20 yr have elapsed since the discovery of the association of the then novel bacterium Helicobacter pylori with different gastroduodenal diseases, including severe active chronic gastritis, gastroduodenal ulcers, adenocarcinoma, and lymphoma (1). The amount of research performed from the clinical to the molecular level is impressive (2). It is presently well established that H. pylori infects the large majority of the human population with a very high prevalence in countries with poor hygienic conditions. H. pylori is human specific and resides in a defined ecological niche comprising the stomach mucus layer and the stomach epithelial lining. The infection is chronic and life lasting if not treated with antibiotics. Only a minority of the infected persons develop gastroduodenal diseases, suggesting that an adverse outcome of the infection depends strongly on the response of the host and/or on the interplay with the genetic background and other factors. As a result, our present knowledge of the molecular and cellular steps of the pathogenesis of the H. pylori–associated diseases is very limited. An additional difficulty is that in vitro experiments must be performed within short time windows of hours to weeks, whereas in vivo the disease develops over years to several decades. Moreover, the pathogenesis in animal models of infection differs substantially with the human disease.
The VacA Toxin.
It is conceivable that a bacterium causing a life-lasting infection has evolved to produce many factors that permit colonization and prolonged persistence in the unique environment of the stomach. Several virulence factors of H. pylori have been identified, and their mechanisms of action at the cellular and tissue level are being actively investigated. One factor that has attracted major attention is the vacuolating cytotoxin (abbreviated VacA), which was first identified as a protein present in H. pylori supernatants that was capable of inducing the formation of membrane-bound vacuoles in cells in culture (3, 4). This toxin is made in the bacterial cytosol as a four-part protein consisting of (a) an inner membrane secretion signal sequence of variable nature, which is responsible for the fact that different H. pylori strains secrete different amounts of VacA; (b) a 37-kD domain (p37) essential for the vacuolating activity; (c) a 58-kD domain (p58) essential for receptor binding; and (c) an autotransporter domain, which drives VacA (p37-p58) across the bacterial outer membrane and is then removed by specific proteolytic cleavage. VacA may remain associated with the bacterial surface or be released in the medium. In water, monomeric VacA has a strong tendency to form flower-shaped oligomers (5) of low vacuolating activity. Oligomers are dissociated by acids or bases leading to a strong increase in activity (6–8).
VacA Functions as an Anion Channel.
VacA binds to a variety of cells in culture with a composite binding curve, suggesting the presence of a limited number of high affinity binding sites and a large number of low affinity binding sites. The high affinity sites may be tentatively attributed to the several cell surface molecules capable of VacA binding described so far—protein tyrosine phosphatases β (8) and α (9), a GPI-anchored protein associated to rafts (10), and the EGF receptor (11)—whereas the nonsaturable low affinity sites are likely to be lipids, since VacA binds to liposomes and to planar lipid bilayers increasing their ionic permeability (12–16). Monomeric VacA has a tendency to insert into membranes where it forms hexameric anion-specific channels (7, 13, 17). Such channels assemble also in the plasma membrane of cells increasing permeability to small anions, including chloride, bicarbonate, and piruvate with consequent membrane depolarization (18). VacA also forms anion-specific channels in the apical membrane of the epithelial cells of the stomach mucosa (19) leading to the suggestion that such VacA activity may benefit the apically residing H. pylori by allowing it to gather anions such as bicarbonate and piruvate, which are both nutrients and pH buffers (20). VacA also appears to act at the apical membrane to increase the paracellular route of transepithelial permeability to ions essential for H. pylori, such as iron and nickel, and to small sugars (21). Though the molecular mechanism of such an activity has not yet been worked out, its significance in terms of promotion of bacterial growth is apparent, particularly if one considers that the mucus layer which covers and protects the stomach epithelial lining is not permeable in the lumen-mucosa direction to protons and many small molecules (20). Therefore, VacA may allow H. pylori to reside in a nutrient-deficient and rather neutral pH environment.
