Abstract
Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL), Nasu-Hakola disease, is a globally distributed recessively inherited disease. PLOSL is characterized by cystic bone lesions, osteoporotic features, and loss of white matter in the brain leading to spontaneous bone fractures and profound presenile dementia. We have earlier characterized the molecular genetic background of PLOSL by identifying mutations in two genes, DAP12 and TREM2. DAP12 is a transmembrane adaptor protein that associates with the cell surface receptor TREM2. The DAP12–TREM2 complex is involved in the maturation of dendritic cells. To test a hypothesis that osteoclasts would be the cell type responsible for the bone pathogenesis in PLOSL, we analyzed the differentiation of peripheral blood mononuclear cells isolated from DAP12- and TREM2-deficient PLOSL patients into osteoclasts. Here we show that loss of function mutations in DAP12 and TREM2 result in an inefficient and delayed differentiation of osteoclasts with a remarkably reduced bone resorption capability in vitro. These results indicate an important role for DAP12–TREM2 signaling complex in the differentiation and function of osteoclasts.
Keywords: bone diseases, central nervous system diseases, osteoporosis, monocytes, dementia
Introduction
Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL), Nasu-Hakola disease, is a genetically heterogeneous, recessively inherited disease. The histological hallmarks of PLOSL are cystic bone lesions, osteoporotic features, and loss of white matter in the brain. The pathological changes lead to bone fractures after minimal trauma, severe dementia, and premature death (1–3). We have recently identified mutations in all PLOSL patients either in DAP12 or TREM2 (4, 5). DAP12 is a transmembrane adaptor molecule that forms a complex with several cell surface receptors depending on the cell type, and is implicated in the activation of myeloid and NK cells (6, 7). On the cell membrane of monocyte-derived dendritic cells, DAP12 is expressed as a complex with TREM2 (8, 9). The interaction between TREM2 and an unidentified ligand results in the phosphorylation of an immunoreceptor tyrosine-based activation motif in the cytoplasmic domain of DAP12. Phosphorylated DAP12 binds the cytoplasmic protein tyrosine kinases SYK and ZAP70. This interaction results in the activation of downstream signal transduction pathways (6, 7, 10).
Although the primary cause of PLOSL has now been characterized, the pathogenic mechanisms behind the lesions in the bone and brain have remained unknown. We have earlier proposed that the cystic bone lesions and loss of trabecular bone in PLOSL could be caused by dysfunction of osteoclasts, the cells responsible for resorption and remodeling of bone (4). To test this hypothesis, we studied the differentiation and bone resorption capability of osteoclasts derived from the PBMC isolated from four Finnish and one German PLOSL patient with homozygous loss of function mutations in DAP12 (TYROBP or KARAP) or TREM2 (4, 5).
Materials and Methods
Patients.
The ethical committee of National Public Health Institute, Helsinki, Finland has approved this study. An informed consent was obtained from all subjects. The mutation analyses were performed as previously described (5). RT-PCR analyses of TREM2 of the German patient were performed using the following primer pairs (sense and antisense): (full-length coding sequence) ATGGAGCCTCTCCGGCTGCT and TCACGTGTCTCTCAGCCCTG, (5′ half of the coding sequence) TCACGTGTCTCTCAGCCCTG and ATCCAGGGGGTCTGCCAGCA, and (3′ half of the coding sequence) TACAACCCCATGATGCGGGTC and TCACGTGTCTCTCAGCCCTG.
Induction of Osteoclasts.
PBMCs were isolated from buffy coat cells over Ficoll-Paque (Amersham Biosciences). The cells were resuspended in α-MEM (GIBCO BRL), FCS, and antibiotics. Samples of 5 × 106 cells were allowed to adhere for 1 h at 37°C in a round cell culture dish 36 mm in diameter containing four round glass coverslips 13 mm in diameter. Adherent cells were stimulated for 1, 3, 7, 14, and 21 d with 25 ng/ml M-CSF (R&D Systems) and 40 ng/ml RANKL (Qbiogene). The media with cytokines was replaced twice a week.
