Abstract
CD4+CD25+ regulatory T cells (Treg) are instrumental in the maintenance of immunological tolerance. One critical question is whether Treg can only be generated in the thymus or can differentiate from peripheral CD4+CD25− naive T cells. In this paper, we present novel evidence that conversion of naive peripheral CD4+CD25− T cells into anergic/suppressor cells that are CD25+, CD45RB−/low and intracellular CTLA-4+ can be achieved through costimulation with T cell receptors (TCRs) and transforming growth factor β (TGF-β). Although transcription factor Foxp3 has been shown recently to be associated with the development of Treg, the physiological inducers for Foxp3 gene expression remain a mystery. TGF-β induced Foxp3 gene expression in TCR-challenged CD4+CD25− naive T cells, which mediated their transition toward a regulatory T cell phenotype with potent immunosuppressive potential. These converted anergic/suppressor cells are not only unresponsive to TCR stimulation and produce neither T helper cell 1 nor T helper cell 2 cytokines but they also express TGF-β and inhibit normal T cell proliferation in vitro. More importantly, in an ovalbumin peptide TCR transgenic adoptive transfer model, TGF-β–converted transgenic CD4+CD25+ suppressor cells proliferated in response to immunization and inhibited antigen-specific naive CD4+ T cell expansion in vivo. Finally, in a murine asthma model, coadministration of these TGF-β–induced suppressor T cells prevented house dust mite–induced allergic pathogenesis in lungs.
Keywords: anergy, IL-10, OVA TCR transgenic, house dust mite, asthma
Introduction
CD4+CD25+ regulatory T cells (Treg) have emerged as a unique population of suppressor T cells that maintain peripheral immune tolerance (1, 2). Although the immunoregulatory ability of Treg is no longer contested, where and how this population of CD4+ T cells is generated and developed still remains largely unknown. The major debate centers on whether Treg are generated only in the thymus from a defined lineage and/or whether these cells represent a stage that different types of CD4+ T cells can acquire. Although most analyses emphasize the thymus as the sole incubator for Treg (3, 4), recent evidence suggests that Treg may also be induced in the periphery (4, 5). One crucial question is whether Treg can be induced or converted from normal peripheral CD4+ T cells and if this occurs, which molecules and/or cytokines are responsible for the transition because activated CD4+ T cells expressing CD25+ under neutral TCR stimulation conditions show no suppressive ability (1, 2).
TGF-β is a critical factor in regulation of T cell–mediated immune responses and in the induction of immune tolerance (for reviews see references 6, 7). When the TGF-β1–mediated inhibitory pathway is abrogated specifically in T cells by restrictive expression of dominant negative TGF-β receptor II (8, 9), these mice develop unchecked T cell proliferation and inflammatory and autoimmune-like diseases, documenting a TGF-β–dependent signal in T cell activation and tolerance in vivo. Although TGF-β regulation of immune responsiveness has been validated in vitro and in vivo, how TGF-β accomplishes its suppressive role in T cell activation remains unclear. A link between TGF-β and induction of Treg has also been suggested in humans (10), but whether TGF-β converts naive T cells to regulatory cells and, most importantly, the underlying molecular mechanisms await revelation.
In exciting new papers, Foxp3, which encodes a transcription factor that is genetically defective in an autoimmune syndrome in humans and mice (11, 12), has been shown to be not only specifically expressed in professional Treg, but also required for their development (13–15). Neither naive nor activated CD4+CD25− responder T cells express Foxp3, distinguishing Foxp3 from other Treg associated molecules (CD25, CTLA-4, and GITR) that can be acquired in CD4+CD25− responder T cells once activated. Foxp3/Scurfin-deficient mice develop massive autoimmune and inflammatory disease, which shares many pathogenetic features of mice deficient in CTLA-4 (16, 17) or TGF-β (18, 19). Significantly, gene transfer of Foxp3 converts naive CD4+CD25− T cells toward a regulatory T cell phenotype similar to that of the professional Treg (13–15). Although the identification of Foxp3 in Treg has greatly expanded our ability to decipher the development and function of this unique population of regulatory T cells, how Foxp3 is controlled and by what physiological inducers remain mysteries to be solved.
In this paper, we present evidence that TGF-β converts naive CD4+CD25− T cells into CD4+CD25+ anergic/suppressor T cells in the periphery. These suppressor T cells not only exhibit unresponsiveness to TCR stimulation, but also suppress normal CD4+ T cell activation and Th1 and Th2 cytokine production in vitro. Moreover, these suppressor T cells also inhibit immune responses in vivo, as evident in their striking prevention of allergic pathogenesis in a house dust mite (HDM)–induced mouse asthma model and in their significant suppression of antigen-specific CD4+ T cell proliferation in an OVA peptide CD4+ transgenic T cell adoptive transfer model. Significantly, we also show that TGF-β conversion of naive CD4+CD25− T cells into CD4+CD25+ suppressor T cells involves induction of Foxp3 expression, the first identification of an inducer of this pathway, which offers an opportunity to control tolerance.
Materials and Methods
Mice.
Normal BALB/c and DO11.10 transgenic mice expressing a TCR with specificity for chicken OVA peptide 323-339 were purchased from The Jackson Laboratory and provided by B. Kelsall (National Institute of Allergy and Infectious Diseases, Bethesda, MD). C57BL/6 mice were obtained from The Jackson Laboratory and from in-house breeding at the National Institute of Dental and Craniofacial Research.
Antibodies and Reagents.
Crystallized chicken OVA, hen egg lysozyme, PMA, and ionomycin were purchased from Sigma-Aldrich. OVA peptide 323-339 (ISQAVHAAHAEINEAGR) was synthesized and purified by reverse phase–HPLC (Synpep Corporation). The purity of the peptides is >98%. The following reagents were obtained from BD Biosciences: purified rat anti–mouse mAbs to IL-2, IL-4, and IFN-γ, anti-CD28, FITC–anti-CD45RB, recombinant mouse IL-2, IL-4, and IFN-γ, PE- or purified anti-CD3 (145-2C11, NA/LE™), PE-anti–CTLA-4, hamster IgG isotypic control, FITC- or biotinylated antimurine CD25 (Clone 7D4), FITC–rat IgM, PE- or purified anti-CD25 (Clone PC61, NA/LE™), and anti-FcRII/III. FITC or PE-anti–mouse CD4, anti-CD8α, and the respective isotypic control mAbs and streptavidin-FITC, PE, or Tricolor were purchased from Caltag. Recombinant human TGF-β1, anti–TGF-β1, 2,3 mAb, biotinylated chicken anti–TGF-β1, and recombinant murine IL-10 were purchased from R&D Systems. 7-amino-actinomycin D (7-AAD) was purchased from Calbiochem.
Cell Preparation.
Spleens were gently minced in complete DMEM containing 10% FBS (BioWhittaker), and CD4+ T cells were purified using a mouse CD4+ T cell column system (R&D Systems; references 20, 21). T cell–depleted or whole spleen cells (irradiated, 3,000 rad) of BALB/c or C57BL6 mice were used as APCs as indicated. To isolate CD4+CD25− T cells, anti-CD25 antibody (PC61, 1 μg/106 cells) was added into the antibody cocktail and incubated with spleen cells before separation on the CD4+ T cell column, to yield a purity of CD4+CD25− T cells >90%. For Treg, the CD4+ T cells were incubated with FITC–anti-CD25 (1 μg/106 cells; antibody depleted of sodium azide by dialysis in PBS overnight) in 2% PBS-FBS for 30 min at 4°C, washed, resuspended in X-Vivo 20 serum-free medium (BioWhittaker), and the Treg and CD4+CD25− T cells were purified using a FACStarPlus™ Cell Sorter (Becton Dickinson). The purity of sorted cells was >95–99%.
Cell Culture and T Cell Proliferation Assay.
For normal C57BL/6 CD4+ T cell primary stimulation, purified CD4+, CD4+CD25−, or Treg were stimulated with 0.5 μg/ml anti-CD3 in the presence of APCs in complete DMEM at 37°C and 5% CO2 for 7–10 d and CD4+ T cells were isolated for further studies. In some studies, 2 ng/ml TGF-β1 was included in the cultures as indicated. In other experiments, CD4+CD25− or CD25+ cells were stimulated with 2 μg/ml of platebound anti-CD3 and 2 μg/ml soluble anti-CD28 in the absence or presence of 0.02, 0.2, 2, or 20 ng/ml TGF-β1, 1 ng/ml IL-10, or 100 U/ml IL-2 for 3 d.
