Abstract
Vascular endothelial growth factor (VEGF) C and VEGF-D stimulate lymphangiogenesis and angiogenesis in tissues and tumors by activating the endothelial cell surface receptor tyrosine kinases VEGF receptor (VEGFR) 2 and VEGFR-3. These growth factors are secreted as full-length inactive forms consisting of NH2- and COOH-terminal propeptides and a central VEGF homology domain (VHD) containing receptor binding sites. Proteolytic cleavage removes the propeptides to generate mature forms, consisting of dimers of the VEGF homology domain, that bind receptors with much greater affinity than the full-length forms. Therefore, proteolytic processing activates VEGF-C and VEGF-D, although the proteases involved were unknown. Here, we report that the serine protease plasmin cleaved both propeptides from the VEGF homology domain of human VEGF-D and thereby generated a mature form exhibiting greatly enhanced binding and cross-linking of VEGFR-2 and VEGFR-3 in comparison to full-length material. Plasmin also activated VEGF-C. As lymphangiogenic growth factors promote the metastatic spread of cancer via the lymphatics, the proteolytic activation of these molecules represents a potential target for antimetastatic agents. Identification of an enzyme that activates the lymphangiogenic growth factors will facilitate development of inhibitors of metastasis.
Keywords: lymphangiogenesis, lymphatics, angiogenesis, proteolysis, metastasis
Introduction
Vascular endothelial growth factor (VEGF) C and VEGF-D are secreted glycoproteins that induce angiogenesis and lymphangiogenesis. For example, overexpression of VEGF-C or VEGF-D in the skin of transgenic mice resulted in lymphatic hyperplasia (1, 2), and adenoviral delivery of VEGF-D in adult tissues induced tissue-specific angiogenesis and lymphangiogenesis (3). Lymphangiogenic growth factors show promise for enhancing tissue fluid drainage for treatment of lymphedema (4, 5). In cancer models, expression of VEGF-C or VEGF-D stimulated growth of tumor lymphatics and, importantly, promoted metastasis via lymphatic vessels (6, 7). Clinical analyses revealed that expression of VEGF-C or VEGF-D in breast, ovarian, and colorectal cancer may be a prognostic indicator associated with lymph node metastasis (8–11). These findings indicate that inhibitors of VEGF-C and VEGF-D may be useful as anticancer agents designed to block metastatic spread, as illustrated by use of a neutralizing VEGF-D antibody to block metastasis via the lymphatics in a mouse model of cancer (7).
The effects of VEGF-C and VEGF-D are mediated by VEGF receptor (VEGFR) 2 and VEGFR-3 (12, 13), cell surface receptor tyrosine kinases predominantly expressed on endothelial cells (for review see reference 14). VEGFR-2 is expressed on blood vessels during embryogenesis and is critical for early vasculogenesis and angiogenesis (14). It can be up-regulated on adult blood vessels in cancer and is thought to signal for tumor angiogenesis (14). VEGFR-3 is initially expressed on the endothelium of blood vessels during embryogenesis and is critical for formation of large vessels in the early embryo (15). In the adult, VEGFR-3 is predominantly restricted to lymphatic endothelium (14), where activation of this receptor induces lymphangiogenesis (2). However, VEGFR-3 can be up-regulated on the endothelium of blood vessels in tumors (14) and during wound healing (16).
VEGF-C and VEGF-D are initially synthesized as full-length forms consisting of NH2- and COOH-terminal propeptides flanking the central VEGF homology domain (VHD) that contains the receptor binding sites (13, 17). Proteolytic processing results in cleavage of the propeptides from the VHD, generating mature VHD homodimers that bind VEGFR-2 and VEGFR-3 with much higher affinity than the full-length form (17, 18). Hence, proteolytic processing activates the lymphangiogenic growth factors but the proteases involved were unknown. Here, we describe an approach for detecting the proteolytic processing of VEGF-D with which we identified the serine protease plasmin as an activator of VEGF-D. Plasmin generated mature, bioactive VEGF-D from full-length material, and also activated VEGF-C. This is the first identification of an enzyme capable of fully activating lymphangiogenic growth factors.
Materials and Methods
Peptide Labeling.
