Abstract
The control of many persistent viral infections by Ag-specific cytolytic CD8+ T cells requires a concurrent virus-specific CD4+ Th cell response. This reflects in part a requirement of activated effector CD8+ T cells for paracrine IL-2 production as a growth and survival factor. In human CMV and HIV infection, the majority of differentiated virus-specific CD8+ T cells notably lose the ability to produce IL-2 but also lose expression of CD28, a costimulatory molecule. Analysis of the fraction of memory CD8+ T cells that continue to express CD28 revealed these cells retain the ability to produce IL-2. Therefore, we examined if IL-2 production by CD28− CD8+ T cells could be restored by introduction of a constitutively expressed CD28 gene. Expression of CD28 in CD28− CD8+ CMV- and HIV-specific CD8+ T cells reconstituted the ability to produce IL-2, which could sustain an autocrine proliferative response after Ag recognition. These results suggest that the loss of CD28 expression during differentiation of memory/effector CD8+ T cells represents a decisive step in establishing regulation of responding CD8+ T cells, increasing the dependence on CD4+ Th for proliferation after target recognition, and has implications for the treatment of viral disease with adoptively transferred CD8+ T cells.
Keywords: CD8+ T cells, CD28, interleukin-2, costimulation, memory response
Introduction
Acute virus infection can stimulate a vigorous CD8+ T cell response, with up to 44% of the resulting CD8+ T cell compartment committed to a single viral epitope (1). After such massive cellular expansion, the population of responding CD8+ T cells must contract to restore lymphocyte homeostasis. This cell death is a consequence of Ag clearance, cytokine withdrawal, and Fas-FasL–mediated apoptosis, leaving a smaller pool of memory CD8+ T cells (2). Some pathogens, such as CMV and EBV, are never eliminated, and maintenance of a high frequency of virus-specific CD8+ T cells is essential to prevent viral reactivation from becoming symptomatic (1, 3).
CD8+ T cells can initially contain many viruses without CD4+ Th cells, but long term control of persistent infection requires CD4+ Th cells (4). CD4+ Th cells are important for conditioning APC through interaction of molecules, such as CD40L with CD40 (5, 6), and may directly help activated CD8+ T cells by providing IL-2 as a growth and survival factor (7). The important role of CD4+ Th cells has been demonstrated in HIV infection (8). Most individuals mount an HIV-specific CD8+ T cell response that contributes significantly to the initial control of HIV viremia (8). However, CD4+ HIV-specific Th cells are selectively eliminated by direct infection (9), and HIV-specific CD8+ T cells are ultimately incapable of containing the virus (8). By contrast, the small subset of infected individuals that do retain a strong HIV-specific CD4+ Th cell response maintain a stronger functional CD8+ T cell response and improved control of the virus (10).
CD28 is an important costimulatory molecule for the generation of CD4+ and CD8+ T cell responses. CD28 interacts with CD80/86 expressed by APC and, in conjunction with a TCR signal, results in tighter formation of the immunological synapse, increased expression of the antiapoptotic protein BcL-XL, enhanced transcription and stability of IL-2 mRNA, and increased IL-2 production (11–14). In humans, CD28 is expressed on all naive T cells and on memory CD4+ Th cells. In contrast, CD28 expression is lost in a subset of human CD8+ T cells, and this subset exhibits reduced proliferation after anti-CD3 stimulation (15). CD28− CD8+ T cells are increased in the elderly (16) and in the CD8+ memory T cell pool specific for persistent viruses, such as CMV and EBV (1, 3). The loss of CD28 is most pronounced in HIV-specific CD8+ T cells, of which >90% can be CD28−, potentially due to continuous Ag stimulation (17).
We have examined the functional consequences of the loss of CD28 on virus-specific CD8+ T cells derived from healthy individuals with chronic infection. CD28+ and CD28− CD8+ T cells were evaluated to determine if the observed differences reflected a multitude of changes for which loss of CD28 expression served as a marker, or if these functions could be restored by reexpression of CD28. The results demonstrated that loss of CD28 expression correlates with the inability to produce IL-2 and proliferate after activation by APC. These functions could be reconstituted by reexpressing an intact signaling CD28 molecule by introduction of the gene under a constitutive promoter.
