Abstract
Bacteriophage Mx9 is a temperate phage that infects Myxococcus xanthus. It lysogenizes the bacteria by integrating into the bacterial chromosome by site-specific recombination at one of two sites, attB1 or attB2. Integration at attB1 results in deletion of DNA between the two attB sites. The attB2 site lies within the 5′ region of the M. xanthus tRNAGly gene. Mx9 integration requires a single protein, Int. Analysis of integration revealed that the phage attachment site (attP) is contained in the int gene and that upon integration, the 3′ end of the int gene is altered. Plasmids containing fusions of the pilA or mgl promoter to lacZ integrated at either Mx9 attB site have higher levels of transcription than the same fusions integrated at the Mx8 attB site.
Mx9 is a general transducing phage that infects the gram-negative bacterium Myxococcus xanthus (9). The phage particle has a polyhedral head and a very short tail. Structurally, this phage resembles Mx8, which also infects M. xanthus.
The integrase gene and attachment site for Mx8 have been characterized (7, 8, 11). Integration of Mx8 by site-specific recombination requires a single phage protein, Int, and the phage attachment site, attP. Unlike the situation in most temperate bacteriophages, the Mx8 attP site is contained in the int gene, and upon insertion into the M. xanthus chromosome, the 3′ end of the int gene is altered. This modified int gene produces a protein, IntX, with lower integrase specific activity (8).
Because no natural replicating plasmids have been identified for M. xanthus or for any other myxobacterium, phage attachment sites provide an efficient and stable alternative way to introduce new genes or add additional copies of existing genes to the cell. With the recent heterologous expression of the epothilone biosynthetic gene cluster in M. xanthus, the ability to engineer the host should prove to be valuable for further optimization of polyketide production (4). The Mx8 int gene and the attachment site can be used to integrate DNA into the chromosome, but expression of many genes is affected by insertion into the Mx8 attB sites; many developmental promoters, as well as two constitutive promoters, mgl and pilA, have reduced activity at the Mx8 sites (2, 6). Therefore, I set out to find another attachment site. Here I describe characterization of the int gene and attP from Mx9, as well as the sites of insertion in the M. xanthus chromosome.
MATERIALS AND METHODS
Bacteria, phage, and plasmids.
DZ1 is a nonmotile strain of M. xanthus and was used for plating Mx9 and for characterization of the Mx9 attachment sites (12). DK816 is a natural M. xanthus isolate lysogenic for Mx9 (9). M. xanthus strains were grown in CYE medium (1) or 1% CTS (1% Casitone, 0.2% MgSO4 · 7H2O, 50 mM HEPES; pH 7.6). Phleomycin (Cayla) was used at a concentration of 30 μg/ml. The Mx9 phage was reisolated from DK816 by growing a culture to the stationary phase, pelleting the cells, and plating dilutions of the supernatant onto DZ1. High-titer stocks of Mx9 were made by coring a plaque and placing it in phage buffer (10 mM morpholinepropanesulfonic acid [MOPS] [pH 7.6], 4 mM MgCl2, 2 mM CaCl2). The eluted phage were diluted and mixed with 0.5 ml of DZ1 in the early stationary phase. After the cells and phage were incubated at room temperature for 20 min, 2.5 ml of top agar was added, and the suspension was poured onto phage plates (1% BBL Trypticase, 0.1% MgSO4 · 7H2O, 1% agar, 10 mM MOPS; pH 7.6). The plates that exhibited confluent lysis after 2 days of incubation at 30°C were overlaid with 5 ml of phage buffer and incubated at 4°C overnight. The eluted phage were stored at 4°C. Phage stocks with concentrations greater than 1 ×109 PFU/ml were obtained by this method. The plasmids used are described in Table 1.
TABLE 1.
Plasmids
| Plasmid | Characteristics |
|---|---|
| pKOS35-117.9.9 | Ampr Kanr ColE1, 4.6-kb fragment from Mx9 |
| pKOS139-29 | Ampr Co1E1, PT7A1 Mx8 int attP− |
| pKOS139-47 | Tcr , p15A, Pmgl lacZ, Mx8 attP |
| pKOS178-86 | Tcr, p15A, PpilA lacZ, Mx8 attP |
| pKOS178-177 | Tcr, p15A, PpilA lacZ, Mx9 int attP |
| pKOS178-188 | Tcr, p15A, Pmgl lacZ, Mx9 int attP |
| pKOS249-31 | Ampr Bleor ColE1, PT7A1 Mx9 int attP |
Isolation of phage DNA.