Immunosuppressive Functions of VacA.
After membrane binding and pore formation, VacA is slowly endocytosed and it reaches the limiting membrane of late endosomal compartments (22, 23). Vacuoles originate from late endosomes and lysosomes and require the proton-pumping activity of the vacuolar-type ATPase pump (v-ATPase) (24, 25). During its journey inside the cell along the endocytic route, VacA is believed to preserve its anion channel activity, since this is not affected by the acidic pH of the lumen of endosomes (14, 15). All the available evidence indicate that the combined actions of the v-ATPase and of the VacA anion channel cause an increase of the osmotic pressure inside late endosomal compartments, which swell to form vacuoles (20). In this way, VacA causes the release of lysosomal hydrolases into the extracellular medium and inhibits the hydrolysis of all molecules which are normally degraded within late endosomes and lysosomes, such as ligands, receptors, and antigens (26).
The antigen-processing compartment of antigen-presenting cells is homologous to late endosomes (27), and VacA was found to profoundly affect the processing of the model antigen tetanus toxin by human antigen-presenting cells in such a way that the proliferation of tetanus toxin-specific T cells was strongly reduced (28). This led to the proposal that VacA could exert a local immunosuppressive activity by inhibiting antigen presentation within the stomach mucosa (28). Given that VacA vacuolates almost any cell type, such inhibition should not be antigen specific. The VacA immunosuppressive activity has not been tested in vivo, but it is in line with the finding that H. pylori infection of mice infected with vaccinia virus reduces the vaccinia-specific cytotoxic T cell response and prolongs the viral infection (29).
Two papers (30, 31) now strongly support the possibility that VacA is immunosuppressive, but the mechanism they describe involves a direct action on T cells rather than antigen-presenting cells. They show that the toxin inhibits the proliferation of T cells induced by polyclonal activators. Purified VacA or different strains of VacA-producing H. pylori, but not isogenic VacA mutants, when added to Jurkat cells or to peripheral blood lymphocytes inhibited their proliferation induced by phytohemagglutinin plus phorbol myristate acetate, or the induction of CD69 expression by CD3 cross-linking. VacA did not distinguish between CD4+ and CD8+ T cells since both were inhibited in the same way (31). T lymphocyte activation and proliferation is strongly promoted secretion of IL-2 in parallel with expression on the cell surface of the high affinity IL-2 receptor. VacA was found to inhibit this autocrine loop as both IL-2 and IL-2 receptor production were reduced. Notably, a single bacterium per Jurkat cell was sufficient to cause a significant reduction of IL-2 secretion (30), implying that even minute amounts of VacA, similar to those that may be present in vivo, are sufficient to cause the effect. This implies that high affinity VacA receptors are present in human lymphocytes, and indeed this was found to be the case (31). Boncristiano et al. (31) noticed that the 58-kD COOH-terminal domain of VacA (p58), which lacks the NH2-terminal p37 domain required for channel formation, was also inhibitory. This questions the involvement of the anion channel activity of VacA in T cell inhibition. In fact, p58 is also devoid of cell vacuolating activity (32, 33).
Two Pathways of T Cell Inhibition.
T cell activation and production of cytokines, including IL-2, is mediated by an increase in cytosolic calcium concentration, and it is under the control of different transcription factors among which NFAT plays a major role (34). In the resting lymphocyte, NFAT is cytosolic, and its translocation into the nucleus follows the dephosphorylation of its nuclear localization signal by calcineurin, a phosphatase activated by calcium and inhibited by the immunosuppressive drugs cyclosporine A and FK506 (35). Boncristiano et al. (31) and Gebert et al. (30) found that VacA inhibits the activation of NFAT, leaving the other nuclear factors unaffected, and prevented its migration into the nucleus. This is caused by the VacA anion channel activity because its effect was blocked by NPPB (18), a potent inhibitor of the VacA channel (31). Moreover, when the calcium ionophore A23187 was used as cell activator the toxin was found to inhibit the rise of cytosolic calcium. This effect can be explained, considering that the VacA depolarizes the plasma membrane (18) and that most of the cytosolic calcium rise triggered by A23187 follows the opening of a plasma membrane located calcium channel, known as CRAC, which is closed upon membrane depolarization.