Histochemistry and Immunofluorescence Stainings.
Staining for tartrate-resistant acid phosphatase (TRAP) was performed using TRAP staining kit (Sigma-Aldrich). The cytoskeletal actin was stained using Alexa Fluor 633 phalloidin reagent (Molecular Probes). The nuclei were visualized using DAPI reagent (Sigma-Aldrich). The staining for cathepsin K was performed using polyclonal anti–human cathepsin K antibody (Santa Cruz Biotechnology, Inc.).
Quantitative RT-PCR Analysis.
Monocytes were stimulated with M-CSF and RANKL for 1, 3, 7, and 21 d as described above. Total RNA was isolated using TRIzol reagent (GIBCO BRL). Quantitative RT-PCR experiments were performed using LightCycler PCR machine (Roche) as previously described (5, 11). Serial dilutions of human DAP12 (sequence data are available from GenBank/EMBL/DDBJ under accession no. AA481924), TREM2 (sequence data are available from GenBank/EMBL/DDBJ under accession no. BF343916), TRAP (sequence data are available from GenBank/EMBL/DDBJ under accession no. J04430), and calcitonin receptor (CALCR; sequence data are available from GenBank/EMBL/DDBJ under accession no. NM_001742) cDNA cloned in a plasmid vector were used to determine the copy number of the amplicon per housekeeping gene cDNA copies (porphobilinogen deaminase [PBGD]; sequence data are available from GenBank/EMBL/DDBJ under accession no. M95623). Probes and human genomic DNA were used to determine the copy number of Cathepsin K, PBGD, and receptor activator of nuclear factor κB (RANK). The sequences for the PCR primers (sense and antisense, respectively) and probes are: (CALCR) TCTCAGGAGTGAAAGCATTGCACATA and AATGCTATGACCGAATGCAGCAGTTA; (DAP12) ATGGGGGGACTTGAACCC and TCATTTGTAATACGGCCTCTGTG; (TRAP) CACACAGCTGTCCTGGCTCAAGAA and CAGGTAGGCAGTGACCCCGTATGT; (TREM2) ATGGAGCCTCTCCGGCTGCT and TCACGTGTCTCTCAGCCCTG; (Cathepsin K) CAGTGAAGAGGTGGTTCAGA and AGAGTCTGGGGCTCTACCTT, (Cathepsin K probe) TCCCGCAGTAATGACACCCTTT; (PBGD) GGGAAACCTCAACACCCGGCT and ACCCGGTTGTGCCAGCCCAT, (PBGD probe) ATCCTGGCAACAGCTGGCCTGCA; and (RANK) GCAAGACCGAGATAGAGGAAGACAGCT and CAGGCTCAGTGAGGAACAGTAACTGGT, (RANK probe) TGTCCATGTATTCATCTTCTGTGGGCATCT.
In Vitro Analysis of Bone Resorption.
In vitro bone resorption analysis was performed by first stimulating PBMCs from three DAP12-deficient and four healthy individuals with M-CSF and RANKL for 7 or 21 d. 4 × 105 cells were then transferred to a well 7.5 mm in diameter containing a dentin slice 5 mm in diameter (Immunodiagnostic Systems). Cells were incubated on dentin in the presence of media with cytokines (replaced twice a week) for 7 d, fixed, and stained for TRAP. The number of multinucleated osteoclasts and nuclei per cell was calculated using light microscope. The cells were then brushed away and the dentin slices were stained with toluidine blue to visualize the resorption pits. The surface area and depth of the resorption pits were determined using AnalySIS 3.2 software (Soft Imaging System) and a confocal microscope, respectively.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 3.0 software (GraphPad Software).
Results and Discussion
DAP12- and TREM2-deficient Monocytes Show a Delayed Differentiation into Osteoclasts.
