Abstract
The Vav family of Rho guanine nucleotide exchange factors is thought to orchestrate signaling events downstream of lymphocyte antigen receptors. Elucidation of Vav function has been obscured thus far by the expression of three highly related family members. We generated mice lacking all Vav family proteins and show that Vav-null mice produce no functional T or B cells and completely fail to mount both T-dependent and T-independent humoral responses. Whereas T cell development is blocked at an early stage in the thymus, immature B lineage cells accumulate in the periphery but arrest at a late “transitional” stage. Mechanistically, we show that the Vav family is crucial for both TCR and B cell receptor (BCR)–induced Ca2+ signaling and, surprisingly, is only required for mitogen-activated protein kinase (MAPK) activation in developing and mature T cells but not in B cells. Thus, the abundance of immature B cells generated in Vav-null mice may be due to intact Ras/MAPK signaling in this lineage. Although the expression of Vav1 alone is sufficient for normal lymphocyte development, our data also reveal lineage-specific roles for Vav2 and Vav3, with the first demonstration that Vav3 plays a critical compensatory function in T cells. Together, we define an essential role for the entire Vav protein family in lymphocyte development and activation and establish the limits of functional redundancy both within this family and between Vav and other Rho–guanine nucleotide exchange factors.
Keywords: thymocyte, antigen receptor, signal transduction, Ca++, mitogen-activated protein kinase
Introduction
T and B lymphocytes differentiate along an ordered program controlled by crucial signals emanating from surface receptors (1). In the T cell lineage, the expression of the pre-TCR induces both differentiation and proliferative expansion of the pool of thymic progenitors, presumably effected by Ras/mitogen activated protein kinase (MAPK) signaling (2–6). In a parallel pathway, the pre–B cell receptor (pre-BCR) induces differentiation and expansion of the pool of pre–B cells (7–9). Since antigen receptors of both immature and mature lymphocytes lack any intrinsic signaling capability, the pre-TCR or pre-BCR, and the TCR or BCR, transmit their signals via associated transmembrane chains (CD3 and TCRζ in T cells or Igα and Igβ in B cells) (10–13) that become tyrosine phosphorylated and recruit adaptor proteins (14, 15) and Vav guanine nucleotide exchange factors (GEFs) (16, 17). Subsequent activation of phosphoinositide 3-OH kinase and phospholipase C (PLC)γ1/2 generates second messengers diacylglycerol (DAG) and inositol triphosphate that induce Ras/MAPK or Ca2+ signaling, respectively (10–13).
The Vav family of three highly conserved Rho-GEFs was initially implicated in signaling downstream of both the TCR and BCR by virtue of inducible tyrosine phosphorylation and the presence of structural domains characteristic of proteins involved in signal transduction (16, 17). Receptor-induced tyrosine phosphorylation and the presence of SH2 and SH3 domains distinguish Vav proteins from the plethora of over 50 known mammalian Rho-GEFs (18). Thus, Vav proteins can interact directly with several tyrosine kinases (through SH2 domain interactions with tyrosine phosphorylation sites) and indirectly with activated tyrosine kinases by binding to adaptor proteins that contain SH2 and/or SH3 binding motifs. Like Vav1 (19, 20), Vav2 and Vav3 are inducibly phosphorylated after stimulation of antigen receptors in immortalized T and B cell lines (21, 22); however, overexpression studies indicate functional differences in the activities of the three family members in coupling to distinct downstream pathways (16, 17). As exchange factors for Rho GTPases, Vav proteins have the potential to regulate antigen receptor–induced actin cytoskeletal rearrangements and other Rho-GTPase–regulated cellular pathways (16, 17). Indeed, loss of Vav1 prevents efficient capping of the TCR through effects on actin polymerization (23, 24). Although the exact mode of Vav regulation in vivo is currently unknown, it is thought that the exchange activity of the DH domain, responsible for regulation of Rho-GTPases, may be controlled by tyrosine phosphorylation of an NH2-terminal autoinhibitory extension (25); however, Vav may have both DH-dependent and DH-independent functions in T cells (26).
The importance of Vav1 in lymphocytes was first demonstrated in mice lacking Vav1 (Vav1ko) which showed activation defects in T and B lymphocytes (27–30) and in NK (31) and γδ T cells (32). However, virtually all of those defects were partial, and Vav1ko mice still developed significant numbers of T cells, whereas development of B cells was nearly intact (27–30). Moreover, Vav1ko lymphocytes retained significant functional ability, presumably due to redundancy between Vav family members. Indeed, analyses of mice deficient in both Vav1 and Vav2 revealed a compensatory function for Vav2 in B lineage cell development and function (33, 34). Although its role in T cell activation has not yet been defined, Vav2 did not appear to have any major function in T cell development (33, 34). Little is known about the function of Vav3 in primary lymphocytes, but analyses of Jurkat T cells (21, 22) and a Vav3-deficient chicken DT40 B cell line (35) suggest that it is involved in both TCR and BCR signaling. Since mice lacking all three Vav proteins have not yet been reported, efforts to define the role of the entire Vav family in lymphocytes have been complicated by the functional redundancy among individual family members.
In this report, we generate mice congenitally lacking all three Vav isoforms and present analyses of lymphocyte development and function. We provide genetic and biochemical evidence that the Vav family is critical and nonredundant in T and B lymphocyte development and function and is essential in the formation of the adaptive immune system. Although the involvement of Vav1 and Vav2 in Ca2+ signaling is well documented (23, 24, 29, 33, 34), our analyses establish that the entire Vav family is indispensable for both TCR- and BCR-induced Ca2+ fluxes. Notably, we observed a dissociation in Vav function at the level of MAPK signaling in which Vav was required in T cells but not in B cells.
Materials and Methods
RNase Protection Assays.
To generate antisense riboprobes, a 198-bp cDNA fragment of murine Vav1 (nucleotides 664–862), a 200-bp cDNA fragment of murine Vav2 (nucleotides 652–852), and a 197-bp cDNA fragment of murine Vav3 (nucleotides 676–873) were generated by RT-PCR using either thymus (Vav1) or spleen RNA (Vav2, Vav3). Each fragment was sequenced and subcloned into pBluescript II SK in antisense orientation with respect to the T7 promoter. Riboprobes were synthesized with T7 RNA polymerase, labeled with α-32P UTP, and PAGE purified. RNase protection assay was performed essentially as described (43) with minor modifications. Briefly, 2.5 μg of total RNA from each sample was hybridized with antisense RNA probes (105 cpm) in 30 μl of 80% formamide, 40 mM PIPES, pH 6.5, 1 mM EDTA, and 0.4 M NaCl at 45°C overnight. The hybrids were digested in 400 μl of 10 mM Tris-HCl, pH 7.5, 5 mM EDTA, 0.3 M NaCl, and 5 μg/ml RNase A at 30°C for 30 min. After proteinase K treatment (125 μg/ml, 37°C, 30 min) and phenol/chloroform extraction, the protected RNA probes were analyzed by 6% PAGE containing 8 M urea. Signal was detected using x-ray film or PhosphorImager scanning with the same exposure time for all samples, and data was analyzed with ImageQuant software (Molecular Dynamics). RNA was isolated using Trizol reagent (Invitrogen).
Generation of Vav3-deficient Mice.
Murine vav3 genomic clones were obtained by screening a 129 strain phage genomic library (Stratagene) with a 438-bp fragment (probe K) corresponding to nucleotides 1,140–1,578 of murine vav3 cDNA, which was generated as described previously (22). This yielded several overlapping vav3 genomic fragments. One of the clones extending toward the 5′ end was used to generate a new probe, which included the 5′ end of DH domain–encoding sequences (probe H). Using both probes, four overlapping clones were characterized that included exons 3–17. A targeting construct was made to replace exon 10, encoding part of the DH domain, with the neomycin-resistance gene in the reverse transcriptional orientation. A thymidine kinase (tk) gene was used for negative selection of cells with randomly integrated constructs. The targeting construct was linearized and transfected into TC-1 ES cells by electroporation. Positive and negative selection of transfectants was performed in media containing G418 (0.4 mg/ml) and gancyclovir (1 μM). Genomic DNA from individual double resistant (G418 and gancyclovir) clones was digested with EcoRI and probed with a 5′ flanking probe on the Southern blot. Several targeted clones were identified by the presence of a 6.8-kb EcoRI band upon hybridization with the 5′ probe, and KpnI digestions were performed to ensure proper homologous recombination at the 3′ end of the targeted region, as identified by hybridization of a 22.0-kb KpnI band with the Neo probe. To rule out any undesired integration events, EcoRI-digested DNA was also hybridized with the Neo probe. Two independently derived heterozygous Vav3 mutant ES cells were subcloned and injected into C57BL/6 blastocysts to generate somatic chimera mice, which were bred with C57BL/6 females to transmit the targeted allele to the germline. F1 heterozygotes were then intercrossed to generate homozygous mutant mice.
