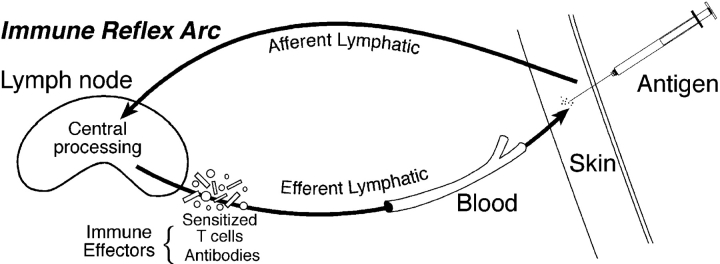
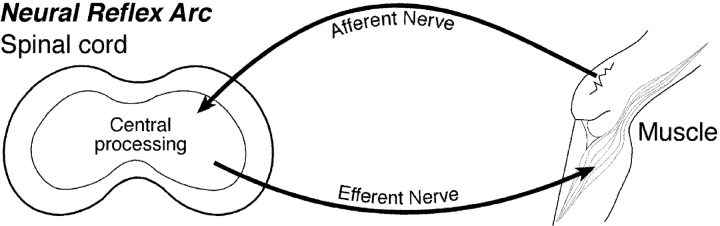
In the past, the neural reflex arc provided immunologists with a useful analogy for understanding the adaptive immune response (Fig. 1) . A revisit to the concept of the immune reflex arc, in which the immune response is divided into an afferent limb, central processing mechanism, and efferent limb, may help us understand where and how various innate immune cells function. Originally, the cells that were thought to be involved in the afferent limb were macrophages (Mϕ) and DCs that had taken up and perhaps processed antigen before transporting the antigen to the secondary lymphoid organ. The central processing mechanism involves the interaction of T and B cells and the antigen-presenting cells (DCs) to generate effector cells and molecules. The efferent limb of the immune reflex arc begins once the effector cells and molecules leave the lymphoid tissue and enter the efferent lymphatics and blood to find the tissue and the site of the antigen.
Figure 1.
(Top) The neural reflex arc. The cartoon depicts the commonly accepted idea of the path that a neurological signal takes from the origin, through the afferent limb, to the central processing mechanism, and its return to the original site of stimulation via the efferent limb. (Bottom) The immune reflex arc. Patterned after the neural reflex arc, the immune reflex arc is shown in cartoon. The antigen enters the body through the tissue, is picked up by antigen-presenting cells (not depicted), and carried through an afferent lymphatics (afferent limb) to the secondary lymphoid tissue where the central processing mechanisms takes place. The central processing mechanisms within the lymphoid tissue involves antigen presentation to lymphocytes that respond by proliferating and differentiating into effector cells that may also produce effector molecules. The efferent limb begins when the effector cells and molecules return to the tissue site of antigen through the efferent lymphatics or the blood and includes the effector arm of the immune response.
Innate cells are involved in all three limbs of the immune reflex arc. During the afferent limb NK cells establish the cytokine milieu that biases the adaptive response toward a T helper type 1 (Th1) response. Mφ and DCs transport the antigen to the lymphoid organ during the afferent limb. The ability of NK cells to lyse tumor cells and bacteria without a prior exposure contributes to the afferent limb by reducing the infectious antigen and allowing for a more effective outcome during the immune reflex arc. NK cells, Mφ, and DCs have a major influence on the central processing mechanism since they are providing the cytokine microenvironment during antigen presentation. Once the effector cells leave the lymphoid organ, the innate cells may again participate during the efferent limb. For example, Mφ and NK cells armed with antibody frequently mediate antibody-dependent cellular cytoxicity.
Invariant NKT Cells.
