Abstract
The linker for activation of T cells (LAT) is an adaptor protein critical for FcɛRI-mediated mast cell activation. LAT is a substrate of the tyrosine kinases activated after TCR and FcɛRI engagement. After phosphorylation of the cytosolic domain of LAT, multiple signaling molecules such as phospholipase C–γ1, Grb2, and Gads associate with phosphorylated LAT via their SH2 domains. The essential role of the four distal tyrosines in TCR-mediated signaling and T cell development has been demonstrated by experiments using LAT-deficient cell lines and genetically modified mice. To investigate the role of these four tyrosines of LAT in FcɛRI-mediated mast cell activation, bone marrow–derived mast cells from LAT-deficient mice were infected with retroviral vectors designed to express wild-type or mutant LAT. Examination of bone marrow–derived mast cells expressing various tyrosine to phenylalanine mutants in LAT demonstrates a differential requirement for these different binding sites. In these studies, assays of biochemical pathways, degranulation, and cytokine and chemokine release were performed. Finally, the role of these tyrosines was also evaluated in vivo using genetically modified animals. Deletion of all four distal tyrosines, and in particular, loss of the primary phospholipase C–γ-binding tyrosine had a significant effect on antigen-induced histamine release.
Keywords: signal transduction, adapter molecules, phosphorylation, Fc epsilon receptor, anaphylaxis
Introduction
Aggregation of the high affinity receptor for IgE (FcɛRI) on mast cells leads, via a complex set of biochemical events, to activation of a number of mast cell effector functions. These include release of granules containing histamine, serotonin, β-hexosaminidase, and mast cell–specific proteases. Additionally, transcription of multiple cytokine and chemokine genes and secretion of these proteins also follows receptor engagement. Among the cytokines, interleukins 1–6, 9, 10, 13, 16, TNF-α, TGF-β, and GM-CSF are known to be produced in response to FcɛRI stimulation. The chemokines MCP-1, MIP-1α, MIP-1β, and regulated on activation, normal T cell–expressed and secreted (RANTES) are also produced in response to FcɛRI stimulation. A major question in the study of FcɛRI-mediated signaling is how the early biochemical events after FcɛRI engagement lead ultimately to granule release and gene transcription (1).
The FcɛRI is comprised of multiple subunits, a ligand-binding α chain, and two chains, β and γ, which function as signal transduction subunits (2). Both of these subunits contain an immunoreceptor tyrosine-based activation motif (ITAM), containing paired tyrosine residues (3). Engagement of this receptor with IgE and polyvalent antigen leads to activation of several Src family protein tyrosine kinases (PTKs). One of these, Lyn, phosphorylates the cytosolic tyrosines of the β and γ ITAMs (4–6). These phosphorylations create a binding site for the tandem SH2 domains of Syk PTK. Interaction of Syk with the phosphorylated ITAMs also activates this enzyme. Syk is known to be a crucial molecule for FcɛRI signaling because Syk-deficient mast cells fail to activate any downstream pathways after FcɛRI engagement (7, 8).
Activation of Syk leads to phosphorylation of at least one important adaptor molecule, the linker for activation of T cells (LAT; reference 9). When phosphorylated, LAT recruits a number of important signaling molecules containing SH2 domains. From works in T cells as well as mast cells, it is known that the LAT-binding proteins include but are not limited to the additional adapters, Grb-2 and Gads (9–11). In turn, these proteins are bound to SOS and SLP-76, respectively. In addition, phosphorylated LAT recruits the enzymes phospholipase C (PLC)–γ1 and PLC-γ2. From this limited list, it is clear that the phosphorylation of LAT leads to recruitment of molecules that are central to activation of small G-proteins (SOS) and to control calcium flux, diacylglycerol production, and activation of protein kinase C (PLC isoforms; reference 12).
The importance of LAT in mast cell function has been revealed in gene inactivation studies. Bone marrow–derived mast cells (BMMCs) from LAT-deficient mice demonstrated severe defects of mast cell function (13). Mast cell degranulation and transcription of multiple cytokine genes was markedly impaired in these studies. Based on work in T cells, it is likely that the distal four tyrosine residues of LAT mediate important LAT-binding events (10). To investigate whether these residues are critical to mast cell function and to determine how various signaling molecules couple to LAT, we reconstituted the function of LAT-deficient BMMCs. Various LAT mutants were introduced into these cells using Moloney murine leukemia retroviral constructs. Additionally, in vivo studies were performed using mice expressing genetically targeted mutations in LAT.
There was no difference in FcɛRI-mediated mast cell activation between BMMCs from LAT+/+ mice and the BMMCs from LAT −/− mice reconstituted with wild-type LAT. There was also no difference in FcɛRI-mediated mast cell activation between BMMCs from LAT−/− mice and the BMMCs from LAT−/− mice that expressed a mutant LAT in which all four distal tyrosines were converted to phenylalanines. These results demonstrate that the four distal tyrosines are critical for LAT-dependent signaling in mast cells after FcɛRI engagement. BMMCs expressing single or combination mutations of LAT demonstrated a differential requirement for the various LAT residues, with the primary PLC-binding site being the most important. The in vivo studies confirmed the critical role of LAT for histamine release. The four distal LAT tyrosines and the principal PLC site are required in this in vivo response to antigen.
Materials and Methods
Mice.
Generation of LAT-deficient mice was reported previously (14). Mice were caged in a specific pathogen-free environment in accordance with National Institutes of Health regulations. All mice were of a C57/BL6 and 129/Sv–mixed background.
Construction of LAT Mutants.
Mouse LAT tyrosine to phenylalanine mutations were made using the Quickchange mutagenesis kit (Stratagene). A NcoI site was made in the start codon of LAT for cloning. Mutations were confirmed by DNA sequencing. The retroviral expression vector used was pMX-puro, which is a chimeric vector of pBabe and MFG (a gift from T. Kitamura, University of Tokyo, Tokyo, Japan). The encephalomyocarditis virus internal ribosomal entry site (IRES) isolated from pCITE-2a was used for bicistronic expression (15). The IRES-EGFP was subcloned at the BamHI and NotI sites of pBluescript. Wild-type or mutant LAT cDNA (a gift from S. Habu, Tokai University School of Medicine, Isehara, Japan) were subcloned downstream of the IRES at the NcoI and NotI sites of this construct. Truncated rat CD2 cDNA with extracellular, transmembrane, and three amino acids of the cytoplasmic region was subcloned upstream of IRES at the SmaI site of this construct. These constructs were digested with XhoI and NotI to make CD2-IRES-LAT as shown in Fig. 1. After treatment with Klenow fragment, these fragments were subcloned into the pMX-puro vector.
Figure 1.
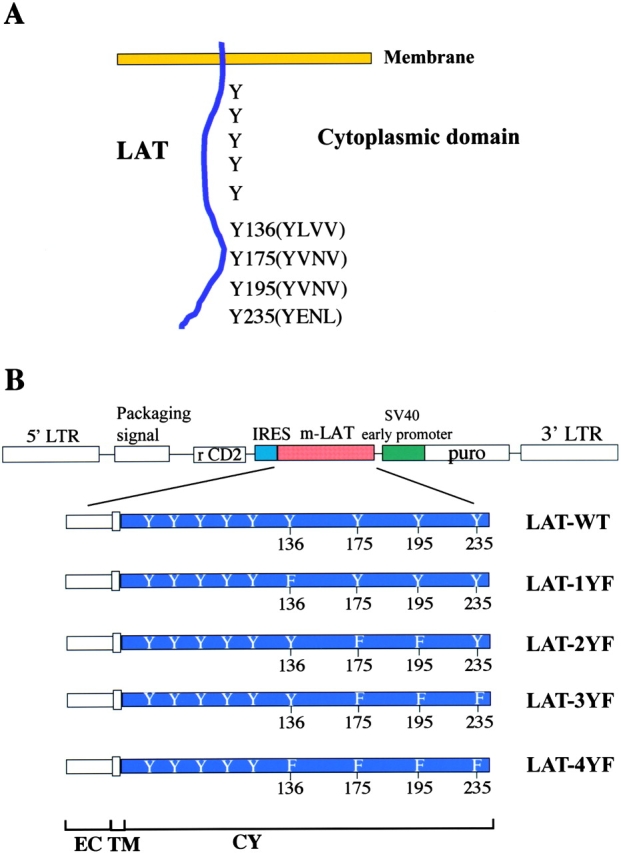
(A) Schematic representation of the mouse LAT molecule. Y denotes tyrosine residues that are potential phosphorylation sites. Y136 (YLVV) is the predicted PLC-γ1–binding site. Y175 and Y195 (YVNV) and 235 (YENL) are the predicted Grb2-binding sites. (B) Retroviral constructions used for coexpression of rat CD2 and LAT in transduced LAT−/− BMMCs. Domains include extracellular (EC), transmembrane (TM), and cytoplasmic (CY). Viruses expressing CD2 alone (CD2-IRES); CD2 with wild-type LAT (CD2-IRES-LAT-WT); the Y136 F mutation (CD2-IRES-LAT-1YF); Y175F and Y195 F mutations (CD2-IRES-LAT-2YF); Y175 F, Y195 F, and Y235 F mutations (CD2-IRES-LAT-3YF); and Y136 F, Y175 F, Y195 F, and Y235 F mutations (CD2-IRES-LAT-4YF) were used to transduce LAT−/− BMMCs.