Materials and Methods
Cell Lines.
B lymphoblastoid cells (LCL) and TAP-deficient, HLA A*0201 T2 cells were maintained in media with 10% FCS. The retroviral packaging cell line Phoenix-GALV was established by transfecting Phoenix GP cells (provided by Dr. G. Nolan, Stanford University, Stanford, CA) with the plasmid pMOV-GaLV Seato env (provided by Dr. A.D. Miller, Fred Hutchinson Cancer Research Center).
Analysis of Cell Surface Receptor Expression.
PBMCs were isolated from 38 healthy HLA A*0201+ donors and stained with an HLA A*0201 PE-labeled tetramer containing the CMVpp65/495–503 epitope NLVPMVATV, αCD8-FITC (Becton Dickinson), and αCD28-Cy (PharMingen). Expression of CD28 on transduced T cell clones was evaluated by staining 5 × 105 T cells with αCD28-PE (Becton Dickinson). All samples were examined on a FACScan™ (Becton Dickinson).
Intracellular Cytokine Staining.
CD8+ T cells were enriched from PBMCs of donors displaying high numbers of tetramer-binding (tet+) T cells with CD8+ Rosette Sep (StemCell Technologies). 2 × 106 enriched CD8+ T cells were incubated for 7 h with 5 × 105 T2 cells alone or pulsed with 10 μM CMVpp65/495–503 peptide in the presence of 10 μg/ml αCD49d mAb (Becton Dickinson). 10 μg/ml brefeldin A (Sigma-Aldrich) was then added for 5 h of incubation.
CD28− CD8+ T cells were isolated from PBMCs, transduced with a retroviral vector encoding CD28, and selected for CD28 expression. 5 × 103 parental and CD28-transduced CD8+ T cells were plated in a 96-well plate previously coated for 12 h at 4°C with αCD3 mAb (10 ng/ml) or with both αCD3 mAb (10 ng/ml) and αCD28 mAb (10 μg/ml). After 6 h, brefeldin A was added, and the cells were incubated for 5 h. Parental and CD28-transduced CD28− CD8+ T cells were also stimulated with 10 ng/ml PMA (Sigma-Aldrich) and 1 μg/ml ionomycin (Sigma) for 5 h followed by the addition of brefeldin A for 2 h. Samples were permeabilized and stained with αIL-2–APC (PharMingen), αCD8-PerCP (Becton Dickinson), and αCD28-PE and analyzed by flow cytometry.
Generation of Human Ag-specific CD8+ T Cells.
The CD8+ CMV-specific T cell clones MT-2 and MT-3 were generated by sorting tet+ CD8+ T cells specific for the pp65495–503 epitope and cloning at 0.3 cells/well. HIV-specific CD8+ T cell clones SM-28A2 and TL-52-H8 were generated from HIV seropositive donors and recognized the HIVgag/20–28 epitope RLRPGGKKK presented by HLA A*0301(18).
Proliferation Assays.
Responder CD8+ T cells were isolated from PBMCs by positive selection (Miltenyi) and FACS®-sorted to achieve >99% purity. An aliquot was also depleted of CD28+ CD8+ T cells by sorting for CD28− CD8+ T cells. Mitomycin C (Sigma-Aldrich)–treated stimulator T2 cells were pulsed with 100 ng/ml of CMVpp65/495–503, washed, and plated at 5 × 104 cells/well in 96-well plates. 2 × 105 responder T cells were added to triplicate wells. 10 μg/ml CTLA-4–Ig fusion protein (provided by Dr. George Georges, Fred Hutchinson Cancer Research Center) or 5 μg/ml anti–IL-2 receptor β mAb (R&D Systems) was added to selected wells at culture initiation. Parental and CD28-transduced HIV-specific CTL clones were stimulated with mitomycin C–treated LCL pulsed with 20 ng/ml HIVgag/20–28 peptide. IL-2 (10 U/ml) was added to selected wells. Cultures were pulsed with 1 μCi 3[H]TdR for the final 18 h of a 90-h assay.
