Abstract
Naturally occurring CD4+CD25+ regulatory T cells appear important to prevent activation of autoreactive T cells. This article demonstrates that the magnitude of a CD8+ T cell–mediated immune response to an acute viral infection is also subject to control by CD4+CD25+ T regulatory cells (Treg). Accordingly, if natural Treg were depleted with specific anti-CD25 antibody before infection with HSV, the resultant CD8+ T cell response to the immunodominant peptide SSIEFARL was significantly enhanced. This was shown by several in vitro measures of CD8+ T cell reactivity and by assays that directly determine CD8+ T cell function, such as proliferation and cytotoxicity in vivo. The enhanced responsiveness in CD25-depleted animals was between three- and fourfold with the effect evident both in the acute and memory phases of the immune response. Surprisingly, HSV infection resulted in enhanced Treg function with such cells able to suppress CD8+ T cell responses to both viral and unrelated antigens. Our results are discussed both in term of how viral infection might temporarily diminish immunity to other infectious agents and their application to vaccines. Thus, controlling suppressor effects at the time of vaccination could result in more effective immunity.
Keywords: regulatory T cells, vaccine, memory, HSV infection, immunopathology
Introduction
The generation of T cell immunity is subject to control by multiple cellular and molecular events. Most is known about circumstances that positively influence immunity such as the role of interacting cells, costimulatory molecules, and cytokines. In fact, until recently, the idea that suppressor cells additionally affect the outcome of immune responses was viewed skeptically. This scenario was changed by the observations of several groups that T cells of a defined phenotype (CD4+CD25+) may down-regulate the severity of lesions caused by autoimmunity and some other immunopathologies (1–3). To date, the focus of regulatory T cell (Treg) research has been on their role in tissue damage events mediated by self-reactive T cells (1, 4). Little is known about the influence of CD4+CD25+ Treg cells on responses to exogenous antigens, especially those expressed by pathogens. Recently, however, it appears that immunopathological and protective immune responses to intracellular parasites are also affected by regulatory T cells (5–7). Currently, the influence of CD4+CD25+ Treg cells in response to viruses is largely unknown. This report examines the role of CD4+CD25+ Treg cells in primary immune response to HSV-1 infection in mice. The model chosen for study was the C57BL/6 (B6) mouse wherein the vast majority of CD8+ T cells react to a known peptide (SSIEFARL), with responses to this peptide alone sufficient for protective immunity in B6 mice (8, 9). Our results show that depletion of CD25+ Treg cells before infection results in an elevated SSIEFARL-specific CD8+ T cell immune response. Furthermore, the responding CD8+ T cells remain activated for a longer period of time, and the memory responses of regulatory T cell–depleted animals was elevated around threefold compared with controls. The adoptive transfer experiments further demonstrated that the suppressive activity was concentrated in CD4+CD25+ Treg cells. Interestingly, HSV infection also served to induce the immunosuppressive function of CD4+CD25+ Treg cells. Our results are discussed in terms of their implication to vaccine design and susceptibility to intercurrent infections in those infected by HSV.
Materials and Methods
Mice and Virus.
Female 5–6-wk-old Thy1.2+ C57BL/6 (B6) and congenic Thy1.1+ B6.PL (H-2b) mice were purchased from Harlan Sprague-Dawley and Jackson Laboratory and housed in the animal facilities at the University of Tennessee. OT-1 mice used were bred and maintained in the Microbiology Department's animal facility. All investigations follow guidelines of the Committee on the Care of Laboratory Animals Resources, Commission on Life Science, National Research Council. HSV-1 strain KOS and KOS (dlsptk) was grown in vero cells obtained from American Type Culture Collection. The viruses were concentrated, titrated, and stored in aliquots at −80°C until use. Titers were measured in vero cells and expressed as PFU per milliliter.
Antibodies and Reagents.
PC61 (anti-CD25) hybridoma was purchased from American Type Culture Collection, and antibody was grown in tissue culture roller bottles. For all injections, an ammonium sulfate cut of PC61 mAb was used. Abs purchased from BD PharMingen were PerCp anti-CD8α, PE–anti-CD25 (PC61); CD62 ligand (CD62L), CD44, CCR5, CD69, CTL-associated antigen (CTLA)-4, IFN-γ, anti-TCR Vβ10, Streptavidin-PE, and FITC-labeled Vβ10, anti-CD25 (7D4), and anti-CD8α. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes. BrdU staining kit was purchased from BD Pharmingen. HSV gB498–505 peptide (SSIEFARL) and chicken ovalbumin (aa 257–264) peptide SIINFEKL were synthesized and supplied by Research Genetics.
Quantitation of HSV-1 in Foot Pad Tissues.
The quantitation of HSV-1 in foot pad (FP) tissue was determined as reported by Jennings et al. (10). Briefly, the mice were killed at the indicated time postinfection (p.i.), the FP surface was cleaned with 70% isopropyl alcohol, and the tissues were removed with a scalpel. The tissues were stored in virus diluent (PBS supplemented with 0.6 mM CaCl2, 0.5 mM MgCl2/H2O, 20 mg Phenol red, and 50 μg gentamicin sulfate per ml) at −80°C. Tissues were disrupted by homogenization in 1 ml ground glass grinders (Wheaton) and centrifuged, and the supernatant was used to assay viral titration on vero cells. Finally, plaques were visualized with neutral red.
Surface and Bromodeoxyuridine Staining.
Surface staining was performed as described previously (11). For bromodeoxyuridine (BrdU) staining, the BrdU kit from BD PharMingen was used according to the manufacturer's instructions. Briefly, HSV-1–infected mice received i.p. injection of BrdU (0.8 mg/ml) 1 d before termination of mice. Spleen cells were stained first with anti-CD8 and Vβ10 mAb and subsequently with labeled anti-BrdU mAb. Samples were analyzed on a FACScan™ cytometer using Cell Quest software (BD Biosciences).
SSIEFARL-specific CD8+ T Cell Proliferation.
CD8+ T cell proliferation was evaluated after in vitro restimulation of splenocytes with MHC class I (H-2b)–restricted SSIEFARL peptide. Briefly, the splenocytes collected from immunized mice were in vitro restimulated with irradiated SSIEFARL peptide (5 μg/ml)-pulsed syngeneic T–depleted splenocytes for 3 d. [3H]Thymidine (1 μCi/well) was added to each well 18 h before harvest. Harvested cells were measured for radioactivity using a β scintillation counter (Inotech).
CTL Activity.
CTL activity was assessed by a standard 4-h 51Cr release assay against labeled target cells as previously described (12). Splenic T cells obtained from immunized mice were in vitro restimulated with SSIEFARL peptide (5 μg/ml)-pulsed syngeneic irradiated T-depleted splenocytes for 5 d and then used as effector cells. The effector cells were then mixed at various ratios with 51Cr-labeled target cells for 4 h. Target cells included SSIEFARL-pulsed MHC-matched MC-38 (H-2b), mismatched EMT6 (H-2d). Spontaneous release of 51Cr was determined by incubating the target cells with medium alone, and maximum release was determined by adding Triton X-100 to a final concentration of 5%. The percentage of specific lysis was calculated as follows:
100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)].
Each experiment was performed twice using triplicate samples.
In Vivo CTL Assay.