1 mg of biotinylated peptide (Auspep) in 200 μl of 200 mM 2-morpholinoethane sulfonic acid (MES), pH 6.0, was mixed with 455 μCi [3H]N-ethylmaleimide (NEM) in pentane (PerkinElmer) and pentane removed under N2. After incubation at room temperature for 10 min, 50 μg NEM in 200 mM MES, pH 6.0, was added and incubated for 1 h at room temperature. An additional 500 μg NEM in 200 mM MES, pH 6.0, was added and incubated for 1 h at room temperature. Labeled peptide was separated from unincorporated label by chromatography on Sephadex G-10 in 150 mM NaCl.
Purification of VEGF-C and VEGF-D.
VEGF-C-FULL-N-FLAG or VEGF-D-FULL-N-FLAG (full-length human VEGF-C or VEGF-D tagged at the NH2 terminus with the FLAG octapeptide), VEGF-DΔNΔC-FLAG (the VHD of human VEGF-D tagged at the NH2 terminus with FLAG; reference 18) and mouse VEGF-D326-FLAG and VEGF-D358-FLAG (full-length isoforms tagged at the COOH termini with FLAG; reference 19) were purified from the conditioned media of transfected 293EBNA cells as described previously (18).
Protease Digests.
Protease digestions were in 10 mM potassium phosphate buffer, pH 7.5, 150 mM NaCl at 37°C for 1 h. Digests contained between 10 and 10−2 U/ml of plasmin from human serum (Calbiochem). α2-antiplasmin (Calbiochem) was incubated with plasmin in PBS for 30 min at room temperature before addition of VEGF-D-FULL-N-FLAG and digestion at 37°C for 1 h. Digests with tissue plasminogen activator (Calbiochem) contained 0.5–50 kU/ml of enzyme. Scintillation proximity assays contained 0.1 U/ml plasmin, 0.1 U/ml thrombin, 5 mU/ml matrix metalloproteinase (MMP) 2, or 9 mU/ml MMP-9 (Calbiochem).
Scintillation Proximity Assay.
After incubation with proteases, 104 cpm of labeled biotinylated peptide was mixed with 200 mg streptavidin scintillant beads (Amersham Biosciences) in 10 mM potassium phosphate buffer, pH 6.0, at room temperature for 20 min, before β counting.
Western Blotting.
After SDS-PAGE and transfer to membranes, proteins were probed with biotinylated antibody to the VHD of mouse VEGF-D (R&D Systems) and HRP-conjugated streptavidin (Zymed Laboratories) and developed using chemiluminescence (Pierce Chemical Co.).
Amino Acid Sequencing.
NH2-terminal amino acid sequencing was with a biphasic NH2-terminal protein sequencer (model G1005A; Agilent Technologies).
Mass Spectrometry.
Peptides were desalted using μC18 ZipTips (Millipore) and cocrystallized onto a 10 × 10 matrix-assisted laser desorption/ionization stainless steel sample plate (Applied Biosystems) with 2,5-dihydroxy benzoic acid matrix (Agilent Technologies) in 0.1% TFA/60% acetonitrile and dried for 10 min. Samples were analyzed on the o-matrix-assisted laser desorption/ionization QStar™ Pulsar mass spectrometer (Applied Biosystems). Positive TOF MS was collected from 700 to 3,000 D for 1 min.
Assays of Receptor Binding and Cross-linking.
Binding assays with VEGF-D-FULL-N-FLAG and soluble receptor-Ig fusion proteins containing the extracellular domains of human VEGFR-2 or VEGFR-3 and the Fc portion of human IgG1 (provided by Y. Gunji, Haartman Institute, Helsinki, Finland and K. Pajusola, Biotechnology Institute, Helsinki, Finland respectively) were performed as described previously, as were bioassays with Ba/F3 cells and ligands at 750 ng/ml (13).
Results
Assay for VEGF-D Processing.
