Abstract
Human macrophages found in juxtaposition to fragmented elastin in vivo express the elastolytic matrix metalloproteinases (MMPs) progelatinase B, prometalloelastase, and promatrilysin. Though MMPs can degrade a range of extracellular matrix components, increasing evidence suggests that preferred targets in vivo include nonmatrix substrates such as chemokines and growth factors. Hence, the means by which MMPs participate in elastin turnover remain undefined as does the identity of the elastolysins. Herein, human macrophage cultures have been established that express a complement of elastolytic proteinases similar, if not identical, to that found in vivo. Under plasminogen-free conditions, macrophages preferentially use metalloelastase to mediate elastolysis via a process that deposits active enzyme on elastin surfaces. By contrast, in the presence of plasminogen, human macrophages up-regulate proteolysis 10-fold by processing promatrilysin to an active elastolysin via a urokinase-type plasminogen activator-dependent pathway. Matrilysin-deficient human macrophages fail to mediate an elastolytic response despite the continued expression of gelatinase B and metalloelastase. Thus, acting in concert with cosecreted cysteine proteinases whose activities are constrained to sites of macrophage-elastin contact (Punturieri, A., S. Filippov, E. Allen, I. Caras, R. Murray, V. Reddy, and S.J. Weiss. 2000. J. Exp. Med. 192:789–799), matrilysin confers macrophages with their most potent MMP-dependent elastolytic system.
Keywords: cysteine proteinase, elastin, macrophage, matrix metalloproteinase, plasminogen
Introduction
Macrophage-derived matrix metalloproteinases (MMPs) play key roles in the elastolytic phenotypes pathognomonic of atherosclerosis, aneurysm formation, and emphysema (1–6). Based largely on studies performed in gene-deleted mice, attention has focused on the role of the MMPs, gelatinase B, and metalloelastase, as important mediators of macrophage-mediated elastin degradation in vivo (3, 7, 8). In these settings, macrophages are thought to secrete latent MMP zymogens that undergo processing to active forms after proteolytic removal of their respective propeptide domains by plasmin, a product of the plasminogen activator-plasminogen system (5, 6). However, although early reports assumed that macrophages use plasmin-activated gelatinase B or metalloelastase as direct acting elastolysins (3, 7, 8), recent studies indicate that these MMPs more likely control elastin turnover indirectly by preferentially regulating proinflammatory events in vivo as a consequence of their ability to (a) proteolyze nonextracellular matrix (ECM) targets including antiproteinases, growth factors, cytokines, chemokines, as well as cell adhesion molecules and (b) regulate the expression and activity of downstream proteolytic systems that might play a more direct role in elastin degradation (9–11).
Given an inherent inability to identify the proteinases directly involved in elastin degradation in vivo, macrophage-elastin cocultures have been used as an alternate means to characterize the elastolytic potential of specific MMPs (8–15). To date, human macrophages have not been reported to use MMPs to degrade elastin efficiently in vitro, thus supporting the contention that these cells may use this class of proteinases in an indirect fashion to support elastolytic events in vivo (9, 12–15). However, as macrophages cultured in vitro seldom recapitulate the full spectrum of elastolytic MMPs expressed in vivo, the significance of these findings has remained unclear (1–6, 12–15). Herein, we have isolated and characterized human monocyte-derived macrophages (MDMs), which express a repertoire of elastin-degrading enzymes similar, if not identical, to those found in vivo. Significantly, these cells display an MMP-dependent elastolytic phenotype hundreds of times greater than that previously reported. Moreover, despite the fact that these macrophages efficiently express gelatinase B as well as metalloelastase, neither MMP played a major role in the elastin-degradative phenotype. Instead, maximal MMP-dependent elastolytic activity was conferred by the plasmin-mediated processing of the metalloproteinase zymogen, promatrilysin, to its catalytically active form. As matrilysin-dependent elastin degradation by MDMs is further complemented by an independent system of cosecreted elastolytic cysteine proteinases (15, 16), we posit that these two proteolytic systems together define the tissue-destructive potential of human macrophages in vivo.
Materials and Methods
Macrophage Preparation.
Human peripheral blood monocytes were either adherence purified as previously described (15, 16) or isolated by negative selection using the MACS Monocyte Isolation Kit (Miltenyi Biotec; reference 17) and cultured for 9 d in RPMI 1640 (Invitrogen) supplemented with 40% autologous serum. Alternatively, monocytes were differentiated atop an ECM-derived substratum deposited by MDCK cells (18) for 9 d and then dislodged from the matrix by washing the cells in Hank's buffered salt solution (without Ca+2 and Mg+2) supplemented with 10 mM EDTA. The cells were then transferred to nontissue culture–treated plates for 24 h before assay. Control cells were treated in an identical fashion except for the fact that the 9-d differentiation process proceeded atop standard bacteriologic culture dishes.
Macrophages were isolated from gelatinase B−/− mice (129/SvEv genetic background; reference 19), metalloelastase−/− mice (mixed 129/SvEv and C57BL6 background; reference 3), or the respective wild-type littermates after the intraperitoneal injection of thioglycollate medium (Becton Dickinson). After 4 d, peritoneal macrophages were harvested, adherence purified, and allowed to further mature for 72 h in RPMI 1640 supplemented with a 1:1 mixture of 40% heat-inactivated (56°C for 30 min) fetal bovine serum (HyClone) and 40% heat-inactivated human serum.
RNA Purification, cDNA Synthesis, In Vitro Transcription, and Array Hybridization.
RNA was isolated with TRIzol reagent (GIBCO BRL) from purified nonadherent human monocytes as well as from cultured cells. Poly A+ RNA was purified with Oligotex (QIAGEN). cDNA was synthesized with a SuperScriptII cDNA synthesis kit (Invitrogen) and used as a template for in vitro transcription using the Megascript system (Ambion). cRNA was labeled and hybridized as previously described (16) using a custom-designed chip to screen for elastases. Biotinylated hybridized cRNA was developed by staining with streptavidin-PE and the arrays were scanned (16).
Determination of Plasminogen Activator and Plasmin Activity Assay.
After 9 d in culture, MDMs were cultured under serum-free conditions for 24 h, cell-free supernatants were recovered, and urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) levels were determined by ELISA (American Diagnostica, Inc.). Plasmin activity was determined in MDM cultures supplemented with 50 μg/ml human glu-type plasminogen (Calbiochem-Novabiochem) under serum-free conditions. After a 24-h incubation, aliquots of cell-free supernatant were assayed for plasmin activity using the SpectrozymePL chromogenic substrate (American Diagnostica, Inc.; reference 20). One unit of activity was defined as the amount of plasmin needed to hydrolyze 1.0 μmol of substrate per minute at 25°C, pH 7.8.
Elastin Preparation and Elastolytic Assay.
Elastin (type E60; Elastin Products Co.) was reductively labeled (800–900 cpm/μg elastin) as previously described (15, 16). Human or mouse macrophages were cocultured with [3H]elastin (8 mg total), which was placed in a transwell insert (3-μm pore size; Corning, Inc.) and suspended above the cell cultures in serum-free RPMI 1640. Alternatively, macrophage-mediated elastin degradation in the presence of cell–substrate contact was assessed by adding 8 mg [3H]elastin directly atop the cell monolayer. Where indicated, macrophage/[3H]elastin cocultures were incubated alone, with 50 μg/ml glu-type human plasminogen, 100 μg/ml mouse-neutralizing antibody against human uPA (American Diagnostica, Inc.), mouse anti–gelatinase B IgG (ab-2; reference 21), 50 μg/ml normal mouse IgG (both from Oncogene Research Products and Calbiochem-Novabiochem), recombinant human pro–MMP-7 (Chemicon International, Inc.), recombinant human pro–gelatinase B and A (R&D Systems), 5 μM of the synthetic MMP inhibitor, BB94 (0.1% DMSO final; provided by British Biotechnology; reference 22), 5 μg/ml endotoxin-free recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2; Fuji Chemical Industries, LTD.; reference 22), 100 μM the pan-specific cysteine proteinase inhibitors, E-64 (0.05% ethanol final; Sigma-Aldrich; reference 16), 100 μg/ml aprotinin (Roche; reference 22), 5 μM the aspartate protease inhibitor, pepstatin (0.05% ethanol final; Roche; reference 23), or solvents (0.1% DMSO and 0.05% ethanol) alone. At 24-h intervals, solubilized [3H]elastin was quantitated in cell-free supernatants by β-scintillation counting. Approximately 1.5% of the total radioactivity associated with the elastin particles could be released by plasmin. [3H]elastin “prestripped” with plasmin was equally sensitive to MDM-dependent proteolysis or elastolysis by purified MMPs (unpublished data). Results are expressed as mean ± SEM.
