Abstract
The P2X1 receptor is a fast ATP-gated cation channel expressed in blood platelets, where its role has been difficult to assess due to its rapid desensitization and the lack of pharmacological tools. In this paper, we have used P2X1 −/− and wild-type mouse platelets, treated with apyrase to prevent desensitization, to demonstrate the function of P2X1 in the response to thrombogenic stimuli. In vitro, the collagen-induced aggregation and secretion of P2X1-deficient platelets was decreased, as was adhesion and thrombus growth on a collagen-coated surface, particularly when the wall shear rate was elevated. In vivo, the functional role of P2X1 could be demonstrated using two models of platelet-dependent thrombotic occlusion of small arteries, in which blood flow is characterized by a high shear rate. The mortality of P2X1 −/− mice in a model of systemic thromboembolism was reduced and the size of mural thrombi formed after a laser-induced vessel wall injury was decreased as compared with normal mice, whereas the time for complete thrombus removal was shortened. Overall, the P2X1 receptor appears to contribute to the formation of platelet thrombi, particularly in arteries in which shear forces are high.
Keywords: platelet, P2 receptors, arterial thrombosis, knockout mice, shear forces
Introduction
Platelet activation, recruitment, and aggregation are crucial events in the maintenance of hemostasis and in the development of an arterial thrombus. ADP and ATP are released from platelets stimulated by agonists such as thrombin or collagen, and the released ADP plays a central part in these processes. Purine nucleotides mediate signaling in platelets through three different P2 receptors (1), the ADP-sensitive G protein–coupled P2Y1 receptor (2, 3), the P2Y12 receptor (4, 5), and the ATP-sensitive ligand-gated P2X1 cation channel (6, 7). The role of the P2X1 receptor in platelet function has long been difficult to assess due to a lack of potent and selective antagonists. In addition, one of the main properties of this receptor is its rapid and lasting desensitization (8). In fact, preparation of platelet-rich plasma or washed platelets for in vitro studies induces leakage of nucleotides susceptible to desensitize platelet responses to ADP (9). Therefore, apyrase (ATP-diphosphohydrolase E.C.3.6.1.5.), an ATP- and ADP-removing enzyme, is commonly used to prevent this drawback (9). However, as described previously (10), it is necessary to add higher concentrations of apyrase at each step of platelet handling to prevent desensitization of the platelet P2X1 receptor. Under these conditions, α,β-methylene-ATP (αβMeATP), a nonmetabolizable P2X1 agonist, induces a rapid calcium influx and transient shape change of human platelets (10). Oury et al. have further suggested that this receptor could be involved in aggregation of human platelets induced by collagen (11). However, the in vivo role of the P2X1 receptor in hemostasis and/or thrombosis has not yet been elucidated.
P2X1-deficient mice (P2X1 −/− mice) have been generated and found to exhibit male infertility, indicating that this receptor is essential for male reproductive function through its role in vas deferens contraction in response to sympathetic nerve stimulation (12). We have used this mouse strain to assess the involvement of the P2X1 receptor in hemostasis and thrombosis.
Materials and Methods
Mouse Strains.
P2X1 −/− mice were generated as described previously (12); these mice of 129Ola-MF1 genetic background were backcrossed with the C57BL/6 mouse strain for nine generations. Hence, the wild-type (WT) and P2X1 −/− mice used in this work were of pure C57BL/6 genetic background. Genotyping was performed on mouse tail DNA by a PCR amplification method (12). Complete blood cell counts were determined as described previously (2).
Bleeding Time.
The bleeding time was measured by severing 3 mm from the distal end of the tail of 8-wk-old WT or P2X1 −/− male mice. If no cessation of blood flow occurred after 30 min, the tail was cauterized and >1,800 s was recorded as the bleeding time.
Washed Platelet Studies.
Washed platelet suspension was prepared as described previously (13) except that 1.8 U/ml apyrase was added at each step of the washing procedure and in the final resuspending buffer to prevent desensitization of the P2X1 receptor. Aggregation, secretion, a platelet-loading procedure with fura-2/AM, and intracellular calcium measurements were performed as described previously (13).
In Vitro Model of Platelet Interaction with Immobilized Collagen in a Flow System.