Multinucleated osteoclasts in humans are formed by the fusion of mononuclear hematopoietic precursor cells circulating in the monocyte fraction (12–14). To generate osteoclasts, we stimulated the PBMCs of four patients with DAP12 mutations, one with a TREM2 mutation and four healthy individuals using a cytokine combination consisting of M-CSF and receptor activator of nuclear factor κB ligand (RANKL), known to induce cells with all morphological and functional characteristics of osteoclasts (14, 15). All Finnish patients carried a homozygous 5.3 kb deletion encompassing exons 1–4 of the five exons of DAP12, resulting in a total loss of expression of DAP12 transcripts or polypeptide (4). The German patient was homozygous for a conversion of nucleotide G 40 to T at the last position of exon 1 of TREM2, resulting in the creation of a premature translation termination codon. We could not detect TREM2 transcripts in the stimulated PBMCs of the German patient in contrast to the control cells, implying the knockout character of this mutation as well (not depicted).
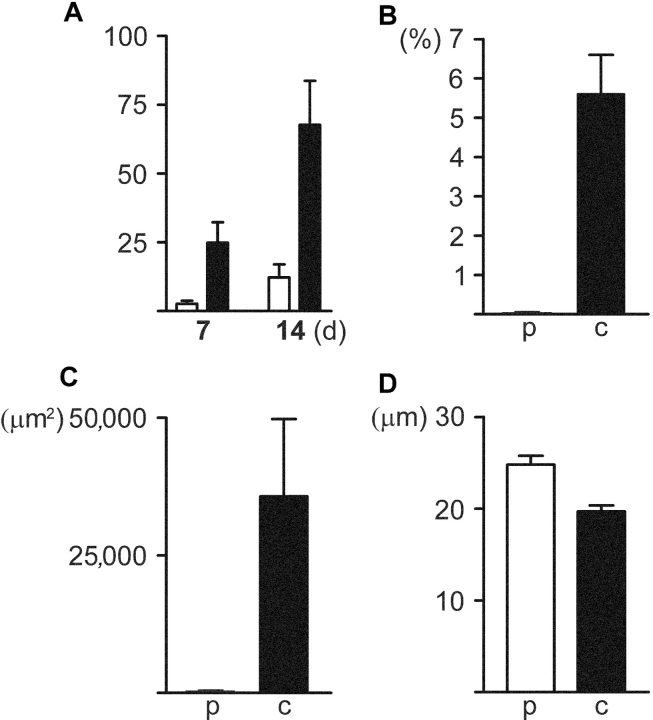
Induction with M-CSF and RANKL generated TRAP+ and cathepsin K+ osteoclasts with 10–20 nuclei in 3–7 d from the PBMCs of healthy individuals. Remarkably, the differentiation of DAP12- and TREM2-deficient PBMCs into multinucleated giant cells was seriously impaired. After 7 d stimulation the number of multinucleated (three or more nuclei) cells generated from DAP12- and TREM2-deficient PBMCs was only 10% of that of control cells (P < 0.01). A vast majority of these genetically deficient multinucleated cells had three or four nuclei. The proportion of cells with five or more nuclei was only 6% of the controls (P = 0.01). After stimulation for 14 d the number of genetically deficient multinucleated cells had increased to 18% (P < 0.01) and the proportion of cells with five or more nuclei increased to 20% of that of controls (P < 0.05; Fig. 1 A). The multinucleation process of DAP12- and TREM2-deficient cells was comparable to each other. DAP12- and TREM2-deficient multinucleated cells were cathepsin K+ and TRAP+ (Fig. 2) . The calculated cell density of cultured DAP12- and TREM2-deficient PBMCs was similar to the controls at all time points, indicating that the genetically deficient cells were able to proliferate in a similar manner as the controls. Taken together, the DAP12–TREM2 complex mediates the differentiation of osteoclasts. Further, in the absence of DAP12/TREM2 signaling, the fusion is very inefficient, but proceeds in the course of time.