Degradation of Aortic Elastin Ex Vivo.
Segments of rat aorta were devitalized after three rounds of freezing (in liquid nitrogen) and thawing. MDMs were cocultured with aortic tissue that was placed into a transwell insert and suspended above the macrophages in the absence or presence of 50 μg/ml plasminogen. After a 10-d culture period, aortic segments were removed, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned.
Western Blot Analysis and Zymography.
After 9 d in culture, macrophages were switched to serum-free medium and cultured either alone (for 48 h) or with elastin in the upper well of a transwell dish for 5 d. Where indicated, 50 μg/ml plasminogen, 0.5 μg/ml recombinant human pro–gelatinase A, and protease inhibitors were added as described above to MDM-elastin cocultures. Aliquots of conditioned media or Triton X-114 extracts (for membrane type 1 [MT1]-MMP; reference 20) were incubated alone or with either 3 mM 4-aminopherylmercuric acetate (Sigma-Aldrich) or 1 μg/ml trypsin (Sigma-Aldrich) for 1 h at 25°C to activate MMP proenzymes (20). To recover proteases bound to the elastin surface, elastin particles were washed in the presence of a protease inhibitor cocktail (Set III; Calbiochem). The elastin was then placed in a boiling solution of elution buffer (62.5 mM Tris-Cl, 2% SDS, 10% vol/vol glycerol, 2 mM dithiothreitol, 0.01% Bromophenol blue, pH 6.8) for 10 min and the elastin-free supernatant was recovered. Equal amounts (10 μg) of protein from the conditioned media or elastin surface were loaded under reducing conditions onto a 12.5% SDS-polyacrylamide gel and transferred to PVDF membrane (Amersham Biosciences; reference 22). The membranes were then incubated with rabbit anti–macrophage metalloelastase polyclonal antisera (4), rat anti–MMP-7 monoclonal antibody αhMat338 (24), or rabbit anti–gelatinase-B polyclonal antisera (provided by H. Welgus, Pfizer Inc., Ann Arbor, MI). The bound primary antibodies were then detected with horseradish peroxidase–conjugated species-specific secondary antibodies (Pierce Chemical Co.) using the SuperSignal West Pico system (Pierce Chemical Co.; references 16 and 22).
For zymography, samples of conditioned media or gelatinase A standards were resolved under nonreducing conditions on 12.5% polyacrylamide gels impregnated with 2 mg/ml gelatin as previously described (23).
Elastolytic Activity of Recombinant Enzymes.
Enzyme concentrations were determined by active site titration with the stoichiometric inhibitor TIMP-2 (25). To assess elastolytic potential, 1.0 pmol active site–titrated gelatinase B, matrilysin, or metalloelastase were incubated alone or in combination with [3H]elastin in serum-free media at 37°C for 5 d. Solubilized [3H]elastin was quantitated as described above.
Results
MMP-dependent Elastolysis.
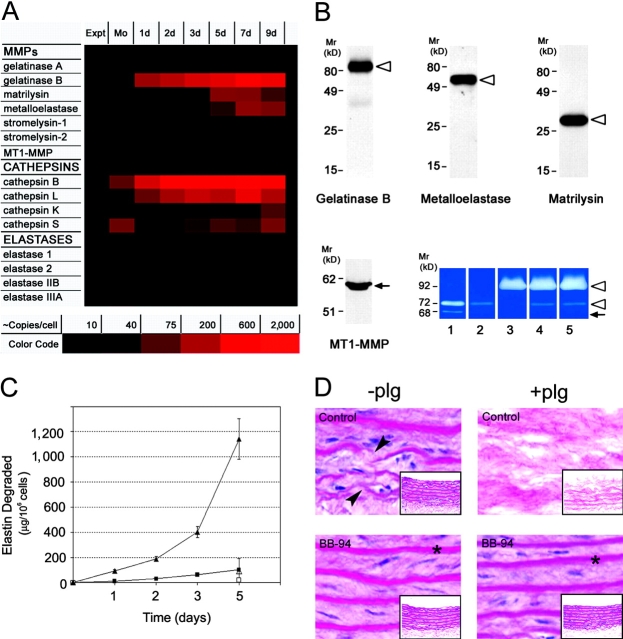
To obtain a comprehensive profile of the elastolytic enzymes expressed by human monocytes during ex vivo differentiation, cRNA from freshly isolated or cultured cells was probed for transcripts belonging to the MMP, cysteine proteinase, or serine proteinase gene families. Although the repertoire of MMPs expressed by freshly isolated monocytes is limited to gelatinase A (MMP-2) and MT1-MMP, the differentiation process triggers the up-regulation of gelatinase B (MMP-9), metalloelastase (MMP-12), and matrilysin (MMP-7; Fig. 1 A). Western blot analysis confirmed the ability of the fully differentiated (i.e., 9-d-old) macrophages to synthesize the proforms of gelatinase B, human metalloelastase, and matrilysin as well as the processed form of MT1-MMP (Fig. 1 B), but not the proforms or active forms of gelatinase A (Fig. 1 B). As previously reported (15, 16), 9-d-old MDMs also express the elastolytic cysteine proteinases, cathepsin L, K, and S (Fig. 1 A). Under these conditions, the expression of elastin-degrading serine proteinases is not detected (Fig. 1 A).
Figure 1.
Expression of elastolytic MMPs and elastolysis by differentiating MDMs. (A) Expression of elastolytic proteinases was assessed in monocytes (Mo) and MDMs after 1, 2, 3, 5, 7, and 9 d in culture by oligonucleotide array analysis. Elastase 1, 2, IIB, and IIIA are pancreatic elastase I, neutrophil elastase, pancreatic elastase II, and pancreatic endopeptidase E, respectively. Results are shown as a color-coded increase in mRNA content relative to that expressed by freshly isolated nonadherent monocytes from a single representative experiment of three performed. (B) Secretory phenotype of MDMs. Mature MDMs were incubated for 48 h in serum-free media. Aliquots of conditioned media or membrane extracts (for MT1-MMP) were resolved by SDS-PAGE and probed with specific antibodies. The ability of MT1-MMP to process gelatinase A was assessed by gelatin zymography (lower right-hand section of panel). MDMs were incubated for 48 h in serum-free media alone (lane 3) or cocultured with 0.5 μg/ml progelatinase A in the absence (lane 4) or presence (lane 5) of BB-94. Standards for the proforms and processed forms of gelatinase A as well as the gelatinase A proenzyme are shown in lanes 1 and 2, respectively. The proforms (open arrowheads) and processed (arrows) forms of enzymes are indicated. Results are shown from a single representative experiment of three performed. (C) MDMs were cocultured with [3H]elastin suspended in the upper well of a transwell insert to prevent direct cell–elastin contact in the absence (▪ and □) or presence (▴ and ▵) of 50 μg/ml exogenous plasminogen. Elastolysis was monitored over the course of a 5-d culture period. Elastolysis was inhibited by BB-94 under both plasminogen-free (□) or plasminogen-supplemented conditions (▵). Results are expressed as the mean ± SEM (n = 5). (D) Human MDMs were cocultured for 10 d with aortic tissue in the absence or presence of 50 μg/ml plasminogen and BB-94. Appearance of the aortic elastin is shown on cross sections by hematoxylin and eosin staining. Arrows indicate zones of elastin fragmentation and asterisks show intact elastic lamina (×100). The appearance of the full thickness sections is shown in the insets (×20).
