Abstract
In B lymphocytes, immunoglobulin (Ig)M receptors drive development and construction of naive repertoire, whereas IgG receptors promote formation of the memory B cell compartment. This isotype switching process requires appropriate B cell activation and T cell help. In the absence of T cell help, activated B cells undergo Fas-mediated apoptosis, a peripheral mechanism contributing to the establishment of self-tolerance. Using Igμ-deficient μMT mouse model, where B cell development is blocked at pro-B stage, here we show an alternative developmental pathway used by isotype-switched B cell precursors. We find that isotype switching occurs normally in B cell precursors and is T independent. Ongoing isotype switching was found in both normal and μMT B cell development as reflected by detection of IgG1 germline and postswitch transcripts as well as activation-induced cytidine deaminase expression, resulting in the generation of IgG-expressing cells. These isotype-switched B cells are negatively selected by Fas pathway, as blocking the Fas/FasL interaction rescues the development of isotype-switched B cells in vivo and in vitro. Similar to memory B cells, isotype-switched B cells have a marginal zone phenotype. We suggest a novel developmental pathway used by isotype-switched B cell precursors that effectively circumvents peripheral tolerance requirements. This developmental pathway, however, is strictly controlled by Fas/FasL interaction to prevent B cell autoimmunity.
Keywords: lymphopoiesis, B cell antigen receptor, immune tolerance, apoptosis, autoimmunity
Introduction
B cell development compartmentalizes processes of generation and selection from memory formation, not only by anatomical sites but also with various phenotypic changes (1–3). Perhaps the most significant and irreversible changes are applied to the B cell antigen receptor (BCR) before entering the memory compartment. Throughout a T cell–dependent process and selection, IgM-expressing B cells undergo somatic mutation and isotype switching to express, in most cases, IgG receptor with increased affinity to the antigen. Some of these cells differentiate into memory B cells and give rise to a high affinity antigen-specific repertoire (for review see references 1, 2, 4, and 5). Thus, although Igμ is preferentially used for B cell development in the BM, Igγ isotypic receptors are associated with formation of the memory compartment (3, 6), whereas Igμ-expressing memory B cells have been described in human and mouse (7, 8).
Maturation of B cells to the periphery is limited by autoimmunity in the BM (9, 10) and subsequent positive selection mediated by BCR signaling (11–14). In the periphery, B cell activation depends on T cell help and positive selection of high affinity clones promotes development into the memory compartment, a process also associated with isotypic changes in the BCR (1, 5, 15). In contrast, lack of T cell help results in Fas-mediated apoptosis, a peripheral mechanism thought to eliminate autoreactive newly formed B cells, and B cells acquiring self-reactivity by somatic mutations during the germinal center reaction (1, 16–19), a paradigm also called “the second window of tolerance” (18). Hence, peripheral activation and the Fas pathway are important in controlling B cell autoimmunity (16, 20–22) and preventing the generation of an autoimmune memory repertoire (23). In contrast, sensitized human memory B cells undergo Fas-independent apoptosis (24). This may suggest that B cell development also compartmentalizes tolerance requirements in Igμ-developing and -mature B cells from an isotype-switched non-Igμ memory compartment.
The requirement of functional μ heavy (H) expression and signaling in promoting B cell development has been shown in several models of mutated mice (13, 25). It is also supported by studies showing that IgG or γH chains fail, in general, to promote B cell development in transgenic (Tg) mice (for review see reference 6). Nevertheless, the process of isotype switching in BM development has not been studied yet. In many μH Tg mice, despite the efficient allelic exclusion mediated by the Tg μH, IgG-expressing B cells are generated, resulting in production of serum IgG (26, 27). Interestingly, lack of Fas in these μH Tg mice generates an autoimmune IgG repertoire (26). These studies may suggest that isotype switching can occur in BM development, although this is difficult to prove in these mice. Hence, γH-driven repertoire may have differential roles in terms of B cell development and tolerance requirements. In this study we use mice lacking the IgM transmembrane tail exons (μMT), in which B cell development is blocked at the pro-B stage (28), to show a novel developmental pathway used by isotype-switched B cell precursors that is T cell independent. These B cells circumvent the peripheral activation requirement and develop directly into the memory compartment. This developmental pathway is tightly controlled by Fas/FasL interaction to prevent B cell autoimmunity. Therefore, we propose that the Fas pathway plays an important role in the establishment of tolerance during BM development.
Materials and Methods
Mice.
Normal C57/BL6 (B6), C57/BL6-Faslpr/Faslpr (B6/lpr), C57/BL6-μMT/μMT (μMT), C57/B6-TCRβ−/−/TCRδ−/− (TCRβ−/−/TCRδ−/−) mice were purchased from The Jackson Laboratory. Mice carrying both lpr and μMT homozygous mutations (μMT/lpr) were generated by crossing B6/lpr and μMT mice. Littermates were typed for μMT and lpr by PCR. To generate μMT/lpr/TCRβ−/−/TCRδ−/− (four knockout) mice, μMT/lpr mice were crossed with TCRβ−/−/TCRδ−/− mice (29, 30). The progeny was screened by PCR analysis to identify TCRδ deficiency (primer sequences as published by The Jackson Laboratory) and by flow cytometry to identify TCRβ deficiency. In some experiments 3-83μδ Tg mice were used (31). All animals were kept under specific pathogen-free conditions. All experiments were approved by the Technion review board.
BM Cell Culture and Cell Sorting.
B cell precursors from normal, μMT, and μMT/lpr mice were grown in vitro as previously described (32). In brief, RBC-depleted BM cells were cultured at a concentration of 2 × 106 cells/ml for 2–5 d in the presence of 50–100 U/ml rIL-7. Cells grown in these primary cultures were used directly for cellular and molecular analysis. In some experiments BM cells were depleted of Ig+ cells using magnetic beads (Miltenyi Biotec), and cells were labeled with CFSE (Molecular Probes), washed, and incubated with IL-7 for 5 d. Cells were then stained for surface markers and analyzed for CFSE labeling by FACS®. In some experiments BM cells were cultured in the presence of 2.5–5 μg/ml hamster anti–mouse Fas antibody (clone Jo2; BD Biosciences) or 25–50 μg/ml recombinant FasIg (33). In other experiments cultured B cells were stained with goat anti–mouse κ-biotin (Southern Biotechnology Associates, Inc.) and Igκ+ cells were sorted using streptavidin MACS microbeads (Miltenyi Biotec). RNA samples from sorted cells were used for RT-PCR. In some experiments B220+ cells were sorted directly from BM and spleen by magnetic beads and used for mRNA preparation.
RT-PCR.
Total RNA was purified from cultured cells or sorted Igκ+ cells. RNA samples were reverse transcribed to cDNA and PCR amplified to detect Fas, FasL, and γH chain expression and for GαS control as previously described (32). PCR conditions for Fas were 30 s at 94°C, 30 s at 52°C, and 60 s at 72°C for 30 cycles. Primer sequences for Fas were: sense, 5′-TGTCCTGCCTCTGGTGCT-3′; antisense, 5′-ACAACCATAGGCGATTTCTGG-3′. PCR conditions for FasL were 30 s at 94°C, 60 s at 52°C, and 90 s at 72°C for 30 cycles. Primer sequences for FasL were: sense, 5′-CATGCAGCAGCCCGTGAATTACCC-3′; antisense, 5′-TTAAAGCTTATATAAGCCAAAAAAAGGT-3′. For detection of γH chain expression we used 5′ oligo specific for VHJ558 family 5′-CGAGTCCTCCAG(AC)ACAGCCTACAT-3′ and γCH1 5′-CGTGTCAGGCTAGCGGGTGTTGTTTTGGC-3′. PCR conditions for γH chain were: 30 s at 94°C, 30 s at 63°C, and 60 s at 72°C for 28 cycles. For detection of ongoing isotype switching we used RT-PCR amplification for IgG germline and postswitch transcripts as well as for activation-induced cytidine deaminase (AID). To detect IgG germline transcripts we used primers at the Iγ1F and constant-γ1R or at Iγ2b and constant-γ2bR. To detect IgG postswitch transcripts we used primers at IμF and constant-γ1R or constant-γ2bR. PCR conditions and primer sequencing were as previously described (34).
