Abstract
Antibodies capable of inhibiting the invasion of Plasmodium merozoites into erythrocytes are present in individuals that are clinically immune to the malaria parasite. Those targeting the 19-kD COOH-terminal domain of the major merozoite surface protein (MSP)-119 are a major component of this inhibitory activity. However, it has been difficult to assess the overall relevance of such antibodies to antiparasite immunity. Here we use an allelic replacement approach to generate a rodent malaria parasite (Plasmodium berghei) that expresses a human malaria (Plasmodium falciparum) form of MSP-119. We show that mice made semi-immune to this parasite line generate high levels of merozoite inhibitory antibodies that are specific for P. falciparum MSP-119. Importantly, protection from homologous blood stage challenge in these mice correlated with levels of P. falciparum MSP-119–specific inhibitory antibodies, but not with titres of total MSP-119–specific immunoglobulins. We conclude that merozoite inhibitory antibodies generated in response to infection can play a significant role in suppressing parasitemia in vivo. This study provides a strong impetus for the development of blood stage vaccines designed to generate invasion inhibitory antibodies and offers a new animal model to trial P. falciparum MSP-119 vaccines.
Keywords: malaria, transfection, P. berghei, merozoite, invasion inhibition
Introduction
It is well established that antibodies targeting merozoite antigens can mediate a degree of immunity to Plasmodium falciparum malaria and this knowledge underpins the development of blood stage vaccines against this devastating pathogen (for review see references 1 and 2). Such antibodies are thought to operate by a number of different mechanisms including the prevention of merozoite release (3), the direct neutralization of merozoites (4, 5), and the induction of monocyte-mediated parasite killing (6). However, the relative contribution of these different mechanisms to controlling blood stage parasitaemia remains unclear. A better understanding of this is particularly important for the development of functional “correlate-of-protection” assays for use in clinical trials of malaria vaccine candidates.
A protective role for merozoite invasion inhibitory antibodies, which are those that act in a manner that is independent of complement or other cellular mediators, has been difficult to formally demonstrate and quantify. There are several reasons for this. First, there has been a lack of robust in vitro inhibition assays that account for confounding factors present in serum that can cause nonspecific inhibitory, or indeed growth-promoting, effects. Although in vitro inhibition assays have been used for some time to assess antibodies to P. falciparum merozoite antigens and have provided a useful guide as to the inhibitory activity of a particular serum or monoclonal antibody, the problems associated with accurate quantification of this activity, especially in whole serum, are well recognized in the field (4, 5, 7, 8). With respect to one important antigen, the 19-kD COOH-terminal domain of P. falciparum merozoite surface protein (MSP)-119, this problem has now been overcome with the development of an assay that allows accurate quantification of MSP-119–specific inhibitory antibodies in whole serum (9, 10). This assay involves a comparison of the inhibitory effect of a given serum on two isogenic parasite lines that differ only in MSP-119. One expresses the P. falciparum domain and the other expresses an antigenically distinct domain from a rodent malaria parasite Plasmodium chabaudi. Using this assay in a previous study, we determined that the invasion inhibitory activity of antibodies present in serum obtained from adults that are clinically immune to malaria, as a result of frequent exposure to P. falciparum, is dominated by those targeting MSP-119 (9). This finding was consistent with many studies that had implicated a protective role for MSP-119–specific antibodies generated in response to infection or vaccination with recombinant proteins (for review see references 11 and 12). Despite the surprising knowledge that antibodies of this single specificity make such an important contribution to the process of invasion inhibition, it remained uncertain whether these antibodies actually contribute significantly to immunity.
The major remaining obstacle to addressing the protective role of inhibitory antibodies is the lack of a simple and robust in vivo challenge system that can be used in parallel with an in vitro inhibition test. In this paper, we have developed such an in vivo model by generating a rodent malaria parasite line that expresses P. falciparum MSP-119 in place of its own domain. Using this model, we show that the level of MSP-119–specific invasion inhibitory antibodies generated in mice that had been repeatedly exposed to this chimeric parasite line correlates with the ability of these animals to control a subsequent blood stage infection. The availability of this novel rodent malaria model also provides an alternative to nonhuman primates for assessing and monitoring P. falciparum MSP-119–based vaccines.
Materials and Methods
Plasmids and Plasmodium berghei Transfection.