To determine the ability of MDMs to use secreted MMPs as elastolytic effectors, [3H]-labeled elastin fragments were placed in a transwell insert and suspended above the cell cultures in the absence or presence of the peptidomimetic MMP inhibitor, BB-94. Despite the fact that MDMs only secrete proforms of the MMPs, ∼80 μg of elastin is degraded over a 5-d culture period via a BB-94–sensitive process (Fig. 1 C). Although elastolysis could potentially be augmented by the MT1-MMP–dependent activation of exogenous gelatinase A (26), MDMs are unable to process recombinant progelatinase A to its active form under these culture conditions despite the expression of processed MT1-MMP (Fig. 1 B).
To characterize the status of the plasminogen activator–plasminogen axis as a potential MMP activating system in macrophages, uPA and tPA expression were monitored as well as the ability of the cells to process plasminogen to plasmin. After 9 d in culture, MDMs synthesize 453 ± 18 pg uPA/106 cells for 24 h while tPA could not be detected. In the presence of 50 μg/ml exogenous plasminogen, 680 ± 16 mU of plasmin activity is generated via a process that can be inhibited completely by either anti-uPA blocking monoclonal antibody or the serine proteinase inhibitor, aprotinin (unpublished data). Consistent with the ability of plasmin to efficiently process pro-MMPs to active forms (8, 27), MDM-mediated elastin degradation increased from 106 ± 18 μg/106 cells for 5 d under plasminogen-free conditions to 1,100 ± 150 μg (n = 5; mean ± 1 SEM) in the presence 50 μg/ml plasminogen (Fig. 1 C). BB-94 inhibited plasminogen-dependent elastolysis by 93 ± 4% (n = 3) without affecting plasmin activity (unpublished data).
Although purified elastin provides a convenient substrate for quantifying elastolytic activity, the elastic lamellae found in situ are more complex structures comprised primarily of elastin as well as microfibrillar and other proteins (8). To determine the tissue-destructive potential of MDMs confronted with a physiologically relevant substrate, cells were cultured with devitalized aortic vessel explants. Although thinning and fragmentation of the elastic lamina of the aortic explants is discerned in MDM cocultures in the absence of plasminogen (Fig. 1 D, top left), almost complete dissolution of the elastin sheets is observed when MDMs are supplemented with plasminogen (Fig. 1 D, top right). As observed with purified elastin, BB-94 completely protects the elastic lamina from MDM-mediated damage without affecting plasmin generation (Fig. 1 D). Thus, macrophages can degrade elastin via MMP-dependent processes in either the absence or presence of plasminogen.
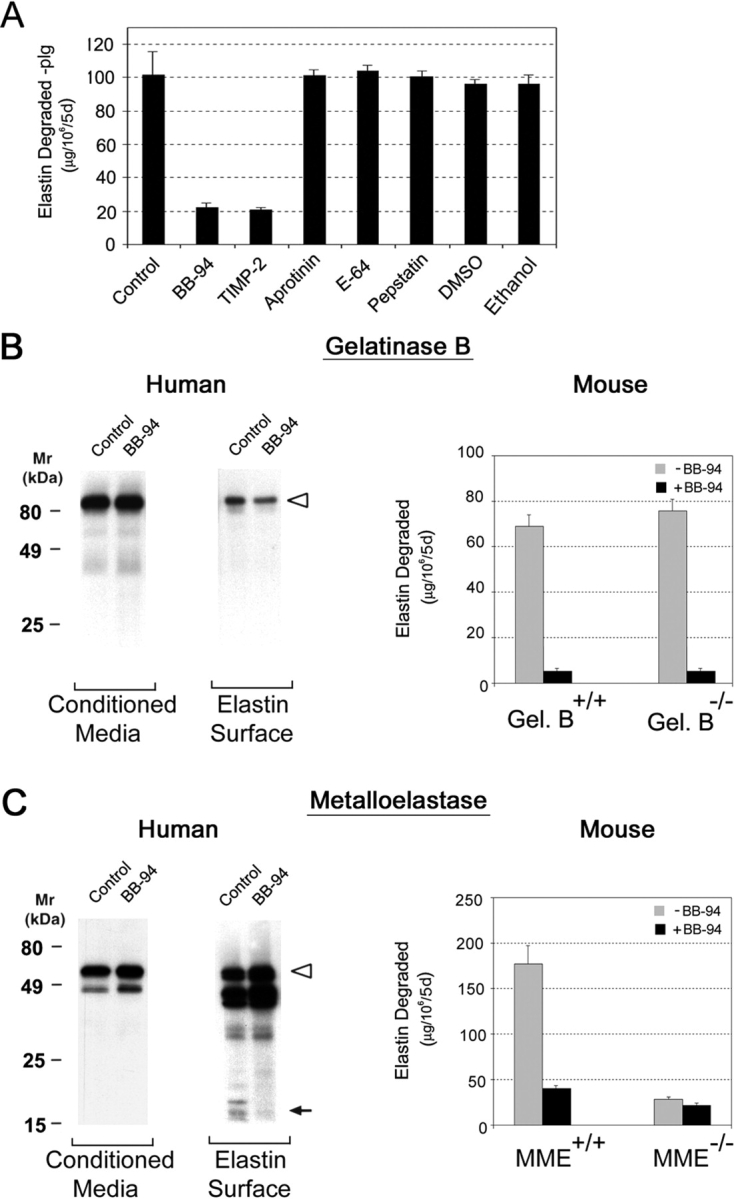
Plasminogen-independent Elastolysis.
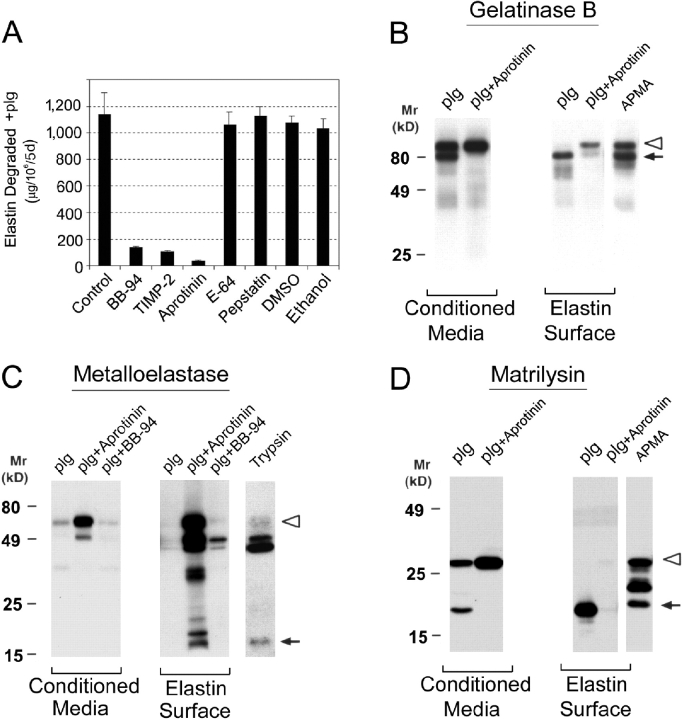
MDMs degrade elastin under plasminogen-free conditions by either mobilizing MMPs alone or using MMPs in collaboration with other proteolytic systems that serve to process latent forms of the metalloproteinases to their catalytically active forms (28). To determine whether MMP-dependent elastolysis involves other proteinase classes, MDMs were cultured with elastin particles in the presence of inhibitors directed against the serine (aprotinin, soybean trypsin inhibitor), cysteine (E-64), or aspartyl (pepstatin) proteinase families. Whereas either BB-94 or the native MMP inhibitor, TIMP-2, block elastin degradation by ∼80%, none of the other inhibitors affect elastolytic activity (Fig. 2 A). Although these results support a direct role for one or more MMPs in elastin degradation, the means by which the MMP zymogens are activated and the identity of the elastolysin(s) remain undefined. Because MMP zymogens may bind to the elastin surface and undergo conformational changes that allow for the expression of proteolytic activity (4, 29, 30), MDMs were cocultured with elastin and the distribution as well as processing of the MMPs were monitored in both the cell-free supernatant as well as the elastin surface. As observed in MDMs cultured alone (Fig. 1 B), progelatinase B (Fig. 2 B) and promatrilysin (unpublished data) are the predominant species detected in the media of MDM-elastin cocultures in either the absence or presence of BB-94. Although only trace amounts of promatrilysin are recovered from the elastin surface (unpublished data), progelatinase B is readily found in association with elastin particles (Fig. 2 B).