The phage from a high-titer stock were pelleted by centrifugation in an SS-34 rotor at 28,000 rpm for 3 h and then resuspended in TE (10 mM Tris [pH 7.6], 1 mM EDTA). The phage proteins were removed by extraction twice with phenol and twice with phenol-chloroform-isoamyl alcohol. The DNA was precipitated and resuspended in TE.
Isolation and sequence of the phage attachment site.
To isolate the phage attachment site, phage DNA was partially cleaved with HinPI, and the fragments were ligated into pKOS35-93 cleaved with AccI. Plasmid pKOS35-93 is pBluescriptII SK+ with the kanamycin resistance from Tn5 ligated into the SmaI and EcoRI sites. One plasmid, pKOS35-117.9.7, integrated efficiently into the chromosome. The insert from this plasmid was sequenced.
Isolation of the bacterial attachment site.
The bacterial attachment site (attB) was isolated by electroporating pKOS35-117.9.7 into DZ1, making chromosomal DNA, and then recovering the plasmid with flanking chromosomal DNA. Six kanamycin-resistant colonies were picked, and chromosomal DNA was prepared from each colony. The DNA was cleaved with either PstI or XhoI, ligated, and then transformed into Escherichia coli. Three colonies from each of the electroporations were picked, and the plasmids recovered were cleaved with PstI or XhoI. One plasmid from each preparation was sequenced by using either primer 183-66.3 (GAAGGAGGCACCATGCACGG) or primer 183-66.4 (CTCACTGAGAGTGAAGCCGC).
PCR amplification of Mx9 attB.
Primers were designed to PCR amplify attB1 and attB2. Primers 183-99.4 (CGAGGTCCGGGACGCGCGCA) and 183-99.6 (TGCCAGGGCTTACGGCTTC) were used to amplify a 285-bp attB1 fragment, and primers 183-99.5 (TATCCCAGCAACCGCCGGAG) and 183-99.4 were used to amplify a 373-bp attB2 fragment. To amplify the native attB1 site, primers 183-99.6 and 249-179.7 (CAGCACGGGTGCAGCAAC) were used to amplify a 250-bp fragment. PCRs were performed by using chromosomal DNA from DZ1 and the FailSafe PCR system from Epicentre. The amplification conditions were 96°C for 2 min and then 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min.
Construction of a minimal integration plasmid.
The int gene was PCR amplified from pKOS35-117.9.7 by using primers 111-74.4 (CCCAATTGGCTCAGGGCAGCGGCTCATT) and 111-82.5 (CCCCATGGCGCTCAGGGGTGCGTCGGACGCC). The PCR amplification conditions were those described above. The amplified fragment was ligated into the EcoRV site of pLitmus 28 (New England Biolabs) to create pKOS249-12. The int gene was removed from this plasmid by cleavage with EcoRI, and the DNA ends were made blunt with the Klenow fragment of DNA polymerase, followed by cleavage with NcoI. The fragment was ligated with pUHE24-2B (3) that was cleaved with PstI, and the DNA ends were made blunt with the Klenow fragment of DNA polymerase I and cleaved with NcoI. The resulting plasmid, pKOS249-23, contained the int gene under control of the E. coli phage T7 A1 promoter that was engineered to contain two LacI binding sites to repress transcription. The bleomycin resistance gene was added to this plasmid by isolating the bleomycin resistance gene from pKOS183-112 as a BamHI-to-HindIII fragment, and the DNA ends were made blunt with the Klenow fragment of DNA polymerase I; then the fragment was ligated with pKOS249-23, which was cleaved with XhoI, and the DNA ends were made blunt with the Klenow fragment of DNA polymerase I. This plasmid was designated pKOS249-31.
β-Galactosidase assays.