For DO11.10 TCR transgenic mice, spleen cells (2 × 106 cells/ml) were stimulated with 100 μg/ml OVA in the absence or presence of 2 ng/ml recombinant TGF-β1 or 1 ng/ml murine IL-10 in complete DMEM for 7–10 d. Cells were harvested and extensively washed, and dead cells were eliminated by centrifugation over Ficoll-Paque (Amersham Biosciences). For secondary stimulation, CD4+ cells purified from the primary cultures were restimulated with OVA or anti-CD3 in the presence of APCs as indicated. For activation with PMA, CD4+ T cells were incubated with 5 ng/ml PMA and 250 ng/ml ionomycin. Cells were cultured at 37°C in 5% CO2 for 72 h and pulsed with 1 μCi [3H]thymidine for the last 6–16 h. Radioactivity incorporated was counted using a flatbed β counter (Wallac). In some experiments, 10 U/ml rIL-2 or 20 μg/ml anti–TGF-β1, 2,3 mAb and its isotypic antibody control were added at the beginning of the secondary culture.
Cytokine Induction and Determination.
For cytokine induction, cells were cultured with antigens or antibodies as indicated in complete DMEM for 24–96 h. Cell-free supernatants were collected for the determination of IL-2, IFN-γ, IL-4, and IL-13 production by ELISA using paired mAbs specific for the corresponding cytokines (BD Biosciences) or the respective ELISA kits (Biosource International and R&D Systems). A standard curve was generated using known amounts of the respective purified recombinant murine cytokines.
Coculture of CD4+ T Cells.
Freshly isolated C57BL/6 or BALB/c CD4+CD25− responder T cells were cultured in 96-well plates with anti-CD3, splenic APCs, and indicated CD4+CD25+ cells for 72 h for proliferation assay. Transwell experiments were performed as described previously (22). In brief, CD4+CD25− responder T cells were cultured in 24-well plates with APCs and 0.5 μg/ml anti-CD3 in the presence or absence of TGF-β–converted or control CD4+CD25+ cells in the Transwell™ (0.4 μM pore size; Costar). For carboxy-fluorescein diacetate succinimidyl ester (CFSE)–labeling assay, 107 cells/ml T cells were incubated with 3 μM CFSE in plain DMEM (without phenol red) at 37°C for 15 min. Cells were washed three times and resuspended in complete DMEM for cell culture.
Flow Cytometry Analysis.
T cells were resuspended in PBS containing 1% BSA (Irvine) and 0.1% sodium azide (Sigma-Aldrich). For the staining of surface antigens, cells were incubated with FITC-, PE-, or Tricolor-conjugated mAbs or their negative control antibodies as indicated for 30 min on ice. For surface active TGF-β staining, cells were stained with biotinylated chicken anti–TGF-β1 and FITC–anti-CD25 for 30 min, followed by PE-streptavidin incubation for an additional 30 min at 4°C. Intracellular staining of CTLA-4 was performed as described previously (23). Intracellular staining of phosphorylated Smad2/3 (P-Smad2/3) was performed as described in the figure legend for Fig. S2 (available at http://www.jem.org/cgi/content/full/jem.20030152/DC1.
Adoptive Transfer Experiments.
Freshly isolated CD4+ KJ1-26+ transgenic T cells were injected i.p. into unirradiated syngeneic BALB/c recipients. Some mice were cotransferred i.p. with control, TGF-β–, or IL-10–pretreated naive CD4+ KJ1-26+ T cells. Animals were immunized s.c. with 100 μg of P323-339 emulsified in IFA (Difco). Cells from draining lymph nodes (inguinal) were harvested at indicated time points and stained ex vivo with Tricolor–anti-CD4, PE–anti-CD25, and FITC–KJ1-26 mAbs to determine the expansion of CD4+KJ1-26+ T cells in vivo. For in vitro restimulation assay, draining lymph node cells were cultured with OVA peptide 323-339 for 3 d to determine T cell proliferation or for 6 h in the presence of GolgiPlug™ (BD Biosciences) to determine intracellular cytokines as described previously (24).
HDM-induced Allergic Pathogenesis.
Allergen-induced asthma was induced as described previously (25) with some modifications. In brief, 6–8-wk-old C57BL/6 mice were immunized by i.p. injection of 10 μg HDM antigen (Greer Laboratories) in 0.1 ml PBS or PBS alone (unpublished data) at days 1 and 7, followed by intratracheal challenge with 100 μg HDM antigen in 40 μl PBS or an equivalent volume of PBS as a control at days 14 and 21, respectively. 4 d after the last challenge, mice were killed, and tissues were harvested for immunohistopathologic analysis or in vitro cultures. The mucin expression in the airways was determined with periodic acid schiff (PAS) staining (25). Where indicated, TGF-β–anergized or control CD4+ T cells were injected i.v. into the mice on day 1 and again on day 14.
Western Blot Analysis.
Western blot analysis was performed as described previously (24) with an antibody to P-Smad2/3 (rabbit polyclonal IgG, 1:200) or to Smad2/3 (goat polyclonal IgG, 1:200; Santa Cruz Biotechnology, Inc.) followed by horseradish peroxidase–conjugated goat anti–rabbit IgG or donkey anti–goat IgG (Santa Cruz Biotechnology, Inc.) as recommended by the manufacturer.
RT-PCR for Foxp3 Expression.
RT-PCR was performed as described previously (13), except the number of cycles was 28 for Foxp3. The primer sequences were as follows and synthesized by Invitrogen: HPRT, 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and 5′-GATTCAACTTGCTCTCATCTTAGGC-3′; and Foxp3, 5′-CAGCTGCCTACAGTGCCCCTAG-3′ and 5′-CATTTGCCAGCAGTGGGTAG-3′. Normalized values for Foxp3 mRNA expression in each sample were calculated as the relative quantity of Foxp3 divided by the relative quantity of hypoxanthineguanine phosphoribosyl transferase (HPRT).
Statistical Analysis.
Student's t tests were used for the significance of data comparison.
Online Supplemental Material.
Fig. S1 shows that TGF-β–converted CD25+ suppressor cells require cell contact to carry out their action, and that IL-10–induced CD25+ T cells fail to suppress normal CD4+ T cell proliferation in vitro. Fig. S2 shows increased P-Smad2/3 expression in TGF-β–converted CD25+ suppressor cells and that anti–TGF-β reverses TGF-β–induced anergy of CD4+ T cells. Fig. S3 shows that cotransfer of TGF-β–converted CD4+ suppressor T cells blocked epithelial cell mucin production in airways in lungs induced by HDM as detected by PAS staining. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20030152/DC1.
Results
TCR and TGF-β Costimulation Induces CD4+CD25− T Cell Anergy, but Fails to Expand Professional Treg.
Although TGF-β suppression of normal CD4+ T cell activation has been validated, it remains unclear whether TGF-β action is through induction of CD4+CD25− T cell anergy or by promoting growth of existing Treg. First, we studied whether TCR and TGF-β costimulation could expand a population of existing Treg in vitro. As expected, freshly isolated Treg were unresponsive to anti-CD3 stimulation, but could be coerced to proliferate upon stimulation with anti-CD3 in the presence of a high dose of exogenous IL-2 (100 U/ml; references 1, 2; unpublished data). However, after primary culture with anti-CD3 only or with anti-CD3 and TGF-β in the presence of APCs for 7 d, most of the Treg died. There was no increase in the number of Treg in TGF-β–treated cultures, but the surviving anti-CD3 plus TGF-β–treated Treg, similar to only anti-CD3–treated cells, remained unresponsive to anti-CD3 restimulation (Fig. 1 A), indicating that exogenous TGF-β in vitro in the absence of other factors, such as IL-2, was insufficient to expand the original Treg.