Figure 2.
Plasminogen-independent proteolysis by human and mouse macrophages. (A) Human MDMs were cocultured with [3H]elastin (suspended in the upper well of a transwell insert) under plasminogen-free conditions in the absence or presence of inhibitors directed against metallo (5 μM BB-94 or 5 μg/ml TIMP-2), serine (100 μg/ml aprotinin), cysteine (100 μM E-64), or aspartate (5 μM pepstatin) proteinases as well as solvent controls (0.1% DMSO and 0.05% ethanol) as indicated. Results are shown as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 6). (B and C) Left: Plasminogen-independent processing and elastolytic activity of MMP zymogens. Human macrophages and elastin were cocultured in the absence or presence of 5 μM BB-94 for 5 d. The processing of progelatinase B (B) and prometalloelastase (C) in the absence of plasminogen (plg) was assessed by Western blot analysis in samples recovered from conditioned media or the elastin surface. The proforms (open arrowheads) and mature (arrows) forms of enzymes are indicated. Results are shown from a single representative experiment of three performed. Right: Mouse macrophages obtained from gelatinase B+/+ (Gel.B+/+) or gelatinase B−/− (Gel.B−/−) mice (B) and metalloelastase+/+ (MME+/+) or MME−/− mice (C) were incubated with [3H]elastin in the absence or presence of BB-94 for 5 d. Results are shown as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3).
Recent studies suggest that the progelatinase B zymogen can express proteolytic activity after binding to the appropriate substrate (30), but gelatinase B–specific inhibitors are not currently available for testing. Hence, the degradative potential of macrophages isolated from gelatinase B+/+ or gelatinase B-null mice was compared directly. As shown in Fig. 2 B, cultured mouse macrophages degrade elastin to a degree comparable to human MDMs under plasminogen-free conditions via a BB-94–sensitive process (Fig. 2 B). However, although mouse macrophages constitutively release gelatinase B (5), gelatinase-null macrophages continue to exert an elastolytic effect indistinguishable from control cells (Fig. 2 B).
Although active forms of neither gelatinase B nor matrilysin were generated in MDM-elastin cocultures, both proforms and processed forms of metalloelastase are found in the conditioned media as well as elutes recovered from the elastin particles (Fig. 2 C). Consistent with a role for elastin in catalyzing the autoactivation process, BB-94 blocked the formation of the ∼18-kD active form on the particle surface (Fig. 2 C). As these findings support a potential role for metalloelastase in plasminogen-independent proteolysis by human cells, the elastolytic potential of metalloelastase+/+ and metalloelastase-null mouse macrophages was assessed. Significantly, the metalloelastase-null cells display little, if any, elastolytic activity despite normal gelatinase B expression (Fig. 2 C and unpublished data). Given the ability of human metalloelastase to bind to elastin and undergo processing to active forms, coupled with the loss of elastolytic activity displayed by metalloelastase-null mouse macrophages, these data imply that metalloelastase, and not gelatinase B, plays a required role in macrophage-mediated elastin degradation under plasminogen-free conditions.
Plasminogen-dependent Elastolysis.
By contrast with the plasminogen-independent system, MDM-dependent elastolysis in the presence of plasminogen is not only sensitive to BB-94 and TIMP-2, but to the plasmin inhibitor, aprotinin, as well. (Consistent with plasmin-dependent release of ∼1–2% of the radioactivity associated with the elastin particles [refer to Materials and Methods], aprotinin inhibited elastolysis slightly more effectively than BB-94 or TIMP-2 alone; Fig. 3 A). Further, plasmin-dependent elastolysis is accompanied by the aprotinin-sensitive processing of the progelatinase B and promatrilysin zymogens to mature forms that comigrate with the active species in both the cell-free conditioned media and at the elastin surface (Fig. 3, B and D). In the case of metalloelastase, plasmin catalyzed the processing of the zymogen to species no longer detectable in the conditioned media or on the elastin surface (Fig. 3 C). Although prometalloelastase and its processed products are recovered when plasmin activity is blocked with aprotinin, the ∼18-kD active form of metalloelastase is not stabilized in the presence of BB-94 (Fig. 3 C). Apparently, the steady state concentration of active metalloelastase is below detectable limits as a consequence of plasmin-dependent turnover.
Figure 3.
Plasminogen-dependent elastolysis and processing of MMP zymogens by human MDMs. (A) Human MDMs were cocultured with [3H]elastin in the upper well of a transwell insert with 50 μg/ml plasminogen (plg) in the absence or presence of the indicated inhibitors. Results are shown as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3). Western Blots: MDMs and elastin were cocultured without contact in the presence of 50 μg/ml plasminogen and/or 100 μg/ml aprotinin for 5 d. The processing of progelatinase B (B), prometalloelastase (C), and promatrilysin (D) in the presence of plasminogen was assessed by Western blot analysis in samples recovered from conditioned media or the elastin surface. The proforms (open arrowheads) and mature (arrows) forms of enzymes are indicated. The processing of the proforms by 4-aminopherylmercuric acetate or trypsin are shown at the far right of each panel with the proforms (open arrowheads) and mature (arrows) forms depicted. Results are shown from a single representative experiment of three performed.
Matrilysin Confers Macrophages with Elastolytic Activity.
To determine the relative roles of gelatinase B, matrilysin, and metalloelastase in plasminogen-dependent elastolysis by human MDMs, the ability of mouse macrophages to degrade elastin in the presence of plasminogen was examined as a prelude to testing the elastolytic potential of the respective MMP-null littermates. Unexpectedly, however, the ability of wild-type mouse macrophages to degrade elastin in the presence of plasminogen was not augmented to the levels observed with human MDMs despite efficient plasmin generation by the mouse cells (Fig. 4 A).
Figure 4.
