Abstract
In humans, the elderly and immunocompromised are at greatest risk for disseminated West Nile virus (WNV) infection, yet the immunologic basis for this remains unclear. We demonstrated previously that B cells and IgG contributed to the defense against disseminated WNV infection (Diamond, M.S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. J. Virol. 77:2578–2586). In this paper, we addressed the function of IgM in controlling WNV infection. C57BL/6J mice (sIgM−/−) that were deficient in the production of secreted IgM but capable of expressing surface IgM and secreting other immunoglobulin isotypes were vulnerable to lethal infection, even after inoculation with low doses of WNV. Within 96 h, markedly higher levels of infectious virus were detected in the serum of sIgM−/− mice compared with wild-type mice. The enhanced viremia correlated with higher WNV burdens in the central nervous system, and was also associated with a blunted anti-WNV IgG response. Passive transfer of polyclonal anti-WNV IgM or IgG protected sIgM−/− mice against mortality, although administration of comparable amounts of a nonneutralizing monoclonal anti-WNV IgM provided no protection. In a prospective analysis, a low titer of anti-WNV IgM antibodies at day 4 uniformly predicted mortality in wild-type mice. Thus, the induction of a specific, neutralizing IgM response early in the course of WNV infection limits viremia and dissemination into the central nervous system, and protects against lethal infection.
Keywords: flavivirus, antibody, innate immunity, encephalitis, risk factor
Introduction
West Nile virus (WNV) is a single-stranded positive-polarity RNA virus and the etiologic agent of West Nile encephalitis. WNV is maintained in a natural cycle between mosquitoes and birds but also infects humans, horses, and other animals. It is endemic in parts of Africa, Europe, the Middle East, and Asia (1), and outbreaks are now occurring annually in North America (2). Humans develop a febrile illness with a subset of cases progressing to a meningitis or encephalitis syndrome (1). Currently, no specific therapy or vaccine has been approved for human use.
Host factors influence the expression of WNV disease in humans (3). Infants, the elderly, and those with impaired immune systems are at greatest risk for severe neurological disease (1, 4, 5). Similarly, in animals, the maturation and integrity of the immune system correlates with resistance to WNV infection (6–8). Through the use of animal models of WNV infection, the immunologic basis for protection is beginning to be understood (9). T and B lymphocytes protect against WNV infection: SCID and RAG1 mice (T and B cell–deficient; references 10, 11) and B cell–deficient mice uniformly succumb to WNV infection (11). Macrophages also have important functions because their depletion increased the neuroinvasiveness of an attenuated strain (12).
Humoral immunity is an essential component of the immune response to WNV and other flaviviruses because neutralizing antibodies limit dissemination of infection. The role of immune IgG in protection has been studied extensively in mouse models of flavivirus infection, including WNV. Passive transfer of polyclonal or monoclonal IgG before infection protects mice against lethal flavivirus challenge (11, 13–21). Immune IgG are speculated to protect against WNV infection by direct neutralization of receptor binding, through Fc-receptor–dependent viral clearance, by complement-mediated lysis of virus or infected cells, and by antibody-dependent cytotoxicity. The importance of antibodies in the protection against WNV infection has been highlighted by recent studies in antibody-deficient mice (11). Mice lacking antibodies developed encephalitis after infection with WNV; high levels of virus and viral RNA were detected both peripherally and in the central nervous system (CNS).
The function of IgM against WNV or other flaviviruses is less well characterized. Low levels of serum IgM antibody against Japanese Encephalitis virus were an independent risk factor for severe neurological deficit in humans (22). Our own studies with B cell–deficient mice demonstrated a ∼500-fold increase in viremia 4 d after infection, a time when only immune IgM was detected in the serum from wild-type mice (11). Because passive transfer of immune IgM against WNV, derived from wild-type mice 4 d after infection, prolonged survival of B cell–deficient mice and completely protected wild-type mice, natural or induced IgM could limit WNV infection by controlling viremia and/or by triggering an adaptive B or T cell response (23, 24).
Natural and induced IgM antibodies against WNV may have important protective functions against WNV. Natural IgM is secreted constitutively by CD5+ B-1 cells without specific stimulation, has widely variable binding avidities (10−3–10−11 M), and represents an initial defense against pathogens (25–27). IgM mediates direct neutralization of some bacteria and viruses in circulation (26, 28, 29), enhances phagocytosis of pathogens (30), and activates complement (27, 31, 32) to prime the immune response. IgM antibody–antigen complexes are efficiently filtered in the spleen and lymph nodes and may diminish hematogenous spread and infection of critical end organ targets such as the brain or spinal cord (24). In this work, we directly assessed the function of secreted IgM in limiting WNV infection. C57BL/6J mice that did not produce secreted IgM (27, 33, 34) uniformly succumbed to WNV infection. Infection in these mice resulted in higher levels of viremia and CNS viral burdens. Passive transfer of induced, but not natural, IgM protected sIgM−/− mice against lethal WNV infection, but administration of a nonneutralizing IgM monoclonal antibody did not improve outcome. A low titer of anti-WNV IgM antibodies at day 4 after infection in wild-type mice uniformly predicted mortality. Thus, the induction of neutralizing anti-WNV IgM early in the immune response blunts viremia and the spread of infection and is required for survival.
Materials and Methods
Cells, Viruses, and Antibodies.
BHK-21 and C6/36 Aedes albopictus cells were cultured as described previously (35, 36). The WNV strain (3000.0259) was isolated in New York in 2000 (37) and obtained from L. Kramer (New York State Department of Health, Albany, NY). All cell culture and in vivo studies used a stock (2 × 108 PFU/ml) of this virus that was propagated (passage 1) once in C6/36 cells. Viruses were diluted in HBSS and 1% heat-inactivated FBS for injection into mice. Hybridoma cells that produce monoclonal antibodies against WNV envelope protein (4G2, IgG2a; reference 38; H5.46, IgM; reference 39) were cultured in DMEM supplemented with 10% FBS. H5.46 is a monoclonal IgM antibody that binds to the E protein of WNV and was used from ascites fluid (39).
Mouse Experiments.
