Abstract
The use of defined protein and peptide antigens can overcome specificity limitations of purified protein derivatives in the detection of bovine tuberculosis when the antigens are used in blood-based tests. Since the use of these specific antigens as skin test reagents could have practical advantages, we investigated the potential of Mycobacterium bovis-specific antigens to stimulate delayed-type hypersensitivity (DTH) responses in cattle experimentally infected with M. bovis. A cocktail of the recombinant antigens ESAT-6, MPB83, and MPB64 failed to stimulate in vivo DTH in cattle that had been experimentally infected with M. bovis despite the fact that the antigens were recognized in vitro by the same animals. However, it was possible to stimulate antigen-specific bovine DTH responses by using ESAT-6 in combination with a synthetic bacterial lipopeptide. This lipopeptide stimulated the release of the proinflammatory cytokine tumor necrosis factor alpha from monocyte-derived bovine dendritic cells in vitro, thereby providing a possible mechanism for its DTH-enhancing properties.
Bovine tuberculosis (TB) is a disease of economic and zoonotic importance caused by the intracellular bacterium Mycobacterium bovis. In the United Kingdom, there has been a steady rise in the incidence of bovine TB during the last decade, and the cost of control measures currently exceeds £25 million per annum. Bovine TB is a zoonotic disease, and prior to introduction of milk pasteurization and disease control measures in England, up to 6% of human deaths (2,500 people per annum) resulting from TB were attributed to M. bovis infection (19). Furthermore, the zoonotic impact of bovine TB remains an important consideration in developing countries in which limited or no control measures are in place (26, 27). The early and specific diagnosis of this disease in cattle is therefore of vital importance.
The control of bovine TB in the United Kingdom relies on detection of infected animals by the single comparative intradermal tuberculin test (SCITT) (32). This test detects the development of a delayed-type hypersensitivity (DTH) response to intradermal injection of a purified protein derivative (PPD) prepared from an M. bovis culture. The reaction to bovine PPD (PPD-B) is compared to the skin reaction induced by a Mycobacterium avium-derived PPD to provide a measure of sensitization to environmental mycobacteria. Cattle presenting with a positive skin test are slaughtered (test and slaughter control policy).
Recent advances in the detection of bovine and human TB have demonstrated that the use of defined protein and peptide antigens can overcome specificity limitations of PPD; for example, this can occur in populations that have been vaccinated with M. bovis BCG (bacillus Calmette-Guérin). In cattle, recent studies have demonstrated that defined M. bovis antigens, such as ESAT-6, MPB64, MPB70, and MPB83, can distinguish BCG-vaccinated animals from M. bovis-infected animals while still providing high levels of sensitivity for diagnosing M. bovis infection (8, 43).
To date, in such studies and studies in which the diagnostic applications of defined protein antigens against bovine TB have been investigated (14, 35, 36) the workers have used in vitro blood-based assays to assess the performance of the candidate antigens. Compared to the traditional skin test, blood-based diagnostic assays have advantages that include the requirement for only a single farm visit and no retest interval restrictions, unlike the recommended 60-day retest interval associated with the SCITT (37). However, skin testing remains a simple, cheap, robust, and widely accepted diagnostic tool whose application could still prove to be useful when field applications of more specific defined diagnostic reagents are considered.
The purpose of the present study was to investigate the diagnostic potential of defined M. bovis antigens as skin tests reagents in cattle. We report here that a protein cocktail comprising recombinant forms of the ESAT-6, MPB64, and MPB83 antigens was found to be a potent DTH-inducing antigen combination in a guinea pig model, yet it failed to induce DTH responses in M. bovis-infected cattle despite the fact that it was recognized in vitro in the same animals. However, the failure of the defined mycobacterial antigens to induce bovine DTH responses was overcome by coadministration of a synthetic bacterial lipopeptide with proinflammatory properties.
MATERIALS AND METHODS
Bacterial strains.
M. bovis strain AN5 was supplied by the Tuberculin Production Unit, Veterinary Laboratories Agency (VLA), Addlestone, United Kingdom. M. bovis stain AF2122/97 was supplied by the TB Diagnostic Unit (VLA). Both strains were stored frozen in Middlebrook 7H9 culture media (Becton Dickinson, Oxford, United Kingdom). The number of viable CFU was determined by plating an aliquot of a strain on Middlebrook 7H10 agar (Becton Dickinson) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (Becton Dickinson). Agar plates were also supplemented with 0.2% (vol/vol) glycerol (Sigma, Poole, United Kingdom) for growth of AN5 and with 4.16 g of pyruvic acid (Sigma) per liter for growth of AF2122/97.
