Abstract
Interleukin (IL) 23 is a heterodimeric cytokine composed of a p19 subunit and the p40 subunit of IL-12. IL-23 affects memory T cell and inflammatory macrophage function through engagement of a novel receptor (IL-23R) on these cells. Recent analysis of the contribution of IL-12 and IL-23 to central nervous system autoimmune inflammation demonstrated that IL-23 rather than IL-12 was the essential cytokine. Using gene-targeted mice lacking only IL-12 (p35−/−) or IL-23 (p19−/−), we show that the specific absence of IL-23 is protective, whereas loss of IL-12 exacerbates collagen-induced arthritis. IL-23 gene-targeted mice did not develop clinical signs of disease and were completely resistant to the development of joint and bone pathology. Resistance correlated with an absence of IL-17–producing CD4+ T cells despite normal induction of collagen-specific, interferon-γ–producing T helper 1 cells. In contrast, IL-12–deficient p35−/− mice developed more IL-17–producing CD4+ T cells, as well as elevated mRNA expression of proinflammatory tumor necrosis factor, IL-1β, IL-6, and IL-17 in affected tissues of diseased mice. The data presented here indicate that IL-23 is an essential promoter of end-stage joint autoimmune inflammation, whereas IL-12 paradoxically mediates protection from autoimmune inflammation.
Keywords: rheumatoid arthritis, collagen-induced arthritis, IL-23 gene–deficient mice, IL-17, IFN-γ
Introduction
IL-12 and IL-23 share a common p40 subunit, yet they comprise unique p35 and p19 subunits, respectively (1). Studies have defined IL-12 as an important factor for the differentiation of naive T cells into IFN-γ–producing Th1 cells (2). Furthermore, gene-targeted mice, which specifically lack IL-12 signaling capabilities through IL-12R-β2 mutations (3) or deletion of the IL-12p35 gene (4), exhibit heightened susceptibility to bacterial infections. This is probably due to poor IFN-γ production in these mice, given the important role IFN-γ plays in antimicrobial immunity (5). Thus, an important role for IL-12 in the generation of Th1 cell–mediated immunity has been well established.
The role for IFN-γ during induction of organ-specific autoimmune diseases is less clear (6–9), and the absolute requirement for IL-12–mediated Th1 responsiveness in autoimmune inflammation has been questioned in several recent papers (10–12). Collagen-induced arthritis (CIA), a rodent model of the human disease rheumatoid arthritis, is induced by immunization with heterologous type II collagen (CII) emulsified in CFA (13). Development of CIA is dependent on both cell-mediated and humoral responses (14) and is characterized by cellular infiltration and synovitis of the joints, resulting in severe swelling of the paws and progressive destruction of bone and cartilage. Loss of IFN-γ signaling has been reported to increase severity of CIA (7, 8).
To determine the relative roles of IL-12 and IL-23 in the establishment and maintenance of autoimmune inflammation, we have analyzed the production of inflammatory cytokines and collagen-specific antibodies after the induction of CIA in p19−/− (IL-23–deficient) mice, p35−/− (IL-12–deficient) mice, and p40−/− (IL-12– and IL-23–deficient) mice. Consistent with the dominant role for IL-23 in central nervous system (CNS) autoimmune inflammation (12), IL-23–deficient mice were resistant to CIA. In contrast, IL-12–deficient mice showed enhanced joint swelling and joint pathology, and, within affected joint tissue, increased mRNA levels encoding for inflammatory mediators, such as IL-17 and TNF. Absence of IL-12, alone (p35−/−) or in combination with IL-23 (p40−/− mice), also resulted in increased anticollagen antibody production, but decreased mRNA encoding for IFN-γ in joints of CIA-affected IL-12–deficient mice relative to arthritic WT mice, an outcome consistent with a key role for IL-12 in IFN-γ induction. An increased number of IL-17–producing CD4+ T cells was evident in cultured draining LN (DLN) cells obtained from IL-12–deficient mice. Significantly, these potentially pathogenic T cells were absent in mice lacking IL-23. Thus, as in CNS inflammation (12), IL-23 is an essential mediator of joint inflammation, with at least one of its activities being to support production of IL-17–producing CD4+ T cells. In contrast, IL-12 is not critical for disease generation, but is the key factor in IFN-γ induction. IL-12 appears to regulate the inflammatory process, possibly through the induction of IFN-γ.