All mice used in these experiments were derived from or backcrossed onto the inbred C57BL/6J strain. Mice that lack the ability to secrete IgM due to a targeted mutation that disrupts expression of the secreted but not membrane form of IgM have been described previously (33) and were obtained from M. Carroll (The Center for Blood Research, Harvard Medical School, Boston, MA) and J. Chen (Massachusetts Institute of Technology, Cambridge, MA). The wild type C57BL/6J was purchased from Jackson ImmunoResearch Laboratories. 8–12-wk-old mice were used for all studies and inoculated subcutaneously with WNV by footpad injection after anesthetization with xylazine and ketamine. In some experiments, mice were phlebotomized (0.2 ml of blood) at day 4 after infection. Mouse experiments were approved and performed according to the guidelines of the Washington University School of Medicine Animal Safety Committee. Differences in survival times and outcome were assessed by the Kaplan-Meier analysis and the log-rank test.
Passive Antibody Transfer Experiments.
Serum was isolated from naive or infected (day 4 after infection) mice, heat-inactivated for 30 min at 56°C, and stored at −80°C. An aliquot was reserved to confirm levels of specific IgM against WNV. Ascites fluid that contained the H5.46 anti-WNV IgM monoclonal antibody was heat-inactivated, and diluted in HBSS with 1% heat-inactivated FBS. For passive transfer experiments, mice were administered 0.5 ml of serum or H5.46 antibody intraperitoneally 1 d before and after inoculation with 102 PFU of WNV. Purified human γ-globulin with reactivity against WNV was obtained commercially (I. Nur, Omrix Biopharmaceuticals Ltd, Kiryat Ono, Israel) from a WNV endemic region (Israel) that has had repeated outbreaks in recent years. Several batches of pooled human γ globulin (50 mg/ml IgG) were obtained and tested positive by ELISA against WNV antigens and by plaque reduction neutralization titer (PRNT) assay (11, 40). 50 mg/ml IgG negative human γ globulin was obtained from a nonendemic region (Midwestern United States, 2000); purified preparations lacked immune antibodies as judged by ELISA and PRNT assays. In experiments with human γ globulin, mice were administered a single dose of immune or nonimmune purified IgG by intraperitoneal injection at the same time as footpad inoculation of WNV.
Quantitation of Viral Burden in Mice.
For analysis of virus in tissues of infected mice, organs were recovered after cardiac perfusion with PBS and dissection, cooled on ice, weighed, homogenized using a Bead-beater apparatus (BioSpec Products, Inc.), and titrated for virus by plaque assay on BHK-21 cells as described previously (11, 36). Serum was obtained from whole blood by phlebotomy of the axillary vein immediately before being killed.
Quantitation of Antibodies.
The titer of neutralizing antibodies was determined by a standard plaque-reduction neutralization assay (11, 41). Experiments were performed in duplicate and plaques were scored visually. Results were plotted and the PRNT for 50% inhibition (PRNT50) was determined. In some experiments, IgM was depleted by immunologic means (11). Serum or diluted ascites were incubated twice with an equal volume of anti-IgM–specific agarose for 1 h at 4°C. After centrifugation, the supernatant (1:4 dilution of original sample) was titrated for PRNT. ELISA confirmed isotype depletion.
The overall titer of antibodies was determined using an ELISA assay against purified WNV antigen. In brief, soluble WNV E protein that was generated from baculovirus-infected SF9 cells (unpublished data) was adsorbed overnight at 4°C to microtiter plates (Maxi-Sorp; Nunc). Nonspecific binding was blocked after incubation with blocking buffer (PBS, 0.05% Tween 20, 3% BSA, and 3% horse serum) for 1 h at 37°C. Plates were incubated with serial dilutions of heat-inactivated serum from infected mice for 1 h at 4°C. After extensive washing, plates were incubated with biotin-conjugated goat anti–mouse IgG (Sigma-Aldrich) and horseradish peroxidase–conjugated streptavidin (Sigma-Aldrich) at 4°C, and developed after the addition of tetramethylbenzidine substrate (Sigma-Aldrich). Optical densities were determined with an automatic ELISA plate reader at 450 nm (Molecular Devices). Optical densities that were twice background (0.06–0.075) binding to BSA were considered positive. The quantitation of antibodies by isotype was performed similarly with some modifications. Serum was obtained from wild-type or sIgM−/− mice at day 10 after infection was used at a standard 1:40 dilution. Dilutions of isotype-specific antibodies (Southern Biotechnology Associates, Inc.) at a previously optimized concentration (1:2,000–1:4,000) were used. Optical densities were measured at 450 nm, and after subtraction of background binding, a value for each isotype was obtained and directly compared.
Statistical Analyses.
For survival analysis, Kaplan–Meier survival curves were plotted using Prism software (GraphPad). Mortality curves were analyzed by the log rank test and average survival times were evaluated using the Mann-Whitney test.
Results
Decreased Survival of sIgM−/− Mice after Infection with WNV
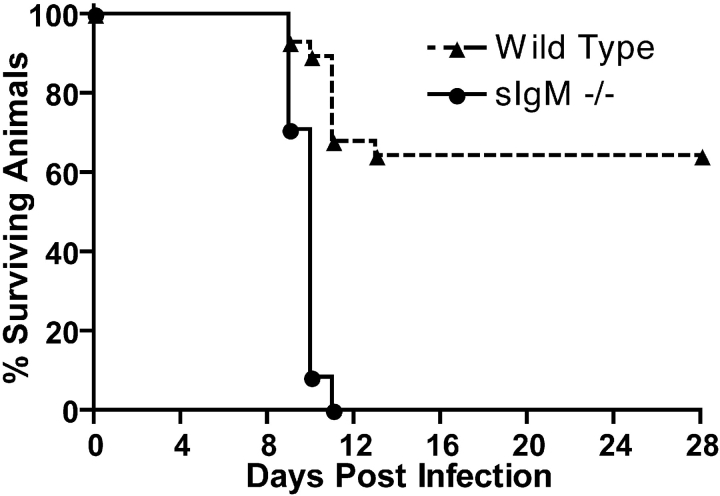
Previous studies in our laboratory with B cell–deficient μMT C57BL/6J mice indicated that B cells and antibody had a critical function in preventing the dissemination of WNV into the CNS. Because a 500-fold difference in viremia between μMT and congenic wild-type mice was observed within 4 d of infection, a time when only specific IgM against WNV was detected in the serum of wild-type mice, we postulated that newly generated anti-WNV IgM limited infection. To address this directly, we compared WNV infection in wild-type and congenic sIgM−/− C57BL/6 mice that lack the ability to secrete IgM, but retain a membrane form of IgM and the ability to class switch and secrete IgG (33). Our previously described mouse model of infection was used in which a low passage WNV isolate was inoculated subcutaneously into the footpad of adult mice to mimic natural infection and facilitate an analysis of viral replication and spread (11). After infection with 102 PFUs of WNV, ∼70% of adult wild-type mice survived (Fig. 1) . All animals showed clinical evidence of infection with fur ruffling, hunchback posture, and weight loss (unpublished data). In contrast, infection with 102 PFU of WNV resulted in 100% mortality of the sIgM−/− mice (P < 0.0001). Similar results were observed with a higher (106 PFU) dose of WNV (unpublished data).
