Abstract
The cellular source of B cell activation factor (BAFF) required for peripheral B cell survival/maturation is unknown. To determine the nature of BAFF-producing cells we established and analyzed reciprocal bone marrow (BM) chimeras with wild-type (WT) and BAFF-deficient mice. The results revealed that BAFF production by radiation-resistant stromal cells is completely sufficient to provide a necessary signal for B cell survival/maturation, as BAFF−/− BM cells transferred into lethally irradiated WT mice gave rise to normal numbers of follicular (FO) and marginal zone (MZ) B cell subpopulations. On the other hand, transfer of WT BM into BAFF−/−lethally irradiated mice resulted only in minimal reconstitution of mature FO B cells and no restoration of MZ B cells. Thus, in the absence of BAFF+/+stromal cells, BAFF production by BM-derived cells, presumably by macrophages, dendritic cells, and/or neutrophils, was not at all sufficient to support normal B cell homeostasis. Interestingly, immunization of both types of chimeras stimulated high levels of antigen-specific antibody secretion, indicating that either stromal cell– or hematopoietic cell–derived BAFF is sufficient for B cell antibody responses.
Keywords: BAFF, B cells, homeostasis, bone marrow, stromal cells
Introduction
B cell activation factor (BAFF; also known as BLyS, TALL-1, THANK, and zTNF4; 1–4) has been recently shown to play a critical role in B cell development and homeostasis. Specifically, mice rendered BAFF deficient through homologous recombination or by treatment with BAFF-blocking reagent (e.g., BCMA-Fc) have a dramatically reduced B cell compartment (5, 6). BAFF deficiency most severely affected mature follicular (FO; CD21interm CD23high) and marginal zone (MZ; CD21high CD23interm) B cell subpopulations, indicating that BAFF is required for their maturation and/or survival. BAFF is further required for humoral immune responses, as mice lacking the protein display severely reduced antibody titers in response to both T-dependent and T-independent antigens (6–8).
Although KO animals have unequivocally demonstrated requirement for BAFF in B cell homeostasis and function, the cellular source(s) of BAFF production required for these functions has remained unknown. Macrophages, DCs, and neutrophils have all been shown to be capable of BAFF production in vitro (4, 9–11). The level of BAFF expression by these cells is especially notable upon stimulation with proinflammatory stimuli such as IFN-γ or IFN-β. However, their ability to produce physiologically relevant levels of BAFF in vivo has not been determined. We have previously shown that BAFF plays a crucial role in B cell homeostasis in the absence of any notable inflammation and hence IFN production, as BAFF-deficient mice have only ∼10% of normal B cell numbers (6). Furthermore, because in the spleen both macrophages and DCs are localized around the B cell area (in MZ and in T cell area) but not directly in the B cell follicle (12), and hence not in the immediate vicinity of most B cells, it is not clear whether BAFF production by macrophages and DCs in vivo under noninflammatory conditions is sufficient for maintenance of B cell homeostasis.
To identify the cells producing BAFF required for B cell homeostasis in vivo, we have used WT and BAFF-deficient animals to generate reciprocal BM chimeras. Lethally irradiated BAFF − / − mice reconstituted with WT BM contain cells of hematopoietic origin (e.g., macrophages, DCs, and neutrophils) that are capable of producing BAFF, whereas the reciprocal chimeras contain only radiation-resistant cells such as stromal cells, which are capable of BAFF production. By analyzing B cells from all of these mice we demonstrated that radiation-resistant stromal cells are the major in vivo source of BAFF required for B cell homeostasis.
Materials and Methods
Mice and BM Chimeras.
All mice used in our studies have been housed at Biogen Inc.'s animal facility under specific pathogen-free conditions. Animal experiments were approved by the institutional animal care and use committee. BAFF-deficient mice were derived as previously described (6) and backcrossed with C57BL6 mice (The Jackson Laboratory) for at least four generations before intercrossing them to obtain BAFF−/−mice. BAFF+/+littermates were used as WT controls. B6.SJL (CD45.1) mice were obtained from The Jackson Laboratory and bred at Biogen Inc.'s animal facility. BM chimeras were constructed by i.v. injection of 6.5 × 106 donor-derived BM cells into the recipient mice lethally irradiated with 950 rads at least 4–8 h prior. Mice were kept on a regular diet and acidified water for 8 wk and used for the experiments 8–16 wk after the BM transfer.
FACS® Analyses.
Single cell suspensions from spleens or mesenteric lymph nodes were prepared by grinding the organs in between the frosted sides of glass slides (Fisher Scientific), except when staining for macrophages and DCs had to be performed. To maximize the output of such cells, lymphoid organs were dissociated by treatment with 200 U/ml collagenase D (Roche) for 60 min at 37°C. For staining, cells were resuspended in FACS® staining buffer (3% FBS, 0.1% Na3N PBS, pH 7.6) and after a brief blocking step with anti-CD16/32 Fc-specific antibodies (BD Biosciences), cells were stained with a mixture of specific antibodies. All antibodies were from BD Biosciences. Cells were fixed with Cytofix solution (BD Biosciences) before analyses on BD Caliber (Becton Dickinson). FloJo® (Tristar) software package was used to analyze the data.