Seed cultures of two isolates for each integration site were grown in 1% CTS (5 ml) to the mid-log to late log phase. To start an assay culture, 35 ml of 1% CTS was inoculated with 1 ml of a seed culture at an optical density at 600 nm (OD600) of 0.073. β-Galactosidase assays were performed by removing an aliquot of cells and adding them to Z buffer to obtain a combined volume of 1 ml. The cells were lysed by adding 1 drop of 0.1% sodium dodecyl sulfate and 2 drops of chloroform and vortexing the sample for 5 s. The assay was initiated by adding 0.1 ml of o-nitrophenyl β-d-galactopyranoside (8 mg/ml) and mixing. The reactions were stopped by adding 0.5 ml of 1 M Na2CO3. The OD600 of the cell culture and the OD420 of the enzyme reaction mixtures were determined with a SpetraMax 250 plate reader. Miller units were determined as previously described (10).
Nucleotide sequence accession numbers.
The Mx9 sequence has been deposited under GenBank accession number AY247757. The accession numbers for attB1 and attB2 are AY297770 and AY297771, respectively.
RESULTS
Identification of the Mx9 int gene and attachment site.
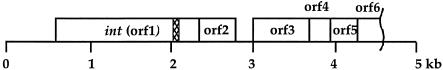
To identify the int gene and attachment site, a library of 5- to 8-kb fragments of Mx9 was made, and a clone that was able to integrate into the M. xanthus chromosome was identified. The insert in this plasmid, pKOS35-117.9.7, was sequenced. Five complete open reading frames (ORFs) and one partial ORF were identified in the 4.6-kb fragment (Fig. 1). ORF 1 was the only reading frame whose product exhibited amino acid similarity with the products of other known integrase genes and therefore was designated int. The other ORFs resembled ORFs from Mx8; ORF 2, ORF 3, ORF 4, ORF 5, and ORF 6 showed similarity to P15, P14, P16, P17, and P18, respectively, from Mx8. Based on the degrees of similarity of these ORFs in Mx8 and Mx9, it appears that Mx8 and Mx9 are very similar phages.
FIG. 1.
Physical map of the int region of Mx9. The boxes represent putative ORFs. The cross-hatched box in int indicates the position of attP.
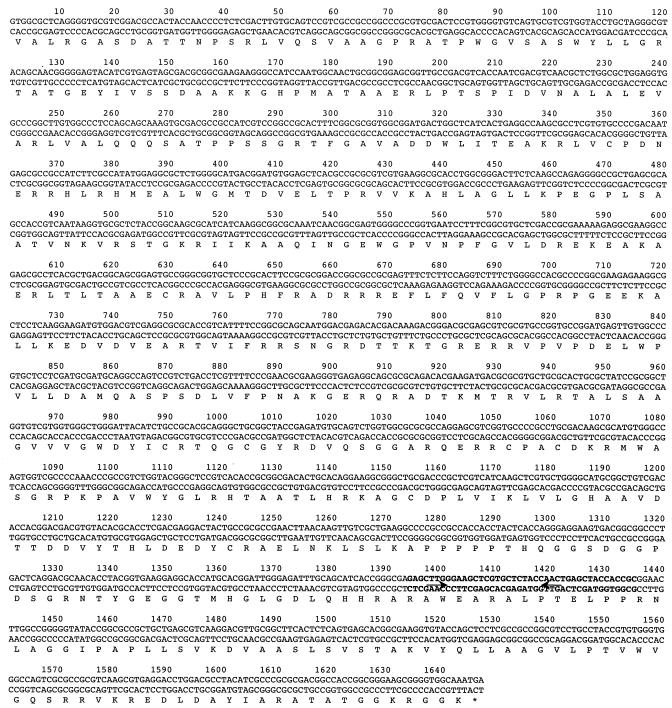
Because the Mx8 attachment site is located within the Mx8 int gene, the Mx9 int gene was examined for sequences which indicate that there is an attachment site. This analysis revealed a DNA segment within the int gene (nucleotides 1397 to 1428 [Fig. 2]) that exhibited sequence similarity to tRNAGly from various organisms. Since Mx8 integrates into the tRNAAsp gene of M. xanthus, the sequence that showed similarity to tRNAGly was predicted to serve as the site of integration for Mx9.