Figure 1.
Survival data for C57BL/6J mice inoculated with WNV. Wild-type and sIgM−/− mice were inoculated via footpad with the indicated doses of WNV and followed for 28 d. The survival curves were constructed using data from three to five separate experiments. The number of animals were n = 28 for wild-type mice and n = 24 for sIgM−/− mice. The mean survival times (in days) were 21.8 ± 1.6 for wild-type and 9.8 ± 0.1 for sIgM−/− mice (P < 0.0001).
Increased Viral Load in sIgM−/− Mice
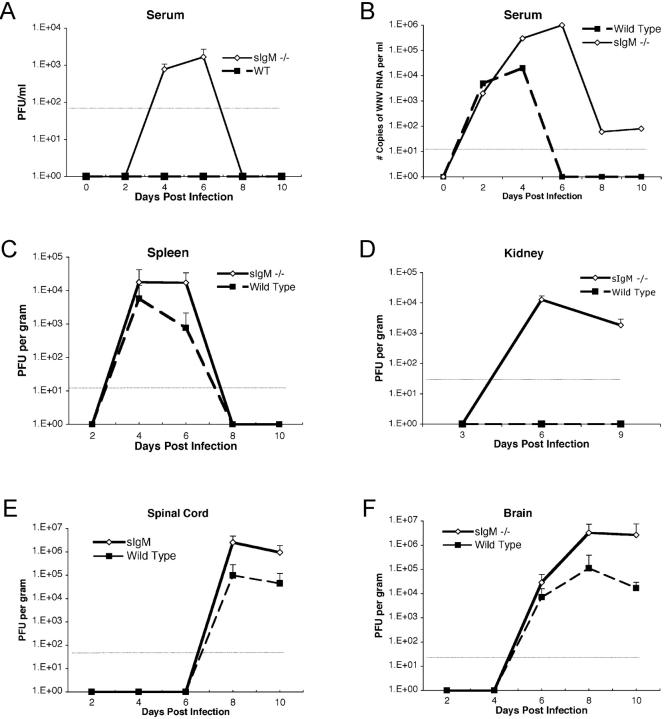
To begin to understand the mechanism by which a deficiency in secreted IgM made mice vulnerable to infection by WNV, the levels of infectious virus were measured from serum, spleen, kidney, brain, and spinal cord using plaque assays (11, 42). A time course was performed in wild-type and sIgM−/− mice after inoculation with 102 PFU to determine the effect of IgM against WNV on the kinetics and magnitude of infection (Fig. 2) .
Figure 2.
WNV burden in peripheral and CNS tissues of adult wild-type and sIgM−/− C57BL/6J mice. (A) Infectious virus levels in serum. Serum was harvested at the indicated days after subcutaneous footpad inoculation with 102 PFU of WNV and virus levels were measured using a viral plaque assay in BHK21 cells. Data are shown as the mean of PFU per milliliter of serum and reflect from 6 to 10 mice per time point. The dotted line represents the limit of sensitivity of the assay. (B) WNV RNA levels in serum. Viral RNA levels were determined from serum of wild-type or sIgM−/− mice after WNV infection at the indicated days using a real-time fluorogenic RT-PCR assay. Data are expressed as genomic equivalents of WNV RNA per milliliter of serum and reflect the mean of five independent mice per time point. (C) Infectious virus levels in the spleen. Virus levels were determined from spleen homogenates by plaque assay and normalized for the weight of the tissue sample. Data are the mean of 10 animals per time point. (D) Infectious virus levels in the kidney. Virus levels were determined from kidney homogenates by plaque assay and normalized for the weight of the tissue sample. Data are the mean of 10 kidneys per time point. (E and F) Infectious virus levels in the CNS. Virus levels were determined from spinal cord (E) or brain (F) as described in Fig. 2 C.
Viremia.
In wild-type mice, viremia was below the level of detection by infectious plaque assay throughout the time course. In contrast, 103–104 PFU/ml of infectious virus was detectable in sIgM−/− mice between days 4 and 6 after infection (Fig. 2 A, P < 0.001), and subsequently waned to levels below detection. However, when viral RNA in serum was measured by a more sensitive fluorogenic RT-PCR assay (11, 42), additional information was obtained (Fig. 2 B). At day 2 after infection, similar levels of WNV RNA were detected (∼5,000 copies of WNV RNA/ml) in both wild-type and sIgM−/− mice. At day 4 after infection, a time when anti-WNV IgM was first observed in the serum of wild-type mice (11), sIgM−/− mice had 50-fold higher levels of WNV RNA in serum (P < 0.01). By day 6, WNV RNA was no longer detected in the serum of wild-type mice; in contrast, ∼106 copies/ml were observed in serum from sIgM−/− mice. By day 8, a time when immune IgG was first measured at significant levels (see Fig. 3); only low levels (∼100 copies WNV RNA/ml) were detected in the serum of sIgM−/− mice. Collectively, these studies suggest that a lack of development of specific IgM after WNV infection results in a delayed clearance of virus from serum.
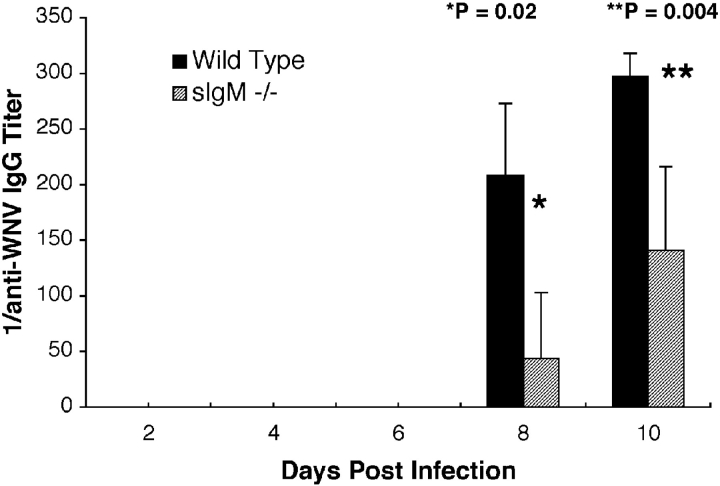
Figure 3.