For the assessment of Ag-driven T cell expansion, parental and CD28-transduced CMV-specific T cell clones were plated at 2 × 106 cells/well in a 24-well plate with 2 × 106 mitomycin C–treated T2 cells pulsed with 100 ng/ml of CMVpp65/495–503 peptide. After 5 d of culture, T cells were counted by trypan blue dye exclusion. In some experiments, parental and CD28-transduced T cells were labeled with CFSE before stimulation. CTLs were washed with PBS and resuspended at 107 cells/ml in PBS. CFSE was added to a final concentration of 20 μM, and cells were mixed and incubated at 23°C for 10 min. Labeling was quenched by the addition of 10% FCS, and the cells were washed and resuspended in media containing 10% FCS. 106 CFSE-labeled CTLs were mixed with 106 mitomycin C–treated T2 cells pulsed with pp65495–503 peptide (100 nM) and incubated at 37°C for 3 d. CFSE dilution was analyzed on a FACSCalibur flow cytometer.
Construction of CD28 and CD28 Mutant Retroviral Vectors.
RNA was isolated from CD4+ T cells using Qiagen RNAeasy (QIAGEN) and reverse transcribed into cDNA by oligo-dt15 primers (Roche) and M-MLV reverse transcriptase (GIBCO BRL). The full-length CD28 cDNA was amplified by PCR with unique 5′ and 3′ primers. A point mutation of the tyrosine at position 200 to phenylalanine was introduced into the CD28 cDNA by PCR with a codon-modified 3′ primer. PCR products were ligated into the Zero Blunt Topo cloning vector (Invitrogen) and sequenced. The confirmed CD28 and CD28 mutant cDNAs were cloned into pLZRSBMN (provided by Dr. G. Nolan, Stanford University, Stanford, CA) downstream of the viral 5′ LTR, transfected into Phoenix GALV cells, and supernatant was harvested from puromycin-resistant cells.
Transduction of T Cells.
CD28− CD8+ T cells were purified from PBMCs by immunomagnetic depletion of CD4+ CD19+, CD14+, CD56+, and CD28+ T cells. The purity of the remaining CD28− CD8+ T cells was >99% as assessed by staining with αCD8-FITC (Becton Dickinson) and αCD28-Cy (PharMingen). Polyclonal CD28 CD8+, T cells and CMV- or HIV-specific CD8+ clones were stimulated with αCD3 mAb (30 ng/ml) as described (18), harvested 1 d later, and resuspended in phosphate-deficient media (GIBCO BRL) with 10% human serum and 50 U/ml IL-2. On day 2, cells were washed, resuspended in retroviral supernatant with 50 U/ml IL-2 and 8 μg/ml polybrene, spun at 1,000 g for 1 h at 32°C, and incubated for 23 h. T cells were then washed, cultured in media containing 50 U IL-2/ml, and restimulated every 14 d.
Cytotoxicity Assays.
Parental and CD28-transduced HIV-specific CTLs were assayed in a chromium release assay with HLA A*0301+ LCL target cells infected with a vaccinia recombinant virus expressing either HIVgag or, as a control, expressing CMVpp65.
IL-2 Production.
IL-2 production was assessed after stimulation with HLA-matched LCL pulsed for 4 h with either 20 ng CMVpp65/495–503 or HIVgag/20–28 peptide. Responder cells (2 × 105 cells/well) and stimulator cells (105/well) were added to 96-well plates. CTLA-4–Ig (10 μg/ml) was added to selected wells, supernatants were harvested after 36 h, and IL-2 production was analyzed by ELISA (R&D Systems).
Results
CD28 Expression of CMV pp65-specific CD8+ T cells in Healthy Donors.