Generation of Vav3 Antibodies.
To generate polyclonal Vav3 antibodies, we immunized rabbits with a fusion protein comprising glutathione S-transferase and a Vav3 fragment, including the DH and PH domains (aa 357–525) expressed in Escherichia coli, using standard methods. The specificity of these antibodies for Vav3 and the lack of cross-reactivity with other family members was confirmed by Western blotting of cell lysates of 293T cells transiently transfected with either an empty vector or expression constructs encoding GFP fusions of Vav1, Vav2, or Vav3. mAbs against murine Vav3 were raised by producing a recombinant glutathione S-transferase–Vav3 fusion protein (encompassing 230 aa including the PH and the NH2-terminal SH3 domains) expressed in E. coli and purified over a glutathione-Sepharose column (Amersham Biosciences), which was used for immunization of Vav3-deficient mice. Splenocytes of the immunized mouse were fused with P3U1 mouse myeloma cells, and hybridoma clones were selected and screened for binding to a maltose binding protein–Vav-3 fusion protein by ELISA. One positive hybridoma clone, 6D42, IgG1 isotype, was established and used for production of mAbs, which showed no cross-reactivity with Vav1 or Vav2.
Mice, Cell Suspensions, Antibodies, and Flow Cytometry.
Germline Vav1−/− and Vav2−/− mice were described previously (29, 34) and were gifts from Dr. Tybulewicz (National Institute for Medical Research, London, UK) and Dr. Turner (The Babraham Institute, Cambridge, MA). These mice were bred with Vav3-deficient mice in the mixed B6/129 background and maintained in the SPF facility of Washington University School of Medicine. All mice analyzed in this report were littermates derived from mating heterozygous animals. Cell suspensions were prepared, counted, and stained with antibodies following standard procedures. The following antibody conjugates were used (BD Biosciences): phycoerythrin (PE)-H129.19 (anti-CD4), fluorescein (FITC)-, PE-, cytochrome C (CyC)–, or biotin–53–6.7 (anti-CD8α), PE-3C7, or FITC-7D4 (anti-IL2Rα), FITC-145–2C11 (anti-CD3ɛ), CyC-RA3–6B2 (anti-B220), PE-B3B4 (anti-CD23), and FITC-7G6 (anti-CD21/35). Biotinylated antibodies were detected with streptavidin (SAV)-PE or SAV-CyC (BD Biosciences). Subsets of double negative (DN) thymocytes were analyzed based on expression of CD44 and CD25 after gating out all cells staining with a cocktail of biotinylated antibodies to CD4, CD8, B220, Mac-1, and Gr-1, followed by Streptavidin Cy-Chrome (BD Biosciences). For intracellular staining of TCRβ, cells were first stained with PE-CD4 and Cy-Chrome-CD8α. Subsequently, cells were fixed, permeabilized in 1% saponin, and stained with FITC-labeled anti-Cβ–specific antibodies (H57 clone) and analyzed on FACSCalibur flow cytometer (Becton Dickinson) with CellQuest or FlowJo software. Data are displayed as histograms or dot plots with logarithmic scale. Each plot represents analysis of ≥2 × 105 events collected as listmode files.
T and B Cell Stimulation and Proliferation Assays.
T cells were purified from LN cell suspensions by magnetic sorting and removal of B cells with anti-Ig–coated Dynabeads (Dynal) to avoid receptor cross-linking during the purification process using standard procedures. The purity of resulting T cells exceeded 90% as confirmed by FACS® analysis. B cells were purified from spleen cell suspensions by magnetic removal of cells stained with biotinylated antibodies anti-Thy1.2, CD4, CD8, CD3, Mac-1, and Gr-1 using streptavidin-coated Dynabeads (Dynal). Purity of B cells was confirmed in each preparation by FACS® analysis and exceeded 85%. Lymphocytes were cultured in DME medium supplemented with 10% FCS, pen/strep, l-glutamine, Na-pyruvate, nonessential amino acids, and 2-mercaptoethanol. T cells were stimulated at 5 × 106 cells/ml with either soluble or plate-bound anti-CD3ɛ antibodies (2C11; a gift from Dr. Kanagawa, Washington University, St. Louis, MO) (0.01–10 μg/ml as indicated) and/or anti-CD28 (2 μg/ml) (NA/LE formula; BD Biosciences) as indicated, and B cells were stimulated with soluble anti-IgM Fab2 fragments (10 μg/ml) (Jackson ImmunoResearch Laboratories) for the indicated times at 37°C. For proliferation assays, purified T and B lymphocytes were cultured at a concentration of 5 × 104 cells/100 μl in 96-well flat-bottom tissue culture plates as indicated. Cells were pulsed with 1 μCi 3H-thymidine at 48 h for an additional 12 to 16 h and then collected and scintillation counted. The data are displayed as raw cpm values. All assays were conducted in triplicates.
Immunizations and Antigen-specific Ig ELISA.
To determine the T-independent type 2 (TI-2) immune response, 6–8-wk-old mice were immunized intraperitoneally with 100 μg trinitrophenyl (TNP)-Ficoll (Biosearch Technologies) in alum (Pierce Chemical Co.). T-dependent (TD) immune response was determined by injection of 100 μg TNP-KLH (Biosearch Technologies) in alum. Mice were bled before immunization (day 0) and at days 7 and 14. After boost with 100 μg antigen at day 14, mice were bled at days 21 and 28. Concentrations of TNP-specific serum antibody isotypes were determined by ELISA. ELISA plates (Nunc Maxisorp) were coated at 100 μl/well with 5 mg/ml TNP-BSA (Biosearch Technologies) or with PBS only, as a control, and incubated overnight at 4°C. After overnight incubation, plates were washed three times with wash buffer (Tris-Cl, pH 7.6, plus 2% Triton X-100) and blocked in 1% FCS in PBS (blocking buffer) for 2 h at 4°C. Serum samples were serially diluted in blocking buffer and applied to the ELISA plates in triplicate. After a 2-h incubation at room temperature with gentle rocking, ELISA plates were washed three times with wash buffer. TNP-bound serum antibodies were detected using 100 μg/well HRP-conjugated isotype-specific antibodies (Southern Biotechnology Associates, Inc.) diluted 1:5,000 in blocking buffer. Plates were incubated at room temperature for 1 h with gentle rocking, washed, and developed using ABTS (Sci-Mart) containing H2O2 as a substrate. Optical densities (OD450) were read at 405 nm (Titertek). For each isotype, a standard curve was generated based on OD450 values using sera from a series of immunized WT mice. Subsequently, the experimental samples were serially diluted such that at least two values fell into the linear range of the standard curve. Concentrations of TNP-specific antibodies are shown as relative units compared with the standard curve determined for each specific isotype.
Cell Culture, Transfections, and Immunoblotting.
Cells were cultured in DMEM with 10% FCS. For immunochemistry, cells were plated in coverslips at 5 × 104 cells per well in 24-well plates. For immunoprecipitation and immunoblotting, cells were plated on 100-mm dish at the density of 1.8 × 106 cells per dish, and cells were transfected using SuperFect (QIAGEN) according to manufacturers instructions. At 36 h after transfection, cells were washed with ice cold PBS and then lysed in cold NP-40 lysis buffer (50 mM Tris-Cl, pH 7.6, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 10% glycerol, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml each of leupeptin, aprotinin, and pepstatin) for 10 min at 4°C. Plates were then scraped and crude lysates cleared by centrifugation at 14,000 g for 10 min at 4°C. For immunoprecipitation, total lysates were incubated with 2 μg of antibodies and a 50:50 slurry of protein A–conjugated Sepharose beads (Amersham Biosciences) with rotation for 3 h at 4°C. Immunoprecipitates were washed four times with cold NP-40 lysis buffer, resuspended in 2× SDS sample buffer, and analyzed by Western blotting following standard procedures. Primary antibodies were developed with HRP-conjugated secondary antibodies (Bio-Rad Laboratories, Inc.) and visualized by chemiluminescence (Amersham Biosciences). For analyses of tyrosine phosphorylation events, stimulated cells were lysed in RIPA buffer (PBS, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 10 mM EDTA) supplemented with a protease and phosphatase inhibitor cocktail (Boehringer). Postnuclear lysates were prepared, resolved by SDS-PAGE, and analyzed with antibodies to phosphotyrosine (4G10; Upstate Biotechnology). Immune complexes were detected by enhanced chemiluminescence (Amersham Biosciences).