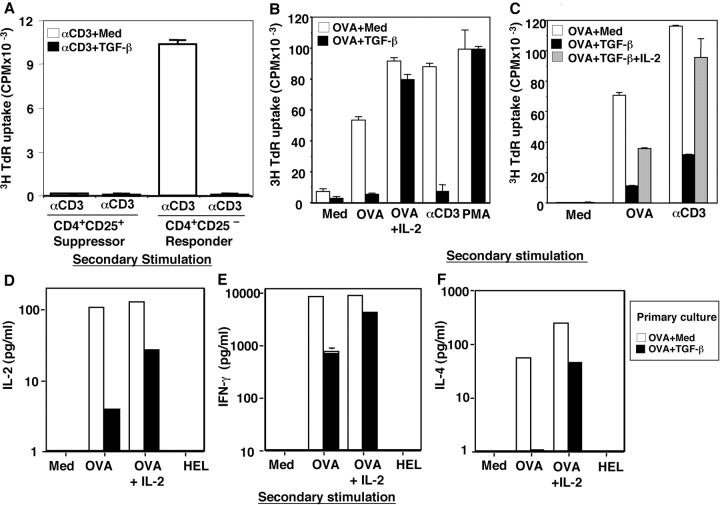
Figure 1.
Costimulation of TCR and TGF-β induces CD4+CD25− T cell anergy, but fails to expand existing Treg. (A) C57BL/6 CD25− naive cells or Treg (5 × 104) were cultured (primary) with anti-CD3 and APCs (2 × 106) in the absence (αCD3 + med) and presence (αCD3 + TGF-β) of 2 ng/ml TGF-β for 7 d. 3 × 104 harvested viable CD4+ responder T cells or 5 × 103 Treg were restimulated with anti-CD3 and APCs for 72 h to monitor their proliferation. The data are representative of three separate experiments. (B–F) TGF-β induces OVA TCR transgenic CD4+ T cells (KJ1-26+) anergy. 2 × 106 cells/ml spleen cells were cultured with OVA in the presence or absence of TGF-β for 7–10 d (primary). Viable CD4+ T cells were purified and restimulated with 100 μg/ml OVA, 100 μg/ml hen egg lysozyme (HEL), 1 μg/ml anti-CD3 mAb, or 10 U/ml IL-2 as indicated in the presence of BALB/c APCs or with PMA and ionomycin (secondary stimulation). The values are expressed as mean ± SD of triplicate wells for 3H incorporation (CPM, 5 × 104 T cells) (B and C) or of duplicate wells of the ELISA (D–F, 2 × 104 T cells). (B) TGF-β induces transgenic CD4+ TCR-specific anergy. (C) Inclusion of exogenous IL-2 in primary cultures blocks TGF-β–induced CD4+ T cell anergy. (D–F) TGF-β induces both Th1 and Th2 cell anergy. Cytokine levels of IL-2 (D), IFN-γ (E), and IL-4 (F) in secondary culture supernatants (after 24–48 h) were determined by ELISA. The data shown were repeated from two to six times with similar results.
Alternatively, to determine whether TGF-β could anergize CD4+CD25− naive T cells, purified CD4+CD25− T cells from spleens of normal C57BL/6 (Fig. 1) or BALB/c (not depicted) mice were cultured with anti-CD3 and APCs in the presence and absence of TGF-β for 1 wk, harvested, washed, and restimulated with anti-CD3 and APCs. Consistent with previous papers (1, 2), control CD4+ T cells, precultured with anti-CD3 and APCs (neutral stimulation condition), proliferated vigorously in response to TCR restimulation (Fig. 1 A). In contrast, anti-CD3 plus TGF-β–pretreated cells were unresponsive to TCR restimulation (Fig. 1 A) without increased apoptosis (not depicted), consistent with TGF-β induction of CD4+CD25− T cell anergy.
Extending this study to CD4+ T stimulation with a specific peptide antigen in TCR transgenic mice, similar results were obtained. Using TCR transgenic mice DO11.10 in which the majority of the peripheral CD4+ T cells express clonotypic TCR (KJ1-26+, Vβ 8.2) recognizing the OVA peptide 323-339, of TGF-β inhibited OVA-specific activation of these TCR transgenic T cells in primary culture in a dose-dependent manner (unpublished data). When TGF-β was included in primary spleen cell cultures with OVA for 1 wk and washed, and the purified transgenic CD4+KJ1-26+ T cells were rechallenged to monitor their secondary responses, the TGF-β–pretreated CD4+ T cells exhibited profound antigen-specific unresponsiveness to OVA or anti-CD3 in the presence of syngeneic (BALB/c) APCs (Fig. 1 B), in contrast to the vigorous proliferation of control cells (Fig. 1 B, OVA + Medium). However, these TGF-β–treated transgenic CD4+ T cells proliferated normally to signals from PMA and ionomycin stimulation (Fig. 1 B). Both OVA-specific production of Th1 (Fig. 1, D and E, IL-2 and IFN-γ) and Th2 (Fig. 1 F, IL-4) cytokines were blunted in TGF-β–pretreated transgenic CD4+ T cells. This antigen-specific T cell unresponsiveness was not due to cell death because TGF-β–treated transgenic T cells manifested similar numbers of apoptotic cells compared with control as determined by DNA dye 7-AAD (20, 21) after 16-h restimulation with OVA and APCs (7-AAD+ OVA + Medium [13%] vs. OVA + TGF-β [17%]). These data suggest that TGF-β1 induces both antigen-specific Th1 and Th2 cell anergy in transgenic CD4+ T cells.
Because exogenous IL-2 reportedly blocks T cell anergy (26), we included IL-2 in secondary restimulation cultures and found that it reversed the antigen-specific suppression of T cell proliferation (Fig. 1 B) and Th1 and Th2 cytokine production in TGF-β–anergized T cells (Fig. 1, D–F). To study IL-2 effects on the induction phase of the TGF-β–induced anergy, IL-2 was included in the primary culture with OVA plus TGF-β for 7 d, and cells were washed and restimulated with OVA or anti-CD3 in the absence of IL-2. Under these conditions, TGF-β–induced suppression of TCR-specific T cell proliferation was abrogated (Fig. 1 C), documenting that IL-2 not only reverses TGF-β–induced anergy, but also abolishes the development of TGF-β–induced CD4+ T cell anergy. Thus, in two discrete systems, TGF-β was uniquely able to induce CD4+CD25− cells into an anergic state.
TCR and TGF-β Converts CD4+CD25− T Cells to CD4+CD25+ Suppressor T Cells In Vitro.