Figure 1.
Impaired multinucleation and bone resorption capability of genetically deficient osteoclasts. (A) The number of multinucleated (three or more nuclei) DAP12- or TREM2-deficient (open bars) osteoclastic cells per 106 adherent PBMCs calculated at day 1 was lower than that of controls (solid bars; P < 0.01) after stimulation with M-CSF and RANKL for 7 and 14 d. (B) The total surface area per dentine slice resorbed by DAP12-deficient osteoclasts (p) after incubation on dentine for 7 d was significantly reduced compared with the controls (c; P < 0.01). (C) The resorbed surface area per DAP12-deficient osteoclast (p) was markedly smaller than that of the controls (c; P < 0.05). (D) The resorption pits generated by DAP12-deficient osteoclasts were significantly deeper compared with the controls (P < 0.0001). The error bars indicate the standard error of the mean.
Figure 2.

TRAP staining of DAP12-, TREM2-deficient, and control osteoclastic cells differentiated from PBMCs. DAP12- (A) and TREM2-deficient (B) osteoclastic cells are intensely TRAP+ and much smaller than the control osteoclasts (C) after stimulation for 7 d. Only occasional genetically deficient cells contain two to three nuclei. Note the numerous processes in DAP12-deficient osteoclastic cells.
DAP12- and TREM2-deficient Osteoclastic Cells Show an Aberrant Morphology.
To characterize the morphology of the induced osteoclastic cells in more detail, we stained the cells for cytoskeletal actin. An actin ring, consisting mostly of F-actin filaments, is a functional characteristic of resorbing osteoclasts and delineates the bone resorption area between an osteoclast and bone (16–18). After 7 d the F-actin filaments in the control osteoclasts formed the typical single large actin ring, whereas even after stimulation for 14 d the multinucleated DAP12-deficient osteoclastic cells had several small, unorganized granular actin clusters. The small TREM2-deficient osteoclastic cells showed only one granular, ring-like actin staining pattern after 14 d (Fig. 3) . The transmembrane adaptor molecule DAP12 forms a complex with several different activating receptors depending on the cell type (7). In DAP12-deficient cells, all DAP12-associated cell surface receptors are likely to be inactive. The slightly different morphology of actin rings in DAP12- and TREM2-deficient osteoclastic cells could be explained by the potential costimulatory function of other DAP12-associated cell surface receptors in TREM2-deficient cells.
Figure 3.
Morphological characteristics of DAP12- and TREM2-deficient osteoclastic cells and control osteoclasts after stimulation for 14 d. (A–C, same visual field) DAP12-deficient osteoclastic cells are small and cathepsin K+ (A). Staining for DAPI demonstrates that only occasional cells have a few nuclei (B). Phalloidin staining for actin demonstrates numerous small unorganized actin clusters in DAP12-deficient osteoclastic cells (C). (D–F, same visual field) TREM2-deficient osteoclastic cells are small and cathepsin K+ (D). Majority of the cells are mononuclear (E) and show a single granular ring-like actin staining pattern (F). (G–I, same visual field) The control cells are large and cathepsin K+ (G), contain multiple nuclei (H), and a single large actin ring (I). (J) A high magnification of two DAP12-deficient mononuclear osteoclastic cells demonstrates several granular, unorganized actin clusters. (K) A high magnification of a mononuclear TREM2-deficient cell shows a single granular ring-like actin staining pattern.
DAP12-deficient Osteoclastic Cells Resorb Bone.