FIG. 2.
Nucleotide sequence of the Mx9 int gene and the deduced amino acid sequence. The amino acids are indicated by single letters under the DNA sequence. Stop codons are indicated by an asterisk. The sequence in boldface type is the Mx9 attP sequence. The arrows indicate inverted repeats.
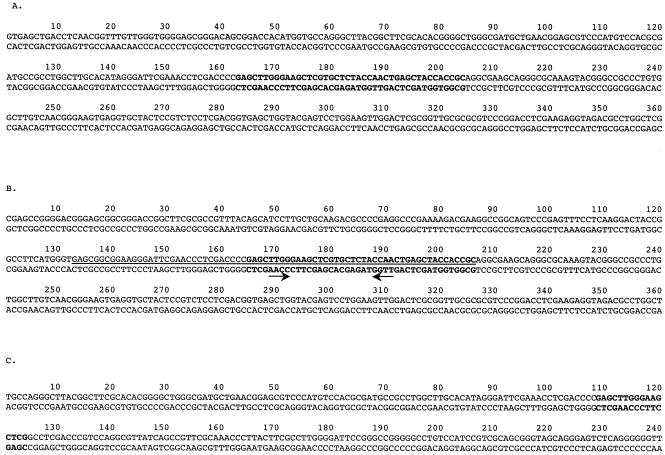
To test this prediction, chromosomal DNA from six integrants containing pKOS35-117.9.7 were cleaved with restriction enzymes, ligated, and transformed into E. coli to recover the plasmid along with flanking chromosomal DNA. Sequencing performed with primers adjacent to the proposed attachment site revealed that the point of recombination was indeed that of the putative tRNAGly. Furthermore, the sequence of flanking chromosomal DNA showed that there were two attB sites. It appeared from the number of integrants at each site (three for attB1 and three for attB2) that the two sites served equally well as the insertion site (Fig. 3).
FIG. 3.
(A) Nucleotide sequence of the reconstituted Mx9 attB1 site. (B) Nucleotide sequence of the Mx9 attB2 site. The arrows indicate an inverted repeat in attB2. (C) Nucleotide sequence of the native Mx9 attB1. Boldface type indicates the core att site. The underlined nucleotides encode tRNAGly.
Structure of the two attB sites.
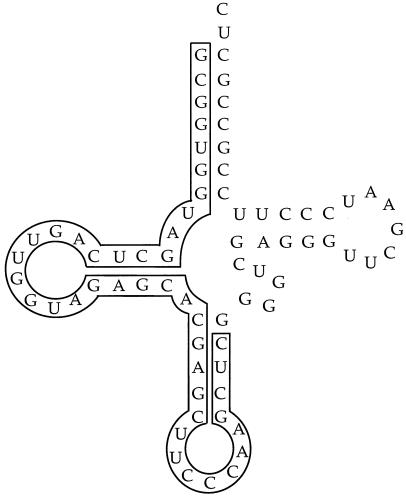
Figure 3 shows 360 bp from each of the attB sites. The two sites have a common 42-bp core sequence that is also found in the Mx9 int gene. In addition, there are 22 bp 5′ to both attB sites that are identical at 21 positions. There is a putative inverted repeat that may play a role in integrase protein binding at the attB and attP sites (Fig. 3B). The site of integration within attB2 lies in the 5′ end of tRNAGly gene, as shown in Fig. 3B. However, the sequence of attB1 does not contain a complete tRNAGly gene. Figure 4 shows the predicted folding of this segment of attB2 into a corresponding tRNA.
FIG. 4.
Predicted cloverleaf secondary structure for tRNAGly from M. xanthus. The bases that are within the core attB sequence are outlined.
Analysis of the attR and attL half-sequences for both attB sites revealed that the two attR sequences are identical, whereas the attL sequences differ. This is also the case with the two Mx8 attB sites (7). Plasmids containing the Mx8 int gene preferentially integrate at attB1, and this integration often is accompanied by a deletion between attB1 and attB2 (8).