To create pPb-PfM19, 1.3 Kb P. berghei MSP-1 targeting sequence was fused in frame to the MSP-119 region of P. falciparum (see Fig. 1). PCR was performed on P. berghei ANKA and P. falciparum D10 genomic DNA (gDNA) using oligonucleotides PbF (5′-CGGGGTACCATCGATAAATACTTTACCTCTGAAGCTGTTCC) and PbR1 (5′- TACATGCTTAGGGTCTATACCTAATAAATC), and PbPfF (5′-GGTATAGACCCTAAGCATGTATGCGTAAAAAAACAATGTCCAGAA) and PfR (5′-TGCTCTAGATTAAATGAAACTGTATAATATTAAC), respectively, and sewing products together via PCR using the primers PbF and PfR. The resulting fragment was cloned into the KpnI/XbaI sites of pGem4Z (Promega) that harbored the hsp86 3′ untranslated region (UTR; reference 13). The MSP-1/hsp86 3′ sequence was excised with KpnI/HindIII, the HindIII site filled in with Klenow reagent and the fragment cloned into the KpnI/HincII site of pDBDTmΔHDB (14). A 0.55 Kb 3′ targeting sequence was then cloned into the EcoRV/BamHI site of this vector to create pPb-PfM19. The 3′ targeting region comprising the P. berghei MSP-1 3′ UTR was identified by library screen (15) and PCR amplified from P. berghei ANKA gDNA using oligonucleotides PbM3′F (5′-GGCGATATCATAAATTATTGAAATATTTGTTGGA) and PbM3′R (5′-CGCGGATCCTATACAAAACATATACAAC). The plasmid pPb-PbM19 is analogous to that of pPb-PfM19 with the exception that the entire MSP-1 5′ targeting sequence is that of P. berghei. This fragment was amplified from P. berghei ANKA gDNA using the oligonucleotides PbF and PbR2 (5′-TGCTCTAGATTAAAATATATTAAATACAATTAATGTG). Linearized plasmids were transfected into P. berghei ANKA parasites essentially as previously described (16, 17, 18).
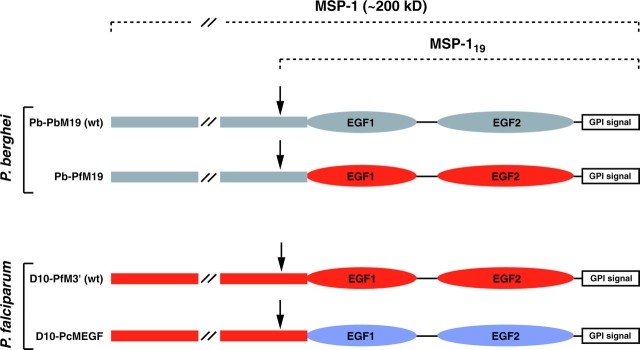
Figure 1.
Schematic representation of P. berghei and P. falciparum MSP-1 chimeras. The MSP-1 sequences of P. berghei (gray), P. falciparum (red), and P. chabaudi (blue) are represented. The arrows indicate the MSP-1 secondary cleavage site.
MSP-119 Glutathione S-Transferase (GST) Fusion Proteins and Generation of Antisera.
The DNA sequence corresponding to amino acids Gly 1672–Ser 1766 of P. berghei MSP-119 was amplified using the oligonucleotides PbM19eF (5′-CGCGGATCCGGTATAGACCCTAAGCATGTATG) and PbM19eR (5′-GGAAGATCTTAGCTACAGAATACACCATCATAAT). The resulting product was ligated into the BamHI site of pGEX-4T-1 and expressed as a GST fusion protein (termed GST-PbM19). Rabbit antisera to GST-PbM19 was derived as previously described (9). Western blot and indirect immunofluorescence assay (IFA) were performed as previously described (9, 19, 20).
Generation of Semi-immune Mice.
Semi-immune BALB/c mice were generated by the administration of 104 erythrocytes infected with either Pb-PbM19 or Pb-PfM19. At 5–10% parasitemia, mice were treated consecutively for 5 d with chloroquine (10 mg/kg body weight, injected i.p.). Recrudescence was typically observed 1 wk after this primary infection, after which mice were administered another five doses of chloroquine. 1 mo later mice were experimentally reinfected and then drug cured as described above. Sera were obtained from individual mice 10 d after the final drug treatment to monitor MSP-119 antibodies. For challenge infections, mice were injected i.p with 5 × 106 Pb-PbM19– or Pb-PfM19–infected erythrocytes and the course of parasitemia was monitored by microscopy.
