitory effect on these responses. FACS® analysis of neutrophils demonstrated increased expression of α6β1 on transmigrated peritoneal neutrophils, as compared with blood neutrophils, in wild-type but not KO mice even though neutrophils from both strains of mice exhibited comparable levels of intracellular expression of α6 as observed by immunofluorescent staining and confocal microscopy. Furthermore, mice deficient in either leukocyte or endothelial cell PECAM-1, as developed by bone marrow transplantation, demonstrated a similar level of reduced neutrophil transmigration and expression of α6β1 on transmigrated neutrophils as that detected in KO mice.
The results demonstrate a role for PECAM-1 homophilic interaction in neutrophil transmigration and increased expression of α6β1 on the cell surface of transmigrated neutrophils in vivo, a response that could contribute to the mechanism of PECAM-1–mediated neutrophil migration through the PBM.
Keywords: inflammation, adhesion molecules, laminin, integrins, intravital microcopy
Introduction
Recruitment of neutrophils into sites of inflammation plays a central role in the pathogenesis of numerous inflammatory conditions. After the generation of local inflammatory mediators, the migration of neutrophils from blood to the extravascular tissue is mediated by a series of sequential neutrophil–endothelial cell interactions leading to the responses of neutrophil rolling and firm adhesion within the vascular lumen, followed by neutrophil migration through vessel walls. In contrast to our increased understanding of the cellular and molecular interactions that mediate the early neutrophil–endothelial cell interactions of rolling and firm adhesion (1), relatively little is known about the mechanisms that mediate penetration of neutrophils through vessel walls. This latter response, termed transmigration, also involves two distinct sequential cellular events, namely migration through endothelial cells and migration through the perivascular basement membrane (PBM).*
Although in vitro studies have implicated a number of adhesion molecules, including the endothelial cell-associated molecules intercellular adhesion molecule (ICAM)-1, ICAM-2, platelet-endothelial cell adhesion molecule (PECAM)-1, CD99, and junctional adhesion molecule (JAM; references 2–4), in migration of leukocytes through endothelial cell junctions, very little is known about the adhesive mechanisms that mediate leukocyte migration through the PBM. In this context, PECAM-1 is of particular interest since there exists evidence from both in vitro and in vivo studies to suggest a role for this molecule in leukocyte migration across both endothelial cells and also through the PBM (5–9). Although the mechanism by which PECAM-1 mediates migration of leukocytes across endothelial cells may be explained by the high expression profile of the molecule at endothelial cell junctions and its ability to mediate homophilic binding with PECAM-1 on transmigrating neutrophils (10), the mechanisms by which PECAM-1 regulates neutrophil migration through the perivascular basement membrane is unclear.
The first indications for the involvement of PECAM-1 in leukocyte migration through the PBM came from the in vitro studies of Liao and colleagues demonstrating that antibodies directed against Ig domain 6 of PECAM-1 had no effect on monocyte transendothelial cell migration but blocked their subsequent migration into the underlying collagen gel (5). In vivo, a study from our group demonstrated that a polyclonal antibody recognizing rat PECAM-1 blocked neutrophil migration through IL-1β–stimulated rat mesenteric venules at the level of the PBM (9). More recently, defects in leukocyte migration through the PBM have been detected in genetically modified mice lacking PECAM-1 (6, 8). Since to date there have been no reports on the ability of PECAM-1 to interact with components of the PBM, the ability of PECAM-1 to mediate leukocyte migration through this barrier may be related to the signaling properties of the molecule. In this regard, in vitro studies have shown that antibody cross-linking of PECAM-1 can activate β1, β2, and β3 integrin functions (11–14), findings that have been extrapolated to suggest that physiological ligation of PECAM-1 may lead to increased expression of integrins thus aiding the direct interaction of transmigrating leukocytes with components of the PBM (e.g., laminin and collagen type IV). Despite these in vitro studies, using protocols that may lead to super-physiological levels of PECAM-1 engagement, there is to date no in vivo evidence for the involvement of PECAM-1 in regulation of cell surface expression of integrins on transmigrating leukocytes, an issue addressed for the first time in the present study.
As laminin is a key component of all mammalian venular basement membranes (15), and in the light of the growing body of in vitro evidence for the potential involvement of the principal leukocyte laminin receptor α6β1 in leukocyte migration through the basement membrane (16–19), the aim of the present study was to investigate the role and regulation of expression of α6β1 during neutrophil transmigration in vivo. The results demonstrate for the first time a PECAM-1–dependent role for α6 integrins in neutrophil migration through the perivascular basement membrane of stimulated venules and provide direct in vivo evidence for a PECAM-1 homophilic interaction leading to both neutrophil transmigration and increased expression of α6β1 on transmigrating neutrophils. Collectively, the present in vivo findings provide a potential mechanism for PECAM-1–mediated migration of neutrophils through the perivascular basement membrane.
Materials and Methods
Animals.
Wild-type C57BL/6 mice (>20 g) were purchased from Harlan-Olac and PECAM-1–deficient mice, backcrossed onto the C57BL/6 background for eight generations, were obtained as a gift from Dr. Tak W. Mak (Amgen Institute, Toronto, Canada).
Intravital Microscopy.
Intravital microscopy on the mouse cremaster muscle was performed as described previously (20). Briefly, male mice were injected intravenously with saline, anti-α6 integrins mAb GoH3 (21) (IgG2a; BD Biosciences), GoH3 F(ab′)2 fragment (all fragmentations were conducted by Cymbus Biotechnology), anti-β1 integrins mAb HMβ1–1 F(ab′)2 (reference 22; Hamster IgG2; gift from Dr. Hideo Yagita, Juntendo University, Tokyo, Japan), or an isotype-matched control mAb (all at 3 mg/kg), 15 min before intrascrotal administration of recombinant murine IL-1β (30 ng). Control groups were injected with intrascrotal saline (400 μl). 4 h later, the mice were anaesthetized by intraperitoneal administration of ketamine (Ketalar; 100 mg/kg; Parke-Davis) and xylazine (Rompun; 10 mg/kg; Bayer) and placed on a custom-built heated (37°C) microscope stage where the surgical procedure was performed. Leukocyte–endothelial cell interactions were observed on a ZEISS Axioskop fixed-stage upright microscope fitted with water-immersion objectives. Video recordings were made using a color video camera and videocassette recorder. Quantifications of rolling flux, adhesion, and transmigration were made as described previously (6). Briefly, using postcapillary venules of 20–40 μm in diameter, rolling leukocytes were defined as those moving slower than the associated blood flow, and rolling flux was quantified as the number of rolling cells moving past a fixed point on the venular wall per minute, averaged over 5 min. Firmly adherent cells were those remaining stationary for 30 s or longer within a given 100-μm segment of venule. Transmigrated leukocytes were those in the perivenular tissue within 50 μm of the 100-μm vessel segment under observation. Several vessel segments (range 3–5) from multiple vessels (range 3–5) were studied for each animal. In some animals, blood samples were taken by tail-tipping and total and differential blood leukocyte counts (in blood smears prepared in a Cytospin-3 cytocentrifuge; Shandon) were determined by Kimura and May-Grunwald/Giemsa stains, respectively. In selected experiments, erythrocyte centreline velocity was determined by means of an Optical Doppler Velocimeter (Microcirculation Research Institute) and blood pressure measurements determined by means of an electronic pressure transducer (Harvard Apparatus).
Electron Microscopy.