Retrovirus Infection and Mast Cell Isolation.
Bone marrow cells taken from LAT+/+ and LAT−/− mouse femurs were incubated in suspension culture in the presence of IL-3 for 4–8 wk. Phoenix E and Bosc 23 were used as packaging cell lines (16). At 24 h before transfection, Phoenix E and Bosc 23 cells were mixed at a 1:9 ratio. Retroviral vectors were transfected into these cells using Lipofectamine Plus (Invitrogen). For retroviral infection, LAT−/− BMMCs cultured for 12 d were mixed with the packaging cell lines transfected with vectors. After 48 h of the mixed culture, the BMMCs were harvested and cultured in IL-3–containing medium. 2 d later, the infection efficiency was measured by anti–rat CD2 staining. 25–30% of the BMMCs were positive for rat CD2. After 2 wk of subsequent puromycin selection, the BMMCs were stained with anti–rat CD2 and IgE + anti-IgE–FITC to confirm expression of LAT protein and to measure the mast cell purity. Mast cell granules were stained with toludine blue.
Flow Cytometric Analysis of IgE Receptor, Rat CD2, and LAT Expression.
BMMCs were preincubated with 5 μg/ml anti-CD16/CD32 (2.4G2; BD Biosciences) and stained for FcɛRI with 2.5 μg/ml anti-DNP IgE (SPE-7 mAb; Sigma-Aldrich), followed by FITC-conjugated rat anti–mouse IgE (BD Biosciences). To detect rat CD2 expression level, BMMCs were treated with FITC-conjugated anti–rat CD2 antibody or FITC-conjugated IgG (BD Biosciences) as a negative control. To detect LAT expression level, BMMCs were fixed with 3.7% paraformaldehyde for 30 min at room temperature and treated with 0.1% Triton X-100 for 4 min. After blocking with 2% goat serum, the BMMCs were stained with rabbit anti-LAT and phycoerythrin-conjugated goat anti–rabbit antibody. FACScan™ (Becton Dickinson) was used for the analysis of stained cells.
Cell Stimulation, Immunoblotting, and Immunoprecipitation.
Cells were preloaded for at least 4 h with 1 μg/ml anti-DNP IgE (17) in medium (without IL-3). Cells were challenged in Tyrode's buffer with 100 ng/ml DNP-HSA (13). Before immunoprecipitation, cells were lysed in buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.6, 4 mM EGTA, 20 μg/ml pepstatin, complete inhibitor cocktail tablets (Roche), 1% Brij or NP-40, 1% n-octyl-β-d-glucoside, 1 mM sodium orthovanadate, 7 mM EDTA for 30 min on ice. The lysis buffer for SLP-76 immunoprecipitation was 150 mM NaCl, 25 mM Tris-HCl, pH 7.6, 4 mM EGTA, 40 μg/ml Bestatin, 2 μg/ml E64, 2.5 μg/ml macroglobulin, 10 μg/ml phosphoramidon, 30 μg/ml pepstatin, complete inhibitor cocktail tablets, 1% Triton X-100, 1% n-octyl-β-d-glucoside, 1 mM sodium orthovanadate, 7 mM EDTA. Antibodies used for immunoprecipitation were anti-LAT antiserum (9), anti–PLC-γ 1 (Upstate Biotechnology), anti-Grb2 and anti–PLC-γ2 (Santa Cruz Biotechnology, Inc.), and anti–SLP-76 (Antibody Solutions). Antibodies used for immunoblotting were 4G10 and SLP-76 (Upstate Biotechnology). Reagents used for detection of antibody in immunoblots were protein A–horseradish peroxidase (HRP) and sheep anti–mouse IgG-HRP (Amersham Biosciences), and protein A/protein G–HRP (Pierce Chemical Co.).
Measurement of Calcium Flux and Degranulation.
Cells were preloaded with 1 μg/ml anti-DNP IgE at 37°C for 4 h. The cells were incubated with 16 μM Fluo-4–AM and 16 μM Fura red in 2% FCS RPMI 1640 medium at 37°C for 45 min. The cells were washed twice with Tyrode's buffer, resuspended, and kept at room temperature for 30 min to allow cleavage of the AM esters. The cells were analyzed by FACScan™ (Becton Dickinson). Intracellular calcium concentration is shown as the Fluo-4/Fura red fluorescence intensity ratio. Degranulation was determined by measuring the release of β-hexosaminidase as described previously (13).
Mitogen-activated Protein (MAP) Kinase Assay.
For ERK and JNK kinase assays, cells were preloaded for 4 h with 1 μg/ml anti-DNP IgE in medium (without IL-3) and stimulated with 100 ng/ml DNP-HSA for 8 or 10 min. For ERK kinase assays, ERK2 immunoprecipitated with rabbit polyclonal serum against ERK2 (Santa Cruz Biotechnology, Inc.) was used to phosphorylate myelin basic protein (MBP; Upstate Biotechnology) for 10 min at room temperature. For JNK assays, JNK1 immunoprecipitated with rabbit polyclonal serum against JNK1 (Santa Cruz Biotechnology, Inc.) was used to phosphorylate the GST-Jun 5–89 fusion protein (c-Jun–GST; Upstate Biotechnology) for 10 min at room temperature. To control for JNK1 levels, this membrane was cut and immunoblotted with anti-JNK1. To detect phosphorylated p38 MAP kinase, cells were preloaded for 4 h with 1 μg/ml anti-DNP IgE in medium (without IL-3) and stimulated with 100 ng/ml DNP-HSA for 5 and 10 min. Total cell lysates for immunoblotting were prepared by boiling in reducing sample buffer, and were separated on a 4–15% acrylamide gel by PAGE. The membrane was immunoblotted with anti–phospho-p38 antibody (New England BioLabs, Inc.). After stripping, the membrane was reblotted with anti-p38 antibody (Santa Cruz Biotechnology, Inc.).
RT-PCR.
(2 × 106 cells were preincubated with 1 μg/ml of DNP-specific IgE at 3 × 106 cells/ml for at least 4 h. Cells were washed three times and stimulated with 100 ng/ml DNP-HSA for 1 h. Total RNA was isolated using the TRI reagent (Molecular Research Center; reference 13). First strand cDNA synthesis was performed using the Superscript RT-PCR kit (Life Technologies). 2.5 μg total RNA was used in reactions primed with oligo dT to obtain cDNA. 7.5 μl of the synthesized cDNA in 150 μl dH20 was used as the template for the cytokine mRNA amplification reactions. PCR conditions were as follows: 94°C for 5 min, 30 cycles for 94°C for 30 s, 57°C for 30 s, 72°C for 1 min, 72°C for 8 min, and soak at 4°C. Primers for IL-2 were obtained from Ambion and primers for GAPDH, used as an internal control, were obtained from CLONTECH Laboratories, Inc.
RNase Protection Assay.
The Multiprobe RNase Protection Assay was performed according to the manufacturer's (BD Biosciences) directions with modifications as follows.