The interaction of platelets with immobilized collagen type I fibrils was studied using an established method (14). Heparin-anticoagulated whole blood, drawn from the retro-orbital vein of mice and containing 1.5 U/ml apyrase, was labeled with 10 μM of the fluorescent dye mepacrine before perfusion at various shear rates through a flow chamber, in which 0.1 mg/ml collagen-coated coverslips formed the lower surface of the flow chamber. The flow chamber, mounted on an epifluorescence microscope (Axiovert model 135M; Carl Zeiss MicroImaging, Inc.), allowed direct visualization of the platelet adhesion and aggregation process, which was recorded with a VCR (model SVO-9500MD; Sony). Thrombus volume was measured in real time by confocal videomicroscopy (model LSM 410; Carl Zeiss MicroImaging, Inc.). Analysis of surface coverage was performed off-line on single frames obtained from the videotape recording (acquisition rate: 30 frames/s) and processed with a software package (Metamorph, Version 5; Universal Imaging Corp.).
In Vivo Models of Thrombosis.
A model of acute systemic vascular occlusion caused by intravascular platelet aggregation induced by infusion of a mixture of collagen and adrenaline was performed as described previously (2, 15). Platelet-independent pulmonary embolism was induced by i.v. injection of a 12.5% suspension of hardened rat RBCs (16). Localized thrombosis of mesenteric arterioles induced by laser injury was performed with male mice weighing 15 g. The mesentery of anesthetized mice was exteriorized and an arteriole with a diameter of 65–100 μm was observed under an inverted microscope (model DMIRB; Leica) coupled to a video camera (DAGE MTI). Localized injury of the luminal surface of the vessel was induced using a 440-nm pulsed nitrogen dye laser applied through the microscope objective with a Micropoint laser system (Photonics Instruments; reference 17). The thrombus development, visualized in real time, triggered in this model mainly involves platelet interactions (18). Six to eight arterioles were targeted over a period of 30 min, only one injury being made on each vessel. The manipulator was unaware of the mouse genotype while performing these experiments.
Results
Hematological Characterization of P2X1 −/− Mice.
The hematological profile of P2X1 −/− mice revealed normal erythrocyte and leukocyte counts and normal hemoglobin and hematocrit. The distribution of leukocyte cell types was also similar between the two genotypes. Platelet counts were indistinguishable between WT and P2X1 −/− mice (1,230 ± 41 × 103 vs. 1,226 ± 52 × 103 platelets/μl, n = 15; unpublished data). No spontaneous bleeding was observed in P2X1 −/− mice and the bleeding time as measured by tail transsection was normal in most of the P2X1 −/− mice as compared with WT mice (Fig. 1) . This result suggests that lack of the P2X1 receptor has a modest impact, if any, on primary hemostasis in mice. Clot retraction evaluated on whole blood was found similar between the two genotypes.
Figure 1.

Tail bleeding time of WT and P2X1 −/− male mice. The bleeding time was normal in most of the P2X1 −/− mice (n = 34, median 107 s) as compared with WT mice (n = 32, median 85 s).
P2X1 −/− Mouse Platelets Exhibit Decreased Aggregation in Response to Collagen.