At the end of selected experiments, the cremaster muscle was dissected and prepared for analysis by transmission electron microscopy (EM) as described previously (6). Briefly, tissues were fixed in a solution containing glutaraldehyde (2.5%), sodium cacodylate (50 mM), hydrochloric acid (4 mM), and calcium chloride (0.18 mM). The samples were then post-fixed in osmium VIII oxide (1%) and after dehydration in methanol, were embedded in araldite resin before sectioning. Ultrathin sections (0.1 μm) of the target area were then mounted on copper mesh grids and stained with uranyl acetate and lead citrate. The position of migrating leukocytes relative to the endothelium and the perivascular basement membrane was assessed using a Hitachi 7000 transmission electron microscope (Hitachi UK), as detailed previously (9). Briefly, for each vessel, the number of leukocytes in each of the following positions was noted: A, within lumen of venule; B, crossing endothelium; C, between endothelium and perivascular basement membrane; D, crossing basement membrane; E, outside venule, but within 50 μm of it. For each venule, the fraction of leukocytes that had crossed the endothelium, but were still inside the basement membrane, was calculated according to the following equation: C/(C+D+E). Tissue samples were analyzed from at least four animals and at least three vessels from each animal studied in detail for each series of experiments.
Quantification of Leukocyte Migration Into the Peritoneal Cavity.
Mice were injected intravenously with saline, mAb GoH3 (whole antibody or F(ab′)2 fragment), or control antibody (all at 3 mg/kg), 15 min before the intraperitoneal administration of IL-1β (10 ng in 1 ml of saline/cavity). Control mice were injected with intraperitoneal saline/PBS. 4 h later, the animals were killed by asphyxiation with CO2, the peritoneal cavity was opened and lavaged with modified PBS solution (containing 0.25% BSA and 2 mM EDTA). Total and differential count of infiltrating leukocytes was performed as detailed above. Neutrophil infiltration is expressed as percentage of total infiltrating leukocytes.
Flow Cytometry.
Whole blood, taken by tail-tipping, and peritoneal leukocytes, from 4-h exudates after injection of IL-1β (as detailed above), were obtained from the same animals. Blood samples, diluted 50:50 with anti-coagulant (20 mM EDTA in PBS), and peritoneal exudates were incubated with primary mAb on ice and the binding of test mAbs was detected after incubation with a F(ab′)2 FITC-conjugated goat anti–rat IgG (Serotec Ltd.). The following rat anti–mouse primary antibodies were used: GoH3 (IgG2a, anti-α6), 9EG7 (IgG2a, anti-β1), GAME-46 (IgG1, anti-β2), MEL-14 (IgG2a, anti–L-selectin), Mec 13.3 (IgG2a, anti-PECAM-1), and isotype-matched control mAbs were obtained from BD Biosciences. PS/2 (IgG2b, anti-α4) was obtained from American Type Culture Collection. After the addition of FACS® lysing reagent (Becton Dickinson), samples were analyzed on an EPICS XL flow cytometer (Beckman Coulter). Gating on neutrophils was based on characteristic forward and side scatter parameters as well as the binding of mAb Gr-1 (BD Biosciences). The binding of primary mAbs, relative to the binding of isotype-matched control mAbs is expressed in terms of relative fluorescence intensity (RFI).
Immunofluorescence Staining and Confocal Analysis of Neutrophils.
Peripheral blood was collected from WT or PECAM-1–deficient mice by cardiac puncture and anti-coagulated with 20 mM EDTA. PMN were purified from whole blood as described previously (6) using a discontinuous Percoll gradient yielding PMN preparations of ∼90% purity. Smears of the freshly isolated PMN were prepared in a cytocentrifuge (Shandon) and air-dried overnight. The cells were then fixed with 4% paraformaldehyde for 15 min followed by permeabilization with 1% Triton X-100 for 30 min, all at room temperature. Nonspecific binding was blocked with 10% normal rabbit serum for 15 min. The primary antibody, anti-α6 integrin mAb, GoH3, was incubated with samples for 1 h, control samples being incubated with PBS only. After two washes, the samples were incubated with the secondary antibody, Alexa Fluor® 633 labeled-goat anti–rat IgG (Molecular Probes Inc.), at 1:100 at room temperature for 1 h. Finally, the slides were washed twice in PBS and observed using a ZEISS laser scanning confocal microscope (LSM5 PASCAL), mounted on a ZEISS Axioskop II FS upright microscope. The 633 nm laser line was generated from a HeNe laser.
Bone Marrow Transplantation.
Transfer of bone marrow cells, as described previously (23), between WT C57BL/6 mice and PECAM-1–deficient mice was performed to generate mice deficient in either leukocyte or endothelial cell PECAM-1. Briefly, recipient mice were lethally irradiated with a dose of 9.5Gy for 25 min using a cesium source (IBL 637; Cis Bio International) 24 h before bone marrow transfer. Bone marrow donors were killed by asphyxiation with CO2 and bone marrow cells were harvested from both femora under sterile conditions. Each recipient was injected with 107 cells by intravenous tail vein injection and was allowed to generate bone marrow–derived cells for at least 8 wk before use. The blood leukocyte phenotype of recipients was determined after 8 wk by indirect immunostaining and FACS® analysis as detailed above.
Statistics.
All results are expressed as mean ± SEM. Statistical significance was assessed by means of Mann-Whitney U test or one way analysis of variance with Neuman-Keuls multiple comparison test, as appropriate. P < 0.05 was considered significant.
Results
An Anti-α6 Integrins mAb, GoH3, Inhibits Neutrophil Migration through the Perivascular Basement Membrane of IL-1β–stimulated Mouse Cremasteric Venules.
The functional role of α6β1 in leukocyte migration through stimulated venules in vivo was investigated using the anti-α6 integrins mAb GoH3 (21). For this purpose, the effect of GoH3 was studied in a model in which we have previously detected PECAM-1–dependent neutrophil migration through the PBM (6), namely leukocyte migration through IL-1β–stimulated mouse cremasteric venules as observed by intravital microscopy. Intrascrotal administration of IL-1β (30 ng, administered 4 h before quantification of responses) had no significant effect on leukocyte rolling flux (unpublished data), but induced a significant increase in leukocyte firm adhesion and transmigration. In animals treated with mAb GoH3 (3 mg/kg, intravenously), whole antibody or F(ab′)2 fragment, leukocyte transmigration, but not firm adhesion, was significantly suppressed as compared with mice treated with an isotype-matched control mAb (78% inhibition, P < 0.001; Fig. 1). Pretreatment of mice with F(ab′)2 of the anti-β1 integrin mAb HMβ1–1 (3 mg/kg, intravenously) also suppressed IL-1β–induced transmigration, as compared with responses obtained in mice treated with a control mAb (71% inhibition, P < 0.05). None of the reagents employed had a significant effect on circulating leukocyte numbers or venular wall shear rates (unpublished data).
Figure 1.