For hybridization, probes were synthesized with [33P]UTP (70–80 μC/full reaction) using the BD Biosciences In Vitro Transcription kit. Probes were purified by gel filtration. 0.5–1.0 × 106 cpm was added to each RNA in a final hybridization volume of 10–20 μl (at least 50% BD Biosciences hybridization buffer).
For the RNase inactivation, a master cocktail containing 200 μl RNase inactivation reagent (Ambion), 50 μl ethanol, 5 μg yeast tRNA, and 1 μl GycoBlue coprecipitate per RNA sample was used to precipitate the protected RNA (Ambion). Pellets were resuspended in 3 μl of sample buffer before electrophoresis (BD Biosciences).
Passive Systemic Anaphylaxis.
Mice were sensitized with intravenous anti-DNP IgE as described previously (13). They were subsequently pseudochallenged by intravenous injection of saline or challenged with either DNP-HSA or 200 μg anti-IgE for 1.5 min, killed with CO2, and blood was obtained immediately by cardiac puncture. Serum histamine levels were assayed using a competitive histamine immunoassay.
Results
Introduction of LAT Mutants into LAT-deficient BMMCs by Retroviral Infection.
We demonstrated previously that LAT was essential for FcɛRI-mediated mast cell activation (13). Mast cell activation after FcɛRI engagement was significantly reduced in LAT-deficient BMMCs. Tyrosine phosphorylation of PLC-γ1, PLC-γ2, and SLP-76, calcium mobilization, degranulation, and cytokine expression were significantly reduced in LAT-deficient mast cells. As shown in Fig. 1 A, the cytosolic domain of LAT contains nine tyrosines. Of the four distal tyrosines, tyrosine 136 (YLVV) is in a potential PLC-γ1–binding motif and tyrosines 175 (YVNV), 195 (YVNV), and 235 (YENL) form classical Grb2-binding motifs (YXN). In T cells, these four distal tyrosines are critical for TCR signaling and T cell differentiation in the thymus (10, 18). To investigate the role of the tyrosines of LAT in mast cells, various LAT mutants were introduced into a Moloney murine leukemia virus vector, pMX-puro (Fig. 1 B; reference 19). This retroviral vector was designed for the bicistronic expression of two products. The first is LAT wild type (LAT-WT) or mutant forms of LAT containing tyrosine to phenylalanine mutations. The second, used as a surface marker to detect successfully infected BMMCs, is truncated rat CD2 containing normal extracellular and transmembrane domains and 3 amino acids of the cytoplasmic domain (15). The encephalomyocarditis virus IRES was used to ensure bicistronic expression. In preliminary experiments, the cDNA of LAT-WT was inserted upstream and the cDNA of truncated rat CD2 was inserted downstream of IRES. With this construct, the infected BMMCs expressed LAT at levels ∼10-fold higher than that found in normal LAT+/+ BMMCs as determined by flow cytometry and blotting experiments using the anti-LAT antibody (unpublished data). We reversed the order of the two DNAs in the vector. The cDNA of LAT was inserted downstream of the IRES and the cDNA for truncated rat CD2 was inserted upstream of the IRES in an attempt to induce lower expression of LAT molecules, because IRES-dependent translation is generally less efficient (20). This change resulted in LAT expression level in the infected BMMCs that was about twofold higher than that expressed in normal LAT+/+ BMMCs (see Fig. 2, C and D). Different LAT cDNAs with specific mutations shown previously to effect T cell activation were inserted downstream of the IRES (10). One cDNA contained the single replacement of the potential PLC-γ1–binding site, Tyr 136 (LAT-1YF). The second cDNA contained a double replacement of both tyrosines 175 and 195, which in the sequence context YVNV both form Grb2-binding sites (LAT-2YF). Mutation of these two tyrosines plus Tyr 235, which also is part of a potential Grb2-binding site (YENL), created the third cDNA (LAT-3YF). Finally, the mutation of all four of these tyrosines was made and inserted (LAT-4YF; Fig. 1 B).
Figure 2.
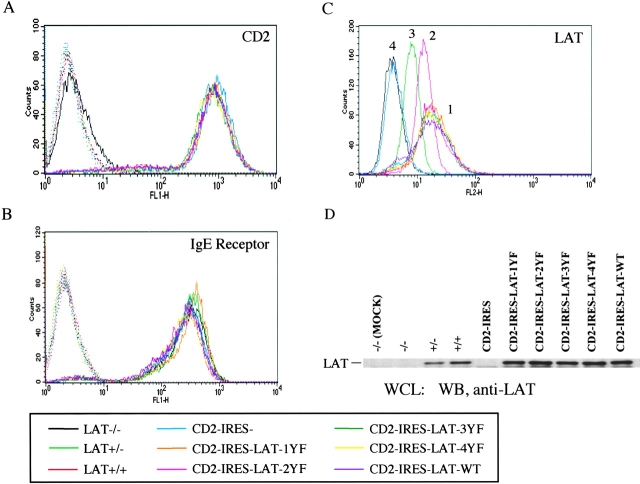
Surface expression level of rat CD2, IgE receptor, and LAT expression level in BMMCs from LAT+/+, LAT+/−, and LAT−/− mice and in the LAT−/− BMMCs infected with retrovirus. (A) Rat CD2 expression level. BMMCs were treated with FITC-conjugated anti–rat CD2 (continuous line) or FITC-conjugated IgG as negative control (dotted line). The rat CD2 expression level on noninfected LAT−/− BMMCs was basal level (continuous line). (B) IgE receptor expression level. BMMCs were treated with anti-DNP IgE mAb after anti-CD16/CD32 treatment, and incubated with FITC-conjugated rat anti–mouse IgE. Control staining was with FITC-conjugated rat anti–mouse IgE alone (dotted line). (C) BMMCs were fixed with 3.7% paraformaldehyde for 30 min and treated with 0.1% Triton X-100 for 4 min. After blocking with 2% goat serum, BMMCs were stained with anti-LAT antiserum and phycoerythrin-conjugated goat anti–rabbit antibody. LAT expression level in BMMCs was divided into four different peaks. Peak 1 shows LAT expression level of the infected CD2-IRES-LAT-1YF, 2YF, 3YF, 4YF, and WT BMMCs. Peak 2 shows LAT expression level of LAT+/+ BMMCs. Peak 3 shows LAT expression level of LAT+/− BMMCs. Peak 4 shows LAT expression level of LAT−/− BMMCs and cells expressing CD2-IRES. (D) Proteins in whole cell lysates from 2 × 105 BMMCs were separated by SDS-PAGE, and immunoblotted with anti-LAT antiserum.
These retroviral constructs as well as a construct lacking any LAT cDNA were individually transfected into mixed cultures of two retrovirus producing cell lines, Bosc 23 and Phoenix E. 16 h later, these transfected cells were mixed with LAT−/− BMMCs cultured for 12 d in medium containing IL-3. After 48 h of this combined culture, the infection efficiency, determined by expression of CD2 was >20%, an efficiency greater than when Bosc 23 or Phoenix E cells were used separately, or when culture supernatants were used. After puromycin selection for >2 wk, the infected LAT−/− BMMCs were stained with anti–rat CD2 antibody. Almost 100% of infected BMMCs expressed rat CD2 at the same level, regardless of which construct was used (Fig. 2 A). The cells were also assayed for FcɛRI expression levels, and receptor levels on the BMMCs after retroviral expression was identical to that of either LAT+/+ or LAT−/− BMMCs (Fig. 2 B). Cells from each of the infected BMMCs contained almost equivalent numbers of toluidine-positive granules, indicating normal mast cell morphology (unpublished data). LAT expression levels in the infected LAT−/− BMMCs were assayed by flow cytometry and blotting with the anti-LAT antibody. Both approaches demonstrated that LAT expression levels in all of the infected BMMCs was about twofold higher than that in LAT+/+ BMMCs (Fig. 2, C and D). Infected LAT−/− BMMCs were generated repeatedly with the same level of CD2 and LAT expression and were used for biochemical and functional analyses of mast cells after FcɛRI engagement.
Tyrosine Phosphorylation of LAT after FcɛRI Engagement.