Serology.
Antibodies reacting with recombinant P. berghei or P. falciparum MSP-119 were detected by ELISA (9). Blood taken from mice before primary infection were used as negative controls. The ELISA endpoint titres were taken as the highest serum dilution that gave an OD reading five times above that of the control sera. Inhibition of invasion assays were performed as previously described (9).
Results
Allelic Replacement of P. berghei MSP-119 with P. falciparum MSP-119: Functional Complementation of Divergent MSP-119 Sequences.
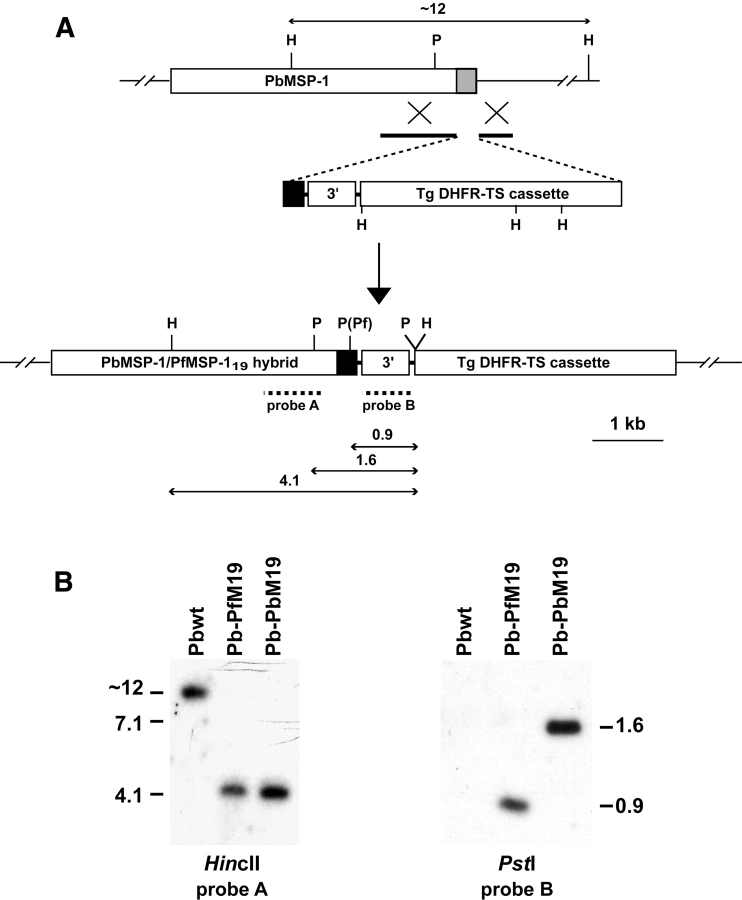
To establish whether P. falciparum MSP-119 can complement the in vivo function of the divergent P. berghei MSP-119 domain, we created a P. berghei MSP-1 chimera that expresses P. falciparum MSP-119 in place of the endogenous molecule (Fig. 1) . The transfection vector pPb-PfM19 was designed to integrate into P. berghei MSP-1 and replace the endogenous sequences encoding epidermal growth factor (EGF) domains 1 and 2, and the GPI recognition sequence, with the corresponding P. falciparum (D10 line) sequence (Figs. 1 and 2 A). A second plasmid, pPb-PbM19, designed to integrate in an identical manner but resulting in a homologous MSP-119 replacement, was also constructed to generate a control transfectant. Both plasmids were electroporated into the P. berghei (ANKA) line and transgenic parasites were cloned by limiting dilution. Southern blot analysis of gDNA showed that integration had occurred in these parasites by the expected double crossover event into MSP-1 (Fig. 2). The resulting P. berghei/P. falciparum chimeric line, which we have termed Pb-PfM19, could be distinguished from a control P. berghei transfection line, termed Pb-PbM19, by digestion with PstI (Fig. 2 B). In addition, PCR amplification of gDNA using oligonucleotides specific for the integration events confirmed the expected integration event (unpublished data).
Figure 2.