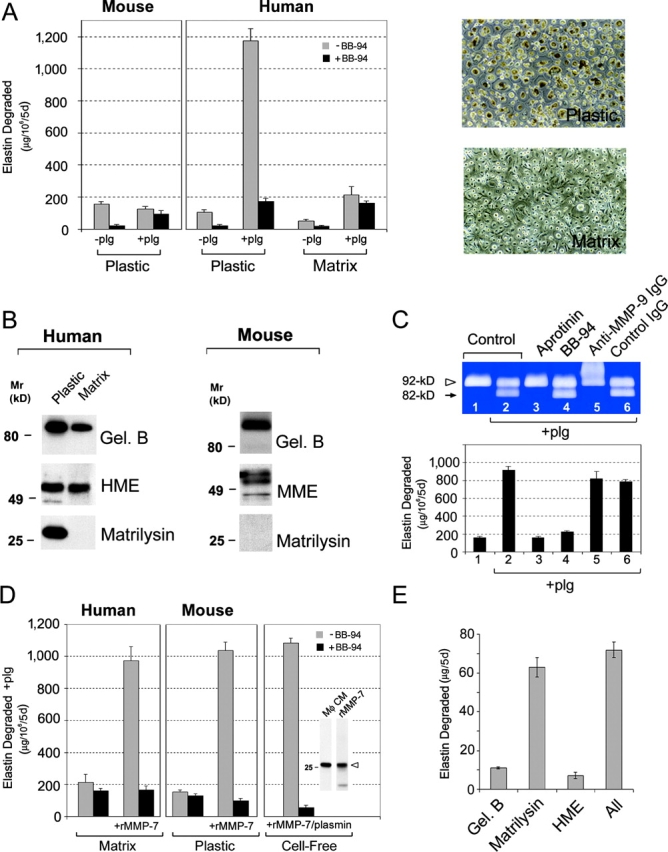
Matrilysin “deficiency” abrogates plasminogen-dependent elastolysis by human MDMs and mouse peritoneal macrophages. (A) Mouse peritoneal macrophages or human monocytes differentiated for 9 d atop the surface of either bacteriologic plastic dishes (Plastic) or a cell-derived ECM (Matrix; phase micrographs are shown on the right of A) were cultured with elastin suspended in transwell inserts as described in Fig. 1 in the absence or presence of 50 μg/ml plasminogen (plg). Where indicated, macrophages were incubated with or without 5 μM BB-94. In the presence of plasminogen and BB-94, the release of radiolabel from elastin is due primarily to plasmin activity and can be reduced to background levels with aprotinin (unpublished data). Results are shown as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3). (B) Secretion of elastolytic MMPs by human MDMs and murine macrophages as a function of in vitro culture conditions. Human MDMs differentiated atop surfaces of bacteriologic plastic (Plastic) or the cell-derived matrix (Matrix) were cultured under serum-free conditions for 48 h. Conditioned media was collected and analyzed by immunoblot for gelatinase B, human metalloelastase (HME), and matrilysin. Mouse macrophages were cultured in bacteriologic plastic dishes for 48 h under serum-free conditions and conditioned media was analyzed for MMPs by immunoblot as described in Fig. 1. (C) Human MDMs were cocultured with [3H]elastin and incubated alone (lane 1), with 50 μg/ml plasminogen (plg; lane 2), or plasminogen in the presence of aprotinin (lane 3), BB-94 (lane 4), anti–gelatinase B antibody (lane 5), or normal mouse IgG (both 50 μg/ml; lane 6). MMP-9 processing was assessed by gelatin zymography (top) and the effects on degradative activity were quantified (bottom). Results are expressed as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3). (D) Human MDMs differentiated atop a cell-derived matrix (Matrix) or mouse peritoneal macrophages cultured under standard conditions (Plastic) were incubated with [3H]elastin and 50 μg/ml plasminogen in the absence or presence of 5 μM BB-94. Where indicated, 20 nM recombinant promatrilysin was added to the culture system in the absence or presence of BB-94. 20 nM recombinant promatrilysin and 25 μg/ml plasmin were also incubated with elastin under cell-free conditions (Cell-Free). Inset shows the relative levels of matrilysin detected in media conditioned by human MDMs (Mφ CM) and the amount of recombinant matrilysin added (rMMP-7). Results are expressed as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3). (E) Equimolar quantities (1.0 pmol) of active site–titrated gelatinase B, human metalloelastase, or matrilysin alone, or all three enzymes together were incubated with [3H]elastin in serum-free media at 37°C. Results are expressed as μg elastin degraded for 5 d (mean ± SEM; n = 3).
As an alternative approach to assign roles to the individual MMPs in macrophage-mediated proteolysis, efforts were initiated to generate an elastolytic-incompetent human macrophage population that would afford the opportunity to characterize critical differences that underlie the acquisition of the destructive phenotype. As monocyte differentiation can be regulated by the physical characteristics of the underlying substratum (31), human monocytes were cultured atop various matrices and elastolytic activity was assessed. Interestingly, when human monocytes are differentiated atop a cell-derived ECM, plasminogen-dependent proteolysis by MDMs is suppressed from ∼1,200 to ∼200 μg elastin degraded (Fig. 4 A). Given that the ability of the matrix-differentiated macrophages to generate plasmin activity remains unchanged (680 vs. 760 mU of plasmin activity generated by plastic- vs. matrix-differentiated MDMs, respectively), MMP expression was monitored by Western blot analysis. Although human metalloelastase synthesis is unaffected, gelatinase B expression is partially depressed whereas matrilysin is no longer produced under these conditions (Fig. 4 B).
The decrease in gelatinase B and/or matrilysin expression by matrix-cultured MDMs correlates with their observed loss in elastin-degrading activity. If gelatinase B plays a required role in the elastolytic process after its plasmin-dependent processing to active species, then blocking antibodies that interfere with the activation process should suppress proteolysis. However, when plasmin-dependent gelatinase B processing is blocked completely with an anti–gelatinase B monoclonal antibody, elastolytic activity is not affected (Fig. 4 C). Furthermore, macrophage-mediated proteolysis is only enhanced modestly by supplementing MDM cultures with 0.5 μg/ml exogenous progelatinase B (a 28% increase relative to control cultures; n = 2).
To next determine if the decrease in matrilysin expression by matrix-cultured MDMs could be linked to the loss in elastolytic activity, matrilysin-deficient macrophages were supplemented with recombinant promatrilysin at levels similar to those expressed by MDMs cultured under standard conditions. In the presence of 20 nM promatrilysin, the ability of matrix-cultured MDMs to degrade elastin is recovered completely (Fig. 4 D). Furthermore, the failure of wild-type mouse macrophages to up-regulate elastolytic activity in the presence of plasminogen (Fig. 4 A) also correlates with the reported inability of these cells to express matrilysin in vitro (Fig. 4 B; reference 32). As in the case of matrix-cultured MDMs, the elastolytic activity of mouse macrophages is similarly augmented by exogenous matrilysin to levels comparable to those expressed by human macrophages (Fig. 4 D).
The fact that matrilysin rescues the depressed proteolytic activity of matrix-cultured MDMs or wild-type mouse macrophages is consistent with the ability of the proteinase to either alter cell function or serve as a direct acting elastolysin (32–35). To determine the relative activities of gelatinase B, metalloelastase, and matrilysin as elastin-degrading proteases, equimolar quantities of the active site–titrated enzymes were incubated with radiolabeled elastin. Although gelatinase B and metalloelastase were able to proteolyze elastin, matrilysin is sixfold more efficient as an elastolysin (Fig. 4 E). Synergistic interactions between the three MMPs are not detected (Fig. 4 E). Further, a mixture of 20 nM recombinant promatrilysin and 25 μg/ml plasmin fully recapitulates the elastolytic activity of intact MDMs (Fig. 4 D). The addition of progelatinase B or prometalloelastase to the cell-free system does not further augment activity (unpublished data). Thus, plasminogen-dependent proteolysis by human MDMs can be largely, if not entirely, ascribed to the plasmin–matrilysin axis.
Cysteine Proteinases Complement MMP-dependent Elastolysis by Human MDMs.
Coincident with an expression of MMPs, human macrophages can constitutively secrete a series of elastolytic cysteine proteinases including cathepsins S, L, and K (15, 16). However, in contrast to MMPs, cysteine proteinase activity is strictly confined to the macrophage–elastin interface within acidified zones maintained by a vacuolar-type H+-ATPase (15, 16). To determine whether MMPs can complement the elastolytic activity of cysteine proteinases, elastin was added to MDM cultures to initiate direct contact between the cell and substrate. In the absence of plasminogen, MDM–elastin contact triggers a large increase in elastin degradation (i.e., 1,200 ± 5 μg/106 cells for 5 d; Fig. 5, A and B) relative to that observed in the absence of cell–substrate contact (∼100 μg; Fig. 1 C). Under these conditions, the bulk of the elastin degradation was sensitive to the specific cysteine proteinase inhibitor, E-64, with only small increases in inhibition observed when MMP activity was blocked with BB-94 (Fig. 5 B). However, in the presence of plasminogen, elastin degradation is increased dramatically to over 2,000 μg/106 cells for 5 d. Although E-64 is only able to decrease elastolysis by ∼50% to 1,092 ± 28 μg/106 cells for 5 d in the presence of plasminogen, the residual activity is mediated by MMPs and proves sensitive to inhibition by BB-94, TIMP-2, or aprotinin (Fig. 5 B). Thus, in the presence of cell–elastin contact, MMPs and cysteine proteinases together arm MDMs with their maximal proteolytic activity.
Figure 5.