Although a minor population of T cells that expressed some NK cell markers was described in the late 1980s, the furor over the function of these cells didn't begin until the middle of the next decade (1, 2). It was shown that whereas the NKT cell exhibited some phenotypic heterogeneity, ∼85% of the mouse NKT cell expressed an invariant TCR (Vα14jα18) that was specific for the class I–like molecule, CD1d (referred to hereafter as iNKT cells). Early investigations suggested that the NKT cell might function early in immune responses to quickly produce the IL-4 needed for the development of Th2 responses (2). It was reasonable to conclude that this minor population of innate cells may act to regulate the pattern of priming of naive T cells. Thus, the NKT cell seemed to function during the afferent limb or during the central processing mechanism of the arc. However, the idea that NKT cells biased the direction of the T helper cell toward a Th2 response was dismantled, publication by publication, until it was conceded that NKT cells only helped to bias Th2 responses under special circumstances such as when anti-IgD induced IgE production (3). CD1d−/− mice that lack iNKT cells were perfectly able to produce normal amounts of IgE (4), and CD1d−/− mice developed airway eosinophilia, a Th2-dependent response, in addition to increased antigen-specific IgE in response to a mouse model of allergic asthma to ovalbumin (5).
iNKT Cells Participate in the Efferent Limb.
Earlier this year, it was shown again that Th2 responses occurred in NKT cell–deficient mice when antigen was given subcutaneously but, surprisingly, not when the antigen was delivered to the lungs of the NKT cell–deficient mice (6, 7). In the analyses of the model, Akbari and colleagues suggest a novel role for iNKT cells in licensing the Th2 effector cells to allow their entry into the lung (7). The exact mechanism by which iNKT cells allow the entry of Th2 cells into the lung remains to be determined. The authors, however, clearly showed that the iNKT cell production of both IL-4 and IL-13 is required for expression of airway hyper reactivity (AHR) in the ovalbumin-induced asthma mouse model, and therefore, the iNKT cell function in this model occurs during the efferent limb of the immune arc.
In this issue, Campos et al. show in another biological model—contact sensitivity (CS)—that iNKT cells can function to promote the effector arm of an immune response; however, in this case, it appears that iNKT cells function during both the afferent and the efferent limbs of the immune reflex arc (8). Previously, Dieli et al. reported that early IL-4 was necessary for the initiation of contact sensitization with the hapten trinitrochlorobenzene (9). They showed that at 1, but not 2 or 3 d post primary immunization, IL-4 was spontaneously released from the draining LNs. Moreover, the release of IL-4 was dependent on a population of double negative (DN, CD4−/CD8−) T lymphocytes that also expressed NK1.1 and the Vα14 Jα18 TCR. These results suggest a role for iNKT cells in the central processing mechanism. The later production of IL-4 in CS was shown to be antigen specific and dependent on a classical CD4+ T cell. Now, Campos and colleagues show that iNKT cell–derived IL-4 is also required for early initiation of the CS efferent response within hours postimmunization and antigen challenge (8).
iNKT Cell-dependent IgM Production.
B-1 cells are classically known as innate B cells that are rapid producers of natural IgM antibodies and are commonly thought to respond only to T-independent antigens and are unable to respond to other antigen immunization (10). However, there are reports that B-1 cells produce specific antibodies to DNP/TNP (11). Previously, Tsuji and colleagues showed that the innate B-1 cell population produce antigen-specific IgM antibodies that played a key role in initiating the efferent CS response (12). Here, Campos et al. suggest that the early release of IL-4 by iNKT cells is required for helping the B-1 cell population to produce the TNP-specific IgM (8). This observation places the iNKT cell in a critical position in the afferent portion of the immune arc during the induction of CS. It is not clear, however, whether this interaction takes place within a lymphoid organ or in tissues. The authors propose that during the efferent limb, the hapten-specific IgM made by the B1 cells enters the circulation and forms immune complexes when it meets specific antigen in the tissue. The immune complexes activate complement resulting in C5a peptides that participate in the activation of mast cells and platelets through their C5a receptors. The subsequent local expression of vasoamines and adhesion molecules supports the recruitment of Th1 effector cells to the challenge site at least 24 h later. The authors also propose a role for iNKT cell in helping the B-1 cell to secrete the antibodies during the efferent limb post antigen challenge. Future experiments will have to be designed to clarify the exact role of the iNKT cell in the immune reflex arc during CS induction and expression.