Analysis of Serum Ig.
Titers of IgG in serum were determined by sandwich ELISA using specific goat anti–mouse polyclonal reagents (Southern Biotechnology Associates, Inc.). Purified IgG was used for the standard curve and for calculation of Ab concentration. In some experiments serum samples were analyzed by SDS-PAGE on a 10% gel. After transfer to nitrocellulose, membrane blots were blocked with 5% milk and detected using goat anti–mouse IgG (γ chain–specific) biotinylated antibody (Southern Biotechnology Associates, Inc.), followed by streptavidin horseradish peroxidase. Visualization of specific bands was performed by enhanced chemiluminescence reagent.
ELISPOT.
Detection of Ab-forming cells by ELISPOT assay was performed as previously described (35). In brief, nitrocellulose filters were placed in 5 ml tissue culture Petri dishes and coated with a mix of 2.5 μg/ml goat anti–mouse κ and goat anti–mouse λ (Southern Biotechnology Associates, Inc.). Filters were then washed (0.1% Tween 20/PBS) and blocked with 5% BSA. Before adding the cells, filters were washed twice with PBS and twice with IMDM (GIBCO BRL). RBC-depleted splenocytes were resuspended in IMDM and cultured on top of filters for 12–16 h. After incubation, filters were extensively washed and incubated with goat anti–mouse IgG (γ chain–specific) biotinylated Ab. Streptavidin conjugated with horseradish peroxidase was used as a secondary probe and signals were generated by the enhanced chemiluminescence reaction.
Flow Cytometry.
Single cell suspension from spleen or BM cultures were stained for surface marker expression using FITC-, PE-, and biotin-conjugated Abs, visualized with streptavidin TriColor (Caltag Laboratories). Abs used for cell staining were: CD19, clone 1D3; CD21, clone 7G6; Fas, clone Jo2; TCRβ, clone H57 (all from BD Biosciences); CD23, clone 2G8; B220, clone RA3-6B2; CD5, clone B19.1; goat anti–mouse κ and goat anti–mouse γ (all from Southern Biotechnology Associates, Inc.). Data for three-color analysis were collected on a FACSCalibur™ and analyzed using CELLQuest™ software (Becton Dickinson). For all analysis forward and side scatter gates were set to include viable cells and exclude dead cells and debris.
Preparation, Microencapsulation, and Injection of FasIg.
FasIg-transfected 3T3 cells engineered to secrete a recombinant form of mouse Fas ligated to human Fc were provided by P. Leder (Harvard Medical School, Boston, MA; reference 33). Recombinant soluble FasIg was purified from cell culture using conventional protein-A column. The eluted FasIg was tested by flow cytometry for its ability to interact with membrane FasL on D4 cells (provided by A. Marshak-Rothstein, Boston University, Boston, MA) and inhibit T cell apoptosis in vitro (33). Soluble FasIg was directly added to BM cultures as described above, or microencapsulated for injection into mice. FasIg-loaded Poly(dl-lactide-coglycolide) PLGA microcapsules were prepared using the double emulsion solvent extraction method as previously described (36). The average concentration of FasIg release from 10 mg microcapsules in culture medium in vitro was 10 ± 1.5 ng/ml/day. Microcapsules were resuspended in sterile PBS and administered to μMT mice by intramuscular injection of 10 mg/mouse (in 100 μl PBS). The first injection was at 2 wk old. Mice were injected with the same dose every 10 d for a period of 40 d (an additional four injections). 2 wk after the last injection the mice were killed for analysis.
Results
Isotype Switching Rescues the Development of μMT B Cells Expressing a Marginal Zone (MZ) Phenotype in Fas-deficient Mice.
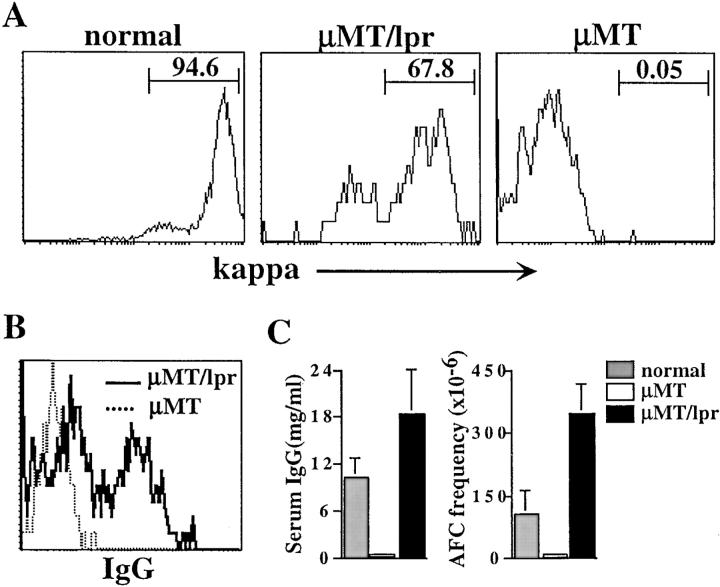
In μMT mice, B cell development is blocked at the pro-B stage (28) and essentially no serum Abs are produced. However, as we previously showed, lack of Fas rescues a significant development of isotype-switched B cells and non-IgM serum Ab production (37). Most of these cells expressed κ (68%; Fig. 1 A) and underwent isotype switching, as indicated by surface staining for γH chain (Fig. 1 B). Despite the small number of mature B cells, these mice developed serum IgG titers and IgG Ab-forming cells that were significantly higher than those of normal mice (Fig. 1 C).
Figure 1.
Lack of Fas rescues development of isotype-switched B cells in μMT mice. (A) Spleen cells from normal, μMT/lpr, and μMT mice were stained for CD19, B220, and κ. Analysis for κ expression was performed on gated B220+/CD19+ cells. (B) Surface expression of IgG. Spleen cells from the indicated mice were stained for CD19, B220, and IgG. Gated B220+/CD19+ cells were analyzed for IgG expression. (C) Serum IgG and frequency of IgG-producing cells in the indicated mice. Serum samples were collected from mice at 8–12 wk old, and titers of IgG (left) were determined by ELISA. Results shown as mean ± SEM of at least five mice in each group. Numbers of IgG-producing cells (right) were determined by ELISPOT assay and are expressed as frequency of IgG-producing cells per 106 spleen cells. Results are mean ± SEM of at least five mice in each group.
Because the development of mature B cells in μMT/lpr mice is abnormal and characterized by an autoimmune reactivity (37), we analyzed B cell populations in spleen. To distinguish between the newly formed (CD21lo/CD23−), follicular (CD21+/CD23+), and MZ (CD21+/CD23−) B cell populations, splenic B cells were stained with antibodies to CD21 and CD23, as previously described (3, 11, 38). The results showed a significant increase of 6–10-fold in the relative size of B cells expressing the MZ phenotype in μMT/lpr mice (Fig. 2 A). Absolute number analysis revealed that 25–30% of κ1 cells in μMT/lpr mice were MZ B cells compared with 3–5% in normal mice (Fig. 2 B). Further analysis revealed that most κ1 cells in μMT/lpr mice were B2 cells, as they did not express CD5 (Fig. 2 C). We conclude that isotype-switched B cells developing in μMT/lpr mice accumulate in the MZ compartment.
Figure 2.