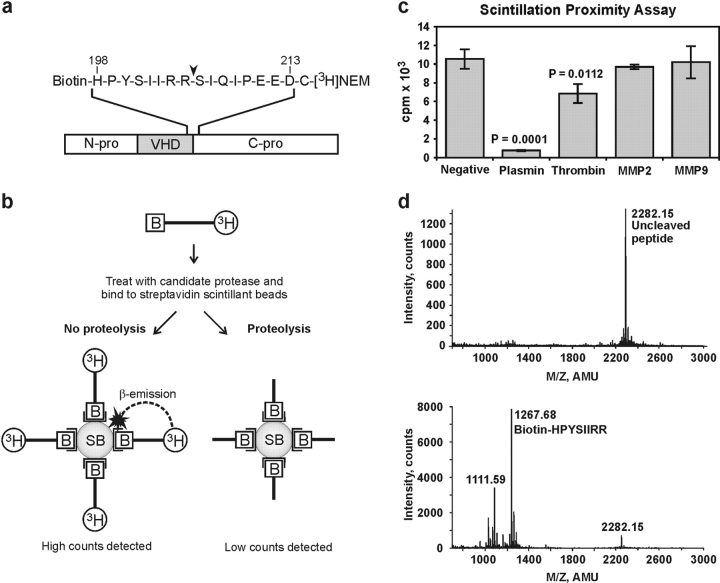
To identify proteases that activate VEGF-D, a scintillation proximity assay (SPA) was developed for monitoring cleavage of the COOH-terminal propeptide from the VHD. The assay was based on the COOH-terminal cleavage because this occurs at a single site in VEGF-D, whereas cleavage of the NH2-terminal propeptide is more complex, occurring at two distinct sites (18). For the assay, a 17-mer peptide (containing residues 198–213 of human VEGF-D) spanning the COOH-terminal cleavage site of VEGF-D, was biotinylated at its NH2 terminus and radiolabeled at its COOH terminus (Fig. 1 a). The principle of the SPA is outlined in Fig. 1 b. After the peptide is treated with proteases, it is bound to streptavidin-conjugated beads impregnated with scintillant. When the peptide is intact, the proximity of the radiolabel at the COOH terminus of the peptide to the scintillant in the beads is sufficient to generate detectable photons. In contrast, there is a dramatic reduction in counts detected when cleavage of the peptide has occurred because the radiolabel is no longer sufficiently close to the scintillant for photons to be generated.
Figure 1.
Scintillation proximity assay. (a) Sequence of the peptide encompassing the site at which the VHD of VEGF-D is cleaved from the COOH-terminal propeptide (C-pro). In VEGF-D from 293EBNA cells, cleavage occurs between arginine 205 and serine 206 (arrowhead; reference 18). Numbers above the amino acid sequence denote positions in human VEGF-D (13). The COOH-terminal cysteine residue, not found in VEGF-D, facilitated radiolabeling. (b) Principle of SPA. Black bars represent biotinylated (B), tritiated VEGF-D peptide that is treated with proteases, and bound to streptavidin-conjugated scintillant beads before β counting. SB denotes scintillant beads and open brackets denote streptavidin moieties conjugated to the beads. (c) SPA results after treatment of VEGF-D peptide with proteases. Values are the average of three replicates ± 1 SD and are representative of duplicate experiments. P-values comparing plasmin- or thrombin-treated samples with negative control were calculated using Student's t test. Negative control is undigested peptide. (d) Mass spectrometric analysis of VEGF-D peptide before (top) and after plasmin digestion (bottom). Identity of the major peak in each panel is shown.
A range of proteases were tested in this assay, including plasmin, thrombin, and MMP-2 and MMP-9. These proteases were chosen because of their involvement in angiogenesis or tumor formation. MMP-2 and MMP-9 had no effect on the counts detected in the SPA, however, plasmin caused a >90% reduction of signal, indicating substantial cleavage of the peptide (Fig. 1 c). Thrombin caused a small reduction of signal. To identify the site at which plasmin hydrolyzed the peptide, samples were analyzed by mass spectrometry. Undigested peptide consisted of a single peak of a molecular mass of 2,282.15 kD, as expected (Fig. 1 d, top). After plasmin treatment, a predominant peak of a molecular mass of 1,267.68 kD was observed, corresponding to Biotin-HPYSIIRR (Fig. 1 d, bottom). This molecular species is an expected product of cleavage of the peptide at the same site as observed in VEGF-D expressed in 293EBNA cells (i.e., between R205 and S206) (Fig. 1 a; reference 18). An alternative cleavage event generating Biotin-HPYSIIR (molecular mass 1,111.59 kD) was also detected.