Ca2+ Fluxes.
Ca2+ signaling was measured by preloading purified B or T cells with either Indo-1-AM or Fluo-4-AM (Molecular Probes) at the concentration of 3–5 μg/ml, 106 cells/ml at 37°C for 45 min, with occasional vortexing. In some experiments, cells were stained with anti–CD4-APC conjugates after loading to allow “gating” on CD4+ T cells during analyses. Cells were washed, resuspended in complete media, and analyzed by flow cytometry by running unstimulated samples for 20 s to establish the baseline, and then cells were stimulated by addition of either anti-IgM antibodies (Jackson ImmunoResearch Laboratories) or anti-CD3 immediately followed by cross-linking with anti–hamster antibodies (BD Biosciences) at the indicated concentrations. Ca2+ fluxes were measured for 8 min followed by the addition of ionomycin for additional 1 min at 0.5 μg/ml.
Intracellular Staining for Phospho-p44/42 MAPK (pErk).
To determine Erk activation in T cells, intracellular staining was done. Thymocyte cell suspensions were prepared directly in prewarmed (37°C) 4% paraformaldehyde (2% final) to fix cells, immediately followed by permeabilization in 100% ice cold methanol for 30 min. As a positive control, fresh cells were stimulated with PMA (20 ng/ml) in PBS for 5 min. Cells were stained with phospho-p44/42 MAPK (pErk) (Thr202/Tyr204, E10 clone; Cell Signaling Technology) in the presence of Fc-block at room temperature for 15–30 min. Anti–mouse IgG1-PE and CD4 CyC were added to each sample and incubated at RT for 15–30 min. Cells were analyzed by flow cytometry and gated electronically based on expression of surface markers as indicated.
Online Supplemental Material.
Fig. S1 depicts analyses of lymphocyte development in Vav2ko and Vav3ko mice. Flow cytometry analyses of thymocytes, splenocytes, LN, and BM cells from WT mice (WT), Vav2ko (Vav2), or Vav3ko (Vav3) mice are shown. Fig. S2 shows the strategy for generation of Vav3-deficient mice. Southern blotting analyses of targeted ES cells used to generate chimeric mice and their offspring and Western blotting analyses of Vav3ko splenocyte lysates with Vav3-specific antibodies showing no full-length or truncated Vav3 protein present are also shown. Fig. S3 presents analyses of humoral TD responses in Vav3-deficient mice. Concentrations of TNP-specific IgM, IgG1, IgG2b, and IgG3 antibodies analyzed at various time points post-primary or secondary immunization with TNP-conjugated keyhole hemagglutinin protein (TNP-KLH) are shown. Fig. S4 presents analyses of cytokine production by Vav-deficient T cells showing defective IL-2 and IFNγ production. Figs. S1–4 are available online at http://www.jem.org/cgi/content/full/jem.20030874/DC1.
Results
All Three Vav Proteins Are Tyrosine Phosphorylated and Recruited by Antigen Receptors in Primary T and B Lymphocytes.
Unlike Vav1, whose expression is predominantly restricted to hematopoietic lineage cells, Vav2 and Vav3 are expressed more broadly (16, 17). To examine their expression in lymphocyte subsets, we used an RNase protection assay and found that Vav1, Vav2, and Vav3 were all expressed in immature and mature populations of T and B cells (Fig. 1 A; unpublished data). Although tyrosine phosphorylation of Vav1 in response to antigen receptor stimulation in lymphocytes is well documented (19, 20), less is known about phosphorylation of Vav2 or Vav3 in T and B lymphocytes (21, 22, 35, 36). To this end, we stimulated freshly isolated LN T cells or splenic B cells with either anti-CD3 or with anti-IgM antibodies, respectively, and found that all three Vav proteins were inducibly tyrosine phosphorylated (Fig. 1 B). Moreover, since Vav1 is implicated in actin cytoskeleton changes that accompany formation of the immunological synapse (23, 24, 37), we examined the localization of all three Vav proteins fused to a GFP tag in anti-CD3–stimulated Jurkat T cells (Fig. 1 C). We found that upon treatment with anti-CD3 antibodies, each one of the Vav fusion proteins colocalized with antigen receptor formed caps and F-actin patches (Fig. 1 C). Together, these results indicate that all three Vav family proteins become tyrosine phosphorylated and colocalize with actin at the TCR caps in lymphocytes.
Figure 1.
Involvement of Vav proteins in antigen receptor signaling in T and B lymphocytes. (A) Expression of the Vav1, Vav2, and Vav3 in lymphocytes. Cells were harvested, and equal amounts of total RNA (5 μg) were analyzed by RNase protection assay with specific riboprobes as indicated. As a loading control, the same preparations of RNAs were hybridized with antisense probe for GAPDH (bottom). This experiment was performed several times using different sets of specific probes with similar results. (B) TCR- and BCR-induced tyrosine phosphorylation of Vav1, Vav2, and Vav3. Freshly isolated LN T cells (top) or splenic B cells (bottom) were either left untreated (−) or treated with stimulatory antigen receptor antibodies (+) for 2 h as indicated. Vav proteins were immunoprecipitated (i.p.) from cell lysates with specific antibodies, and the level of phosphotyrosine was analyzed by immunoblotting with antiphosphotyrosine (anti-PY) antibodies (4G10; Upstate Biotechnology). The mobilities of Vav proteins are indicated. The data are representative of three independent experiments; the relative levels of phosphorylation of individual Vav proteins varied somewhat between experiments. (C) Jurkat T cells were transfected with GFP-tagged full-length Vav1, Vav2, or Vav3 cDNA constructs. Cells were incubated for 16–24 h and then either left untreated or treated with stimulatory anti-CD3 antibodies for 5 h, cytospun, and processed for immunofluorescense microscopy. Staining with rhodamine-phalloidine (red, top) visualized accumulations of F-actin (patches) indicated by white arrows. GFP fluorescence is shown in green (middle). Two colors were merged (bottom) to visualize staining overlap.
Mice with Combined Vav Deficiencies Reveal a Major Compensatory Role for Vav3 in T Cell Development.
Previous studies of Vav1ko mice demonstrated partial defects in T cell development and activation (27–30); however, Vav1ko T cells still retained substantial developmental and functional capability. To determine if this was due to the expression of Vav2 in these cells, we examined mice lacking both Vav1 and Vav2 (Vav1/2ko). As expected based on previous studies (27–30, 33, 34), Vav1ko mice showed diminished numbers of thymocytes and peripheral T cells (approximately two- to threefold; Fig. 2 A), whereas Vav2ko mice had normal T cell development (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20030874/DC1). Vav1/2ko mice had populations of CD4−8− (DN), CD4+8+ (double positive [DP]), and CD4+8− or CD4−8+ (single positive [SP]) thymocytes and peripheral T cells comparable to those in Vav1ko mice (Fig. 2 A). Moreover, subsets of DN thymocytes distinguished based upon expression of CD44 and CD25 (DN1, CD44+25−; DN2, CD44+25+; DN3, CD44−25+; and DN4, CD44−25−) were similar in Vav1ko and Vav1/2ko mice (Fig. 2 A). These experiments suggest that Vav2 has no major function in the development of T lymphocytes in the absence of Vav1.
Figure 2.
Defective lymphocyte development in Vav-deficient mice. (A) Impaired development of Vav-deficient T cells. Single cell suspensions of thymocytes or LN cells from WT mice, Vav1ko (Vav1), Vav1/2ko (Vav1/2), Vav1/3ko (Vav1/3), Vav2/3ko (Vav2/3), or Vav1/2/3ko (Vav1/2/3) mice were counted and stained with fluorescent antibody conjugates and analyzed by flow cytometry as indicated. DN thymocytes were gated as live cells that did not stain with CD4, CD8, B220, γδ TCR, Gr1, and Mac1, as described in Materials and Methods. Total numbers and percentages of gated lymphocyte populations are shown for representative samples (n > 5). (B) Impaired development of Vav-deficient B cells. Cell suspensions of BM or spleen samples were prepared as above and stained and analyzed by flow cytometry as indicated. Total numbers and percentages of gated lymphocyte populations are shown for representative samples (n > 5). Data are displayed as dot plots with logarithmic scale.