Materials and Methods
Mice.
IL-12p35−/−, IL-12p40−/−, and WT control mice on the C57BL/6 background were obtained from the Jackson Laboratory. IL-23p19−/− mice and WT B6 × 129 F2 controls have been described previously (12). p19−/− mice on the C57BL/6 background were used in intracellular cytokine detection experiments.
CIA.
CIA was induced in male and female C57BL/6 and B6 × 129 F2 mice aged 8–16 wk and clinically assessed as described previously (15). Mice were killed at day 42 after primary immunization; the rear paws were removed, fixed, decalcified in Cal-EX II (Fisher Scientific), and processed for paraffin embedding; and the sections were cut and stained with hematoxylin and eosin.
T Cell Proliferation and Cytokine Production.
For T cell proliferation assay, cells were stimulated with 0–200 μg/ml denatured chicken CII (boiled for 10 min) and cultured for 72 h with [3H]thymidine addition during the final 18 h. For cytokine measurement, cells were stimulated with 100 μg/ml denatured CII for 72 h and supernatants were assayed for IFN-γ and IL-4 by ELISA (R&D Systems).
ELISA Detection of CII-specific Antibodies.
Mice were bled and serum was prepared for determination of serum CII-specific Ab levels by ELISA. Samples were added to CII-coated 96-well plates (Chondrex Inc.) and incubated overnight. Horseradish peroxidase–conjugated goat anti–mouse Ig (H + L; total Ig), IgG1, or IgG2a were applied to determine the isotype of CII-specific antibodies. The quantity of CII-specific Ab detected is expressed as OD490 readings minus the relevant blank.
Gene Expression Analysis.
RNA was extracted from hind paws of mice, cDNA was prepared, and mRNA levels were quantitated as described previously (16).
DLN Cultures for Intracellular IL-17 and IFN-γ Detection.
DLNs were harvested from WT, p19−/−, p35−/−, and p40−/− mice (all on C57BL/6 background). For intracellular staining, DLN cells were initially stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of GolgiPlug™ for 3 h and stained with FITC-conjugated anti-CD4. Samples were washed, fixed, permeabilized with Cytofix/Cytoperm™ buffer, and stained with PE- or allophycocyanin-conjugated anti–IL-17 and anti–IFN-γ. All antibodies and buffers unless otherwise stated were purchased from BD Biosciences.
Results and Discussion
IL-23 Rather Than IL-12 Is Essential for Development of Joint Autoimmune Inflammation.