Plasmin Processes VEGF-D.
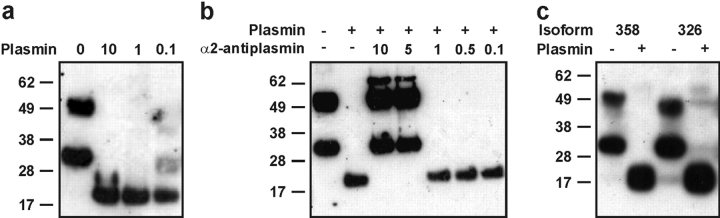
To establish if VEGF-D is a substrate for plasmin, this protease was incubated with full-length human VEGF-D (VEGF-D-FULL-N-FLAG) purified from the medium of transfected 293EBNA cells. A degree of proteolytic processing occurs in the medium of these cells, resulting in VEGF-D preparations containing full-length material (∼50 kD) and a partially processed form (∼31 kD) consisting of the NH2-terminal propeptide and VHD (Fig. 2 a; reference 18). After plasmin digestion, a single ∼21-kD band was detected by Western blotting (Fig. 2 a). This species corresponds to the previously observed mature, fully-processed form of VEGF-D (18), suggesting that plasmin can cleave both the NH2- and COOH-terminal propeptides from the VHD. NH2-terminal amino acid sequencing of this ∼21-kD species revealed two sequences, FAATFYDIE and VIDEE, indicating that cleavage of the NH2-terminal propeptide was occurring at two sites. FAATF (residues 89–93 of VEGF-D) represents the sequence identified as the NH2 terminus of the predominant form of fully-processed, mature VEGF-D purified from the conditioned medium of 293EBNA cells (18). VIDEE (residues 101–105 of VEGF-D) represents an NH2 terminus that is located one residue toward the COOH terminus compared with that of the other form of mature VEGF-D (KVIDEE) detected in the medium of 293EBNA cells (18). Therefore, plasmin cleaves the NH2-terminal propeptide from the VHD at almost identical positions to those described previously (18). In contrast to plasmin, the serine proteases thrombin and tissue plasminogen activator were unable to cleave the propeptides of human VEGF-D from the VHD (unpublished data).
Figure 2.
Analysis of proteolytic processing of VEGF-D by plasmin with Western blotting. (a) Analysis of 100 ng/lane human VEGF-D-FULL-N-FLAG with anti-VHD antibody after digestion with 10, 1, 0.1, or 0 U/ml of plasmin. (b) α2-antiplasmin inhibition of plasmin. Plasmin (1 U/ml; 130 nM) was incubated with a range of α2-antiplasmin concentrations before addition of VEGF-D-FULL-N-FLAG and incubation at 37°C for 1 h. α2-Antiplasmin/plasmin molar ratios are shown above the blot. (c) Analysis of 100 ng/lane mouse VEGF-D isoforms. Mouse VEGF-D358 (358) and VEGF-D326 (326) were treated with 1 U/ml plasmin (+) or remained undigested (–). Sizes of molecular mass markers in kD are shown to the left of each panel.
The plasmin used in this work was purified from human plasma. To eliminate the possibility that the processing of VEGF-D observed was due to a contaminating activity in the plasmin preparation, α2-antiplasmin, a specific inhibitor of plasmin that forms an inactive 1:1 complex with this protease, was incubated with the plasmin sample before digestion of VEGF-D. Analysis of resulting digestion products demonstrated complete inhibition of digestion by α2-antiplasmin when included at a fivefold molar excess to plasmin (Fig. 2 b). Therefore, the observed proteolytic processing of VEGF-D by the plasmin preparation used here was due to plasmin.
Full-length mouse VEGF-D exists as two isoforms, VEGF-D326 and VEGF-D358, that differ in the COOH terminus of the protein (19). Plasmin digestion of the mouse VEGF-D isoforms was performed to analyze the effect of the distinct COOH termini on proteolytic processing. Plasmin treatment of both isoforms produced a ∼21-kD species containing the VHD, as for human VEGF-D, indicating that this enzyme can fully process both isoforms (Fig. 2 c).