Measuring the Antibody Response.
8–12 wk after BM transfer, mice were immunized with 100 μg NP21-CGG conjugate (Biosearch Technologies) precipitated in alum (Pierce Chemical Co.). On day 10 after the immunization, mice were killed and sera and lymphoid organs were collected to be evaluated by ELISA, ELISPOT, and FACS®. The frequencies of antigen-specific antibody-secreting cells (ASCs) in the spleen were estimated by ELISPOT assay. For ELISPOT assay mixed cellulose esters (HA), 96-well plates (Millipore) were coated with NP2-BSA, NP17-BSA, or CGG at 50 μg/ml in PBS overnight at 4°C. Plates were washed twice and blocked by incubation with DMEM supplemented with 5% FBS for 2 h before cell addition. 3 × 105 spleen cells per well were cultured for 20 h at 37°C in 5% CO2 in complete media (DMEM supplemented with 5% FBS, 2-ME, 1% penicillin and streptomycin, and 1% l-glutamine). Plates were washed and spots were visualized using horseradish peroxidase–conjugated goat anti–mouse IgG1 (Southern Biotechnology Associates, Inc.) followed by AEC substrate (Zymogen). The colorimetric reaction was stopped by washing the plates with distilled water. The spots were counted with the aid of a dissecting microscope.
ELISA for the levels of NP2- and NP17-specific IgG1 Igs in sera of immunized mice was performed as previously described (13). A serum from NP21-CGG hyperimmunized mouse was used as a standard. Hyperimmunized mouse serum was obtained from C57BL6 mice 6 d after being boosted with 50 μg NP21-CGG in PBS, 30 d after primary challenge with 100 μg NP17-CGG in alum. The level of antigen-specific Igs was expressed as units of a reciprocal dilution of a standard serum sample.
Measuring BAFF Levels.
Generation of monoclonal murine BAFF-specific antibodies and conditions for BAFF ELISA were described elsewhere (unpublished data). Sensitivity of the ELISA was 5 ng/ml.
Immunofluorescent Staining.
Spleens from different chimeric animals were snap frozen in OCT compound. 0.5-μm thick spleen sections were frozen after brief fixation in acetone and kept at −70°C until used. Frozen sections were defrosted, air dried, and blocked with 0.2% Tween 20 1% BSA PBS, and then stained with several antibody combinations: (a) anti-CD19 FITC mAb and biotinylated anti-CD4 and anti-CD8 followed by staining with Streptavidin-Alexa Red594 conjugate (Molecular Probes), (b) anti-CD11c FITC plus anti-CD11b FITC, anti-IgD PE, and biotinylated anti-IgM followed by Streptavidin Alexa Blue350 conjugate (Molecular Probes), and (c) anti–MOMA-1 FITC and anti-B220 biotin followed with Streptavidin-Alexa Red594 conjugate (Molecular Probes). All antibodies were from BD Biosciences, except for anti–MOMA-1 antibody, which was from Serotec. After washing, slides were embedded in mounting media with anti-fade ProLong compound (Molecular Probes) and examined under Leica DMR microscope. Pictures were taken with a Hammamatsu digital camera at ×200.
Statistics.
Unpaired Student's t test was used to evaluate the difference between two groups. To calculate the statistically significant difference between the antibody titers and ELISPOT data, Mann-Whitney nonparametric test was used. P < 0.050 was considered to be statistically significant for both tests.
Results
Generation of BM Chimeras.
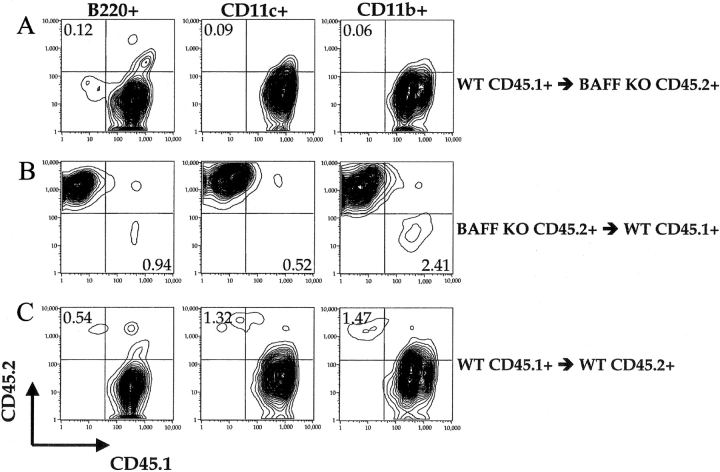
DCs, macrophages, and neutrophils have all been shown to be capable of BAFF production in vitro (4, 9, 10), but previous studies did not establish their contribution to BAFF-dependent B cell development in vivo. To investigate the cellular source of BAFF in vivo, we created reciprocal BM chimeras with WT and BAFF-deficient mice. To distinguish between donor- and recipient-derived cells in chimeric mice, we have used C57BL6 and B6.SJL congenic mice that differ in the expression of the CD45 allele. BM cells from C57BL6.SJL (CD45.1+) mice were transferred into lethally irradiated (950 rads) BAFF−/−(CD45.2+) mice (WT→KO) and vice versa (KO→WT). As a control we have transferred BM from C57BL6.SJL mice (CD45.1+) into lethally irradiated congenic C57BL6 (CD45.2+) mice (WT→WT). 8 wk after transplantation almost all macrophages (CD11b+ CD11c−), DCs (CD11c+), B cells, and T cells were of donor origin (Fig. 1 and unpublished data). Therefore, the chimeric mice we have created are capable of producing BAFF by all BM-derived cells including macrophages, DCs, and neutrophils but not on radioresistant stromal cells (WT→KO mice), or by only radioresistant stromal cells (KO→WT mice) but not by any BM-derived cells. In the control chimeras (WT→WT mice), all cells are competent to produce BAFF.