Development of specific IgG against WNV. Serum was collected from wild-type or sIgM−/− mice at the indicated days after infection. The levels of specific IgG were determined by incubating serum with adsorbed control or purified WNV E protein. Data are the mean of serum from 8 to 12 mice per time point performed in duplicate. p-values and statistical significance are as indicated.
Spleen and Kidney.
In spleens from both wild-type and sIgM−/− mice, similar kinetics of WNV accumulation were observed (Fig. 2 C). Virus levels peaked in the spleen at day 4 after infection, decreased by day 6, and were absent at day 8. Although there was no significant difference in viral burden at day 4 after infection (∼104 PFU/g; P = 0.3), there was a small yet significant increase in virus production in the spleens of sIgM−/− mice at day 6 after infection (∼104 compared with 103 PFU/g; P = 0.01). In contrast, a distinct pattern of WNV infection was observed in a different peripheral organ, the kidney (Fig. 2 D). In wild-type mice, kidney viral burdens were below the level of detection by direct plaque assay at all times measured. However, >103–104 PFU of virus was detected in kidneys from sIgM−/− mice at days 6 and 9 after initial infection (P < 0.00001).
CNS.
The kinetics of CNS dissemination between wild-type and sIgM−/− mice began similarly. Infectious virus was first detected in the brain at day 6 and in the spinal cord at day 8 after infection in wild-type and sIgM−/− mice (Fig. 2, E and F). However, by 8 d after infection, sIgM−/− mice had from 10- to 20-fold higher levels of infectious virus in the brain and spinal cord (P < 0.04), and by day 10, 100-fold higher levels of WNV were measured in the brains of sIgM−/− mice. The difference of CNS viral load between wild-type and sIgM−/− mice was not explained by the bias of the survival curves because, by day 10, no individual wild-type mouse that was morbidly infected had viral titers in the brain that approached those in the sIgM−/− mice (e.g., maximum wild-type brain titer, 2.1 × 104 PFU/g; unpublished data).
IgG Response against WNV in sIgM−/− Mice
IgM is believed to have an important role in triggering the adaptive immune response (24) as mice that lacked secreted IgM had blunted IgG responses after inoculation with influenza virus (27) or protein antigens (33). To determine whether a deficiency of secreted IgM affected the IgG responses against WNV, an ELISA against purified WNV envelope protein was used to measure serum levels of anti-WNV IgG in wild-type and sIgM−/− mice after inoculation with WNV. In agreement with our previous work (11), wild-type mice developed a specific anti-WNV IgM response that was detectable within 4 d of infection, and as expected, there was no specific IgM detected in the sIgM−/− mice (unpublished data). No specific IgG against WNV was detected in either wild-type or sIgM−/− mice until 8 d after infection. The sIgM−/− mice responded to WNV infection but the overall levels of virus-specific IgG were reduced compared with wild-type controls at days 8 and 10 after infection (Fig. 3) . Isotype-specific analysis revealed markedly lower levels of IgG2b in sIgM−/− mice (unpublished data). The results of these experiments suggest that secreted IgM that is generated after WNV infection may trigger an amplified IgG response that facilitates clearance of virus.
Role of Natural and Induced IgM in WNV Protection
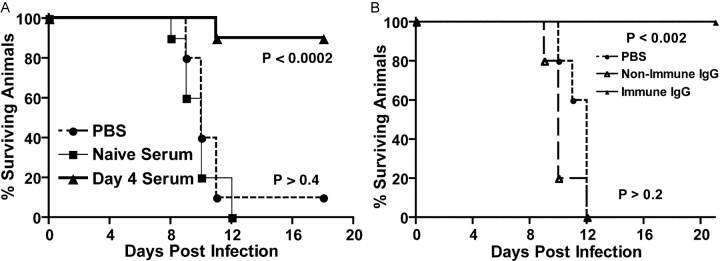
Although our studies demonstrated that sIgM−/− mice were vulnerable to WNV infection, the nature of the protective IgM response remained unclear. We wished to distinguish the protective function of natural and induced IgM. Natural IgM is constitutively secreted by CD5+ B-1 cells without specific stimulation, has widely variable binding avidities (10−3–10−11 M), and represents an initial defense against pathogens (25–27). Induced IgM is secreted after antigen-specific stimulation in the lymph node or spleen; depending on the antigen, this process may occur in a T cell–dependent or T cell–independent manner. Natural and induced IgM may directly neutralize pathogens (26, 28), facilitate clearance via phagocytosis (30), and activate complement (27) to prime the immune response (24, 43). To address the protective nature of natural and induced IgM, sIgM−/− mice were passively administered pooled heat-inactivated serum collected from naive wild-type mice (natural IgM) or mice that were exposed to WNV for 4 d (induced IgM); at this early juncture, serum contains low levels of neutralizing IgM (Table I) but no specific IgG (reference 11 and unpublished data). sIgM−/− mice were inoculated with 0.5 ml of heat-inactivated sera 1 d before and after infection with 102 PFU of WNV (Fig. 4 A). Although similar quantities of naive serum (i.e., natural IgM) protected sIgM−/− mice against infection with influenza virus (27) and vesicular stomatitis virus (26), it had no significant effect on mortality or average survival time (P > 0.4) after WNV infection. In contrast, passive administration of 1.0 ml of heat-inactivated serum that contained induced IgM, but not IgG, provided almost complete protection against infection with 102 PFU of WNV (Fig. 4 A, P < 0.0002). Chemical and immunologic depletion experiments confirmed that IgM was responsible for the protective effect (reference 11 and unpublished data). The results of the reconstitution experiments with sIgM−/− mice contrasted with B cell–deficient mice in which induced IgM increased average survival time but did not diminish WNV-induced mortality (11). In parallel experiments, passive transfer of purified polyclonal human anti-WNV IgG protected wild-type, B cell–deficient, and sIgM−/− mice against lethal WNV challenge (Fig. 4 B; reference 40). Although natural IgM may function in the initial priming of the immune response against WNV, by itself, it cannot sustain control of WNV infection. In contrast, induced IgM, which is produced within the first few days of infection, slows the dissemination of WNV temporarily until an adaptive T and B cell response is produced.