Plasmin Generates Bioactive VEGF-D and VEGF-C.
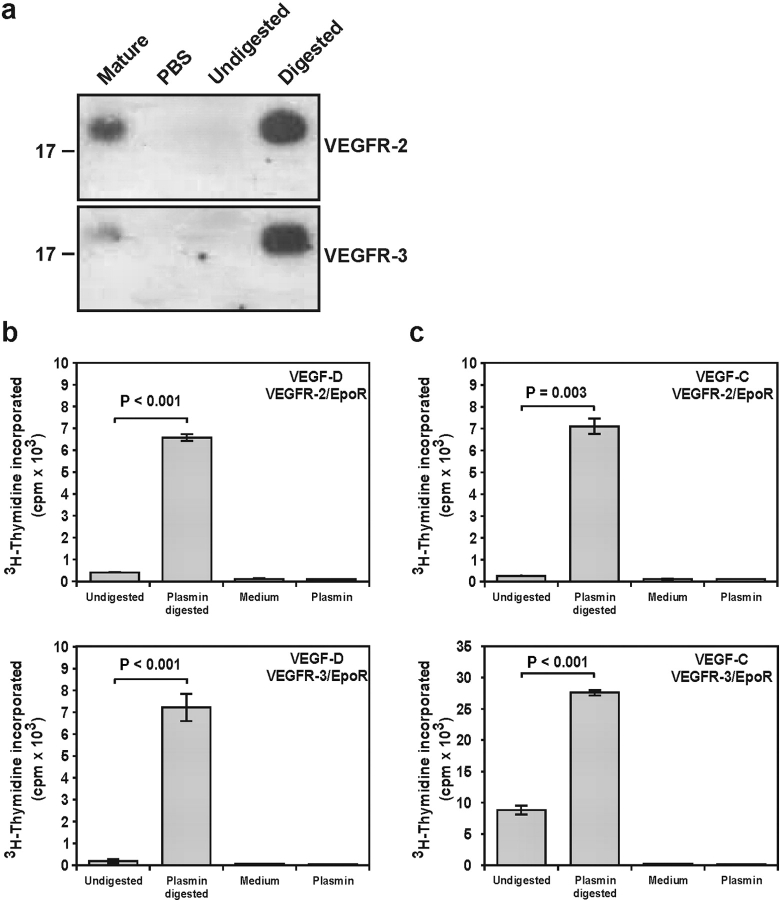
To establish if mature VEGF-D generated by plasmin binds VEGFR-2 and VEGFR-3, we performed immunoprecipitation studies using soluble receptor-Ig fusion proteins containing the extracellular domains of VEGFR-2 or VEGFR-3 (Fig. 3 a). This revealed that the plasmin-generated mature forms bound to both VEGFR-2 and VEGFR-3 extracellular domains.
Figure 3.
Mature growth factors generated by plasmin bind and cross-link receptors. (a) Binding to soluble receptors. Receptor-Ig fusion proteins containing the extracellular domains of human VEGFR-2 or VEGFR-3 were conjugated to protein-A sepharose and incubated with mature recombinant human VEGF-D as positive control (Mature), PBS as negative control, and undigested and plasmin-digested full-length VEGF-D (Undigested and Digested, respectively). (top) VEGFR-2 binding. (bottom) VEGFR-3 binding. Material bound to the receptor-Ig proteins was analyzed by Western blotting using an anti-VHD antibody. Plasmin-generated mature VEGF-D (∼21 kD) is apparent. Sizes of molecular mass standards (kD) are shown to the left. (b and c) Analysis of receptor binding and cross-linking in BaF3 bioassays. Ba/F3 cells expressing chimeric receptors containing the extracellular domains of VEGFR-2 or VEGFR-3 and the cytoplasmic domain of EpoR were treated with plasmin-digested or undigested full-length VEGF-D (b) or VEGF-C (c). (top) VEGFR-2/EpoR bioassays. (bottom) VEGFR-3/EpoR bioassays. Controls were medium lacking growth factor (Medium) or plasmin digests lacking growth factor (Plasmin). Values are the average of duplicates ± 1 SD and are representative of three experiments. P-values comparing results of plasmin-digested with undigested material were calculated using Student's t test.