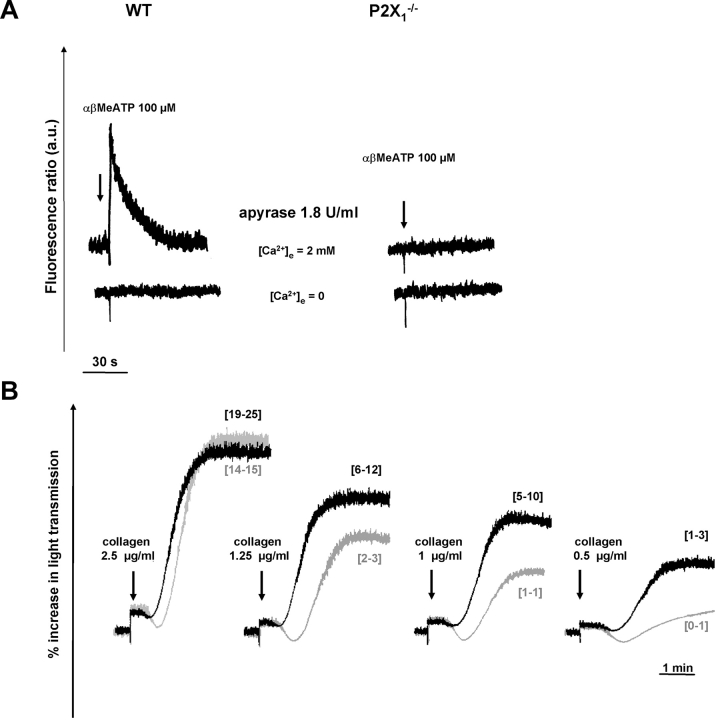
The presence of a functional P2X1 receptor was examined by measuring variations in intracellular calcium ([Ca2+]i) in washed platelets. In the presence of a high concentration of apyrase (1.8 U/ml) at each step of platelet handling to maintain a functional P2X1 receptor, αβMeATP (0.1–100 μM) induced a transient [Ca2+]i increase in WT but not in P2X1 −/− platelets. The calcium rise induced by 100 μM αβMeATP peaked at 91 ± 4 nM (n = 4) above basal levels and returned to these levels after 50 s (Fig. 2 A). It was abolished in the absence of external calcium, indicating that the increase was due to calcium entry from the external medium.
Figure 2.
In vitro evaluation of platelet P2X1 receptor function. (A) [Ca2+]i measurements in washed platelet suspensions. In the presence of 1.8 U/ml apyrase, 100 μM αβMeATP elicited a [Ca2+]i rise in WT but not in P2X1 −/− platelets, which was abolished in the absence of external calcium (0.2 mM EGTA). (B) Aggregation of WT (black lines) and P2X1 −/− (gray lines) platelets in response to various concentrations of collagen. P2X1 −/− platelets showed a diminished response to collagen as compared with WT platelets. The percentage [3H]serotonin secretion, as indicated in brackets, was reduced in P2X1 −/− platelets as compared with WT platelets (values are from two independent experiments). Tracings are from one experiment representative of five independent experiments with identical results.
Under these conditions, at a low collagen concentration (0.5 μg/ml), aggregation of P2X1 −/− platelets was reduced as compared with the WT (Fig. 2 B). This diminished response was still visible at somewhat higher collagen concentrations (1 and 1.25 μg/ml), whereas at concentrations exceeding 2.5 μg/ml, WT and P2X1 −/− platelets displayed comparable aggregation. Similar results were obtained by desensitizing the P2X1 receptor of WT mouse platelets with αβMeATP (unpublished data). The alteration of collagen-induced platelet activation was not due to a difference in collagen receptor or other major platelet glycoprotein density as flow cytometric analyses indicated normal amounts of α2β1 integrin, GPIb–IX, or GPIIbIIIa complexes on WT and P2X1 −/− platelets (unpublished data). Moreover, this decreased reactivity of P2X1 −/− platelets was limited to collagen, because no difference could be observed as compared with the WT when aggregation was triggered by ADP (100 μM, inducing only a weak response in the presence of the high concentration of apyrase) or by low concentrations of agonists such as the 2 μM thromboxane A2 analogue U46619 or 0.05 U/ml thrombin, both of which induce release of nucleotides from the dense granules (unpublished data).
Parallel measurements of the release reaction triggered by collagen in platelets loaded with [3H]serotonin ([3H]5-HT) demonstrated a decreased [3H]5-HT release in P2X1 −/− platelets as compared with WT, consistent with a contribution of P2X1 to this platelet response (Fig. 2 B). In contrast, 1 U/ml thrombin elicited similar [3H]5-HT release in the two genotypes. Altogether, these in vitro studies indicated only a minor role of the P2X1 receptor in platelet aggregation and secretion in response to low concentrations of collagen.
A Role of the P2X1 Receptor in Thrombus Growth on Immobilized Collagen at High Shear Rates.