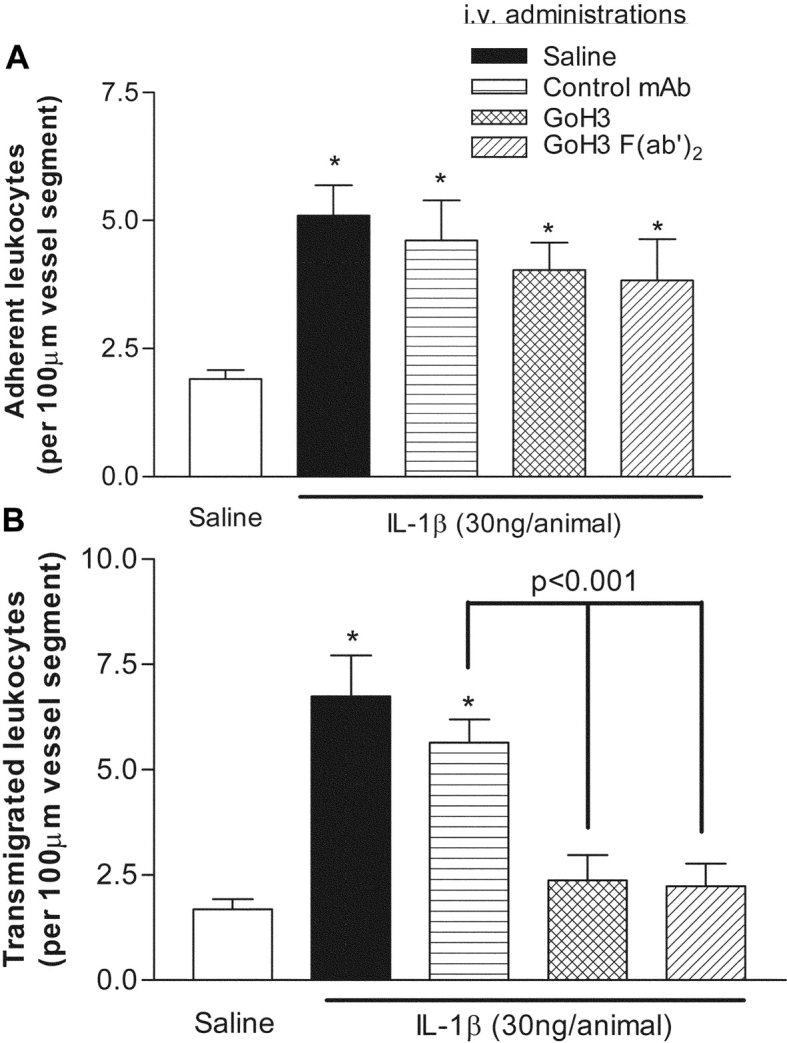
Effect of anti-α6 integrins mAb GoH3 on leukocyte migration through IL-1β–stimulated mouse cremasteric venules, as observed by intravital microscopy. Animals were treated with intrascrotal saline (white bars) or IL-1β (30 ng/animal, black bars) 4 h before the surgical preparation. In additional groups of mice, animals were pretreated with intravenous mAbs GoH3 (anti-α6 integrins), whole antibody or F(ab′)2, or an isotype-matched control mAb, all at the dose of 3 mg/kg, 15 min before the intrascrotal injection of IL-1β. The panels show leukocyte firm adhesion and transmigration from the same experiments. The data represent mean ± SEM, from n = 5–8 mice/group. A significant difference from responses obtained from saline-injected animals is shown by asterisks, *P < 0.05. Additional statistical comparisons are indicated by lines.
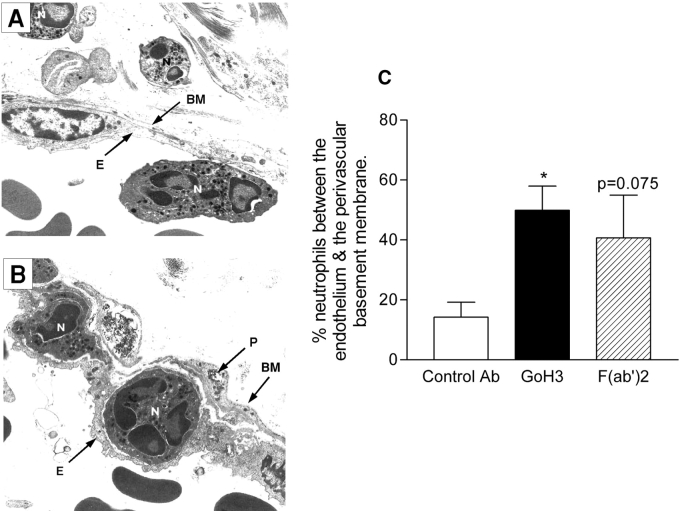
The inhibitory effect of GoH3 on leukocyte transmigration appeared to be at the level of the perivascular basement membrane, as analyzed by transmission. In venular sections from GoH3-treated animals, but not control-antibody treated mice, neutrophils were frequently observed between the endothelium and the perivascular basement membrane (Fig. 2, A and B). Quantitative analysis of these observations indicated that ∼3 times as many neutrophils were trapped in IL-1β–stimulated venules of mice treated with the anti-α6 integrins mAb (whole antibody or F(ab′)2 fragment; Fig. 2).
Figure 2.
Analysis of IL-1β–stimulated cremasteric venules from mice treated with GoH3, by transmission . A and B are representative electron micrographs of IL-1β (30 ng/mouse)-stimulated cremasteric venule (4-h test period) from control antibody-treated or GoH3-treated mice, respectively. The following structures are labeled: endothelial cells (E), neutrophils (N), pericyte (P), and basement membrane (BM), with a magnification of ×5,400. C shows quantified EM observations as the number of neutrophils observed between the venular endothelium and the perivascular basement membrane, expressed as the percentage of the number of neutrophils that had crossed the venular wall (but were within 50 μm of it), in the tissue sections analyzed at the 4-h time point. In all three groups, the antibodies were administered at the dose of 3 mg/kg intravenous 15 min before the intrascrotal administration of IL-1β. The data represent mean ± SEM from 10–23 randomly selected vessel segments from 4–7 mice/group. A significant difference from the control antibody group is shown by an asterisk; *P < 0.05.
GoH3 Inhibits IL-1β–induced Neutrophil Migration in Wild-Type but Not PECAM-1–deficient Mice.
We next examined the effect of mAb GoH3 on neutrophil transmigration in both WT and PECAM-1–deficient mice, using two IL-1β–driven models, namely neutrophil migration through IL-1β–stimulated cremasteric venules and neutrophil migration into IL-1β–stimulated peritoneal cavities. In WT mice, locally administered IL-1β (intrascrotal 30 ng or intraperitoneal 10 ng, administered 4 h before quantification) elicited significant neutrophil transmigration as compared with animals injected with saline (Fig. 3). These responses were almost totally inhibited in mice treated with whole (Fig. 3) or F(ab′)2 fragment of GoH3 (unpublished data). Interestingly, in PECAM-1–deficient mice, while there was a significant suppression of IL-1β–induced neutrophil transmigration in both models (55 and 57% inhibition of neutrophil transmigration in the cremaster muscle and peritoneum, respectively), GoH3 had no additional inhibitory effects (Fig. 3). With respect to the peritonitis model, we have used total and differential leukocyte counts to express the neutrophil migration data as percentage of neutrophil infiltration due to occasional large variations in total leukocyte numbers. However, as the observed suppressed neutrophil migration into IL-1β–stimulated peritoneal cavity of PECAM-1–deficient mice is a novel and important observation, the absolute number of total leukocyte counts, as well as the percent differentials of the infiltrating leukocytes, obtained in response to intraperitoneal PBS or IL-1β, as compared with thioglycollate, in WT and PECAM-1 KO mice, is shown in Table I . The results demonstrate an increase in total infiltrating leukocytes and percentage of neutrophils in response to both IL-1β and thioglycollate in WT mice. In PECAM-1–deficient mice, no significant difference in responses elicited by thioglycollate was observed, as compared with WT mice, in agreement with a previously published report (8). In contrast, a significant suppression of infiltrating neutrophils was detected after intraperitoneal injection of IL-1β, in agreement with the data presented in Fig. 3. Interestingly, the suppression of neutrophil infiltration appeared to be compensated by a significant increase in infiltrating mononuclear leukocytes.
Figure 3.