To compare the capacities of full-length and plasmin-generated mature VEGF-D to bind and cross-link receptors at the cell surface, bioassays were used using Ba/F3 preB cells expressing chimeric receptors consisting of the extracellular domains of human VEGFR-2 or VEGFR-3 and the transmembrane and cytoplasmic domains of the erythropoietin receptor (EpoR; references 13, 20). These cell lines are IL-3–dependent, however, signaling from the EpoR cytoplasmic domain, which occurs when the extracellular domains of the chimeric receptors are cross-linked by ligand, leads to cell survival and proliferation in the absence of IL-3. These bioassays allow comparison of receptor binding and cross-linking and were used to define the receptor interactions of a range of VEGFR-2 and VEGFR-3 ligands (13, 20). Cells were incubated with undigested or plasmin-digested VEGF-D in the absence of IL-3 and the proliferative response assessed by incorporation of [3H]thymidine into DNA. Cells expressing either the VEGFR-2 or VEGFR-3 chimeric receptors, which were exposed to plasmin-digested VEGF-D, exhibited a much greater response than those treated with undigested protein (Fig. 3 b). Therefore, plasmin treatment generates mature forms of VEGF-D that are much better able to bind and cross-link VEGFR-2 and VEGFR-3 at the cell surface than full-length material. Comparable results were observed with VEGF-C (Fig. 3 c), indicating that plasmin activates both of the known lymphangiogenic growth factors.
Discussion
In addition to activating the lymphangiogenic growth factors VEGF-C and VEGF-D, plasmin modulates the effects of the angiogenic protein VEGF. Plasmin cleaves some VEGF isoforms, releasing them from the extracellular matrix or cell surface and, thus, making them available for inducing angiogenesis (21, 22). In a porcine model of cutaneous wound healing, lymphatic vessels were observed to appear concurrently with blood vessels (16), suggesting that angiogenesis and lymphangiogenesis are coordinately regulated. As VEGF-C and VEGF-D are localized on vascular smooth muscle in adult tissues (23, 24) and VEGF levels are elevated in cutaneous wounds (25), these growth factors are available to coordinate angiogenesis and lymphangiogenesis as a result of activation by plasmin during wound healing. Furthermore, plasmin degrades fibrin clots and, therefore, could integrate fibrinolysis and vessel formation during wound healing. Examination of this hypothesis will require (a) analysis of the relative activity of plasmin for VEGF, VEGF-C, and VEGF-D and (b) correlation of local plasmin levels with the timing of angiogenesis and lymphangiogenesis during wound healing.
Clinical analyses revealed that expression of VEGF-C or VEGF-D in breast, ovarian, and colorectal cancer may be an independent prognostic indicator of survival associated with lymph node metastasis (8–11). Moreover, expression of lymphangiogenic growth factors promoted metastatic spread of tumor cells via the lymphatics in animal models (6, 7, 26). Plasmin and other members of the fibrinolytic system have also been associated with tumor growth and metastasis (for review see reference 27) as shown in models in which the fibrinolytic system was manipulated. For example, overexpression of plasminogen activator inhibitor-2 inhibited the metastasis of a human melanoma cell line to both the lymph nodes and the lung (28). Furthermore, plasminogen-null mice displayed fewer regional lymph node metastases than controls when transplanted with Lewis lung carcinoma (29). A contributing factor underlying these observations could be that down-regulation of plasmin production leads to diminished tumor lymphangiogenesis and metastasis via the lymphatics. Therefore, analysis of tumor lymphatics and lymphangiogenesis in these animal models of cancer is required.
Studies in animal models showed that inhibiting the VEGFR-3 signaling pathway blocked tumor lymphangiogenesis and metastasis via the lymphatics (7, 30). Approaches included administration of an anti–VEGF-D antibody that blocked the binding of VEGF-D to VEGFR-2 and VEGFR-3 (7) or the soluble extracellular domain of VEGFR-3 to sequester VEGF-C and VEGF-D (30). Alternatively, this pathway could be targeted at the level of ligand activation using ligand-binding molecules that block interaction with the processing proteases. The finding that plasmin activates lymphangiogenic growth factors provides an opportunity to screen for such inhibitors.