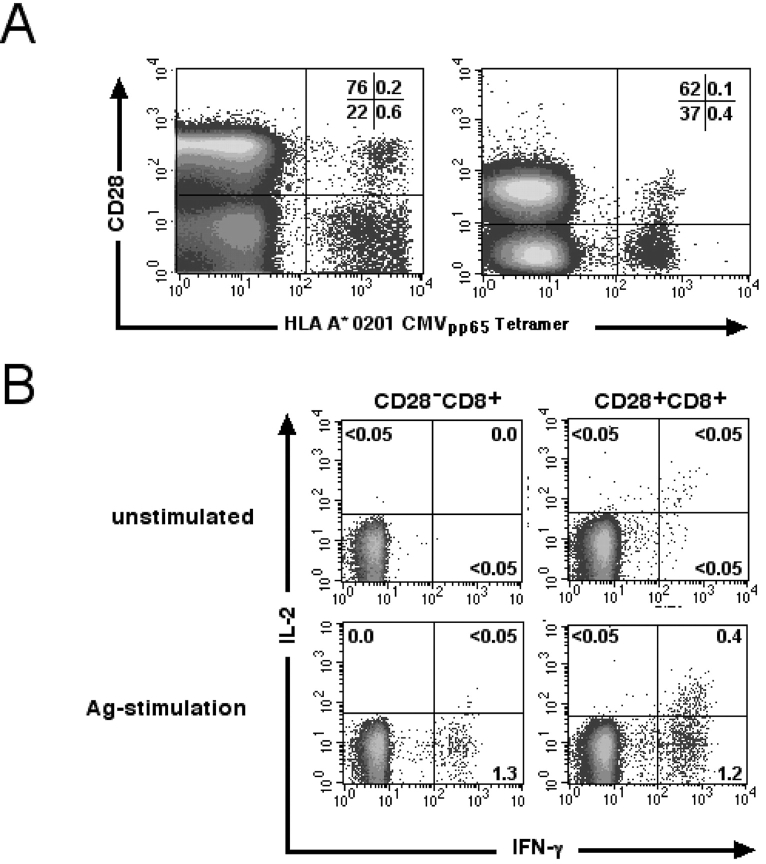
An HLA-A*0201 tetramer was used to determine the frequency and phenotype of CD8+ T cells reacting to the CMVpp65 /495–503 epitope in healthy donors. A mean of 1.27% (range 0.2–6.0%) of all CD8bright T cells were tet+ in 19 subjects with detectable tet+ cells. CD28 expression by the tet+ CD8+ T cells was assessed in seven of the donors, and in five of the seven the majority of tet+ CD8bright T cells were CD28− (Fig. 1 A). The average percentage of CD28− T cells in the CD8+ tet+ population for the seven donors was 60.7% (range 11–91%).
Figure 1.
IL-2 production is confined to the subset of CMVpp65-specific CD28+ CD8+ T cells. (A) CD28 staining of tet+ CD8bright T cells from two representative donors. The percentage of T cells in each quadrant is indicated. (B) Freshly isolated CD8+ T cells were stimulated with either T2 cells or peptide-pulsed T2 cells. Cells were gated to identify CD28− CD8+ and CD28+ CD8+ T cells and assessed for cytokine production. Values indicate the percentage of cells in each quadrant producing IFN-γ or IL-2.
CD28+ and CD28− Ag-specific CD8+ T Cells Are Functionally Distinct.
Since CD28 engagement can modify effector functions, we evaluated if the loss of CD28 expression was associated with changes in CD8+ T cell function. CD8+ T cells were purified from peripheral blood, stimulated with CD80/86-expressing APC pulsed with the CMVpp65 peptide, and IFN-γ and IL-2 production by the CD28+ and CD28− subsets was assessed by intracellular staining. Unstimulated CD28− or CD28+ CD8+ T cells did not express either IFN-γ or IL-2 (<0.05%) (Fig. 1 B). After peptide stimulation, IFN-γ production was detected in both the CD28− and CD28+ CD8+ T cell populations, but IL-2 production was only observed in CD28+ CD8+ T cells and only in those that also produced IFN-γ (Fig. 1 B).