But the finding that p58, which does not form anion channels and does not affect cytosolic calcium, also inhibits T cell activation indicates that this is only part of the picture (31). This is not surprising in the light of the already mentioned complexity of the cell binding of VacA, which is thought to involve lipid rafts and cell surface proteins known to activate tyrosine protein phosphorylation. Indeed VacA and p58 were found by Boncristiano et al. (31) to induce phosphorylation of a subset of protein substrates with a consequent cascade of phosphorylations/activation events leading to Rac activation and to a reorganization of the actin cytoskeleton (31), which could inhibit T cell activation and division. Furthermore, it was shown that Rac activation, which is known to be coupled to stress kinases (36–38), correlates with the selective activation of the stress-activated kinase p38 but not of the related mitogen-activated kinase Erk (31). The finding that antagonist peptides, which inhibit T cell activation, stimulate the Vav/Rac/stress kinase pathway, while failing to stimulate other TCR-mediated signaling pathways, including the Ras/Erk pathway (39, 40), provides a possible explanation for the anion channel–independent immunosuppressive activity of VacA and p58.
New Proinflammatory Activities of VacA.
Boncristiano et al. (31) went further by showing that VacA induces phosphorylation of p38 in neutrophils and macrophages also. This nuclear transcription-activating factor controls, among others, the gene encoding for the proinflammatory enzyme cyclooxygenase (COX)-2, whose expression was found to be increased upon exposure to VacA. RPTP-β is a VacA receptor (8), and it is perhaps relevant in this context that intragastric administration of VacA to RPTP-β knockout mice caused vacuolization with no inflammation, implying that VacA binding to this protein receptor is necessary for its proinflammatory activities but not for vacuolization (41). This is in agreement with the model for VacA-induced vacuolization, described above, which only requires a VacA anion channel activity localized on late endosomal compartments (20).
Toward a Model of VacA Function.
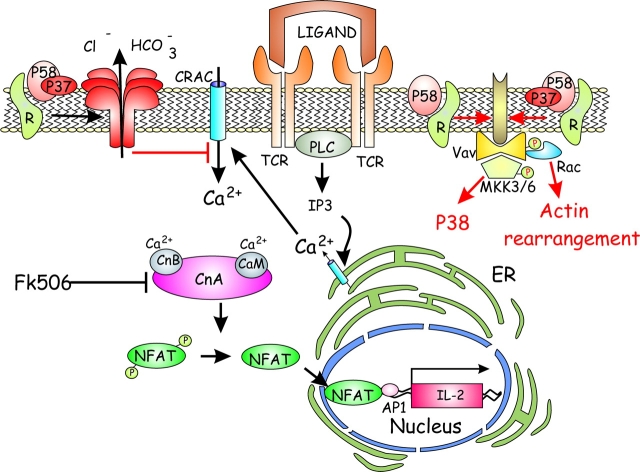
Though some aspects of VacA function require further evidence and experimentation, the ensemble of the current data can be summarized as in Fig. 1 . VacA binds to different protein receptors depending on its concentration and on the cell type. At higher concentrations, it binds to lipids directly. In addition, by local detachment from the protein receptor, VacA may insert into the lipid bilayer, a step expected to be irreversible owing to its tendency to form hexameric anion-selective channels. The different modes of binding are likely to trigger different cellular responses. The toxin channel activity causes membrane depolarization, which interferes with calcium signaling events within the cell. Thus, the rise of cytosolic calcium mediated by the plasma membrane channel, which is operated by calcium released from intracellular calcium stores, is strongly inhibited. This prevents the activation of calcineurin and the consequent dephosphorylation of NFAT, which plays a major role in the activation of the expression of high affinity IL-2 receptor and cytokines, including IL-2. This causes a pronounced inhibition of the activation and proliferation of T cells. In addition, via protein receptors which bind VacA and its COOH-terminal domain p58 with high affinity, protein tyrosine phosphorylation is somehow promoted, leading to a Vav-mediated activation of Rac which induces a cytoskeletal reorganization that interferes with T cell proliferation. At the same time, stress kinases are activated with phosphorylation of p38, in the absence of Erk activation, which has an anergic effect on T cells. This second pathway of T cell inhibition occurs at lower toxin concentration and may, therefore, be of great relevance in vivo (31). A further analysis will provide a more solid ground to this mode of T cell inhibition by VacA.