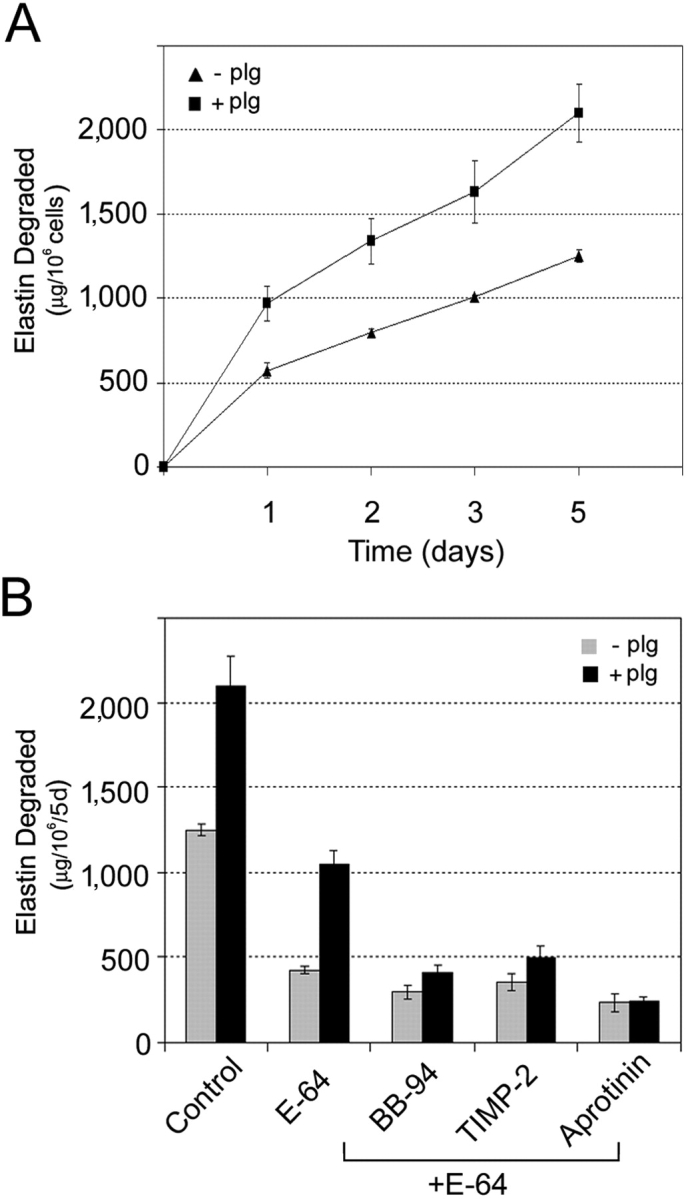
Cysteine proteinases complement MMP-dependent elastolysis by human MDMs. (A) MDMs were incubated with [3H]elastin to allow cell–substrate contact and elastolysis was monitored in the absence (▴) or presence (▪) of 50 μg/ml exogenous plasminogen (plg) over the course of a 5-d culture period. Results are expressed as the mean ± SEM (n = 4). (B) MDMs were cocultured in contact with elastin in the absence (gray bar) or presence (solid bar) of plasminogen for 5 d alone or with either 100 μM E-64 alone or E-64 in combination with either 5 μm BB-94, 5 μg/ml TIMP-2, or 100 μg/ml aprotinin. Results are shown as μg elastin degraded/106 cells for 5 d (mean ± SEM; n = 3).
Discussion
Studies in gene-deleted mice have identified roles for metalloelastase and gelatinase B in cigarette smoke–induced emphysema and macrophage-dependent aneurysm formation, respectively (3, 7, 8). As each of these MMPs express elastolytic activity in cell-free systems, it has been assumed that active metalloelastase and gelatinase B play similar roles in vivo by mediating directly the dissolution of elastin-rich tissues (3, 7, 8). However, these conclusions have been questioned as each of these MMPs has been shown to regulate proinflammatory effects in vivo via largely unexpected mechanisms (9–11). For example, in animal models of emphysema, metalloelastase-deleted macrophages display defects in tumor necrosis factor expression that alter the subsequent recruitment of tissue-destructive polymorphonuclear leukocytes (6). Furthermore, in proinflammatory states characterized by the expression of a complex mixture of MMPs (e.g., IL-13–induced models of chronic obstructive pulmonary disease), metalloelastase-null mice mount a blunted response associated with a failure to up-regulate a series of downstream proteinases (37). Similarly, gelatinase B–deficient mice display altered inflammatory responses after tissue damage (38, 39). Given the multiplicity of changes observed in vivo after the deletion of either metalloelastase or gelatinase B (36–39), attempts to assign specific ECM substrates to either MMP in pathophysiologic settings have been met with limited success. Indeed, where MMPs have been implicated directly in tissue-destructive states in vivo, recent studies identified probable target substrates as plasma-derived proteinase inhibitors or latent proinflammatory mediators, rather than ECM molecules per se (10, 11).
To circumvent the complexities inherent in attempts to identify specific substrates for a given MMP, we turned to an ex vivo model wherein MDMs are cultured with elastin (either in the form of isolated fragments or as native elastic lamina) to identify the proteinases in the macrophage repertoire that are capable of directly mediating elastolytic effects. To more accurately mimic the in vivo state, MDMs were further cultured under conditions where they expressed gelatinase B, metalloelastase, MT1-MMP, and matrilysin, a complement of MMPs similar, if not identical, to that found in macrophages surrounding sites of elastin damage in vivo (1, 2, 9). Under optimal conditions, MDMs were able to use MMPs to degrade elastin in quantities hundred of times greater than previously assigned to this family of proteinases (12–15), thus prompting efforts to identify the key mediators involved.
In vivo studies have identified a role for the plasminogen activator–plasminogen axis in elastolytic states, but the proteolytic activity of this system is dictated by the local concentration of plasminogen activator and plasminogen as well as plasminogen activator and plasmin inhibitors (5, 6, 27). Using plasminogen-free conditions to mimic conditions under which the plasminogen activator–plasminogen axis would be inactive or neutralized, MDMs were found to express limited, but significant, elastolytic activity. Based on recent findings that substrate binding can unmask latent MMP activity (29, 30), we considered the possibility that elastin degradation might be mediated by elastin-bound gelatinase B. However, gelatinase B–null macrophages degraded elastin comparably to wild-type cells and recombinant progelatinase B did not initiate an elastolytic effect when cultured with elastin fragments under cell-free conditions. Instead, elastin degradation was found to correlate with the binding of metalloelastase to elastin and its subsequent processing to an active elastolysin. The proteinase(s) responsible for generating the multiple metalloelastase products found in association with the elastin surface has not yet been identified, but apparently does not belong to the metalloproteinase family as the spectrum of metalloelastase fragments detected in the cocultures was unaffected by the addition of MMP inhibitors. Nonetheless, the final processing of the metalloelastase zymogen to a species with a Mr similar, if not identical, to active metalloelastase was blocked completely by BB-94. Further, by using macrophages isolated from gene-deleted mice, we demonstrated that metalloelastase-null macrophages were unable to degrade elastin under plasminogen-free conditions. Although metalloelastase-dependent elastolysis has not previously been proposed to occur in the absence of plasmin (8, 39), these data are consistent with a model wherein the MMP binds to the elastin surface where it is cleaved by uncharacterized proteinases to generate fragments that undergo autocatalytic processing to generate the active enzyme. We considered the possibility that plasminogen-independent elastolysis might be augmented by the MT1-MMP–mediated activation of gelatinase A (26), but MDMs were unable to process the exogenously supplied proenzyme. Although the mechanism underlying the inability of MT1-MMP to activate gelatinase A was not examined in our study, similar defects have been ascribed to a failure to oligomerize the membrane-anchored MMP at the cell surface (40).