The in vivo CTL assay was done as reported earlier (13). Splenocytes from naive B6 mice were used as target cells and equally split into two populations. One was pulsed with 2.5μg gB498–505 peptide for 45 min at 37°C and then labeled with a high concentration (2.5 μm) of CFSE. The other population was unpulsed and labeled with a low concentration of CFSE (0.25 μm). Equal number of cells from each population (107) were mixed together and adoptively transferred i.v. into naive and HSV-1–infected CD25-depleted and nondepleted B6 mice. In some experiments, splenocytes were collected either 1 or 4 h after adoptive transfer from recipient mice, erythyrocytes were lysed, and cell suspensions were analyzed by FACS® vantage system. Each population was distinguished by their respective fluorescence intensity. Assuming that the number of peptide-pulsed cells injected is equivalent to the number of no peptide-pulsed cells injected, the percentage of killing of target cells in uninfected animals was determined as:
[(Percentage of CFSElow – percentage of CFSEhigh) × 100]/Percentage CFSElow.
The percentage killing of target cells in infected animals was calculated as:
Ratio = (Percentage of CFSElow/percentage of CFSEhigh).
Percentage specific lysis = [1 − (ratio uninfected/ratio infected) × 100].
Intracellular Cytokine Staining.
To enumerate the number of IFN-γ–producing T cells, intracellular cytokine staining was performed as previously described (11). In brief, 106 freshly explanted splenocytes were cultured in U bottom 96-well plates. Cells were left untreated, stimulated with SSIEFARL peptide (1 μg/ml), or treated with PMA (10 ng/ml) and ionomycin (500 ng/ml), and incubated for 6 h at 37°C in 5% CO2. Brefeldin A (10 μg/ml) was added for the duration of the culture period to facilitate intracellular cytokine accumulation. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD PharMingen) in accordance with the manufacturer's recommendations. The Ab used was anti–IFN-γ (clone XMG1.2). The fixed cells were resuspended in PBS and analyzed with Cell Quest.
Single Cell ELISPOT Assay for IFN-γ–secreting Cells.
The enzyme-linked immunospot (ELISPOT) assay was used for quantification of cytokine-producing cells as reported by others (14). Briefly, ELISPOT plates (Millipore) were previously coated with IFN-γ anti–mouse Ab. The HSV-primed T cells (responder cells) were mixed with syngeneic splenocytes (stimulator cells) pulsed with or without gB498–505 (2.5 μg/ml) along with 10 U/well of human IL-2. Coincubation of the responder and stimulator cells was continued for 48 h at 37°C. The ELISPOT plates were washed three times with PBS and three times with PBS/Tween-20 (PBST), and then biotinylated IFN-γ Ab was added to the plates for 1 h at 37°C. The spots were developed using nitro blue tetrazolium (Sigma-Aldrich) and 5-bromo-4-chloro-3-indolylphosphate (Sigma-Aldrich) as a substrate after incubation with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratory) for 1 h and counted 24 h later under a stereomicroscope.
Adoptive Transfer of HSV-1–primed Memory CD8+ T Cells to CD25-depleted B6 Mice.
B6 mice were infected via i.p. route with 2 × 106 PFU of HSV-1 in the presence or absence of CD4+CD25+ Treg cells. CD8+ T cells were purified from the spleen of respective groups 90 d p.i. by MACS column, and 107 viable CD8+ T cells were adoptively transferred to recipient B6 mice, which were challenged within 2 h in the hind FP with 5 × 106 pfu of HSV-1 KOS. 2 d before adoptive transfer, the recipient mice was depleted of Treg cells by PC61 treatment. FP was removed after regular interval of time p.i. and homogenized for viral titration as described earlier.
CD4+CD25+ T Cell Purification and Adoptive Transfer in Syngeneic B6 Mice.
CD4+CD25+ T cells were purified as reported by others (15). Briefly, CD4 columns (R&D Systems) were used for the isolation of CD4+ T cells. CD4+CD25+ T cells were purified by incubating the enriched CD4+ T cells with biotin-conjugated anti-CD25 (15 μg/108 cells) mAb in PBS/2% FCS for 15 min at 4°C. The positive magnetic separation was performed with LS+ columns (Miltenyi Biotec) according to the suggested protocol. The purity of cells ranged from 85 to 95%. In some experiments, CD4+CD25+ T cells purified from naive B6 animals (Thy1.1) were adoptively transfer into B6 (Thy1.2) or Rag−/− mice 24 h before virus infection. Immune response was analyzed 7 and 28 d after virus infection.
Cytokine ELISA.
The culture supernatants from the bulk test cultures without addition of any exogenous cytokines were screened for the presence of IL-10 and IFN-γ. ELISA plates were coated with capture Abs for the respective cytokines and incubated at 4°C overnight. The plates were washed with PBS/Tween 20 and blocked with 3% BSA for 2 h at room temperature. After washing, serially diluted samples and standards were added to the plate and incubated at 4°C overnight. The plates were washed followed by the addition of cytokine-specific detection antibodies for 2 h. Finally, peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratory) was added. The color was developed by adding the substrate (ABTS) solution (Sigma-Aldrich), and the concentration was calculated with an automated ELISA reader (SpectraMAX 340; Molecular Devices).
Statistical Analysis.
All analysis for statistically significant differences were performed with Student's paired t test. P < 0.01 and P < 0.05 were considered significant. Results are expressed as mean ± SD.
Online Supplemental Material.
Figs. S1 and S2, available at http://www.jem.org/cgi/content/full/jem.20030171/DC1, show in vivo depletion and HSV-1 immunization. C57BL/6 mice were given 1 mg PC61 mAb I.P. (i.p.) 3 d before HSV-1 infection. Initial kinetics of depletion was studied in uninfected mice. PC61 treated or nontreated C57BL/6 mice were immunized subcutaneously in each hind FP with 105 PFU HSV-1 KOS (dlsptk) in 50 μl of PBS. At regular intervals after infection, lymphocytes from draining lymph node (DLN) and spleen were isolated for phenotypic and functional studies.
Results
In Vivo Depletion of CD4+CD25+ Treg Cells Before HSV-1 Infection Enhances Virus-specific CD8+ T Cell Responses.
To study the in vivo role of CD4+CD25+ Treg cells in regulating the HSV-specific CD8+ T cell immune responses, B6 mice were treated once with 1 mg of anti-CD25 mAb PC61 i.p. The kinetics of depletion was studied from day 3 to 21 in spleen of depleted mice. Almost total depletion was observed by day 3, but by day 21 CD25+ regulatory T cell levels were ∼80% normal (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20030171/DC1). No significant depletion of CD25+ Treg cells was observed in mice treated with Rat Ig control (unpublished data).
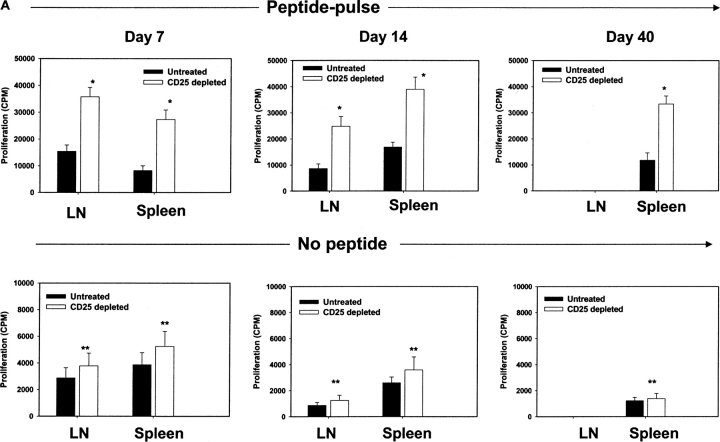
3 d postdepletion, mice were FP infected with HSV-1 virus, and animals were killed at regular intervals to measure SSIEFARL-specific CD8+ T cell responses in the draining popliteal LNs and spleen. As is evident in Fig. 1 , peptide-specific CD8+ T cell numbers were elevated in the CD25-depleted mice compared with controls as measured by four separate assays. Thus, at day 7 p.i., peptide-specific proliferation in CD25-depleted animals was elevated more than threefold and twofold in splenocytes and DLN cells, respectively (Fig. 1 A). By day 40 in the memory phase, significant differences were still evident in the splenocyte proliferation response between depleted and nondepleted animals (Fig. 1 A).