Platelet aggregation in response to collagen in an aggregometer cuvette may not represent a reflection of events as they occur in vivo, where platelets establish contact only with insoluble collagen in extracellular matrices under flow. Therefore, we studied platelet thrombus formation on a collagen-coated surface, under flow conditions generating wall shear rates between 800 and 6,000 s−1 to cover the range encountered in larger arteries as well as arterioles (19). At a relatively low shear rate (800 s−1), there was no difference in surface coverage by thrombi between WT and P2X1 −/− blood (P = 0.088, n = 3; Fig. 3 A). At a higher shear rate (2,000 s−1), surface coverage by thrombi tended to be lowered in P2X1 −/− blood as compared with WT, but the difference failed to reach statistical significance (P = 0.067, n = 9). In contrast, at the highest shear rate tested (6,000 s−1), thrombus formation was significantly decreased in the P2X1 −/− blood as compared with WT (*, P = 0.032, n = 15; Fig. 3 A). The distribution of adherent and aggregated platelets was not homogeneous in the P2X1 −/− blood, and the platelet thrombi that formed in the absence of P2X1 function grew to a normal size in the areas where they developed (Fig. 3 B). For this reason, the direct measurements of thrombus volume, which were performed on predetermined areas before the beginning of perfusion, showed a considerable variability, and the difference observed between normal and P2X1 −/− blood, although clear, failed to reach statistical significance (P = 0.36, n = 6). For technical reasons, thrombus volume could only be measured on surfaces that correspond to 1/16 of the areas shown in Fig. 3 B; thus, with the P2X1 −/− blood, the values obtained could range from normal to markedly decreased depending on the randomly selected position on the surface.
Figure 3.
Role of the platelet P2X1 receptor in thrombus growth on a collagen-coated surface at high shear rates. (A) Surface coverage by platelet thrombi formed on a surface coated with 0.1 mg/ml type I collagen fibrils. Blood was perfused for 90–240 s at the indicated shear rates. Surface coverage by thrombi was significantly decreased in P2X1 −/− blood only at the shear rate of 6,000 s−1 (*, P = 0.032, n = 15). (B) Volume of thrombi formed on a 0.1-mg/ml collagen-coated surface after perfusion for 150 s at 6,000 s−1 wall shear rate with either WT or P2X1 −/− blood (P = 0.36, n = 6). Two single frame images at low magnification from a real time recording of one such experiment are also shown. Thrombi appeared as bright objects rendered fluorescent by the incorporation of mepacrine into platelet granules.
P2X1 −/− Mice Are Resistant to Thrombosis In Vivo.
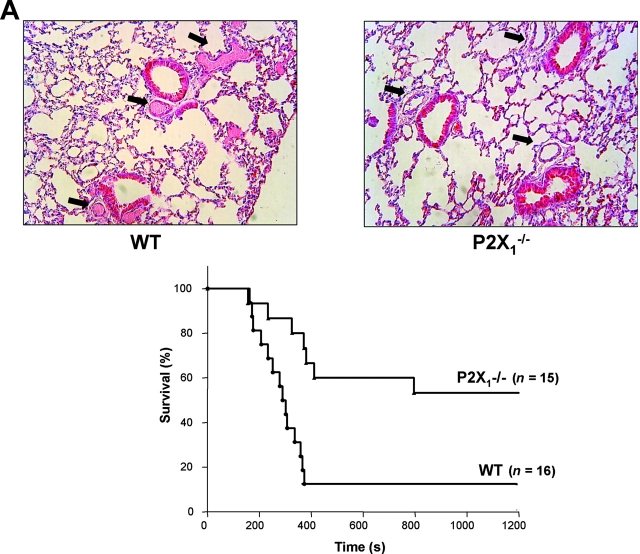
The results of in vitro experiments showed that platelet interaction with a collagen-coated surface was defective in P2X1 −/− blood perfused at high shear rates. However, the difference between WT and P2X1 −/− platelets could have been underestimated in our in vitro studies, due to incomplete recovery by added apyrase of desensitized receptors in WT platelets and/or to an inappropriate environment even under flow conditions. For this reason, to avoid platelet handling and desensitization of the P2X1 receptor, we chose to evaluate further the role of this receptor in vivo using two models of thrombosis in which platelet activation is critical. The first was a model of acute vascular occlusion due to intravascular platelet aggregation induced by infusion of a mixture of collagen and adrenaline into the jugular vein (2, 15). This led to death in 14 of 16 WT mice within 6 min, which was due to extensive obstruction of the lung microcirculation with occlusive thrombi (Fig. 4 A). In contrast, among 15 P2X1 −/− mice, 6 died within 6 min and 1 after 13 min, whereas the 8 