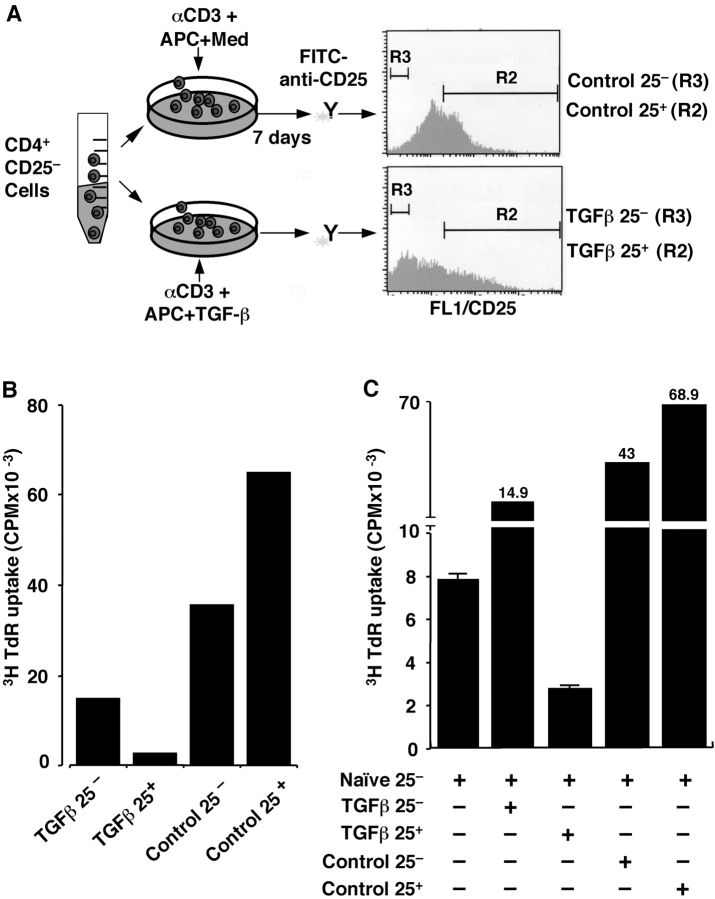
We studied whether the TGF-β–anergized CD4+ T cells were functionally active and able to suppress responder T cell proliferation. Freshly isolated CD4+CD25− naive T cells were cultured with anti-CD3 and APCs in the absence and presence of TGF-β. After 1 wk, viable CD4+ T cells were stained with FITC–anti-CD25 and sorted with flow cytometry into four populations (Fig. 2 A): Control CD4+CD25− (control 25−) and CD4+CD25+ (control 25+), and TGF-β–treated CD4+CD25− (TGF-β 25−) and CD4+CD25+ (TGF-β 25+). The individual populations were restimulated with anti-CD3 and APCs (Fig. 2 B). Both control 25− (3H uptake mean CPM = 35,661) and control 25+ (mean CPM = 65,057) cells proliferated vigorously (Fig. 2 B). In contrast, TGF-β 25− exhibited reduced TCR-triggered proliferation (mean CPM = 14,684) as did TGF-β 25+ cells (Fig. 2 B, mean CPM = 2,471). Most importantly, when these individual populations were cocultured with freshly isolated CD4+CD25− naive responder T cells, only the TGF-β 25+ cells inhibited the anti-CD3–induced responder T cell proliferation (Fig. 2 C; Fig. S1 H, available at http://www.jem.org/cgi/content/full/jem.20030152/DC1). Neither control 25+ nor control 25− had any suppressive action (Fig. 2 C; Fig. S1 H). Interestingly, TGF-β–treated cells that remained 25− cells also lacked suppressive action to normal T cell proliferation, despite their anergy to TCR stimulation (Fig. 2 B). Similar results were obtained in normal BALB/C mice (unpublished data). When CD4+CD25− responder T cells were labeled with CFSE and cocultured with TGF-β 25+ or control 25+ T cells, only TGF-β 25+, but not control 25+ T cells, blocked CFSE-labeled reduction as a marker of proliferation in CD25− responder cells (Fig. S1, A–D). The immunosuppressive action in vitro was dependent on cell contact because no inhibition occurred when TGF-β–converted suppressor T cells were separated from the responder cells in Transwell™ plates (Fig. S1, E and F). If TGF-β was replaced with IL-10, another potent immunoregulatory cytokine, in parallel cultures of naive CD4+CD25− T cells for 7 d, the resultant CD4+CD25+ T cells (IL-10 25+) exhibited no suppression to normal CD4+ T cell proliferation in vitro (Fig. S1, G and H). The data support a role for TGF-β in the conversion of CD4+CD25− naive/responder into CD4+CD25+ anergic/suppressor T cells.
Figure 2.
TGF-β converts naive CD4+CD25− T cells to CD4+ CD25+ anergic/suppressor T cells. (A) Schematic for the experiment. Freshly isolated B6 CD4+CD25− T cells were stimulated with anti-CD3 and APCs in the absence and presence of TGF-β for 1 wk. Viable CD4+ T cells were stained with FITC–anti-CD25 mAb, and four populations of cells (control 25−, control 25+, TGF-β 25−, and TGF-β 25+) were sorted by FACStarPlus™ Cell Sorter. (B) The individual populations (5 × 104) were restimulated with anti-CD3 and APCs (2 × 105) to monitor their proliferative response. The data are shown as mean of duplicate wells of 3H incorporation (CPM). (C) 1.5 × 104 freshly isolated CD4+CD25− responder T cells were stimulated with anti-CD3 and APCs in the absence (naive CD25− alone) or presence of the four individual populations of cells (5 × 104) to examine their suppressive ability for naive responder T cell activation. The data are representative of three experiments.
Phenotype of TGF-β–induced Anergic/Suppressor CD4+ T Cells.
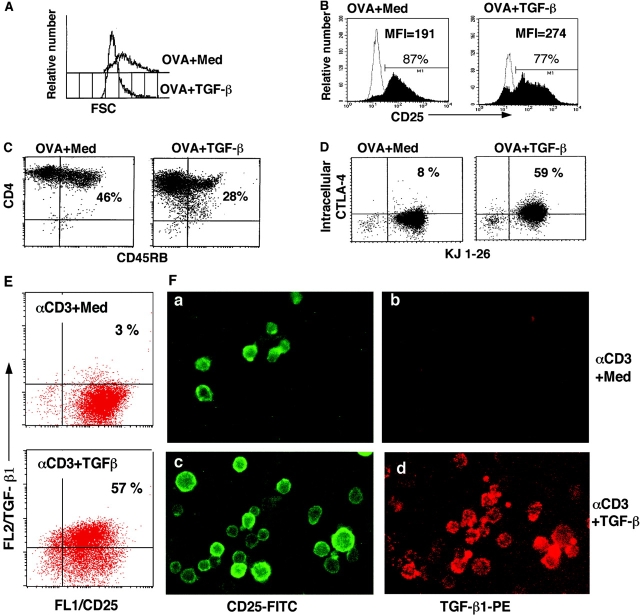
We determined whether TGF-β–induced anergic/suppressor T cells exhibited a phenotype comparable to that of Treg (e.g., CD25+, CD45RB−/low) and intracellular CTLA-4+(1, 23). OVA TCR transgenic CD4+ T cells were stimulated with OVA in the presence or absence of TGF-β. After 1 wk, TGF-β–anergized viable CD4+ transgenic T cells were found to be smaller in size (Fig. 3 A), to express CD25+ (Fig. 3 B), and to show reduced CD45RB (Fig. 3 C, CD45RB−/low).
Figure 3.
Phenotype of TGF-β–induced anergic/suppressor T cells. (A–D) DO11.10 TCR transgenic spleen cells were stimulated with OVA in the absence (OVA + Med) and presence of TGF-β (OVA + TGF-β) for 7 d. CD4+ T cells were purified and stained with PE–anti-CD4 and FITC–anti-CD25, or FITC–anti-CD45RB. CD4+ T cells (>98% KJ1-26+) were gated, and histogram profiles of cell size on FSC (A) and CD25 (B) are displayed. Profile of dual CD4 and CD45RB expression (C) CD4+ T cells purified at day 7 after the primary cultures were rested in complete DMEM for 56 h. Viable CD4+ T cells were stained with FITC-anti–KJ1-26 and intracellular PE-anti–CTLA-4. (D) TGF-β–induced anergic/suppressor T cells express membrane-bound TGF-β (E and F). B6 CD4+CD25− T cells were cultured with anti-CD3 and APCs in the absence (panels a and b, αCD3 + Med) or presence of TGF-β (panels c and d, αCD3 + TGF-β) for 3 d. After extensive washes, cells were stained with FITC–anti-CD25 and biotinylated chicken anti–TGF-β1, followed by streptavidin-PE. Cells were analyzed on flow cytometry and under immunofluorescence microscopy.
Next, we focused on the expression of CTLA-4 associated with Treg (23, 27, 28). After primary culture for 7 d, viable transgenic CD4+ T cells were isolated and maintained in complete DMEM for up to 56 h without addition of any growth factors or cytokines to rest the T cells completely. The CD4+ T cells were doubly stained with anti–CTLA-4 antibody together with KJ1-26, and both surface and intracellular CTLA-4 levels were examined. Although almost all surviving control CD4+ transgenic T cells lost their intracellular (8%) and surface (1.5%) CTLA-4 as typical resting T cells (Fig. 3 D), TGF-β–anergized CD4+ KJ1-26+ T cells retained intracellular CTLA-4+ (56%), albeit not on the surface (2.2%; Fig. 3 D and not depicted). Similar results were obtained when normal B6 and BALB/c CD4+ T cells were cultured with anti-CD3 plus TGF-β (unpublished data). Thus, TGF-β–induced anergic/suppressor CD4+ T cells exhibit a similar phenotype to Treg.
TGF-β–induced Anergic/Suppressor CD4+ T Cells Express Cell Membrane–bound Active TGF-β.