Figure 1.
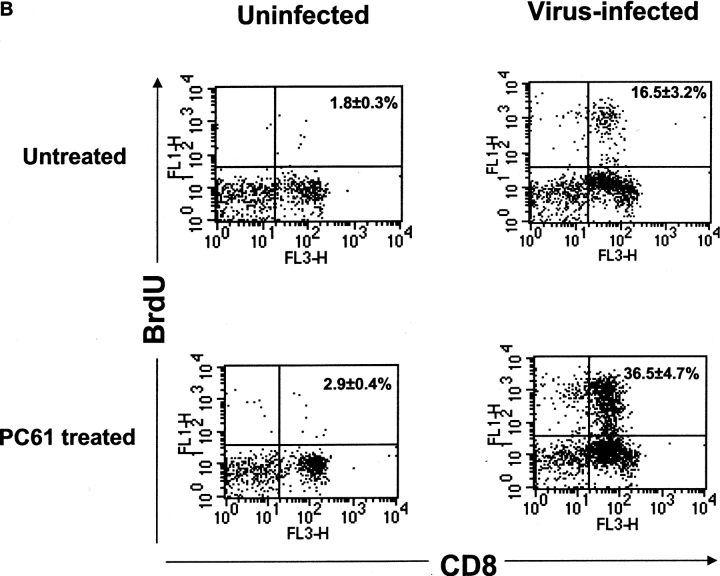
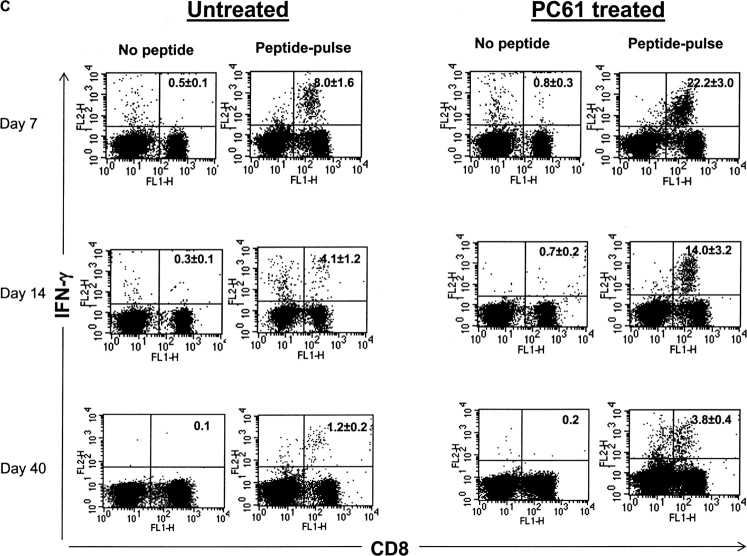
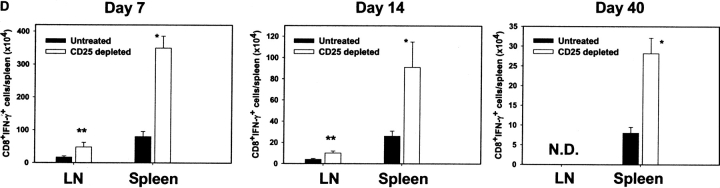
Enhancement of antigen-specific CD8+ T cell responses after in vivo depletion of CD4+CD25+ Treg cells before HSV-1 infection. (A) The kinetics of SSIEFARL-specific CD8+ T cell proliferation was measured in vitro in both depleted and nondepleted mice by [3H]thymidine incorporation. The results are expressed as cpm. *P < 0.01 compared with nondepleted HSV-infected B6 mice. (N.D., not done). The experiment shown is representative of two similar experiments, and error bars reflect the mean ± SD of four mice per group. The cpm of nondepleted and CD25+-depleted noninfected groups was always <1,000 with no significant difference observed between the two groups. (B) In vivo proliferation of Vβ10+CD8+ T cells was measured by BrdU incorporation assay. Mice infected with HSV-1 3 d postdepletion were injected i.p. with 100 μl (1 mg) of BrdU solution in PBS 1 d before sacrifice. Mice were terminated 7 d p.i. to examine the in vivo proliferation of CD8+Vβ10+T cells. Cells were gated on Vβ10+ cells, and the percentage of BrdU+CD8+Vβ10+ T cells is represented in the upper right quadrant. The data in B are representative of two identical experiments. (C) Kinetics of HSV-1–specific immune response shows that SSIEFARL-specific CD8+ T cells increased in number in PC61-treated mice are also functional as evident by intracellular IFN-γ staining. The experiment shown is representative of two additional experiments. The values shown in these FACS® plots are the mean of four mice per group. (D) Increase in IFN-γ–secreting CD8+ T cells in B6 mice devoid of CD4+CD25+ T cells were also measured by standard ELISPOT assay. On different days post HSV infection, LNs and spleen cells were analyzed for the number of IFN-γ–secreting CD8+ T cells in response to SSIEFARL peptide. The bars represent the mean ± SD of three different mice of same group and are representative of two similar experiments. *P < 0.01 and **P < 0.05 compared with untreated HSV-infected B6 mice. (N.D., not done). Without peptide stimulation, there was no significant difference in CD8/IFN-γ–producing cells in nondepleted and CD25-depleted HSV-infected mice, and the maximum number obtained at any time point was always <5,000 per spleen.
Approximately 65% of gB498–505-specific CD8+ T cells in B6 mice express a highly conserved Vβ10+ TCR, and these SSIEFARL-specific Vβ10+CD8+ T cells actively participate in CTL responses against HSV (16). To seek direct in vivo evidence for the influence of CD4+CD25+ regulatory T cells on antigen-specific proliferative responses, infected animals in both groups were pulsed with BrdU at day 6 p.i, and 1 d later lymphoid populations were analyzed by FACS® for evidence of BrdU incorporation. Vβ10+ cells were gated on FACS® plot, and the assay revealed an average 2.5-fold increase in SSIEFARL-specific CD8+ T cells in splenocytes of the CD25-depleted group (Fig. 1 B).
For protection against HSV infection, T cell–derived IFN-γ is considered a crucial event (17). Analysis of the intracellular IFN-γ response of splenic CD8+ T cells stimulated by the immunodominant SSIEFARL peptide revealed significantly enhanced immune responses in CD25+-depleted animals (Fig. 1 C). Furthermore, the splenocyte cytokine response of depleted animals, as measured by ELISPOT at 7 d p.i., was around fourfold that of undepleted animals (Fig. 1 D). Similarly, the LN response of CD25-depleted animals was approximately threefold that of nondepleting animals. Notably, even after 40 d p.i., when levels of regulators had returned to normal, the IFN-γ ELISPOT response in splenocytes (a measure of CD8+ T cell memory) was still elevated around threefold in depleted animals compared with nondepleted controls (Fig. 1 D). Together, these results demonstrate that the immunodominant CD8+ T cell response became elevated in both the acute and memory phase of HSV-infected animals if CD4+CD25+ Treg cells were depleted before infection.
Removal of CD4+CD25+ Regulatory T Cells In Vivo Enhances HSV-1–specific CD8+ T Cell Cytotoxic Immune Response.