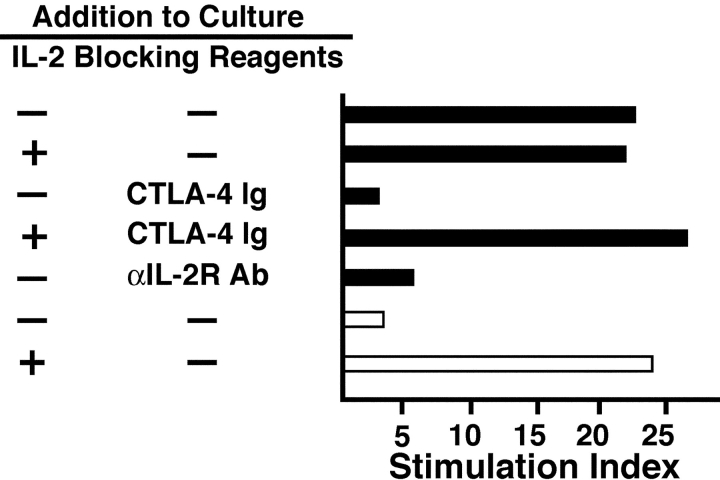
IL-2 produced by CD28+ CD8+ T cells after target recognition might serve as an autocrine growth factor and provide this subset with the ability to sustain a proliferative response. Incubation of purified CD8+ T cells with APC pulsed with 100 ng of CMVpp65/495–503 peptide elicited a strong proliferative response with a stimulation index of 22.3 (Fig. 2) . Since it was not possible to isolate sufficient CD28+ tet+ cells for direct analysis, the contribution of the CD28+ population to the proliferative response was assessed by blocking and depletion studies. The observed proliferation required specific interaction between CD28 and CD80/86, since addition of CTLA-4–Ig, which has a 10-fold higher affinity for CD80/86 than does CD28 (14), abrogated the response (Fig. 2). Addition of a mAb to IL-2 receptor β suppressed the response, indicating that IL-2 was responsible for the observed proliferation (Fig. 2). Depletion of CD28+ T cells from the purified CD8+ T cells also abolished proliferation, but the response from the remaining CD28− cells was rescued by the addition of IL-2 (10 U/ml) at culture initiation (Fig. 2). These findings suggest IL-2 production is essential for autocrine proliferation and that alternative costimulatory receptors to CD28, such as 4–1BB and LFA-1, which may be expressed on CD28− CD8+ T cells, are not sufficient for proliferation or IL-2 production under these conditions.
Figure 2.
Proliferation of CMV-specific CD8+ T cells is dependent on the CD28+ CD8+ subset. Purified CD8+ T cells (black bars) or CD28-depleted CD8+ T cells (white bars) were stimulated with peptide-pulsed T2 cells and proliferation assessed. Indicated wells were supplemented with IL-2, CTLA-4–Ig, or αIL–2R-β mAb at culture initiation. All values represent the means stimulation index of triplicate samples. The results are representative of assays from three donors.
CD28− T Cells Transduced To Express CD28 Produce IL-2.