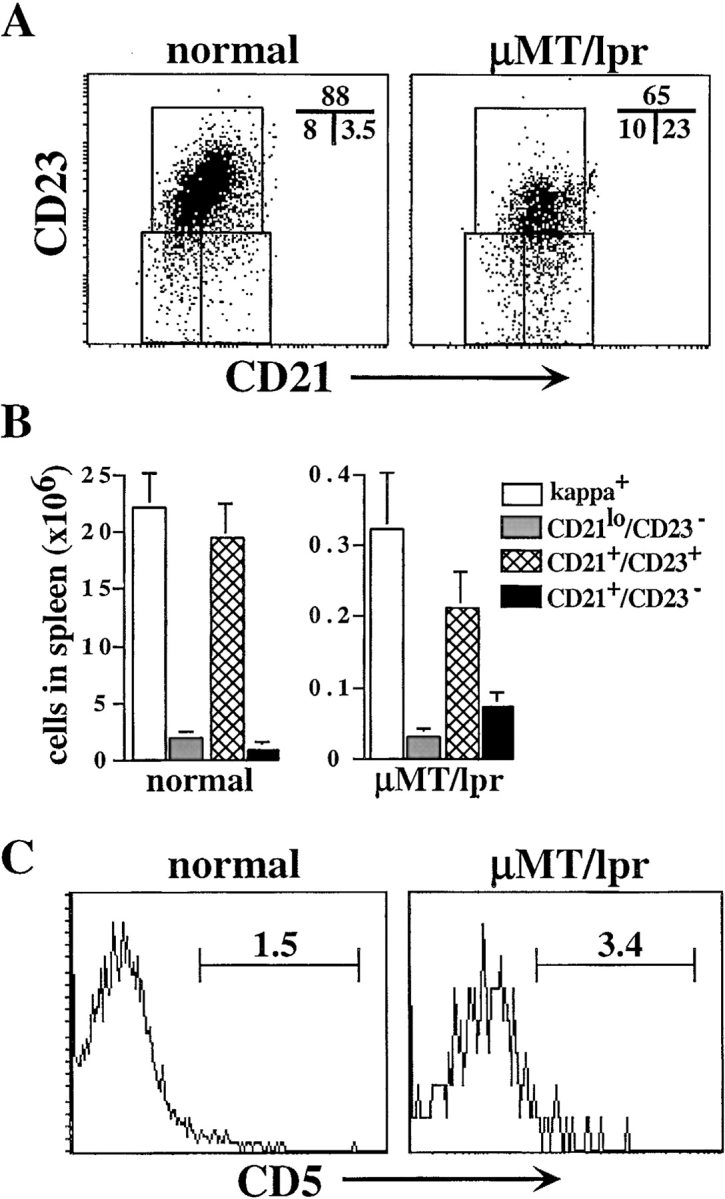
Accumulation of isotype-switched B cells in MZ of spleen in μMT/lpr mice. (A) Spleen cells from normal and μMT/lpr mice were stained for CD21, CD23, and κ. FACS® analysis for expression of CD21 and CD23 was performed on gated κ1 cells. (B) Absolute numbers of newly formed (CD21lo/CD23−), follicular (CD21+/CD23+), and MZ (CD21+/CD23−). Results are mean ± SEM of three mice in each group. (C) FACS® analysis for CD5 expression in gated CD19+/κ1 spleen cells from the indicated mice.
Lack of FasL Allows Development of Isotype-switched μMT B Cells In Vitro.
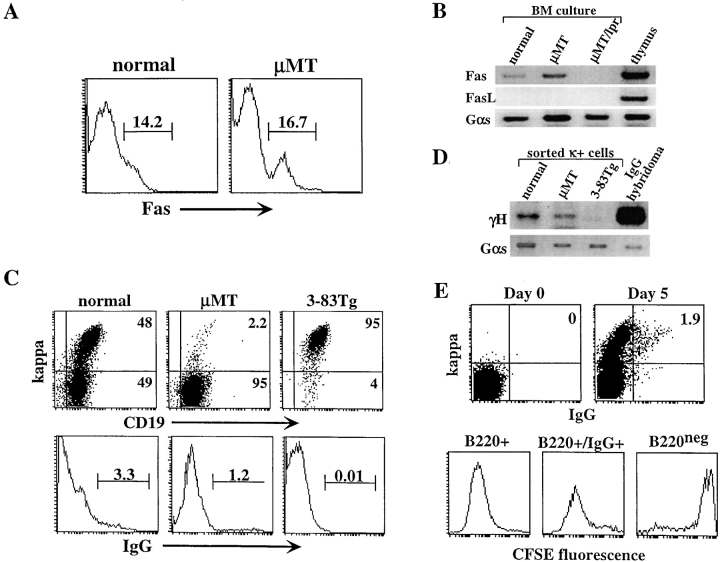
The generation of mature B cells in μMT/lpr mice suggests that Fas has a direct role in negative selection of isotype-switched B cells. This hypothesis predicts that isotype switching occurs in μMT and normal mice but such cells are deleted by Fas-dependent mechanisms. To test this hypothesis, we used our IL-7–driven BM culture system. Surface staining revealed that 15–20% of developing μMT and normal B cells in culture expressed Fas receptor (Fig. 3, A and B) , thereby supporting a few earlier studies showing Fas expression in developing B cells in vivo (39, 40). As expected, functional Fas expression was not detectable in μMT/lpr cultures (Fig. 3 B). In contrast, FasL expression could not be detected in either culture, probably due to the lack of T cells, which are the major source of FasL (Fig. 3 B).
Figure 3.
Lack of FasL allows development of isotype-switched B cell precursors in vitro. BM cells from normal and μMT mice were cultured for 5 d in the presence of IL-7. (A) Cells grown in culture were stained for B220 and Fas receptor and analyzed by FACS®. Expression of Fas was determined in gated B220+ cells. (B) RNA samples from normal μMT and μMT/lpr BM cultures were analyzed by RT-PCR for Fas and FasL gene expression and for Gαs internal control. Thymus RNA sample was used for positive control. In μMT/lpr sample, no product was observed in the PCR for Fas because oligonucleotides used flank the deleted DNA fragment that is found in lpr mice. (C) Surface expression of κ and IgG in BM cultures. BM cells from normal, μMT, and 3-83μδ Tg mice were cultured in vitro, and cells were stained for B220, CD19, κ, and IgG. Analysis for κ (top) and surface IgG expression was performed on 5,000 gated B220+ cells. (D) RT-PCR amplification for detection of γH expression in BM cultures. κ-expressing cells from the indicated BM cultures were sorted using magnetic beads. mRNA samples from the sorted cells were subjected to RT-PCR amplification using oligonucleotides specific for VHJ558 and γCH1. mRNA from IgG-producing hybridoma was used as control. (E) Normal BM cells were depleted of Ig+ cells using magnetic beads, labeled with CFSE on day 0, washed, and cultured for 5 d with IL-7. Cells were stained and analyzed for IgG and κ coexpression on days 0 and 5 (top). Cells grown in the culture were stained for B220 and IgG and analyzed for CFSE fluorescence (bottom). Analysis was performed on gated B220+ cells (left), B220+/IgG+ cells (middle), or B220− cells (right). Results are representative of three different experiments.
The possibility that lack of FasL would allow development of isotype-switched μMT B cells was directly tested by cell staining. Strikingly, we found a significant population of 1.5–2.5% κ-expressing B cells in μMT BM cultures (Fig. 3 C, top). Further surface staining for IgG revealed the development of 1–2% of isotype-switched B cells in the μMT cultures (Fig. 3 C, bottom). These cells are B220+/CD43− (not depicted), suggesting that they progressed to the Ig+ immature stage. To confirm these results, κ-expressing cells were sorted by magnetic beads and mRNA samples were subjected to RT-PCR amplification using oligonucleotides specific for VHJ558 and the first five amino acids of the γCH1 regions (AKTTP). As shown in Fig. 3 D, the expression of γH chain was evident in μMT cultures. Importantly, γH could also be detected in normal BM cultures, implicating the biological relevance of isotype switching to normal B cell development. In control cultures of 3–83μδ Tg mice, γH expression was not detectable by surface staining (Fig. 3 C, bottom) or by RT-PCR (Fig. 3 D). To confirm that the IgG/κ-expressing cells in the normal cultures are not residual memory or plasma cells that may populate the BM, Ig+ cells were depleted and cells were labeled with CFSE, washed, and cultured in vitro for 5 d. Cells were then analyzed for κ and IgG coexpression (Fig. 3 E, top) and CFSE fluorescence (Fig. 3 E, bottom). The results clearly show that no IgG/κ-expressing cells are present at the initiation of the cultures, but a significant population of 2% is evident on day 5. These cells are probably culture derived, as they have minimal CFSE fluorescence as the total B220+ cells in the culture (Fig. 3 E, bottom, compare left to middle), due to multiple divisions during the culture period. In contrast, the nondividing B220− cells present in the culture, which are B220−, are stained for CFSE and correspond to the initial labeling (Fig. 3 E, bottom right). These results suggest that isotype switching occurs during BM development of μMT and normal mice, and that maturation of switched B cells is controlled by Fas/FasL pathway.