To determine if Vav3 compensates for the loss of Vav1 in Vav1ko T cells, we generated Vav3-deficient (Vav3ko) mice using standard gene targeting approaches (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20030874/DC1). Although Vav3ko mice showed normal T cell development (Fig. S1 A), the lack of Vav3 significantly exacerbated the T cell phenotype of Vav1ko mice (Fig. 2 A). Thus, populations of DN4, DP and SP thymocytes, and peripheral T cells were all significantly reduced in Vav1/3ko mice compared with Vav1ko mice (Fig. 2 A), indicating that Vav3 is critical for development of T cells lacking Vav1.
Vav-null T Cells Are Developmentally Blocked at the DN Stage.
To establish the role of the entire family of Vav proteins in lymphocytes, we generated mice lacking Vav1, Vav2, and Vav3 (Vav1/2/3ko). These mice were viable and showed no gross abnormalities of internal organs upon histological examination at 4–6 wk of age (unpublished data). Vav1/2/3ko mice had drastically reduced thymocyte numbers (∼50- to 100-fold compared with WT) with few DP and SP cells or peripheral T cells (which were reduced by ∼100-fold compared with WT) (Fig. 2 A). The block in thymocyte development occurred at the DN3 to DN4 transition, since Vav1/2/3ko mice showed predominantly DN3 cells and only few DN4 cells (Fig. 2 A). In addition, large DN4 cells were essentially absent in Vav1/2/3ko mice (<2% compared with 15–20% in WT; unpublished data). These results clearly demonstrate that Vav proteins are indispensable in T cell development, although we could still detect few DP cells in the thymus and few T cells in the peripheral lymphoid organs of Vav-null mice suggesting some “leakiness” of the developmental block (Fig. 2 A and Fig. 3 B).
Figure 3.
Late developmental block in Vav-null B cells. (A) Late developmental block at the T1/T2 stage and accumulation of NF cells in Vav-null mice. Splenocyte suspensions from WT mice or Vav1/2/3ko (Vav1/2/3) were stained and analyzed by flow cytometry as indicated. Percentages of gated lymphocyte populations are shown for representative samples (n = 3). Total numbers of corresponding populations are shown in Table I. Data are displayed as dot plots with logarithmic scale. (B) Differential requirement for Vav family proteins in T and B lymphocyte development and MAP kinase activation. Schematic representation of developmental defects of lymphoid cells observed in Vav1/2/3ko (Vav1/2/3) mice. See Results and Discussion for details.
Notably, mice lacking Vav2 and Vav3 (Vav2/3ko) showed no obvious T cell abnormalities (Fig. 2 A), indicating that expression of Vav1 alone is sufficient to support T cell development. Thus, these results suggest a hierarchy of Vav proteins in T cell development with Vav1 playing a major role that can be compensated for, at least in part, by Vav3. On the other hand, a compensatory function of Vav2 is only apparent in developing T cells lacking both Vav1 and Vav3.
Vav-null B Cells Show a “Leaky” Developmental Block at the T1/T2 Stage.
In contrast to T cells, immature populations of B cells in BM including progenitors (B220low CD43hiIgM−), precursors (B220+CD43lowIgM−/low), and immature (B220+CD43lowIgM+) B cells appeared to develop normally in mice with single or combined Vav deficiencies (Fig. 2 B; Fig. S1 B; unpublished data). Notably, Vav1/2ko, Vav1/3ko, and Vav1/2/3ko mice showed reduced populations of “recirculating” BM B220hiIgMlo B cells (Fig. 2 B). Mature IgMloIgDhi B cells appeared normal in Vav2ko, Vav3ko, and Vav2/3ko mice and marginally decreased in Vav1ko and Vav1/3ko mice (Fig. 2 B and Fig. S1 B) but were more significantly decreased in Vav1/2ko and Vav1/2/3ko mice (approximate two- to threefold reduction compared with WT; Fig. 2 B). To more precisely determine the developmental defect in B cells lacking all Vav proteins, we used anti-CD21/35 and anti-CD23 antibodies to subdivide B cells into populations of newly formed (NF or B220+ CD21−CD23−), transitional 1 (T1 or CD21loIgMhiIgDlo), transitional 2 (T2 or CD21hiIgMhiIgDhi), follicular (FO or CD21intCD23hiIgMloIgDhi), and marginal zone (MZ or CD21hiCD23lo) cells. Strikingly, these analyses revealed drastically reduced subsets of FO and MZ B cells in Vav1/2/3ko mice compared with WT (Fig. 3 A and Table I). Moreover, T2 cells appeared slightly reduced (approximately twofold) in Vav1/2/3ko mice, whereas the numbers of T1 and NF cells were increased, indicating a “leaky” developmental block at T1/T2 stage (Fig. 3, A and B; Table I).
Table I.
B Lymphocyte Populations in Vav-null Mice
| Total | Newly formed | T1 | T2 | Follicular | MZ | |
|---|---|---|---|---|---|---|
| Wild type | 94.5 ± 38.3 | 4.5 ± 1.4 | 5.3 ± 2.9 | 6.3 ± 2.0 | 12.9 ± 8.6 | 1.9 ± 1.3 |
| Vav null | 88.7 ± 7.6 | 20.6 ± 3.9 | 15.6 ± 3.5 | 2.5 ± 1.1 | 0.7 ± 0.3 | 0.5 ± 0.1 |
Cells from spleens of WT and Vav1/2/3ko (Vav-null) mice were counted and stained for cell surface expression of B220, IgM, IgD, CD23, and CD21/35, as shown in Fig. 3 A, and analyzed by flow cytometry. Numbers of indicated populations are shown for newly formed (NF or B220+CD21−CD23−), transitional 1 (T1 or B220+CD21loIgMhiIgDlo), transitional 2 (T2 or B220+CD21hiIgMhiIgDhi), follicular (FO or B220+CD21intCD23hiIgMloIgDhi), and marginal zone (MZ or B220+CD21hiCD23lo) cells. Data are presented as means ± SDs and represent multiples of 106 cells (n = 3). Total cells were counted by gating live lymphoid cells.
Vav-null Mice Fail To Mount Humoral Responses to TD and TI Antigens.
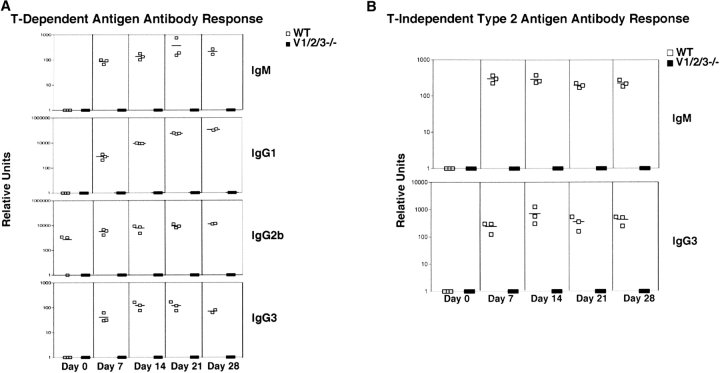
To determine if lymphocytes generated in Vav-null mice are functional in vivo, we immunized Vav1/2/3ko or WT mice with the TD antigen, trinitrophenyl (TNP)-conjugated keyhole hemagglutinin (TNP-KLH), and measured the concentrations of TNP-specific IgM, IgG1, IgG2b, and IgG3 antibodies at various time points post-primary and secondary immunization (Fig. 4 A). In this regard, although Vav1ko and Vav2ko mice were shown previously to have impaired production of switched antibodies against TD antigens, their IgM responses were completely normal (33, 38). In contrast, Vav3ko mice showed robust humoral responses and the ability to isotype switch (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20030874/DC1). Strikingly, Vav1/2/3ko mice completely failed to produce any detectable anti-TNP–specific IgM antibodies at any time point after immunization (Fig. 4 A). Moreover, Vav1/2/3ko mice generated no detectable IgG1, IgG2b, or IgG3 anti-TNP antibodies (Fig. 4 A). These data demonstrate that the entire Vav family is absolutely required for generation of antigen-specific TD humoral responses.
Figure 4.
Failure to mount antigen-specific humoral responses in Vav-null mice. (A) TD responses. WT or Vav1/2/3ko mice were immunized with trinitrophenyl (TNP)-conjugated keyhole haemaglutinin protein (TNP-KLH), and the concentrations of TNP-specific IgM, IgG1, IgG2b, and IgG3 antibodies were analyzed at various time points post-primary or secondary immunization by antigen-specific ELISA as described in Materials and Methods. Each symbol represents an individual mouse, and mean values are indicated by horizontal bars. WT mice (□) and Vav1/2/3ko mice (▪). (B) T-independent type 2 (TI-2) responses. WT or Vav1/2/3ko mice were immunized with a TI-2 antigen, TNP-Ficoll, and the concentrations of TNP-specific IgM and IgG3 antibodies were analyzed as in A. Each symbol represents an individual mouse and mean values are indicated by horizontal bars. WT mice (□) and Vav1/2/3ko mice (▪).