Figure 1.
Hematopoietic cells in BM chimeras are of donor origin. Lethally irradiated mice were reconstituted with 6.5 × 106 BM cells. 8–12 wk after reconstitution, single cell suspensions were prepared by collagenase digestion of spleens from B6.SJL BM→BAFF KO C57BL/6 (A), BAFF KO C57BL/6 BM→B6.SJL (B), and B6.SJL BM→C57BL/6 (C) chimeric mice. After gating, B220+ (left), CD11c+ (middle), or CD11b+ CD11c− (right) cells were examined for CD45.1 and CD45.2 expression. Graphs are representative of six mice per group examined.
Requirement for Radiation-resistant Stromal Cell–derived BAFF in B Cell Homeostasis.
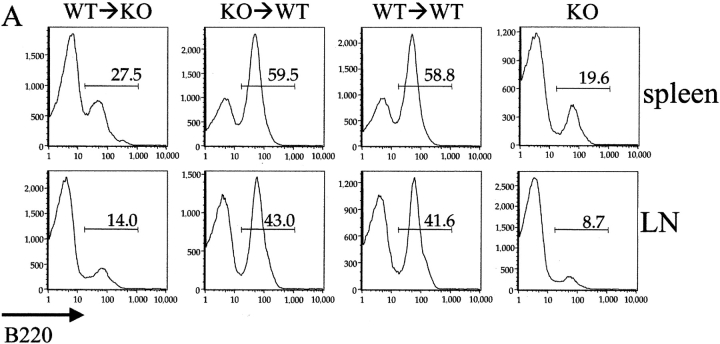
Analysis of B cells from spleen and mesenteric lymph nodes from the chimeric mice revealed (Table I and Fig. 2) that BAFF expression only by radiation-resistant stromal cells (KO→WT mice) is completely sufficient to restore normal B cell numbers. In contrast, B cell homeostasis was still severely impaired in the absence of BAFF expression by stromal cells (WT→KO mice), despite the presence of BAFF+/+macrophages and DCs. Specifically, the proportion of B cells in the spleen and lymph nodes from these mice was reduced relative to control (WT→WT) mice (Fig. 2 A). Consistent with the reduction in the percentage of B220+ cells in the secondary lymphoid organs, total B cell numbers in WT→KO mice were 3.5-fold lower than in control WT→WT mice (Table I).
Table I.
Cellular Subset Distribution in Different Chimeric and BAFF−/− Mice
| WT→KO | KO→WT | WT→WT | KO | |
|---|---|---|---|---|
| Total | 35.21 ± 4.95a | 91.75 ± 10.9b | 86.76 ± 5.98b | 44.50 ± 9.32a |
| B220+ | 14.28 ± 1.78a | 64.26 ± 5.01b | 50.15 ± 4.06b | 8.65 ± 0.78a |
| CD4+ and CD8+ | 16.47 ± 2.40 | 25.82 ± 2.52 | 23.94 ± 1.60 | 21.93 ± 2.13 |
| CD11c+ | 0.54 ± 0.10a | 1.30 ± 0.25b | 1.16 ± 0.15b | 0.44 ± 0.02a |
| CD11b+ | 2.10 ± 0.66 | 1.78 ± 0.22 | 1.70 ± 0.17 | 1.52 ± 0.26 |
| FO (CD21interm CD23high) | 8.04 ± 1.03a , b | 45.45 ± 3.82b | 38.93 ± 4.18b | 1.70 ± 0.39a |
| MZ (CD21high CD23low) | 0.41 ± 0.12a | 3.41 ± 0.42b | 3.68 ± 0.37b | 0.14 ± 0.04a |
| NF (CD21− CD23−) | 2.89 ± 0.53a | 10.79 ± 1.87 | 4.99 ± 0.56 | 4.58 ± 1.55 |
8–12 wk after the reconstitution of lethally irradiated (950 rads) mice with the BM, spleen cells were analyzed by FACS®. Mean cell numbers ×106 ± SD for each population are derived from 8–14 mice per group.