Generation of P. berghei chimera lines containing either P. berghei or P. falciparum MSP-1 19. (A) Schematic diagram of the P. berghei MSP-1 locus, the transfection vector (pPb-PfM19) used to replace the endogenous MSP-119 molecule, and the predicted MSP-1 locus of the Pb-PfM19 chimeric line after integration. The gray box represents endogenous P. berghei MSP-1 19 sequence and the black box represents P. falciparum MSP-1 19 sequence (or P. berghei MSP-1 19 sequence for creation of the Pb-PbM19 chimeric line). The solid lines in pPb-PfM19 depict targeting sequence. TgDHFR-TS, selectable marker cassette; 3′, HSP86 3′ UTR. The expected sizes of fragments resulting from digestion with either HincII (H) or PstI (P) are shown. (B) Southern blot analysis of digested genomic DNA from P. berghei wild-type and chimeric lines.
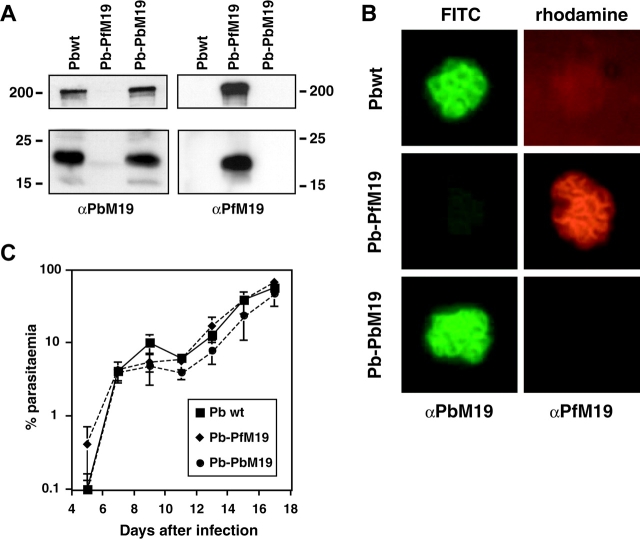
To determine whether the Pb-PfM19 and Pb-PbM19 lines expressed the expected MSP-119 domains, Western blot analysis was performed on late stage parasite extracts using specific anti–MSP-119 antibodies (Fig. 3 A). P. falciparum MSP-119 antibodies recognized both MSP-119 and an ∼200-kD band corresponding to full-length MSP-1 in Pb-PfM19 parasites but not in Pb-PbM19 parasites. In contrast, antibodies specific for P. berghei MSP-119 only recognized MSP-119 and full-length MSP-1 in wild-type P. berghei and the transfection control line, Pb-PbM19. This demonstrates that P. falciparum MSP-119 can be correctly expressed and processed in P. berghei and that the endogenous MSP-119 gene is no longer expressed in Pb-PfM19 parasites. The localization of MSP-119 in P. berghei lines was also assessed by double-labeling IFA. Characteristic merozoite surface labeling was observed in both chimera lines, with Pb-PfM19 parasites reacting only with the P. falciparum–specific monoclonal antibody 4H9/19, whereas P. berghei wild-type and Pb-PbM19 chimeric parasites reacted only with rabbit anti–P. berghei MSP-119 antibodies (Fig. 3 B). This confirms that the appropriate MSP-119 domain is correctly localized in both Pb-PbM19 and Pb-PfM19 parasite lines. The growth rates of the transfected lines were also examined and compared with the wild-type parasite line (Fig. 3 C). All mice injected with 104 parasites succumbed to infection over a similar time frame, regardless of which parasite line they were given. These results extend our previous finding that the function of MSP-119 during in vitro culture is conserved across divergent Plasmodium species (9, 20) to show that MSP-119 function is also conserved during the erythrocytic cycle in vivo.
Figure 3.
Phenotypic analysis of P. berghei chimeric lines. (A) Western blot analysis of late stage parasite extracts using rabbit αPbM19 or αPfM19 antibodies (both diluted 1/4,000) detecting both full-length MSP-1 (∼200 kD) and MSP-119 (∼19 kD). (B) Localization of MSP-119 by IFA. Schizont stage parasites were incubated with a mixture of rabbit αPbM19 (1/1,000) and 4H9/19 (αPfM19; 1/100) antibodies, followed by a mixture of FITC-conjugated anti–rabbit and rhodamine-conjugated anti–mouse immunoglobulins (both diluted 1/200). (C) Course of blood parasitemia in mice after infection at day 0 with P. berghei wild-type, Pb-PbM19, or Pb-PfM19. Shown is the mean ± SD of the parasitemia observed in five mice.