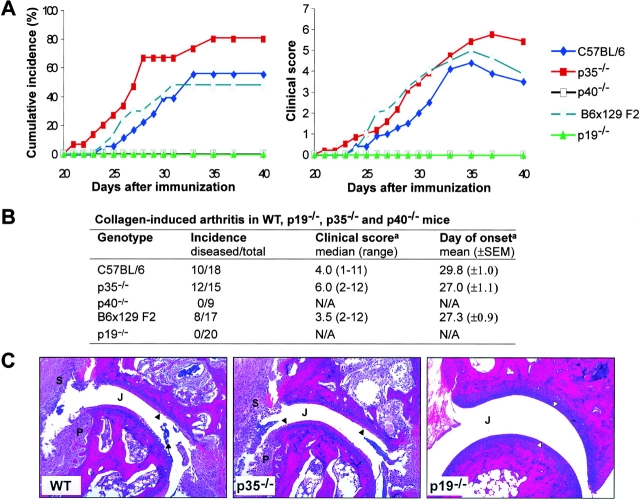
To study the differential functions of IL-12 and IL-23 in joint autoimmune inflammation, we compared susceptibility of mice lacking only IL-23 (p19−/−), only IL-12 (p35−/−), or both cytokines (p40−/−) to CIA. Overall incidence of disease was similar in WT C57BL/6 and B6 × 129 F2 mice (Fig. 1, A and B , ∼50%) as reported previously (17). In contrast, mice specifically lacking IL-23 (p19−/−) and those animals lacking both IL-12 and IL-23 (p40−/−) were resistant to CIA induction (Fig. 1, A and B). Histological examination of joints revealed no pathology in p40−/− (reference 18; not depicted) and p19−/− mice (Fig. 1 C). Unexpectedly, mice specifically lacking only IL-12 (p35−/−) were highly susceptible to CIA with 80% of mice developing clinical signs of disease (Fig. 1, A and B). Histologically, disease was indistinguishable from that of similarly affected WT animals (Fig. 1 C). However, disease severity (Fig. 1 B) appeared to be more intense in p35−/− mice (median clinical score of mice with disease = 6; 12 out of 15 mice developed disease) compared with WT C57BL/6 mice (median clinical score of mice with disease = 4; 10 out of 18 mice developed disease). Disease recovery was also delayed in mice lacking only IL-12 (Fig. 1 A). Thus, IL-23, but not IL-12, is necessary for the development of joint autoimmune inflammation, and the absence of IL-12 may result in a more severe disease.
Figure 1.
IL-23, and not IL-12, is required for induction of CIA. (A) Cumulative incidence and the mean clinical score of mice that developed clinical disease are plotted against days after primary immunization with CII/CFA. (B) Disease incidence (number of diseased mice/total mice in group), disease severity (median maximum disease score attained and range of clinical scores), and mean day of onset of clinical disease are shown for mice of all genotypes. a, Only mice that developed clinical signs of disease were analyzed. N/A, not applicable. (C) Histopathology of decalcified paws at day 42 after primary CII immunization from WT, p35−/−, and p19−/− mice. Hematoxylin and eosin staining of tarsal joints from both WT and p35−/− mice frequently showed severe pathology with articular cartilage erosion (▴), synovial inflammation (S), and pannus (P) formation. p19−/− joints showed no pathology, characterized by smooth, intact articular cartilage (▵) and no cellular infiltrate in the joint space (J). Original magnification, 100.
Reduced IFN-γ and Elevated CII-specific Antibodies in IL-12–deficient Mice.
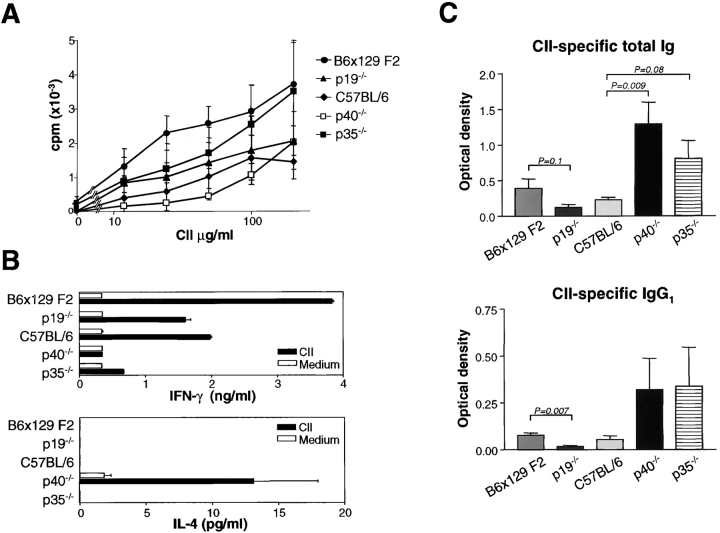
CIA development is dependent on both T and B cell responses (14, 19), and the nature of the T cell response can have an important impact in initiating CIA (20). T cell development was first examined by CII-specific proliferation, and cytokine production in DLN cells was taken from mice 10 d after immunization with CII/CFA. WT C57BL/6, WT B6 × 129 F2, p19−/−, p35−/−, and p40−/− mice had similar CII-specific proliferative responses, regardless of susceptibility to CIA (Fig. 2 A), indicating that induction of CII-reactive T cells did not depend on either IL-12 or IL-23. DLN cells from p19−/− mice secreted WT levels of IFN-γ, but undetectable levels of IL-4, in response to CII stimulation, indicating that classical Th1 cells were