To assess the bone resorption capability of DAP12-deficient multinucleated cells, we performed an in vitro bone resorption assay. We first induced DAP12-deficient and control PBMCs with M-CSF and RANKL for 7 or 21 d and then transferred the cells on dentin slices for 7 d. Multinucleated DAP12-deficient cells stimulated for 7 d before transferring on dentine slices were capable of bone resorption (Fig. 4) . Although the same number of DAP12-deficient and control cells were transferred per well, the total surface area per dentine slice resorbed by DAP12-deficient osteoclasts was reduced, being only 0.03% of that of control osteoclasts (P < 0.01; Fig. 1 B). Similarly, we observed a significant difference in the average resorbed surface area per multinucleated osteoclast between DAP12-deficient and control osteoclasts (36 μm2 and 35,700 μm2, respectively, P < 0.05; Fig. 1 C). Surprisingly, the surface area per resorption pit generated by DAP12-deficient and control osteoclasts showed no difference. However, the resorption pits generated by DAP12-deficient osteoclasts were 25% deeper compared with the controls (25 μm and 20 μm, respectively, P < 0.0001; Fig. 1 D). Neither DAP12-deficient nor control osteoclasts were capable of bone resorption in resorption assays initiated after stimulation for 21 d. Taken together, DAP12-deficient multinucleated cells can resorb mineralized bone and they fulfill the criteria for osteoclasts. The lower resorption rate of these osteoclasts could either be due to an impaired actin ring formation or to a loss of controlled cyclic changes in the actin cytoskeleton required for osteoclast movement (17). Abnormal function of the actin ring or cytoskeleton in the genetically defective osteoclasts could potentially lead to a decrease in resorbed surface area, prolonged resorption process, and abnormally deep resorption pits.
Figure 4.

DAP12-deficient osteoclasts are capable of bone resorption in vitro. (A) Control osteoclasts generated long continuous resorption pits. (B) The average surface area per resorption pit generated by DAP12-deficient osteoclasts is similar to controls. The resorption pits generated by genetically deficient osteoclasts are deeper compared with controls (toluidine blue staining).
Quantitative RT-PCR Analysis.
To study the molecular basis for resorption capability and osteoclastic character, we performed a time course study of mRNA of the stimulated cells for cathepsin K, RANK (19), TRAP, and CALCR (20) using quantitative RT-PCR (Table I). Cathepsin K transcripts were expressed at a very low level at days 1–3, but became strongly up-regulated after stimulation for 7–21 d (P < 0.001). There was no difference between DAP12/TREM2-deficient and control cells. Transcripts of the gene encoding RANK, the receptor for RANKL, were expressed in both genetically deficient and control cells after stimulation for 1 d. No significant difference was observed between the patient and control cells. TRAP transcript level was initially low but became up-regulated after stimulation for 3–7 d. No significant differences between the patients and controls were observed. Both DAP12- and TREM2-deficient cells differentiating along the osteoclastic lineage expressed CALCR, the receptor regulating the resorption by osteoclasts. Reliable quantitation could not be performed due to a low transcript level. However, there were no obvious differences between genetically deficient and control cells during the stimulation for 1–21 d. The transcript levels of DAP12 and TREM2 in control cells showed a progressive increase during the stimulation for up to 21 d. A fivefold increase in DAP12 (P < 0.01) and a 39-fold increase in TREM2 transcript level (P < 0.001) was observed between stimulations for 1 and 21 d. Taken together, the delayed differentiation and impaired resorption function of genetically deficient osteoclasts is not due to loss of RANK, CALCR, or TRAP expression. There were no significant differences between DAP12/TREM2-deficient and control cells, suggesting that the steady-state expression levels of the genes studied are not dependent on DAP12 and TREM2. TREM2 polypeptide has been shown to be undetectable in macrophages derived by stimulation of monocytes for up to 14 d with M-CSF alone (9). Thus, osteoclasts are likely to express DAP12 and TREM2 because the expression of DAP12 and TREM2 in PBMCs differentiating along the osteoclastic lineage became progressively up-regulated parallel with the increasing number of multinucleated osteoclasts.
Table I.