Mycobacterial antigens.
The recombinant M. bovis antigens ESAT-6, MPB70, and MPB83 were produced as previously described (43). Recombinant MPB64 was kindly provided by D. Bakker, Animal Health Science, Boxtel, The Netherlands. PPD-B and avian PPD (PPD-A) were produced and supplied by the Tuberculin Production Unit (VLA) according to procedures prescribed by the European Pharmacopoeia (12). For assessment of the purity of antigens, samples were separated on 8 to 18% precast gel sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels (Amersham-Pharmacia Biotech, Little Chalfont, United Kingdom), and electrophoresis was performed by using a Multiphor II system (Amersham-Pharmacia Biotech). Sample purity was assessed by performing total-protein staining of gels with a commercial silver staining kit (Amersham-Pharmacia Biotech) and by using a standard Western blot procedure to probe for the presence of contaminating Escherichia coli proteins with rabbit anti-E. coli polyclonal antibody sera (Dako Ltd., Ely, United Kingdom).
Protease treatment of PPD-B was performed with a lyophilized aliquot of international standard bovine tuberculin PPD (NIBSC, Potters Bar, United Kingdom). The PPD-B standard was reconstituted at a concentration of 1 mg/ml and then incubated (1-ml aliquot) in the presence of 5 μl of a 10-mg/ml solution of proteinase K (Sigma) for 5 min at 37°C. The sample was then incubated at 65°C for 5 min to heat inactivate the enzyme. The proteinase K treatment was repeated two more times. The sample was then incubated in the presence of 10 μl of a 10-mg/ml solution of pronase (Roche Diagnostics Ltd., Lewes, United Kingdom) for 90 min at 25°C. Finally, the pronase was heat inactivated at 80°C for 10 min.
Protein concentrations of noncommercially derived materials were determined with a bicinchoninic acid protein assay kit (Perbio Science UK Ltd., Tattenhall, United Kingdom).
Cattle and guinea pig experimental infections.
Guinea pigs that weighed 350 to 400 g and were free of intercurrent infection were obtained from Charles River UK Ltd., Margate, United Kingdom. The guinea pigs were infected with 100 CFU of M. bovis AN5 in 0.25 ml of phosphate-buffered saline (PBS) by intramuscular injection into the flexor muscles of the crus region of the right hind leg. The route of infection and the strain of M. bovis used were in accordance with the recommendations of the European Pharmacopoeia for evaluation of the potency of PPD-B (12).
Cattle that were approximately 6 months old were obtained from United Kingdom herds free of bovine TB. Calves were infected with 5 × 104 CFU of M. bovis AF2122/97 by endobronchial challenge as described previously (7). The infection status of all animals was confirmed by the presence of tuberculous lesions at postmortem and by culture of M. bovis from isolated tissues. A total of 16 infected calves were used in this study.
DTH skin tests.
Guinea pig skin tests were performed with animals 5 weeks following experimental infection with M. bovis AN5. Both flanks of a guinea pig were shaved and depilated prior to intradermal injection of 0.1 ml of antigen. Up to four sites on each side of the guinea pig were used. The extent of the erythematous reaction was measured with calipers 24 h later and was expressed as the mean of two measurements taken at right angles to each other delineating the reaction area. Statistical analysis of the responses induced by each of the protein cocktails was performed by using repeated measures of analysis variance with Tukey-Kramer posttest analysis, while a measure of the strength of the responses was represented by the mean ± standard deviation.
Cattle skin tests were performed with infected animals 16 to 20 weeks following experimental infection with M. bovis AF2122/97. The skin test was performed in accordance with the standard SCITT procedure (13). Each antigen preparation (0.1 ml), supplied coded to the veterinary surgeon performing the test, was injected intradermally into the neck of the animal. Up to four sites on each side of the neck were used. The normal skin thickness at the injection site was measured with calipers prior to antigen administration and then again 72 h later. The results were expressed as the difference between the measurements obtained at time zero and 72 h.