induced in these mice (Fig. 2 B). In contrast, DLN cells from p40−/− mice exhibited a Th2 phenotype with low IFN-γ and high IL-4 levels. DLN cells from highly CIA susceptible p35−/− mice produced low levels of IFN-γ (Fig. 2 B) in accordance with the known role for IL-12 in driving this cytokine (2, 21), yet did not produce IL-4, consistent with small amounts of IFN-γ seen in these cultures (Fig. 2 B). Our ongoing studies, as well as recent published work (22), indicate that mouse IL-23 does not induce IFN-γ production in T cells, but rather induces TNF and IL-17 (see the IL-23 Promotes the Development…section).
Figure 2.
Type II collagen (CII)–specific T and B cell responses in IL-23– and IL-12–deficient mice. (A) Normal CII-specific T cell proliferation for both IL-23– and IL-12–deficient mice from DLN cells taken day 10 after CII/CFA immunization (n = 3–4/group) were cultured individually in triplicate. Data show the mean proliferative response of DLN cells for each genotype ± SEM. (B) DLN cells (day 10 after CII immunization) from individual mice were cultured and assayed as described in Materials and Methods. Data represent the mean response of 3–4 mice ± SEM. (C) Divergent humoral responses by p19−/− mice versus p35−/− and p40−/− mice. Sera levels of CII-specific total Ig and IgG1 at day 42 after primary CII immunization (n = 5/group) were determined by ELISA. Differences in Ig levels between relevant groups are indicated by p-values as determined by the unpaired Student's t test. Data represent the mean ± SEM.
p19−/− mice generated a lower CII-specific antibody response compared with WT B6 × 129 F2 controls, suggesting that their resistance to CIA may be due in part to impaired humoral responses (Fig. 2 C). Thus, even though p19−/− mice have normal T cell response with normal capacity to produce IL-12 and IFN-γ (Fig. 2 B; reference 12), their ability to elicit collagen-specific autoantibodies is impaired. By contrast, p35−/− mice had increased CII-specific total Ig and IgG1 levels (Fig. 2 C) compared with WT C57BL/6 mice, suggesting that specific absence of IL-12 leads to increased autoantibody production. At this stage, it is not known whether this has a causative role in contributing to increased disease severity in p35−/− mice. Low and comparable levels of CII-specific IgG2a were detected in WT C57BL/6, WT B6 × 129 F2, p19−/−, p35−/−, and p40−/− mice (unpublished data). p40−/− mice also exhibited increased CII-specific total Ig and IgG1, even though these mice were completely resistant to CIA. This result is in contrast to others that have reported reduced CII-specific antibody responses in p40−/− mice backcrossed onto the DBA/1 background (18), and in anti–IL-12p40–treated DBA/1 mice immunized to induce CIA (23). This may be due to differences in mouse strains used in the studies. It has also been shown in several infectious disease models that p40−/− mice on a C57BL/6 background produce dramatically elevated levels of IgG1 (24) and IgE (25). Overall, the results suggest that IL-12 may be an important regulator of autoantibody responses, especially of those isotypes commonly associated with a Th2 or allergic phenotype. Significantly, the presence of high CII-specific antibody levels in p40−/− mice was not sufficient to induce arthritis in the absence of IL-23 and IL-12.