Quantitative RT-PCR Analysis of RANKL/M-CSF–stimulated PBMCs of DAP12- and TREM2-deficient and Healthy Individuals
| Time of stimulation
|
||||
|---|---|---|---|---|
| Gene | 1 d | 3 d | 7 d | 21 d |
| DAP12 (controls)a | 260 ± 132 | 365 ± 95 | 450 ± 161 | 1,304 ± 254 |
| TREM2 (controls)a | 14 ± 6 | 65 ± 8 | 76 ± 25 | 541 ± 125 |
| TRAP (controls)b | 9 ± 4 | 63 ± 27 | 63 ± 11 | 76 ± 43 |
| TRAP (patients) | 5 ± 1 | 36 ± 8 | 59 ± 16 | 16 ± 4 |
| Cathepsin K (controls)c | 0.3 ± 0.2 | 1 ± 0.9 | 374 ± 360 | 324 ± 183 |
| Cathepsin K (patients) | 16 ± 16 | 15 ± 13 | 963 ± 710 | 533 ± 155 |
| RANK (controls)b | 8 ± 3 | 33 ± 17 | 5 ± 3 | 25 ± 15 |
| RANK (patients) | 71 ± 29 | 71 ± 13 | 8 ± 3 | 54 ± 11 |
The numbers indicate the transcript copy number per housekeeping gene copies (PBGD) ± SEM. Note: DAP12, TREM2, TRAP, and cathepsin K copy number is presented as (the absolute copy number)/(PBGD copy number), and RANK expression as 1,000 × (the absolute copy number)/(PBGD copy number).
n (controls) = 4, n (patients) = 5.
DAP12 and TREM2 expression increased significantly in stimulated PBMCs (P < 0.01; Bonferroni's multiple comparison test).
No difference between controls and patients (P > 0.05).
A significant up-regulation in patients and controls (P < 0.001; Kruskal-Wallis test), but no difference between patients and controls.
DAP12- and TREM2-deficient PBMCs Migrate Efficiently.
To determine if the fusion of genetically deficient osteoclast precursor cells is delayed due to reduced motility we performed a motility assay. We plated 10 × 106 DAP12-, TREM2-deficient, or control PBMCs per well 36 mm in diameter, each well containing four round coverslips 13 mm in diameter. A cylindrical silicone object 5 mm in diameter attached to the center of each coverslip before plating was removed after 24 h stimulation. After 7 d, the genetically deficient cells effectively migrated to the center of the coverslip, and multinucleated osteoclasts were found at the center. A notable difference was observed in the control cell density between the center and surrounding areas (P < 0.01), whereas there was no significant difference in the density of genetically deficient cells between these areas (P > 0.05). This implies that DAP12- and TREM2-deficient PBMCs migrate efficiently and that the delayed fusion of prefusion osteoclasts is not caused by impaired motility.
The receptors and signals required for the fusion of osteoclast precursor cells during osteoclast maturation are not fully understood. Our results demonstrate that DAP12/TREM2 signaling mediates the differentiation of osteoclasts and that DAP12/TREM2-deficiency results in an aberrant osteoclast morphology and a severely delayed maturation process in vitro, as indicated by the inefficient fusion and impaired actin ring formation of the immature osteoclasts.
Kaifu et al. (21) have recently reported a defect in osteoclast differentiation in DAP12-deficient mice. Interestingly, their mouse model presents a mild osteopetrosis and no cystic bone lesions. This observation is in contradiction with human PLOSL where the pathological hallmarks are cystic bone cavities and osteoporosis.