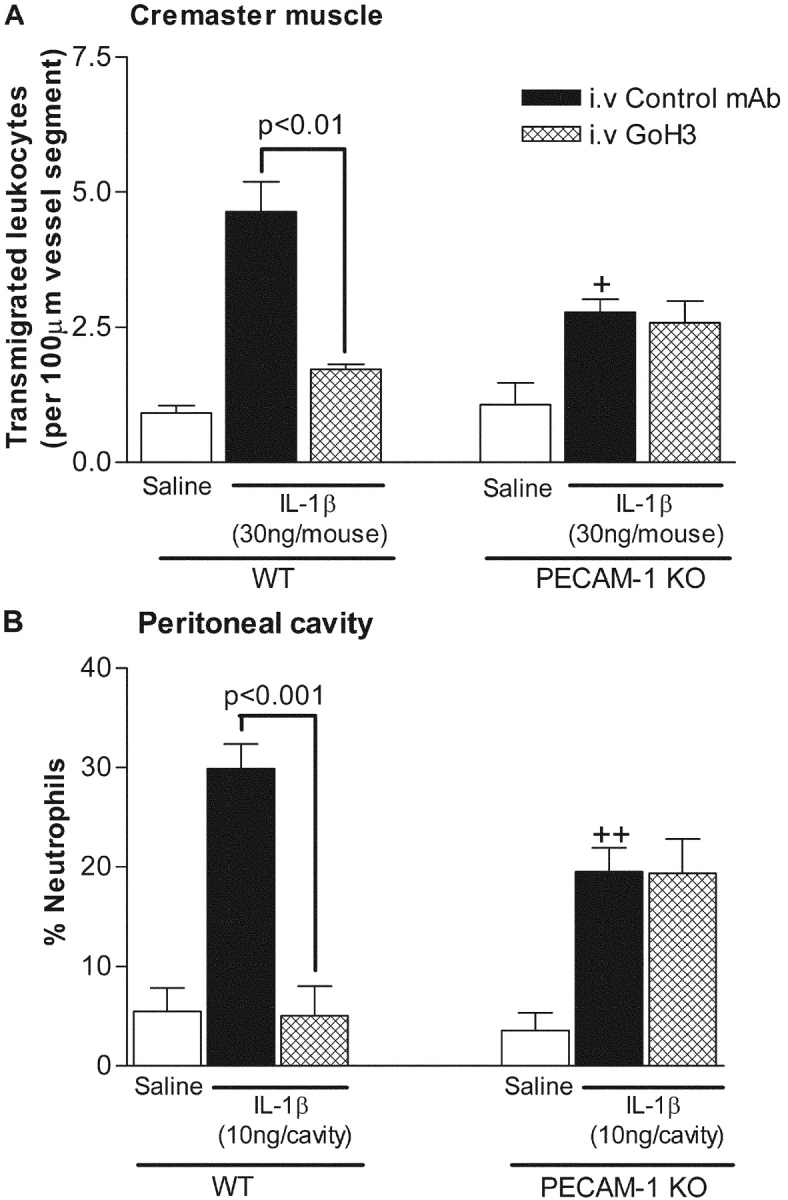
Effect of GoH3 on (A) leukocyte transmigration through IL-1β–stimulated cremasteric venules and (B) neutrophil infiltration into IL-1β–stimulated peritoneal cavity of WT and PECAM-1–deficient mice. Wild-type or PECAM-1–deficient mice were injected with intra-scrotal (A) or intraperitoneal (B) saline (white bars) or intrascrotal or intraperitoneal IL-1β (30 ng/mouse and 10 ng/cavity, respectively), 4 h before quantification. In the IL-1β–injected groups, the mice were pretreated with an isotype-matched control antibody or GoH3 (both at 3 mg/kg intravenously) 15 min before administration of IL-1β. The data represent mean ± SEM from n = 3–6 mice/group in A and n = 5–10 in B. Significant differences between control IL-1β elicited responses in WT and PECAM-1–deficient mice are indicated by +P < 0.05 and ++P < 0.01. Additional statistical comparisons are indicated by lines.
Table I.
IL-1β and Thioglycollate-induced Leukocyte Migration into the Peritoneum of WT and PECAM-1–deficient (KO) Mice
| Stimulus | Total leukocytes | Neutrophils | Eosinophils | Mononuclear leukocytes | |
|---|---|---|---|---|---|
| (×106) | % | % | % | ||
| PBS | WT | 3.33 ± 0.27 | 4.15 ± 0.83 | 1.25 ± 0.48 | 94.6 ± 0.83 |
| KO | 4.46 ± 1.00 | 3.36 ± 1.81 | 1.12 ± 0.38 | 95.5 ± 2.15 | |
| IL-β | WT | 5.03 ± 0.48 | 24.92 ± 1.92 | 1.27 ± 0.37 | 73.86 ± 2.02 |
| (10 ng) | KO | 5.70 ± 1.04 | 12.60 ± 2.05** | 1.48 ± 0.71 | 85.93 ± 2.39** |
| Thioglycollate | WT | 14.00 ± 1.59 | 62.18 ± 5.59 | 0.88 ± 0.34 | 36.94 ± 5.82 |
| (4%) | KO | 10.85 ± 1.71 | 48.63 ± 6.49 | 0.93 ± 0.21 | 50.43 ± 6.47 |
Mice were injected intraperitoneally with PBS (1 ml/cavity), IL-1β (10 ng/cavity), or thioglycollate (1 ml of 4% solution/cavity) 4 h prior to quantification. Total and differential leukocyte counts were determined as detailed in Materials and Methods. The data represent mean ± SEM of samples obtained from n = 4–13 animals/group. Significant differences between samples from WT and KO animals are indicated by
P < 0.01.
Increased Expression of α6β1 on Transmigrated Neutrophils In Vivo Is PECAM-1 Dependent.
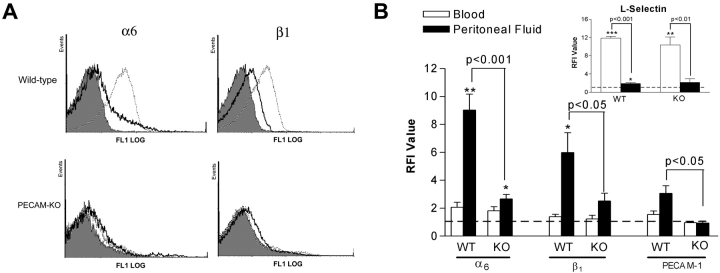
As GoH3 inhibited neutrophil migration in WT but not PECAM-1–deficient mice, we investigated the expression of α6β1 on the cell surface of neutrophils in the two strains of animals. With respect to blood neutrophils, low levels of cell surface α6 and β1 were detected in WT and PECAM-1–deficient mice (Fig. 4, A and B). Similarly, low levels of PECAM-1 (Fig. 4 B) and α4 integrins (unpublished data) were detected, although high levels of L-selectin (Fig. 4 B) and β2 integrins were observed (unpublished data). Overall, no marked differences in expressions of adhesion molecules were detected between WT and PECAM-1–deficient blood neutrophils. With respect to IL-1β–induced peritoneal neutrophils, a significantly enhanced level of α6 and β1 expression was detected on cells obtained from WT but not PECAM-1–deficient mice (Fig. 4, A and B), indicating an association between cell surface expression of α6β1 on transmigrated neutrophils and PECAM-1, in vivo. By comparison, cell surface expression of L-selectin was equally reduced on transmigrated neutrophils, as compared with blood neutrophils, in both WT and KO mice (Fig. 4 B).
Figure 4.
Cell surface expression of α6β1, PECAM-1, and L-selectin on blood and IL-1β–elicited peritoneal neutrophils in WT and PECAM-1 deficient (KO) mice. Panel A shows representative fluorescence histograms comparing cell surface expressions of α6 and β1 on blood and IL-1β–induced peritoneal neutrophils in WT and PECAM-1 deficient mice, as indicated. The filled tracings are from blood samples incubated with an isotype-matched control mAb (the same low binding was found with peritoneal samples) and the solid and dashed line tracings are from blood and peritoneal samples, respectively, incubated with mAbs GoH3 (anti-α6) or 9EG7 (anti-β1), as indicated. B shows pooled relative fluorescence intensities of samples stained with specific antibodies and analyzed and quantified by FACS® as detailed in Materials and Methods. The data represent mean ± SEM of samples obtained from n = 3–10 animals/group. Significant binding of primary mAbs is indicated by asterisks, *P < 0.05, **P < 0.01 and other statistical comparisons are indicated by lines.