To determine if Vav-null mice could mount TI-2 responses, we immunized WT or Vav1/2/3ko mice with TNP-conjugated Ficoll (TNP-Ficoll) and determined concentrations of TNP-specific IgM and IgG3 antibodies after primary or secondary immunization. In this regard, Vav1ko mice were shown previously to generate normal responses to TI-2 antigens (38), although Vav2ko and Vav1/2ko mice had defective TI-2 responses (33, 34). Strikingly, Vav1/2/3ko mice completely failed to produce any detectable anti-TNP antibodies in response to TI-2 antigen at any time point after immunizations (Fig. 4 B). Together, these data show a strict requirement for Vav proteins in mounting both TD and TI humoral responses.
Vav3 Functionally Compensates for the Loss of Vav1 in T Cell Proliferative Responses.
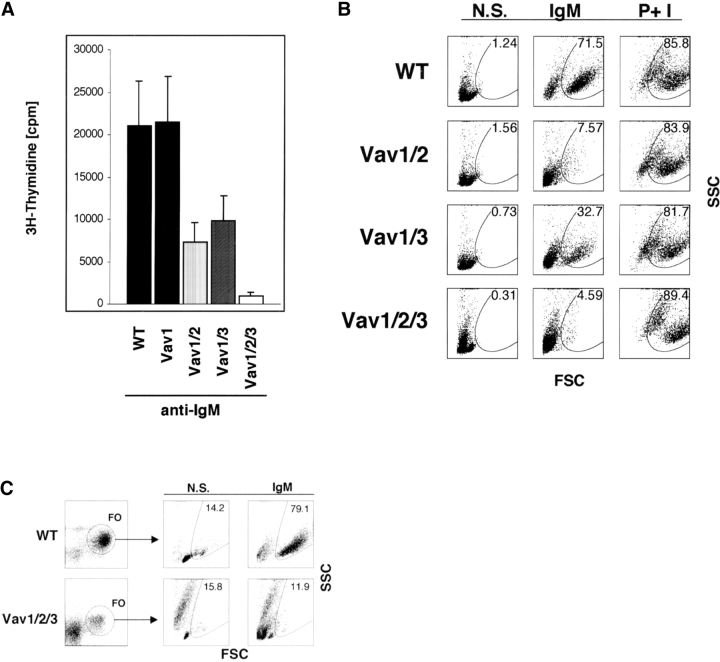
Next, we examined in vitro proliferative responses of T cells lacking Vav proteins. Consistent with previous reports (27–30), Vav1ko T cells showed a partial defect in proliferative responses to stimulation with anti-CD3 with or without anti-CD28 antibodies as measured by 3H-thymidine incorporation (Fig. 5 A). Notably, Vav1/2ko T cells proliferated equally to Vav1ko T cells, indicating that Vav2 does not substantially compensate for the loss of Vav1 (Fig. 5 A). Strikingly, however, T cells from Vav1/3ko or Vav1/2/3ko mice virtually failed to incorporate 3H-thymidine in response to stimulation with anti-CD3 and anti-CD28 antibodies compared with WT (Fig. 5 A). These data demonstrate that, unlike Vav2, Vav3 appears critical for function of T cells that do not express Vav1. T cells from Vav2ko or Vav3ko mice showed normal proliferative responses, indicating that neither of these proteins is essential in T cells expressing Vav1 (unpublished data).
Figure 5.
Defective proliferative responses and blast formation by Vav-deficient T cells. (A) Impaired proliferation of antigen receptor–stimulated T cells lacking Vav proteins. Purified LN T cells from WT (black bars), Vav1ko (Vav1, white dotted bars), Vav1/2ko (Vav1/2, black dotted bars), Vav1/3ko (Vav1/3, white bars), Vav2/3ko (Vav2/3, horizontal striped bars), or Vav1/2/3ko (Vav1/2/3, gray bars) mice were cultured in either media alone or in the presence of stimulatory anti-CD3 and/or anti-CD28 antibodies as indicated. Proliferation was determined at 48 h by 3H-thymidine incorporation and scintillation counting. The data are displayed as raw cpm values. The results are representative of three independent experiments. All assays were conducted in triplicates, and SDs are shown as vertical lines. (B) Defective blast formation of antigen receptor–stimulated Vav-deficient T cells. LN T cells from indicated mice were cultured in either media alone or in the presence of stimulatory anti-CD3 and anti-CD28 antibodies, or with phorbol myristyl acetate (PMA) and ionomycin (I) for 36 h as described in Materials and Methods. Cells were then harvested and analyzed by flow cytometry. Forward scatter dot blots of CD4+ gated cells in representative samples are shown (n > 5) and numbers represent percentages of blasting cells.
As an alternative measure of T cell proliferation, we also assayed for the production of large (blasting) cells. In this assay, nonstimulated fresh LN T cells remained small at various time points in culture (indicated by their low forward and side scatter; Fig. 5 B, left). On the other hand, cultures of WT T cells stimulated with anti-CD3 and anti-CD28 antibodies contained predominantly blasting cells (Fig. 5 B, middle). In contrast, the ability of Vav1ko or Vav1/2ko T cells to generate blasts was reduced by approximately three- to fourfold (Fig. 5 B), whereas T cells from Vav1/3ko or Vav1/2/3ko mice virtually failed to produce blasts in response to anti-CD3 and anti-CD28 antibody stimulation. Thus, unlike Vav1ko T cells, which retain a significant proliferative capacity, T cells lacking both Vav1 and Vav3 have severely disrupted proliferative responses. All T cells responded normally to PMA and ionomycin (Fig. 5 B; unpublished data), indicating no intrinsic cell cycle defects in Vav-deficient T cells.
In addition, we examined cytokine production by T cells in response to TCR stimulation and found that the production of IL-2 and IFNγ appeared equally diminished in Vav1ko and Vav1/2ko T cells compared with WT but was virtually undetectable in Vav1/3ko and Vav1/2/3ko T cells (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20030874/DC1). Together, these results demonstrate that in contrast to mice lacking individual Vav proteins no functional T cells can be generated in mice lacking all Vav family members. Moreover, individual Vav proteins contribute unevenly in this lineage, since Vav3 but not Vav2 has a major compensatory function.
Vav-null B Cells Fail To Proliferate in Response to Ig Receptor Stimulation.
To determine if B cells developing in Vav-deficient mice still retain their ability to proliferate in vitro in response to stimulation with anti-Ig antibodies, we used 3H-thymidine incorporation assay. Although responses of Vav1ko B cells to Ig cross-linking were comparable to WT, proliferation of Vav1/2ko B cells was severely reduced (Fig. 6 A). Thus, consistent with previous reports (33, 34), Vav1 and Vav2 appear to overlap functionally in B cells. Vav1/3ko B cells also showed diminished proliferation, whereas Vav1/2/3ko B cells neither incorporated 3H-thymidine nor produced blasting cells in response to anti-Ig stimulation (Fig. 6, A and B). Notably, all B cells proliferated vigorously to stimulation with PMA and ionomycin (Fig. 6 B).
Figure 6.
Defective proliferative responses and blast formation by Vav-deficient B cells. (A) Impaired proliferation of antigen receptor–stimulated B cells lacking Vav proteins. Purified spleen B cells from WT (black bars), Vav1ko (Vav1, white dotted bars), Vav1/2ko (Vav1/2, black dotted bars), Vav1/3ko (Vav1/3, diagonal striped bars), or Vav1/2/3ko (Vav1/2/3, white bars) mice were cultured in either media alone or in the presence of stimulatory anti-Ig antibodies as indicated (and as described in Materials and Methods). Proliferation was determined at 48 h by 3H-thymidine incorporation and scintillation counting. The data are displayed as raw cpm values. The results are representative of three independent experiments. All assays were conducted in triplicates, and SDs are shown as vertical lines. (B) Defective blast formation of antigen receptor–stimulated Vav-deficient B cells. B cells from indicated mice were cultured in either media alone, or in the presence of stimulatory anti-Ig antibodies, or with phorbol myristyl acetate (PMA) and ionomycin (I) for 36 h as described in Materials and Methods. Cells were then harvested and analyzed by flow cytometry. Forward scatter dot blots of B220+-gated cells in representative samples are shown (n > 5), and numbers represent percentages of blasting cells. (C) Defective proliferation of Vav-null FO B cells. B cells from indicated mice were cultured in either media alone or in the presence of stimulatory anti-Ig antibodies and analyzed as in B. Forward scatter blots of gated FO cells are shown with percentages of blasting cells. The results are representative of three independent experiments.