The symptoms of PLOSL appear approximately at age 20 as skeletal pain. Spontaneous fractures in the bones of the extremities occur a few years later due to bone cavities filled with membranous lipid material (3, 22). The lesions are found in all limb bones, especially in the bones of the wrists, hands, ankles and feet. In the long tubular bones, the lesions are typically located in the distal end of the bones. In addition, the bones show osteoporotic features, i.e., severe loss of trabecular bone. The development of the bone lesions is slowly progressive and the age at which the lesions begin to develop is unknown (23). The normal average height and the macroscopic structure of the bones in PLOSL patients, apart from the lesion cavities and trabecular bone loss, indicate that the resorptive phase of bone development and growth is not severely affected. As expected, multinucleated, TRAP+ and CD68+ osteoclasts were found in the bones of DAP12-deficient PLOSL patients. The size of osteoclasts appeared normal (not depicted). Although the bone resorption capability of DAP12-deficient osteoclasts was impaired in vitro, the bones of PLOSL patients are osteoporotic rather than osteopetrotic, as is seen in severe osteoclastic failure (24). Our finding of an impaired resorption capability of genetically deficient osteoclasts in vitro is somewhat contradictory with the local osteolytic process in PLOSL patients. Current knowledge of DAP12/TREM2 signaling does not provide self-evident explanations for this paradox. The locally increased bone loss in vivo could be explained by systemic or local factors (endocrine, paracrine, etc.) affecting the differentiation or activation of osteoclasts in situ.
The reason why the bone lesions in DAP12- and TREM2-deficient PLOSL patients are found only in the limb bones, and especially in the distal segment of the bones, remains unclear. Bones develop either by endochondral or intramembranous ossification. In endochondral ossification a cartilage model serves as the precursor of bone, whereas intramembranous ossification occurs without an intervening cartilage precursor (25). Interestingly, all bones affected by PLOSL are formed by endochondral ossification. No lesions have been found in the bones developed by intramembranous ossification (e.g., the skull, clavicle, and mandible; 23).
So far, the function of DAP12 and TREM2 has been thought to be limited to transmitting activating signals to cells of the immune system. Our findings provide, for the first time, direct evidence that DAP12 and TREM2 in humans also play a key role in the normal functions of cells not directly involved in immune responses. Further characterization of the role of DAP12/TREM2 signaling in common disorders of bone, such as osteoporosis, should be stimulated by our findings. Finally, despite the characterization of a defective osteoclast maturation and function caused by DAP12/TREM2 deficiency, understanding the role of osteoclasts in the bone pathogenesis of PLOSL in detail still requires additional studies in vitro and in vivo.
Acknowledgments
We thank Jari Salo for helpful comments on the manuscript and Tuula Manninen and Mika Hukkanen for technical help.
This study was supported by the Academy of Finland, the Ulla Hjelt Fond of the Foundation for Pediatric Research, Helsinki Biomedical Graduate School, the Finnish Cultural Foundation, the Finnish Medical Foundation, the Oskar Öflund Foundation, the Emil Aaltonen Foundation, and Maud Kuistila Foundation.
J. Paloneva and J. Mandelin contributed equally to this work.
References
- 1.Hakola, H.P. 1972. Neuropsychiatric and genetic aspects of a new hereditary disease characterized by progressive dementia and lipomembranous polycystic osteodysplasia. Acta. Psychiatr. Scand. Suppl. 232:1–173. [PubMed] [Google Scholar]
- 2.Verloes, A., P. Maquet, B. Sadzot, M. Vivario, A. Thiry, and G. Franck. 1997. Nasu-Hakola syndrome: polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J. Med. Genet. 34:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paloneva, J., T. Autti, R. Raininko, J. Partanen, O. Salonen, M. Puranen, P. Hakola, and M. Haltia. 2001. CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology. 56:1552–1558. [DOI] [PubMed] [Google Scholar]