A Key Role for MSP-119–specific Invasion Inhibitory Antibodies in Protection Elicited by Repeated Infection/Drug Cure.
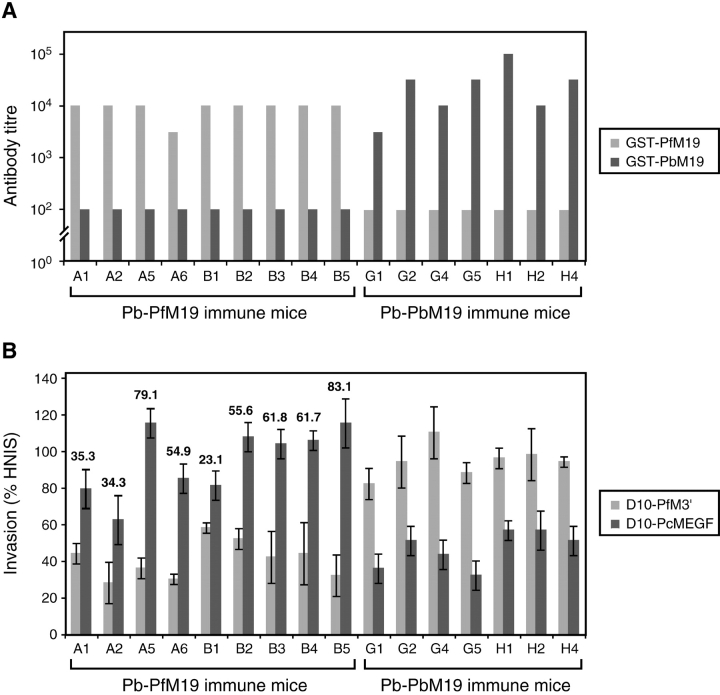
Sera from BALB/c mice that were rendered semi-immune to either Pb-PfM19 or Pb-PbM19 as a result of a low dose infection/drug cure regimen were tested for total MSP-119 antibodies in ELISA and for MSP-119–specific invasion inhibitory antibodies. Using MSP-119 GST fusion proteins as antigen in an ELISA, all mice generated a strong MSP-119 antibody response that was specific for the relevant MSP-119 domain (Fig. 4 A). This data highlights the immunogenicity of this domain in the context of a low dose blood stage infection procedure and validates the expression of the appropriate MSP-119 domains in the transfected P. berghei lines.
Figure 4.
Mice repeatedly infected with P. berghei transfectants elicit MSP-119–specific inhibitory antibodies. (A) Reciprocal anti–MSP-119 antibody endpoint titres of serum from Pb-PfM19 and Pb-PbM19 immune mice against recombinant P. falciparum and P. berghei MSP-119–GST fusion proteins. (B) Invasion inhibition assay of D10-PfM3′ and D10-PcMEGF parasite lines in the presence of individual serum from Pb-PfM19 and Pb-PbM19 immune mice. Invasion is expressed as a percentage of the invasion observed in parasites cultured in human nonimmune sera (HNIS). The numbers shown represent the P. falciparum MSP-119–specific invasion inhibitory activity of a given serum, calculated by subtracting the invasion rate of D10-PfM3′ from that of D10-PcMEGF.
For the in vitro inhibition assay, the ability of a given serum to inhibit the invasion of RBC by two isogenic P. falciparum lines, D10-PfM3′ and D10-PcMEGF (Fig. 1), was compared. D10-PcMEGF expresses the antigenically diverse P. chabaudi MSP-119 polypeptide and so is not recognized by P. falciparum MSP-119–specific antibodies. This line was used as an alternative to a P. falciparum chimera expressing P. berghei MSP-119 because the latter is currently not available. Hence, P. falciparum MSP-119–specific invasion inhibitory activity of a given serum can be calculated by determining the difference in invasion rates of D10-PfM3′, which uses the wild-type P. falciparum MSP-119 domain for invasion, and D10-PcMEGF in the presence of the test serum. All sera from Pb-PfM19 mice inhibited D10-PfM3′ parasites far more effectively than D10-PcMEGF parasites (Fig. 4 B). Conversely, all sera from Pb-PbM19 immune mice inhibited D10-PcMEGF more effectively than wild-type P. falciparum. Because P. chabaudi and P. berghei are closely related rodent parasites with somewhat conserved MSP-119 domains (73% identity), a degree of antigenic cross-reactivity was expected here. However, because many epitopes differ between the MSP-119 domains of rodent malaria parasites (21), the invasion inhibitory activity of these sera cannot be accurately determined.