In normal mice, only mature B cells respond to Ig receptor cross-linking with proliferation, whereas the majority of immature cells undergo cell death. Because Vav1/2/3ko B cells show a reduction of mature (FO) B cells and a relative increase in the proportion of immature (NF) cells, it is possible that the failure of such cells to proliferate and to incorporate 3H-thymidine is a reflection of these developmental abnormalities. To directly test the ability of mature (FO) Vav1/2/3ko B cells to proliferate, we used flow cytometry and showed that the residual FO B cells present in Vav1/2/3ko mice failed to respond to stimulation with anti-Ig antibodies (Fig. 6 C), demonstrating that the Vav family proteins are essential for in vitro B cell proliferative responses to anti-Ig stimulation.
The Vav Family Proteins Are Indispensable for Ca2+ Signaling in Both T Cells and B Cells.
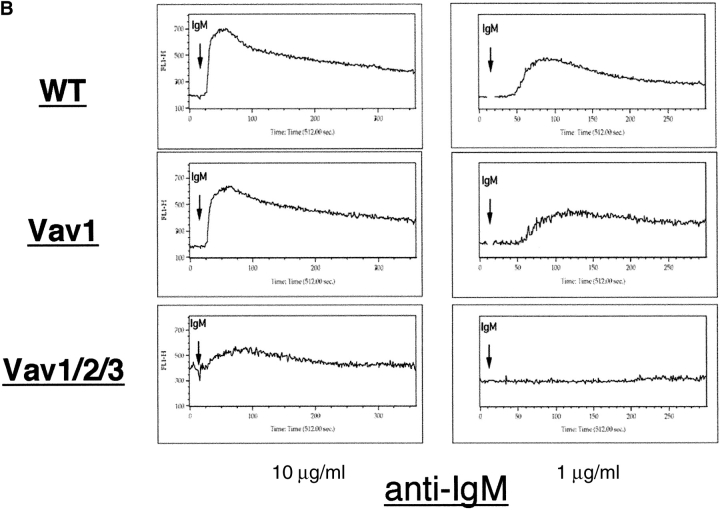
To determine if intracellular signaling was impaired by the loss of Vav function, we examined the ability of Vav-deficient T and B cells to mobilize Ca2+ ions. Ca2+ fluxes induced by anti-CD3 antibody cross-linking were diminished in Vav1ko T cells (Fig. 7 A), consistent with previously published data (23, 24, 29). However, Ca2+ fluxes were totally disrupted in Vav1/2/3ko T cells even under maximal stimulation conditions (Fig. 7 A). In contrast, Vav1ko, Vav1/2ko, Vav1/3ko, Vav2ko, Vav3ko, and Vav2/3ko B cells had Ca2+ fluxes comparable to WT when stimulated with maximal doses of anti-Ig antibodies (Fig. 7 B; unpublished data), whereas Vav1ko, Vav1/2ko, and Vav1/3ko B cell Ca2+ fluxes were only minimally reduced at lower doses of stimulatory antibodies (Fig. 7 B; unpublished data). Notably, anti-Ig induced Ca2+ responses were severely disrupted in Vav1/2/3ko B cells even under maximal stimulation conditions (Fig. 7 B). Together, these data indicate that the Vav family is indispensable for Ca2+ signaling downstream of both the TCR and BCR.
Figure 7.
Disruption of Ca2+ signaling in Vav-null lymphocytes. (A) LN T cell and spleen B cells (B) were loaded with Fluo4-AM as described in Materials and Methods. T cell suspensions were also surface stained with anti-CD4 antibodies conjugated to APC. Cell suspensions were analyzed by flow cytometry. Samples were run at ∼103 cells/s for 15 s to determine background, and then for an additional 7 min after stimulation. T cells were stimulated by the addition of anti-CD3 antibodies (2C11) immediately followed by anti–hamster antibodies; B cells were stimulated by the addition of anti-IgM antibodies at indicated concentrations. Data was analyzed using FlowJo software and displayed as histograms of relative fluorescence intensity over time (in seconds). Representative analyses are shown (n > 3).
Vav-null Mice Reveal a Differential Requirement for Vav Proteins in Erk Activation in T Cells and B Cells.
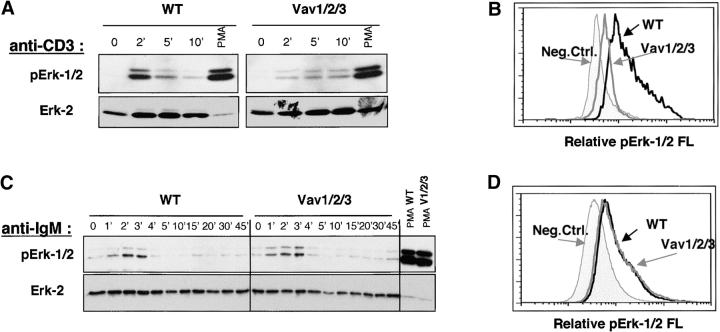
Activation of Ras and MAP kinases is crucial for the induction of lymphocyte proliferation and cytokine production. To examine if the loss of Vav perturbed the ability of T cells to activate the Ras/MAPK pathway, we probed immunoblots with phospho-specific antibodies that recognize active forms of the p44 and p42 MAP kinases (Erk-1 and Erk-2). In this regard, Vav1ko T cells showed attenuated Erk-1/2 activation in response to anti-CD3 antibodies (39 and unpublished data), whereas Vav1/2/3ko T cells virtually failed to activate Erk-1/2 in response to this treatment (Fig. 8 A). Importantly, Erk-1/2 could still be induced in Vav1/2/3ko T cells by PMA treatment, indicating a defect upstream of DAG production (Fig. 8 A). Surprisingly, anti-Ig cross-linking resulted in normal Erk-1/2 activation in Vav1/2/3ko B cells and in B cells lacking any other combination of Vav proteins (Fig. 8 C; unpublished data). Thus, it appears that in contrast to T cells, Vav proteins are not required for Erk-1/2 activation in B cells, indicating a fundamentally distinct mechanism of antigen-receptor signal transduction in cells of the T and B lineages.
Figure 8.
Disruption of Erk-1/2 activation in Vav1/2/3ko T cells but not B cells. (A) Impaired TCR induced Erk-1/2 phosphorylation in WT and Vav1/2/3ko T cells. Freshly isolated LN T cells were either left untreated (0′) or treated with anti-CD3 antibodies immediately followed by anti–hamster antibodies for the indicated period of time. PMA was used as a positive control at 100 ng/ml for 5 min. Cell lysates were prepared by direct lysis with SDS buffer followed by boiling and sonication and then analyzed by immunoblotting with anti–phospho-p42/p44 Erk-1/2 antibodies (as described in Materials and Methods). Protein loading was verified by stripping and reprobing blots with anti–Erk-2 antibodies. The data are representative of three independent experiments. (B) Disruption of Erk-1/2 activation in thymocytes undergoing “β selection.” Thymocytes from WT or Vav1/2/3ko mice were surface stained with CyC-labeled anti-CD4 and anti-CD8 and FITC-labeled anti-CD25 antibodies, and were permeabilized and stained with PE-labeled anti–phospho-p42/p44 Erk-1/2 antibodies as described in Materials and Methods. Histograms representing phospho–Erk-1/2 staining intensity are shown for gated populations of CD4−CD8−CD25+ “large” cells from WT or Vav1/2/3ko mice as indicated. (C) Normal BCR induced Erk-1/2 activation in Vav1/2/3ko B cells. Freshly isolated spleen B cells were either left untreated (0′) or treated with anti-Ig antibodies or PMA and analyzed as in A. (D) Normal Erk-1/2 activation in large pro–B cells. BM cells from WT or Vav1/2/3ko mice were surface stained with CyC-labeled anti-B220 and FITC-labeled anti-CD43 antibodies and then were permeabilized and stained with PE-labeled anti–phospho-p42/p44 Erk-1/2 antibodies as described in Materials and Methods. Histograms representing phospho–Erk-1/2 staining intensity are shown for gated populations of B220loCD43+ large cells from WT or Vav1/2/3ko mice as indicated.