The model suggested by Campos et al. is very stimulating and raises many questions (8). Could iNKT cell help for B-1 cells also be required for NKT cell licensing of the expression of AHR? Interestingly, although both IL-4 and IL-13 are involved in iNKT cell function in the AHR model, Campos et al. found that only iNKT cell–derived IL-4 was needed for the initiation of CS. Another unique finding of the Campos paper is the role of liver iNKT cells in the initiation of CS. The authors propose that the hapten quickly activates the liver iNKT cells which in turn helps the B-1 cell (mostly found in the peritoneal cavity) to produce its antigen-specific IgM. It is not clear how cells from these respective sites get together. One possibility is that liver iNKT cells and B-1 cells might be recruited to the lymphoid tissue for central processing. Alternatively, concerning the B-1 cells, there may be small but sufficient numbers of B-1 cells existing throughout the body. A question is raised as to whether the liver might be a source of iNKT cells that participate in the “arc” after exposure to many antigens or only when the antigen is a hapten? All small reactive chemicals that induce CS express an adequate amount of toxicity, which might direct this response to the liver. In summary, this study clearly shows that innate cells are required during various limbs of the immune reflex arc and may be recruited from tissues like the liver and the peritoneal cavity in order to perform their role in the arc (Fig. 2) .
Figure 2.
iNKT cells and the immune reflex arc. A simplistic view of what we know about the iNKT cell and its influence on the immune reflex arc is depicted by a cartoon in an overlay of the immune reflex arc graphic (gray). The cartoon is based on the models discussed in the commentary. In the center of the drawing is the liver (red) where the iNKT cell (green cell with hands) initiates CS response by helping the B-1 (B1-B) cell to make antigen-specific IgM antibodies (not depicted) during the afferent limb of the arc. The specific IgM antibodies eventuate into immune complexes (yellow spiked circle) that help the effector cells (blue circles) and molecules (yellow rectangles) into the site of antigen in the tissue (shown here as skin). The iNKT cell facilitates the development of efferent T regulatory (Tr) cells (pink cell with STOP signs) during the central processing mechanism (shown in the lymphoid tissue) after antigen is inoculated into the eye (tissue not depicted). The possibility exists that the iNKT cells may promote the development of effector cells (small blue circles) and effector molecules (yellow rectangles) during the central processing mechanism and is shown as an iNKT cell with a GO sign. The iNKT cell sitting in the lung depicts the iNKT cell function of licensing or allowing effector cells into the organ for the development of AHR during the efferent limb of the immune reflex arc.
iNKT Cells Function during Afferent Limb.
In a methylcholanthrene (MCA)-induced 4T1 mammary carcinoma tumor model where there is an initial regression of the tumor followed by a progressive growth of the tumor, Terabe and colleagues showed that CD1d-restricted NKT cells were needed for the promotion of the growth of the 4T1 mammary carcinoma. The promotion of tumor growth suggests that the immune response may be suppressed by unknown mechanisms (13, 14). If the iNKT cell facilitates the development of T regulatory cells in the 4T1 model, iNKT cell would be functioning during the afferent limb of the arc. Another idea in relation to tumor immunology is promoted by Dunn and colleagues (15) who recently reported that T lymphocytes might contribute to the survival of MCA-induced sarcomas (like 4T1) by modulating their immunogenic surface molecules so that they are less antigenic. This process has recently been termed immunoediting (15). Whether a tumor has been immunoedited or not may be tested by assessing the immunogenicity of tumor cells (harvested from immunocompetent or immunoincompetent hosts) after transfer into naive mice. In contrast to growth pattern of tumors grown in immunocompetent animals, the majority of MCA-induced tumors from T lymphocyte–deficient Rag2−/− and iNKT cell–deficient Ja218−/− mice grew more slowly when transplanted in the WT recipients (15, 16). The experiments performed in the iNKT cell–deficient mice show that the T cells that play a role in the process may be iNKT cells since tumors harvested from iNKT cell–deficient mice grew more slowly. Thus, in this instance the iNKT cell may be suppressing the afferent limb of the arc by modulating potential antigens. The mechanisms involved in immunoediting are currently being studied but could involve epigenetic mutations or establishment of immune environments that promote tolerance to tumor antigens.