Table I.
Correlation of Anti-WNV IgM Neutralization Activity with Protection against WNV Infection
| ELISA Binding Activity to Purified E Protein a | |||||
|---|---|---|---|---|---|
| 1:33 | 1:100 | 1:300 | |||
| Day 4 serum after infection (pooled) | 0.251 | 0.103 | NS | ||
| H5.46 monoclonal IgM (1:10 ascites) | 0.505 | 0.330 | 0.210 | ||
| H5.46 monoclonal IgM (1:40 ascites) | 0.325 | 0.254 | 0.125 | ||
| Neutralizing Titers (PRNT 50 ) b | |||||
| No treatment |
IgM preclear |
||||
| Day 4 serum after infection (pooled) | 1:30 | ND | |||
| H5.46 monoclonal IgM (1:10 ascites) | ND | NT | |||
| Protection of sIgM −/− Mice with Anti-IgMAntibody Reconstitution c | |||||
| Survival at 21 d | |||||
| Day 4 serum after infection (pooled, 1 ml) | 90% (n = 10) | ||||
| H5.46 monoclonal IgM (1:10 ascites, 1 ml) | 0% (n = 10) | ||||
| H5.46 monoclonal IgM (1:40 ascites, 1 ml) | 0% (n = 10) | ||||
| No antibody | 0% (n = 6) | ||||
An ELISA assay against solid phase purified WNV E protein was performed with the indicated dilutions of polyclonal or monoclonal IgM against WNV. Optical density readings are given for one representative experiment of two performed in duplicate. NS, not significant.
Neutralizing titers were determined by plaque reduction assay in BHK21 cells. No treatment indicates that the diluted heat-inactivated serum or ascites were mixed directly with 100 PFU of WNV. IgM preclear indicates that serum or ascites were precleared with anti-IgM Sepharose before the plaque reduction assay. Results are representative of three independent experiments. ND, none detected; NT, not tested.
0.5 ml of heat-inactivated day 4 serum or H5.46 monoclonal IgM (1:10 or 1:40 dilution of ascites) was given intraperitoneally to sIgM−/− 1 d before and after subcutaneous infection with 102 PFU of WNV. Results were obtained from two independent experiments.
Figure 4.
Passive administration of serum or purified antibodies to sIgM−/− mice. (A) Serum was collected from naive mice, or mice that were infected with WNV for 4 d. After heat-inactivation, 0.5 ml of serum was administered to sIgM−/− mice 1 d before and after infection with 102 PFU of WNV. Data reflect two independent experiments with five mice per condition, and p-values are indicated next to each curve. (B) 10 mg of purified human immune or nonimmune IgG were administered as a single dose via an intraperitoneal route immediately before administration of 102 PFU of WNV via footpad inoculation. Data are from two independent experiments with five mice per condition, and p-values are indicated next to each curve.
The Role of Neutralizing IgM in Protection
Because the absence of secreted IgM resulted in increased viremia at day 4, we speculated that induced IgM might limit WNV infection by neutralizing virus before the development of an adaptive IgG response. As mentioned, pooled serum that was obtained from mice at 4 d after infection contained neutralizing IgM (PRNT50 ≈ 1/30) and conferred protection; the neutralizing activity could be precleared with anti-IgM but not anti-IgG Sepharose (Table I; reference 11). To assess whether neutralizing activity of IgM was required for protection, we passively transferred H5.46, a monoclonal IgM antibody that recognized the E protein of WNV (39) and lacked neutralizing activity (Table I). An ELISA assay against purified E protein confirmed that similar, if not greater, amounts of the H5.46 antibody were administered. Interestingly, passive transfer of either dilution (1:10 and 1:40 of ascites) of H5.46 anti-WNV IgM to sIgM−/− mice did not alter the survival time, mortality rate, or viral burden in peripheral or CNS compartments when compared with untreated controls (Table I and unpublished data). Thus, for protection to be transferred, anti-WNV IgM required neutralizing activity.
The IgM Response against WNV as a Predictor of Survival
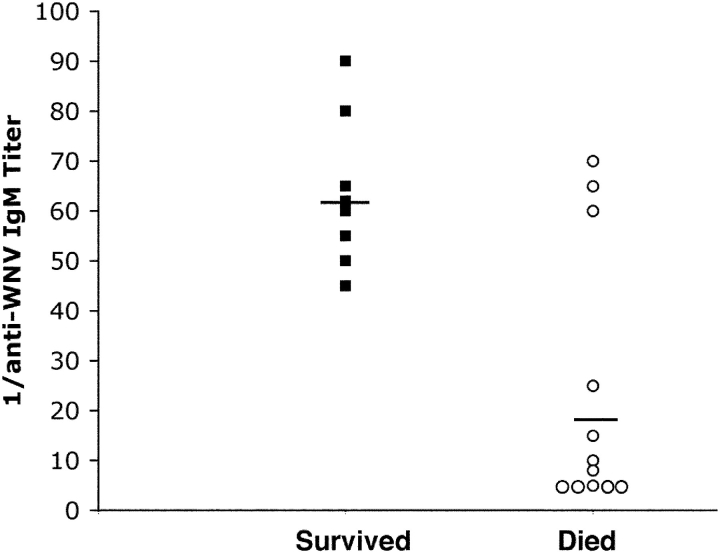
Because a genetic deficiency of secreted IgM was associated with lethality after WNV infection, we questioned whether variability in anti-WNV IgM levels correlated with survival in wild-type animals. To address this, 7-wk-old wild-type C57BL/6J mice were infected with 102 PFU of WNV. At 4 d after infection, mice were phlebotomized (0.2 ml of blood) and followed prospectively for survival, and the serum was analyzed for anti-WNV IgM levels by an isotype-specific ELISA against affinity-purified recombinant WNV E protein (Fig. 5) . Mice that survived had anti-WNV IgM titers of ≥1:45 at day 4 after infection. In contrast, all mice died that had anti-WNV IgM titers ≤1:25 at day 4 after infection. Thus, the presence of a low titer of anti-WNV IgM early in the course of WNV infection uniformly predicted mortality.
Figure 5.