others survived the challenge for the 90-min observation period. The effect of genotype on survival was significant by log-rank test (P = 0.0026), and the percentage of P2X1 −/− mice that survived for 90 min was significantly greater than the percentage of WT mice that survived (P = 0.0151, χ2 test). Histological analysis of the lungs of P2X1 −/− mice that recovered showed that only a few vessels were obstructed and that the thrombi were rarely occlusive (Fig. 4 A). Thus, P2X1 −/− mice were resistant to the fatal occlusion of the microvasculature, indicating a role of the P2X1 receptor in this model. To assess whether vascular P2X1 receptors, which have been shown to play a role in vasoconstriction of arteries (20), contributed to the observed phenotype, mice were tested in a model of mechanical pulmonary embolism, induced by i.v. injection of hardened rat RBCs, that is independent of platelet activation (16). Only 18% of WT mice were still alive 45 min after the infusion, and there was no significant difference in mortality for P2X1 −/− mice (log-rank test NS, P = 0.3909; Fig. 4 B). In contrast, there was a 100% survival rate after vasodilation of WT mice with the calcium channel antagonist nifedipine (100 mg/kg), (significantly different from WT or P2X1 −/− mice, P = 0.0003 and P = 0.0076 by log-rank test, respectively). This implies that any vasodilation due to the deficiency of vascular P2X1 receptor does not contribute significantly to the resistance of P2X1 −/− mice to fatal thromboembolism.
Figure 4.
Resistance of P2X1 −/− mice to acute systemic intravascular thromboembolism. (A) Representative histological sections of lungs of WT or P2X1 −/− mice challenged with collagen and adrenaline (top). Frequent occurrence of occlusive intravascular thrombi in lungs of WT mice (left, arrows) compared with unobstructed vessels in lungs of P2X1 −/− mice (right, arrows). Time from 0.3 mg/kg collagen and 60 μg/kg adrenaline injection to death of mice (bottom). Results are expressed as the percentage of mice alive as a function of time. The effect of genotype on survival was significant by log-rank test (P = 0.0026). (B) Mechanical thromboembolism induced by i.v. infusion of hardened rat RBCs. 100 mg/kg nifedipine was administered per os 3 h before i.v. challenge with hardened RBCs. The effect of nifedipine treatment on survival of WT mice was significant by log-rank test ( P = 0.0003) as was the survival of nifedipine-treated WT mice as compared with P2X1 −/− mice (P = 0.0076). In contrast, the survival of WT mice was not significantly different as compared with that of P2X1 −/− mice (NS, P = 0.3909).
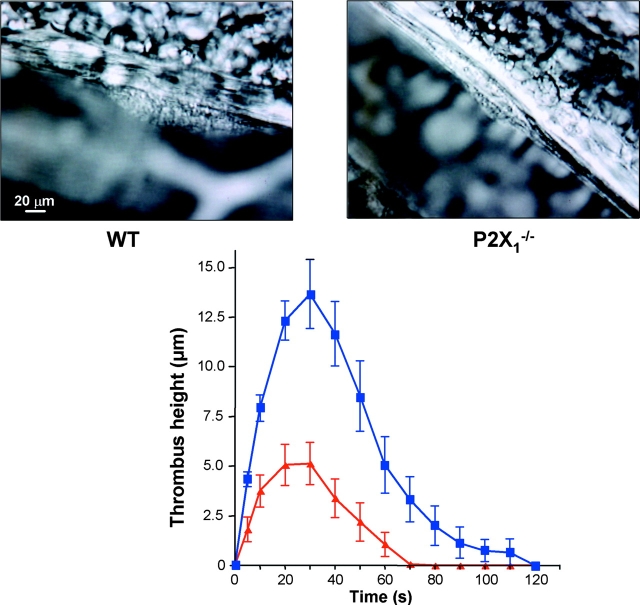
Other experiments were performed in a model of localized thrombosis in mesenteric arterioles triggered by laser-induced vessel wall injury. The time course of thrombus formation in WT mice showed that platelets accumulated rapidly, a maximum height of 13.7 ± 1.7 μm (n = 15 vessels in four different mice) being reached within 30 s of injury and diminishing until complete removal of the thrombus after 120 s (Fig. 5) . The size of the thrombus in P2X1 −/− mice was markedly reduced, reaching only 5.1 ± 1.1 μm (n = 16 vessels in four different mice; P = 0.0005, Wilcoxon signed rank test) with complete removal after 70 s. Thus, P2X1 −/− mice failed to develop an adequate thrombotic mass and the thrombi that formed were easily dispersed. Vasoconstriction was not reduced as measurement of vessel diameters before and after laser-induced injury (percentage of vasoconstriction) was found similar between WT (36 ± 7%, n = 15) and P2X1 −/− (29 ± 5%, n = 16) mice (P = 0.64, Mann-Whitney test). These findings indicate that the P2X1 receptor may contribute to modulate the formation and reversal of localized arteriolar thrombus after laser-induced vessel injury.