IL-23 Is Required for Induction of Joint Inflammatory Mediators, Including IL-17 and TNF.
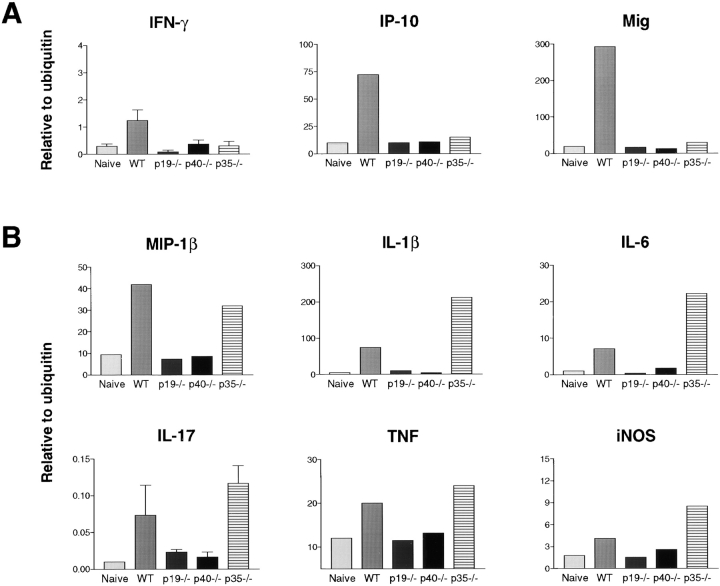
To further analyze the means through which IL-23 deficiency mediates its protection in CIA, and in contrast, how the absence of IL-12 results in more severe disease, we adopted a gene expression strategy. At day 42 after primary immunization with CII/CFA (∼1 wk after peak of disease in WT mice), IFN-γ mRNA levels were elevated above naive levels only in WT paws (Fig. 3 A) and in accordance with this, the IFN-γ–inducible chemokines, monokine induced by IFN-γ (Mig) and IFN-γ–inducible protein 10 (IP-10; reference 26) were also elevated only in WT samples (Fig. 3 A). Notably, these factors were not elevated in p35−/− samples. Also, although IL-23–deficient mice generate a normal antigen-specific Th1 response characterized by IFN-γ production by DLN cells (Fig. 2 B), this response does not develop in the joints, as indicated by the absence of mRNA encoding both IFN-γ and chemokines induced by IFN-γ (Fig. 3 A).
Figure 3.
Reduced IFN-γ, but elevated inflammatory cytokine and chemokine expression by p35−/− mice. Cytokine and chemokine mRNA levels in hind paws day 42 after primary CII/CFA immunization were determined by quantitative real-time PCR and expressed in units relative to ubiquitin mRNA levels. (A) mRNA expression levels for IFN-γ and the IFN-γ–inducible chemokines IP-10 and Mig. (B) mRNA expression of genes encoding proinflammatory factors. RNA from 3 to 5 individual paw samples for each genotype were pooled for analysis, except for IFN-γ and IL-17, where the data represent the mean ± SEM; n = 3. The abundance of mRNA encoding IFN-γ and IL-17 were very low and, hence, were confirmed using expression data from individual mice. (Naive) Paws from unimmunized mice. WT samples, both inflamed and naive, consist of pooled RNA from both C57BL/6 and B6 × 129 F2 mice.
mRNA levels for the proinflammatory cytokines IL-1β, IL-6, TNF, and IL-17, as well as a range of chemokines including MIP-1β (Fig. 3 B), MIP-1α, and MCP-6 (not depicted) remained elevated in WT and p35−/− paws at day 42, but were not elevated above unimmunized WT (naive) levels in p19−/− and p40−/− paws (Fig. 3 B). These factors, levels of IL-1β and IL-6 in particular, were substantially higher in paws of p35−/− mice, which is indicative of the more chronic disease in these animals relative to arthritic WT controls (Fig. 3 B). Nitric oxide synthase 2 gene expression, which is associated with activated macrophages (a major component of the synovial cellular infiltrate), was also elevated in p35−/− mice relative to arthritic WT animals (Fig. 3 B). These results suggest that the specific absence of IL-12, associated with reduced levels of IFN-γ and increased expression of IL-1β, IL-6, IL-17, TNF, and nitric oxide synthase 2, exacerbates autoimmune joint inflammation.