Tyrosine phosphorylation of LAT is critical for LAT function. To analyze whether various tyrosine mutations of LAT molecules expressed in infected LAT−/− BMMCs could be tyrosine-phosphorylated after FcɛRI engagement, the infected BMMCs were sensitized by incubation with monoclonal anti-DNP IgE and were stimulated with 100 ng/ml DNP-HSA for 1 min. Tyrosine phosphorylation of LAT was maximal at 30 s–2 min after stimulation (13). Wild-type and mutant LAT molecules were immunoprecipitated with anti-LAT antibody, and tyrosine phosphorylation was detected by antiphosphotyrosine blotting. As shown in Fig. 3 A, the LAT-WT molecule expressed in infected LAT−/− BMMCs was phosphorylated at a level similar to endogenous LAT phosphorylation in LAT+/+ BMMCs. Mutation of Y136 (LAT-1YF) had a minimal effect on phosphorylation, though consistently levels of phosphorylation were less than with LAT-WT. Phosphorylation of the LAT-2YF molecule was decreased, and mutation of all three Grb2-binding sites had a dramatic effect on the level of phosphorylation. Finally, mutation of all four distal tyrosines, LAT-4YF, almost entirely inhibited phosphorylation of the molecule. The results demonstrate that the four distal tyrosines are the major phosphorylation sites of LAT in mast cells.
Figure 3.
Protein tyrosine phosphorylation of LAT, PLC-γ1, PLC-γ2, and SLP-76 in BMMCs after antigen stimulation. BMMCs were sensitized with 1 μg/ml anti-DNP IgE and stimulated with 100 ng/ml DNP-HSA for 1 min. Lysates (2–4 × 107 cells) were immunoprecipitated with anti-LAT (A), anti–PLC-γ1 (B), anti–PLC-γ2 (C), anti–SLP-76 (D). Immunoprecipitated proteins were resolved by electrophoresis and transferred to nitrocellulose membranes. (A) Tyrosine phosphorylation of LAT and Grb2 association with LAT. After LAT immunoprecipitation, the membrane was immunoblotted with antiphosphotyrosine and anti-Grb2 antibody, and after stripping was reblotted with anti-LAT antiserum. (B) Tyrosine phosphorylation of PLC-γ1 and association of PLC-γ1 and LAT. After PLC-γ1 immunoprecipitation, the membrane was immunoblotted with antiphosphotyrosine, and after stripping, it was reblotted with anti–PLC-γ1. (C) Tyrosine phosphorylation of PLC-γ2 and association PLC-γ2 and LAT. After PLC-γ2 immunoprecipitation, the membrane was immunoblotted with antiphosphotyrosine, and after stripping, it was reblotted with anti–PLC-γ2. (D) Tyrosine phosphorylation of SLP-76. After SLP-76 immunoprecipitation the membrane filter was immunoblotted with antiphosphotyrosine, and after stripping, it was reblotted with anti–SLP-76. Representative data from three or four independent experiments are shown.
Association of Grb2 with LAT.
Phosphorylated LAT is associated with multiple signaling molecules, including the adaptor molecule Grb2. LAT has several potential Grb2-binding sites as described in Fig. 1. To analyze the Grb2 association with LAT in the reconstituted LAT−/− BMMCs, activated cells were lysed, WT or mutant LAT molecules were immunoprecipitated with anti-LAT antibody, and Grb2 association was detected by anti-Grb2 blotting. As shown in Fig. 3 A, the level of association between the LAT-WT molecule expressed in LAT−/− BMMCs and Grb2 was comparable to that of endogenous LAT and Grb2 in activated LAT+/+ BMMCs. The amount of Grb2 associated with LAT-1YF was actually higher than that observed with the LAT-WT and Grb2 association. The association of Grb2 with LAT-2YF was markedly depressed, and neither LAT-3YF nor LAT-4YF showed any interaction with Grb2. Reciprocal Grb2 immunoprecipitation experiments supported these results. LAT-WT and LAT-1YF molecules were coprecipitated with the anti-Grb2 antibody from activated cells. However, in this experiment, LAT-2YF did not coprecipitate with Grb2 (unpublished data). These results all demonstrate that the predicted Grb2-binding sites, tyrosines 175, 195, and 235 are, in fact, essential for Grb2 and LAT association.
PLC-γ1– and Grb2-binding Binding to LAT Are Essential for the Tyrosine Phosphorylation of PLC-γ1 and PLC-γ2.
Previously, we demonstrated that LAT is essential for optimal tyrosine phosphorylation, activation of PLC-γ1 and PLC-γ2, and, thus, for calcium mobilization (13). To explore which tyrosine residues in LAT bind PLC-γ1 and PLC-γ2, these proteins were immunoprecipitated from activated, reconstituted LAT−/− BMMCs with anti–PLC-γ1 and anti–PLC-γ2 antibodies. As shown in Fig. 3 (B and C), tyrosine phosphorylation of PLC-γ1 and PLC-γ2 was detected by antiphosphotyrosine blotting and LAT association was determined. PLC-γ1 was tyrosine phosphorylated in cells expressing LAT-WT after stimulation. Tyrosine phosphorylation of PLC-γ1 in infected BMMCs expressing LAT-2YF was decreased by ∼50%. Tyrosine phosphorylation of PLC-γ1 in cells expressing LAT-1YF, LAT-3YF, or LAT-4YF was detected at a level comparable to cells not expressing any LAT (CD2 only) or LAT−/− BMMC. In this experiment, LAT coprecipitation with PLC-γ1 was difficult to detect and was only seen in cells expressing LAT-WT or LAT-2YF. The subtle defect in PLC phosphorylation in the 2YF, which might be more impressive were it not for the twofold-higher level of protein expression (Fig. 2 C) and the more complete block in the 3YF, indicates that, in addition to the predicted PLC-γ site at Y136, the Grb2-binding sites in LAT are also essential for PLC-γ1. In similar experiments, LAT coprecipitation with PLC-γ2 was difficult to detect (unpublished data). However, results qualitatively similar to those seen with PLC-γ1 were seen when tyrosine phosphorylation of PLC-γ2 was measured in cells expressing the various LAT mutants. From these results, it is clear that phosphorylation, and, thus, activation of both PLC isoforms is dependent on the same LAT tyrosine residues.
That the association of PLC isoforms with LAT also depends on Grb2-binding sites may reflect the observations made in T cell systems that the adaptor SLP-76 is also required for LAT interaction with PLC. SLP-76 is brought to LAT via the interaction of Gads, a Grb2-related protein (21, 22). Gads-binding sites on LAT include, at least, Tyr 175 and 195 (10). In this model, cooperative binding events occur and PLC binds LAT both directly and indirectly via SLP-76 (23). In a previous work, we reported that phosphorylation of SLP-76 was decreased in LAT−/− BMMCs after FcɛRI engagement (13). To demonstrate SLP-76 phosphorylation in the infected BMMCs, SLP-76 was immunoprecipitated with anti–SLP-76 antibody after activation. We observed a robust phosphorylation of SLP-76 molecule in LAT-WT expressing infected BMMCs after 1-min stimulation (Fig. 3 D). Loss of two Grb2 and Gads-binding sites in LAT-2YF cells resulted in a 50–55% decrease in SLP-76 phosphorylation (specific activity of phosphorylation), and loss of all three Grb2 and Gads-binding sites in LAT-3YF cells resulted in a decrease of SLP-76 tyrosine phosphorylation of ∼87%. Removal of the PLC-binding site also had an effect. SLP-76 phosphorylation decreased by ∼70% in LAT-1YF cells compared with that in LAT-WT expressing BMMCs. Removal of all four distal tyrosines resulted in nearly complete loss of SLP-76 tyrosine phosphorylation in LAT-4YF and CD2 only expressing infected LAT−/− BMMCs cells. These results are compatible with the aforementioned idea of cooperative binding.
The Role of LAT Tyrosines in Calcium Mobilization and Degranulation.