Significantly (P < 0.01) different from the WT→WT mice.
Significantly (P < 0.01) different from the KO mice.
Figure 2.
BAFF+/+ stromal cells are necessary and sufficient for complete reconstitution of the B cell compartment. (A) B cell reconstitution of secondary lymphoid organs was evaluated by FACS® analysis of spleens and mesenteric lymph nodes from different chimeric mice 8–12 weeks after BM transplantation. (B) Spleen cells from these mice were also evaluated for the expression of IgM and IgD (B), as well as CD21 and CD23 (C). CD21 and CD23 expression on B cell after gating on B220+ cells is also shown (D). Numbers in the quadrants or next to the boxes indicate percentages of the indicated population. MZ, FO, and NF denote the gates used to determine correspondingly MZ, FO, and newly formed B cell populations. Dot plots are representative of four mice per group. The experiment was repeated five times with similar results.
Although the total number of B cells in WT→KO mice was only slightly higher (1.8-fold) than in mice completely deficient in BAFF (KO), the numbers of specific B cell subpopulations were differentially effected. Thus, FO B cell (CD21interm CD23high) numbers were fivefold higher than in KO mice. In contrast, MZ B cell (CD21high CD23low) numbers are still just as deficient as in KO. To get a better understanding of B cell differentiation in the different chimeras and KO mice, we performed a FACS® analysis for CD21 and CD23 expression on B220+ B cells. Notably, as seen from Fig. 2 D, despite their reduced total numbers and percentage within spleen or lymph nodes, FO B cells in WT→KO chimeras represent a normal proportion among remaining B cells in these mice. Therefore, in the absence of stromal cell–derived BAFF, it appears that BAFF production by macrophages, DCs, neutrophils, or other BM-derived cells is insufficient to maintain homeostasis of MZ B cells and the majority of FO B cells.
Immunofluorescent staining of spleen sections from chimeric and BAFF-deficient mice (Fig. 3) supported the data derived by FACS® analysis. BAFF production by stromal cells alone (KO→WT) leads to reconstitution of the white pulp with a normal proportion of B and T cells. In the presence of only the BM cell–derived BAFF (WT→KO), as a consequence of the reduced B cell zone the white pulp area is smaller than normal, and mostly consists of T cells similar to that observed in BAFF-deficient mice. IgD+ B cells are clearly more prevalent in WT→KO chimeras than in BAFF-deficient mice, whereas IgMhigh MZ B cells were just as scant. Interestingly, all mature IgD+ B cells in WT→KO chimeras are aligned along BAFF+/+macrophages and DCs (CD11c+ and CD11b+) that surround the follicle. Staining for MZ B cells using markers for B cells (B220) and metallophilic macrophages (MOMA-1), which delineates the MZ from FO area, confirms the paucity of MZ B cells in WT→KO mice (Fig. 3, bottom).
Figure 3.
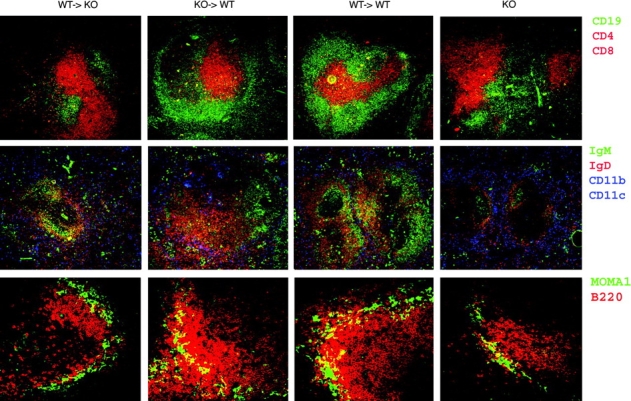
Organization of the splenic B cell follicle in spleen requires BAFF+/+ stroma. Frozen sections of spleens from different chimeras were stained with anti-CD19 (green) and anti-CD4 plus anti-CD8 (red; top), anti-IgM (green), anti-IgD (red), and anti-CD11c plus CD11b (blue; middle), or anti–MOMA-1 (green) and anti-B220 (red). Images of one representative section out of five spleens per group are shown. ×200.
BAFF Production by Macrophages and DCs Is Sufficient for B Cell Activation.