Figure 1.
VacA inhibits T cell activation and proliferation via two mechanisms. The first is linked to the anion-selective channel activity of this toxin which depolarizes the plasma membrane and prevents the opening of the CRAC calcium channel, which is operated by the calcium released from intracellular stores. At low cytosolic calcium, the phosphatase calcineurin does not dephosphorylate NFAT, thus preventing its translocation into the nucleus and the activation of the expression of IL-2 and IL-2 receptor necessary for proliferation. In addition, low doses of VacA or its COOH-terminal domain p58 (top right side) inhibit T cell activation by inducing a cascade of phosphorylation events involving a still unidentified protein (brown), Vav, and MKK3/6, resulting in an increase of the active form of p38, but not of Erk, which may induce anergy. In addition Vav induces actin rearrangement through the small GTPase Rac, which leads to inhibition of T cell proliferation.
What is already clear from the present work is that VacA promotes the expression of the proinflammatory enzyme COX-2, not only in T cells but also in neutrophils and macrophages. That VacA may have proinflammatory activity is also suggested by the finding that it activates mast cells to produce proinflammatory cytokines such as TNF and IL-6 (42). Proinflammatory activities and immunosuppressive effects are only apparently contradictory. In fact, they can be rationalized considering that H. pylori has evolved to reside in a nutrient poor ecological niche and to give a chronic infection. The localized and moderate inflammation of the stomach mucosa caused by the presence of H. pylori promotes the release from the tissue of nutrients needed for its growth and this necessity, apparently, cannot be dealt with in other ways, given the very limited permeability of the stomach mucus layer and of the epithelial lining. On the other hand, a persistent mucosal infection may benefit from an inhibition of the local immune response. Although different H. pylori molecules have proinflammatory activities, the same virulence factor, VacA, appears to be both proinflammatory and immunosuppressive depending on the type of cells it binds to.
In conclusion, all the findings and considerations discussed in this commentary make sense in terms of cell and tissue biology, but it remains to be established how and to what an extent they apply to the in vivo situation. We may have reached the point where we need to investigate in detail and in quantitative terms the tissue localization of VacA and the relative amounts found above, inside and below epithelial cells. We may also have to develop methods to deliver VacA, or any other H. pylori virulence factor, at precise cellular sites within the stomach mucosa and to determine its effects.
References
- 1.Warren, J.R., and B.J. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1:1273–1275. [PubMed] [Google Scholar]
- 2.Suerbaum, S., and M. Achtman. 2001. Helicobacter pylori: Molecular and Cellular Biology. Horizon Scientific Press, Norfolk, UK. 330 pp.