Detection of Ongoing Isotype Switching in μMT and Normal BM Cultures and in BM In Vivo.
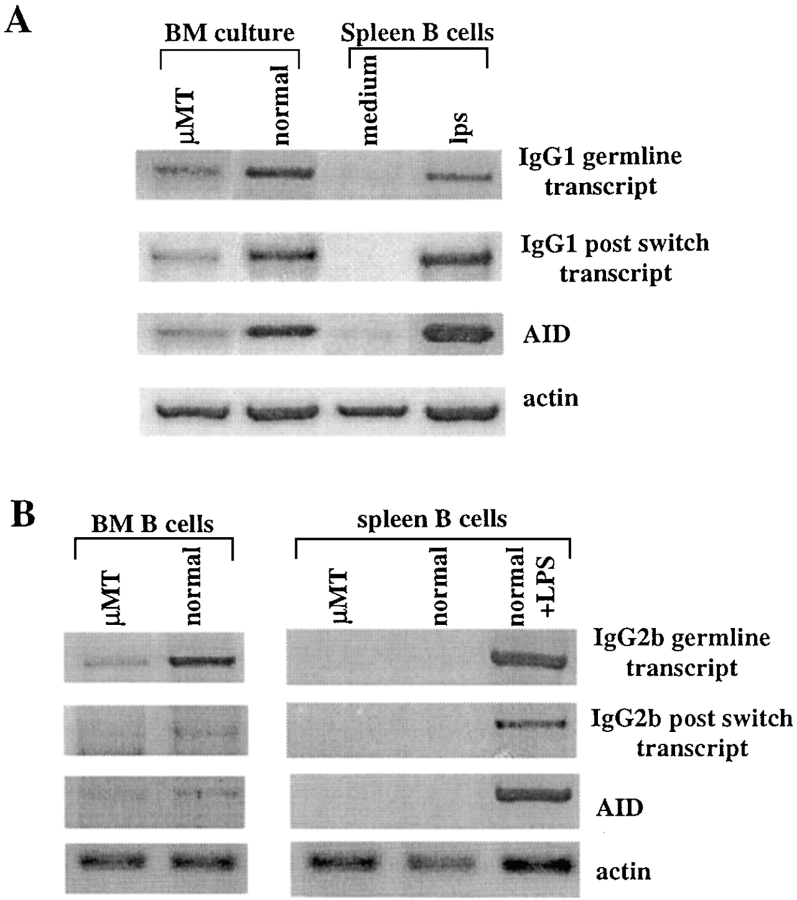
To directly confirm that isotype switching occurs during normal and μMT B cell development in vitro, we used RT-PCR amplification for well-described markers indicating ongoing isotype switching such as IgG germline and postswitch transcripts and expression of AID, as previously described (34). The results in Fig. 4 A clearly show detection of all three markers that indicate ongoing isotype switching in BM cultures of normal and μMT mice. Thus, AID is significantly expressed in developing B cells and detection of IgG1 germline transcript suggests that these loci are accessible for recombination. The detection of IgG1 postswitch transcript implicates that isotype switching does occur in both the normal and the μMT cultures. These markers were undetected in control mature splenic B cells but were induced in these cells upon LPS treatment as has been shown (34), thereby indicating the specificity of the assay.
Figure 4.
Ongoing isotype switching in normal and μMT BM cultures and BM in vivo. (A) BM cells from normal and μMT mouse were cultured in vitro for 5 d in the presence of IL-7. mRNA samples from the cultured cells were subjected to RT-PCR amplification for IgG1 germline and postswitch transcript as well as for AID expression. Normal splenic cells either unstimulated or stimulated with LPS were used as negative and positive controls, respectively. The results shown are representative of three to five different experiments and a total of five to eight mice in each group. (B) B220+ cells were sorted directly from BM or spleen of the indicated mice and mRNA samples were subjected to RT-PCR amplification for AID and IgG2b germline and postswitch transcripts. Representative blots from three mice in each group are shown.
To confirm for the biological relevance, ongoing isotype switching was tested in B220+ cells sorted directly from μMT and normal BM or spleen. We found low but significant expression of AID and IgG2b germline transcript in both μMT and normal BM, but not in spleen B cells, suggesting that this spontaneous isotype switching is restricted to the BM. We were able to find detectable expression of IgG2b postswitch transcript only in normal BM B cells. This may result from increased frequency of isotype switching in normal BM as also reflected in our BM cultures (about twofold more IgG-expressing cells shown in Fig. 3 C, and increased levels of isotype switching markers shown in Fig. 4 A). These results confirm that isotype switching is an ongoing process during normal and μMT B cell development in BM cultures and in the BM in vivo, resulting in the expression of IgG BCR shown in Fig. 3.
Isotype-switched B Cell Precursors Are Eliminated by Fas/FasL Pathway.
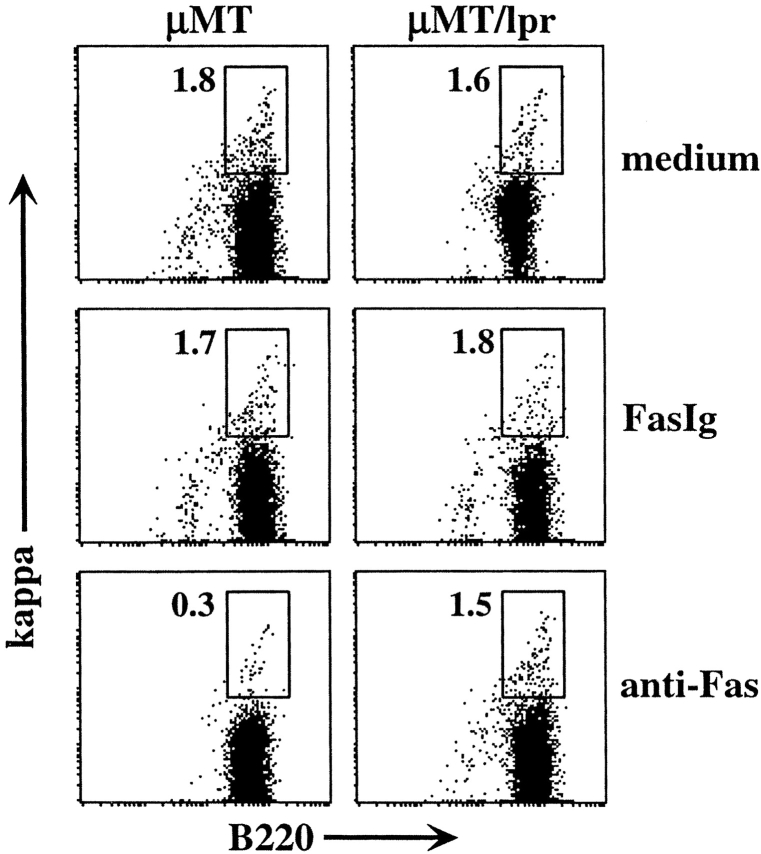
To test whether isotype-switched B cell precursors are eliminated by Fas-dependent mechanisms, we treated cultured cells with anti-Fas antibodies during the last 48 h of culture. In these experiments we followed the appearance and/or disappearance of κ-expressing cells using monoclonal reagents to ensure specificity of the staining. The results in Fig. 5 clearly show the appearance of a significant population of 1.5–2% of isotype-switched κ1 cells (average of three experiments, 1.7 ± 0.4) in both μMT and μMT/lpr cultures (Fig. 5, top). However, upon addition of anti-Fas antibodies, the κ1 B cell population was significantly reduced in μMT cultures by five- to sixfold, and only 0.3% κ1 cells (average of three experiments, 0.35 ± 0.1) could be detected (Fig. 5, bottom). In contrast, anti-Fas antibodies had no effect in μMT/lpr cultures, which are deficient in functional Fas receptors. The addition of FasIg, a recombinant form of soluble Fas that is capable of binding FasL and neutralizing the Fas/FasL interaction (33), had no effect in either culture (Fig. 5, middle). Thus, we conclude that isotype-switched B cell precursors are susceptible to Fas-mediated deletion.