To determine if the identical attR sites are due to the presence of two attB sites containing identical attR sites or due to deletion of the DNA between the two attB sites after integration into one of them, PCR analysis was performed either with primers 183-99.4 and 183-99.6 for attB1 or with primers 183-99.4 and 183-99.5 for attB2. A PCR fragment was detected by using the primers specific for attB2, but no fragment was detected by using the primers specific for attB1 (data not shown). This suggests that a deletion may occur upon integration of attB1, but to be certain that the lack of a PCR product was not due to a failure to PCR amplify the DNA fragment, further experiments were performed.
Next, the genomic sequence of M. xanthus strain DK1622, generated by Monsanto and available at The Institute for Genome Research web site, was examined for the two attB sites (www.TIGR.org). The attB2 sequence was almost identical to the sequence identified previously (Fig. 3B), but only the first 178 bp of the attB1 site shown in Fig. 3A was present before the sequence diverged. By using this sequence information for attB1, a primer was designed that was approximately 100 bp downstream from the point at which the sequence diverged (primer 249-179.7). By using this primer along with 183-99.6, the primer 5′ to the attB1 site, and DZ1 genomic DNA, a PCR product that was approximately 250 bp long was isolated and sequenced. This PCR product was identical to that obtained from the DK1622 genomic sequence (Fig. 3C). Analysis of the sequence revealed that only 16 bp of the 42-bp core att site was present in the native attB1 site.
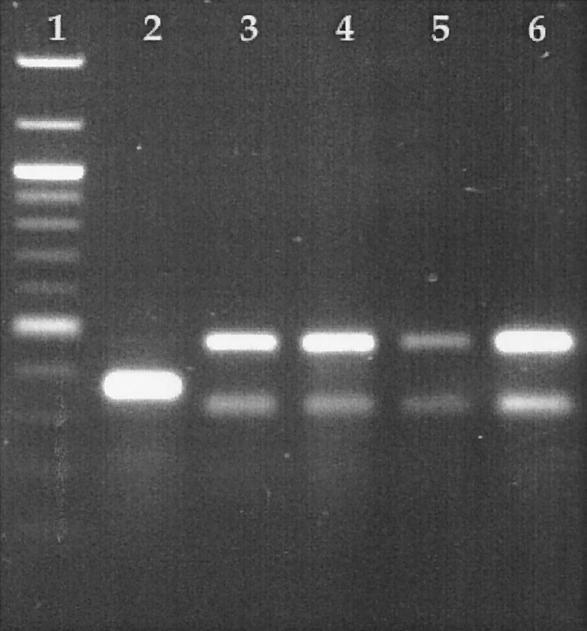
Final proof that a deletion does occur between attB1 and attB2 is shown in Fig. 5. By using primers 183-99.4 and 183-99.5, the primers that amplify the attB2 site, PCR amplification was performed with genomic DNA from the wild-type strain or strains harboring a plasmid integrated at either attB1 or attB2. By using chromosomal DNA from DZ1, a strain with no plasmid integrated at either attB site, a 372-bp PCR product containing the attB2 site was detected (Fig. 5, lane 2). Two strains that had insertions at attB2 (Fig. 5, lanes 5 and 6) did not produce the 372-bp band and should not have amplified attB2 due to the presence of a plasmid integrated at that site. If a deletion did occur between attB1 and attB2, there should have been no detectable amplification of attB2 when a plasmid integrated at attB1. The results showed that no attB2 PCR product was detected, indicating that there was a deletion of DNA between attB1 and attB2 when integration occurred at attB1 (Fig. 5, lanes 3 and 4).
FIG. 5.
Agarose gel containing PCR-amplified DNA fragments. Lane 1, 100-bp ladder from New England Biolabs; lane 2, PCR amplification for detection of attB2 in wild-type strain DZ1; lanes 3 and 4, PCR amplification for detection of attB2 in two independent isolates that contain a plasmid integrated at attB1; lanes 5 and 6, PCR amplification for detection of attB2 in two independent isolates that contain a plasmid integrated at attB2.
Integration results in alteration of the carboxy terminus of the Mx9 Int protein.
Because attP lies within the int gene, integration into the chromosome alters the 3′ end of the int gene. In the 1,160 bp of attR that has been sequenced, no stop codon has been identified (data not shown). Thus, 70 amino acids should be removed from Int, and more than 389 amino acids should be added to the Int protein that is synthesized after integration into the chromosome. These additional amino acids presumably reduce the enzymatic activity of Int because the IntX protein of Mx8 has lost 112 residues and contains 13 added amino acids and is less active in site-specific recombination (8).