Acknowledgments
We thank C. Parish for assistance with the SPA, D. Frecklington for mass spectrometry, and G. Reid, I. Smith, and T. Burgess for helpful comments.
This work was supported by the National Health and Medical Research Council of Australia (NH and MRC) and The Cancer Council Victoria. S.A. Stacker is supported by a Senior Research Fellowship from the Pharmacia Foundation and C. Freeman by an NH and MRC program grant.
Abbreviations used in this paper: EpoR, erythropoietin receptor; MES, 2-morpholinoethane sulfonic acid; MMP, matrix metalloproteinase; NEM, [3H]N-ethylmaleimide; SPA, scintillation proximity assay; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; VHD, VEGF homology domain.
The current address of M.E. Baldwin is the Dept. of Molecular Oncology, Genentech Inc., South San Francisco, CA 94080.
References
- 1.Jeltsch, M., A. Kaipainen, V. Joukov, X. Meng, M. Lakso, H. Rauvala, M. Swartz, D. Fukumura, R.K. Jain, and K. Alitalo. 1997. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 276:1423–1425. [DOI] [PubMed] [Google Scholar]
- 2.Veikkola, T., L. Jussila, T. Makinen, T. Karpanen, M. Jeltsch, T.V. Petrova, H. Kubo, G. Thurston, D.M. McDonald, M.G. Achen, et al. 2001. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byzova, T.V., C.K. Goldman, J. Jankau, J. Chen, G. Cabrera, M.G. Achen, S.A. Stacker, K.A. Carnevale, M. Siemionow, S.R. Deitcher, and P.E. DiCorleto. 2002. Adenovirus encoding vascular endothelial growth factor-D induces tissue-specific vascular patterns in vivo. Blood. 99:4434–4442. [DOI] [PubMed] [Google Scholar]
- 4.Szuba, A., M. Skobe, M.J. Karkkainen, W.S. Shin, D.P. Beynet, N.B. Rockson, N. Dakhil, S. Spilman, M.L. Goris, H.W. Strauss, et al. 2002. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 16:1985–1987. [DOI] [PubMed] [Google Scholar]
- 5.Karkkainen, M.J., A. Saaristo, L. Jussila, K.A. Karila, E.C. Lawrence, K. Pajusola, H. Bueler, A. Eichmann, R. Kauppinen, M.I. Kettunen, et al. 2001. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA. 98:12677–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandriota, S.J., L. Jussila, M. Jeltsch, A. Compagni, D. Baetens, R. Prevo, S. Banerji, J. Huarte, R. Montesano, D.G. Jackson, et al. 2001. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacker, S.A., C. Caesar, M.E. Baldwin, G.E. Thornton, R.A. Williams, R. Prevo, D.G. Jackson, S.-I. Nishikawa, H. Kubo, and M.G. Achen. 2001. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 7:186–191. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura, Y., H. Yasuoka, M. Tsujimoto, Q. Yang, A. Tsukiyama, S. Imabun, M. Nakahara, K. Nakao, M. Nakamura, I. Mori, and K. Kakudo. 2003. Clinicopathological significance of vascular endothelial growth factor-C in breast carcinoma with long-term follow-up. Mod. Pathol. 16:309–314. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama, Y., D.S. Charnock-Jones, D. Licence, A. Yanaihara, J.M. Hastings, C.M. Holland, M. Emoto, M. Umemoto, T. Sakamoto, S. Sato, et al. 2003. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br. J. Cancer. 88:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White, J.D., P.W. Hewett, D. Kosuge, T. McCulloch, B.C. Enholm, J. Carmichael, and J.C. Murray. 2002. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 62:1669–1675. [PubMed] [Google Scholar]
- 11.Nakamura, Y., H. Yasuoka, M. Tsujimoto, Q. Yang, S. Imabun, M. Nakahara, K. Nakao, M. Nakamura, I. Mori, and K. Kakudo. 2003. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin. Cancer Res. 9:716–721. [PubMed] [Google Scholar]