Previous papers have shown that the Treg express cell membrane–bound TGF-β, which is involved in Treg cell contact–dependent immunosuppression (references 29, 30; unpublished data). We examined whether TGF-β–induced anergic/suppressor T cells express increased TGF-β. Naive C57BL/6 CD4+ CD25− T cells were stimulated with anti-CD3 and APCs in the presence or absence of TGF-β for 3 d, washed extensively, and stained doubly with antibody to active TGF-β and anti-CD25. As expected, most of the CD4+ T cells (>90%) expressed CD25 after TCR stimulation for 3 d. The striking finding was that the majority of TGF-β–treated CD25+ T cells (>50–80%) exhibited cell surface active TGF-β, whereas only a few CD25+ T cells (<3–5%) in control cultures (anti-CD3 alone) were positive for surface TGF-β, determined by flow cytometry (Fig. 3 E) and immunofluorescence microscopy (Fig. 3 F). To establish a link between their increased surface TGF-β and functional anergy to TCR stimulation, anti-CD3 restimulation showed increased phosphorylation of Smad2/3, which are key mediators of TGF-β signaling (6, 7) and which is indicative of TGF-β signal transduction, by Western blot analysis (Fig. S2 A) or intracellular immunofluorescence staining (Fig. S2 B). Furthermore, when anti–TGF-β neutralizing monoclonal antibody was included in secondary restimulation cultures of these anergic T cells, anti–TGF-β, but not isotypic control antibodies, reversed their proliferation (Fig. S2 C), as well as IL-2, IFN-γ, and IL-4 production (not depicted). Anti–TGF-β had no apparent effects on control CD4+ T cells (Fig. S2 C, OVA + Med), consistent with their lack of surface TGF-β (Fig. 3, E and F). Thus, TGF-β–induced anergic/suppressor CD4+ T cells expressed increased TGF-β, which likely mediates their own anergic state, as well as their suppression of normal T cell activation.
TGF-β Induces Foxp3 Expression in CD4+CD25− Naive/Responder T Cells.
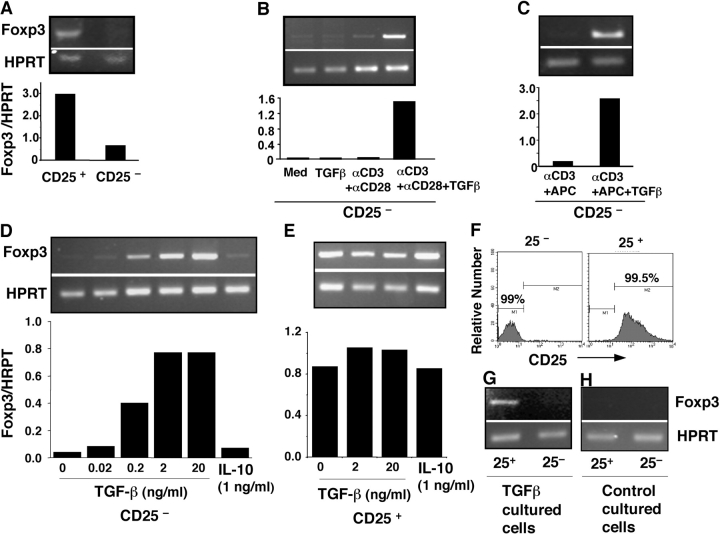
Although TGF-β converts peripheral naive CD4+CD25− T cells into anergic/suppressor T cells, the molecular mechanisms by which TGF-β drives this transition are unknown. Transcription factor Foxp3 is a recently described gene associated with the development of regulatory T cells, and retroviral gene transfer of Foxp3 into naive responder T cells converts them toward a regulatory T cell phenotype (13–15). The physiological inducers for Foxp3 gene expression remain a mystery, but we reasoned whether TGF-β might trigger Foxp3 expression in CD4+ CD25− naive/responder T cells, switching them toward a regulatory phenotype.
Freshly purified (>99%) Treg(CD25+), but not CD4+ CD25− (CD25−) naive responder T cells, in normal C57BL/6 mice express Foxp3 (Fig. 4 A; references 13–15). When naive responder T cells were stimulated with anti-CD3 and anti-CD28, the Foxp3 gene was not up-regulated (Fig. 4 B), consistent with previous findings (13–15). Significantly, stimulation of naive responder T cells with anti-CD3 and anti-CD28 (Fig. 4 B) or with anti-CD3 and APCs (Fig. 4 C) in the presence of active TGF-β dramatically induced Foxp3 expression, which was dependent on the levels of TGF-β (Fig. 4 D), whereas TGF-β treatment alone failed to elicit Foxp3 (Fig. 4 B). Similar results were observed in BALB/c mice (unpublished data), indicating a generality of the effect. By comparison, stimulation of Foxp3-expressing Treg with anti-CD3 and anti-CD28 and a high dose of IL-2 (100 U/ml), a regimen for their proliferation (1), did not increase constitutive Foxp3 (Fig. 4 E; reference 13), nor did the addition of TGF-β (Fig. 4 E). Separation of TGF-β and anti-CD3–cultured CD4+CD25− cells (day 7) into CD25+ suppressor and CD25− nonsuppressor cells by FACS® (Fig. 2 A and Fig. 4 F) revealed that the TGF-β–converted CD25+ subset had much higher levels of Foxp3 than did the CD25− subpopulation (Fig. 4 G). As expected, both CD25+ and CD25− subsets from control cultures had undetectable Foxp3 (Fig. 4 H). On the other hand, IL-10 failed to induce Foxp3 expression in naive CD4+CD25− cells (Fig. 4 D) or to further enhance Foxp3 in Treg (Fig. 4 E). Thus, costimulation of Foxp3-negative CD4+CD25− naive responder T cells with TCR engagement in the presence of TGF-β induces de novo expression of Foxp3, concomitant with conversion to a population of CD4+CD25+ Foxp3 + T cells phenotypically and functionally indistinguishable from professional Treg.
Figure 4.
TGF-β and TCR costimulation induces Foxp3 expression in CD4+CD25− naive responder T cells. (A) B6 spleen cells were sorted into CD4+CD25− (CD25−) and Treg (CD25+) populations. cDNA from each population was subjected to nonsaturating PCR using Foxp3 or HPRT-specific primers, and data are presented as Foxp3/HPRT ratio. (B) CD25− cells were cultured with medium or 2 ng/ml TGF-β1 (24 h) or stimulated with platebound anti-CD3 and soluble anti-CD28 in the absence or presence of TGF-β1 (72 h) and assessed for the expression of Foxp3 by RT-PCR. (C) CD25− cells were activated with soluble anti-CD3 and APCs with or without TGF-β for 3 d and assessed for Foxp3 expression. (D) Dose dependence of TGF-β and failure of IL-10 on Foxp3 induction in CD25− naive T cells. CD25− cells were cultured as in B in the presence of indicated concentrations of TGF-β or recombinant murine IL-10. (E) Both TGF-β and IL-10 failed to further enhance Foxp3 expression in Treg. Freshly isolated Treg were activated with platebound anti-CD3, soluble anti-CD28, and IL-2 (100 U/ml) with or without TGF-β or IL-10, and Foxp3 expression was assessed by RT-PCR. (F and G) Flow cytometry analysis (F) and Foxp3 expression (G) of CD25+ or CD25− T cells sorted from TGF-β– and anti-CD3–costimulated naive CD25− T cells (day 7). (H) No Foxp3 expression in both CD25+ and CD25− subsets purified from control (anti-CD3 only) stimulated CD25− naive cells (day 7). The data in the figure are representative of at least three experiments.
TGF-β–induced CD4+ Transgenic Anergic/Suppressor T Cells Suppress OVA-specific T Cell Proliferation In Vivo.