iNKT Cell Function during Central Processing Mechanism.
iNKT cells were first shown to have a role in the central processing mechanism during the induction of tolerance after inoculation of antigen into the eye (17). In this model of tolerance, which is known as anterior chamber immune deviation, or ACAID, the generation of CD8+ T regulatory (Tr) cells is dependent on the presence and interaction of iNKT cells, lymphocytes, and APC in the splenic marginal zone after antigen inoculation into the eye (18, 19). The development of CD8+ Tr cells in this model has now been clearly shown to depend on a distinct population of iNKT cells that make IL-10 (20), TGFβ (21), but not IL-4 or IL-13 (unpublished data). Although CD4+ molecules expressed by the iNKT cell are required for the generation of the Tr cells, neither conventional CD4+ T cells nor MHC class II molecules are required for the process (22). Interestingly, the tolerance generated in ACAID suppresses IL-13 and IgE production in a Th2 OVA mouse asthma model, which provides further evidence that tolerance is unlikely to be the promotion of Th2 responses by NKT cells (23). The numbers of iNKT cells that increase in the spleen during ACAID raises the possibility that they are either recruited from other tissues (like the thymus or the liver) to the spleen or proliferate in situ for their role the central processing mechanism. Although there is no evidence that proliferation takes place, blocking of MIP-2 or the absence of CxCr2 (chemokine receptor for MIP-2) prevents accumulation of iNKT cells in the spleen and thus supports a role for recruitment in the model (18).
Recently, Faunce et al. showed that CD1d-restricted NKT cells are required to produce the IL-4 at the onset of the generalized suppression that occurs subsequently to burn injury (24). The authors suggest that NKT cell–derived IL-4 establishes a microenvironment that suppresses subsequent immune inflammatory responses to experimental antigens administered. Other reports of thermal injury show the prominent presence of early IL-10 (and TGFβ) (25). The suppressive cytokine environment occurs early postthermal injury and regulates the type of immune response that develops during central processing.
Do iNKT Cell Subsets with Unique Properties Exist?
An issue not addressed by Campos and colleagues (8) or by Dieli and colleagues (9) is the nature of the subpopulation of iNKT cells that is needed for the initiation of the response or licensing of the effector cells, respectively. Dieli et al. showed that DN iNKT cells were required for the production of IL-4 in LNs by 1 d postimmunization for CS (9). In the human, it seems clear that CD4+, CD8+, and DN NKT subpopulations demonstrate distinct cytokine profiles after activation with the artificial CD1 ligand α galactosylceramide (αGalCer) (26). αGalCer is a glycolipid, isolated from a marine sponge, which has stimulatory properties for the iNKT cell in both mouse and humans. These studies and others (27, 28) suggest that the markers on iNKT cells denote distinct cell populations that are linked to discreet cytokine production. Alternatively, there is the possibility that the iNKT cell is a chameleon, able to change both its external phenotype and, in this instance, its internal synthetic process depending on the microenvironment in which it finds itself. If this is true, then the nature of the APC that expresses the CD1d molecule recognized by invariant TCR on the iNKT cell might influence the behavior of the iNKT cell.
iNKT Cells and the Immune Reflex Arc.