Scatter plot of the relationship between specific anti-WNV IgM titer at day 4 after infection and survival. 7-wk-old wild-type mice were infected with 102 PFU of WNV. At day 4 after infection, animals were phlebotomized and followed clinically for survival. An ELISA was used to evaluate serum for specific IgM titer against purified WNV E protein using a biotin-conjugated goat anti–mouse IgM secondary antibody. 20 animals were included in the study. 8 survived and 12 died after infection. Five of the animals that died had identical titers of 1:5. Solid lines indicate the mean titer.
Discussion
We demonstrated previously that antibody played a critical role in preventing the dissemination of WNV into the CNS (11). Because B cell–deficient mice that lacked antibodies demonstrated a ∼500-fold increase in viremia 4 d after infection, a time when only immune IgM was detected in the serum from wild-type mice, we postulated a critical role for IgM in controlling the early phases of WNV infection. In this paper, we directly addressed the function of secreted IgM in limiting the spread of WNV infection. C57BL/6J sIgM−/− mice that were deficient in the production of secreted IgM but capable of secreting other immunoglobulin isotypes were highly vulnerable to lethal infection even after infection with low doses of WNV.
To our knowledge, this is the first paper to show a definitive protective role for secreted IgM against flavivirus infection. Although previous papers have demonstrated that passive administration of IgG or immune serum prevents encephalitis caused by flaviviruses (11, 13, 14, 21, 44–46) and nonflaviviruses (47–54), none demonstrated a definitive role for IgM. Induced IgM does have an important protective role against other pathogens. An absence of virus-induced secreted IgM resulted in 40% and 50% excess mortality in mice after infection with influenza A virus (27) or gastrointestinal bacteria (34), respectively. In comparison, the absence of secreted IgM resulted in 100% lethality in response to WNV infection across a wide (4 log) range of inoculating doses.
The role of natural IgM in protection against viral infection remains controversial. Reconstitution experiments demonstrated that IgM that was induced after virus infection was sufficient to confer protection against WNV, whereas natural IgM provided no protection. These results agree with our previous observations with wild-type and B cell–deficient mice (11): induced IgM prevented mortality in wild-type mice and delayed mortality in B cell–deficient mice, whereas natural IgM had no effect on survival time or rate in B cell–deficient mice. These results contrast with prior reconstitution experiments with other pathogens as follows: transfer of natural IgM conferred protection against influenza A (27) and vesicular stomatitis (26) viruses, and peritonitis induced by cecal ligation and puncture (34). Despite our negative data, we speculate that natural IgM may still function to link the innate and adaptive immune responses against WNV (24). However, unlike other pathogens, administration of natural IgM alone does not adequately control WNV infection nor compensate for the production of antigen-triggered induced IgM.
Compared with congenic wild-type mice, no significant difference in WNV infection was observed in sIgM−/− mice during the first 2 d after infection. However, by 4 d after infection, markedly higher levels of infectious virus and viral RNA were detected in the serum of sIgM−/− mice compared with wild-type mice. Thus, the early anti-WNV IgM response functions, in part, to limit infection by preventing hematogenous spread. Indeed, the enhanced viremia and absence of induced IgM correlated with higher WNV viral burdens in peripheral and CNS tissues at different times after infection. Importantly, significantly higher viral burdens were observed in the kidneys of sIgM−/− mice at day 6 after infection, a time that precedes the development of specific anti-WNV IgG antibodies in wild-type mice. The induction of specific IgM against WNV appeared to directly limit spread to peripheral nonlymphoid organs. Although IgM could inhibit WNV dissemination early in the course of infection by preventing virus attachment and entry or by binding C1q and directing complement-mediated lysis of viral particles (24, 43), the experiments with the H5.46 monoclonal IgM antibody suggest that the neutralizing activity of IgM confers protection. The experiments with H5.46 also suggest that IgM binding to WNV in vivo may not result in clinically significant complement-mediated lysis of viral particles, results that agree with in vitro studies that showed that in the presence of complement, nonneutralizing IgM facilitated rather than inhibited viral infection in macrophages, presumably by enhancing viral entry through complement receptor 3 (55, 56). Based on these experiments, we conclude that one critical function of induced IgM is to limit WNV infection by neutralizing virus before the development of an adaptive IgG response.
Nonetheless, the lack of secreted anti-WNV IgM may predispose to lethal infection by additional mechanisms. In addition to neutralizing pathogens in circulation (24, 26, 28), IgM inhibits infection by directly enhancing phagocytosis in lymphoid tissue (30), activating complement (27, 32, 43), and by priming the adaptive immune response (24). Indeed, some of our data suggest that secreted IgM against WNV contributes to the priming of the adaptive immune response. Despite the differences in viremia between wild-type and sIgM−/− mice that were observed at day 4 after infection, the levels of virus detected in the brain at day 6 were not significantly different; thus, enhanced viremia was associated with but did not directly correlate with increased CNS viral burden. In addition, by day 8 after infection, a time when ∼10–20-fold higher levels of WNV were observed in the CNS of sIgM−/− mice, differences in the anti-WNV IgG response were observed: decreased levels of anti-WNV IgG were consistently detected in the sIgM−/− mice. These results agree with others that have observed blunted IgG levels in response to viral or protein antigens in mice that lack secreted IgM (27, 33). Because virus eradication in neurons may depend on specific IgG (47, 57), the depressed specific IgG response in sIgM−/− mice may contribute to WNV spread in the CNS and injury of neurons.
The importance of induced IgM early in the course of WNV infection was confirmed in wild-type mice by prospective analysis. Mice that had high (≥1:45) anti-WNV IgM titers at day 4 after infection had an improved (70% for IgMhi vs. 40% overall) survival rate. More dramatically, mice that had low (≤1:25) anti-WNV IgM titers at day 4 after infection uniformly died. Thus, the absence of a strong anti-WNV IgM early in the course of infection, by itself, predicted mortality. To our knowledge, this is the first work that definitively demonstrates that a depressed or delayed IgM response against a pathogen in wild-type animals is an independent risk factor for poor outcome. A recent retrospective clinical analysis supports an important role of IgM in flavivirus infections: low levels of anti–Japanese Encephalitis virus IgM antibody were an independent risk factor for neurological disease in humans (22). Although IgM appears to have an important function in limiting WNV infection, it is clear from published works (9–11, 58) that other aspects of the innate (e.g., interferon and complement) and adaptive (e.g., T cells) immune system also control WNV infection.