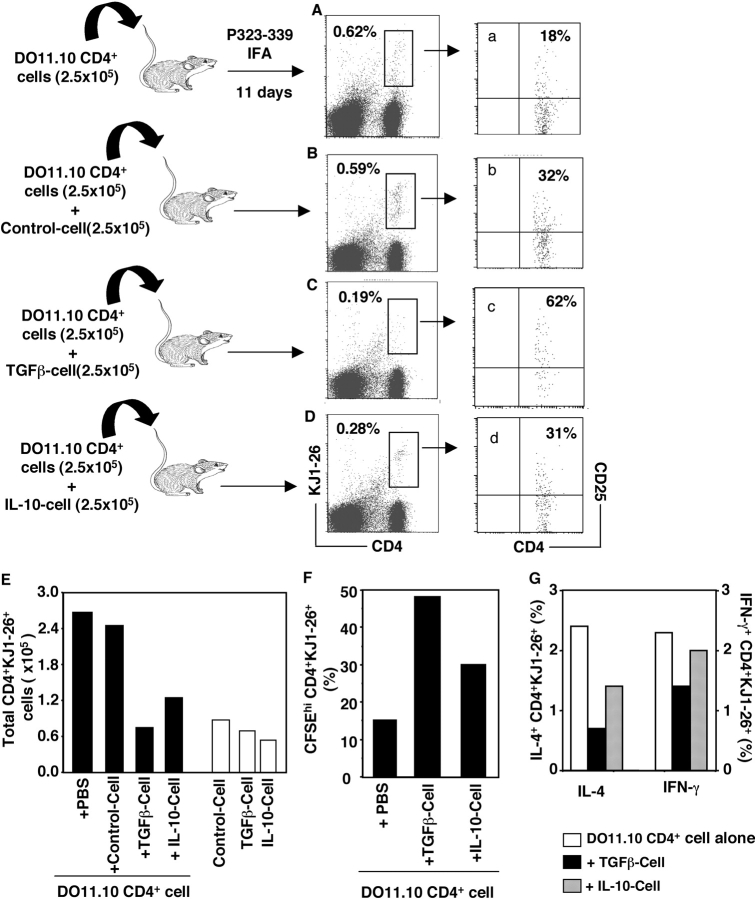
Extending these studies in vivo, first we used an adoptive transfer model (31, 32) in which transgenic CD4+ KJ1-26+ T cells were transferred into syngeneic BALB/c mice followed by OVA peptide immunization. The proliferation of the transgenic CD4+ T cells in host mice was monitored using clonotypic antibody KJ1-26 (Fig. 5) . In contrast to their anergic state in vitro, the TGF-β–converted CD4+CD25+ transgenic suppressor T cells (TGF-β Cell) expanded at similar levels as control CD4+ transgenic T cells (Control Cell) in vivo after OVA peptide 323-339 immunization (Fig. 5 E and not depicted; without immunization, recovered CD4+KJ1-26+ T cells were <0.1 × 105 in draining lymph nodes in all groups). Significantly, the recovered CD4+ KJ1-26+ T cells from draining lymph nodes receiving TGF-β Cell expressed high levels of CD25 (>70%) and preserved their specific unresponsiveness to OVA peptide 323-339 restimulation in vitro (unpublished data). CD4+KJ1-26+ T cells recovered from mice receiving Control Cell had much lower levels of CD25 (28%) and proliferated vigorously to OVA peptide 323-339 (unpublished data). When cotransferred with naive/responder CD4+ transgenic T cells, the TGF-β Cell, but not Control Cell, blocked antigen-specific expansion of naive CD4+KJ1-26+ T cells in draining lymph nodes (Fig. 5, A–C and E). Importantly, the recovered CD4+KJ1-26+ T cells from the mice injected with naive CD4+KJ1-26+ cells plus TGF-β Cell also expressed much higher levels of CD25 (Fig. 5 c) compared with that in mice injected with naive CD4+ T cells alone (Fig. 5 a) or plus Control Cell (Fig. 5 b). Interestingly, in contrast to their nonsuppressive features in vitro, IL-10–induced CD4+ transgenic T cells (IL-10 Cell) suppressed antigen-driven CD4+KJ1-26+ T cell proliferation in vivo (Fig. 5, D and E). However, the recovered CD4+KJ1-26+ T cells from mice receiving IL-10 cells expressed reduced CD25 (Fig. 5 d) compared with those from mice receiving TGF-β Cell (Fig. 5 c).
Figure 5.
TGF-β–converted CD4+ suppressor T cells inhibited antigen-specific expansion of transgenic CD4+ T cells in vivo. Freshly isolated CD4+ KJ1-26+ T cells (DO11.10 CD4+ cells) or with equal number of OVA only (Control cell), plus 2 ng/ml TGF-β (TGF-β cell) or plus 1 ng/ml IL-10–converted (IL-10 cell) CD25+ KJ1-26+ T cells were injected i.p into normal BALB/c mice. Some mice were injected with 2.5 × 105 of Control cell, TGF-β cell, or IL-10 cell alone. Mice were immunized with Peptide 323-339 emulsified with IFA on day 2. On day 11, draining lymph nodes (inguinal) were harvested, and cells were stained with Tricolor–anti-CD4, FITC–KJ1-26, and PE–anti-CD25 and analyzed on FACSCalibur™. (A–D) Live cells were gated, and the profiles between CD4 versus KJ1-26 are displayed. The numbers represent CD4+KJ1-26+ cell frequency within lymph node cells. The numbers in the parentheses are injected cells. Each group contained two mice and the lymph node cells were pooled before staining. (a–d) CD4+KJ1-26+ T cells in corresponding A–D were gated, and CD25+ frequency is shown. (E) Total CD4+KJ1-26+ T cells in draining lymph nodes per mouse (percentage of CD4+KJ1-26+ × the number of total lymph node cells/2). (F) 2 × 106 lymph node cells labeled with 3 μM CFSE were cultured with 1 μg/ml Peptide 323-339 in 24-well plates for 72 h. Cells were stained with Tricolor–anti-CD4 and PE–KJ1-26 and analyzed on FACSCalibur™. CD4+KJ1-26+ cells were gated, and the CFSEhi cells are shown. The marker was set according to CFSE fluorescence of the live CD4+ KJ1-26+ T cells in parallel cultures with medium only. (G) 2 × 106 lymph node cells were cultured with Peptide 323-339 in the presence of GolgiPlug™ for 5–6 h. Cells were stained with Tricolor–anti-CD4 and FITC–KJ1-26 antibodies before being fixed and intracellularly stained for IL-4 or IFN-γ cytokines with respective antibodies (PE-anti–IL-4 or PE-anti–IFN-γ, 0.5 μg/106 cells). 40,000–80,000 cells were acquired on FACSCalibur™, and percentages of KJ1-26+ IL-4+ or IFN-γ+ cells within CD4+ T cells are shown. The experiments were repeated twice with similar results.
When draining lymph node cells were restimulated in vitro with OVA peptide 323-339, the antigen-specific T cell proliferation from mice cotransferred with TGF-β Cell was significantly inhibited compared with that of mice receiving naive CD4+KJ1-26+ T cells alone (Fig. 5 F). The antigen-specific IL-4 and IFN-γ production in recovered CD4+KJ1-26+ T cells was also inhibited in mice cotransferred with TGF-β suppressor cells (Fig. 5 G). The recovered CD4+KJ1-26+ T cells from mice cotransferred with IL-10 Cell exhibited less inhibition of OVA-specific T cell proliferation and cytokine production (Fig. 5, F and G). Clearly, TGF-β–converted CD25+ T cells have regulatory ability for antigen-induced naive CD4+ T cell activation in vivo, consistent with the functional features of Treg (31, 32), but distinct from IL-10–induced CD25+ T cells.
TGF-β–induced CD4+ Anergic/Suppressor T Cells Prevent HDM-induced Allergic Pathogenesis In Vivo.
The immunoregulatory effect of TGF-β–induced anergic/suppressor CD4+ T cells in vivo was further assessed in an antigen-induced allergic model. C57BL/6 CD4+CD25− naive T cells were cultured with anti-CD3 and APCs in the absence or presence of TGF-β for 3–4 d, washed extensively, and rested in complete medium containing IL-2 for an additional 3 d. Viable cells were harvested and delivered intravenously into mice immunized and challenged with HDM as depicted in Fig. 6 A. After HDM intraperitoneal immunization and intratracheal challenge, massive inflammatory cell infiltrates were evident with mucus obstruction of the airway in the lung (Fig. 6 C), compared with the lung tissues in naive or PBS-injected mice (Fig. 6 B and not depicted). Administration of control CD4+ T cells (Control Cell) pretreated with anti-CD3 alone failed to reduce the inflammatory cell infiltration significantly, although mucus was less obvious in the airways (Fig. 6 D). In contrast, administration of TGF-β–converted CD4+ suppressor T cells (TGF-β cell) dramatically reduced HDM-induced inflammatory cell infiltration and preserved the integrity of airway structures (Fig. 6 E). Cotransfer of TGF-β–converted CD4+ suppressor T cells blocked epithelial cell mucin production in airways induced by HDM as detected by PAS staining, whereas control CD4+ T cells did not (Fig. 6, B–E; Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20030152/DC1). Spleen T cells from mice treated with HDM and TGF-β–induced anergic/suppressor CD4+ T cells produced much lower levels of HDM-specific, as well as anti-CD3 driven, Th1 (IFN-γ) and Th2 (IL-4 and IL-13) cytokines compared with mice receiving HDM alone (Fig. 6, F–H). In contrast, spleen T cells from mice treated with HDM and control CD4+ T cells produced higher levels of IFN-γ (Fig. 6 F), although these cells produced reduced levels of IL-4 and IL-13 (Fig. 6, G and H). Thus, TGF-β–induced anergic/suppressor CD4+ T cells dampen inflammation and associated mucin production in allergic immune responses in vivo.