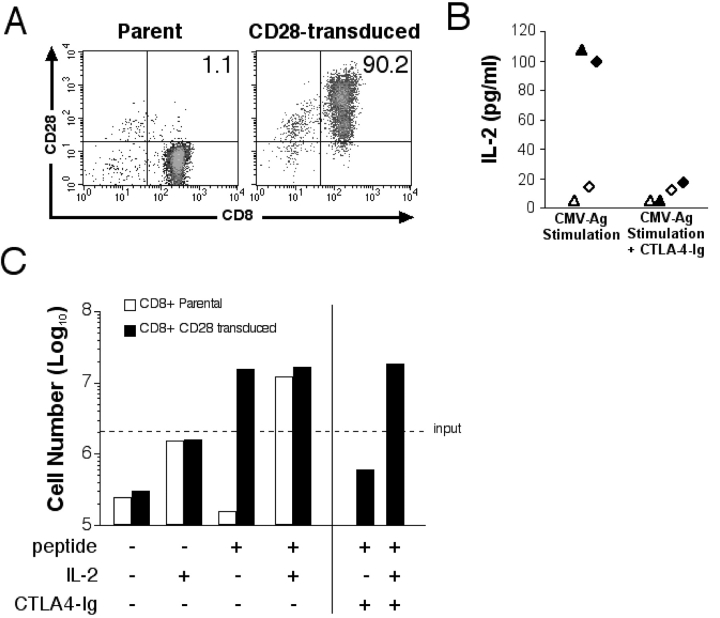
The ability of the CD28+ subset to produce IL-2 and the inability of the CD28− CD8+ subset to do so suggested that failure to express and deliver signals through this costimulatory molecule might be responsible for the loss of function. However, CD28− CD8+ T cells could also have undergone other changes that could interfere with the ability to produce IL-2. Therefore, we expressed CD28 in CMV-specific CD8+ CTL clones and in primary CD28− T cells using a retrovirus encoding CD28 and assessed function. On day 12 after transduction, >90% of the transduced T cells expressed both CD28 and CD8, whereas the parental cells remained CD28− CD8+ (Fig. 3 A). CD28 expression was maintained for >8 wk in CD28-transduced T cells restimulated every 14 d (unpublished data).
Figure 3.
Reconstitution of CD28 restores Ag-triggered IL-2 production and T cell proliferation. (A) CD28 expression was assessed before and after transduction of a CMV-specific CD28− CD8+ T cell clone, MT-2 with the CD28 gene. The percentage of cells positive for both CD8 and CD28 is indicated and is representative of five experiments. (B) IL-2 production by parental and CD28-transduced CMV-specific CD8+ T cells after Ag stimulation. HLA-matched peptide-pulsed LCLs (CD80/86+) were incubated with parental (⋄ and ▵) or CD28-transduced (♦ and ▴) CMV-specific CD8+ T cell clones MT-2 (▴ and ▵) and MT-3 (♦ and ⋄). CTLA-4–Ig was added to indicated wells. Data points represent the means of two samples for each clone. (C) Proliferation of CD28− parental and CD28-transduced CD8+ CMV-specific T cells. 2 × 106 parental (white bar) and CD28-transduced (black bar) T cells were plated with 2 × 106 mitomycin C–treated T2 cells alone or pulsed with 100 nM of pp65495–503 peptide. IL-2 (10 U/ml) and CTLA-4–Ig were added to selected wells. Cell growth was assessed at day 5 by counting viable T cells.
Parental and CD28-transduced CTL clones were then stimulated with HLA A*0201+ LCL pulsed with 20 ng/ml of the CMVpp65/495–503 peptide. No IL-2 production was detected from unstimulated parental or transduced CD8+ CTLs (data not shown). Parental CD28− CD8+ CTLs stimulated with peptide-pulsed APC produced no detectable or very low levels of IL-2 (Fig. 3 B). In contrast, the CD28-transduced CTLs produced substantial IL-2 after stimulation (Fig. 3 B). Addition of CTLA-4–Ig at the initiation of cultures to block CD28 engagement by CD80/86 abrogated IL-2 production by the CD28-transduced CD8+ T cells (Fig. 3 B), confirming that the IL-2 production directly resulted from expression of CD28.
We next evaluated whether IL-2 production by CD28-transduced CD8+ CMV-specific T cell clones was sufficient for Ag-driven autocrine proliferation. Stimulation of parental CD8+ CD28− CMV-specific T cells with peptide-pulsed APC in the absence of exogenous IL-2 led to a decrease in cell number over 5 d in culture, although addition of IL-2 induced a sevenfold expansion (Fig. 3 C). By contrast, stimulation of CD28-transduced T cells resulted in a ninefold increase in cell number without addition of IL-2 (Fig. 3 C). The proliferation of the CD28-transduced T cells was blocked by CTLA-4–Ig, confirming the requirement for CD28 signaling for cell expansion (Fig. 3 C). Additional experiments in which both the parental and CD28-transduced T cells were labeled with CFSE before peptide stimulation demonstrated that the CD28-transduced T cells, but not the CD28− parental cells, proliferated and remained viable 4 d after stimulation (not depicted).