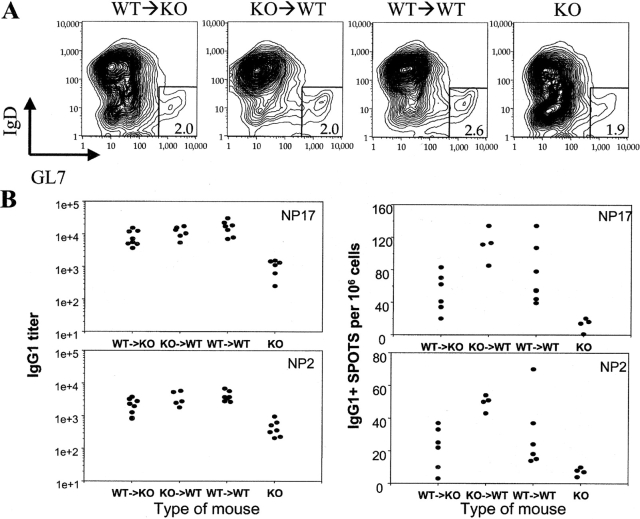
BAFF−/−mice are severely deficient in mounting antibody responses to T cell–dependent antigens (6). Although BAFF production by macrophages and DCs was not sufficient to maintain normal numbers of mature B cells in WT→KO mice, it is possible that BAFF production by these cells will still be crucial in providing help to B cells during their activation in vivo, as they can be found in the close proximity to activated B cells (14). To investigate whether BAFF production by either stromal cells or macrophages and DCs is sufficient to facilitate B cell activation and differentiation, we immunized the BM chimeras with NP21-CGG absorbed in alum 8–10 wk after BM reconstitution. BAFF KO mice were used as a control. 10 d later sera and spleens were taken from these mice and analyzed (Fig. 4) . As can be seen from Fig. 4 A, all immunized mice have similar frequencies of germinal center (GC) B cells (GL7+ IgD− B cells) among B220+ cells in their spleens, although absolute numbers of these cells in spleens of WT→KO chimeras and KO mice were still 3.5-fold and 6-fold lower than in spleens of WT→WT or KO→WT mice. Despite the similar frequencies of GC B cells in all mice, the frequencies of antigen-specific IgG1 ASCs in spleens of BAFF KO mice were the lowest as determined with ELISPOT assay (Fig. 4 B). The low frequency of ASCs in the spleens of KO mice is in complete agreement with low titers of antigen-specific IgG1 antibodies in sera of these mice (Fig. 4 B, left; reference 6). Interestingly, although the frequency of ASCs and titers of antigen-specific antibodies in WT→KO mice were lower than in WT→WT mice, they were at least 10 times higher than in BAFF KO animals. High affinity (as measured by their reactivity with NP2 antigen) antibodies were also much higher in WT→KO mice than in KO mice, indicating that affinity maturation can also be restored by hematopoietic cell–derived BAFF. These data demonstrate that BAFF production by DCs and macrophages is sufficient to provide the level of BAFF necessary for the differentiation of B cells into ASCs and consequently for antibody production. BAFF production by stromal cells was also sufficient for both of these activities as the levels of ASCs and antibodies in KO→WT mice were comparable to those in WT→WT mice.
Figure 4.
Both BM-derived BAFF+/+ and stroma BAFF+/+ cells can provide BAFF signal during antigen-specific antibody response. (A) 10 d after the immunization with 100 μg NP21-CGG in alum, spleen cells from various chimeras were analyzed for the presence of GC B cells. Numbers in the squares represent percent of GC (GL7+ IgD−) B cells among B220+ cells in spleens of the chimeric mice. Graphs representative of six mice per each group are shown. No significant number of GL7+ IgD− B220+ cells could be detected in the absence of immunization. (B) Spleen cells from the mice immunized as in A were assayed for the frequency of NP2- and NP17-specific ASCs per 9 × 105 splenocytes using ELISPOT assay (right). NP2- and NP17-specific titers of IgG1 antibodies in sera of different immunized chimeric mice were determined by ELISA and expressed as a reciprocal of standard sera dilution giving the same OD450 as the sample (left). 6–10 mice per group were used in the experiment. The values for all chimeric mice were statistically significantly higher than the values from BAFF−/− (KO) mice (P < 0.05).
Stromal Cell Production of Systemic Levels of BAFF.
Previously it has been demonstrated that sera from healthy people have detectable levels of BAFF (15), suggesting that some of the homeostatic functions of BAFF might be mediated by soluble rather than cell surface–expressed BAFF. Because we observed that in WT→KO mice the mature IgD+ B cells are located in a thin layer next to BAFF-competent CD11b+ and CD11c+ cells (Fig. 3), we speculated that CD11c+ and CD11b+ cells are capable of producing BAFF only locally but not systemically, which might be required to sustain a larger B cell follicle. To test the hypothesis that only stromal radiation–resistant cells are the major source of soluble systemic BAFF production, we checked the level of BAFF in various generated chimeras 8–10 wk after reconstitution. As seen from Fig. 5 , a high systemic level of BAFF in sera is achieved through the production of BAFF by stromal cells (KO→WT mice) and not through BM-derived macrophages, DCs, or neutrophils (WT→KO).
Figure 5.
Production of systemic levels of secreted BAFF in sera requires BAFF+/+ stromal cells. Sera from different chimeric mice 8–12 wk after their reconstitution were assayed for BAFF levels by ELISA. Sera were collected from 6–10 mice per group. *, statistically significant (P < 0.05) difference relative to the WT→WT mice. Limit of detection of this ELISA was 5 ng/ml.