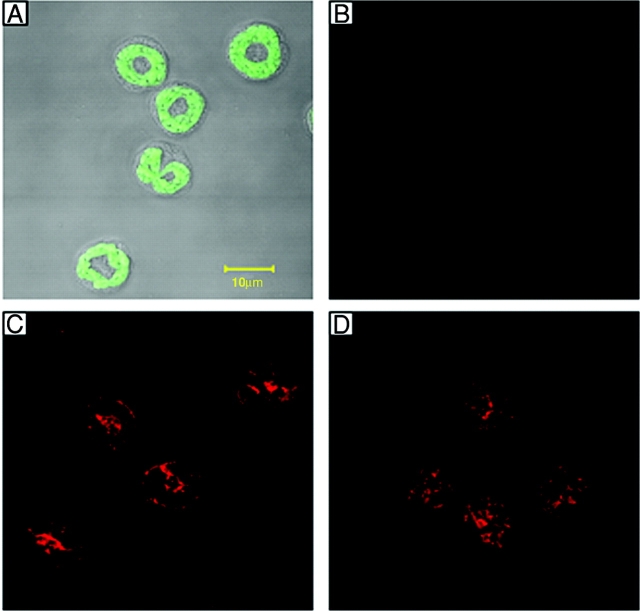
Since in human neutrophils α6β1 is reportedly stored within intracellular granules and up-regulated to the cell surface after in vitro transendothelial cell migration (24), one possible explanation for our findings is reduced intracellular stores of the integrin in PECAM-1 KO neutrophils. However, immunofluorescent staining of fixed and permeabilized mouse neutrophils, as observed by confocal microscopy, indicated comparable levels of intracellular α6 expression in neutrophils from both WT and PECAM-1–deficient mice (Fig. 5). Of interest, stimulation of mouse blood neutrophils with PMA (100 ng/ml for 20 min) failed to enhance the cell surface expression of α6β1 but as expected significantly elevated the cell surface expression of β2 and caused the shedding of L-selectin (unpublished data).
Figure 5.
Confocal microscopy images of mouse blood neutrophils immunostained for α6 expression. Cytospins of purified mouse neutrophils were prepared, fixed, and permeabilized as detailed in Materials and Methods. Cell samples were then incubated with or without the primary antibody, anti-α6 integrin mAb GoH3, and binding was detected using an Alexa Fluor® 633-labeled secondary Ab before being observed using a ZEISS confocal microscope. Panel A shows autofluorescence image of neutrophils (green channel) superimposed on DIC of the same cells. B shows control cells stained with the secondary Ab in the absence of mAb GoH3. C and D show positive staining of neutrophils from WT and PECAM-1–deficient mice, respectively, for α6 integrin. The images are representative of cells obtained from three different mice/group.
Neutrophil Transmigration and Increased Expression of α6β1 on Transmigrated Neutrophils In Vivo Is Mediated by a Homophilic Interaction between Leukocyte and Endothelial Cell PECAM-1.
Having found a role for PECAM-1 in increased expression of α6β1 on transmigrated neutrophils, we next sought to determine the relative contributions of leukocyte and endothelial cell PECAM-1 in this response. For this purpose, chimeric mice deficient in either endothelial cell (EC−/−) or leukocyte (L−/−) PECAM-1, but expressing WT leukocyte and endothelial cell PECAM-1, respectively, were developed by bone marrow transplantation (phenotypes were confirmed by FACS® analysis; Fig. 6), and used in the IL-1β peritonitis model. Interestingly, a similar suppression of neutrophil transmigration and lack of cell surface expression of α6 on transmigrated neutrophils was detected using PECAM-1–deficient mice and chimeric mice lacking either endothelial cell (EC−/−) or leukocyte (L−/−) PECAM-1 (Fig. 7), findings that suggest PECAM-1 homophilic interaction mediates both neutrophil transmigration and integrin up-regulation in vivo.
Figure 6.
Cell surface expression of PECAM-1 on neutrophils from chimeric mice having undergone bone marrow transfer. The figure shows representative fluorescence histograms demonstrating the binding of an anti-PECAM-1 mAb (Mec 13.3) to blood neutrophils. The filled tracings are from blood samples incubated with an isotype-matched control mAb and the solid line tracings are blood samples incubated with Mec 13.3 from (A) a lethally irradiated WT mouse injected with bone-marrow from a WT mouse, (B) a lethally irradiated PECAM-1–deficient (KO) mouse injected with bone marrow from a KO mouse, (C) a lethally irradiated KO mouse injected with bone marrow from a WT mouse, and (D) a lethally irradiated WT mouse injected with bone marrow from a KO mouse.
Figure 7.
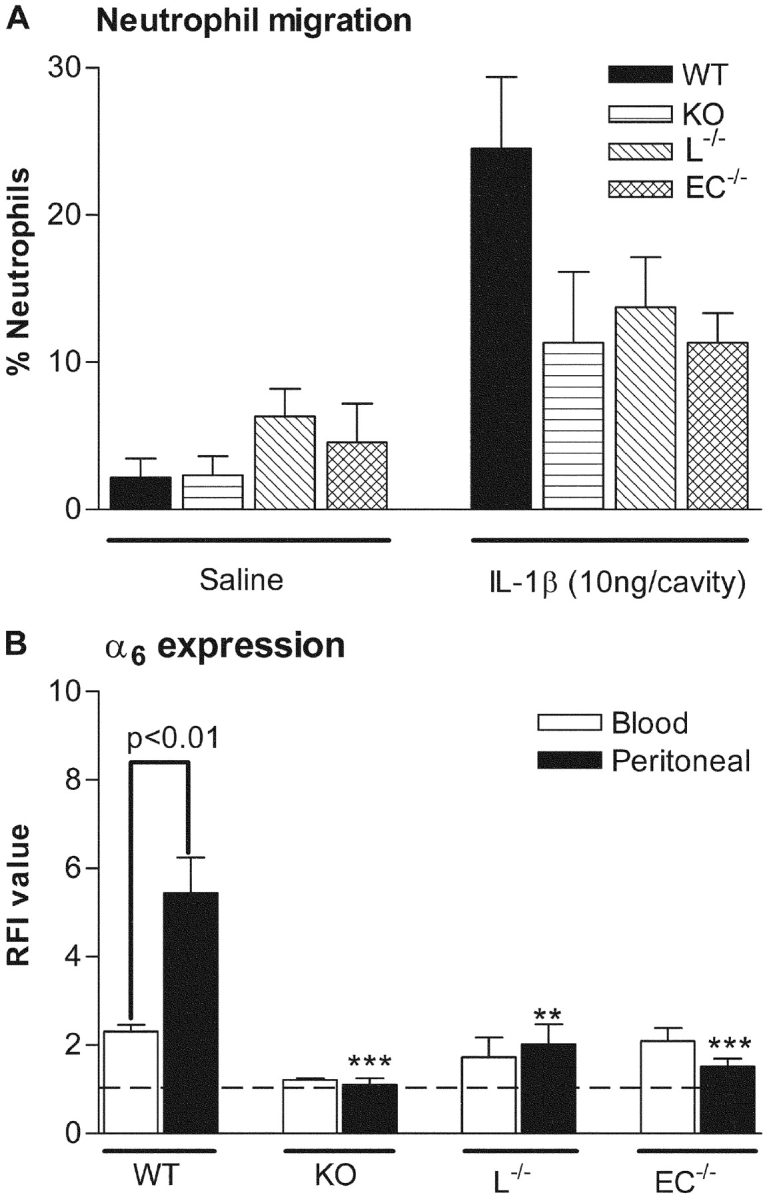
Neutrophil migration and cell surface expression of α6 on blood and peritoneal neutrophils in an IL-1β–elicited peritonitis model in WT, PECAM-1–deficient (KO), and chimeric mice deficient in either leukocyte (L−/−) or endothelial cell (E−/−) PECAM-1. Mice were injected via the intraperitoneal route with IL-1β (10 ng/animal) and 4 h later, blood or peritoneal lavage was collected. The lavage fluid was used to quantify neutrophil infiltration (A). Lavage and blood samples were also analyzed for the binding of GoH3 (anti-α6) as quantified by FACS® (B), as detailed in Materials and Methods. The data represent mean ± SEM of samples obtained from n = 4–7 animals/group. Significant differences between peritoneal samples from WT mice and KO, L−/−, or E−/− mice are indicated by **P < 0.01 and ***P < 0.001. Other statistical differences are indicated by lines.