Bovine lymphocyte transformation assay.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by gradient centrifugation on Histopaque 1077 (Sigma) and were cultured in RPMI 1640 Glutamax-1 (Life Technologies, Paisly, United Kingdom) supplemented with 5% complete protein serum replacement 1 (Sigma), nonessential amino acids (Sigma), 5 μM 2-mercaptoethanol (Sigma), penicillin (100 U/ml; Life Technologies), and streptomycin sulfate (100 μg/ml; Life Technologies). PBMC were cultured in triplicate at a concentration of 2 × 105 cells/well (volume, 0.2 ml) at 37°C with 5% CO2 for 6 days in flat-bottom 96-well culture plates in the presence of antigen, and they were labeled during the final 18 h of culture with 37 kBq of [3H]thymidine (Amersham-Pharmacia Biotech) per well. The cultures were harvested on glass fiber filters and counted with a TopCount NXT beta counter (Perkin Elmer Life Sciences, Zarentem, Belgium). Responses to defined protein antigens were considered positive when the stimulation index (SI) (counts per minute with antigen/counts per minute without antigen) was ≥2 and the signal strength was >1,000 cpm. When the responses to PPD-B were compared with the responses to PPD-A, a response was considered positive when the SI with PPD-B was greater than that with PPD-A and the PPD-B-induced SI was >3 with a signal strength >1,000 cpm.
Bovine monocyte-derived DC culture.
Bovine dendritic cells (DC) were derived from CD14+ monocytes that were isolated from PBMC and cultured with granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) as previously described by Hope et al. (23). After 3 days of culture, the synthetic bacterial lipopeptide Pam3CSK4 (EMC Microcollections, Tubingen, Germany) (42, 45), where Pam3C is a palmitoyl-Cys[(RS)-2,3-di(palmitoyloxy)-propyl] residue, was added to the DC. Supernatants were harvested 48 h later and assayed for the presence of bovine tumor necrosis factor alpha (TNF-α) by using a luminometric enzyme-linked immunosorbent assay (ELISA) protocol.
TNF-α luminometric ELISA.
Antibodies to TNF-α were generated by immunization of mice with recombinant bovine TNF-α and hybridomas generated in a manner similar to that described previously for IL-12 (22). A pair of monoclonal antibodies, designated CC327 and biotinylated CC328, were shown to recognize recombinant and native bovine TNF-α in ELISA (J. C. Hope, L. S. Kwong, and C. J. Howard, unpublished data) when a protocol similar to that developed for assaying IL-12 (22) was used. U-bottom black 96-well plates (Porvair, Shepperton, United Kingdom) were coated with 2 μg of CC327 per ml overnight at room temperature. The plates were washed with PBS containing 0.01% Tween 20 (wash buffer) and then blocked by using sodium casein (1 mg/ml in PBS) for 1 h at room temperature. The plates were washed, and DC culture supernatant or a COS cell-derived TNF-α standard (Institute for Animal Health) was added to the appropriate wells. Following a 1-h incubation at room temperature, the plates were washed again and then incubated in the presence of biotinylated CC328 (1 μg/ml) for an additional 1 h. The plates were washed and then incubated in the presence of horseradish peroxidase-labeled streptavidin (Amersham-Pharmacia Biotech) for 45 min at room temperature. Following the final wash, the Super Signal ELISA femto maximum-sensitivity substrate (Perbio) was added to each well, and the number of relative light units was determined 1 min later with an Anthos LUCY 1.0 luminometer (AnthosLabtec, Salzburg, Austria). The number of biological units of TNF-α per milliliter was calculated by comparison with the bovine standard.
RESULTS
Screening of candidate antigens in a guinea pig DTH model.
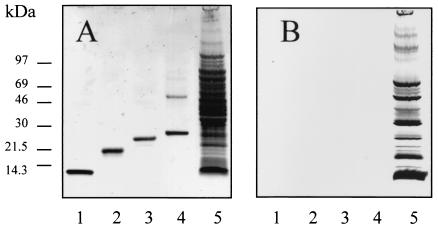
The homogeneity of recombinant antigens ESAT-6, MPB64, MPB70, and MPB83 was confirmed prior to investigation in animals by SDS-PAGE analysis (Fig. 1). While ESAT-6, MPB64, and MPB70 produced single bands at the expected molecular masses, an additional band at a molecular mass of 50 kDa was obtained for MPB83 along with the expected product at a molecular mass of 25 kDa (Fig. 1A, lane 4). However, this band, as well as the 25-kDa product, reacted with an MPB83-specific monoclonal antibody (data not shown) but not with anti-E. coli antibodies (Fig. 1B, lane 4). We therefore concluded that this 50-kDa product was a dimeric form of recombinant MPB83.
FIG. 1.
Assessment of the purity of recombinant mycobacterial antigens. The homogeneity of recombinant mycobacterial antigens was determined by separation on an SDS-PAGE gel and staining for protein by a silver staining procedure (A) or by transferring the protein to a nitrocellulose membrane and probing with polyclonal anti-E. coli rabbit sera to detect contaminating E. coli proteins (B). Lanes 1 to 5 contained ESAT-6 (0.5 μg), MPB64 (0.5 μg), MPB70 (0.5 μg), MPB83 (0.5 μg), and E. coli cell sonicate (10 μg), respectively.