Discussion
Our results clearly demonstrate that the major source of BAFF required for normal B cell homeostasis is a radioresistant cell population(s). BAFF production only by BM-derived macrophages, DCs, or neutrophils is insufficient to maintain normal B cell numbers. However, BM cell–derived BAFF can drive differentiation of some immature newly formed B cells into mature FO, but not MZ, B cells. Interestingly, BAFF production by BM-derived cells as well as radioresistant cells could support B cell activation and antibody production in response to immunization with a T-dependent antigen.
Mice deficient in BAFF have greatly reduced numbers of mature B cells in their lymphoid organs (6). Treatment of mice with BAFF-blocking reagents like the soluble BCMA-Fc receptor fusion molecule also leads to the disappearance of mature B cells from the lymphoid organs (5). These data indicate that there is a constant requirement for BAFF-mediated signaling during B cell maturation as well as for survival of mature B cells. Earlier in vitro studies showed that monocytes and DCs can secrete BAFF and also express it on their surface, particularly after activation by proinflammatory stimuli including type I and II IFNs (4, 9). Similarly, G-CSF is capable of inducing BAFF production by neutrophils (10). However, the macrophages, polymorphonuclear granulocytes, and DCs, which are found around the splenic white pulp and in the red pulp, do not localize precisely with most splenic B cells (12). Rather, the distributions of these cells overlap mostly with the edges of the MZ. Perhaps reflecting this, in spleens of WT→KO chimeras (which contain BAFF+/+BM-derived macrophages, DCs, and neutrophils) we observed B cell areas almost as small as in KO mice, and mature IgD+ B cells aligned in a narrow area along the surrounding CD11c+ and CD11b+ cells. By contrast, the size of B cell areas in KO→WT chimeras appeared normal, although all hematopoietic cells including macrophages, DCs, and neutrophils in these mice are BAFF −/−. These findings cannot be explained by the continued presence of BM-derived host cells because we unequivocally established the origin and genotype of hematopoietic cells through staining for CD45.1 and CD45.2 antigens at the time of analysis. 8 wk after transplantation, >97% of macrophages (CD11b+ CD11c−) and DCs (CD11c+) in all chimeric mice were donor derived (Fig. 1). Although spleens of mice whose radiation-resistant cells were BAFF−/−(i.e., WT→KO and KO mice) contained approximately twofold fewer DCs than normal (Table I), the reduction is, most likely, secondary to the B cell deficiency in such mice. In fact, previous studies have shown that B cells are required for normal repopulation of spleens by DCs (16). Furthermore, in vitro cultures of BM cells from BAFF−/−and WT mice gave rise to similar numbers of DCs (unpublished data).
It is very clear from our studies that even in the complete absence of BAFF production by DCs, but in the presence of BAFF+/+radiation-resistant stromal cells, the B cell compartment is normal. Therefore, cells other than macrophages, DCs, and neutrophils contribute the majority of BAFF production required for normal B cell homeostasis. Splenic stromal cells, given their radioresistance and close proximity to all cells in the spleen including developing B lymphocytes (17, 18), are plausible candidates for production of BAFF. Analysis of LTβ receptor (LTβR)-deficient mice and radiation chimeras derived from these animals demonstrated a crucial role for one type of stromal cell, FO DCs, in chemokine production required to maintain normal architecture of B and T cell zones in the spleen (for review see reference 19). It is tempting to speculate that interaction of B cells and stromal cells (whereupon the B cells provide LTβ to LTβR-expressing stromal cells) is responsible not only for proper B cell localization in secondary lymphoid organs, but also for BAFF-dependent B cell maturation and survival. Interestingly, injection of LTβR agonist antibodies into mice leads to a dramatic increase of BAFF mRNA expression in the spleen (20). However, there must be an LTβ-independent source of BAFF production, as LTβ-deficient mice have normal numbers of B cells (21, 22). It is also important to mention in this regard that it remains formally possible that radiation-resistant cells outside of secondary lymphoid organs may contribute substantially to circulating levels of BAFF required for maintenance of B cells. This would be consistent with our observation of circulating BAFF only in mice with BAFF+/+radiation-resistant cells (Fig. 5).
Despite a major requirement for additional BAFF from other sources, its production solely by BM-derived cells does play a role in normal immune responses. Our FACS® and immunohistochemical analysis of radiation chimeras whose macrophages, DCs, and neutrophils could produce BAFF showed that signaling induced by these cells was sufficient to induce maturation of some newly formed B cells to mature FO B cells. Immature B cells constantly emerge from the BM in large numbers, migrate to peripheral lymphoid organs, and then mostly die soon thereafter unless they can receive a maturation and survival signal (23). Depending on the nature of that signal(s), they differentiate into either FO B cells (CD21low CD23high) or MZ B cells (CD21high CD23low; references 24 and 25). BAFF plays a crucial role in that process. The lymphoid organs of BAFF-deficient mice contain nearly normal numbers of immature B cells, but almost completely lack FO and MZ B cell compartments (6). In the presence of BAFF production by BM-derived cells only (WT→KO chimeras), total B cell numbers were still drastically reduced by comparison to WT animals. However, the number of mature FO B cells was significantly higher than in BAFF-deficient mice and even comprised a normal proportion of FO B cells within the remaining B cells (Fig. 2 D). Whether this small number of cells represents a subpopulation requiring less BAFF for complete maturation remains unknown.