Figure 6.
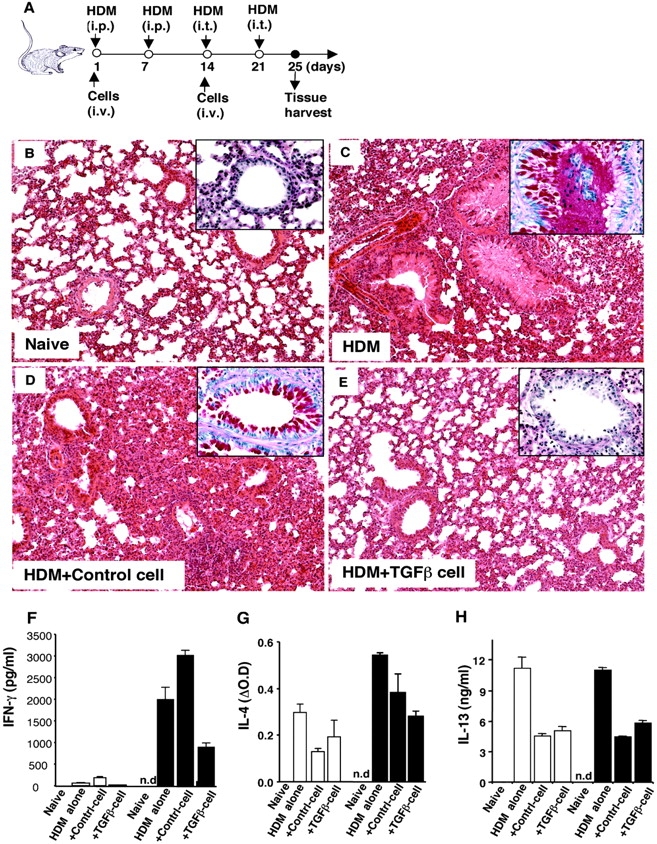
TGF-β–converted-CD4+ suppressor T cells prevent HDM-induced allergic pathogenesis in vivo. B6 CD4+CD25− T cells were cultured with anti-CD3 and APCs in the absence (Control cell) and presence of TGF-β (TGF-β cell) for 3–5 d, extensively washed and rested in DMEM complete medium containing 10 U/ml of IL-2 for an additional 3 d. Viable cells were harvested, washed, and resuspended in PBS for injection. For induction of allergic immune responses in lungs, B6 mice were injected with HDM allergen as depicted in schematic plan (A). 4 d after the last HDM intratracheal challenge, mice were killed, and lungs were immediately fixed with 10% PBS-Formalin for immunohistological staining (H&E). One representative lung from each group (three to five mice) is shown. The magnification of the images is 20. (B) Naive lung. (C) HDM-injected lung. (D) HDM-injected lung coinjected with Control cell (106 on day 1 and 5 × 105 on day 14, i.v.). (E) HDM-injected lungs coinjected with TGF-β cell (106 on day 1 and 5 × 105 on day 14, i.v.). (inset) Boxed images are mucin staining in airways by PAS (40×). The red staining represents mucin positive cells. (F–H) Spleen cells were harvested and pooled from three to five mice per group. 4 × 105 spleen cells were cultured with 100 μg/ml HDM (white bars) or 0.5 μg/ml anti-CD3 (black bars) for 24–96 h, the cell-free supernatants were collected to measure IFN-γ (F, 48 h), IL-4 (G, 24 h), and IL-13 (H, 96 h), respectively, by ELISA. IL-4 levels are shown as ΔOD (OD value of test supernatants − OD value of culture medium; OD = 0.107). n.d., not done.
Discussion
Of many unresolved questions regarding Treg, where and how they are generated remains significant (2, 33). In this report, we have provided evidence that Treg can be induced/converted from peripheral CD4+CD25− naive responder T cells through costimulation of TCR and TGF-β signaling. Several important conclusions can be drawn from the current work. First, TGF-β treatment in the presence of TCR stimulation converts naive CD4+CD25− responder T cells to anergized CD4+CD25+ T cells, but fails to promote growth of existing Treg, at least in vitro. The effect of TGF-β on the fate of T cell activation is determined not only by TGF-β concentration, but also by the level of IL-2. Importantly, exogenous IL-2 not only reverses unresponsiveness to TCR restimulation of TGF-β–anergized CD4+ T cells but also antagonizes the induction of CD4+ T cell anergy by TGF-β.
Second, TGF-β1–converted anergic CD4+ T cells are suppressor cells. Phenotypically, TGF-β–converted anergic/suppressor T cells retain surface CD25+ and exhibit CD45RB−/low, similar to professional Treg (2, 34). Another key finding is that TGF-β–anergized CD4+CD25+ T cells express intracellular CTLA-4 as do Treg (23, 27, 28). TGF-β regulation of intracellular CTLA-4 in CD4+ T cells does not appear to involve induction of CTLA-4 synthesis, but rather prevention of its degradation (unpublished data). Functionally, when cocultured with normal CD4+ T cells, TGF-β1–anergized T cells inhibit proliferation and cytokine production of the target T cells. This suppressive activity is mainly attributed to the CD25+ subpopulation of anergized cells, because TGF-β–anergized cells lacking CD25, at least in vitro, have no obvious suppressive ability. Neither CD25− nor CD25+ T cells from control cultures (without TGF-β) have suppressive activity, consistent with previous observations (1, 2, 34, 35). The level of immunosuppression is dependent on the cell number and reversible by exogenous IL-2. Consistent with Treg, TGF-β–converted CD25+ suppressor T cells also require cell contact to carry out their suppression, which can be mediated by cell membrane–bound TGF-β (29, 30).
The data that TGF-β–converted CD4+CD25+ T cells express cell surface TGF-β in active form provide further evidence that the professional Treg can be converted by TGF-β and TCR costimulation from peripheral naive CD4+ T cells. Although not unanimous, increasing evidence has demonstrated that Treg produce/express higher levels of TGF-β, which plays a significant role in immune regulation in vitro and in vivo (27, 29, 30, 36, 37). That TGF-β–converted CD25+ suppressor T cells express cell surface TGF-β not only supports the concept that these suppressor cells possess the same phenotype as the professional Treg but also offers an explanation for how professional Treg are developed. Although how these TGF-β suppressor T cells acquire surface TGF-β remains elusive, it is conceivable that TGF-β enhances its own production as evident by exogenous TGF-β up-regulation of its own mRNA expression in CD4+ T cells (reference 7; unpublished data) and/or TGF-β binds onto its cognate receptors on these suppressor T cells, because TCR stimulation enhances TGF-β receptor expression in CD4+ T cells (unpublished data). The cell membrane–bound TGF-β is likely involved in unresponsiveness to TCR engagement and suppression of normal T cells by these TGF-β anergic/suppressor T cells because restimulation of the suppressor cells drives a higher level of phosphorylation of Smad2/3, and anti–TGF-β antibody reverses their anergy and abrogates their suppressive action.
The strongest evidence that TGF-β converts CD4+ CD25− naive T cells to CD25+ regulatory cells comes from our surprising findings that TGF-β and TCR costimulation induces transcription factor Foxp3 gene expression in CD4+CD25− T cells. The observation that TGF-β induces Foxp3 expression in CD4+CD25− naive T cells has led to several unprecedented conclusions. First, TGF-β can induce Foxp3 expression in CD4+ CD25− T cells under conditions involving either anti-CD3 and anti-CD28 costimulation in the absence of APCs or with anti-CD3 in the presence of APCs, suggesting the dispensability of APCs. The Foxp3 induction is dependent on the levels of TGF-β, suggesting the causal influence of TGF-β. Second, in the absence of TCR stimulation, TGF-β itself fails to induce or enhance Foxp3 expression, indicating the necessity of TCR engagement. Moreover, when CD25+ and CD25− subsets were isolated from TGF-β– and TCR-costimulated cultures, CD25+ T cells exhibited much higher levels of Foxp3 than the CD25− subset, supporting the notion that Foxp3 may be involved in Treg immunoregulation (38). Lastly, TGF-β had no further enhancement on existing Foxp3 gene in professional Treg, consistent with the evidence that Foxp3 expression is stable in Treg (13). The failure of IL-10 to induce Foxp3 in CD25− naive T cells indicates that IL-10 is not a physiological inducer for Foxp3 expression. Although mechanistically elusive, the observation that IL-10–induced CD25+ T cells lack characteristic suppression in vitro, but are functional in vivo suggests that these cells are distinct from TGF-β–converted CD25+ regulatory cells. The data are consistent with the notion that IL-10 is more likely a switch for CD4+ T cells into Trl cells (39). These discriminating results not only provide a molecular mechanism for the TGF-β–driven transition of naive T cells toward regulatory T cells, but also unveil the first physiological inducer for Foxp3 expression in CD4+ naive/responder T cells, which may enable manipulation of this specific population of regulatory cells.