Mx9 Int is the only phage protein required for integration.
To determine whether int is necessary and sufficient for integration, the int gene was PCR amplified and ligated into an E. coli expression vector that uses an engineered phage T7 A1 promoter. When plasmid pKOS249-31 was electroporated into DZ1, it integrated efficiently into the chromosome; approximately 1 × 104 colonies per μg of DNA were obtained. Thus, the Mx9 int gene is the only phage-encoded protein required for integrative recombination into the bacterial chromosome.
Transcription from the pilA and the mgl promoters integrated at the two Mx9 attB sites.
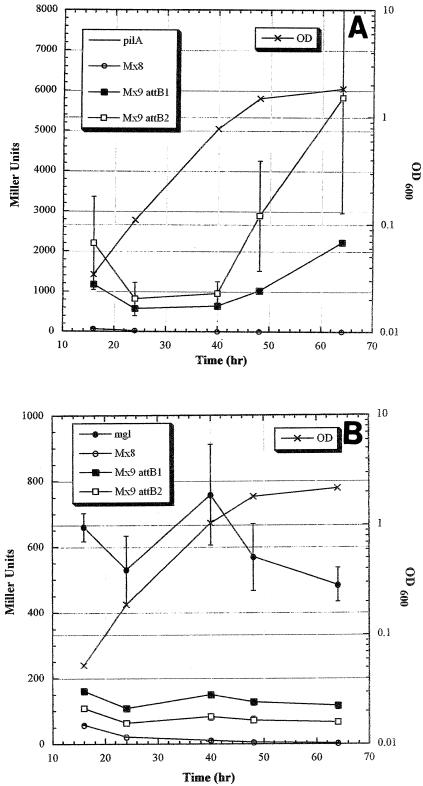
Because the interest in Mx9 integration was to find a phage attachment site on the M. xanthus chromosome that supports efficient expression of genes from a variety of promoters, fusions of lacZ to the mgl or pilA promoters were constructed, and transcription from these promoters at the two Mx9 attB locations, the Mx8 attB location, and the native chromosomal location was analyzed. Figure 6A shows the levels of expression of the pilA promoter (PpilA) at the four different locations. Surprisingly, there was little transcription when the PpilA plasmid was integrated by homologous recombination at the pilA location (pKOS178-86). This suggests that there may be a deletion in the pilA promoter region that eliminates activation of the pilA promoter in DZ1 since there was no expression in several isolates that were examined. As observed previously, little transcription from PpilA is seen when it is integrated at the Mx8 attB site (pKOS178-86 plus pKOS139-29). However, the Mx9 sites showed high levels of transcription from PpilA (pKOS178-177), and the levels were fairly similar at the two sites, although attB2 had high variability of expression in the two isolates examined. In addition, the regulation at the two sites was similar; transcription from PpilA increased during the late log and stationary phases.
FIG. 6.
(A) lacZ gene transcribed from the pilA promoter integrated at either the pilA chromosomal location, Mx9 attB1 or attB2, or the Mx8 attB sites. (B) lacZ gene transcribed from the mgl promoter integrated at either the mgl chromosomal location, Mx9 attB1 or attB2, or the Mx8 attB sites.
The results of transcription from the mgl promoter (Pmgl) are shown in Fig. 6B. Transcription from Pmgl at the two Mx9 attB (pKOS178-188) sites was better than transcription at the Mx8 site (pKOS139-47 plus pKOS139-29), but it was not as good when the promoter was integrated by homologous recombination at the chromosomal mgl location (pKOS139-47). However, the lower level of expression at the two Mx9 sites may have been vector dependent. When a plasmid that contained only the attP site was used and integrated by supplying the int gene in trans, Pmgl functioned just as well at both Mx9 sites as it did at the chromosomal mgl location (data not shown). The data from these studies indicate that the Mx9 attB sites are good sites for expression of foreign or native genes.