MZ B cells, despite their proximity to BM-derived BAFF+/+CD11b+ cells in chimeric (WT→KO) mice, were very poorly reconstituted for reasons that remain unclear. Lack of MZ B cells cannot simply be explained by slow kinetics of reconstitution for these cells as we did not detect any significant increase in the numbers of these cells in WT→KO mice even 4 mo after BM reconstitution (unpublished data). Their absence may rather reflect an increased requirement for circulating BAFF by this subpopulation, a need for additional circulating BAFF-dependent costimulatory factors, or some specific requirement by MZ cells for direct cell–cell interaction with radiation-resistant stromal cells that produce BAFF.
The importance of BAFF in supporting antigen-specific B cell responses is further clarified by our studies. It was previously known that BAFF deficiency or inhibition with soluble receptor decoy molecules (BCMA-Fc and TACI-Fc) significantly reduces antibody responses to a variety of specific antigens (6–8). Our data demonstrate that BAFF production by radiation-resistant cells or by BM-derived cells is sufficient to support B cell responses. Both types of chimeras created using BAFF−/−cells (WT→KO and KO→WT) responded to NP21-CGG immunization with frequencies of ASCs and antibody levels that significantly exceeded those observed in BAFF−/−mice. Although the frequency of ASCs and the level of antibodies in WT→KO chimeras were smaller than in control (WT→WT) mice, the reduced numbers of total mature B cells in these mice is likely to account for this finding.
The antibody responses were fully supported only by radiation-resistant cell-produced BAFF, suggesting that BAFF production by BM-derived cells is redundant. However, because we did not assay all aspects of the immune response, e.g., memory B cell generation and maintenance, we cannot completely exclude the possibility that BM-derived cells represent an important and nonredundant source of BAFF for maintenance of some of the immune responses. Our data do not allow direct identification of a single population of BAFF-producing cells in the spleen, which is crucial to normal antibody responses. However, the findings are consistent with previous observations showing DCs in close contact with T and B cells during the antigen response in vivo (26, 27). This may reflect a need for (B cell–expressed) BAFF receptor, BAFF-R, to directly engage BAFF expressed on the DC surface. This need would be similar to the requirement for direct interaction between B cells and FO DCs during normal GC development. Alternately, relatively close proximity of B lymphocytes to DCs producing locally high concentrations of soluble BAFF may mediate B cell survival and differentiation after antigen challenge. Both of these possibilities are completely consistent with the demonstrated role of DC-produced BAFF for plasmablast generation (14) and during Ig class switching (11).
The studies of radiation chimeras described here establish that the most important source of BAFF for B cell homeostasis originates from radiation-resistant stromal cells. These findings may have significant implications for treatment of certain B cell–mediated diseases. For example, autologous BM transplantation is being considered for treatment of SLE on the premise of “resetting” the immune system (28). If some cases of lupus result from dysregulated BAFF production, then those patients will likely fail to benefit from the BM transplant as such a therapy would not directly reset BAFF production by radiation-resistant cells. Therefore, careful revision of these strategies may need to be considered.
Acknowledgments
We are grateful to Drs. Jeff Browning and Paula Hochman for critical reading of the manuscript and helpful discussions. We wish to thank Dr. Fabienne Mackay for anti-muBAFF mAbs, Sarah Bixler, Jeff Thompson, and Margot Brickelmaier for help with the BAFF ELISA and reagent preparation, and Akos Szilvasi and Juanita Campos-Rivera for help with FACS® analysis.
Abbreviations used in this paper: ASC, antibody-secreting cell; BAFF, B cell activation factor; FO, follicular; GC, germinal center; MZ, marginal zone.