The DTH-inducing potential of these antigens was first tested in a guinea pig skin test model to help define an optimized antigen combination that could be used for further investigations in cattle. Each of the antigens stimulated DTH in a proportion of the guinea pigs tested, and ESAT-6 induced DTH responses most frequently (Fig. 2) (for MPB64 there were responses in three of eight animals; for MPB70 and MPB83 there were responses in four of eight animals; and for ESAT-6 there were responses in six of eight animals). The combination of ESAT-6 and MPB83 stimulated DTH responses in all of the guinea pigs (12.7 ± 0.5 mm) (Fig. 2). However, the optimal protein cocktail contained ESAT-6, MPB64, and MPB83 since this cocktail induced significantly stronger DTH responses (P > 0.05) than the ESAT-6-MPB83 combination (14.5 ± 1.5 mm). Addition of MPB70 to this cocktail resulted in no significant increase in the strength of the responses observed (14.4 ± 1.7 mm). In contrast to PPD-B, which gave an increase in skin thickness of 15.6 ± 1.3 mm, none of these proteins induced skin test responses in BCG-vaccinated guinea pigs when the proteins were tested individually or in combination (data not shown), thereby confirming their high specificity.
FIG. 2.
DTH responses to mycobacterial antigens in guinea pigs. Antigen-stimulated DTH was tested in guinea pigs that had been infected with 100 CFU of live M. bovis by the intramuscular route. Intradermal administration of antigens either individually (2 μg) or in combination (2 μg of each constituent) was performed 5 weeks postinfection, and the erythematous DTH reaction was read 24 h later. E6, ESAT-6; 83, MPB83; 64, MPB64; 70, MPB70.
DTH response to mycobacterial antigens in cattle.
The most promising protein combination identified in guinea pigs (ESAT-6, MPB64, and MPB83) was then tested as a skin test reagent in five calves experimentally infected with M. bovis. These animals were selected because we observed in vitro recognition of the protein cocktail by T cells isolated from each of them, as demonstrated by the proliferative responses shown in Fig. 3A. After injection of the skin test reagents, all cattle exhibited a strong DTH response to PPD-B, which was at least 4 mm greater than the response induced by PPD-A, thereby confirming the tuberculin skin test-positive status of the animals (Fig. 3B). In contrast, in comparison to the PBS control in the same animals, the protein cocktail induced no DTH response in three of the calves and a poor response (2 mm) in the remaining calves (Fig. 3B).
FIG. 3.
Lymphocyte transformation assay and DTH responses to mycobacterial antigens in cattle. Lymphocyte transformation assay (A) and skin test (B) responses were tested in calves experimentally infected with 5 × 104 CFU of M. bovis approximately 16 weeks postinfection. PBMC were cultured in the presence of protein cocktail (5 μg of each component protein per ml), PPD-B, or PPD-A (10 μg/ml) for 6 days, and incorporation of [H3]thymidine was determined during the final 18 h of culture. Responses to the protein cocktail were considered positive if the SI (antigen-stimulated proliferative response/no-antigen response) was ≥2 and proliferation was >1,000 cpm. Skin test responses in the same animals were determined by intradermal administration of the protein cocktail (20 μg of each component antigen) or PPD-B plus PPD-A (100 μg). The increase in skin induration was measured 72 h later.
Bacterial lipopeptide can promote antigen-specific DTH responses in cattle.
We postulated that since we had determined that antigen-specific T cells were present in the peripheral blood of our M. bovis-infected cattle (Fig. 3A), the inability of the protein cocktail to induce bovine DTH was likely to be due in part to a failure to recruit effector T cells to the site of antigen administration due to an inability to induce proinflammatory cytokines. Therefore, we investigated whether potential proinflammatory stimuli were able to promote the DTH responses induced by recombinant mycobacterial protein antigens. As PPD itself has been shown to induce proinflammatory cytokines during DTH responses (9), we first assessed whether nonprotein components of PPD were able to enhance specific DTH responses to ESAT-6, MPB64, and MPB83. We therefore formulated these proteins together with protease-treated PPD-B, which by itself does not stimulate DTH in M. bovis-infected cattle or guinea pigs (data not shown), and we tested the DTH-inducing properties of this antigen formulation in three M. bovis-infected calves. However, protease-treated PPD-B failed to promote DTH responses to the three recombinant antigens in these calves (data not shown).