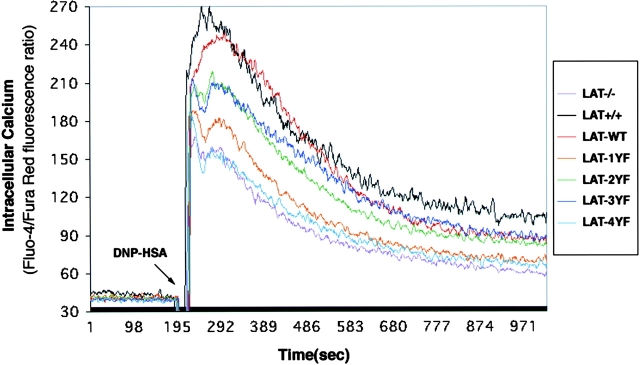
Elevation of intracellular calcium is a well-known consequence of FcɛRI stimulation. To test the effect of LAT mutations on calcium mobilization in mast cells after FcɛRI stimulation, the reconstituted BMMCs were loaded with the calcium-sensitive dyes Fluo-4–AM and Fura red-AM. The data shown in Fig. 4 compare the calcium response of each reconstituted cell type compared with LAT+/+ and LAT−/− BMMCs. It should be noted that, as demonstrated previously, LAT−/− BMMCs show a modest calcium response. Reconstitution of LAT−/− BMMCs with LAT-WT restored virtually normal calcium responses. Expression of LAT-2YF and LAT-3YF resulted in a partial calcium response. Cells expressing the point mutation at Y136 demonstrated a much lower calcium response. Loss of all four distal tyrosines had a major inhibitory effect, and the resulting calcium response was comparable to the LAT−/− cells and those expressing only the control CD2 marker (unpublished data). In this experiment, the calcium responses after 1-μM thapsigargin stimulation were comparable in all cells (unpublished data). The responses to FcɛRI aggregation support the conclusion that the Y136 site is most important for the calcium response, but a supportive role is played by the three other distal tyrosines. These functional results are consistent with aforementioned evidence revealing the cooperative interactions required for PLC binding and phosphorylation.
Figure 4.
Ca2+ mobilization in BMMCs after antigen stimulation. BMMCs were sensitized with 1 μg/ml anti-DNP IgE for 6 h and loaded with Fluo-4–AM and Fura red–AM. BMMCs were stimulated with 100 ng/ml DNP-HSA at the indicated time. Fluo-4 and Fura red fluorescence intensity after antigen stimulation was measured by flow cytometry. Intracellular Ca concentration is indicated by the ratio of Fluo-4/Fura red fluorescence intensity. Representative data from five independent experiments are shown.
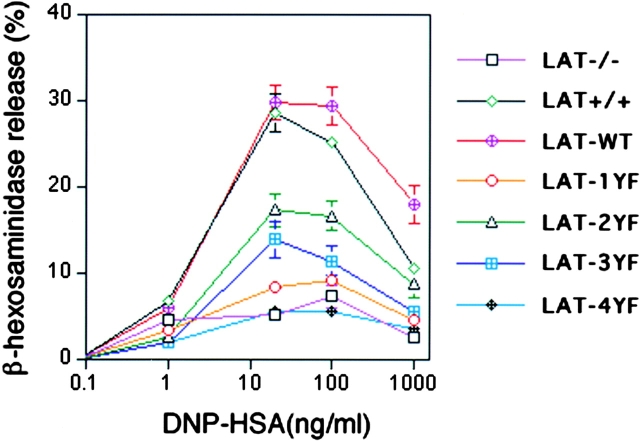
Continuing the functional analysis of the cells expressing LAT mutations, we analyzed mast cell degranulation using the β-hexosaminidase release assay (Fig. 5) . Again, for the various reconstituted cells, degranulation was compared with LAT+/+ and LAT−/− BMMCs. LAT-WT fully reconstituted LAT−/− BMMCs. The degranulation of cells expressing LAT-2YF and LAT-3YF was partially inhibited. In contrast the LAT-1YF, LAT-4YF and control cells (unpublished data) released β-hexosaminidase at the same level as LAT−/− BMMCs. Thapsigargin stimulation–induced degranulation was comparable in all infected BMMCs and noninfected BMMCs (unpublished data). Consistently in the assays described in Figs. 3 and 4, the loss of the four distal tyrosines ablates a response. In degranulation, the loss of the primary PLC-binding site alone is equally destructive.
Figure 5.
The level of degranulation was determined by measuring the release of β-hexosaminidase. 2 × 106/ml BMMCs were sensitized for 6 h with 1 μg/ml anti-DNP IgE in medium (without IL-3), and stimulated with the indicated concentration of DNP-HSA or 1 mM thapsigargin for 7 min in Tyrode's buffer. β-Hexosaminidase enzymatic activity was measured in supernatants and cell pellets solubilized with 0.5% Triton X-100 in Tyrode's buffer. Total percentage of the β-hexosaminidase release was calculated by dividing absorbance in the supernatant by the total absorbance in the supernatants plus cell pellets. Standard errors reflect three experiments. Spontaneous release ranged from 8.7 to 12%.
Regulation of MAP Kinases via the Four Distal Tyrosines of LAT.
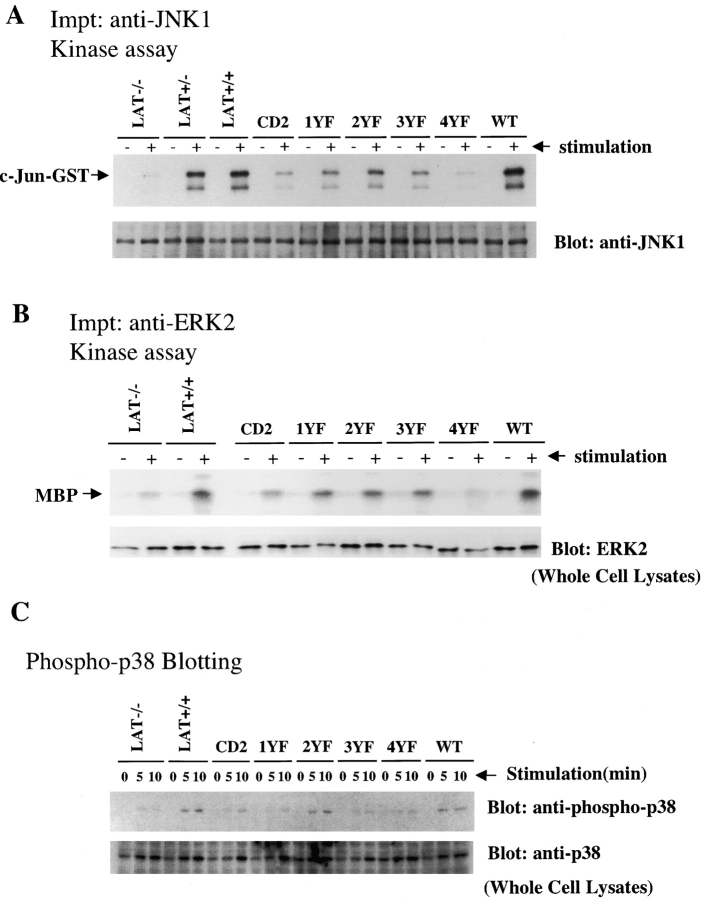
MAP kinase activation is central to transcriptional activation of cytokines and chemokines in mast cells. We reported previously that mast cells deficient in LAT demonstrated impaired ERK and JNK activation (13). JNK1 activation in the reconstituted BMMCs was measured by in vitro kinase assay using c-Jun–GST as a substrate. As shown in Fig. 6 A, JNK1 activation in BMMCs expressing LAT-WT was robust and comparable or greater than in LAT+/+ BMMCs. JNK1 activation was comparable in cells expressing LAT-4YF and LAT−/− BMMCs. JNK1 activation was not fully reconstituted by expression of any of the four mutant LAT constructs. JNK1 activation in cells expressing LAT-2YF was decreased by 55–60% compared with those cells with LAT-WT. JNK1 activation in cells expressing LAT-1YF and LAT-3YF was decreased by ∼78%. JNK1 activation in BMMCs with LAT-4YF was decreased by 90%. These results demonstrate that the four distal tyrosines are critical for JNK1 activation and that both the Y136-binding site and the three Grb2-binding sites are essential for JNK1 activation after FcɛRI engagement.
Figure 6.