By contrast with the limited elastolytic effect observed under plasminogen-free conditions, the ability of MDMs to degrade elastin was increased over 10-fold via the uPA-dependent activation of plasminogen to plasmin. As expected, plasminogen-dependent elastolysis was accompanied by the processing of the gelatinase B, metalloelastase, and matrilysin zymogens (6, 27). In an effort to identify the relative roles of each of these MMPs in the elastin-degradative phenotype, initial efforts focused on using macrophages recovered from the respective gene-deleted mice. However, mouse macrophages were unable to up-regulate their elastolytic potential in the presence of plasminogen, despite efficient plasmin generation. Although these findings appear to contradict earlier studies, it should be noted that murine macrophages have been previously reported to express a much more limited degradative phenotype than that reported here (i.e., in the presence of plasminogen, murine macrophages only degrade 5 μg/106 cells for 24 h), presumably due to an in vitro culture protocol that blunts the elastolytic potential of mouse macrophages.
As an alternate to using MMP-null murine macrophages, human monocytes were cultured atop an ECM-coated surface to generate elastolytically incompetent cells. Previous studies have demonstrated that the culture substratum used to support monocyte differentiation can regulate cell phenotype (31). Although matrix-cultured MDMs no longer degrade elastin effectively relative to the standard culture system used here, the uPA–plasmin axis was unaffected and metalloelastase was synthesized at normal levels. However, gelatinase B and matrilysin levels were depressed, though to different degrees. Given our inability to implicate gelatinase B in plasminogen-dependent elastin degradation, attention focused on the fact that the depressed elastolytic activity of matrix-cultured MDMs correlated with the loss of matrilysin expression. Likewise, the failure of mouse macrophages to augment elastolytic activity in the presence of plasminogen was also linked to the apparent inability of these cells to express matrilysin in vitro (32). Significantly, when either matrilysin-“deficient” human MDMs or mouse macrophages were supplemented with physiological concentrations of promatrilysin, elastolytic activity was reconstituted to the levels observed for fully active human MDMs. As matrilysin has been shown to play key roles in host defense and inflammation by virtue of its ability to process antibacterial factors and shed membrane-anchored growth factors, cell adhesion molecules, cytokines, or proteoglycans (32–35), these findings seemed most consistent with the possibility that matrilysin affected elastolytic activity by altering macrophage function. However, of the three MMPs tested, matrilysin proved to be the most effective elastolysin, and after plasmin activation, physiologically relevant concentrations of matrilysin degraded elastin comparably to intact MDMs. Consistent with our analyses of macrophage-mediated elastin degradation, the elastolytic potential of matrilysin under cell-free conditions was not enhanced significantly by either gelatinase B or metalloelastase. Thus, we conclude that the uPA–plasminogen–matrilysin axis confers macrophages with their most potent MMP-dependent elastolytic activity.
In addition to the ability of human macrophages to mobilize MMPs, recent studies have highlighted the importance of an independently functioning family of elastolytic cysteine proteinases (15, 16). During the differentiation process, MDMs not only up-regulate the expression of a triad of elastolytic cysteine proteinases including cathepsins L, S, and K, but also acquire the ability to secrete active forms of these proteinases into the extracellular milieu (15, 16). In the pericellular environment, cathepsin S, L, and K activities are restricted to the cell–substrate interface where an acidified microenvironment is generated to support the catalytic activity of the acidophilic cysteine proteinases (15, 16). Additional studies with gene-deleted mouse macrophages have identified cathepsin L and S as the major cysteine proteinases responsible for this effect (unpublished data). Thus, in the presence of macrophage–elastin contact, the bulk of the elastolytic phenotype observed under plasminogen-free conditions can be linked to these two cysteine proteinases. By contrast, in the presence of plasminogen, elastin degradation was increased dramatically with both cysteine proteinases and MMPs working in concert to arm macrophages with the ability to degrade milligram quantities of elastin. Although we attempted to determine the degree to which matrilysin participates in the elastolytic process in the presence of macrophage–elastin contact, the ECM-differentiated MDMs no longer bound the elastin particle tightly (unpublished data), a prerequisite condition for cysteine proteinase–dependent proteolysis (15, 16). Nonetheless, the relatively modest elastolytic activity assigned to gelatinase B or metalloelastase in either our intact cell system or under cell-free conditions, makes it unlikely that either of these MMPs play a major role in the presence of macrophage–elastin contact. This issue notwithstanding, depending on the absence or presence of macrophage–elastin contact and the local concentration of plasmin in the pericellular milieu, our findings demonstrate that macrophages can mediate elastolysis by mobilizing either cysteine proteinases or MMPs, alone or in combination.
In this study, efforts have focused on identifying elastolytic mediators mobilized by human or murine macrophages, but these results should not be misconstrued to suggest that proteinases such as gelatinase B or metalloelastase play less important roles in tissue-destructive states in vivo. By hydrolyzing antiproteinases, chemokines, growth factors, and cytokines, many MMP family members appear to exert their most important effects by targeting non-ECM substrates (9–11). Nonetheless, although in vivo studies can provide critical insights into the role played by individual proteinases in complex outcomes, only indirect correlations can be established between an expressed or deleted proteinase and an observed effect (9). Using ex vivo models that faithfully recapitulate specific components of the inflammatory process, tissue-destructive mediators can be identified unequivocally and targeted for further testing. Indeed, although caution must be exercised in extrapolating results from the mouse macrophage to its human counterpart (e.g., reference 41), or to assuming the direct actions of a given proteinase in an in vivo setting (9), cysteine proteinases and MMPs have recently been linked to tissue-destructive effects in mouse models of atherosclerosis, chronic obstructive pulmonary disease, or pulmonary fibrosis (42–44). The demonstration that human macrophages are armed not only with a subset of tissue-destructive MMPs, but also a series of elastolytic cysteine proteinases as well, underscores the complex interplay that exists between distinct proteolytic systems and suggests that therapeutics aimed at select members of both gene families might be necessary to ameliorate elastolytic effects in vivo.
Acknowledgments
We thank M. Gyetko (University of Michigan) for assistance with the plasminogen activator determinations.
This work was supported by the National Institutes of Health grant AI21301.
Abbreviations used in this paper: ECM, extracellular matrix; MDM, monocyte-derived macrophage; MMP, matrix metalloproteinase; MT1, membrane type 1; TIMP-2, tissue inhibitor of metalloproteinases-2; tPA, tissue-type plasminogen activator; uPA, urokinase-type plasminogen activator.