We next investigated the potential DTH-enhancing properties of a synthetic bacterial lipopeptide, Pam3CSK4. This model lipopeptide contains acyl moieties that mimic those identified in bacterial lipoproteins (47), and it has been shown to be capable of inducing TNF-α from murine and human monocytes (4, 21), a proinflammatory cytokine important in the in vivo recruitment of T cells (25). This lipopeptide was tested in combination with ESAT-6, the immunodominant antigen of the protein cocktail, in seven calves experimentally infected with M. bovis. Each of these calves exhibited positive recognition of ESAT-6 in vitro, as determined by the T-cell proliferation assay (median SI, 5.9; SI range, 2.0 to 50.6). As expected, ESAT-6 alone failed to induce a DTH response in any of these calves. However, when ESAT-6 was coadministered with the lipopeptide, DTH responses were induced in four of the animals (Fig. 4A). The modulation of ESAT-6-induced DTH responses by the lipopeptide in these infected calves was demonstrated to be antigen specific since the lipopeptide control in these calves induced no measurable response (Fig. 4A) and, furthermore, when ESAT-6 and the lipopeptide were administered alone or in combination, they stimulated no measurable DTH response in four SCITT-negative uninfected calves (reaction sizes, 0 mm).
FIG. 4.
Synthetic bacterial lipopeptide promotes enhanced bovine DTH and stimulates TNF-α production from bovine DC. (A) Skin test responses to the lipopeptide Pam3CSK4 and ESAT-6 were tested in cattle that had been infected with 5 × 104 CFU of live M. bovis. Intradermal administration of ESAT-6 (100 μg) either alone or in the presence of synthetic lipopeptide (20 μg) was performed 16 weeks postinfection, and the increase in skin induration was read 72 h later (all cattle exhibited strong PPD-B responses). (B) TNF-α-inducing properties of Pam3CSK4 were tested by using bovine monocyte-derived DC. Bovine CD14+ monocytes were cultured in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 for 3 days and then for an additional 2 days in the presence of Pam3CSK4. The TNF-α in culture supernatants was determined by using a luminometric ELISA and was quantified by comparison with a bovine TNF-α standard. The data are representative of the results of one of three independent experiments.
To investigate a molecular mechanism by which the synthetic lipopeptide could stimulate enhanced inflammatory responses, we quantified the presence of the proinflammatory cytokine TNF-α in culture supernatants of monocyte-derived bovine DC stimulated with Pam3CSK4. As shown in Fig. 4B, Pam3CSK4 was able to induce bovine DC to produce TNF-α in a dose-dependent manner.
DISCUSSION
Defined antigens of M. bovis have demonstrated much promise in overcoming specificity limitations of PPD for diagnosing bovine TB when they are used in blood-based in vitro assay formats (8, 14, 35, 36, 43). However, investigation of such antigens as bovine skin test reagents has not been reported previously and therefore was the subject of the present study. ESAT-6, MPB64, and MPB70 have previously been shown to be capable of stimulating DTH responses in guinea pigs that were infected or sensitized with either live Mycobacterium tuberculosis, M. bovis BCG, or heat-killed M. bovis strains (1, 11, 15, 18, 31, 34). The demonstration that recombinant forms of these antigens also induced DTH in M. bovis-infected guinea pigs in the present study confirmed that DTH-inducing antigens may be useful as skin test reagents in cattle. In addition, we also identified MPB83 as another promising DTH-inducing antigen whose potential for diagnosing bovine TB through detection of cellular immune responses has been demonstrated previously only in vitro (14, 43).
Having defined an antigen cocktail that induced DTH responses in guinea pigs that were comparable to those induced by PPD-B, we tested the ability of this cocktail to detect M. bovis infection in the target host. However, this cocktail failed to stimulate measurable DTH in infected calves. These calves were not deficient in the ability to mount DTH responses since PPD-B induced strong skin responses in each of them. This observation is analogous to the results of a clinical study in which the workers investigated MPT64 as a skin test reagent in TB patients. Like the proteins tested in the present study, MPT64 was selected for a clinical investigation due to its ability to induce specific and potent DTH responses in M. tuberculosis-infected guinea pigs (18, 20). However, it also failed to induce DTH in the majority of the patients tested (46). We considered that the explanation for the exquisite sensitivity of guinea pigs to induce DTH compared with the sensitivities of other hosts may be related to differences in the cellular compositions of the dermis and epidermis. The cells present in the skin that have an important role in mediating DTH include mast cells, which increase vascular permeability through the release of vasoactive amines (2, 16), and Langerhans cells, which are potent antigen-presenting cells of the DC lineage (39). However, a review of literature indicated that the densities of Langerhans cells in the epidermis of guinea pigs and in the epidermis of cattle are equivalent (6, 38), while the densities of mast cells in cattle dermis and human dermis are actually greater than the density observed in guinea pigs (17, 28). Therefore, while the differences in sensitivity between guinea pig and cattle (and human) DTH remain unclear, these data suggest that caution should be used when attempting to extrapolate quantitative DTH data between these species.