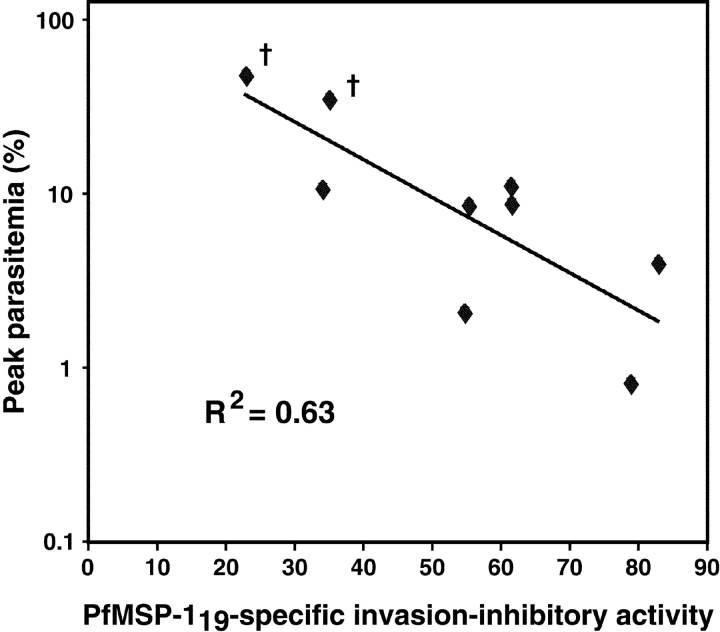
To determine if there is an association between the levels of MSP-119–specific invasion inhibitory antibodies present in mouse serum and degree of protection from a subsequent parasite challenge, Pb-PfM19 semi-immune mice were administered a high dose (5 × 106) of Pb-PfM19–infected erythrocytes 3 d after they had been bled for the serological analyses. After challenge, the course of parasitemia was determined and plotted against levels of P. falciparum MSP-119–specific invasion inhibitory antibodies (Fig. 5) . Strong evidence of regression was observed (R2 = 0.63; P = 0.01 by ANOVA), implicating a substantial role for MSP-119–specific inhibitory antibodies in controlling a blood stage infection. A similarly significant regression curve was evident when invasion inhibition was plotted against cumulative parasitemia in these mice (R2 = 0.56; P = 0.02). The apparent linear relationship shown in Fig. 5 might be explained by the fact that P. berghei growth in BALB/c mice in our hands has two periods of almost log phase expansion (see Fig. 3 C). We also performed a two-sided rank correlation test, a more stringent analysis that does not assume a linear relationship between two parameters. This analysis also demonstrated significance for peak parasitemia versus MSP-119–specific invasion inhibition (P = 0.05). Importantly, all mice had very similar anti–PfMSP-119 ELISA antibody endpoint titres when measured against MSP-119 GST fusion proteins (Fig. 4 A) or a baculovirus-expressed P. falciparum MSP-142 antigen (MAD20 allele). The latter antigen is likely to represent MSP-119 in a more appropriate conformation and the reciprocal endpoint titres obtained in this case ranged from 104.5–105 for Pb-PfM19 mice and ≥102 for Pb-PbM19 mice. Hence, no association between IgG levels and protection were observed.
Figure 5.
Evidence that MSP-119–specific inhibitory antibodies control a blood stage infection. MSP-119–specific invasion inhibitory activity of serum from individual Pb-PfM19 immune mice plotted against peak parasitemia attained after challenging corresponding mice with Pb-PfM19.