References
- 1.Shu, H., W. Hu, and H. Johnson. 1999. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J. Leukoc. Biol. 65:680–683. [PubMed] [Google Scholar]
- 2.Schneider, P., F. MacKay, V. Steiner, K. Hofmann, J.L. Bodmer, N. Holler, C. Ambrose, P. Lawton, S. Bixler, H. Acha-Orbea, et al. 1999. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 189:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukhopadhyay, A., J. Ni, Y. Zhai, G.-L. Yu, and B.B. Aggarwal. 1999. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappa B, and c-Jun NH2-terminal kinase. J. Biol. Chem. 274:15978–15981. [DOI] [PubMed] [Google Scholar]
- 4.Moore, P.A., O. Belvedere, A. Orr, K. Pieri, D.W. LaFleur, P. Feng, D. Soppet, M. Charters, R. Gentz, D. Parmelee, et al. 1999. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 285:260–263. [DOI] [PubMed] [Google Scholar]
- 5.Thompson, J.S., P. Schneider, S.L. Kalled, L. Wang, E.A. Lefevre, T.G. Cachero, F. MacKay, S.A. Bixler, M. Zafari, Z.Y. Liu, et al. 2000. BAFF binds to the tumor necrosis factor receptor–like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J. Exp. Med. 192:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiemann, B., J.L. Gommerman, K. Vora, T.G. Cachero, S. Shulga-Morskaya, M. Dobles, E. Frew, and M.L. Scott. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 293:2111–2114. [DOI] [PubMed] [Google Scholar]
- 7.Yu, G., T. Boone, J. Delaney, N. Hawkins, M. Kelley, M. Ramakrishnan, S. McCabe, W.R. Qiu, M. Kornuc, X.Z. Xia, et al. 2000. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat. Immunol. 1:252–256. [DOI] [PubMed] [Google Scholar]
- 8.Yan, M., S.A. Marsters, I.S. Grewal, H. Wang, A. Ashkenazi, and V.M. Dixit. 2000. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat. Immunol. 1:37–41. [DOI] [PubMed] [Google Scholar]
- 9.Nardelli, B., O. Belvedere, V. Roschke, P.A. Moore, H.S. Olsen, T.S. Migone, S. Sosnovtseva, J.A. Carrell, P. Feng, J.G. Giri, et al. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 97:198–204. [DOI] [PubMed] [Google Scholar]
- 10.Scapini, P., B. Nardelli, G. Nadali, F. Calzetti, G. Pizzolo, C. Montecucco, and M.A. Cassatella. 2003. G-CSF–stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litinskiy, M.B., B. Nardelli, D.M. Hilbert, B. He, A. Schaffer, P. Casali, and A. Cerutti. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metlay, J.P., M.D. Witmer-Pack, R. Agger, M.T. Crowley, D. Lawless, and R.M. Steinman. 1990. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 171:1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koni, P.A., and R.A. Flavell. 1999. Lymph node germinal centers form in the absence of follicular dendritic cell networks. J. Exp. Med. 189:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balazs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352. [DOI] [PubMed] [Google Scholar]
- 15.Roschke, V., S. Sosnovtseva, C.D. Ward, J.S. Hong, R. Smith, V. Albert, W. Stohl, K.P. Baker, S. Ullrich, B. Nardelli, et al. 2002. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169:4314–4321. [DOI] [PubMed] [Google Scholar]
- 16.Ngo, V.N., R.J. Cornall, and J.G. Cyster. 2001. Splenic T zone development is B cell dependent. J. Exp. Med. 194:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Berg, T.K., E.A. Dopp, J.J. Breve, G. Kraal, and C.D. Dijkstra. 1989. The heterogeneity of the reticulum of rat peripheral lymphoid organs identified by monoclonal antibodies. Eur. J. Immunol. 19:1747–1756. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida, K., N. Tamahashi, N. Matsuura, T. Takahashi, and T. Tachibana. 1991. Antigenic heterogeneity of the reticular meshwork in the white pulp of mouse spleen. Cell Tissue Res. 266:223–229. [DOI] [PubMed] [Google Scholar]
- 19.Cyster, J.G., K.M. Ansel, K. Reif, E.H. Ekland, P.L. Hyman, H.L. Tang, S.A. Luther, and V.N. Ngo. 2000. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176:181–193. [DOI] [PubMed] [Google Scholar]
- 20.Dejardin, E., N.M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z.W. Li, M. Karin, C.F. Ware, and D.R. Green. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 17:525–535. [DOI] [PubMed] [Google Scholar]
- 21.Koni, P.A., R. Sacca, P. Lawton, J.L. Browning, N.H. Ruddle, and R.A. Flavell. 1997. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 6:491–500. [DOI] [PubMed] [Google Scholar]
- 22.Alimzhanov, M.B., D.V. Kuprash, M.H. Kosco-Vilbois, A. Luz, R.L. Turetskaya, A. Tarakhovsky, K. Rajewsky, S.A. Nedospasov, and K. Pfeffer. 1997. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA. 94:9302–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 24.Cariappa, A., M. Tang, C. Parng, E. Nebelitskiy, M. Carroll, K. Georgopoulos, and S. Pillai. 2001. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 14:603–615. [DOI] [PubMed] [Google Scholar]
- 25.Niiro, H., and E.A. Clark. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945–956. [DOI] [PubMed] [Google Scholar]
- 26.Ingulli, E., A. Mondino, A. Khoruts, and M.K. Jenkins. 1997. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 185:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wykes, M., A. Pombo, C. Jenkins, and G.G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313–1319. [PubMed] [Google Scholar]
- 28.Van Laar, J.M., and A. Tyndall. 2003. Intense immunosuppression and stem-cell transplantation for patients with severe rheumatic autoimmune disease: a review. Cancer Control. 10:57–65. [DOI] [PubMed] [Google Scholar]