The calves used in the present study were considered suitable for investigating the DTH-inducing properties of the defined protein cocktail since we were able to demonstrate that in each animal, T cells present in PBMC recognized the cocktail in vitro. However, while in vitro assays demonstrate that antigen-specific T cells have been generated, such assays do not model the inflammatory process that is required to recruit antigen-experienced T cells to sites of local antigen exposure in vivo. It has long been recognized that injection of protein antigens in the absence of a suitable adjuvant results in poor humoral and cell-mediated immune responses. In a recent review of the in vivo recruitment of CD4+ T cells (24), the workers proposed that the role of the adjuvant is to stimulate the release of proinflammatory cytokines, such as TNF-α and IL-1, which promote migration of DC both to the site of antigen administration and subsequently to the draining lymph nodes after digestion of the antigen. The presentation and recognition of the antigen by memory T cells in turn lead to an influx of monocytes, macrophages, and T cells to the site of antigen administration. In the absence of an adjuvant stimulus, presentation of the antigen should be insufficient due to the low level of DC migration that occurs under noninflammatory conditions (24). Therefore, we concluded that the poor performance of the protein cocktail as a skin test reagent in our cattle was probably due in part to the absence of a suitable proinflammatory stimulus.
PPD is itself a potent DTH-inducing antigen that, due to its crude method of manufacture, can contain up to 15% nonprotein components, including DNA and cell wall-derived lipids and carbohydrates (30). Since mycobacterial cell wall components and DNA are both known to be potent adjuvants for cell-mediated responses (3, 40), we speculated that the nonprotein constituents of PPD might provide an important component of the proinflammatory signal required to promote the antigen-specific component of DTH responses. However, PPD-B depleted only of its protein component by treatment with proteases failed to promote bovine DTH responses to the protein cocktail.
Bacterial lipoproteins and lipopeptides from a diverse range of bacteria, including E. coli, Borrelia burgdorferi, Treponema pallidum, Mycoplasma fermentans, and M. tuberculosis, have been shown to induce proinflammatory cytokine production from monocytes and macrophages through triggering of the innate pattern recognition signal Toll-like receptor 2 (5, 29). Similarly, a synthetic lipopeptide containing an acyl moiety that models that of bacterial lipoproteins (47) has also been shown to stimulate the production of proinflammatory cytokines, including TNF-α (4, 21, 33). Therefore, we investigated this synthetic bacterial lipopeptide, Pam3CSK4, in combination with ESAT-6 and demonstrated that it was capable of promoting antigen-specific DTH responses in more than one-half of the calves tested. The immunomodulatory influence of this lipopeptide was not observed in vitro since Pam3CSK4 did not enhance ESAT-6-stimulated T-cell proliferation when the two reagents were cocultured in the presence of PBMC (data not shown), although this result may reflect the in vitro culture conditions used rather than indicate a complete inability of the lipopeptide to enhance T-cell responses in vitro. Since we were then able to demonstrate that Pam3CSK4 stimulated the production of TNF-α from monocyte-derived DC, we propose that the molecular mechanism for the DTH-enhancing properties of this compound involves an ability to induce TNF-α and other proinflammatory cytokines in vivo, thereby promoting enhanced migration of DC and infiltration of monocytes, macrophages, and T cells. However, to be able to consider formulations based on defined antigens as skin test reagents in cattle for general field applications, further optimization is required. In addition, it is necessary to ensure that the diagnostic sensitivity of such a skin test is at least as good as that of an equivalent blood-based assay by, for example, inclusion of additional antigens, such as CFP-10, which have been shown to be useful in diagnosing bovine TB (10, 41, 44).
In summary, our data indicate that the development of a skin test for bovine TB in which defined antigens are used presents many more challenges than the development of blood-based tests. However, we demonstrated that the limitations of defined mycobacterial antigens to induce DTH responses in cattle may be overcome by using them in combination with a suitable proinflammatory stimulus, although further test optimization, such as optimization of antigen concentrations or the time that the reactions are read, is necessary if practical application of defined antigens as skin test reagents for bovine TB is to be realized.
Acknowledgments
We are grateful to Douwe Bakker, Institute for Animal Health and Science, Boxtel, The Netherlands, for his kind gift of the recombinant MPB64 antigen used in this study and to Paul Cockle, VLA-Weybridge, for his assistance with the guinea pig experiments and for the support of the Animal Services Unit at VLA-Weybridge.