Figure 5.
A key role of the platelet P2X1 receptor in arterial thrombosis. Representative photographs of the thrombus formed after laser-induced injury of the wall of mesenteric arterioles in WT and P2X1 −/− mice (top). Arterioles with a diameter of 65–100 μm were targets for injury. Kinetics of thrombus formation in mesenteric arterioles of WT (closed squares) and P2X1 −/− (closed triangles) mice (top). Values are the mean thrombus height (±SEM) at each time point in four WT mice (n = 15 vessels) and four P2X1 −/− mice (n = 16 vessels), and time 0 corresponds to the time of injury.
Discussion
We report here that under optimal in vitro conditions, the P2X1 receptor contributes to the response of platelets exposed to collagen under flow conditions characterized by elevated shear stress, and enhances thrombosis in injured small arteries in vivo. Although the bleeding time was normal in most of the P2X1 −/− mice, indicating that lack of the P2X1 receptor has a modest impact if any on primary hemostasis in the microcirculation, this pattern was very similar to what it had been observed with the P2Y1 −/− mice (2) and in the GPVI−/− mice, opening the “intriguing possibility that such target may be more relevant for pathological thrombus formation and less essential for normal hemostasis” as pointed out by Kato et al. (21). This is very different from mice with GPIIbIIIa or with P2Y12 deficiency, for example, where the bleeding time is dramatically prolonged (5, 22). P2X1 −/− mice displayed resistance to a model of acute thromboembolism that normally leads to massive occlusion of the pulmonary microvessels with platelet aggregates. In a model of mechanical embolism, the vascular P2X1 receptor did not appear to contribute significantly to the phenotype, whereas survival of the P2X1 −/− mice was slightly increased as compared with that of WT mice. Moreover, in a model of localized arterial thrombosis of the mesenteric arterioles triggered by laser-induced vessel wall injury, the extent of thrombus development was reduced in P2X1 −/− mice, pointing to a role of the P2X1 receptor in thrombus formation and growth at sites of endothelial damage of vessels in which blood flow generates high shear forces.