Figure 5.
Fas receptor ligation eliminates Isotype-switched B cell precursors in vitro. BM cells from μMT and μMT/lpr mice were cultured for a total of 5 d in the presence of IL-7. During the last 48 h, cultured cells were treated with 25 μg/ml FasIg or 2.5 μg/ml anti-Fas antibodies. Cultured cells were stained for CD19, B220, and κ. FACS® analysis was gated to viable CD19+ cells. The plots shown are representative of three different experiments.
Blocking of the Fas/FasL Interaction In Vivo Allows Development of Isotype-switched B Cells in μMT Mice.
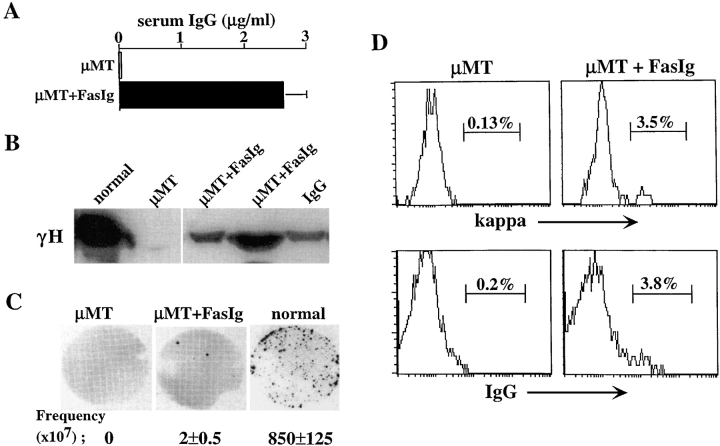
To test whether blocking the Fas/FasL interaction in vivo would rescue isotype-switched B cell precursors, PLGA microcapsules containing recombinant FasIg were injected into μMT mice. As described in Materials and Methods, these capsules allowed continuous slow release of FasIg at a constant amount of 10 ng/ml/day. 10 d after the last FasIg microcapsule injection, mice were tested for the presence of mature B cells and antibody production. The results in Fig. 6 clearly show that inhibition of Fas/FasL interaction by continuous presence of FasIg rescued isotype-switched B cell precursors in μMT mice. Serum analysis revealed a low, but significant amount of IgG production in μMT mice treated with FasIg (Fig. 6 A). This was in contrast to the control μMT mice, which fail to produce any serum IgG. The presence of IgG in sera was confirmed by Western blotting and independent detection of γH chain (Fig. 6 B). The specificity of the ELISA and the Western blot assays was confirmed and no reactivity was found between the anti–mouse IgG reagent and human IgG or purified FasIg (not depicted). Using an ELISPOT assay we were able to detect low frequency of IgG-producing cells in the FasIg-treated μMT mice (total of 10 ± 4/spleen) relative to control (Fig. 6 C). Finally, staining of spleen cells for κ and IgG expression revealed a very low population of mature, isotype-switched B cells in μMT mice treated with FasIg (total of 1,750 ± 585/spleen), but not in the control μMT group (Fig. 6 D). Thus, administration of FasIg to μMT mice rescues isotype-switched B cells from Fas-mediated apoptosis allowing maturation and serum IgG production in these mice, as confirmed by several experimental techniques.
Figure 6.
Blocking the Fas/FasL interaction in vivo rescues isotype-switched B cells in μMT mice. A neutralizing recombinant FasIg protein was purified and engineered into slow releasing PLGA microcapsules. The microcapsules were administered to μMT mice by intramuscular injection of 10 mg/mouse every 10 d for 8 wk. Mice were first injected at 2 wk of age. Control μMT mice were injected with empty microcapsules. 10 d after the last injection, mice were killed. (A) Serum samples from the mice were assayed for the presence of IgG by ELISA. Results are mean ± SEM of three mice in each group. (B) Detection of γH chain in serum samples by Western blotting. Normal serum sample dilution is 1:10. Samples of μMT mice (control and FasIg-treated) were not diluted. Purified IgG was used as control. Line indicates where irrelevant lane was removed digitally. (C) Quantitation of IgG-producing cells in spleens of μMT mice treated with FasIg relative to μMT and normal mice by ELISPOT. Representative ELISPOT membranes and the calculated frequency of IgG-producing cells per 107 spleen cells are shown. Results are mean ± SEM of three mice in each group. (D) FACS® analysis for κ and IgG expression. Spleen cells from the treated mice were stained for CD19, B220, κ, and IgG. Analysis for κ and IgG expression was performed on 10,000 gated CD19+/B220+ cells. The results shown are representative of eight injected mice in three different experiments.
Developmental Pathway of Isotype-switched B Cell Precursors Is T Cell Independent.
To test for the role of T lymphocytes in regulating the developmental pathway of isotype-switched B cell precursors, we crossed the Fas-deficient μMT mice with mice deficient in TCRβ and TCRδ. Results in Fig. 7 show that lack of T lymphocytes did not abolish the development of isotype-switched B cells in μMT/lpr mice. We found a low, but significant amount of 0.4–1.2 mg/ml IgG in serum samples collected from μMT/lpr/TCRβδ−/− mice at different ages (8–16 wk; Fig. 7 A). The presence of IgG in the serum was confirmed by Western blotting (Fig. 7 C) and IgG-producing plasma cells were visualized and quantitated at a significant frequency by ELISPOT assay (Fig. 7, A and B). In addition, low numbers of κ-expressing cells are detected in μMT/lpr/TCRβδ−/− spleens by flow cytometry (0.5–0.8%; unpublished data). Furthermore, 2–3% κ-expressing cells could be detected in BM culture prepared from μMT/lpr/TCRβδ−/− mice (Fig. 7 D), frequencies that are similar to those found in μMT and μMT/lpr BM cultures (Figs. 3 and 5). Thus, the developmental pathway and maturation of isotype-switched B cell precursors is T cell independent. However, because levels of serum IgG and frequency of IgG-producing cells in the μMT/lpr/TCRβδ−/− mice are significantly reduced relative to the μMT/lpr mice, it is possible that T lymphocytes are important in selection and expansion of particular isotype-switched B cell clones, as has been proposed (41).
Figure 7.
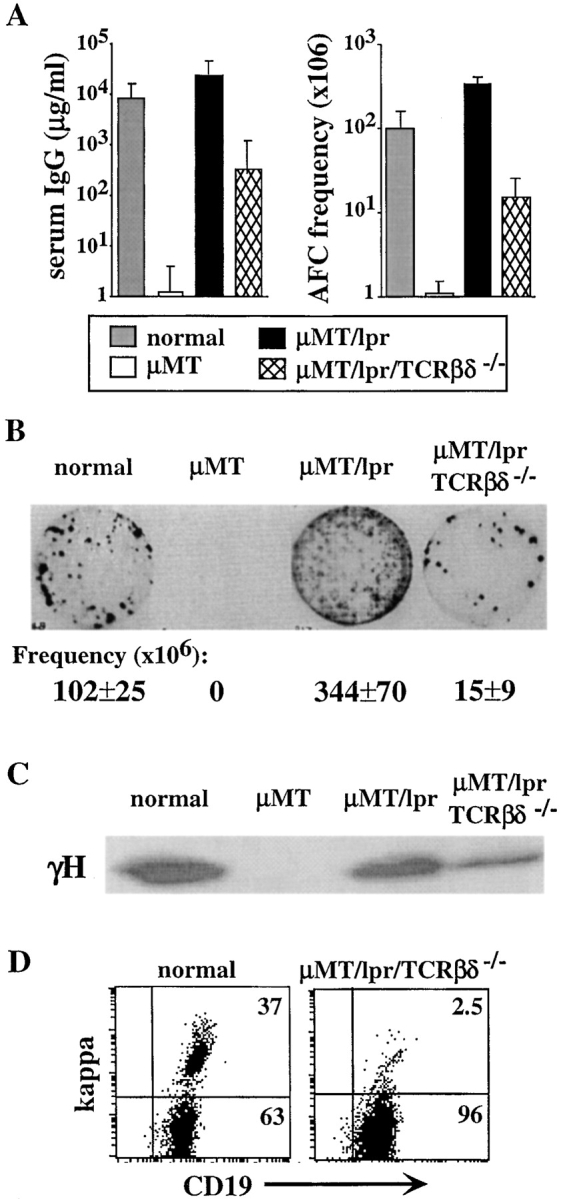
Development of isotype-switched B cell precursors is T cell independent. Normal, μMT, μMT/lpr, and μMT/lpr/TCRβδ−/− mice were analyzed for development of isotype-switched B cells and production of serum IgG. (A) Titers of serum IgG were measured by ELISA (left) and are expressed as milligram/milliliter. Frequency of IgG-producing cells in spleens of these mice was quantitated by ELISPOT. The results are mean ± SEM of at least five mice in each group. (B) Representative ELISPOT membrane. Frequencies of plasma cells are mean ± SEM of at least five mice in each group. (C) Detection of γH chain in serum samples by Western blotting. Normal serum sample dilution is 1:10 and μMT/lpr serum dilution is 1:20. Samples of μMT and μMT/lpr/TCRβδ−/− are not diluted. (D) Expression of κ-expressing cells in μMT/lpr/TCRβδ−/− BM cultures. Cells grown in BM cultures prepared from the indicated mice were stained for B220, CD19, and κ. Analysis for κ expression is performed on 5,000 gated B220+ cells.