Since Ras/MAPK signaling is implicated in the DN to DP transition in the thymus and pre–B cell differentiation and BM egress (2–9), we investigated if Erk-1/2 activity could be detected in large DN3 cells and in large pro–B cells in WT and Vav1/2/3ko mice. In this regard, intracytoplasmic staining of ex vivo BM cells revealed increased levels of activated Erk-1/2 in large pro–B cells in both WT and Vav1/2/3ko mice, whereas large DN3 cells in Vav1/2/3ko mice had significantly reduced levels of activated Erk-1/2 compared with WT (Fig. 8 B). Phospho–Erk-1/2 staining was equivalent in all cells treated with PMA (unpublished data). Collectively, these data indicate that the loss of Vav proteins leads to a disruption of Ras/MAP kinase signaling in both immature and mature T cells but not in developing or mature B cells.
Discussion
In this report, we examined the roles of Vav Rho–guanine nucleotide exchange factors in lymphocytes. We describe the generation and analyses of mice in which all known members of the Vav family, Vav1, Vav2, and Vav3, have been inactivated via gene targeting. We show that in the lymphoid system this combined Vav deficiency prevents the development of functionally mature T or B cells and disrupts signaling downstream of both T and B cell antigen receptors.
In primary lymphocytes, all three Vav proteins are functionally coupled to signaling events downstream of the TCR and the BCR as indicated by their inducible tyrosine phosphorylation and recruitment to structures associated with the immunological synapse. Despite their common ancestry, conserved structural features, and the ability of each Vav protein to couple with lymphocyte antigen receptors, individual Vav proteins have both common and specialized functions in lymphocytes in vivo. For example, our analyses of Vav1/2ko mice indicate that Vav2 does not functionally compensate for Vav1 in T cells as it does in B cells (33, 34). Thus, Vav2 may be specifically involved in signaling downstream of the BCR but not the TCR. Nevertheless, Vav2 is able to substitute for some functions of Vav3, since there is a more severe developmental block of Vav1/2/3ko thymocytes compared with Vav1/3ko. On the other hand, analyses of lymphocytes in Vav2/3ko mice indicate that Vav1 itself is sufficient to support apparently normal development and function of T and B cells, suggesting mainly auxiliary role(s) for Vav2 and Vav3. Thus, although we have not yet identified any strictly nonredundant functions for any of the individual Vav proteins, genetic evidence presented in this report clearly demonstrates that of the ∼50 Rho-GEFs that exist in mammalian cells (18) the Vav family Rho-GEFs play a role that is both specialized and nonredundant in T and B lymphocytes (Fig. 3 B).
Developing T and B cells receive differentiation and expansion signals from distinct classes of surface receptors, such as cytokine receptors and the pre-TCR or pre-BCR, all of which could conceivably involve Vav proteins. For example, Vav has been implicated in signaling by receptors sharing the common γ-chain, which have crucial functions in lymphocyte ontogeny (1). In this regard, normal development of DN1, DN2, and DN3 thymocytes as well as pro–B and pre–B cells in Vav1/2/3ko mice indicates that Vav proteins are not required in signaling by receptors such as the IL-7R. In fact, both WT and Vav1/2/3ko BM cells proliferate in response to IL-7 treatment in vitro (unpublished data). Moreover, the accumulation of immature populations of B cells, including NF and T1 (Table I), in Vav1/2/3ko mice indicates that Vav proteins are not required for signaling downstream of the pre-BCR, yet they appear essential for the transition to more mature developmental stages that may require stronger antigen receptor signals. Clearly, our data indicate that in B lineage cells Vav proteins are superfluous in Ras/MAPK activation through the BCR (Fig. 8, C and D) and thus may allow for development of all immature populations of B cells in Vav1/2/3ko mice despite the uncoupling of BCR signaling from Ca2+ fluxes. In this regard, our data are consistent with previously published reports by us and other groups that the activation of Ras/MAPK pathway alone is sufficient to promote development of B lineage cells beyond the pre–B cell stage (7–9).
On the other hand, our observations indicate a critical role for Vav in signaling by the pre-TCR (Fig. 8 B). Specifically, we show that Vav deficiency results in developmental arrest at the DN3 to DN4 transition. This block is not due to defects in assembly and/or expression of TCRβ chain genes, since a significant fraction of Vav1/2/3ko DN3 cells expressed cytoplasmic TCRβ chains (unpublished data). Rather, drastically reduced numbers of DP thymocytes and large (blasting) DN3/DN4 thymocytes in Vav1/2/3ko mice compared with WT suggest a pivotal role for Vav proteins in pre-TCR signaling.
Several studies have implicated the activation of the Ras/MAPK pathway as a crucial event in the induction of differentiation and expansion of both T and B cell precursors (2–9). Given the crucial role for Ras/MAPK signaling in the development of both T and B lineage cells (2–5, 7–9) and the strikingly different requirement for Vav in these cells, it is possible that Vav may not be required for regulation of MAPK, or alternatively, T and B cells may utilize diverse mechanisms of MAPK activation. Indeed, two lines of experimental evidence presented in this report support the latter possibility. First, analyses of MAP kinase (Erk-1/2) activity in antigen receptor–stimulated T cells and B cells indicated a complete disruption of Erk-1/2 signaling in Vav1/2/3ko T cells, which is in contrast to normal activation of Erk-1/2 in Vav1/2/3ko B cells. Second, whereas WT “large” DN3 thymocytes showed significantly increased Erk-1/2 activity (presumably resulting from pre-TCR signaling), Erk-1/2 activity was drastically reduced in the equivalent population of Vav1/2/3ko pro–B cells. Yet, both WT and Vav1/2/3ko “large” pro–B cells showed similarly increased Erk-1/2 activity. Together, these results support a view in which the regulation of Ras/MAPK signaling in T and B lineage cells is fundamentally different. In this context, recent evidence suggests that DAG-dependent Ras guanine nucleotide release protein (Ras-GRP1) may be specifically required in the activation of Erk-1/2 in T cells, but not in B cells (40). In fact, the defects in Erk-1/2 activation in Vav1/2/3ko T cells resemble those observed in RasGRP1ko T cells (Fig. 8) (40), suggesting that TCR induction of Erk-1/2 maybe regulated by Vav via PLCγ1 and RasGRP1. Thus, it is plausible that the TCR complex may require DAG-dependent pathway for Erk-1/2 activation, whereas the BCR could, conceivably, use a DAG-independent mechanism, such as the one involving Grb2/SOS. Indeed, Grb2 haplo-insufficiency in T cells resulted in decreased activity of JNK and p38 but not Erk-1/2 (41), consistent with such a view. Our data also indicate that the induction of Ras/MAPK in B cells does not depend on Ca2+ signaling and, presumably, PLCγ activity.
In this paper, we also extended our previous studies of Vav function in Ca2+ signaling (23). We found that unlike Vav1ko lymphocytes, which show only partial Ca2+ defects, Vav1/2/3ko T and B cells have disrupted Ca2+ signaling downstream of their antigen receptors. These data are consistent with observations that Vav may be involved in the activation of PLCγ1 (42), although the exact mechanism is still unknown. Nevertheless, our observations establish that Vav family proteins are essential to determine the signaling output of antigen receptors in both T cells and B cells, although they appear to differentially link the TCR and BCR with Ras/MAPK induction.
Acknowledgments
We thank Drs. Ken Murphy, Andrey Shaw, Marco Colonna, Barry Sleckman, and Albert Shaw for critical review of the manuscript.
W. Swat is a recipient of the American Cancer Society Award. F.W. Alt is an investigator of the Howard Hughes Medical Institute. This work was supported in part by the Howard Hughes Medical Institute Institutional Research Award (to W. Swat), National Institutes of Health grant HL059561 (F.S. Rosen), National Cancer Institute (CA78773) (J.S. Brugge), and the Leukemia Society of America (S.L. Moores).
K. Fujikawa and A.V. Miletic contributed equally to this work.
The online version of this article contains supplemental material.
Abbreviations used in this paper: BCR, B cell receptor; DAG, diacylglycerol; DN, double negative thymocyte; DP, double positive thymocyte; GEF, guanine nucleotide exchange factor; FO, follicular B cell; IP3, inositol triphosphate; MAPK, mitogen-activated protein kinase; MZ, marginal zone B cell; NF, newly formed B cell; PI3K, phosphoinositide 3-OH kinase; PLC, phospholipase C; SP, single positive thymocyte; TD, T-dependent; TI-2, T-independent type 2; TNP, trinitrophenyl.