As we become aware of more biological models that require the participation of iNKT cells, we can make an attempt to determine which limbs of the immune reflex arc require iNKT cell participation. The iNKT cell does not require proliferation and differentiation and, therefore, this innate cell may influence cells in the periphery on their way to or through the immune loop. There are many disease states that have been reported to diminish or worsen when iNKT cells are absent (29–31). But many of these studies have not dealt with mechanisms. Importantly, the biological models that I chose to discuss in light of the findings reported by Campos et al. (8) were shown to be dependent on iNKT cells in the absence of exogenously added αGalCer. In each of the models discussed in which iNKT cells are required, we can define their contribution to the immune reflex arc. First, iNKT cells have been shown to be involved in the afferent limb in the suppression of a tumor regression model either by release of cytokines and or by yet to be defined immunoediting process. Second, the CD4+ iNKT cell is firmly ensconced during the central processing mechanism after antigens are inoculated into the eye, since the iNKT cell facilitates CD8+ T regulatory cell generation in the splenic marginal zone. Third, iNKT cells are part of the efferent limb of the reflex arc when they license classical Th2 cells to enter the lung and contribute to the increase in AHR. Finally, in this issue Campos et al. (9) nicely show that iNKT cells are needed to initiate the expression of CS early during the effector stage, placing them in the efferent limb. However, since the iNKT cell–dependent IgM response appears to be required within hours of the first immunization and subsequent challenge, the early iNKT cell–derived IL-4 may contribute to both the afferent and the efferent limbs of the response. Each of the models represents a novel way in which iNKT cells are essential and involved in the immune reflex arc of the adaptive immune response. As more mechanisms are revealed, we will be able to build a more complete scenario of where the iNKT cell assists the adaptive immune response and novel therapies may be developed to control this critical cell, which has been shown to enable both immune inflammation and its suppression.
References
- 1.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimoto, T., and W.E. Paul. 1994. CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W.E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 270:1845–1847. [DOI] [PubMed] [Google Scholar]
- 4.Smiley, S.T., M.H. Kaplan, and M.J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 275:977–979. [DOI] [PubMed] [Google Scholar]
- 5.Korsgren, M., C.G.A. Persson, F. Sundler, T. Bjerke, T. Hansson, B.J. Chambers, S. Hong, L. Van Kaer, H.G. Ljunggren, and O. Korsgren. 1999. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J. Exp. Med. 189:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisbonne, M., S. Diem, A. de Castro Keller, J. Lefort, L.M. Araujo, P. Hachem, J.-M. Fourneau, S. Sidobre, M. Kronenberg, M. Taniguchi, et al. 2003. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171:1637–1641. [DOI] [PubMed] [Google Scholar]
- 7.Akbari, O., P. Stock, E. Meyer, M. Kronenberg, S. Sidobre, T. Nakayama, M. Taniguchi, M.J. Grusby, R.H. DeKruyff, and D.T. Umetsu. 2003. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9:582–588. [DOI] [PubMed] [Google Scholar]
- 8.Campos, R.A., M. Szczepanik, A. Itakura, M. Akahira-Azuma, S. Sidobre, and M. Kronenberg. 2003. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198:1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieli, F., M. Taniguchi, G.L. Asherson, G. Sireci, N. Caccamo, E. Scire, C.T. Bonanno, and A. Salerno. 1998. Development of hapten-induced IL-4-producing CD4+ T lymphocytes requires early IL-4 production by αβ T lymphocytes carrying invariant Vα14 TCR α chains. Int. Immunol. 10:413–420. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa, K., R.R. Hardy, L.A. Herzenberg, A.D. Steinberg, and L.A. Herzenberg. 1983. Ly-1 B: a functionally distinct B-cell subpopulation. Progress in Immunology V, from Proceedings of Fifth International Congress of Immunology, Kyoto, Japan. Y. Yamamura and T. Tada, editors. Academic Press, Tokyo, Japan. 661–668.