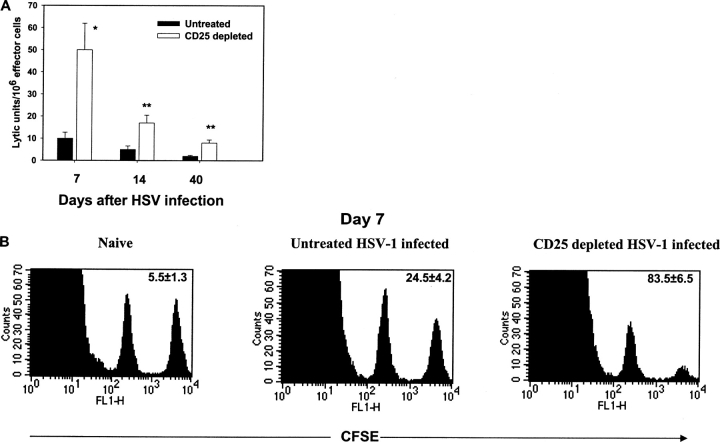
Additional assays were performed to compare the effector functions of CD8+ T cells after HSV infection of Treg-depleted and control mice. Two approaches were used. First, peptide-specific CTL responses were compared in splenocytes at different times p.i. By this assay, CTL responses of CD25+-depleted animals were around fourfold and threefold compared with those of controls in the acute and memory phase, respectively (Fig. 2 A). A second approach employed to compare control versus CD25-depleted mice was the in vivo CTL assay (13). This method measures lysis of specific peptide pulsed target cells adoptively transferred into infected animals that have effector CD8+ T cells. The assay was performed at 7 d p.i. The results shown in Fig. 2 B, indicate that CD25 depletion resulted in a mean 83% lysis of specific targets in the spleen compared with 25% lysis of targets in nondepleted animals when measured 1 h postadoptive transfer of peptide-pulsed targets. In uninfected animals, the lysis of peptide-pulsed targets was always ∼5–8%. Since the in vivo CTL assay does not require in vitro expansion, this approach is considered to be direct evidence that regulatory T cells function in vivo to diminish the extent of CD8+ T cell response to HSV infection.
Figure 2.
Increased antigen-specific CD8+ T cell cytotoxicity in CD25-depleted B6 mice after HSV-1 infection. (A) Splenocytes collected from WT and CD25-depleted B6 mice at different time points after HSV infection were expanded in vitro with irradiated SSIEFARL-pulsed syngeneic splenocytes for 5 d and used as effectors in a 4-h 51Cr release assay. The targets included SSIEFARL-pulsed, MHC-matched MC-38 and unpulsed MC-38. The figure shows only the data for SSIEFARL peptide-pulsed MC-38 targets in lytic units. One lytic unit (LU) is the number of lymphocytes required to give 30% lysis. The lysis in control targets was insignificant and below the limit for calculation of lytic units. The experiments were performed using four mice, and data shown above are mean ± SD of four mice from one experiment. *P < 0.01 and **P < 0.05 compared with untreated HSV-infected B6 mice. (B) To analyze the difference of in vivo SSIEFARL-specific cytotoxicity in vivo in CD25-depleted and nondepleted B6 mice 7 d post HSV-1 infection, syngeneic spleen cells were pulsed with gB498–505 peptide and labeled with CFSE (2.5 μm). To control for antigen specificity, unpulsed syngeneic spleen cells were labeled with a lower concentration of CFSE (0.25 μm). A 1:1 mixture of each target cell population was injected i.v. into both groups of mice. 1 h p.i., spleen cells were prepared from individual mice and acquired on a FACS® Vantage system. The percentage of specific lysis was determined as mentioned in Materials and Methods. The number shown in each plot is the mean of percent antigen specific lysis observed in four mice per group.
Removal of CD4+CD25+ Regulatory T Cells Influences the Course of Viral Infection.
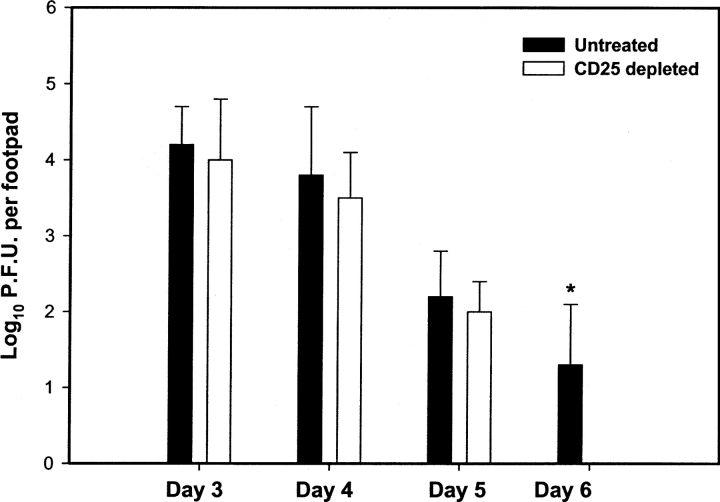
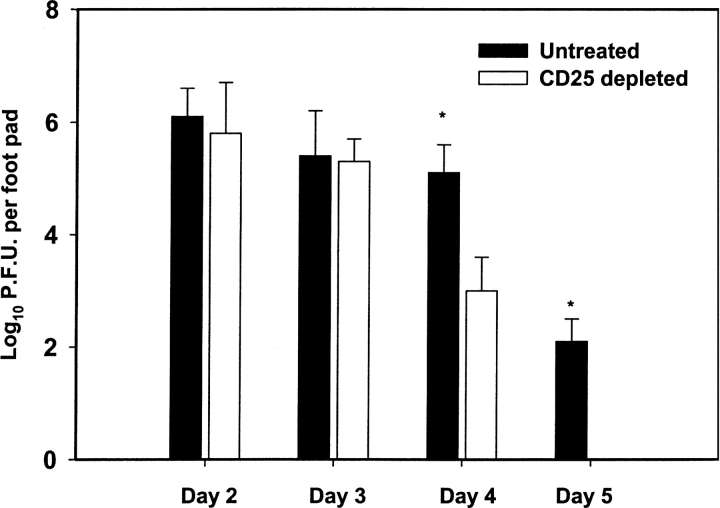
The clearance of acute cutaneous HSV-1 infection is dependent on T lymphocyte–mediated immune function, and IFN-γ plays an important role in this process (18). We monitored whether the enhancement of the SSIEFARL-specific CD8+ T cell immune response observed in the absence of Treg cells affected the course of viral infection. Our data imply that the acute cutaneous viral load in the FP was comparable in both CD25-depleted and nondepleted B6 mice until day 5. However, no viral plaques were observed in the CD25-depleted group on day 6, whereas a significant viral load was still present in the control nondepleted group (Fig. 3) . By day 8, no virus was detected in the FP of both groups (data not depicted).
Figure 3.
Increased rate of viral clearance from the FP of CD25-depleted HSV-1–infected mice during the primary immune response. Mice were infected in the hind FPs with 106 pfu HSV-1, and on days 3 to 6 the hind feet were removed and homogenized in virus diluent. The resulting supernatant was used in a vero cell based viral plaque assay to determine the virus titer. The results are expressed as number of pfu of virus per FP. Each error bar represents the mean and SD of six individual FPs. *P < 0.01 compared with CD25-depleted HSV-infected B6 mice.
Depletion of CD25+ Regulatory T Cells Enhances the Expression of Cell Surface Activation Markers on Virus-specific CD8+ T Cells.