- 3.Leunk, R.D., P.T. Johnson, B.C. David, W.G. Kraft, and D.R. Morgan. 1988. Cytotoxin activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93–99. [DOI] [PubMed] [Google Scholar]
- 4.Cover, T.L., and M.J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570–10575. [PubMed] [Google Scholar]
- 5.Reyrat, J.M., R. Rappuoli, and J.L. Telford. 2000. A structural overview pf the Helicobacter cytotoxin. Int. J. Med. Microbiol. 290:375–379. [DOI] [PubMed] [Google Scholar]
- 6.de Bernard, M., E. Papini, V. de Filippis, E. Gottardi, J. Telford, R. Manetti, A. Fontana, R. Rappuoli, and C. Montecucco. 1995. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J. Biol. Chem. 270:23937–23940. [DOI] [PubMed] [Google Scholar]
- 7.Cover, T.L., P.I. Hanson, and J.E. Heuser. 1997. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating toxin, reveals its pattern of assembly. J. Cell Biol. 138:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yahiro, K., T. Niidome, M. Kimura, T. Hatakeyama, H. Aoyagi, H. Kurazono, K. Imagawa, A. Wada, J. Moss, and T. Hirayama. 1999. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 274:36693–36699. [DOI] [PubMed] [Google Scholar]
- 9.Yahiro, K., A. Wada, M. Nakayama, T. Kimura, K. Ogushi, T. Niidome, H. Aoyagi, K. Yoshino, K. Yonezawa, J. Moss, and T. Hirayama. 2003. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J. Biol. Chem. 278:19183–19189. [DOI] [PubMed] [Google Scholar]
- 10.Ricci, V., A. Galmiche, A. Doye, V. Necchi, E. Solcia, and P. Boquet. 2000. High sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell. 11:3897–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seto, K., Y. Hayashi-Kuwabara, T. Moneta, H. Suda, and H. Tamaki. 1998. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 431:347–350. [DOI] [PubMed] [Google Scholar]
- 12.Moll, G., E. Papini, R. Colonna, D. Burroni, J.L. Telford, R. Rappuoli, and C. Montecucco. 1995. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur. J. Biochem. 234:947–952. [DOI] [PubMed] [Google Scholar]
- 13.Molinari, M., C. Galli, M. de Bernard, N. Norais, J.M. Ruysschaert, R. Rappuoli, and C. Montecucco. 1998. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem. Biophys. Res. Commun. 248:334–340. [DOI] [PubMed] [Google Scholar]
- 14.Tombola, F., C. Carlesso, I. Szabò, M. de Bernard, J.M. Reyrat, J.L. Telford, R. Rappuoli, C. Montecucco, E. Papini, and M. Zoratti. 1999. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: possible implications for the mechanism of cellular vacuolation. Biophys. J. 76:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamoto, H., D.M. Czajkowsky, T.L. Cover, G. Szabo, and Z. Shao. 1999. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 450:101–104. [DOI] [PubMed] [Google Scholar]
- 16.Pagliaccia, C., X.M. Wang, F. Tardy, J.L. Telford, J.M. Ruysschaert, and V. Cabiaux. 2000. Structure and interaction of Vac of Helicobacter pylori with a lipid membrane. Eur. J. Biochem. 267:104–109. [DOI] [PubMed] [Google Scholar]
- 17.Czajkowsky, D.M., H. Iwamoto, T.L. Cover, and Z. Shao. 1999. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. USA. 96:2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabò, I., S. Brutsche, F. Tombola, M. Moschioni, B. Satin, J.L. Telford, R. Rappuoli, C. Montecucco, E. Papini, and M. Zoratti. 1999. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 18:5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debellis, L., E. Papini, C. Montecucco, and S. Curci. 2001. Helicobacter pylori cytotoxin VacA increases alkaline secretion in gastric epithelial cells. Am. J. Physiol. 281:G1440–G1448. [DOI] [PubMed] [Google Scholar]
- 20.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Cell Biol. 2:457–466. [DOI] [PubMed] [Google Scholar]
- 21.Papini, E., B. Satin, N. Norais, M. de Bernard, J.L. Telford, R. Rappuoli, and C. Montecucco. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Invest. 102:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLain, M.S., W. Schraw, V. Ricci, P. Boquet, and T.L. Cover. 2000. Acid activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol. Microbiol. 37:433–442. [DOI] [PubMed] [Google Scholar]