Discussion
The migration of leukocytes through stimulated venular walls involves their penetration across two distinct barriers, namely endothelial cells and its associated perivascular basement membrane (PBM). In contrast to our increased understanding of the molecular and cellular events that mediate leukocyte–endothelial cell interactions, the mechanisms employed by leukocytes in penetrating the perivascular basement membrane remain unresolved. An important and relatively recent development in this field has been the demonstration that PECAM-1 plays a functional role in mediating/regulating leukocyte migration through this barrier (5, 6, 8, 9), though the mechanisms by which PECAM-1 may be mediating this response is at present unclear. In this regard, the findings of the present study suggest a PECAM-1–dependent functional role for the laminin receptor α6β1 in leukocyte migration through the PBM and for the first time demonstrate a direct in vivo link between PECAM-1 and increased expression of α6β1 on the cell surface of transmigrating neutrophils. Furthermore, the data indicate the involvement of a PECAM-1 homophilic interaction in both neutrophil transmigration and α6 integrin upregulation in vivo. Collectively the findings of our study add a new step to the final stage of the currently accepted multistep paradigm of leukocyte migration by providing evidence to suggest that the interaction of neutrophil PECAM-1 with endothelial cell PECAM-1 at endothelial cell junctions stimulates enhanced expression of α6β1 on the cell surface of transmigrating neutrophils, thus aiding their migration through the perivascular basement membrane via interactions with the laminin component of this barrier.
The integrin α6β1 was first described on human platelets (25) but is now known to be expressed by numerous cell types, including neutrophils (16) and endothelial cells (26). This molecule is the principal leukocyte receptor for the basement membrane protein laminin and as such anti-α6β1 antibodies have been shown to block neutrophil adhesion to the purified protein (16, 18) as well as suppressing neutrophil transmigration through endothelial cells cultured on laminin (19). Such in vitro findings, in conjunction with the fact that laminin is a major constituent of the basement membrane (27), have led to suggestions that leukocyte α6β1 may play a significant role in mediating leukocyte migration through the PBM though to date there have been no in vivo evidence in support of this possibility. In the first part of the present study the role of α6β1 in neutrophil migration in vivo was investigated using the anti-α6 integrin mAb GoH3. For this purpose, we employed two IL-1β–driven inflammatory models, namely leukocyte migration through IL-1β–stimulated cremasteric venules (>90% being neutrophils, as determined by analysis) and neutrophil migration into IL-1β–stimulated peritoneal cavities. Furthermore, as one of the principal objectives of the study was to address the potential association of α6β1 with PECAM-1 during neutrophil transmigration, as well as investigating WT mice, the effect of GoH3 on neutrophil transmigration was also investigated in PECAM-1 deficient animals. Of relevance, although the first paper to report on the generation and characteristics of PECAM-1–deficient mice indicated no overall defects in total leukocyte migration in a number of inflammatory models in these mice, the study did report a defect in neutrophil migration through the perivascular basement membrane of mesenteric venules (8). Using the same mouse strain, in agreement with the findings of Duncan et al., we observed normal infiltration of both total leukocytes and neutrophils into the peritoneum of PECAM-1 KO mice following intraperitoneal administration of thioglycollate. However, although in agreement with Duncan et al. we found normal infiltration of total leukocytes into IL-1β–stimulated peritoneum of PECAM-1–deficient mice, differential quantification of the infiltrating leukocytes demonstrated a significant suppression of neutrophil infiltration. These results are consistent with our previous observations, demonstrating a stimulus-specific defect in neutrophil transmigration through cremasteric venules of PECAM-1–deficient mice (6). Interestingly, in addition to exhibiting anti-inflammatory profiles, PECAM-1–deficient mice have recently been reported to exhibit enhanced proinflammatory responses of increased vascular permeability and an early onset of EAE (28), adding to the increasing list of phenotypes associated with this strain of mice.
Pretreatment of control WT mice with mAb GoH3 resulted in a significant blockade of neutrophil transmigration through IL-1β–stimulated cremasteric venules, as observed by intravital microscopy, a blockade that appeared to be at the level of the perivascular basement membrane as analyzed by transmission EM. GoH3 also blocked neutrophil infiltration into IL-1β–stimulated peritoneal cavity of WT mice. Interestingly, however, although in PECAM-1–deficient mice a significant suppression of IL-1β–induced neutrophil transmigration was quantified in both models, GoH3 had no additional inhibitory effects, suggesting that the functional importance of α6 integrins in neutrophil migration requires PECAM-1. Hence in PECAM-1 knockout mice, the observed neutrophil migration is both independent of PECAM-1 and α6 integrins, presumably, as a result of developmental redundancy, as previously reported in a number of other cell adhesion molecule deficient mice (29, 30). For example, neutrophil emigration during endotoxin-induced pneumonia was reduced by anti–ICAM-1 mAb or ICAM-1 antisense oligonucleotide, but was not affected in ICAM-1–deficient mice (31) and anti-αvβ3–integrin blockers inhibit angiogenesis in WT mice while mice deficient in αv or β3 exhibit no defect in angiogenesis or vasculogenesis (32). Furthermore, by using a direct quantification procedure, Eppinhimer et al., reported that compared with WT mice, CD18-, ICAM-1–, and P-selectin–deficient mice exhibit an altered expression of E- and P-selectin under basal and/or cytokine-stimulated conditions (33). The nature of the compensatory mechanisms developed in the PECAM-1–deficient mice leading to PECAM-1- and α6 integrins-independent migration of neutrophils through the PBM is at present unknown but will be the subject of future investigations within our laboratory. Hence, as GoH3 had no inhibitory effects on neutrophil migration in the PECAM-1–deficient mice, and in the light of the previous in vitro studies showing that PECAM-1 ligation can lead to increased expression of integrins, including β1 integrins (11), we hypothesized that the dependency of neutrophil migration on α6β1 and PECAM-1 may be functionally associated. To investigate this possibility, we next examined the cell surface expression of α6β1 on blood and transmigrated neutrophils in both WT and PECAM-1–deficient animals.
Blood neutrophils from WT and PECAM-1 KO mice exhibited similar profiles of expression of selected adhesion molecules, as quantified by flow cytometric analysis, a technique that also confirmed the absence of leukocyte PECAM-1 in the latter. Specifically, neutrophils from the two strains of mice showed comparable and significant levels of L-selectin and β2 integrins, but low levels of α6 or β1 were detected. The low expression of α6β1 on blood neutrophils detected in the present study is in agreement with previous reports (21, 34, 35). In contrast to blood neutrophils, IL-1β–elicited peritoneal neutrophils exhibited a significantly enhanced level of α6β1 in WT mice, a response that was not detected in PECAM-1–deficient animals. Increased expression of α6 and/or β1 on transmigrated human neutrophils in vitro (24, 36) and increased expression of β1 integrins on transmigrated human and rat neutrophils in vivo (34, 37), have previously been demonstrated, results that are in agreement with our findings. However, using a clean model of genetic deletion, the present study extends these observations by providing evidence for a direct association between PECAM-1 and increased expression of α6β1. Of importance, since in human neutrophils there is indirect evidence for expression of α6β1 within intracellular granules, a store that is up-regulated to the cell surface after in vitro transendothelial cell migration (24), one possible explanation for the low level of α6β1 on the cell surface of transmigrated neutrophils in the PECAM-1–deficient mice is an overall lower level of intracellular stores of the integrin in PECAM-1–deficient neutrophils. However, direct analysis of α6 expression in permeabilized mouse blood neutrophils immunostained by GoH3 and analyzed by confocal microscopy indicated comparable levels of intracellular α6 integrins in neutrophils obtained from WT and PECAM-1–deficient mice. Of interest, in agreement with a previous report (18), stimulation of mouse neutrophils with PMA did not lead to increased cell surface expression of α6, suggesting that regulation of expression of α6β1 is mediated through adhesive events, as discussed below.