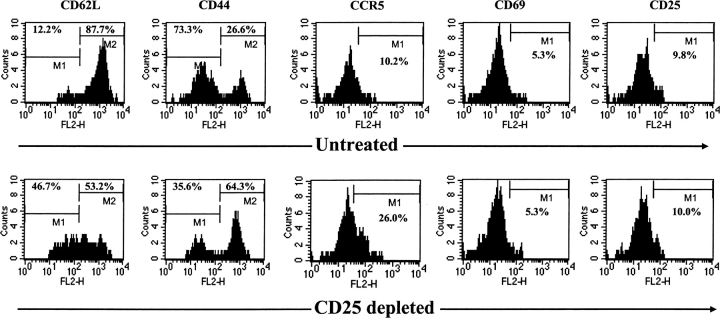
The results reported above indicate that a consequence of CD25 depletion is that the CD8+ T cell response to the immunodominant peptide SSIEFARL is significantly elevated. Additional assays were performed to define if the population of peptide-specific T cells isolated from CD25-depleted animals differed in phenotypic expression of activation markers compared with control undepleted animals at a time when effector responses were declining. As shown in Fig. 4 , 14 d p.i. an approximately twofold increase in percentage of cells expressing CCR5 and CD44 on Vβ10+CD8+ T cells (which account for the majority of SSIEFARL-specific CD8+ T cells, [9]) was found in the Treg-depleted group. Furthermore, ∼88% of Vβ10+CD8+ T cells were CD62L high in nondepleted group compared with 53% in CD25-depleted mice. Differences were not evident in CD69 and CD25 expression. Expression of activation markers were also compared day 7 and 21 p.i.; however, no significant differences were observed on these time points (unpublished data). We interpret these observations to indicate that CD25 regulators might regulate the contraction phase of an immune response, and their absence allowed the recently activated CD8+ T cell population to remain in an effector state for an extended duration.
Figure 4.
Removal of CD4+CD25+ T cells before HSV infection enhances the expression of activation markers on virus-specific CD8+ T cells. Expression of activation markers on T cells gated on with double positive CD8+Vβ10+ cells in spleen of CD25-depleted and nondepleted mice were examined. Splenocytes from both groups were collected on day 14 post HSV infection and stained with anti–mouse CD8α and anti–mouse Vβ10 mAb in combination with an antibody against on activation marker (anti-CD44, CD62L, CD69, CD25, CCR5) and the appropriate isotype control using three color FACS® analysis. Histograms are representative of four individual mice from one such experiment, and values reflect the percentage of cells expressing the respective marker.
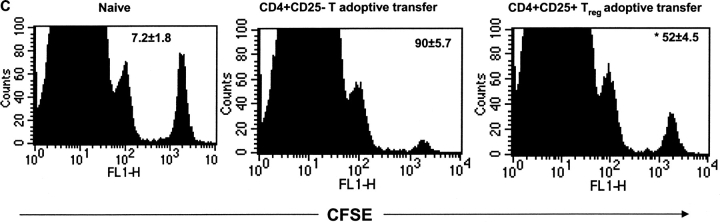
Inhibition of Virus-specific CD8+ T Cell Responses by CD4+CD25+ Regulatory Cells in an Adoptive Transfer Model.
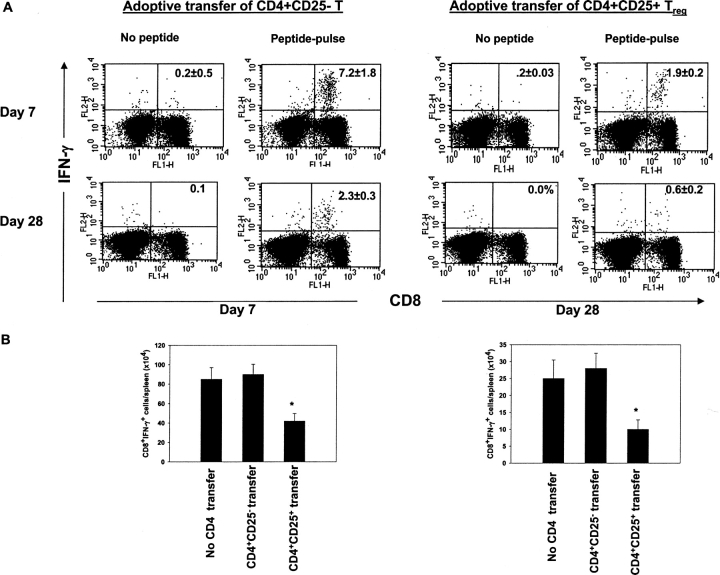
To further establish the role of regulatory T cells in suppressing virus-specific CD8+ T cell immune responses, an adoptive transfer system was used. Accordingly, CD4+CD25+ and CD4+CD25− T cells were isolated from naive Thy1.1 mice and adoptively transferred into Thy1.2 B6 mice 24 h before HSV-1 infection. At 7 and 28 d p.i., the SSIEFARL-stimulated CD8+ T cell IFN-γ production in splenocytes was measured. As shown in Fig. 5 , the transfer of CD4+CD25+ (but not CD4+CD25− T cells) significantly impaired the intracellular IFN-γ and ELISPOT CD8+ T cell responses to HSV-1 (Fig. 5 A). Reduction was between three and fourfold for intracellular IFN-γ staining (ICG) (Fig. 5 A) and was ∼2.5-fold for ELISPOT (Fig. 5 B). Similarly, the in vivo CTL assay also revealed that adoptive transfer of CD4+CD25+ T cells significantly suppressed the in vivo cytolytic activity of SSIEFARL-specific CD8+ T cells (Fig. 5 C). Together, these results demonstrate that the CD4+CD25+ regulatory T cells significantly inhibit the virus-specific CD8+ T cell immune response. Similar results were obtained with the adoptive transfer of purified CD4+CD25+ Treg cells with CD25 depleted total SPC (1:5) in a Rag−/− mice where SSIEFARL-specific CD8/IFN-γ production was measured 7 d p.i. (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20030171/DC1).
Figure 5.
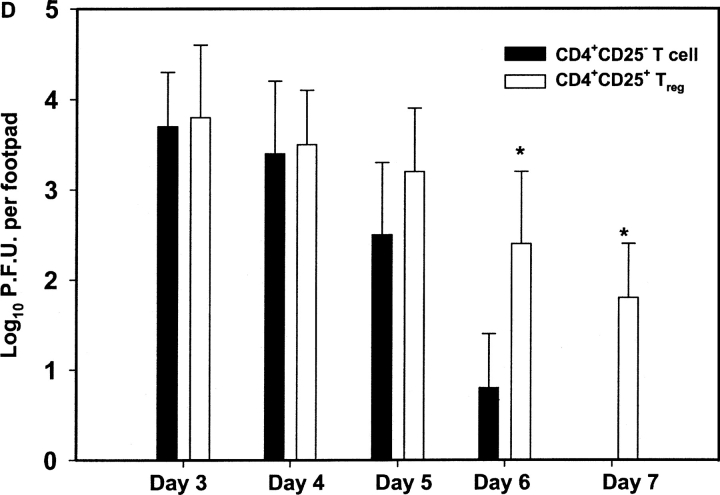
Adoptive transfer of CD4+CD25+ T cells inhibits HSV-1–specific CD8+ T cell responses. Purified CD4+CD25+ and CD4+CD25− T cells (2 × 106/mouse) were adoptively transferred into WT B6 mice 24 h before HSV infection, and the immune response was measured on days 7 and 28 p.i. (A) On days 7 and 28 p.i., spleen cells were incubated with gB498–505, and CD8/IFN-γ production was measured by intracellular staining. The number shown in each plot is the mean percentage of IFN-γ–producing CD8+ T cells obtained from four mice per group. (B) The resulting decrease in IFN-γ–secreting CD8+ T cells in B6 mice after adoptive transfer of CD4+CD25+ T cells were also measured by a standard ELISPOT assay. On days 7 and 28 post HSV infection, spleen cells were analyzed for the number of IFN-γ–secreting CD8+ T cells in response to SSIEFARL peptide. The error bars represent the mean ± SD of four different mice in the same group. *P < 0.05 compared with HSV-infected B6 mice receiving no adoptive transfer. Without peptide stimulation, there was no significant difference in CD8/IFN-γ–producing cells between HSV-infected mice receiving either CD4+CD25+ or CD4+CD25− T cells. The maximum number of cells obtained at both time points was always <4,000 per spleen. (C) gB-specific cytotoxicity was also measured by an in vivo CTL assay to analyze differences in SSIEFARL-specific cytotoxicity after adoptive transfer of CD25+ or CD25− CD4+ T cells. On day 7 post HSV-1 infection, syngeneic spleen cells were pulsed with gB498–505 peptide and labeled with CFSE (2.5 μm). Unpulsed syngeneic spleen cells were labeled with a lower concentration of CFSE (0.25 μm). A 1:1 mixture of each target cell population was injected i.v. into both groups. 4 h p.i., spleen cells were prepared from individual mice and acquired on a FACS® vantage system. Specific lysis was calculated as mentioned in Materials and Methods. The number shown in each plot is the mean percent antigen specific lysis observed in four mice per group. *P < 0.05 compared with group with CD4+CD25− T cell adoptive transfer. (D) Adoptive transfer of CD25+ Treg delays the viral clearance in HSV-infected mice. Each bar represents mean and SD of six individual FPs. *P < 0.05 compared with HSV-infected B6 mice receiving CD4+CD25− T cells.