- 11.Silverman, G.J., S.P. Cary, D.C. Dwyer, L. Luo, R. Wagenknecht, and V.E. Curtiss. 2000. A B cell superantigen-induced persistent “hole” in the B-1 repertoire. J. Exp. Med. 192:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji, R.F., M. Szczepanik, I. Kawikova, V. Paliwal, R.A. Campos, A. Itakura, M. Akahira-Azuma, N. Baumgarth, L.A. Herzenberg, and P.W. Askenase. 2002. B cell–dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J. Exp. Med 196:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. [DOI] [PubMed] [Google Scholar]
- 14.Crowe, N.Y., M.J. Smyth, and D.I. Godfrey. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 196:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn, G.P., A.T. Bruce, H. Ikeda, L.J. Old, and R.D. Schreiber. 2002. Cancer immunoediting: from immuno-surveillance to tumor escape. Nat. Immunol. 3:991–998. [DOI] [PubMed] [Google Scholar]
- 16.Smyth, M.J., K.Y. Thia, S.E. Street, E. Cretney, J.A. Trapani, M. Taniguchi, T. Kawano, S.B. Pelikan, N.Y. Crowe, and D.I. Godfrey. 2000. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonoda, K.-H., M. Exley, S. Snapper, S. Balk, and J. Stein-Streilein. 1999. CD1 reactive NKT cells are required for development of systemic tolerance through an immune privileged site. J. Exp. Med. 190:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faunce, D.E., K.-H. Sonoda, and J. Stein-Streilein. 2001. MIP-2 recruits NKT cells to the spleen during tolerance induction. J. Immunol. 166:313–321. [DOI] [PubMed] [Google Scholar]
- 19.Faunce, D.E., and J. Stein-Streilein. 2002. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J. Immunol. 169:31–38. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda, K.-H., D.E. Faunce, M. Taniguchi, M. Exley, S. Balk, and J. Stein-Streilein. 2001. NKT cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J. Immunol. 166:42–50. [DOI] [PubMed] [Google Scholar]
- 21.Stein-Streilein, J., and J.W. Streilein. 2002. Anterior chamber associated immune deviation (ACAID); regulation, biological relevance, and implications for therapy. Int. Rev. Immunol. 21:123–152. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, T., K.H. Sonoda, D.E. Faunce, J. Gumperz, T. Yamamura, S. Miyake, and J. Stein-Streilein. 2003. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an immune privileged site. J. Immunol. 171:1266–1271. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri, K., J. Zhang-Hoover, J.S. Mo, J. Stein-Streilein, and J.W. Streilein. 2002. Using tolerance induced via the anterior chamber of the eye to inhibit Th2-dependent pulmonary pathology. J. Immunol. 169:84–89. [DOI] [PubMed] [Google Scholar]
- 24.Faunce, D.E., R.L. Gamelli, M.A. Choudhry, and E.J. Kovacs. 2003. A role for CD1d-restricted NKT cells in injury-associated T cell suppression. J. Leukoc. Biol. 73:747–755. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, J.L., A. Lyons, C.C. Soberg, J.A. Mannick, and J.A. Lederer. 1997. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 122:146–152. [DOI] [PubMed] [Google Scholar]
- 26.Gumperz, J.E., S. Miyake, T. Yamamura, and M.B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond, K.J., S.B. Pelikan, N.Y. Crowe, E. Randle-Barrett, T. Nakayama, M. Taniguchi, M.J. Smyth, I.R. van Driel, R. Scollay, A.G. Baxter, and D.I. Godfrey. 1999. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29:3768–3781. [DOI] [PubMed] [Google Scholar]
- 28.Zeng, D., G. Gazit, S. Dejbakhsh-Jones, S.P. Balk, S. Snapper, M. Taniguchi, and S. Strober. 1999. Heterogenity of NK1.1+ T cells in the bone marrow: divergence from the thymus. J. Immunol. 163:5338–5345. [PubMed] [Google Scholar]
- 29.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]
- 30.Bendelac, A. 1995. Mouse NK1+ T cells. Curr. Opin. Immunol. 7:367–374. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald, H.R. 1995. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J. Exp. Med. 182:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]