Having demonstrated a functional role for PECAM-1 in increased expression of α6β1 on the cell surface of transmigrated neutrophils in vivo, in a final series of experiments we sought to investigate the relative roles of neutrophil and endothelial cell PECAM-1 in this response. For this purpose, chimeric mice deficient in either leukocyte or endothelial cell PECAM-1 were generated by bone marrow transfer between PECAM-1–deficient and WT animals. Using these mice in the IL-1β–elicited peritonitis model, a similar suppression of neutrophil migration was detected in mice deficient in either leukocyte or endothelial cell PECAM-1 as that detected in the KO mice. Furthermore, the results demonstrated that increased expression of α6 on transmigrating neutrophils required both leukocyte and endothelial cell PECAM-1. Collectively the present results provide the first direct evidence for a homophilic PECAM-1–PECAM-1 interaction mediating integrin up-regulation in vivo. Hence, in conjunction with previous observations, the present results suggest that while PECAM-1–PECAM-1 interaction at endothelial cell junctions supports leukocyte migration through endothelial cell junctions, through its ability to stimulate increased expression of α6β1, this interaction also mediates leukocyte migration through the PBM. At first glance this conclusion may appear in conflict with the in vitro observations of Liao et al. (5) demonstrating that while homophilic PECAM-1 interactions (via domain 1 and 2) support monocyte migration across endothelial cells, heterophilic PECAM-1 interactions (via its domain 6) support monocyte migration through basement membrane like collagen gels. However, it is possible that in the study of Liao and colleagues, blockade of homophilic PECAM-1–PECAM-1 interactions by mAbs directed against domains 1 and/or 2 as well as inhibiting the migration of monocytes across endothelial cells may also have blocked integrin upregulation, though the latter was not quantified and would not have been easily evident within the experimental protocol used. The mechanism by which blockade of domain 6 (believed to support heterophilic PECAM-1 interactions) inhibited monocyte migration through collagen gels is still unclear though this possible discrepancy between our results and those of Liao et al. may involve different PECAM-1–dependent mechanisms employed by different leukocyte subtypes, e.g. monocytes as compared with neutrophils (investigated in this study) and/or differences between the species or models investigated. Furthermore, although in vitro studies have demonstrated or suggested that PECAM-1 can support both homotypic and heterotypic interactions (10), to our knowledge there is no evidence for the occurrence of the latter in vivo. Indeed, previous in vivo investigations support our findings by providing evidence to suggest the involvement of PECAM-1 homophilic interaction in neutrophil migration in vivo (38–40).
The mechanism by which neutrophil PECAM-1 ligation mediates increased expression of α6β1 is at present unclear but may involve translocation of the integrin from granules, as has been suggested from in vitro studies using human neutrophils (24). To our knowledge however, a correlation between leukocyte PECAM-1 ligation and leukocyte degranulation has not been reported but is currently under investigation in our laboratory. Furthermore, with respect to α6β1, very little is known about the physiological mechanisms that regulate the expression/activation of this integrin on leukocytes (18, 41). In this context, the present in vivo findings strongly suggest transmigration as an important regulator of α6β1 expression, and perhaps activation, a response that may determine the efficiency with which the leukocytes subsequently interact with components of the perivascular basement membrane and/or extravascular tissue. Of relevance, transmigration has previously been shown to be an effective regulator of expression of other β1 integrins such as α4β1 (36) and α2β1 (34). The stimulatory effect of transmigration and the apparent lack of effect of soluble neutrophil chemoattractants or PMA on expression of β1 integrins (18, 34, 37) suggest the requirement of adhesion-mediated signaling events in the regulation of expression of these family of integrins, as has been demonstrated by Werr and colleagues (42) and indicated in the present study.
In summary, we have demonstrated a functional role for α6 integrins in neutrophil migration through the perivascular basement membrane, a response that appears to be PECAM-1 dependent. Furthermore, the study provides direct in vivo evidence for the involvement of a homophilic interaction between leukocyte and endothelial cell PECAM-1 in neutrophil transmigration and enhanced expression of α6β1 on transmigrated neutrophils, collectively suggesting a role for PECAM-1 homophilic interaction in both neutrophil migration through endothelial cell junctions and through the perivascular basement membrane. Indeed, lack of defects in leukocyte transendothelial cell migration but defects in leukocyte migration through the PBM in PECAM-1–deficient animals (6, 8) suggests a key role for PECAM-1 in regulation of leukocyte migration through the latter barrier. However, even in this regard, there is evidence for PECAM-1–independent mechanisms as the observed defect in leukocyte migration through the PBM in PECAM-1–deficient mice is transient and stimulus specific (6) and the lack of inhibitory effect of α6 integrin blockade in the PECAM-1 KO mice indicates the existence of PECAM-1– and α6β1-independent mechanisms of leukocyte transmigration. Future characterization of such mechanisms will enhance our understanding of the molecular events involved in leukocyte emigration as well as identifying targets for the development of novel antiinflammatory therapies.
Acknowledgments
This work was funded by grants from the British Heart Foundation (PG/98016) and The Wellcome Trust, UK (058260/Z/99/Z/JMW/TH/JF).
J. Dangerfield and K.Y. Larbi contributed equally to this work.
Footnotes
Abbreviations used in this paper: EM, electron microscopy; ICAM, intercellular adhesion molecule; PBM, perivascular basement membrane; PECAM, platelet-endothelial cell adhesion molecule.