References
- 1.Galis, Z.S., G.K. Sukhova, M.W. Lark, and P. Libby. 1994. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94:2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpert, I., U.I. Sires, J.D. Roby, S. Potter-Perigo, T.N. Wight, S.D. Shapiro, H.G. Welgus, S.A. Wickline, and W.C. Parks. 1996. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc. Natl. Acad. Sci. USA. 93:9748–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hautamaki, R.D., D.K. Kobayashi, R.M. Senior, and S.D. Shapiro. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 4.Curci, J.A., S. Liao, M.D. Huffman, S.D. Shapiro, and R.W. Thompson. 1998. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Invest. 102:1900–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet, P., L. Moons, R. Lijnen, M. Baes, V. Lemaitre, P. Tipping, A. Drew, Y. Eeckhout, S. Shapiro, F. Lupu, and D. Collen. 1997. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 17:439–444. [DOI] [PubMed] [Google Scholar]
- 6.Heymans, S., A. Luttun, D. Nuyens, G. Theilmeier, E. Creemers, L. Moons, G.D. Dyspersin, J.P.M. Cleutjens, M. Shipley, A. Angellilo, et al. 1999. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat. Med. 5:1135–1142. [DOI] [PubMed] [Google Scholar]
- 7.Pyo, R., J.K. Lee, J.M. Shipley, J.A. Curci, D. Mao, S.J. Ziporin, T.L. Ennis, S.D. Shapiro, R.M. Senior, and R.W. Thompson. 2000. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 105:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo, G.M., W. Xiong, T.C. Greiner, Y. Zhao, N. Fiotti, and B.T. Baxter. 2002. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks, W.C. 2002. A confederacy of proteinases. J. Clin. Invest. 110:613–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egeblad, M., and Z. Werb. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2:161–174. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Otin, C., and C.M. Overall. 2002. Protease degradomics: a new challenge for proteomics. Nat. Rev. Mol. Cell Biol. 3:509–519. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, H.A., Jr., O.L. Stone, and Z. Vavrin. 1984. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J. Clin. Invest. 73:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman, H.A., Jr., and O.L. Stone. 1984. Comparison of live human neutrophil and alveolar macrophage elastolytic activity in vitro. Relative resistance of macrophage elastolytic activity to serum and alveolar proteinase inhibitors. J. Clin. Invest. 74:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senior, R.M., N.L. Connolly, J.D. Cury, H.G. Welgus, and E.J. Campbell. 1989. Elastin degradation by human alveolar macrophages. Am. Rev. Respir. Dis. 139:1251–1256. [DOI] [PubMed] [Google Scholar]
- 15.Reddy, V.Y., Q.Y. Zhang, and S.J. Weiss. 1995. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA. 92:3849–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punturieri, A., S. Filippov, E. Allen, I. Caras, R. Murray, V. Reddy, and S.J. Weiss. 2000. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K–deficient human macrophages. J. Exp. Med. 192:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geissmann, F., P. Revy, A. Regnault, Y. Lepelletier, M. Dy, N. Brousse, S. Amigorena, O. Hermine, and A. Durandy. 1999. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 162:4567–4575. [PubMed] [Google Scholar]
- 18.Tammi, R.H., M.I. Tammi, V.C. Hascall, M. Hogg, S. Pasonen, and D.K. MacCallum. 2000. A preformed basal lamina alters the metabolism and distribution of hyaluronan in epidermal keratinocyte “organotypic” cultures grown on collagen matrices. Histochem. Cell Biol. 113:265–277. [DOI] [PubMed] [Google Scholar]
- 19.Betsuyaku, T., J.M. Shipley, Z. Liu, and R.M. Senior. 1999. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am. J. Respir. Cell Mol. Biol. 20:1303–1309. [DOI] [PubMed] [Google Scholar]
- 20.Gardell, S.J., L.T. Duong, R.E. Diehl, J.D. York, T.R. Hare, R.B. Register, J.W. Jacobs, R.A. Dixon, and P.A. Friedman. 1989. Isolation, characterization, and cDNA cloning of a vampire bat salivary plasminogen activator. J. Biol. Chem. 264:17947–17952. [PubMed] [Google Scholar]
- 21.Ramos-DeSimone, N., E. Hahn-Dantona, J. Sipley, H. Nagase, D.L. French, and J.P. Quigley. 1999. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 274:13066–13076. [DOI] [PubMed] [Google Scholar]
- 22.Hotary, K.B., I. Yana, F. Sabeh, X.-Y. Li, K. Holmbeck, H. Birkedal-Hansen, E.D. Allen, N. Hiraoka, and S.J. Weiss. 2002. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP–dependent and –independent processes. J. Exp. Med. 195:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotary, K., E. Allen, A. Punturieri, I. Yana, and S.J. Weiss. 2000. Regulation of cell invasion and morphogenesis in a 3-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2 and 3. J. Cell Biol. 149:1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shattuck-Brandt, R.L., L.W. Lamps, K.J. Heppner Goss, R.N. DuBois, and L.M. Matrisian. 1999. Differential expression of matrilysin and cyclooxygenase-2 in intestinal and colorectal neoplasms. Mol. Carcinog. 24:177–187. [PubMed] [Google Scholar]
- 25.Knight, C.G., F. Willenbrock, and G. Murphy. 1992. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 296:263–266. [DOI] [PubMed] [Google Scholar]
- 26.Shankavaram, U.T., W.-C. Lai, S. Netzel-Arnett, P.R. Mangan, J.A. Ardans, N. Caterina, W.G. Stetler-Stevenson, H. Birkedal-Hansen, and L.M. Wahl. 2001. Monocyte membrane type 1-matrix metalloproteinase. J. Biol. Chem. 276:19027–19032. [DOI] [PubMed] [Google Scholar]
- 27.Lijnen, H.R., and D. Collen. 1999. Matrix metalloproteinase system deficiencies and matrix degradation. Thromb. Haemost. 82:837–845. [PubMed] [Google Scholar]
- 28.Brinckerhoff, C.E., and L.M. Matrisian. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 3:207–214. [DOI] [PubMed] [Google Scholar]
- 29.Emonard, H., and W. Hornebeck. 1997. Binding of 92 kDa and 72 kDa progelatinases to insoluble elastin modulates their proteolytic activation. Biol. Chem. 378:265–271. [DOI] [PubMed] [Google Scholar]
- 30.Bannikov, G.A., T.V. Karelina, I.E. Collier, B.L. Marmer, and G.I. Goldberg. 2002. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J. Biol. Chem. 277:16022–16027. [DOI] [PubMed] [Google Scholar]
- 31.Maxeiner, H., J. Husemann, C.A. Thomas, J.D. Loike, J. El Khoury, and S.C. Silverstein. 1998. Complementary roles for scavenger receptor A and CD36 of human monocyte-derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H2O2. J. Exp. Med. 188:2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haro, H., H.C. Crawford, B. Fingleton, K. Shinomiya, D.M. Spengler, and L.M. Matrisian. 2000. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-α in a model of herniated disc resorption. J. Clin. Invest. 105:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, C.L., A.J. Ouellette, D.P. Satchell, T. Ayabe, Y.S. Lopez-Boado, J.L. Stratman, S.J. Hultgren, L.M. Matrisian, and W.C. Parks. 1999. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 286:113–117. [DOI] [PubMed] [Google Scholar]
- 34.Powell, W.C., B. Fingleton, C.L. Wilson, M. Boothby, and L.M. Matrisian. 1999. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9:1441–1447. [DOI] [PubMed] [Google Scholar]
- 35.Li, Q., P.W. Park, C.L. Wilson, and W.C. Parks. 2002. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 11:635–646. [DOI] [PubMed] [Google Scholar]
- 36.Churg, A., R.D. Wang, H. Tai, X. Wang, C. Xie, J. Dai, S.D. Shapiro, and J.L. Wright. 2003. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via TNF-alpha release. Am. J. Respir. Crit. Care Med. 167:1083–1089. [DOI] [PubMed] [Google Scholar]
- 37.Lanone, S., T. Zheng, A. Zhu, W. Liu, C.G. Lee, B. Ma, Q. Chen, R.J. Homer, J. Wang, L.A. Rabach, et al. 2002. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J. Clin. Invest. 110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan, R., S.K. Chintala, J.C. Jung, W.V.L. Villar, F. McCabe, L.A. Russo, Y. Lee, B.E. McCarthy, K.R. Wollenberg, J.V. Jester, et al. 2002. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 277:2065–2072. [DOI] [PubMed] [Google Scholar]
- 39.Shipley, J.M., R.L. Wesselschmidt, D.K. Kobayashi, T.J. Ley, and S.D. Shapiro. 1996. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA. 93:3942–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehti, K., J. Lohi, M.M. Juntunen, D. Pei, and J. Keski-Oja. 2002. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 277:8440–8448. [DOI] [PubMed] [Google Scholar]
- 41.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M.T. Ochoa, M. Engele, P.A. Sieling, P.F. Barnes, M. Rollinghoff, P.L. Bolcskei, M. Wagner, et al. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 291:1544–1547. [DOI] [PubMed] [Google Scholar]
- 42.Zheng, T., Z. Zhu, Z. Wang, R.J. Homer, B. Ma, R.J. Riese, Jr., H.A. Chapman, Jr., S.D. Shaprio, and J.A. Elias. 2000. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Invest. 106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo, F., N. Kaminski, E. Eugui, J. Allard, Z. Yakhini, A. Ben-Dor, L. Lollini, D. Morris, Y. Kim, B. DeLustro, et al. 2002. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc. Natl. Acad. Sci. USA. 99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sukhova, G.K., Y. Zhang, J.-H. Pan, Y. Wada, T. Yamamoto, M. Naito, T. Kodama, S. Tsimikas, J.L. Witztum, M.L. Lu, et al. 2003. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 111:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]