References
- 1.Borowski, C., C. Martin, F. Gounari, L. Haughn, I. Aifantis, F. Grassi, and H. von Boehmer. 2002. On the brink of becoming a T cell. Curr. Opin. Immunol. 14:200–206. [DOI] [PubMed] [Google Scholar]
- 2.Swat, W., Y. Shinkai, H.L. Cheng, L. Davidson, and F.W. Alt. 1996. Activated Ras signals differentiation and expansion of CD4+8+ thymocytes. Proc. Natl. Acad. Sci. USA. 93:4683–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton, T., K.C. Gilmour, and M.J. Owen. 1996. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 86:243–251. [DOI] [PubMed] [Google Scholar]
- 4.Gartner, F., F.W. Alt, R. Monroe, M. Chu, B.P. Sleckman, L. Davidson, and W. Swat. 1999. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity. 10:537–546. [DOI] [PubMed] [Google Scholar]
- 5.Iritani, B.M., J. Alberola-Ila, K.A. Forbush, and R.M. Perimutter. 1999. Distinct signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity. 10:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aifantis, I., F. Gounari, L. Scorrano, C. Borowski, and H. von Boehmer. 2001. Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nat. Immunol. 2:403–409. [DOI] [PubMed] [Google Scholar]
- 7.Iritani, B.M., K.A. Forbush, M.A. Farrar, and R.M. Perlmutter. 1997. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16:7019–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw, A.C., W. Swat, L. Davidson, and F.W. Alt. 1999. Induction of Ig light chain gene rearrangement in heavy chain-deficient B cells by activated Ras. Proc. Natl. Acad. Sci. USA. 96:2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw, A.C., W. Swat, R. Ferrini, L. Davidson, and F.W. Alt. 1999. Activated Ras signals developmental progression of recombinase-activating gene (RAG)–deficient pro-B lymphocytes. J. Exp. Med. 189:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane, L.P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242–249. [DOI] [PubMed] [Google Scholar]
- 11.Acuto, O., and D. Cantrell. 2000. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18:165–184. [DOI] [PubMed] [Google Scholar]
- 12.DeFranco, A.L. 2000. B-cell activation. Immunol. Rev. 176:5–9. [PubMed] [Google Scholar]
- 13.Gauld, S.B., J.M. Dal Porto, and J.C. Cambier. 2002. B cell antigen receptor signaling: roles in cell development and disease. Science. 296:1641–1642. [DOI] [PubMed] [Google Scholar]
- 14.Samelson, L.E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371–394. [DOI] [PubMed] [Google Scholar]
- 15.Koretzky, G.A., and P.S. Myung. 2001. Positive and negative regulation of T-cell activation by adaptor proteins. Nat. Rev. Immunol. 1:95–107. [DOI] [PubMed] [Google Scholar]
- 16.Bustelo, X.R. 2001. Vav proteins, adaptors and cell signaling. Oncogene. 20:6372–6381. [DOI] [PubMed] [Google Scholar]
- 17.Turner, M., and D.D. Billadeau. 2002. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2:476–486. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt, A., and A. Hall. 2002. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16:1587–1609. [DOI] [PubMed] [Google Scholar]
- 19.Bustelo, X.R., J.A. Ledbetter, and M. Barbacid. 1992. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 356:68–71. [DOI] [PubMed] [Google Scholar]
- 20.Margolis, B., P. Hu, S. Katzav, W. Li, J.M. Oliver, A. Ullrich, A. Weiss, and J. Schlessinger. 1992. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 356:71–74. [DOI] [PubMed] [Google Scholar]
- 21.Movilla, N., and X.R. Bustelo. 1999. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell. Biol. 19:7870–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moores, S.L., L.M. Selfors, J. Fredericks, T. Breit, K. Fujikawa, F.W. Alt, J.S. Brugge, and W. Swat. 2000. Vav family proteins couple to diverse cell surface receptors. Mol. Cell. Biol. 20:6364–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holsinger, L.J., I.A. Graef, W. Swat, T. Chi, D.M. Bautista, L. Davidson, R.S. Lewis, F.W. Alt, and G.R. Crabtree. 1998. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8:563–572. [DOI] [PubMed] [Google Scholar]
- 24.Fischer, K.D., Y.Y. Kong, H. Nishina, K. Tedford, L.E. Marengere, I. Kozieradzki, T. Sasaki, M. Starr, G. Chan, S. Gardener, et al. 1998. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8:554–562. [DOI] [PubMed] [Google Scholar]
- 25.Aghazadeh, B., W.E. Lowry, X.Y. Huang, and M.K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 102:625–633. [DOI] [PubMed] [Google Scholar]
- 26.Kuhne, M.R., G. Ku, and A. Weiss. 2000. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J. Biol. Chem. 275:2185–2190. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, R., F.W. Alt, L. Davidson, S.H. Orkin, and W. Swat. 1995. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 374:470–473. [DOI] [PubMed] [Google Scholar]
- 28.Tarakhovsky, A., M. Turner, S. Schaal, P.J. Mee, L.P. Duddy, K. Rajewsky, and V.L. Tybulewicz. 1995. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 374:467–470. [DOI] [PubMed] [Google Scholar]
- 29.Turner, M., P.J. Mee, A.E. Walters, M.E. Quinn, A.L. Mellor, R. Zamoyska, and V.L. Tybulewicz. 1997. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 7:451–460. [DOI] [PubMed] [Google Scholar]
- 30.Fischer, K.D., A. Zmuldzinas, S. Gardner, M. Barbacid, A. Bernstein, and C. Guidos. 1995. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 374:474–477. [DOI] [PubMed] [Google Scholar]
- 31.Colucci, F., E. Rosmaraki, S. Bregenholt, S.I. Samson, V. Di Bartolo, M. Turner, L. Vanes, V. Tybulewicz, and J.P. Di Santo. 2001. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J. Exp. Med. 193:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swat, W., R. Xavier, A. Mizoguchi, E. Mizoguchi, J. Fredericks, K. Fujikawa, A.K. Bhan, and F.W. Alt. 2003. Essential role for Vav1 in activation, but not development, of gammadelta T cells. Int. Immunol. 15:215–221. [DOI] [PubMed] [Google Scholar]
- 33.Doody, G.M., S.E. Bell, E. Vigorito, E. Clayton, S. McAdam, R. Tooze, C. Fernandez, I.J. Lee, and M. Turner. 2001. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2:542–547. [DOI] [PubMed] [Google Scholar]
- 34.Tedford, K., L. Nitschke, I. Girkontaite, A. Charlesworth, G. Chan, V. Sakk, M. Barbacid, and K.D. Fischer. 2001. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2:548–555. [DOI] [PubMed] [Google Scholar]
- 35.Inabe, K., M. Ishiai, A.M. Scharenberg, N. Freshney, J. Downward, and T. Kurosaki. 2002. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med. 195:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doody, G.M., D.D. Billadeau, E. Clayton, A. Hutchings, R. Berland, S. McAdam, P.J. Leibson, and M. Turner. 2000. Vav-2 controls NFAT-dependent transcription in B- but not T-lymphocytes. EMBO J. 19:6173–6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wulfing, C., A. Bauch, G.R. Crabtree, and M.M. Davis. 2000. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc. Natl. Acad. Sci. USA. 97:10150–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulbranson-Judge, A., V.L. Tybulewicz, A.E. Walters, K.M. Toellner, I.C. MacLennan, and M. Turner. 1999. Defective immunoglobulin class switching in Vav-deficient mice is attributable to compromised T cell help. Eur. J. Immunol. 29:477–487. [DOI] [PubMed] [Google Scholar]
- 39.Costello, P.S., A.E. Walters, P.J. Mee, M. Turner, L.F. Reynolds, A. Prisco, N. Sarner, R. Zamoyska, and V.L. Tybulewicz. 1999. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc. Natl. Acad. Sci. USA. 96:3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dower, N.A., S.L. Stang, D.A. Bottorff, J.O. Ebinu, P. Dickie, H.L. Ostergaard, and J.C. Stone. 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1:317–321. [DOI] [PubMed] [Google Scholar]
- 41.Gong, Q., A.M. Cheng, A.M. Akk, J. Alberola-Ila, G. Gong, T. Pawson, and A.C. Chan. 2001. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat. Immunol. 2:29–36. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds, L.F., L.A. Smyth, T. Norton, N. Freshney, J. Downward, D. Kioussis, and V.L. Tybulewicz. 2002. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase–dependent and –independent pathways. J. Exp. Med. 195:1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujikawa, K., Y. Inoue, M. Sakai, Y. Koyama, S. Nishi, R. Funada, F.W. Alt, and W. Swat. 2002. Vav3 is regulated during the cell cycle and effects cell division. Proc. Natl. Acad. Sci. USA. 99:4313–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]