Additional experiments were performed to measure if diminished immune responses resulting from the transfer of CD25+ Treg had any effect on viral replication after infection of the FPs of recipient mice. As is evident in Fig. 5 D, measurement of viral clearance in the infected FP of transferred recipient revealed no significant difference over the first 5 d. However, the transfer of CD4+CD25+ Treg resulted in enhanced susceptibility to infection, with mice failing to clear the virus by day 7. In contrast, the control group receiving CD4+CD25− T cells cleared the virus by day 7, and viral titers were significantly diminished on day 6. These experiments indicate that the transfer of additional CD25+ Treg impairs the viral clearance rate, but this is evident at a time when clearance is mediated by adaptive immune responses.
HSV Infection Activates CD4+CD25+ Regulatory T Cells.
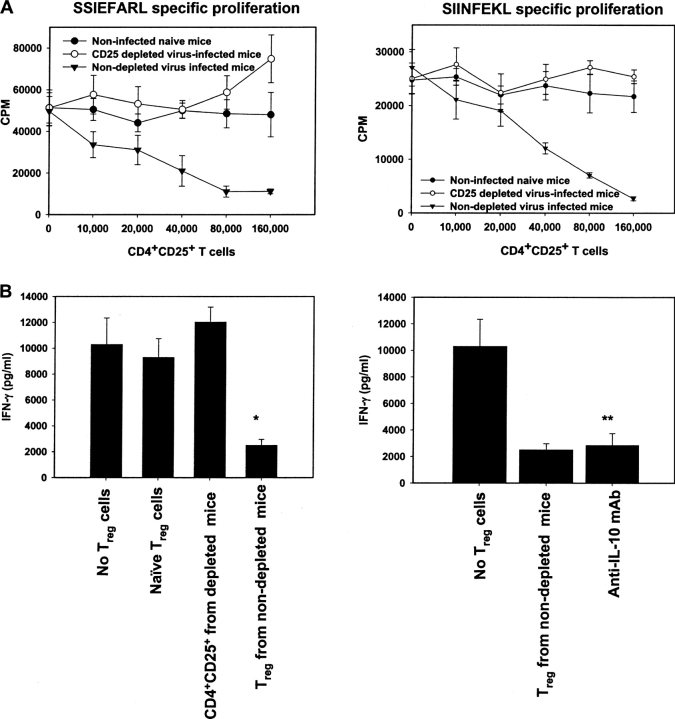
Usually CD4+CD25+ Treg cells require in vitro activation via their TCR to exert their inhibitory functions (15). How such cells become activated in vivo remains unresolved. The results shown in Fig. 6 indicate that one consequence of HSV-1 infection may be potentiation of Treg cell function. In these experiments, the inhibitory effect of CD4+CD25+ T cells was measured in vitro against CD8+ T cell responses to peptides. Three populations of CD4+CD25+ T cells isolated from different groups of animals were evaluated. These were prepared from naive animals, animals infected 10 d previously with HSV-1 and from mice depleted of CD25+ T cells before HSV infection. As shown in Fig. 6 A, CD4+CD25+ T cells from naive animals and from Treg-depleted virus-infected mice failed to inhibit in vitro proliferative responses of SSIEFARL- and SIINFEKL-specific CD8+ T cells. In contrast, a dose-dependent inhibition of both SSIEFARL-specific and SIINFEKL-specific CD8+ T cell proliferation occurred when CD4+CD25+ T cells isolated from HSV-infected nondepleted animals were added to cultures. Results measuring the suppressive effects of the three series of CD4+CD25+ T cells against peptide-induced IFN-γ production measured by ELISA also revealed that only the population from the undepleted virus infected mice showed significant inhibitory effects (Fig. 6 B). The suppressive effect mediated by virus-activated CD25+ T cells appeared not to involve IL-10, since neither significant levels of IL-10 were detected in cocultures (unpublished data), nor did the addition of anti-IL-10 mAb to cultures abrogate the suppressive function of the virus-activated Treg cells (Fig. 6 B).
Figure 6.
CD4+CD25+ T cells isolated from HSV-1–infected nondepleted B6 mice suppress CD8+ T cells proliferation to both HSV-specific and unrelated antigens. (A) HSV-1–primed CD8+ T cells or OT-1 CD8+ T cells (2 × 106/ml) were stimulated with either SSIEFARL or SIINFEKL peptide-pulsed, T-depleted splenocytes (106/ml) in the presence of CD4+CD25+ T cells isolated from either naive, nondepleted, or CD25-depleted B6 mice 10 d post HSV-1 infection. The proliferation was measured in vitro by [3H]thymidine incorporation assay, and the results are expressed as cpm. The basal level of proliferation of CD4+CD25+ T cells isolated from each group was always >5,000 with maximum proliferation observed in CD4+CD25+ T cells purified from CD25-depleted virus-infected mice. (B) CD4+CD25+ T cells isolated from naive and virus-infected mice were cocultured (106/ml) with HSV-1–primed CD8+ T cells (2 × 106/ml) stimulated with SSIEFARL-pulsed APCs (2 × 106/ml), and IFN-γ secretion in cultures was measured by ELISA as mentioned in Materials and Methods. Anti–IL-10 mAb (5 μg/ml) was also added in some of the cocultures to determine the effect on IFN-γ production. No spontaneous IFN-γ production was observed in CD4+CD25+ T cells isolated from either group. *P < 0.01 when compared with wells containing no Treg cells and **P > 0.05 when compared with wells containing Treg. Results from one representative experiment are shown.