DISCUSSION
The Mx9 int gene and attachment site were identified, along with the site of integration into the M. xanthus chromosome. The analysis revealed remarkable similarity to the int gene and attachment site in the myxophage Mx8 (7, 8, 11). Both phages contain attP within the int gene and integrate within a tRNA gene. They both have two attB sites, and it appears that adjacent chromosomal DNA is deleted when integration occurs at one of the sites. For both, Int is the only phage-encoded protein needed for integration.
One difference between the Mx8 and Mx9 phage integration systems is the length of the core sequences. The core sequence for Mx8 integration is smaller, composed of 29 bp. The attB2 site has two nucleotides that differ at one end, which may account for the preference of Mx8 for inserting at attB1. The att core region of Mx9 is 42 bp long, but only one of the two integration sites, attB2, contains all 42 bases. The attB1 site contains only 16 bases of the core sequence. The lack of a complete core sequence in attB1 may explain why there is always a deletion between attB1 and attB2 when integration occurs at attB1. The Int protein may bind to the inverted repeat within the 42-bp core. Binding of the λ Int protein to its att sites has been demonstrated (5). Since attB1 contains one-half of the inverted repeat, only one-half of the necessary protein complex can form; however, once it has assembled, it may interact with the complementary half of proteins formed from attB2 to allow integration. This should result in a looping out of the DNA between attB1 and attB2 and its subsequent loss upon integration of DNA.
In PCRs to detect attB1 with primers 183-99.4 and 183-99.6, the conditions were such that if the distance between attB1 and attB2 was less than 2 kb, then a PCR product should have been detected. Since no product was observed, the results suggest that the distance between the two sites is greater than 2 kb. Analysis of the DK1622 sequence showed that the two attB sites are 6.7 kb apart. Analysis of this sequence revealed two ORFs that have sequence similarity to transposase genes, suggesting the presence of a transposon. The product of another ORF that was identified exhibited high levels of sequence similarity to proteins whose functions are unknown. Clearly, the ORFs between the two attB sites are not critical for growth under laboratory conditions since strains with integrations at attB1 have no visible growth defects.
Acknowledgments
I thank Monsanto for use of the M. xanthus sequence.
REFERENCES
- 1.Campos, J. M., and D. R. Zusman. 1975. Regulation of development in Myxococcus xanthus: effect of 3′:5′-cyclic AMP, ADP, and nutrition. Proc. Natl. Acad. Sci. USA 72:518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisseha, M., M. Gloudemans, R. E. Gill, and L. Kroos. 1996. Characterization of the regulatory region of a cell interaction-dependent gene in Myxococcus xanthus. J. Bacteriol. 178:2539-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Julien, B., and R. Calendar. 1995. Purification and characterization of the bacteriophage P4 delta protein. J. Bacteriol. 177:3743-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julien, B., and S. Shah. 2002. Heterologous expression of the epothilone biosynthetic genes in Myxococcus xanthus. Antimicrob. Agents Chemother. 46:2772-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landy, A. 1989. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu. Rev. Biochem. 58:913-949. [DOI] [PubMed] [Google Scholar]
- 6.Li, S. F., and L. J. Shimkets. 1988. Site-specific integration and expression of a developmental promoter in Myxococcus xanthus. J. Bacteriol. 170:5552-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magrini, V., C. Creighton, and P. Youderian. 1999. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: genetic elements required for integration. J. Bacteriol. 181:4050-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magrini, V., M. L. Storms, and P. Youderian. 1999. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: regulation of integrase activity by reversible, covalent modification. J. Bacteriol. 181:4062-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, S., E. Sodergren, T. Masuda, and D. Kaiser. 1978. Systematic isolation of transducing phages for Myxococcus xanthus. Virology 88:44-53. [DOI] [PubMed] [Google Scholar]
- 10.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Tojo, N., K. Sanmiya, H. Sugawara, S. Inouye, and T. Komano. 1996. Integration of bacteriophage Mx8 into the Myxococcus xanthus chromosome causes a structural alteration at the C-terminal region of the IntP protein. J. Bacteriol. 178:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zusman, D. R., D. M. Krotoski, and M. Cumsky. 1978. Chromosome replication in Myxococcus xanthus. J. Bacteriol. 133:122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]