The fact that the effect of P2X1 was more evident in in vivo studies could be ascribed to the persistence of some desensitization of the receptor in WT platelets, despite our attempts to rescue P2X1 function with high concentrations of apyrase. In vivo, P2X1 desensitization is prevented, because platelets circulate in the vicinity of endothelial cells, which maintain a nonthrombogenic surface by expressing in particular the ectoenzyme CD39 (23). CD39 converts circulating extracellular nucleotides, such as ATP and ADP, into AMP and, thus, protects the platelet P2 receptors from desensitization as highlighted by a paper on mice deficient in CD39, which are resistant to thrombosis as a result of desensitization of the P2Y1 receptor (24). Furthermore, AMP is further metabolized by the endothelial 5′-nucleotidase to adenosine, another powerful inhibitor of platelet activation. In addition, prostacyclin (PGI2) and nitric oxide (NO), produced by endothelial cells, contribute to the thromboregulatory properties of the endothelium by inducing vasodilation and inhibition of platelet activation, especially when the cells are exposed to high shear conditions (23). Interestingly, the contribution of the P2X1 receptor was found to be more important when levels of cAMP or cGMP were elevated in platelets, as would be the case in vivo in platelets exposed to the endothelial products PGI2 and NO (25). Thus, the mechanisms displayed by endothelial cells to inhibit the interactions of platelets with the vessel wall are also involved in maintaining high and fast responsiveness of platelets. Rapid calcium entry through the P2X1 cation channel (within 10 ms) and the subsequent calcium response triggered by stimulation of the P2Y1 receptor (200 ms) displayed marked synergy, thereby accelerating and amplifying the overall peak increase in [Ca2+]i (25, 26). Hence, it can be hypothesized that at sites of injury in vessels subjected to high shear conditions, release of ATP, stored with ADP in the dense granules, induces a rapid calcium influx through activation of the P2X1 receptor, which will synergize with the slower calcium increase triggered by ADP-induced activation of the P2Y1 receptor, leading to platelet aggregation and thrombus formation. Therefore, the more rapid response when the P2X1 receptor is functional should be of importance in determining whether or not platelets are activated quickly enough to form a platelet plug in vivo. The results of our in vitro studies on collagen-coated surfaces clearly indicate that the P2X1 receptor may be required to initiate thrombus formation on surfaces exposed to rapidly flowing blood. The functional defect of P2X1 is likely to result in impaired platelet recruitment into a growing thrombus because the platelets that attach to the surface are not fully activated. This may also cause some impairment of initial adhesion, because irreversible platelet attachment to a reactive substrate is also dependent on activation. Hence, the P2X1 receptor may contribute in a significant manner to the initiation of thrombosis at sites of stenosis in atherosclerotic arteries, particularly in response to relatively mild vascular insults (19).
In summary, besides the G protein–coupled ADP receptors, P2Y1 and P2Y12, known to be critically involved in hemostasis and thrombosis, our results point to a role of the P2X1 receptor in arterial thrombosis under high shear conditions.
Acknowledgments
The authors would like to thank J.N. Mulvihill for reviewing the manuscript and N. Lantz for histomorphological analysis.
This work was supported by INSERM, Association de Recherche et de Développement en Médecine et Santé Publique, EFS-Alsace, Fondation de France (no. 2002005149), the National Institutes of Health (HL-31950), and the Wellcome Trust.
References
- 1.Gachet, C. 2001. ADP receptors of platelets and their inhibition. Thromb. Haemost. 86:222–232. [PubMed] [Google Scholar]
- 2.Léon, C., B. Hechler, M. Freund, A. Eckly, C. Vial, P. Ohlmann, A. Dierich, M. LeMeur, J.P. Cazenave, and C. Gachet. 1999. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J. Clin. Invest. 104:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabre, J.E., M. Nguyen, A. Latour, J.A. Keifer, L.P. Audoly, T.M. Coffman, and B.H. Koller. 1999. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat. Med. 5:1199–1202. [DOI] [PubMed] [Google Scholar]
- 4.Hollopeter, G., H. Jantzen, D. Vincent, G. Li, L. England, V. Ramakrishan, R. Yang, P. Nurden, A. Nurden, D. Julius, and P. Conley. 2001. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 409:202–207. [DOI] [PubMed] [Google Scholar]