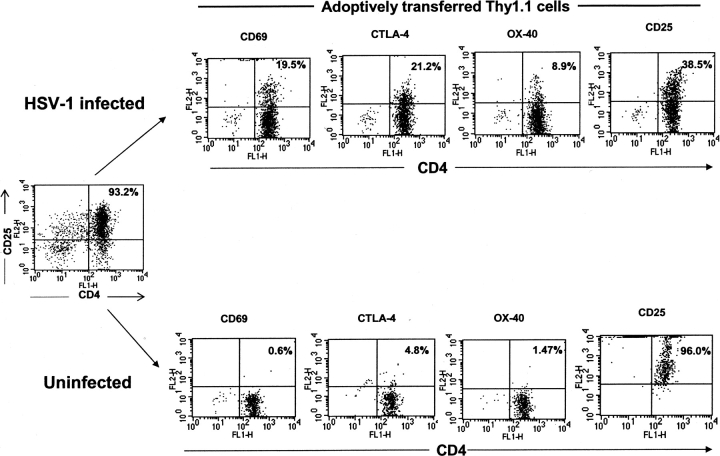
The Treg activation resulting from HSV infection was further analyzed in an adoptive transfer assay in which CD4+CD25+ Treg purified from naive Thy1.1 mice were adoptively transferred (106/mouse) into congenic Thy1.2 B6 recipients. The purity of CD4+CD25+ Treg was 93%. One group of animals was virus infected and the other acted as noninfected controls. Analysis of spleen cells isolated from recipient mice on day 7 p.i. revealed a threefold increase in the donor Thy1.1+ cells (2.4% ± 0.4) compared with the uninfected control group (0.7% ± 0.1). Furthermore, virus infected animals also demonstrated a four- to fivefold increase in Treg cells expressing OX-40 and CTLA-4. Around 20% of adoptively transferred Treg cells expressed CD69 (Fig. 7) . Interestingly, in virus-infected animals more than half of the donor Treg cells lost their CD25 marker, and the percentage of CD4+CD25+ T cells was only 40%, whereas in noninfected animals ∼95% of Treg cells were still CD25 positive. These results add further evidence that HSV infection leads to activation of Treg function.
Figure 7.
Activation of CD4+CD25+ T cells by HSV infection in an adoptive transfer model. CD4+CD25+ T cells purified from Thy1.1 B6 mice were adoptively transferred into congenic Thy1.2 B6 recipients. One group was infected 14 h posttransfer and the other acted as noninfected controls. On day 7, spleen cells isolated from both groups were gated on the Thy1.1 population and analyzed for the expression of the activation markers such as OX-40, CTLA-4, CD69, and CD25 on CD4+ T cells.
Memory CD8+ T Cells Generated in the Absence of CD4+CD25+ T Cells Confer Greater Protection upon Rechallenge.
It has been shown earlier that HSV-specific CTL provide a level of protection against acute HSV infection (10). To evaluate the ability of HSV-specific memory CD8+ T cells generated in the presence or absence of CD4+CD25+ Treg cells to mediate protection, CD8+ T cells were purified 90 d p.i. from splenocytes and transferred to naive recipients that were then infected via the FP with virus. Animals were killed at intervals to measure viral clearance from FPs. The experiment revealed that the protection was better in the recipient of CD8+ T cells taken from CD25-depleted animals on days 4 and 5 when compared with control group (Fig. 8) . Furthermore, by day 5, virus could only be demonstrated in the recipients of CD8+ T cells from untreated animals, and all animals were negative by day 6. These results suggest that CD25+ Treg cells may indeed exert long term effects on the resulting memory CD8+ T cell pool.
Figure 8.
Adoptive transfer of HSV-specific memory CD8+ T cells generated in the absence of Treg results in enhanced viral clearance than those generated in the presence of CD25+ regulatory T cells. Splenic MACS purified CD8+ T cells isolated from depleted and nondepleted animals 90 d p.i. were adoptively transferred into CD25-depleted naive B6 mice. 24 h posttransfer, mice were challenged in the hind FPs with 5 × 106 pfu of HSV-1 KOS. The level of infectious HSV in the FP was observed from day 2 to 5 postchallenge. *P < 0.05. A higher virus load was always observed in CD25-depleted, HSV-infected group without any adoptive transfer compared with the groups with adoptive transfer (data not depicted).
Discussion
This report demonstrates that the magnitude of a T cell–mediated immune response to an acute viral infection is subject to control by CD4+CD25+ Treg. Accordingly, if Treg were depleted with specific anti-CD25 antibody before infection with HSV, the resultant CD8+ T cell response to the immunodominant peptide SSIEFARL was significantly enhanced. This was shown by several in vitro measures of CD8+ T cell reactivity and by assays that directly determine CD8+ T cell function, such as proliferation and in vivo cytotoxicity. The enhanced responsiveness in CD25+ T cell–depleted animals was between three- and fourfold with the effect evident both in the acute and memory phases of the immune response. Moreover, CD8+-specific T cells retained an activation phenotype for longer periods in depleted animals. Other experiments demonstrated that HSV infection itself appeared to act as a means of inducing and/or activating Treg. Our results imply that procedures that minimize the effects of Treg at the time of immunization could benefit HSV vaccination procedures. Given the poor track record of past vaccines against HSV, modulating suppression could be a maneuver worth considering to improve such vaccines.
Since the concept of regulatory cells was reawakened by the observations of several groups (1, 15, 19, 20), it has become evident that the intensity of many immune inflammatory reactions are subject to control by Treg. The initial focus was on organ-specific autoimmunity, but immunopathological reactions to foreign exogenous antigens, including those expressed by microorganisms, are also influenced by Treg (3, 5, 6, 21). So far, studies on microorganisms have focused on nonviral intracellular pathogens and emphasized immunopathology.
This report analyzes the role of CD4+CD25+ Treg in a virus infection and addresses their effects on resultant immunoprotective responses. Both by removing Treg and by adoptive transfer studies, our results clearly showed that the CD8+ T cell–mediated immune responses to HSV were significantly limited by the action of naturally occurring CD25+ T cells. This was shown by measures of proliferation, cytokine production, and cytotoxicity. In the B6 mouse strain used for such studies, the CD8+ T cell response is overwhelmingly dominated by a reactivity to the peptide SSIEFARL (9). Moreover, responses to SSIEFARL alone can account for functional systemic and mucosal immunity to HSV in B6 mice (12). Most striking was the observation, using the recently described in vivo cytotoxicity assay, a direct measure of T cell function that cytotoxicity in the absence of Treg in infected, depleted animals was markedly higher than in nondepleted controls tested under the same conditions.
Further, support for the influence of CD25+ Treg on the level of immunity to infection came from adoptive transfer experiments. These results showed that the transfer of CD25+ cells into naive B6 mice and Rag−/− recipients resulted in suppressed CD8+ T cell responses after virus infection. Finally, mice depleted of CD25+ T cells before infection cleared virus from infected FPs more effectively than did nondepleted controls. Together, the results of both depletion and adoptive transfer experiments indicate that the extent of virus-specific immunity is limited by CD4+CD25+ Treg cells.
Currently, it is not clear how regulatory cells are induced and act to modulate effector cell function, especially of CD8+ T cells. Work in well-studied systems indicates that CD25+ Treg are induced in the thymus and express TCR that recognize self-peptides (22–25). Activation requires TCR interaction, but suppression itself is nonspecific and in some systems appears mediated by TGF-β, CTLA-4, or IL-10 (19, 26–28). A possible mechanism by which the immune response to pathogens might be modulated by Treg might involve either direct or indirect reactivity with pathogen specific antigens. This could be achieved, for example, by the pathogen possessing superantigen activity. Such a scenario was suggested for mouse mammary tumor virus (29), which expresses a superantigen. However, no known superantigen activity is encoded by HSV. Moreover, it is unlikely that under normal infection conditions HSV would interact directly with more than a minute fraction of the population of CD25+ regulatory T cells. Hence, the means by which HSV induces Treg activation is more likely to represent a paracrine event mediated by cytokines (such as IL-12/IL-18), stress proteins, or other activation molecules.