MAP kinase activity in BMMCs after antigen stimulation. (A and B) ERK and JNK activities in BMMCs after antigen stimulation. BMMCs were sensitized with 1 μg/ml anti-DNP IgE and stimulated with 100 ng/ml DNP-HSA for 10 min. (A) JNK1 kinase activity was measured by in vitro kinase assay. (top) An autoradiograph of 32P-incorporated into c-Jun–GST after its phosphorylation by JNK1 immunoprecipitates from BMMCs. The same filter was probed with an anti-JNK1 antibody. (bottom) The JNK1 protein amount is shown. (B) ERK2 kinase activity was measured by in vitro kinase assay. (top) An autoradiograph of 32P-incorporated into myelin basic protein (MBP) after its phosphorylation by ERK2 immunoprecipitates from BMMCs. (bottom) The amount of ERK2 protein in the same cell lysates by anti-ERK2 immunoblot. (C) p38 phosphorylation in BMMCs after antigen stimulation. BMMCs were sensitized with 1 μg/ml anti-DNP IgE and stimulated with 100 ng/ml DNP-HSA for 5 and 10 min. Proteins in cell lysates from 2 × 105 BMMCs were separated by SDS-PAGE, and were transferred to nitrocellulose membrane filters. The membrane was immunoblotted with anti–phospho-p38 antibody. After stripping, the same membrane was immunoblotted with anti-p38. Representative data from three independent experiments are shown.
To detect ERK activity in the BMMCs, ERK2 was immunoprecipitated from LAT−/− BMMCs, LAT+/+ BMMCs and the reconstituted BMMCs. ERK2 activity was measured by in vitro kinase assay using MBP as a substrate (Fig. 6 B). LAT-WT reconstituted the LAT−/− cells. Reconstitution with the 1YF, 2YF, or 3YF constructs was partial. Expression of the LAT-4YF construct failed to reconstitute ERK2 activation to a level higher than seen in control reconstituted cells. As shown in Fig. 6 C, activated p38 was detected by blotting lysates with a specific anti-phospho–p38 antibody. An increase in phosphorylation of the p38 kinase was observed in activated LAT+/+ BMMCs as well as BMMCs expressing LAT-2YF and LAT-WT. However p38 MAP kinase phosphorylation was decreased significantly in LAT−/− BMMCs, and LAT−/− cells expressing only CD2, LAT-1YF, LAT-3YF, or LAT-4YF. Thus, for all the tested MAP kinases, complete reconstitution requires all four distal LAT tyrosine residues.
Cytokine and Chemokine Expression Are Regulated by the Predicted PLC-γ1– and Grb2-binding Sites in the Distal Four Tyrosines of LAT.
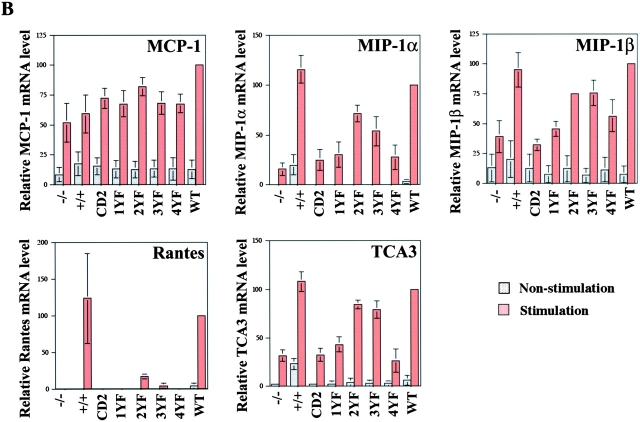
To detect cytokine and chemokine mRNA expression after FcɛRI engagement, BMMCs were stimulated with 100 ng/ml DNP-HSA for 60 min. RNA purified from the BMMCs was analyzed by RT-PCR for IL-2 detection or by RNA protection assay using specific primers or probes. Seven cytokines (IL-1β, IL-2, IL-6, IL-13, LIF, M-CSF, and TNF-α) and five chemokines (MCP-1, MIP-1α, MIP-1β, RANTES, and TCA3) were evaluated (Fig. 7) . By comparing the response of LAT−/− cells reconstituted with WT or the four mutant forms of LAT, three qualitative patterns of response were detected. For all but two of the products (IL-1β and MCP-1), a similar hierarchy of response was observed. For all of these chemokines and cytokines, the analysis revealed the same hierarchy of mutant effects: 2YF < 3YF < 1YF < 4YF. The effect of the loss of two or three of the Grb2-binding sites was less than the effect of losing Y136. The loss of all four distal tyrosines was most pronounced, and was roughly comparable to the LAT−/− cells. Within this order of response however, the actual level of activity varied from nearly complete LAT dependence (RANTES and IL-2) to examples in which the response was nearly half that of the LAT+/+ cells (MIP-1α and TCA3).
Figure 7.
Expression of cytokine and chemokine mRNAs after antigen stimulation in BMMCs. (A) 2 × 106 BMMCs were sensitized with 1 μg/ml anti-DNP IgE and stimulated with 100 ng/ml DNP-HSA for 1 h. mRNA was purified from BMMCs, and cytokine mRNAs, except for IL-2, were detected by RNA protection assay using specific primers or template sets for each cytokine. IL-2 results were obtained by RT-PCR. (B) Chemokine mRNAs were also detected with RNA protection assay. Standard errors in RNA protection assays reflect three to five experiments.
The analysis of IL-1β production showed a slightly different phenotype. In this case the effects of the 2YF and 3YF mutations was greater than that of the 1YF, suggesting more of a role for pathways coupled to Grb2 or related molecules compared with PLC. Nonetheless, expression of the 4YF mutation did bring the level of cytokine production down to that produced by the LAT−/− cells. Finally, analysis of MCP-1 production revealed virtually no role for LAT. There was no difference in expression between the LAT−/− and LAT+/+ cells, and, thus, the expression of the various LAT mutations did not affect levels of MCP-1 production.
Passive Systemic Anaphylaxis in Mice with LAT Mutations.
The preceding series of experiments provide further evidence of the function of LAT in various signaling cascades and cellular responses in BMMC. Reconstitution with the various LAT mutants confirm the importance of these sites in the signal transduction process. To analyze the function of these LAT tyrosine residues in the intact animal, we used an assay for passive systemic anaphylaxis. Normal mice were pretreated with monoclonal anti-DNP IgE and challenged with polyvalent DNP-HSA to aggregate mast cell FcɛRI. A massive histamine release can be measured minutes after antigen delivery. In a previous paper (13), we showed that LAT-deficient mast cells failed to release histamine under these conditions. In the current analysis, the wild-type response was compared with two mice in which LAT mutations that showed the most pronounced defect in PLC binding replaced the wild-type LAT allele (18, 24). Mice lacking all four distal LAT tyrosines showed a marked decrease in histamine release after administration (Fig. 8) . Mice expressing only LAT protein with the Y136F (1YF) mutation showed no measurable histamine release.
Figure 8.
Mice were sensitized and challenged as described in Materials and Methods. The serum histamine levels of four or six mice of the indicated genotype either pseudochallenged or challenged with DNP-HSA or anti-IgE is shown. The median responses are indicated by the horizontal lines.
Mice expressing the Y136F mutation exhibit a lymphoproliferative disorder characterized by expansion of CD4+ T cells that elaborate Th2 lymphokines (24). One of many consequences is that levels of circulating IgE are very high. Such IgE could saturate mast cell FcɛRI, thereby blocking the binding of the anti-DNP IgE and preventing mast cell activation via multivalent antigen. To bypass that possibility, WT and 1YF mice were infused with IgE and treated with anti-IgE as a cross-linking reagent. Mast cells from anti-IgE–treated WT mice release histamine at levels comparable to antigen-treated mice. In contrast, cross-linked mast cells in the 1YF mice fail to release significant amounts of histamine. This result demonstrates the critical function of the LAT Y136 residue in vivo.