This work was funded by the Department for Environment, Food and Rural Affairs, Great Britain.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Andersen, A. B., L. Ljungqvist, K. Haslov, and M. W. Bentzon. 1991. MPB 64 possesses ‘tuberculosis-complex'-specific B- and T-cell epitopes. Scand. J. Immunol. 34:365-372. [DOI] [PubMed] [Google Scholar]
- 2.Askenase, P. W., S. Bursztajn, M. D. Gershon, and R. K. Gershon. 1980. T cell-dependent mast cell degranulation and release of serotonin in murine delayed-type hypersensitivity. J. Exp. Med. 152:1358-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekiererkunst, A. 1984. Adjuvanticity of mycobacteria and their glycolipid components, wax D and cord factor, p. 761-786. In G. P. Kubica and W. Kubica (ed.), The mycobacteria: a sourcebook, part B. Marcel Dekker Inc., New York, N.Y.
- 4.Belge, K. U., F. Dayyani, A. Horelt, M. Siedlar, M. Frankenberger, B. Frankenberger, T. Espevik, and L. Ziegler-Heitbrock. 2002. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J. Immunol. 168:3536-3542. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 6.Bryan, L. A., P. J. Griebel, D. M. Haines, W. C. Davis, and J. R. Allen. 1988. Immunocytochemical identification of bovine Langerhans cells by use of a monoclonal antibody directed against class II MHC antigens. J. Histochem. Cytochem. 36:991-995. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, C. Q., M. Field, E. Andrew, D. Haskard, M. Feldmann, and R. N. Maini. 1992. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin. Exp. Immunol. 90:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhay, M. J., T. Oettinger, and P. Anderson. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Council. 1997. Tuberculin purified protein derivative, bovine, p. 1680-1681. In European pharmacopoeia, 3rd ed. Council of Europe Publishing, Strasbourg, France.
- 13.European Economic Community. 1980. EEC directive 80/219, amending directive 64/432 annexe B. Off. J. Eur. Union L047:25-32.
- 14.Fifis, T., L. A. Corner, J. S. Rothel, and P. R. Wood. 1994. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand. J. Immunol. 39:267-274. [DOI] [PubMed] [Google Scholar]
- 15.Fifis, T., P. Plackett, L. A. Corner, and P. R. Wood. 1989. Purification of a major Mycobacterium bovis antigen for the diagnosis of bovine tuberculosis. Scand. J. Immunol. 29:91-101. [DOI] [PubMed] [Google Scholar]
- 16.Gershon, R. K., P. W. Askenase, and M. D. Gershon. 1975. Requirement for vasoactive amines for production of delayed-type hypersensitivity skin reactions. J. Exp. Med. 142:732-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godlewski, A., J. Dobek-Smiech, and A. Zielinski. 1996. Comparative studies on mast cell distribution in the skin of humans and laboratory animals. A preliminary report. Folia Histochem. Cytobiol. 34(Suppl. 1):47-48. [PubMed] [Google Scholar]
- 18.Haga, S., R. Yamaguchi, S. Nagai, K. Matsuo, A. Yamazaki, and R. M. Nakamura. 1995. Delayed-type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovis BCG. J. Leukoc. Biol. 57:221-225. [DOI] [PubMed] [Google Scholar]
- 19.Hardie, R. M., and J. M. Watson. 1992. Mycobacterium bovis in England and Wales: past, present and future. Epidemiol. Infect. 109:23-33. [PMC free article] [PubMed] [Google Scholar]
- 20.Haslov, K., A. Andersen, S. Nagai, A. Gottschau, T. Sorensen, and P. Andersen. 1995. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect. Immun. 63:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauschildt, S., P. Hoffmann, H. U. Beuscher, G. Dufhues, P. Heinrich, K. H. Wiesmuller, G. Jung, and W. G. Bessler. 1990. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur. J. Immunol. 20:63-68. [DOI] [PubMed] [Google Scholar]
- 22.Hope, J. C., L. S. Kwong, G. Entrican, S. Wattegedera, H. M. Vordermeier, P. Sopp, and C. J. Howard. 2002. Development of detection methods for ruminant interleukin (IL)-12. J. Immunol. Methods 266:117-126. [DOI] [PubMed] [Google Scholar]