More importantly, these TGF-β1–converted CD4+ CD25+ suppressor cells also inhibit T cell responses when transferred in vivo. Evidence from the OVA transgenic adoptive transfer model document that TGF-β–converted CD25+ suppressor cells resemble Treg. First, TGF-β–converted CD25+ suppressor T cells proliferate in vivo upon specific antigen immunization, yet preserve their antigen specific anergy when recultured in vitro. Second, transgenic CD4+KJ1-26+ T cells recovered from mice receiving TGF-β–converted suppressor cells expressed elevated CD25. Significantly, TGF-β–converted KJ1-26+ CD25+ suppressor cells inhibit antigen-driven CD4+ T cell growth in vivo. Again, the recovered CD4+KJ1-26+ cells from the suppressed draining lymph nodes show high levels of CD25 and fail to respond to specific antigen stimulation. All these phenotypic and functional features of TGF-β–converted suppressor T cells are consistent with those of professional Treg (31, 32). Moreover, TGF-β–converted suppressor T cells may carry out their suppression in vivo in a nonspecific manner because TGF-β–converted BALB/c CD25+ T cells harvested from the primary cultures exhibit the inhibition of antigen-driven expansion of the transferred CD4+KJ1-26+ T cells (unpublished data). In HDM-induced allergic immune responses in lungs, TGF-β–converted suppressor T cells ameliorate allergen-driven inflammatory cell infiltration and mucin production in airways. Anergic/suppressor T cells appear to inhibit both Th1 and Th2 responses, whereas administration of control CD4+ cells enhanced Th1 IFN-γ, which might contribute to the inhibition of Th2 cytokines, but failed to significantly limit the inflammatory response in the lungs, indicating the complexity of the Th1 effector T cell role in allergic lung inflammation (40). Interestingly, we also observed an increase in TGF-β production in the TGF-β suppressor cell–treated mice (unpublished data), which reflects previous findings that the professional Treg require TGF-β in vivo to carry out their suppressive activity (27), and supports our evidence that TGF-β and TCR costimulation convert naive CD4+ T cells toward a regulatory T cell phenotype mimicking professional Treg.
In summary, we have provided evidence for TGF-β1 as a critical factor in the development of peripheral Treg. The present paper has not only demonstrated that TGF-β converts CD4+CD25− naive T cells toward a suppressor T cell phenotype similar to that of Treg but also uncovered that TGF-β induces transcription factor Foxp3 expression in CD25− naive T cells to enforce transition to regulatory T cells. Functionally, TGF-β–converted suppressor T cells not only suppress T cell proliferation and Th1 and Th2 cytokine production in vitro but also inhibit antigen-driven CD4+ T cell expansion and block allergen-driven lung inflammation in vivo in a similar manner as the professional CD4+CD25+ regulatory cells (31, 32). Thus, our findings provide additional mechanisms for how Treg are generated and developed. It becomes conceivable for the first time to design strategies to embellish the limited and/or inadequate numbers of these suppressor T cells in the periphery, as needed, for therapeutic intervention in autoimmune diseases, transplantation, cancer, and HIV infection.
Acknowledgments
We thank Drs. N. McCartney-Francis and N. Vazquez for critically reviewing the manuscript. We also thank M. Frank and J. Lin for technical assistance.
This work was performed at the National Institute of Dental and Craniofacial Research, National Institutes of Health.
The online version of this article includes supplemental material.
Abbreviations used in this paper: 7-AAD, 7-amino-actinomycin D; CFSE, carboxy-fluorescein diacetate succinimidyl ester; HDM, house dust mite; HPRT, hypoxanthineguanine phosphoribosyl transferase; PAS, periodic acid schiff; P-Smad2/3, phosphorylated Smad2/3; Treg, CD4+CD25+ regulatory T cells.
References
- 1.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 2.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 3.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 4.Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, X., L. Izikson, L. Liu, and H.L. Weiner. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253. [DOI] [PubMed] [Google Scholar]
- 6.Gorelik, L., and R.A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46–53. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., and S.M. Wahl. 2002. TGF-beta: receptors, signaling pathways and autoimmunity. Curr. Dir. Autoimmun. 5:62–91. [DOI] [PubMed] [Google Scholar]
- 8.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 9.Lucas, P.J., S.J. Kim, S.J. Melby, and R.E. Gress. 2000. Disruption of T cell homeostasis in mice expressing a T cell–specific dominant negative transforming growth factor β II receptor. J. Exp. Med. 191:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagiwa, S., J.D. Gray, S. Hashimoto, and D.A. Horwitz. 2001. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166:7282–7289. [DOI] [PubMed] [Google Scholar]
- 11.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 12.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 13.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 14.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4(+)CD25(+) T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4(+) CD25(+) regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 16.Tivol, E., F. Borriello, A. Schweitzer, W. Lynch, J. Bluestone, and A. Sharpe. 1995. Loss of CTLA4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction. Immunity. 3:541–547. [DOI] [PubMed] [Google Scholar]
- 17.Waterhouse, P., J. Penninger, E. Timms, A. Wakeham, A. Shahinian, K. Lee, C. Thompson, H. Griesser, and T. Mak. 1995. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 270:985–988. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni, A.B., C.-H. Huh, D. Becker, A. Gerser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor-β null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shull, M.M., I. Ormsby, A.B. Kier, S. Pawlowski, R.J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, W., W. Jin, and S.M. Wahl. 1998. Engagement of cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4(+) T cells. J. Exp. Med. 188:1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, W., W. Jin, H. Tian, P. Sicurello, M. Frank, J.M. Orenstein, and S.M. Wahl. 2001. Requirement for transforming growth factor β1 in controlling T cell apoptosis. J. Exp. Med. 194:439–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 24.Chen, W., M.E. Frank, W. Jin, and S.M. Wahl. 2001. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 14:715–725. [DOI] [PubMed] [Google Scholar]
- 25.Wills-Karp, M., J. Luyimbazi, X. Xu, B. Schofield, T.Y. Neben, C.L. Karp, and D.D. Donaldson. 1998. Interleukin-13: central mediator of allergic asthma. Science. 282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz, R.H. 1996. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 184:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T.W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 192:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact–dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface–bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, W., and S.M. Wahl. 2003. TGF-beta: the missing link in CD4(+)CD25(+) regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 14:85–89. [DOI] [PubMed] [Google Scholar]
- 31.Walker, L.S., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein, L., K. Khazaie, and H. Von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. [DOI] [PubMed] [Google Scholar]
- 34.Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. [DOI] [PubMed] [Google Scholar]
- 35.Chatenoud, L., B. Salomon, and J.A. Bluestone. 2001. Suppressor T cells–they're back and critical for regulation of autoimmunity! Immunol. Rev. 182:149–163. [DOI] [PubMed] [Google Scholar]
- 36.Jonuleit, H., E. Schmitt, H. Kakirman, M. Stassen, J. Knop, and A.H. Enk. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo, E.Y., H. Yeh, C.S. Chu, K. Schlienger, R.G. Carroll, J.L. Riley, L.R. Kaiser, and C.H. June. 2002. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168:4272–4276. [DOI] [PubMed] [Google Scholar]
- 38.Ramsdell, F. 2003. Foxp3 and natural regulatory T cells. Key to a cell lineage? Immunity. 19:165–168. [DOI] [PubMed] [Google Scholar]
- 39.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. d. Vries, and M.G. Roncarolo. 1997. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 40.Herrick, C.A., and K. Bottomly. 2003. To respond or not to respond: T cells in allergic asthma. Nat. Rev. Immunol. 3:405–412. [DOI] [PubMed] [Google Scholar]