Discussion
Aggregation of the FcɛR1 leads to activation of multiple PTKs and subsequent phosphorylation of adaptor molecules capable of recruiting multiple signaling molecules (1, 25, 26). Much effort has been directed at characterization of one such pathway dependent on Lyn, Syk, and the phosphorylation of the adaptor molecules, LAT and SLP-76 (4, 13, 27, 28). The significance of this pathway has been confirmed by the significant defects in mast cell activation that follows genetic disruption of genes encoding these molecules (7, 13, 29, 30). However, a number of papers demonstrate that loss of one of these signaling molecules does not ablate mast cell signaling completely. As an example, our previous work documenting the essential role of LAT in mast cell signaling still provided evidence that LAT−/− mast cells had partial, residual function (13). Recent analyses provide evidence for an alternative signaling pathway in mast cells. The adaptor molecule Gab2 has been shown to be critical to mast signaling due to its ability to recruit PI-3 kinase (31). Gab2 phosphorylation and, thus, its ability to recruit additional molecules appears to be dependent on the Fyn PTK (32). Our current studies focus primarily on LAT-associated pathways, which we demonstrate to be complex and interactive.
Previous studies in T cells demonstrated that all four distal tyrosines of LAT were necessary for optimal signaling (10). These studies, performed in LAT-deficient Jurkat cells, showed that cells reconstituted with a mutant lacking these four residues failed to signal at all. The work also confirmed that the distal three tyrosine residues bind Grb2 and that residues homologous to Tyr 175 and 195 are the primary sites of Gads–SLP-76 binding. PLC-γ1 binding and activation in Jurkat cells was primarily dependent on the site homologous to the murine Y136 site, but both binding and activation were decreased in cells reconstituted with the LAT-3YF mutation. LAT mutations have also been introduced into the murine genome, and the subsequent effects on T cell development, also a LAT-dependent process, have been studied. No T cell development occurs in LAT−/− mice nor in mice expressing only the LAT 4YF mutation (14, 18). Thymocytes of mice expressing only the LAT-1YF mutation are defective in their ability to flux calcium. These mice develop an interesting lymphoproliferative and autoimmune phenotype (24, 33).
The current studies were initiated in an attempt to further define the role of LAT in mast cell function. LAT-dependent binding and signaling were assayed in mast cells derived from WT or LAT−/− mice or from LAT−/− mast cells reconstituted using retroviral vectors with one of four LAT mutants. Coimmunoprecipitation studies were used to test the effects of these mutations. The detection of a coimmunoprecipitated protein was difficult, and in our experiments, presence of the more easily detected phosphoprotein was used as evidence for binding. In coprecipitation studies, loss of the Y136 site had no effect on Grb2 binding, as expected. However, the other binding studies showed some indication of more complex interactions. Coprecipitation with LAT of phosphorylated forms of two PLC isoforms and SLP-76 was affected both by loss of the Y136 site as well as loss of the three Grb2-binding sites. The loss of all four tyrosines resulted in a further decrease in coprecipitated phosphorylated protein. Evidence that multiple tyrosines on LAT regulate binding of PLC and SLP-76 is consistent with evidence in T cells that binding of Gads, SLP-76, and PLCγ is highly cooperative (23). In this model, both Gads and PLC bind LAT directly and additional evidence for SLP-76/PLCγ-binding has been provided.
Our functional experiments provide further support for the role of protein interaction at LAT. Both calcium flux, which is dependent on PLC binding and activation, as well as degranulation are highly dependent on LAT. No reconstitution of the response in LAT−/−mast cells is shown when cell expressing LAT-4YF were tested, indicating that the role of LAT in these functions is wholly dependent on these tyrosines. Partial reconstitution is observed with the other LAT mutants (1YF, 2YF, and 3YF). The calcium response is more dependent on Y136, but loss of the Grb2- or Gads-binding sites has an effect supporting the aforementioned model of complex interactions. Similar results are found with degranulation, though the 1YF mutation was essentially identical to LAT−/− and 4YF mutants. These data may reflect the added complexity that degranulation is dependent both on calcium flux as well as other signaling pathways. It is possible that ERK is coupled to LAT by virtue of Grb2 recruiting the activator SOS, but as is true in T cells, additional Ras GEFs such as RasGRP may be involved (34–37). JNK and p38 are also known to be activated by a cascade of proximal serine/threonine kinases, but the actual means of coupling these cascades to LAT is not known. Regardless, the LAT dependence of all of these pathways also seems complex and similar to the pathways described in Figs. 3–5. All the LAT-4YF is most strongly inhibited whereas the other mutants provide only partial reconstitution.
We demonstrated recently that weak stimulation of FcɛRI caused a stronger induction of Fyn/Gab2- versus LAT-mediated signaling and skewed the lymphokine response toward a more potent chemokine response (38). Analysis, under weak stimulation, of mast cells from gene-inactivated mice showed a difference in the relative importance of Gab2 or LAT for chemokines or cytokines, respectively. This difference was narrowed when the stimulus strength was increased. However, consistent with the present results, MCP-1 mRNA was relatively unaffected by LAT deficiency, regardless of stimulus strength. In contrast, Gab2 was required for a normal MCP-1 response. The transcriptional events we measured in the present work demonstrate still further complexity. Evidence for at least partial LAT dependence is observed for nearly all lymphokines when a strong stimulus is used. Again, wherever a role for LAT was shown, the 4YF mutation ablated all LAT-dependent effects. The other three mutations had less effect. These results offer further evidence for the idea that multiple pathways converge at LAT and that these interactions are needed for optimal response.
BMMCs were used as a means to study the effects of LAT tyrosine mutations on signaling and cellular events that follow FcɛRI engagement. As noted, these tyrosine residues are critical to these pathways and to mast cell activation. However, it is difficult to judge the significance of some of the quantitative differences between cells expressing the various sets of mutations. To more critically evaluate the function of the distal four tyrosines and the Y136F mutation, in particular, we decided to determine the effect of these mutations on in vivo histamine release. Previous work had revealed the requirement for LAT in this pathophysiologic response (13). The experiments described in this paper indicate that the mice bearing either the 4YF or the 1YF LAT mutations are severely compromised in the passive systemic anaphylaxis response. In view of the high level of circulating IgE observed in the 1YF mouse, anti-IgE cross-linking was used as a means of bypassing potentially saturated receptors. The response was the same under these conditions. In view of the fact that these mice are systemically ill, a reasonable caveat for this result is that there may be other unknown factors contributing to the complete absence of response. The low level of histamine release in some of the 4YF mice does suggest that there may be some compensatory mechanism in these mice, but not in the totally LAT-deficient mice that allow for some response.
In addition to our work with LAT-deficient BMMCs, works on the function of BMMCs deficient in SLP-76, Vav, and Gab2 have been published. The phenotype of the LAT- and SLPL-76–deficient mast cells are nearly identical, suggesting, as suspected, that these molecules lie in the same pathway (13, 30). In Vav-deficient cells, most responses are similar to LAT-deficient cells, but degranulation, systemic anaphylaxis, and IL-3 expression were not as severely compromised in Vav-deficient cells (39). TNFα expression and ERK activation were not significantly affected and PI3 kinase activation was more affected in the Vav-deficient cells. The general similarity of response in these cells suggests that Vav and LAT are in the same pathway for many responses but are independent for others. Some of the loss of Vav effects are also observed in Gab2-deficient cells, which differ most notably from LAT-deficient cells in that Gab2 is required for a PI3 kinase response whereas loss of LAT has no effect on the PI3 kinase pathway (31). JNK and p38 kinases are more adversely affected by loss of Gab2 than by loss of LAT. The results from these various studies indicate that several pathways are coupled to the FcɛR1. These include Gab2- and LAT-coupled responses (32). Additionally, our current work demonstrates the interaction of pathways coupled via different tyrosine residues on LAT. More detailed understanding of these various pathways will require careful analysis of BMMCs designed to be deficient in multiple adapters and signaling molecules.
Acknowledgments
The authors thank S. Krebs and M. Sanford for cytokine and chemokine analysis.
S. Saitoh was a fellow of the Japan Society for the Promotion of Science.
Abbreviations used in this paper: BMMC, bone marrow–derived mast cells; HRP, horseradish peroxidase; IRES, internal ribosomal entry site; ITAM, immunoreceptor tyrosine-based activation motif; LAT, linker for activation of T cells; MAP, mitogen-activated protein; MBP, myelin basic protein; PLC, phospholipase C; PTK, protein tyrosine kinase; RANTES, regulated on activation, normal T cell–expressed and secreted; WT, wild type.