It is intriguing to consider that severe human WNV infection, which is heavily biased toward an elderly and immunocompromised population, occurs, in part, because of a dysfunctional IgM response against WNV early during infection. The elderly frequently have delayed antibody production and shortened durations of protective immunity after viral challenge or immunization (59–61). Although WNV causes neurological disease, it does so in a small subset of cases. As therapies become available, it will be important to target high-risk populations. Natural history studies in humans are currently being designed to identify both clinical and laboratory risk factors for severe WNV disease. Based on the studies presented here, the delayed development of specific, neutralizing anti-WNV IgM is likely to be an independent risk factor for morbidity and mortality.
Acknowledgments
The authors thank T. Chambers, A. Pekosz, D. Leib, L. Morrison, P. Olivo, P. Stuart, and their laboratories for experimental advice. The authors thank J. Chen and M. Carroll for the sIgM−/− mice, and M. Carroll, K. Whitby, M. Samuel, S. Stanley, and P. Olivo for critical reading of the manuscript. The authors thank I. Nur and Omrix Biopharmaceuticals Ltd. for the gift of purified human γ globulin.
The work was supported by grants from the Edward Mallinckrodt Jr. Foundation and by the Center for Disease Control and Prevention (U50/CCU720545 and U50/CCU620539).
Abbreviations used in this paper: CNS, central nervous system; PRNT, plaque reduction neutralization titer; WNV, West Nile virus.
References
- 1.Hubalek, Z., and J. Halouzka. 1999. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanciotti, R.S., J.T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K.E. Volpe, M.B. Crabtree, J.H. Scherret, et al. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 286:2333–2337. [DOI] [PubMed] [Google Scholar]
- 3.Camenga, D.L., N. Nathanson, and G.A. Cole. 1974. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J. Infect. Dis. 130:634–641. [DOI] [PubMed] [Google Scholar]
- 4.Asnis, D.S., R. Conetta, A.A. Teixeira, G. Waldman, and B.A. Sampson. 2000. The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin. Infect. Dis. 30:413–418. [DOI] [PubMed] [Google Scholar]
- 5.Tsai, T.F., F. Popovici, C. Cernescu, G.L. Campbell, and N.I. Nedelcu. 1998. West Nile encephalitis epidemic in southeastern Romania. Lancet. 352:767–771. [DOI] [PubMed] [Google Scholar]
- 6.Eldadah, A.H., N. Nathanson, and R. Sarsitis. 1967. Pathogenesis of West Nile Virus encephalitis in mice and rats. 1. Influence of age and species on mortality and infection. Am. J. Epidemiol. 86:765–775. [DOI] [PubMed] [Google Scholar]
- 7.Eldadah, A.H., and N. Nathanson. 1967. Pathogenesis of West Nile Virus encepahlitis in mice and rats. II. Virus multiplication, evolution of immunofluorescence, and development of histological lesions in the brain. Am. J. Epidemiol. 86:776–790. [DOI] [PubMed] [Google Scholar]
- 8.Weiner, L.P., G.A. Cole, and N. Nathanson. 1970. Experimental encephalitis following peripheral inoculation of West Nile virus in mice of different ages. J. Hyg. (Lond.). 68:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, M.S., B. Shrestha, E. Mehlhop, E. Sitati, and M. Engle. 2003. Innate and adaptive immune responses determine protection against disseminated infection by West Nile Encephalitis virus. Viral Immunol. 16:259–278. [DOI] [PubMed] [Google Scholar]
- 10.Halevy, M., Y. Akov, D. Ben-Nathan, D. Kobiler, B. Lachmi, and S. Lustig. 1994. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch. Virol. 137:355–370. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, M.S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Nathan, D., I. Huitinga, S. Lustig, N. van Rooijen, and D. Kobiler. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141:459–469. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Nathan, D., S. Lustig, G. Tam, S. Robinzon, S. Segal, and B. Rager-Zisman. 2003. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating west nile virus infection in mice. J. Infect. Dis. 188:5–12. [DOI] [PubMed] [Google Scholar]
- 14.Kimura-Kuroda, J., and K. Yasui. 1988. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J. Immunol. 141:3606–3610. [PubMed] [Google Scholar]
- 15.Henchal, E.A., L.S. Henchal, and J.J. Schlesinger. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 69:2101–2107. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger, J.J., M.W. Brandriss, and E.E. Walsh. 1985. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 135:2805–2809. [PubMed] [Google Scholar]
- 17.Schlesinger, J.J., and S. Chapman. 1995. Neutralizing F(ab')2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 76:217–220. [DOI] [PubMed] [Google Scholar]
- 18.Heinz, F.X., R. Berger, W. Tuma, and C. Kunz. 1983. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology. 126:525–537. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman, B.M., P.L. Summers, D.R. Dubois, W.H. Cohen, M.K. Gentry, R.L. Timchak, D.S. Burke, and K.H. Eckels. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41:576–580. [DOI] [PubMed] [Google Scholar]
- 20.Mason, P.W., J.M. Dalrymple, M.K. Gentry, J.M. McCown, C.H. Hoke, D.S. Burke, M.J. Fournier, and T.L. Mason. 1989. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J. Gen. Virol. 70:2037–2049. [DOI] [PubMed] [Google Scholar]
- 21.Roehrig, J.T., L.A. Staudinger, A.R. Hunt, J.H. Mathews, and C.D. Blair. 2001. Antibody prophylaxis and therapy for flaviviral encephalitis infections. Ann. NY Acad. Sci. 951:286–297. [DOI] [PubMed] [Google Scholar]
- 22.Libraty, D.H., A. Nisalak, T.P. Endy, S. Suntayakorn, D.W. Vaughn, and B.L. Innis. 2002. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans. R. Soc. Trop. Med. Hyg. 96:173–178. [DOI] [PubMed] [Google Scholar]
- 23.Ochsenbein, A.F., D.D. Pinschewer, B. Odermatt, M.C. Carroll, H. Hengartner, and R.M. Zinkernagel. 1999. Protective T cell–independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochsenbein, A.F., and R.M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 21:624–630. [DOI] [PubMed] [Google Scholar]
- 25.Casali, P., and A.L. Notkins. 1989. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol. Today. 10:364–368. [DOI] [PubMed] [Google Scholar]
- 26.Ochsenbein, A.F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R.M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159. [DOI] [PubMed] [Google Scholar]