To assess the overall contribution of MSP-119 to blood stage immunity, in parallel with the homologous challenge experiment described above, groups of Pb-PbM19 and Pb-PfM19 immune mice were cross-challenged with the heterologous line. In both cases the data suggested that the groups challenged with the homologous parasite line were better protected. When homologous and heterologous challenge groups were combined for analysis, a difference was observed for mean peak parasitemia (14.8% in the homologous groups compared with 18.8% in the heterologous groups), cumulative parasitemia (586% compared with 1,033%), and time until parasite clearance (<0.01%; 12.6 compared with 15.1 d). However, although this trend was consistent across all parameters measured, only the latter approached statistical significance (P = 0.056 using log-rank test). Thus, to further explore the overall importance of MSP-119 antibodies to immunity, sera from P. berghei immune mice was pooled and transferred into two groups of five BALB/c mice (150 μl serum/mouse on days −1 and 0), which were subsequently challenged (on day 0) with 104 Pb-PbM19 or Pb-PfM19 parasites. Mice challenged with the homologous line were significantly better protected than those challenged with Pb-PfM19 when comparing the parasitemia of the mice in the two groups over the first 10 d after challenge (P = 0.05 using a one-sided permutation test) or when comparing the time taken to reach 2% parasitemia (P < 0.05 by log-rank test). Together, these data are consistent with a significant role for MSP-119 antibodies in the control of blood stage parasites.
Discussion
Although antibodies directed against merozoite antigens are known targets of protective immunity, the relative importance of such antibodies to clinical immunity and their mode of action remain uncertain. In this paper we provide strong evidence implicating a key role for merozoite invasion inhibitory antibodies in reducing the parasite burden in animals previously exposed to blood stage parasites. In addition, consistent with our previous findings (9), we demonstrate that antibodies that are specific for the EGF domains of MSP-119 comprise a large component of the inhibitory activity of the sera from these animals.
This study involved the generation of a P. berghei parasite line (Pb-PfM19) that expresses the P. falciparum MSP-119 domain in place of its own domain and an experiment that demonstrates that the in vivo function of MSP-119 is fully conserved across distantly related Plasmodium species. It is now clear that the extensive amino acid differences between rodent and human (P. falciparum) malaria MSP-119 domains, including an absent disulfide bond in the first EGF domain of the rodent malaria form, are not involved in species-specific erythrocyte invasion events either in vivo (this study) or in vitro (9, 20).
Our observation that the level of inhibitory antibodies specific for MSP-119 correlates with the control of parasitaemia upon subsequent blood stage challenge has a number of important implications. First, the dominant protective role for antibodies of this single specificity provides strong support for the development of MSP-119–based vaccines. Second, our findings validate the quantification of MSP-119–specific inhibitory antibodies as a useful correlate-of-protection assay. It should be stressed that the measurement of total MSP-119–specific immunoglobulins is not particularly useful in this regard as some B cell epitopes formed by this protein are not protective (7, 22) and indeed we found no correlation between IgG levels and either invasion inhibition or protection.
The model developed here may prove to be a useful tool to test and monitor the potency of P. falciparum MSP-119–based vaccines. At present there are numerous candidate vaccines based on this domain that are undergoing preclinical testing including different recombinant forms, conformers, and vaccine combinations that incorporate MSP-119 as one component. MSP-142, a protein that incorporates MSP-119 and MSP-133, is the most advanced of these candidates. It appears that the protective antibodies elicited by MSP-142 are directed against the MSP-119 fragment (the MSP-133 fragment may contain T cell epitopes) and hence the animal model developed here should be useful to analyze this vaccine. Currently, the only P. falciparum challenge model available to test MSP-119 antigens involves the use of nonhuman primates. Although vaccine testing in these animals is probably an important precursor to human trials, it is clearly substantially less practical for routine efficacy testing than the simple rodent challenge model described here.
Acknowledgments
We thank Drs. Chris Janse and Andy Waters for helpful advice on P. berghei transfection and for provision of the P. berghei cloning plasmid, Drs. Carole Long, Anthony Stowers, and Louis Miller for the baculovirus-expressed MSP-142 antigen, and Drs. Louis Schofield and Diana Hanson for helpful discussion. We also acknowledge the expert technical services provided by Lynn Buckingham. We are grateful to the Australian Red Cross Blood Bank for the provision of human blood and serum.
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia. T.F. de Koning-Ward, R.A. O'Donnell, and D.R. Drew are recipients of postdoctoral training awards from the NHMRC. B.S. Crabb is an International Research Scholar of the Howard Hughes Medical Institute.
Abbreviations used in this paper: EGF, epidermal growth factor; GST, glutathione S-transferase; IFA, immunofluorescence assay; MSP, merozoite surface protein; UTR, untranslated region.