References
- 1.Benhamou, M., J.S. Gutkind, K.C. Robbins, and R.P. Siraganian. 1990. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc. Natl. Acad. Sci. USA. 87:5327–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank, U., C. Ra, L. Miller, K. White, H. Metzger, and J.P. Kinet. 1989. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 337:187–189. [DOI] [PubMed] [Google Scholar]
- 3.Reth, M. 1989. Antigen receptor tail clue. Nature. 338:383–384. [PubMed] [Google Scholar]
- 4.Eiseman, E., and J.B. Bolen. 1992. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 355:78–80. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita, T., S.Y. Mao, and H. Metzger. 1994. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc. Natl. Acad. Sci. USA. 91:11251–11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouvin, M.H., M. Adamczewski, R. Numerof, O. Letourneur, A. Valle, and J.P. Kinet. 1994. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J. Biol. Chem. 269:5918–5925. [PubMed] [Google Scholar]
- 7.Costello, P.S., M. Turner, A.E. Walters, C.N. Cunningham, P.H. Bauer, J. Downward, and V.L. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 13:2595–2605. [PubMed] [Google Scholar]
- 8.Zhang, J., E.H. Berenstein, R.L. Evans, and R.P. Siraganian. 1996. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 184:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R.P. Trible, and L.E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 92:83–92. [DOI] [PubMed] [Google Scholar]
- 10.Zhang, W., R.P. Trible, M. Zhu, S.K. Liu, C.J. McGlade, and L.E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355–23361. [DOI] [PubMed] [Google Scholar]
- 11.Paz, P.E., S. Wang, H. Clarke, X. Lu, D. Stokoe, and A. Abo. 2001. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 356:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finco, T.S., T. Kadlecek, W. Zhang, L.E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 9:617–626. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh, S., R. Arudchandran, T.S. Manetz, W. Zhang, C.L. Sommers, P.E. Love, J. Rivera, and L.E. Samelson. 2000. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 12:525–535. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, W., C.L. Sommers, D.N. Burshtyn, C.C. Stebbins, J.B. DeJarnette, R.P. Trible, A. Grinberg, H.C. Tsay, H.M. Jacobs, C.M. Kessler, et al. 1999. Essential role of LAT in T cell development. Immunity. 10:323–332. [DOI] [PubMed] [Google Scholar]
- 15.Hozumi, K., R. Ohtsuka, D. Suzuki, K. Ando, M. Ito, T. Nishimura, M. Merkenschlager, and S. Habu. 2000. Establishment of efficient reaggregation culture system for gene transfection into immature T cells by retroviral vectors. Immunol. Lett. 71:61–66. [DOI] [PubMed] [Google Scholar]
- 16.Pear, W.S., G.P. Nolan, M.L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 90:8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, F.T., J.W. Bohn, E.L. Ferry, H. Yamamoto, C.A. Molinaro, L.A. Sherman, N.R. Klinman, and D.H. Katz. 1980. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J. Immunol. 124:2728–2737. [PubMed] [Google Scholar]
- 18.Sommers, C.L., R.K. Menon, A. Grinberg, W. Zhang, L.E. Samelson, and P.E. Love. 2001. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J. Exp. Med. 194:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi, M., S. Kinoshita, Y. Morikawa, A. Shibuya, J. Phillips, L.L. Lanier, D.M. Gorman, G.P. Nolan, A. Miyajima, and T. Kitamura. 1996. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24:324–329. [PubMed] [Google Scholar]
- 20.Mizuguchi, H., Z. Xu, A. Ishii-Watabe, E. Uchida, and T. Hayakawa. 2000. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 1:376–382. [DOI] [PubMed] [Google Scholar]
- 21.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, S.K., and C.J. McGlade. 1998. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene. 17:3073–3082. [DOI] [PubMed] [Google Scholar]
- 23.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol. Cell. Biol. 21:4208–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommers, C.L., C.S. Park, J. Lee, C. Feng, C.L. Fuller, A. Grinberg, J.A. Hildebrand, E. Lacana, R.K. Menon, E.W. Shores, et al. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 296:2040–2043. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami, T., N. Inagaki, M. Takei, H. Fukamachi, K.M. Coggeshall, K. Ishizaka, and T. Ishizaka. 1992. Tyrosine phosphorylation is required for mast cell activation by Fc epsilon RI cross-linking. J. Immunol. 148:3513–3519. [PubMed] [Google Scholar]
- 26.Rivera, J., R. Arudchandran, C. Gonzalez-Espinosa, T.S. Manetz, and S. Xirasagar. 2001. A perspective: regulation of IgE receptor-mediated mast cell responses by a LAT-organized plasma membrane-localized signaling complex. Int. Arch. Allergy Immunol. 124:137–141. [DOI] [PubMed] [Google Scholar]
- 27.Minoguchi, K., M. Benhamou, W.D. Swaim, Y. Kawakami, T. Kawakami, and R.P. Siraganian. 1994. Activation of protein tyrosine kinase p72syk by Fc epsilon RI aggregation in rat basophilic leukemia cells. p72syk is a minor component but the major protein tyrosine kinase of pp72. J. Biol. Chem. 269:16902–16908. [PubMed] [Google Scholar]
- 28.Hendricks-Taylor, L.R., D.G. Motto, J. Zhang, R.P. Siraganian, and G.A. Koretzky. 1997. SLP-76 is a substrate of the high affinity IgE receptor-stimulated protein tyrosine kinases in rat basophilic leukemia cells. J. Biol. Chem. 272:1363–1367. [DOI] [PubMed] [Google Scholar]
- 29.Hibbs, M.L., D.M. Tarlinton, J. Armes, D. Grail, G. Hodgson, R. Maglitto, S.A. Stacker, and A.R. Dunn. 1995. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 83:301–311. [DOI] [PubMed] [Google Scholar]
- 30.Pivniouk, V.I., T.R. Martin, J.M. Lu-Kuo, H.R. Katz, H.C. Oettgen, and R.S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Invest. 103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- 31.Gu, H., K. Saito, L.D. Klaman, J. Shen, T. Fleming, Y. Wang, J.C. Pratt, G. Lin, B. Lim, J.P. Kinet, and B.G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature. 412:186–190. [DOI] [PubMed] [Google Scholar]
- 32.Parravicini, V., M. Gadina, M. Kovarova, S. Odom, C. Gonzalez-Espinosa, Y. Furumoto, S. Saitoh, L.E. Samelson, J.J. O'Shea, and J. Rivera. 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3:741–748. [DOI] [PubMed] [Google Scholar]
- 33.Aguado, E., S. Richelme, S. Nunez-Cruz, A. Miazek, A.M. Mura, M. Richelme, X.J. Guo, D. Sainty, H.T. He, B. Malissen, and M. Malissen. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 296:2036–2040. [DOI] [PubMed] [Google Scholar]
- 34.Buday, L., and J. Downward. 1993. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 73:611–620. [DOI] [PubMed] [Google Scholar]
- 35.Egan, S.E., B.W. Giddings, M.W. Brooks, L. Buday, A.M. Sizeland, and R.A. Weinberg. 1993. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 363:45–51. [DOI] [PubMed] [Google Scholar]
- 36.Ebinu, J.O., S.L. Stang, C. Teixeira, D.A. Bottorff, J. Hooton, P.M. Blumberg, M. Barry, R.C. Bleakley, H.L. Ostergaard, and J.C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood. 95:3199–3203. [PubMed] [Google Scholar]
- 37.Yang, Y., L. Li, G.W. Wong, S.A. Krilis, M.S. Madhusudhan, A. Sali, and R.L. Stevens. 2002. RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J. Biol. Chem. 277:25756–25774. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Espinosa, C., S. Odom, A. Olivera, J. Peyton Hobson, M.E.C. Martinez, A. Oliveira-dos-Santos, L. Barra, S. Spiegel, J.M. Penninger, and J. Rivera. 2003. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J. Exp. Med. 197:1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manetz, T.S., C. Gonzalez-Espinosa, R. Arudchandran, S. Xirasagar, V. Tybulewicz, and J. Rivera. 2001. Vav1 regulates phospholipase cgamma activation and calcium responses in mast cells. Mol. Cell. Biol. 21:3763–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]