IL-23 Promotes the Development of IL-17–producing T Cells.
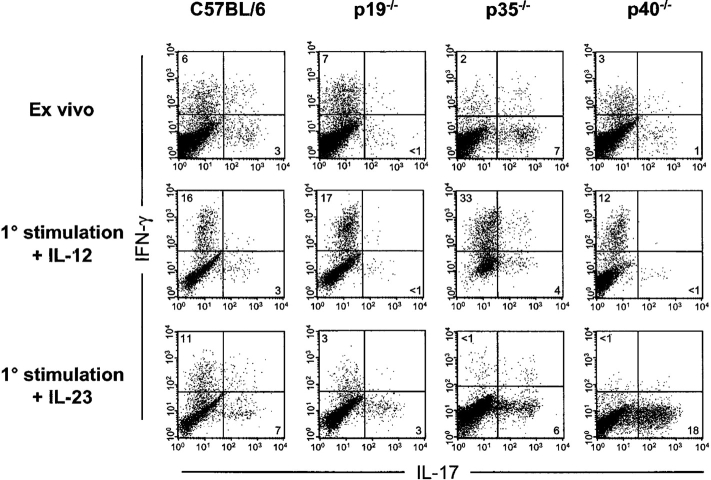
IL-17 is a potent proinflammatory cytokine produced by PBMCs/T cells and by cells isolated from synovial fluid of rheumatoid arthritis patients (27). To determine whether there is a correlation between IL-23 and the development of IL-17–producing cells, we analyzed DLN cell cultures derived from mice inoculated s.c. with CFA (Fig. 4) . Ex vivo analysis of DLN cells from WT C57BL/6 mice showed two relatively discrete populations of IFN-γ and IL-17–producing CD4+ T cells. In contrast, p19−/− mice (C57BL/6 background) showed an absence of IL-17–producing CD4+ cells, as did p40−/− mice, which also lack IL-23 (Fig. 4). Identical results were observed with p19−/− versus WT mice on the B6 × 129 F2 background (unpublished data). IL-12–deficient (p35−/−) mice showed elevated numbers of IL-17–producing T cells relative to WT controls. DLN cells from WT and p19−/− mice both produced IFN-γ (Fig. 4), which is consistent with their ability to produce CII-specific IFN-γ (Fig. 2 B). Culture of DLN cells for 5 d in the presence of IL-12 caused skewing of CD4+ T cells toward IFN-γ production. Conversely, culture with IL-23 skewed toward IL-17 production, and this effect was especially dramatic in p35−/− and p40−/− animals in which IFN-γ production is minimal (Fig. 4). Preliminary studies indicate that IFN-γ neutralization in vitro enables outgrowth of IL-17–producing CD4+ T cells (unpublished data). These results indicate that IL-23 promotes the development of IL-17–producing cells, whereas IL-12 drives IFN-γ production.
Figure 4.
IL-23 promotes the production of IL-17, which is regulated by the IL-12 pathway. Intracellular detection of IFN-γ and IL-17 protein by CD4+ T cells from DLN cells at day 10 after CFA immunization. Cells were either analyzed immediately after isolation (ex vivo) or after 5 d of culture in the presence of rIL-12 or rIL-23, both at 10 ng/ml. All plots are gated on live CD4+ T cells and are representative of four separate experiments.