- 4.Paloneva, J., M. Kestila, J. Wu, A. Salminen, T. Bohling, V. Ruotsalainen, P. Hakola, A.B. Bakker, J.H. Phillips, P. Pekkarinen, et al. 2000. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 25:357–361. [DOI] [PubMed] [Google Scholar]
- 5.Paloneva, J., T. Manninen, G. Christman, K. Hovanes, J. Mandelin, R. Adolfsson, M. Bianchin, T. Bird, R. Miranda, A. Salmaggi, et al. 2002. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanier, L.L., B.C. Corliss, J. Wu, C. Leong, and J.H. Phillips. 1998. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 391:703–707. [DOI] [PubMed] [Google Scholar]
- 7.Lanier, L.L., and A.B. Bakker. 2000. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today. 21:611–614. [DOI] [PubMed] [Google Scholar]
- 8.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995. [DOI] [PubMed] [Google Scholar]
- 9.Bouchon, A., C. Hernandez-Munain, M. Cella, and M. Colonna. 2001. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 194:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McVicar, D.W., L.S. Taylor, P. Gosselin, J. Willette-Brown, A.I. Mikhael, R.L. Geahlen, M.C. Nakamura, P. Linnemeyer, W.E. Seaman, S.K. Anderson, et al. 1998. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 273:32934–32942. [DOI] [PubMed] [Google Scholar]
- 11.Konttinen, Y.T., M. Takagi, J. Mandelin, J. Lassus, J. Salo, M. Ainola, T.F. Li, I. Virtanen, M. Liljestrom, H. Sakai, et al. 2001. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. J. Bone Miner. Res. 16:1780–1786. [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa, Y., J.M. Quinn, A. Sabokbar, J.O. McGee, and N.A. Athanasou. 1996. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 137:4058–4060. [DOI] [PubMed] [Google Scholar]
- 13.Heymann, D., J. Guicheux, F. Gouin, N. Passuti, and G. Daculsi. 1998. Cytokines, growth factors and osteoclasts. Cytokine. 10:155–168. [DOI] [PubMed] [Google Scholar]
- 14.Suda, T., N. Takahashi, N. Udagawa, E. Jimi, M.T. Gillespie, and T.J. Martin. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20:345–357. [DOI] [PubMed] [Google Scholar]
- 15.Quinn, J.M., J. Elliott, M.T. Gillespie, and T.J. Martin. 1998. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 139:4424–4427. [DOI] [PubMed] [Google Scholar]
- 16.Vaananen, H.K., and M. Horton. 1995. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J. Cell Sci. 108:2729–2732. [DOI] [PubMed] [Google Scholar]
- 17.Salo, J., P. Lehenkari, M. Mulari, K. Metsikko, and H.K. Vaananen. 1997. Removal of osteoclast bone resorption products by transcytosis. Science. 276:270–273. [DOI] [PubMed] [Google Scholar]
- 18.Teitelbaum, S.L. 2000. Bone resorption by osteoclasts. Science. 289:1504–1508. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa, N., M. Kinosaki, K. Yamaguchi, N. Shima, H. Yasuda, K. Yano, T. Morinaga, and K. Higashio. 1998. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 253:395–400. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi, M., A.M. Inzerillo, B.S. Moonga, P.J. Bevis, and C.L. Huang. 2002. Forty years of calcitonin-where are we now? A tribute to the work of Iain Macintyre, FRS. Bone. 30:655–663. [DOI] [PubMed] [Google Scholar]
- 21.Kaifu, T., J. Nakahara, M. Inui, K. Mishima, T. Momiyama, M. Kaji, A. Sugahara, H. Koito, A. Ujike-Asai, A. Nakamura, et al. 2003. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasu, T., Y. Tsukahara, and K. Terayama. 1973. A lipid metabolic disease-”membranous lipodystrophy”-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol. Jpn. 23:539–558. [DOI] [PubMed] [Google Scholar]
- 23.Makela, P., O. Jarvi, P. Hakola, and P. Virtama. 1982. Radiologic bone changes of polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy. Skeletal Radiol. 8:51–54. [DOI] [PubMed] [Google Scholar]
- 24.de Vernejoul, M.C., and O. Benichou. 2001. Human osteopetrosis and other sclerosing disorders: recent genetic developments. Calcif. Tissue Int. 69:1–6. [DOI] [PubMed] [Google Scholar]
- 25.Ross, H.M., L.J. Romrell, and G.I. Kaye. 1995. Bone. In Histology: A Text and Atlas. P.A. Coryell, editor. Williams & Wilkins, Baltimore. 150–187.