Discussion
This study attempts to investigate the role of isotype switching in promoting B cell development and its regulation. The process of isotype switching has been linked, so far, with mature B cell activation in response to T-dependent antigens (1, 2, 5). Here we show for the first time that isotype switching occurs normally during BM development of B cell precursors and that development of these cells is regulated by Fas signaling. Therefore, we suggest that an alternative developmental pathway is used by isotype-switched B cell precursors. The possibility that this pathway is involved in B cell autoimmunity is discussed below.
In μMT mice, both V(D)J recombination and isotype switching should occur to allow the development of mature, IgG-producing cells. Because V(D)J recombination is not altered in μMT mice (28), there are two possible explanations for lack of B cells in μMT mice: (a) B cell precursors are not undergoing isotype switching, and (b) isotype-switched B cells fail to develop and/or are negatively selected. Recent studies have shown that isotype-switched B cells can develop in μMT mice deficient of functional Fas (μMT/lpr; reference 37), and development of some IgA-secreting cells was evident in μMT mice responding to intestinal bacteria (42). By using our in vitro culture system, here we show that isotype switching to γH occurs in developing μMT B cell precursors as well as in normal B cell precursors. The physiological relevance of this phenomenon was further confirmed by detection of ongoing isotype switching in developing B cells directly in BM and not in the spleen of both μMT and normal mice (Fig. 4 B). This suggests that spontaneous isotype switching is restricted to developing B cells in the BM and is not artificially induced in vitro. Ongoing class switching was revealed at the RNA level by detection of AID IgG1 and IgG2b germline and postswitch transcripts (Fig. 4), all evident for the occurrence of class switch recombination (34). This is further confirmed by the detection of IgG-expressing cells (Fig. 3) in the normal and the μMT cultures. Detection of class switching in the normal cultures and directly in the BM is important in linking the biological and the physiological relevance of isotype switching to normal B cell development, rather than to being artificially driven by the neo gene inserted into the μMT mouse genome. In addition, under appropriate stimulation, RAG−/− precursors have also been shown to undergo isotype switching (43). What triggers isotype switching in B cell precursors is unclear. It is tempting to speculate that this process is T cell dependent. This possibility is unlikely because B cells develop normally in T cell–deficient mice (44) and only few T cell can be found in our BM cultures (<3%), and, as shown here, isotype-switched B cells develop in μMT/lpr/TCRβδ−/− mice. Therefore, it is possible that isotype switching can occur spontaneously in normal B cell development, as has been described in several B cell lines and tumor cells (45), though at a low frequency, as we show here. This may suggest that γH-driven B cell development may have differential regulation and/or tolerance requirement relative to the well-described μH-driven development. One of these differential regulations that we show here is the sensitivity to Fas-mediated apoptosis.
How do we reconcile the facts that isotype switching occurs in developing B cells, but these cells fail to mature in μMT mice? One possible explanation is that γH fails to promote B cell development and maturation (46). Data collected from several γH Tg mice clearly showed that B cell development and maturation and the establishment of allelic exclusion are severely impaired (for review see reference 6). The inability of γH in supporting B cell development may be attributable to isotype-specific differences in membrane proximal regions and to stage-specific signaling requirements (6, 47). An alternative explanation we raise here is that isotype-switched B cells are negatively selected in the BM. A possible mechanism of such negative selection is apoptosis, induced by Fas signaling (17). The data we present here clearly support this hypothesis, showing that lack of Fas signaling rescues isotype-switched B cell precursors in μMT/lpr mice, in μMT BM cultures in vitro, and in mice injected with FasIg slow-releasing microcapsules. Fas expression was detected in developing BM B cells in vivo (39, 40) and in BM cultures in vitro (Fig. 3), and activation of the Fas receptor resulted in increased cell death (unpublished data) and elimination of most of the Ig+ cells in μMT cultures (Fig. 5). This is in contrast to earlier studies, which failed to demonstrate a role for Fas receptor in negative selection of self-reactive Ig Tg B cell precursors in lpr mice (26, 48). Importantly, B cell development in these Ig Tg mouse models is driven by Tg μH, which may lead to Fas-independent pathway. Interestingly, lack of Fas signaling in these mouse models allowed the development of isotype-switched B cells secreting high titers of anti-DNA serum IgG (26). The ability of isotype-switched IgG-expressing B cells to develop in these μH-Tg/lpr mice (26), as well as in μMT/lpr mice (37), in μMT BM cultures, and in FasIg-treated μMT mice, may suggest that γH can support B cell development. However, as we show here, cells developing in this pathway are sensitive to negative selection imposed by Fas signaling.
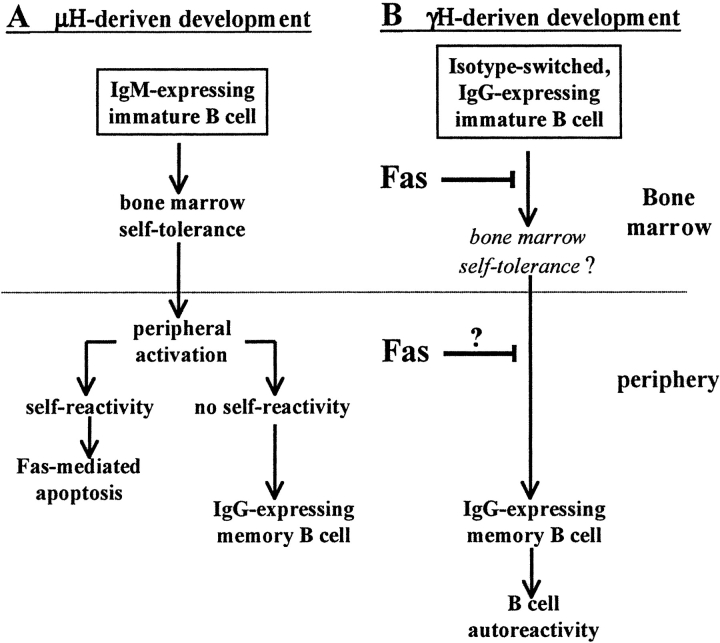
Our interpretation of the results is illustrated in Fig. 8 , suggesting that different isotypic BCRs use different developmental pathways in B cell hematopoiesis. Most of B cell development is driven by μH (Fig. 8 A; reference 13). In this pathway, IgM-expressing immature B cells are subjected to negative selection imposed by immune tolerance, where both clonal deletion and receptor editing are major mechanisms (9, 10). Data collected indicate that Igμ-driven B cell development and tolerance in the BM is Fas independent (26, 48). However, upon maturation and peripheral activation, B cells express Fas and are sensitive to Fas-mediated apoptosis. This has been attributed to newly formed cells encountering self-antigens in the periphery and to cells acquiring self-reactivity during the germinal center reaction and somatic mutation (1, 16, 17). Such cells undergo Fas-mediated apoptosis, but can be rescued upon appropriate T cell help provided by CD40L (1). In lpr mice, lack of Fas signaling promotes selection of autoreactive B cells (20–22), thereby implying a major role of this molecule in maintaining peripheral tolerance. In contrast, nonself–B cells undergo affinity maturation and isotype switching to enter the memory long-lived compartment (1, 2, 5).