We have shown previously that IL-23 is required for CNS autoimmunity (12), and here we show that IL-23 is also essential in joint autoimmune inflammation and, therefore, possibly other organ-specific autoimmune diseases. Thus, IL-23, but not IL-12, is necessary for disease development. Moreover, the absence of IL-12 results in more severe disease, reflected in elevated and prolonged expression of proinflammatory cytokines such as IL-1β, IL-6, IL-17, and TNF (Fig. 3 B). These results are in agreement with other papers (10–12) demonstrating that mice selectively lacking only IL-12 (p35−/− mice) or IL-12Rβ, the specific IL-12 signaling chain (28), exhibit increased severity of experimental autoimmune encephalomyelitis compared with WT animals.
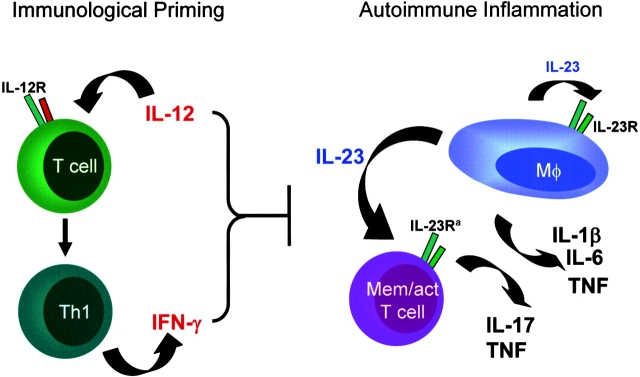
Together, these studies are consistent with the view that IL-12, on the one hand, is a key factor driving Th1 responses and IFN-γ production in the initial phases of an immune response; on the other hand, IL-12 may play a subsequent immunoregulatory role in late-stage inflammation at a point when IL-23 strongly supports the inflammatory process (Fig. 5) . Interplay between IL-23 and IL-17 production may be a critical immune pathway, potentially regulated via an IL-12–induced IFN-γ pathway (Figs. 4 and 5; reference 22). Therefore, specific therapeutic blockade of IL-23 (rather than IL-12 neutralization) may provide a preferred approach for the treatment of a range of inflammatory autoimmune diseases.
Figure 5.
A model proposing the respective roles of IL-12/IFN-γ and IL-23 in T cell-priming events versus end-stage autoimmune inflammation. The precise mechanism through which IL-12/IFN-γ may down-regulate the magnitude of the inflammatory response (i.e., reducing IL-17 production) remains to be defined. It is recognized that IFN-γ may have dual roles during late-stage inflammation (i.e., antiinflammatory actions through termination of activated T cell responses [references 9, 29] while also stimulating macrophage function [reference 5]). a, The IL-23R is present on CD4+ CD45RBlo “memory” T cells (30), but this cell phenotype may also include activated CD4+ T cells that are not strictly memory T cells.
Acknowledgments
We thank C. Ollinger for histology assistance.
DNAX Research Inc. is supported by the Schering-Plough Corporation.
C.A. Murphy and C.L. Langrish contributed equally to this work.