- 12.Joukov, V., K. Pajusola, A. Kaipainen, D. Chilov, I. Lahtinen, E. Kukk, O. Saksela, N. Kalkkinen, and K. Alitalo. 1996. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt-4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 13.Achen, M.G., M. Jeltsch, E. Kukk, T. Mäkinen, A. Vitali, A.F. Wilks, K. Alitalo, and S.A. Stacker. 1998. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk-1) and VEGF receptor 3 (Flt-4). Proc. Natl. Acad. Sci. USA. 95:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veikkola, T., M. Karkkainen, L. Claesson-Welsh, and K. Alitalo. 2000. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 60:203–212. [PubMed] [Google Scholar]
- 15.Dumont, D.J., L. Jussila, J. Taipale, A. Lymboussaki, T. Mustonen, K. Pajusola, M. Breitman, and K. Alitalo. 1998. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 282:946–949. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen, K., P. Puolakkainen, L. Jussila, T. Jahkola, and K. Alitalo. 2000. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 156:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joukov, V., T. Sorsa, V. Kumar, M. Jeltsch, L. Claesson-Welsh, Y. Cao, O. Saksela, N. Kalkkinen, and K. Alitalo. 1997. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16:3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacker, S.A., K. Stenvers, C. Caesar, A. Vitali, T. Domagala, E. Nice, S. Roufail, R.J. Simpson, R. Moritz, T. Karpanen, et al. 1999. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 274:32127–32136. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin, M.E., S. Roufail, M.M. Halford, K. Alitalo, S.A. Stacker, and M.G. Achen. 2001. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 276:44307–44314. [DOI] [PubMed] [Google Scholar]
- 20.Achen, M.G., S. Roufail, T. Domagala, B. Catimel, E.C. Nice, D.M. Geleick, R. Murphy, A.M. Scott, C. Caesar, T. Makinen, et al. 2000. Monoclonal antibodies to vascular endothelial growth factor-D block interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem. 267:2505–2515. [DOI] [PubMed] [Google Scholar]
- 21.Houck, K.A., D.W. Leung, A.M. Rowland, J. Winer, and N. Ferrara. 1992. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267:26031–26037. [PubMed] [Google Scholar]
- 22.Plouët, J., F. Moro, S. Bertagnolli, N. Coldeboeuf, H. Mazarguil, S. Clamens, and F. Bayard. 1997. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J. Biol. Chem. 272:13390–13396. [DOI] [PubMed] [Google Scholar]
- 23.Achen, M.G., R.A. Williams, M.P. Minekus, G.E. Thornton, K. Stenvers, P.A.W. Rogers, F. Lederman, S. Roufail, and S.A. Stacker. 2001. Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumour angiogenesis. J. Pathol. 193:147–154. [DOI] [PubMed] [Google Scholar]
- 24.Partanen, T.A., J. Arola, A. Saaristo, L. Jussila, A. Ora, M. Miettinen, S.A. Stacker, M.G. Achen, and K. Alitalo. 2000. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 14:2087–2096. [DOI] [PubMed] [Google Scholar]
- 25.Yao, F., S. Visovatti, C.S. Johnson, M. Chen, J. Slama, A. Wenger, and E. Eriksson. 2001. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 9:371–377. [DOI] [PubMed] [Google Scholar]
- 26.Skobe, M., T. Hawighorst, D.G. Jackson, R. Prevo, L. Janes, P. Velasco, L. Riccardi, K. Alitalo, K. Claffey, and M. Detmar. 2001. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7:192–198. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen, P.A., R. Egelund, and H.H. Petersen. 2000. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 57:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller, B.M., Y.B. Yu, and W.E. Laug. 1995. Overexpression of plasminogen activator inhibitor 2 in human melanoma cells inhibits spontaneous metastasis in scid/scid mice. Proc. Natl. Acad. Sci. USA. 92:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bugge, T.H., K.W. Kombrinck, Q. Xiao, K. Holmback, C.C. Daugherty, D.P. Witte, and J.L. Degen. 1997. Growth and dissemination of Lewis lung carcinoma in plasminogen-deficient mice. Blood. 90:4522–4531. [PubMed] [Google Scholar]
- 30.He, Y., K. Kozaki, T. Karpanen, K. Koshikawa, S. Yla-Herttuala, T. Takahashi, and K. Alitalo. 2002. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 94:819–825. [DOI] [PubMed] [Google Scholar]