- 27.Baumgarth, N., O.C. Herman, G.C. Jager, L.E. Brown, L.A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell–derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gobet, R., A. Cerny, E. Ruedi, H. Hengartner, and R.M. Zinkernagel. 1988. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 56:175–180. [DOI] [PubMed] [Google Scholar]
- 29.Barrington, R., M. Zhang, M. Fischer, and M.C. Carroll. 2001. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 180:5–15. [DOI] [PubMed] [Google Scholar]
- 30.Navin, T.R., E.C. Krug, and R.D. Pearson. 1989. Effect of immunoglobulin M from normal human serum on Leishmania donovani promastigote agglutination, complement-mediated killing, and phagocytosis by human monocytes. Infect. Immun. 57:1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid, R.R., S. Woodcock, A. Shimabukuro-Vornhagen, W.G. Austen, Jr., L. Kobzik, M. Zhang, H.B. Hechtman, F.D. Moore, Jr., and M.C. Carroll. 2002. Functional activity of natural antibody is altered in Cr2-deficient mice. J. Immunol. 169:5433–5440. [DOI] [PubMed] [Google Scholar]
- 32.Austen, W.G., Jr., L. Kobzik, M.C. Carroll, H.B. Hechtman, and F.D. Moore, Jr. 2003. The role of complement and natural antibody in intestinal ischemia-reperfusion injury. Int. J. Immunopathol. Pharmacol. 16:1–8. [DOI] [PubMed] [Google Scholar]
- 33.Boes, M., C. Esau, M.B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776–4787. [PubMed] [Google Scholar]
- 34.Boes, M., A.P. Prodeus, T. Schmidt, M.C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond, M., T. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond, M.S., D. Edgil, T.G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814–7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebel, G.D., A.P. Dupuis II, K. Ngo, D. Nicholas, E. Kauffman, S.A. Jones, D. Young, J. Maffei, P.Y. Shi, K. Bernard, and L.D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt, W.E., J.M. McCown, M.K. Gentry, and P.K. Russell. 1982. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect. Immun. 36:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould, E.A., A. Buckley, S. Higgs, and S. Gaidamovitch. 1990. Antigenicity of flaviviruses. Arch. Virol. 1:S137–S152. [Google Scholar]
- 40.Engle, M., and M.S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile Virus infection in wild type and immunodeficient mice. J. Virol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halstead, S.B., C.N. Venkateshan, M.K. Gentry, and L.K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 132:1529–1532. [PubMed] [Google Scholar]
- 42.Lanciotti, R.S., A.J. Kerst, R.S. Nasci, M.S. Godsey, C.J. Mitchell, H.M. Savage, N. Komar, N.A. Panella, B.C. Allen, K.E. Volpe, et al. 2000. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll, M.C. 1998. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 16:545–568. [DOI] [PubMed] [Google Scholar]
- 44.Broom, A.K., M.J. Wallace, J.S. Mackenzie, D.W. Smith, and R.A. Hall. 2000. Immunisation with gamma globulin to Murray Valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against Australian encephalitis: evaluation in a mouse model. J. Med. Virol. 61:259–265. [DOI] [PubMed] [Google Scholar]
- 45.Mathews, J.H., and J.T. Roehrig. 1984. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J. Immunol. 132:1533–1537. [PubMed] [Google Scholar]
- 46.Kreil, T.R., and M.M. Eibl. 1997. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J. Virol. 71:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin, D., B. Levine, W. Tyor, S. Ubol, and P. Despres. 1997. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 159:155–161. [DOI] [PubMed] [Google Scholar]
- 48.Tyor, W.R., S. Wesselingh, B. Levine, and D.E. Griffin. 1992. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149:4016–4020. [PubMed] [Google Scholar]
- 49.Griffin, D.E., B. Levine, W.R. Tyor, and D.N. Irani. 1992. The immune response in viral encephalitis. Semin. Immunol. 4:111–119. [PubMed] [Google Scholar]
- 50.Stanley, J., S.J. Cooper, and D.E. Griffin. 1986. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J. Virol. 58:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews, A.E., S.R. Weiss, M.J. Shlomchik, L.G. Hannum, J.L. Gombold, and Y. Paterson. 2001. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J. Immunol. 167:5254–5263. [DOI] [PubMed] [Google Scholar]
- 52.Bergmann, C.C., C. Ramakrishna, M. Kornacki, and S.A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575–1583. [DOI] [PubMed] [Google Scholar]
- 53.Ramakrishna, C., S.A. Stohlman, R.D. Atkinson, M.J. Shlomchik, and C.C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 168:1204–1211. [DOI] [PubMed] [Google Scholar]
- 54.Seiler, P., U. Kalinke, T. Rulicke, E.M. Bucher, C. Bose, R.M. Zinkernagel, and H. Hengartner. 1998. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J. Virol. 72:2253–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardosa, M.J., S. Gordon, S. Hirsch, T.A. Springer, and J.S. Porterfield. 1986. Interaction of West Nile virus with primary murine macrophages: role of cell activation and receptors for antibody and complement. J. Virol. 57:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardosa, M.J., J.S. Porterfield, and S. Gordon. 1983. Complement receptor mediates enhanced flavivirus replication in macrophages. J. Exp. Med. 158:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griffin, D.E., S. Ubol, P. Despres, T. Kimura, and A. Byrnes. 2001. Role of antibodies in controlling alphavirus infection of neurons. Curr. Top. Microbiol. Immunol. 260:191–200. [DOI] [PubMed] [Google Scholar]
- 58.Wang, T., E. Scully, Z. Yin, J.H. Kim, S. Wang, J. Yan, M. Mamula, J.F. Anderson, J. Craft, and E. Fikrig. 2003. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J. Immunol. 171:2524–2531. [DOI] [PubMed] [Google Scholar]
- 59.Vikerfors, T., M. Grandien, M. Johansson, and C.A. Pettersson. 1988. Detection of an immunoglobulin M response in the elderly for early diagnosis of respiratory syncytial virus infection. J. Clin. Microbiol. 26:808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saurwein-Teissl, M., T.L. Lung, F. Marx, C. Gschosser, E. Asch, I. Blasko, W. Parson, G. Bock, D. Schonitzer, E. Trannoy, and B. Grubeck-Loebenstein. 2002. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893–5899. [DOI] [PubMed] [Google Scholar]
- 61.Weksler, M.E., and P. Szabo. 2000. The effect of age on the B-cell repertoire. J. Clin. Immunol. 20:240–249. [DOI] [PubMed] [Google Scholar]