References
- 1.Holder, A.A. 1999. Malaria vaccines. Proc. Natl. Acad. Sci. USA. 96:1167–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richie, T.L., and A. Saul. 2002. Progress and challenges for malaria vaccines. Nature. 415:694–701. [DOI] [PubMed] [Google Scholar]
- 3.Lyon, J.A., A.W. Thomas, T. Hall, and J.D. Chulay. 1989. Specificities of antibodies that inhibit merozoite dispersal from malaria-infected erythrocytes. Mol. Biochem. Parasitol. 36:77–85. [DOI] [PubMed] [Google Scholar]
- 4.Brown, G.V., R.F. Anders, G.F. Mitchell, and P.F. Heywood. 1982. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 297:591–593. [DOI] [PubMed] [Google Scholar]
- 5.Brown, G.V., R.F. Anders, and G. Knowles. 1983. Differential effect of immunoglobulin on the in vitro growth of several isolates of Plasmodium falciparum. Infect. Immun. 39:1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman, M.J., H.-G. Heidrich, S. Donachie, J.S. McBride, and A.A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red blood cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan, A., P. Burghaus, P. Druilhe, A. Holder, and E. Riley. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133–139. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell, R.A., T.F. de Koning-Ward, R.A. Burt, M. Bockarie, J.C. Reeder, A.F. Cowman, and B.S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saul, A., and L.H. Miller. 2001. A robust neutralization test for Plasmodium falciparum malaria. J. Exp. Med. 193:F51–F54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diggs, C.L., W.R. Ballou, and L.H. Miller. 1993. The major merozoite surface protein as a malaria vaccine target. Parasitol. Today. 9:300–302. [DOI] [PubMed] [Google Scholar]
- 12.Holder, A.A., J.A. Guevara Patino, C. Uthaipibull, S.E. Syed, I.T. Ling, T. Scott-Finnigan, and M.J. Blackman. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia. 41:409–414. [PubMed] [Google Scholar]
- 13.Crabb, B.S., T. Triglia, J.G. Waterkeyn, and A.F. Cowman. 1997. Stable transgene expression in Plasmodium falciparum. Mol. Biochem. Parasitol. 90:131–144. [DOI] [PubMed] [Google Scholar]
- 14.van Spaendonk, R.M.L., J. Ramesar, A. van Wigcheren, W. Eling, A.L. Beetsma, G.-J. van Gemert, J. Hooghof, C.J. Janse, and A.P. Waters. 2001. Functional equivalence of structurally distinct ribosomes in the malaria parasite, Plasmodium berghei. J. Biol. Chem. 276:22638–22647. [DOI] [PubMed] [Google Scholar]
- 15.Pace, T., C. Birago, C.J. Janse, L. Picci, and M. Ponzi. 1998. Developmental regulation of a Plasmodium gene involves the generation of stage-specific 5′ untranslated sequences. Mol. Biochem. Parasitol. 97:45–53. [DOI] [PubMed] [Google Scholar]
- 16.Janse, C.J., B. Mons, J.J.A.B. Croon, and H.J. van der Kaay. 1984. Long term in vitro cultures of Plasmodium berghei and preliminary observations on gametocytogenesis. Int. J. Parasitol. 14:317–320. [DOI] [PubMed] [Google Scholar]
- 17.de Koning-Ward, T.F., C.J. Janse, and A.P. Waters. 2000. The development of genetic tools for dissecting the biology of malaria parasites. Annu. Rev. Microbiol. 54:157–185. [DOI] [PubMed] [Google Scholar]
- 18.Menard, R., and C.J. Janse. 1997. Gene targeting in malaria parasites. Methods. 13:148–159. [DOI] [PubMed] [Google Scholar]
- 19.Cooper, J.A., L.T. Cooper, and A.J. Saul. 1992. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol. Biochem. Parasitol. 51:301–312. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell, R.A., A. Saul, A.F. Cowman, and B.S. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91–95. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin, P.A., I.T. Ling, G. Clottey, L.M. Spencer Valero, S.A. Ogun, S.L. Fleck, D. Walliker, W.D. Morgan, B. Birdsall, J. Feeney, et al. 1999. Antigenic and sequence diversity at the C-terminus of the merozoite surface protein-1 from rodent malaria isolates, and the binding of protective monoclonal antibodies. Mol. Biochem. Parasitol. 104:147–156. [DOI] [PubMed] [Google Scholar]
- 22.Patino, J.A., A.A. Holder, J.S. McBride, and M.J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]