- 23.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285-291. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins, M. K., A. Khoruts, E. Ingulli, D. L. Mueller, S. J. McSorley, R. L. Reinhardt, A. Itano, and K. A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23-45. [DOI] [PubMed] [Google Scholar]
- 25.Kalaaji, A. N., K. McCullough, and J. B. Hay. 1989. The enhancement of lymphocyte localization in skin sites of sheep by tumor necrosis factor alpha. Immunol. Lett. 23:143-147. [DOI] [PubMed] [Google Scholar]
- 26.Kazwala, R. R., C. J. Daborn, J. M. Sharp, D. M. Kambarage, S. F. H. Jiwa, and N. A. Mbembati. 2001. Isolation of Mycobacterium bovis from human cases of cervical adenitis in Tanzania: a cause for concern? Int. J. Tuber. Lung Dis. 5:87-91. [PubMed] [Google Scholar]
- 27.Kidane, D., J. O. Olobo, A. Habte, Y. Negesse, A. Aseffa, G. Abate, M. A. Yassin, K. Bereda, and M. Harboe. 2002. Identification of the causative organism of tuberculous lymphadenitis in Ethiopia by PCR. J. Clin. Microbiol. 40:4230-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuther, K., L. Audige, P. Kube, and M. Welle. 1998. Bovine mast cells: distribution, density, heterogeneity, and influence of fixation techniques. Cell Tissue Res. 293:111-119. [DOI] [PubMed] [Google Scholar]
- 29.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 30.Llandi, S. 1984. Production and standardisation of tuberculin, p. 505-535. In G. P. Kubica and W. Kubica (ed.), The mycobacteria: a sourcebook, part A. Marcel Dekker Inc., New York, N.Y.
- 31.Miura, K., S. Nagai, M. Kinomoto, S. Haga, and T. Tokunaga. 1983. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect. Immun. 39:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 33.Muller, M. R., S. D. Pfannes, M. Ayoub, P. Hoffmann, W. G. Bessler, and K. Mittenbuhler. 2001. Immunostimulation by the synthetic lipopeptide P3CSK4: TLR4-independent activation of the ERK1/2 signal transduction pathway in macrophages. Immunology 103:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai, S., K. Miura, T. Tokunaga, and M. Harboe. 1986. MPB70, a unique antigenic protein isolated from the culture filtrate of BCG substrain Tokyo. Dev. Biol. Stand. 58:511-516. [PubMed] [Google Scholar]
- 35.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 36.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 37.Radunz, B. L., and A. W. Lepper. 1985. Suppression of skin reactivity to bovine tuberculin in repeat tests. Aust. Vet. J. 62:191-194. [DOI] [PubMed] [Google Scholar]
- 38.Sueki, H., C. Gammal, K. Kudoh, and A. M. Kligman. 2000. Hairless guinea pig skin: anatomical basis for studies of cutaneous biology. Eur. J. Dermatol. 10:357-364. [PubMed] [Google Scholar]
- 39.Teunissen, M. B. 1992. Dynamic nature and function of epidermal Langerhans cells in vivo and in vitro: a review, with emphasis on human Langerhans cells. Histochem. J. 24:697-716. [DOI] [PubMed] [Google Scholar]
- 40.Tokunaga, T., T. Yamamoto, and S. Yamamoto. 1999. How BCG led to the discovery of immunostimulatory DNA. Jpn. J. Infect. Dis. 52:1-11. [PubMed] [Google Scholar]
- 41.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von den Esche, U., M. Ayoub, S. D. Pfannes, M. R. Muller, M. Huber, K. H. Wiesmuller, T. Loop, M. Humar, K. F. Fischbach, M. Strunkelnberg, P. Hoffmann, W. G. Bessler, and K. Mittenbuhler. 2000. Immunostimulation by bacterial components. I. Activation of macrophages and enhancement of genetic immunization by the lipopeptide P3CSK4. Int. J. Immunopharmacol. 22:1093-1102. [DOI] [PubMed] [Google Scholar]
- 43.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiesmuller, K. H., W. Bessler, and G. Jung. 1983. Synthesis of the mitogenic S-[2,3-bis(palmitoyloxy)propyl]-N-palmitoylpentapeptide from Escherichia coli lipoprotein. Hoppe-Seyler's Z. Physiol. Chem. 364:593-606. [DOI] [PubMed] [Google Scholar]
- 46.Wilcke, J. T., B. N. Jensen, P. Ravn, A. B. Andersen, and K. Haslov. 1996. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber. Lung. Dis. 77:250-256. [DOI] [PubMed] [Google Scholar]
- 47.Wu, H. C., and T. M. Tokunaga. 1986. Biogenesis of lipoproteins in bacteria. Curr. Top. Microbiol. Immunol. 125:127-157. [DOI] [PubMed] [Google Scholar]