- 5.Foster, C.J., D.M. Prosser, J.M. Agans, Y. Zhai, M.D. Smith, J.E. Lachowicz, F.L. Zhang, E. Gustafson, F.J. Monsma, Jr., M.T. Wiekowski, et al. 2001. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Invest. 107:1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacKenzie, A.B., M.P. Mahaut-Smith, and S.O. Sage. 1996. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J. Biol. Chem. 271:2879–2881. [DOI] [PubMed] [Google Scholar]
- 7.Vial, C., B. Hechler, C. Léon, J.P. Cazenave, and C. Gachet. 1997. Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thromb. Haemost. 78:1500–1504. [PubMed] [Google Scholar]
- 8.North, R.A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82:1013–1067. [DOI] [PubMed] [Google Scholar]
- 9.Baurand, A., A. Eckly, N. Bari, C. Léon, B. Hechler, J.P. Cazenave, and C. Gachet. 2000. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb. Haemost. 84:484–491. [PubMed] [Google Scholar]
- 10.Rolf, M.G., C.A. Brearley, and M.P. Mahaut-Smith. 2001. Platelet shape change evoked by selective activation of P2X1 purinoceptors with alpha,beta-methylene ATP. Thromb. Haemost. 85:303–308. [PubMed] [Google Scholar]
- 11.Oury, C., E. Toth-Zsamboki, C. Thys, J. Tytgat, J. Vermylen, and M.F. Hoylaerts. 2001. The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen. Thromb. Haemost. 86:1264–1271. [PubMed] [Google Scholar]
- 12.Mulryan, K., D.P. Gitterman, C.J. Lewis, C. Vial, B.J. Leckie, A.L. Cobb, J.E. Brown, E.C. Conley, G. Buell, C.A. Pritchard, and R.J. Evans. 2000. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 403:86–89. [DOI] [PubMed] [Google Scholar]
- 13.Ohlmann, P., A. Eckly, M. Freund, J.P. Cazenave, S. Offermanns, and C. Gachet. 2000. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Galphaq. Blood. 96:2134–2139. [PubMed] [Google Scholar]
- 14.Savage, B., F. Almus-Jacobs, and Z.M. Ruggeri. 1998. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 94:657–666. [DOI] [PubMed] [Google Scholar]
- 15.DiMinno, G., and M.J. Silver. 1983. Mouse antithrombotic assay: a simple method for the evaluation of antithrombotic agents in vivo. Potentiation of antithrombotic activity by ethyl alcohol. J. Pharmacol. Exp. Ther. 225:57–60. [PubMed] [Google Scholar]
- 16.Guarneri, L., A. Molinari, F. Casacci, E. Pacei, C. Cerletti, and G. de Gaetano. 1988. A new model of pulmonary microembolism in the mouse. J. Pharmacol. Methods. 20:161–167. [DOI] [PubMed] [Google Scholar]
- 17.Falati, S., P. Gross, G. Merrill-Skoloff, B.C. Furie, and B. Furie. 2002. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8:1175–1181. [DOI] [PubMed] [Google Scholar]
- 18.Rosen, E.D., S. Raymond, A. Zollman, F. Noria, M. Sandoval-Cooper, A. Shulman, J.L. Merz, and F.J. Castellino. 2001. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am. J. Pathol. 158:1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross, J.M., B.R. Alevriadou, and L.V. McIntire. 1998. Rheology. In Thrombosis and Hemorrhage. J.W. Pine, editor. Williams & Wilkins, Baltimore, MD. 405–421.
- 20.Vial, C., and R.J. Evans. 2002. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol. Pharmacol. 62:1438–1445. [DOI] [PubMed] [Google Scholar]
- 21.Kato, K., T. Kanaji, S. Russell, T.J. Kunicki, K. Furihata, S. Kanaji, P. Marchese, A. Reininger, Z.M. Ruggeri, and J. Ware. 2003. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 10.1182/blood-2003-03-0717. [DOI] [PubMed]
- 22.Hodivala-Dilke, K., K. McHugh, D. Tsakiris, H. Rayburn, D. Crowley, M. Ullman-Cullere, F. Ross, B. Coller, S. Teitelbaum, and R.O. Hynes. 1999. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus, A., M. Broekman, J. Drosopoulos, D. Pinsky, N. Islam, R. Gayle III, and C. Maliszewski. 2001. Thromboregulation by endothelial cells: significance for occlusive vascular diseases. Arterioscler. Thromb. Vasc. Biol. 21:178–182. [DOI] [PubMed] [Google Scholar]
- 24.Enjyoji, K., J. Sevigny, Y. Lin, P.S. Frenette, P.D. Christie, J.S. Esch II, M. Imai, J.M. Edelberg, H. Rayburn, M. Lech, et al. 1999. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5:1010–1017. [DOI] [PubMed] [Google Scholar]
- 25.Sage, S.O., E.H. Yamoah, and J.W. Heemskerk. 2000. The roles of P(2X1)and P(2T AC)receptors in ADP-evoked calcium signalling in human platelets. Cell Calcium. 28:119–126. [DOI] [PubMed] [Google Scholar]
- 26.Vial, C., M.G. Rolf, M.P. Mahaut-Smith, and R.J. Evans. 2002. A study of P2X(1) receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br. J. Pharmacol. 135:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]