Figure 8.
Suggested models for alternative developmental pathways driven by different Ig heavy chain isotypes. The prevailing view of B cell development is shown in A. Most of B cell development is driven by μH chain, and IgM-expressing immature B cells are subjected to self-tolerance in the BM. Mature peripheral B cells express IgM and IgD and become activated upon encountering antigen. Published data suggest that peripheral activation is an important checkpoint to extinguish self-reactivity, mediated by lack of T cell help. In contrast, appropriate T cell help (such as CD40L expression) promotes high affinity, nonself– B cells to develop in the germinal center and undergo isotype switching and differentiate into the memory compartment. The alternative developmental pathway of isotype-switched B cell precursors is shown in B. According to this model, IgG receptor drives IgG-expressing B cells directly into the memory compartment. Because such development circumvents the requirement for peripheral activation, it may confer severe autoimmune risk. In the normal (non-lpr) mouse, such cells are negatively selected by the Fas pathway to prevent their development into the memory compartment and participation in an autoimmune response. Thus, the Fas/FasL pathway is a fail-safe mechanism for elimination of isotype-switched B cell precursors and has a major role in controlling autoimmunity during BM development.
Isotype-switched B cells use the alternative developmental pathway shown in this model (Fig. 8 B). Accordingly, the γH-driven developmental pathway allows isotype-switched B cell precursors to develop directly into the memory compartment as a result of the IgG isotypic BCR. The validity of this developmental pathway is supported by earlier studies by Martin and Goodnow (3) showing that IgG membrane tail signaling directs IgG and IgM/G-expressing cells into the memory compartment, and that generation of memory B cells (49) and acquisition of somatic mutations (21) can occur independently of germinal center formation. Such cells accumulate in the MZ subset in the spleen, as this subset contains many of the memory B cells in immunized animals (6, 50). Our results are in agreement with these studies by showing accumulation of mature B cells expressing an MZ phenotype in μMT/lpr mice because only isotype-switched B cells can develop in mice carrying the μMT mutation. Also, mature μMT/lpr B cells in spleen express relatively low levels of BCR (Fig. 1 A and reference 37), a characteristic of isotype-switched memory B cells in normal mice in vivo (6, 50). Thus, isotype-switched B cell precursors can develop directly into the memory compartment, effectively circumventing the requirement for peripheral activation. Such a pathway confers severe autoimmune risk, as these memory cells are not subjected to peripheral tolerance mechanisms that are linked with activation (Fig. 8 A; references 1, 16, and 17). In support of this are several studies showing that tolerance is maintained in anti–self-IgM Tg mice (10, 31), whereas autoantibodies are produced in anti–self-IgG Tg mice (51, 52). However, as we show here, Fas signaling strictly controls the development of isotype-switched B cell precursors in non-Tg mice. We believe that this negative selection aims to prevent the generation of autoimmune memory B cell population originating from isotype-switched B cell precursors, as Fas-deficient μMT mice generate an autoimmune IgG repertoire (37). It is now interesting to find whether isotype-switched B cell precursors undergo self-tolerance in the BM, or perhaps are negatively selected regardless of their BCR specificity. Such studies should consider the possibility that IgM- and IgG-expressing immature B cells might have different signaling requirements for establishment of tolerance (10, 31, 51, 52). Although it is clear from our data that isotype switching occurs during B cell development, we cannot exclude the possibility that self-reactivity is promoted in the μMT/lpr mice because of peripheral lymphopenia. This, however, does not exclude the role of Fas in negative selection of isotype-switched B cells as we show here in vivo and in vitro using Fas-sufficient mice. Whether autoimmunity in the μMT/lpr mice is a result of lymphopenia or a direct effect of Fas deficiency in selection of self-reactive clones is yet to be determined. It is worth noting in this regard that at least with T lymphocytes, it is still unclear whether lymphopenia promotes autoimmunity or autoimmunity causes lymphopenia.
Although T lymphocytes are key regulators for the induction of isotype switching in activated Igμ B cells (1, 2, 5), isotype switching in B cell precursors is T independent. However, the low amounts of serum IgG and IgG-secreting cells in μMT/lpr/TCRβδ−/− mice suggest a role for T lymphocytes in positive selection and expansion of isotype-switched B cell clones (1). This is supported by several studies showing that development of activated B cells into the MZ and the memory compartment depends on proper signaling and positive selection of high affinity clones (3, 11, 15, 53).
Finally, isotype-switched B cell precursors that circumvent limitation of tolerance may generate an autoimmune memory repertoire as found in μMT/lpr mice and in anti–self-IgG Tg mice (37, 51, 52). In the non-Tg mouse, the development of these cells depends exclusively on lack of Fas functionality (37). Recent data suggest an additional role of Fas signaling in promoting selection and activation of self-reactive cells and in memory formation (21–23). If not deleted, such as in mice carrying lpr or gld (nonfunctional FasL) mutations, isotype-switched B cells may develop into the memory compartment. In a T-dependent response, these cells can be positively selected and activated to participate in B cell autoimmunity. Hence, although Fas functionality in controlling mature B cell activation and tolerance is well described, this study implies a major role for this pathway in negative selection and in controlling autoimmunity at early stages of B cell development.
Acknowledgments
This research is supported by The Israel Science Foundation, the German-Israel Foundation for Scientific Research and Development, Young Scientists' Program, and the Colleck Research Fund.
Abbreviations used in this paper: AID, activation-induced cytidine deaminase; BCR, B cell antigen receptor; H, heavy; MZ, marginal zone; Tg, transgenic.