References
- 1.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 2.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542–550. [DOI] [PubMed] [Google Scholar]
- 3.Wu, C., X. Wang, M. Gadina, J.J. O'Shea, D.H. Presky, and J. Magram. 2000. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 165:6221–6228. [DOI] [PubMed] [Google Scholar]
- 4.Lankford, C.S., and D.M. Frucht. 2003. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 73:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Shtrichman, R., and C.E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251–259. [DOI] [PubMed] [Google Scholar]
- 6.Willenborg, D.O., S. Fordham, C.C. Bernard, W.B. Cowden, and I.A. Ramshaw. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157:3223–3227. [PubMed] [Google Scholar]
- 7.Vermeire, K., H. Heremans, M. Vandeputte, S. Huang, A. Billiau, and P. Matthys. 1997. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J. Immunol. 158:5507–5513. [PubMed] [Google Scholar]
- 8.Guedez, Y.B., K.B. Whittington, J.L. Clayton, L.A. Joosten, F.A. van de Loo, W.B. van den Berg, and E.F. Rosloniec. 2001. Genetic ablation of interferon-gamma up-regulates interleukin-1beta expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 44:2413–2424. [DOI] [PubMed] [Google Scholar]
- 9.Chu, C.Q., S. Wittmer, and D.K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon-γ–deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becher, B., B.G. Durell, and R.J. Noelle. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gran, B., G.X. Zhang, S. Yu, J. Li, X.H. Chen, E.S. Ventura, M. Kamoun, and A. Rostami. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169:7104–7110. [DOI] [PubMed] [Google Scholar]
- 12.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 13.Courtenay, J.S., M.J. Dallman, A.D. Dayan, A. Martin, and B. Mosedale. 1980. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 283:666–668. [DOI] [PubMed] [Google Scholar]
- 14.Seki, N., Y. Sudo, T. Yoshioka, S. Sugihara, T. Fujitsu, S. Sakuma, T. Ogawa, T. Hamaoka, H. Senoh, and H. Fujiwara. 1988. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J. Immunol. 140:1477–1484. [PubMed] [Google Scholar]
- 15.Campbell, I.K., M.J. Rich, R.J. Bischof, A.R. Dunn, D. Grail, and J.A. Hamilton. 1998. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 161:3639–3644. [PubMed] [Google Scholar]
- 16.Murphy, C.A., R.M. Hoek, M.T. Wiekowski, S.A. Lira, and J.D. Sedgwick. 2002. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J. Immunol. 169:7054–7062. [DOI] [PubMed] [Google Scholar]
- 17.Campbell, I.K., J.A. Hamilton, and I.P. Wicks. 2000. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur. J. Immunol. 30:1568–1575. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre, K.W., D.J. Shuster, K.M. Gillooly, R.R. Warrier, S.E. Connaughton, L.B. Hall, L.H. Arp, M.K. Gately, and J. Magram. 1996. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur. J. Immunol. 26:2933–2938. [DOI] [PubMed] [Google Scholar]
- 19.Stuart, J.M., and F.J. Dixon. 1983. Serum transfer of collagen-induced arthritis in mice. J. Exp. Med. 158:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauri, C., R.O. Williams, M. Walmsley, and M. Feldmann. 1996. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur. J. Immunol. 26:1511–1518. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri, G., M. Wysocka, A. D'Andrea, M. Rengaraju, M. Aste-Amezaga, M. Kubin, N.M. Valiante, and J. Chehimi. 1992. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog. Growth Factor Res. 4:355–368. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 23.Malfait, A.M., D.M. Butler, D.H. Presky, R.N. Maini, F.M. Brennan, and M. Feldmann. 1998. Blockade of IL-12 during the induction of collagen-induced arthritis (CIA) markedly attenuates the severity of the arthritis. Clin. Exp. Immunol. 111:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su, Z., and M.M. Stevenson. 2002. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 168:1348–1355. [DOI] [PubMed] [Google Scholar]
- 25.Anderson, S., V.L. Shires, R.A. Wilson, and A.P. Mountford. 1998. In the absence of IL-12, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur. J. Immunol. 28:2827–2838. [DOI] [PubMed] [Google Scholar]
- 26.Luster, A.D., J.C. Unkeless, and J.V. Ravetch. 1985. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 315:672–676. [DOI] [PubMed] [Google Scholar]
- 27.Ziolkowska, M., A. Koc, G. Luszezykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832–2838. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, G.X., B. Gran, S. Yu, J. Li, I. Siglienti, X. Chen, M. Kamoun, and A. Rostami. 2003. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J. Immunol. 170:2153–2160. [DOI] [PubMed] [Google Scholar]
- 29.Refaeli, Y., L. Van Parijs, S.I. Alexander, and A.K. Abbas. 2002. Interferon-γ is required for activation-induced death of T lymphocytes. J. Exp. Med. 196:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parnham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K.P. Singh, F. Vega, et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708. [DOI] [PubMed] [Google Scholar]