Curiously, our results did unearth evidence that HSV infection resulted in the activation and potentiation of Treg cell function. Accordingly, CD25+ T cells taken from infected animals after the peak of effector activity had enhanced regulatory activity against CD8+ T cell proliferative responses in vitro. This regulatory effect was not confined to HSV antigens, since the inhibition, which did not require additional in vitro viral Ag stimulation, was demonstrated against CD8+ T cell proliferative responses to the unrelated OVA408–419 peptide. Further, we believe that CD4+CD25+ T cells purified from CD25-depleted HSV-infected animals were enriched in effector CD4+ T cells and did not have immunosuppressive activity. Currently, we lack a mechanistic explanation for the induction or expression of the regulation, but the effect appeared not to involve IL-10. In our system, use of anti–IL-10 mAb under in vitro conditions failed to inhibit the CD25+ Treg-mediated immunosuppressive activity. However, we cannot rule out the possibility that CTLA-4 or TGF-β (membrane bound or secreted) mediated the suppression. Conceivably, the induction of regulatory cells may be a common event with several virus infections and may account for the transient immunosuppression that often occurs during or after infection (30–33). The process and its implication for resistance to secondary infection require further study.
Our results indicate that a regulatory response by CD4+CD25+ T cells serves to limit the immunodominant CD8+ T cell response to infection by HSV. The effect was evident and approximately equal in magnitude in both the acute and memory phases. Such observations may support the hypothesis that the generation of the memory pool is regulated by the effector response (34, 35). However, some differences in average phenotype of specific CD8+ T cells were observed between Treg-depleted and nondepleted animal in the acute population. Accordingly, in CD25+-depleted animals, effector CD8+ T cells appeared to remain activated for a longer duration. Our results indicate that the Treg function, activated as a consequence of HSV infection, may contribute to the contraction phase of the immune response. In turn, the absence of Tregs may allow activated effector cells to exist for an extended period. Studies also revealed that the increased pool of memory CD8+ T cells generated in CD25+-depleted animals had enhanced cytotoxicity and conferred increased protection against viral challenge with HSV-1 in comparison to memory CD8+ T cells generated in the presence of Treg cells.
Finally, our results may have an impact on vaccine design, especially against agents such as HSV, for which satisfactory vaccines are still unavailable. These studies infer that immunity can be elevated if existing Treg responses are inhibited before immunization/infection. Clearly, it would not be practical to limit the function of Treg with antibody, the approach used in our experiments, but other more practical procedures might be developed to limit Treg function during vaccination. Furthermore, it could be that postvaccinal Treg suppression would be an effective procedure to enhance immunity as was most recently implied by the studies of Mittrucker et al. (36). It would also seem that vaccines should be evaluated in terms of their susceptibility to inhibitory Treg responses and whether this affects the potency of the resulting T cell functional immunity. We are currently evaluating such ideas in our laboratory.
Acknowledgments
We thank Nancy Neilsen for excellent technical support with flow cytometry.
Work supported by grants AI 14981 and AI 46462 from the National Institutes of Health.
The online version of this article contains supplemental material.
References
- 1.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 2.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 3.Maloy, K.J., L. Salaun, R. Cahill, G. Dougan, N.J. Saunders, and F. Powrie. 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHugh, R.S., E.M. Shevach, and A.M. Thornton. 2001. Control of organ-specific autoimmunity by immunoregulatory CD4(+)CD25(+) T cells. Microbes Infect. 3:919–927. [DOI] [PubMed] [Google Scholar]
- 5.Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 7.Xu, D., H. Liu, M. Komai-Koma, C. Campbell, C. McSharry, J. Alexander, and F.Y. Liew. 2003. CD4(+) CD25(+) Regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 170:394–399. [DOI] [PubMed] [Google Scholar]
- 8.Blaney, J.E., Jr., E. Nobusawa, M.A. Brehm, R.H. Bonneau, L.M. Mylin, T.M. Fu, Y. Kawaoka, and S.S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace, M.E., R. Keating, W.R. Heath, and F.R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 73:7619–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonneau, R.H., and S.R. Jennings. 1989. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J. Virol. 63:1480–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumaraguru, U., and B.T. Rouse. 2000. Application of the intracellular gamma interferon assay to recalculate the potency of CD8(+) T-cell responses to herpes simplex virus. J. Virol. 74:5709–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gierynska, M., U. Kumaraguru, S.K. Eo, S. Lee, A. Krieg, and B.T. Rouse. 2002. Induction of CD8 T-cell-specific systemic and mucosal immunity against herpes simplex virus with CpG-peptide complexes. J. Virol. 76:6568–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coles, R.M., S.N. Mueller, W.R. Heath, F.R. Carbone, and A.G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834–838. [DOI] [PubMed] [Google Scholar]
- 14.Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J.D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. [DOI] [PubMed] [Google Scholar]
- 15.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, C.M., S.C. Cose, J.M. McNally, S.R. Jennings, W.R. Heath, and F.R. Carbone. 2000. Diminished secondary CTL response in draining lymph nodes on cutaneous challenge with herpes simplex virus. J. Gen. Virol. 81:407–414. [DOI] [PubMed] [Google Scholar]
- 17.Geiger, K.D., T.C. Nash, S. Sawyer, T. Krahl, G. Patstone, J.C. Reed, S. Krajewski, D. Dalton, M.J. Buchmeier, and N. Sarvetnick. 1997. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 238:189–197. [DOI] [PubMed] [Google Scholar]
- 18.Smith, P.M., R.M. Wolcott, R. Chervenak, and S.R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology. 202:76–88. [DOI] [PubMed] [Google Scholar]
- 19.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor, P.A., R.J. Noelle, and B.R. Blazar. 2001. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aseffa, A., A. Gumy, P. Launois, H.R. MacDonald, J.A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232–3241. [DOI] [PubMed] [Google Scholar]
- 22.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 23.Bensinger, S.J., A. Bandeira, M.S. Jordan, A.J. Caton, and T.M. Laufer. 2001. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J. Exp. Med. 194:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 25.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 28.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J. Autoimmun. 16:115–123. [DOI] [PubMed] [Google Scholar]
- 29.Papiernik, M. 2001. Natural CD4+ CD25+ regulatory T cells. Their role in the control of superantigen responses. Immunol. Rev. 182:180–189. [DOI] [PubMed] [Google Scholar]
- 30.Halford, W.P., and P.A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants To establish wild-type levels of latency in vivo. J. Virol. 74:5957–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leist, T.P., E. Ruedi, and R.M. Zinkernagel. 1988. Virus-triggered immune suppression in mice caused by virus-specific cytotoxic T cells. J. Exp. Med. 167:1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarozinski, C.C., J.M. McNally, B.L. Lohman, K.A. Daniels, and R.M. Welsh. 2000. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J. Virol. 74:3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider-Schaulies, S., S. Niewiesk, J. Schneider-Schaulies, and V. ter Meulen. 2001. Measles virus induced immunosuppression: targets and effector mechanisms. Curr. Mol. Med. 1:163–181. [DOI] [PubMed] [Google Scholar]
- 34.Hu, H., G. Huston, D. Duso, N. Lepak, E. Roman, and S.L. Swain. 2001. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat. Immunol. 2:705–710. [DOI] [PubMed] [Google Scholar]
- 35.Opferman, J.T., B.T. Ober, and P.G. Ashton-Rickardt. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 283:1745–1748. [DOI] [PubMed] [Google Scholar]
- 36.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S.H. Kaufmann, and H.W. Mittrucker. 2002. Regulatory CD4(+)CD25(+) T cells restrict memory CD8(+) T cell responses. J. Exp. Med. 196:1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]