References
- 1.Carlos, T.M., and J.M. Harlan. 1994. Leukocyte-endothelial adhesion molecules. Blood. 84:2068–2101. [PubMed] [Google Scholar]
- 2.Muller, W.A. 1995. Migration of leukocytes across the vascular intima. Molecules and mechanisms. Trends Cardiovasc. Med. 5:15–20. [DOI] [PubMed] [Google Scholar]
- 3.Schenkel, A.R., Z. Mamdouh, X. Chen, R.M. Liebman, and W.A. Muller. 2002. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3:143–150. [DOI] [PubMed] [Google Scholar]
- 4.Johnson-Leger, C., M. Aurrand-Lions, and B.A. Imhof. 2000. The parting of the endothelium: miracle, or simply a junctional affair? J. Cell Sci. 113:921–933. [DOI] [PubMed] [Google Scholar]
- 5.Liao, F., H.K. Huynh, A. Eiroa, T. Greene, E. Polizzi, and W.A. Muller. 1995. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 182:1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson, R.D., K.E. Noble, K.Y. Larbi, A. Dewar, G.S. Duncan, T.W. Mak, and S. Nourshargh. 2001. Platelet endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 97:1854–1860. [DOI] [PubMed] [Google Scholar]
- 7.Muller, W.A., S.A. Weigl, X. Deng, and D.M. Phillips. 1993. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan, G.S., D.P. Andrew, H. Takimoto, S.A. Kaufman, H. Yoshida, J. Spellberg, J.L. De la Pomapa, A. Elia, A. Wakeham, B. Karan-Tamir, et al. 1999. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J. Immunol. 162:3022–3030. [PubMed] [Google Scholar]
- 9.Wakelin, M.W., M.-J. Sanz, A. Dewar, S.M. Albelda, S.W. Larkin, N.K. Boughton-Smith, T.J. Williams, and S. Nourshargh. 1996. An anti-PECAM-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J. Exp. Med. 184:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller, W.A. 1995. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J. Leukoc. Biol. 57:523–528. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka, Y., S.M. Albelda, K.J. Horgan, G.A. Van Seventer, Y. Shimizu, W. Newman, J. Hallam, P.J. Newman, C.A. Buck, and S. Shaw. 1992. CD31 expressed on distinctive T cell subsets is a preferential amplifier of β1 integrin-mediated adhesion. J. Exp. Med. 176:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman, M.E., and W.A. Muller. 1995. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18). J. Immunol. 154:299–307. [PubMed] [Google Scholar]
- 13.Varon, D., D.E. Jackson, B. Shenkman, R. Dardik, I. Tamarin, N. Savion, and P. Newman. 1998. Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood. 91:500–507. [PubMed] [Google Scholar]
- 14.Pellegatta, F., S.L. Chierchia, and M.R. Zocchi. 1998. Functional association of platelet endothelial cell adhesion molecule-1 and phosphoinositide 3-kinase in human neutrophils. J. Biol. Chem. 273:27768–27771. [DOI] [PubMed] [Google Scholar]
- 15.Timpl, R. 1996. Macromolecular organization of basement membranes. Curr. Opin. Cell Biol. 8:618–624. [DOI] [PubMed] [Google Scholar]
- 16.Bohnsack, J.F. 1992. CD11/CD18-independent neutrophil adherence to laminin is mediated by the integrin VLA-6. Blood. 79:1545–1552. [PubMed] [Google Scholar]
- 17.Rieu, P., P. Lesavre, and L. Halbwachs-Mecarelli. 1993. Evidence for integrins other than β2 on polymorphonuclear neutrophils: expression of α6β1 heterodimer. J. Leukoc. Biol. 53:576–582. [DOI] [PubMed] [Google Scholar]
- 18.Sixt, M., R. Hallmann, O. Wendler, K. Scharffetter-Kochanek, and L.M. Sorokin. 2001. Cell adhesion and migration properties of β2-integrins negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J. Biol. Chem. 276:18878–18887. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama, J., A. Hidemura, H. Saito, and H. Nagawa. 2000. Shear stress affects migration behaviour of polymorphonuclear cells arrested on endothelium. Cell. Immunol. 203:39–46. [DOI] [PubMed] [Google Scholar]
- 20.Ley, K., D.C. Bullard, M.L. Arbones, R. Bosse, D. Vestweber, T.F. Tedder, and A.L. Beaudet. 1995. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181:669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnenberg, A., H. Janssen, F. Hogervorst, J. Calafat, and J. Hilgers. 1987. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J. Biol. Chem. 262:10376–10383. [PubMed] [Google Scholar]
- 22.Noto, K., K. Kato, K. Okumura, and H. Yagita. 1995. Identification and functional characterisation of mouse CD29 with a mAb. Int. Immunol. 7:835–842. [DOI] [PubMed] [Google Scholar]
- 23.Harari, O.A., J.F. McHale, D. Marshall, S. Ahmed, D. Brown, P.W. Askenase, and D.O. Haskard. 1999. Endothelial cell E- and P-selectin up-regulation in murine contact sensitivity is prolonged by distinct mechanisms occurring in sequence. J. Immunol. 163:6860–6866. [PubMed] [Google Scholar]
- 24.Roussel, E., and M.-C. Gingras. 1997. Transendothelial migration induces rapid expression on neutrophils of granule-release VLA-6 used for tissue infiltration. J. Leukoc. Biol. 62:356–362. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg, A., P.W. Modderman, and F. Hogervorst. 1988. Laminin receptor on platelets is the integrin VLA-6. Nature. 336:487–489. [DOI] [PubMed] [Google Scholar]
- 26.Terpe, H.J., H. Stark, P. Ruiz, and B.A. Imhof. 1994. Alpha 6 integrin distribution in human embryonic and adult tissues. Histochemistry. 101:41–49. [DOI] [PubMed] [Google Scholar]
- 27.Timpl, R. 1989. Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 180:487–502. [DOI] [PubMed] [Google Scholar]
- 28.Graesser, D., A. Solowiej, M. Bruckner, E. Osterwei, A. Juedes, S. Davis, N.H. Ruddle, B. Engelhardt, and J.A. Madri. 2002. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 109:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward, P.A. 1995. Adhesion molecule knockouts: one step forward and one step backward. J. Clin. Invest. 95:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etzioni, A., C.M. Doerschuk, and J.M. Harlan. 1999. Of man and mouse: Leukocyte and endothelial adhesion molecule deficiencies. Blood. 94:3281–3288. [PubMed] [Google Scholar]
- 31.Kumasaka, T., W.M. Quinlan, N.A. Doyle, T.P. Condon, J. Sligh, F. Takei, A.L. Beaudet, C.F. Bennett, and C.M. Doerschuk. 1996. Role of the intracellular adhesion molecule-1 (ICAM-1) in endotoxin-induced Pneumonia evaluated using ICAM-1 antisense oligonucleotides, anti-ICAM-1 monoclonal antibodies, and ICAM-1 mutant mice. J. Clin. Invest. 97:2362–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet, P. 2002. Integrin indecision. Nat. Med. 8:14–16. [DOI] [PubMed] [Google Scholar]
- 33.Eppihimer, M.J., J. Russell, D.C. Anderson, B.A. Wolitzky, and D.N. Granger. 1997. Endothelial cell adhesion molecule expression in gene-targeted mice. Am. J. Physiol. 273:H1903–H1908. [DOI] [PubMed] [Google Scholar]
- 34.Werr, J., J. Johansson, E.E. Eriksson, P. Hedqvist, E. Ruoslahti, and L. Lindbom. 2000. Integrin α2β1 (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 95:1804–1809. [PubMed] [Google Scholar]
- 35.Frieser, M., R. Hallmann, S. Johansson, D. Vestweber, S.L. Goodman, and L. Sorokin. 1996. Mouse polymorphonuclear granulocytes binding to extracellular matrix molecules involves β1 integrins. Eur. J. Immunol. 26:3127–3136. [DOI] [PubMed] [Google Scholar]
- 36.Kubes, P., X.F. Niu, C.W. Smith, M.E. Kehrli, P.H. Reinhardt, and R.C. Woodman. 1995. A novel β1-dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J. 9:1103–1111. [PubMed] [Google Scholar]
- 37.Werr, J., X. Xie, P. Hedqvist, E. Ruoslahti, and L. Lindbom. 1998. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J. Exp. Med. 187:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao, F., J. Ali, T. Greene, and W.A. Muller. 1997. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J. Exp. Med. 185:1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christofidou-Solimidou, M., M.T. Nakada, J. Williams, W.A. Muller, and H.M. DeLisser. 1997. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J. Immunol. 158:4872–4878. [PubMed] [Google Scholar]
- 40.Nakada, M.T., K. Amin, M. Christofidou-Solimidou, C.D. O'Brien, J. Sun, I. Gurubhagavatula, G.A. Heavner, A.H. Taylor, C. Paddock, Q.-H. Sun, et al. 2000. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J. Immunol. 164:452–462. [DOI] [PubMed] [Google Scholar]
- 41.Wei, J., L.M. Shaw, and A.M. Mercurio. 1997. Integrin signaling in leukocytes: lessons from the α6β1 integrin. J. Leukoc. Biol. 61:397–407. [DOI] [PubMed] [Google Scholar]
- 42.Werr, J., E.E. Eriksson, P. Hedquist, and L. Lindbom. 2000. Engagement of β2 integrins induces surface expression of β1 integrin receptors in human neutrophils. J. Leukoc. Biol. 68:553–560. [PubMed] [Google Scholar]