References
- 1.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 2.Klinman, N.R. 1997. The cellular origins of memory B cells. Semin. Immunol. 9:241–247. [DOI] [PubMed] [Google Scholar]
- 3.Martin, S.W., and C.C. Goodnow. 2002. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 3:182–188. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. [DOI] [PubMed] [Google Scholar]
- 5.Han, S., B. Zheng, Y. Takahashi, and G. Kelsoe. 1997. Distinctive characteristics of germinal center B cells. Semin. Immunol. 9:255–260. [DOI] [PubMed] [Google Scholar]
- 6.Pogue, S.L., and C.C. Goodnow. 2000. Gene dose-dependent maturation and receptor editing of B cells expressing immunoglobulin (Ig)G1 or IgM/IgG1 tail antigen receptors. J. Exp. Med. 191:1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White, H., and D. Gray. 2000. Analysis of immunoglobulin (Ig) isotype diversity and IgM/D memory in the response to phenyl-oxazolone. J. Exp. Med. 191:2209–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed, D., R.J. Benschop, J.C. Cambier, and D. Nemazee. 1998. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 92:173–182. [DOI] [PubMed] [Google Scholar]
- 10.Hartley, S.B., M.P. Cooke, D.A. Fulcher, A.W. Harris, S. Cory, A. Basten, and C.C. Goodnow. 1993. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 72:325–335. [DOI] [PubMed] [Google Scholar]
- 11.Martin, F., and J.F. Kearney. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 12:39–49. [DOI] [PubMed] [Google Scholar]
- 12.Meffre, E., R. Casellas, and M.C. Nussenzweig. 2000. Antibody regulation of B cell development. Nat. Immunol. 1:379–385. [DOI] [PubMed] [Google Scholar]
- 13.Rolink, A.G., C. Schaniel, J. Andersson, and F. Melchers. 2001. Selection events operating at various stages in B cell development. Curr. Opin. Immunol. 13:202–207. [DOI] [PubMed] [Google Scholar]
- 14.Shivtiel, S., N. Leider, O. Sadeh, Z. Kraiem, and D. Melamed. 2002. Impaired light chain allelic exclusion and lack of positive selection in immature B cells expressing incompetent receptor deficient of CD19. J. Immunol. 168:5596–5604. [DOI] [PubMed] [Google Scholar]
- 15.Shih, T.A., E. Meffre, M. Roederer, and M.C. Nussenzweig. 2002. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat. Immunol. 3:570–575. [DOI] [PubMed] [Google Scholar]
- 16.Rathmell, J.C., M.P. Cooke, W.Y. Ho, J. Grein, S.E. Townsend, M.M. Davis, and C.C. Goodnow. 1995. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 376:181–184. [DOI] [PubMed] [Google Scholar]
- 17.Krammer, P.H. 2000. CD95's deadly mission in the immune system. Nature. 407:789–795. [DOI] [PubMed] [Google Scholar]
- 18.Klinman, N.R. 1996. The “clonal selection hypothesis” and current concepts of B cell tolerance. Immunity. 5:189–195. [DOI] [PubMed] [Google Scholar]
- 19.Nossal, G.J. 1994. Negative selection of lymphocytes. Cell. 76:229–239. [DOI] [PubMed] [Google Scholar]
- 20.Brard, F., M. Shannon, E.L. Prak, S. Litwin, and M. Weigert. 1999. Somatic mutation and light chain rearrangement generate autoimmunity in anti–single-stranded DNA transgenic MRL/lpr mice. J. Exp. Med. 190:691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.William, J., C. Euler, S. Christensen, and M.J. Shlomchik. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 297:2066–2070. [DOI] [PubMed] [Google Scholar]
- 22.Mandik-Nayak, L., S.J. Seo, C. Sokol, K.M. Potts, A. Bui, and J. Erikson. 1999. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti–double-stranded DNA B cells. J. Exp. Med. 189:1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi, Y., H. Ohta, and T. Takemori. 2001. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 14:181–192. [DOI] [PubMed] [Google Scholar]
- 24.Berard, M., M. Casamayor-Palleja, G. Billian, C. Bella, P. Mondiere, and T. Defrance. 1999. Activation sensitizes human memory B cells to B-cell receptor-induced apoptosis. Immunology. 98:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niiro, H., and E.A. Clark. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945–956. [DOI] [PubMed] [Google Scholar]
- 26.Kench, J.A., D.M. Russell, and D. Nemazee. 1998. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J. Exp. Med. 188:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlomchik, M.J., D. Zharhary, T. Saunders, S.A. Camper, and M.G. Weigert. 1993. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int. Immunol. 5:1329–1341. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin u chain gene. Nature. 350:423–426. [DOI] [PubMed] [Google Scholar]
- 29.Mombaerts, P., A.R. Clarke, M.A. Rudnicki, J. Iacomini, S. Itohara, J.J. Lafaille, L. Wang, Y. Ichikawa, R. Jaenisch, M.L. Hooper, et al. 1992. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 360:225–231. [DOI] [PubMed] [Google Scholar]
- 30.Itohara, S., P. Mombaerts, J. Lafaille, J. Iacomini, A. Nelson, A.R. Clarke, M.L. Hooper, A. Farr, and S. Tonegawa. 1993. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 72:337–348. [DOI] [PubMed] [Google Scholar]
- 31.Russell, D.M., Z. Dembic, G. Morahan, J.F. Miller, K. Burki, and D. Nemazee. 1991. Peripheral deletion of self-reactive B cells. Nature. 354:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melamed, D., J.A. Kench, K. Grabstein, A. Rolink, and D. Nemazee. 1997. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J. Immunol. 159:1233–1239. [PubMed] [Google Scholar]
- 33.Hildeman, D.A., T. Mitchell, T.K. Teague, P. Henson, B.J. Day, J. Kappler, and P.C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563. [DOI] [PubMed] [Google Scholar]
- 35.Spira, G., P.D. Gregor, and M.D. Scharff. 1993. The use of chemiluminescence and the ELISA spot assay to identify and enumerate rare immunoglobulin switch variants. J. Immunol. Methods. 165:263–268. [DOI] [PubMed] [Google Scholar]
- 36.Okochi, H., and M. Nakano. 2000. Preparation and evaluation of w/o/w type emulsions containing vancomycin. Adv. Drug Deliv. Rev. 45:5–26. [DOI] [PubMed] [Google Scholar]
- 37.Melamed, D., E. Miri, N. Leider, and D. Nemazee. 2000. Unexpected autoantibody production in membrane Ig-m-deficient/lpr mice. J. Immunol. 165:4353–4358. [DOI] [PubMed] [Google Scholar]
- 38.Loder, F., B. Mutschler, R.J. Ray, C.J. Paige, P. Sideras, R. Torres, M.C. Lamers, and R. Carsetti. 1999. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 190:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carsetti, R., G. Kohler, and M.C. Lamers. 1995. Transitional B cells are the target of negative selection in the B cell compartment. J. Exp. Med. 181:2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandik, L., K.A. Nguyen, and J. Erikson. 1995. Fas receptor expression on B-lineage cells. Eur. J. Immunol. 25:3148–3154. [DOI] [PubMed] [Google Scholar]
- 41.Seery, J.P., E.C. Wang, V. Cattell, J.M. Carroll, M.J. Owen, and F.M. Watt. 1999. A central role for alpha beta T cells in the pathogenesis of murine lupus. J. Immunol. 162:7241–7248. [PubMed] [Google Scholar]
- 42.Macpherson, A.J., A. Lamarre, K. McCoy, G.R. Harriman, B. Odermatt, G. Dougan, H. Hengartner, and R.M. Zinkernagel. 2001. IgA production without mu or delta chain expression in developing B cells. Nat. Immunol. 2:625–631. [DOI] [PubMed] [Google Scholar]
- 43.Rolink, A., F. Melchers, and J. Andersson. 1996. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 5:319–330. [DOI] [PubMed] [Google Scholar]
- 44.Mombaerts, P., E. Mizoguchi, H.G. Ljunggren, J. Iacomini, H. Ishikawa, L. Wang, M.J. Grusby, L.H. Glimcher, H.J. Winn, A.K. Bhan, et al. 1994. Peripheral lymphoid development and function in TCR mutant mice. Int. Immunol. 6:1061–1070. [DOI] [PubMed] [Google Scholar]
- 45.Kadowaki, N., R. Amakawa, T. Hayashi, T. Akasaka, K. Yabumoto, H. Ohno, S. Fukuhara, and M. Okuma. 1995. Immunoglobulin heavy chain class switching, mu to gamma, in a human lymphoma cell line FL-318 carrying a t(14;18)(q32;q21) chromosomal translocation. Leukemia. 9:1139–1143. [PubMed] [Google Scholar]
- 46.Storb, U., P. Roth, and B.K. Kurtz. 1994. γ2b transgenic mice as a model for the rule of immunoglobulins in B cell development. Immunol. Res. 13:291–298. [DOI] [PubMed] [Google Scholar]
- 47.Roth, P.E., L. Doglio, J.T. Manz, J.Y. Kim, D. Lo, and U. Storb. 1993. Immunoglobulin gamma 2b transgenes inhibit heavy chain gene rearrangement, but cannot promote B cell development. J. Exp. Med. 178:2007–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathmell, J.C., and C.C. Goodnow. 1994. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J. Immunol. 153:2831–2842. [PubMed] [Google Scholar]
- 49.Toyama, H., S. Okada, M. Hatano, Y. Takahashi, N. Takeda, H. Ichii, T. Takemori, Y. Kuroda, and T. Tokuhisa. 2002. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 17:329–339. [DOI] [PubMed] [Google Scholar]
- 50.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 51.Tsao, B.P., K. Ohnishi, H. Cheroutre, B. Mitchell, M. Teitell, P. Mixter, M. Kronenberg, and B.H. Hahn. 1992. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J. Immunol. 149:350–358. [PubMed] [Google Scholar]
- 52.Battegay, M., P. Fiedler, U. Kalinke, F. Brombacher, R.M. Zinkernagel, H.H. Peter, G. Kohler, and H. Eibel. 1996. Non-tolerant B cells cause autoimmunity in anti-CD8 IgG2a-transgenic mice. Eur. J. Immunol. 26:250–258. [DOI] [PubMed] [Google Scholar]
- 53.Reed, A.J., M.P. Riley, and A.J. Caton. 2000. Virus-induced maturation and activation of autoreactive memory B cells. J. Exp. Med. 192:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]