- 23.Ricci, V., P. Sommi, R. Fiocca, M. Romano, E. Solcia, and U. Ventura. 1997. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J. Pathol. 183:453–459. [DOI] [PubMed] [Google Scholar]
- 24.Papini, E., B. Satin, C. Bucci, M. de Bernard, J.L. Telford, R. Manetti, R. Rappuoli, M. Zerial, and C. Montecucco. 1997. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 16:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papini, E., E. Gottardi, B. Satin, M. de Bernard, J. Telford, P. Massari, R. Rappuoli, S.B. Sato, and C. Montecucco. 1996. The vacuolar ATPase proton pump is present on intracellular vacuoles induced by Helicobacter pylori. J. Med. Microbiol. 44:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Satin, B., N. Norais, J.L. Telford, R. Rappuoli, M. Murgia, C. Montecucco, and E. Papini. 1997. Vacuolating toxin of Helicobacter pylori inhibits maturation of procathepsin D and degradation of epidermal growth factor in HeLa cells through a partial neutralization of acidic intracellular compartments. J. Biol. Chem. 272:25022–25028. [DOI] [PubMed] [Google Scholar]
- 27.Watts, C. 2000. Antigen processing in the endocytic compartment. Curr. Opin. Immunol. 13:26–31. [DOI] [PubMed] [Google Scholar]
- 28.Molinari, M., M. Salio, C. Galli, N. Norais, R. Rappuoli, A. Lanzavecchia, and C. Montecucco. 1998. Selective inhibition of Li-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirai, M., T. Arichi, T. Nakazawa, and J.A. Berzofsky. 1998. Persistent infection by Helicobacter pylori down-modulates virus-specific CD8+ cytotoxic T cell response and prolongs viral infection. J. Infect. Dis. 177:72–80. [DOI] [PubMed] [Google Scholar]
- 30.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T-lymphocyte activation. Science. 301:1099–1102. [DOI] [PubMed] [Google Scholar]
- 31.Boncristiano, M., S. Rossi Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M.M. D'Elios, J.L. Telford, and C.T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T-cell activation by two independent mechanisms. J. Exp. Med. 198:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bernard, M., D. Burroni, E. Papini, R. Rappuoli, J.L. Telford, and C. Montecucco. 1998. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect. Immun. 66:6014–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye, D., D.C. Willhite, and S.R. Blanke. 1999. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 274:9277–9282. [DOI] [PubMed] [Google Scholar]
- 34.Rao, A., C. Luo, and P.G. Logan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707–747. [DOI] [PubMed] [Google Scholar]
- 35.Hogan, P.G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205–2232. [DOI] [PubMed] [Google Scholar]
- 36.Coso, O.A., M. Chiariello, J.C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J.S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signalling pathway. Cell. 81:1137–1146. [DOI] [PubMed] [Google Scholar]
- 37.Bagrodia, S., B. Derijard, R.J. Davis, and R.A. Cerione. 1995. Cdc42 and PAK-mediated signalling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995–27998. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, S., J. Han, M.A. Sells, J. Chernoff, U.G. Knaus, R.J. Ulevitch, and G.M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934–23936. [DOI] [PubMed] [Google Scholar]
- 39.Huang, J., D. Tilly, A. Altman, K. Sugie, and H.M. Grey. 2000. T-cell receptor antagonists induce Vav phosphorylation by selective activation of Fyn kinase. Proc. Natl. Acad. Sci. USA. 97:10923–10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang, J., K. Sugie, D.M. La Face, A. Altman, and H.M. Grey. 2000. TCR antagonist peptides induce formation of APC-T cell comjugates and activate a Rac signalling pathway. Eur. J. Immunol. 30:50–58. [DOI] [PubMed] [Google Scholar]
- 41.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukada, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, et al. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33:375–381. [DOI] [PubMed] [Google Scholar]
- 42.Supajatura, V., H. Ushio, A. Wada, K. Yahiro, K. Okumura, H. Ogawa, T. Hirayama, and C. Ra. 2002. VacA, a vacuolating cytotoxin of Helicobacter pylori directly activates mast cells for migration and production of pro-inflammatory cytokines. J. Immunol. 168:2603–2607. [DOI] [PubMed] [Google Scholar]