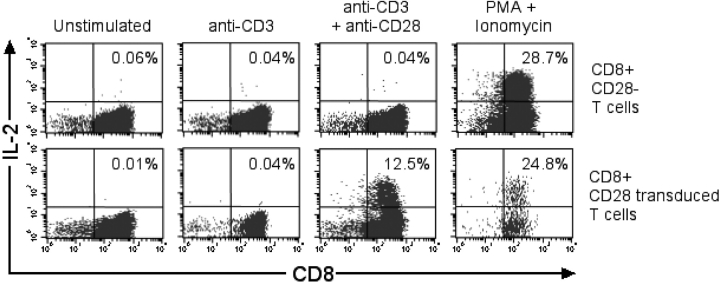
The restoration of IL-2 production in the T cell clones by the expression of CD28 might be an aberration from long term culture and not apply to CD28− T cells in the blood. Therefore, CD28 was expressed by retroviral transduction on CD28− CD8+ T cells isolated from PBMC. Aliquots of parental and CD28-transduced T cells were then stimulated with αCD3mAb alone, with both αCD3 and αCD28 mAbs or with PMA and ionomycin, and evaluated for IL-2 production by intracellular staining. No IL-2 was detected in the parental or CD28-transduced CD8+ T cells after stimulation with αCD3mAb. However, a fraction of the CD28-transduced but not the parental CD8+ T cells produced IL-2 after stimulation with both αCD3 and αCD28 mAbs (Fig. 4) . PMA and ionomycin stimulation, which bypasses the requirement for TCR and costimulatory signals, was capable of inducing IL-2 production in both the parental CD28− CD8+ and CD28-transduced T cells (Fig. 4). These results demonstrate that expression of CD28 in CD28− peripheral blood T cells was sufficient to restore IL-2 production.
Figure 4.
Reconstitution of CD28 expression in CD28− CD8+ T cells provides costimulation for IL-2 production. CD28− CD8+ T cells were isolated from PBMCs, and an aliquot was transduced with the CD28 retrovirus. After selection, >80% of the transduced T cells expressed CD28. CD28-transduced (bottom panels) and nontransduced (top panels) CD28− CD8+ T cells were stimulated with αCD3 mAb (10 ng/ml), with both αCD3 mAb (10 ng/ml) and αCD28 mAb (10 μg/ml), or with PMA and ionomycin, and then stained for intracellular IL-2.
Expression of CD28 in CD28− HIV-specific CD8+ CTLs Results in Ag-triggered Autocrine Proliferation.
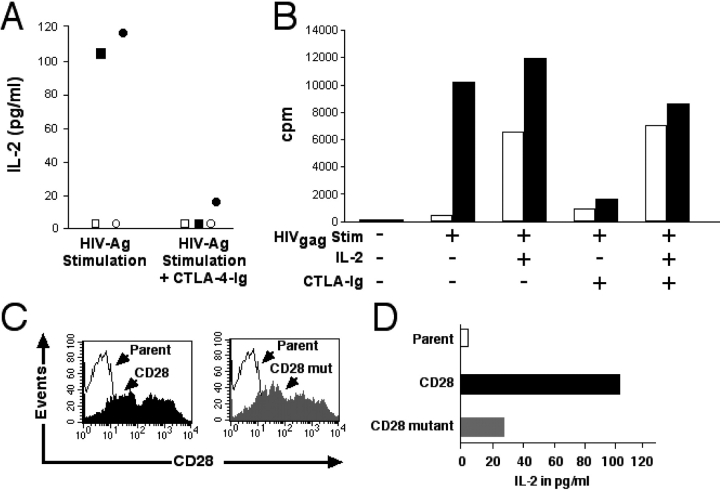
One hallmark of HIV infection is the loss of virus-specific CD4+ Th cells, which normally can provide IL-2 as a paracrine growth factor for responding CD8+ T cells. The majority (70–90%) of HIV-specific CD8+ T cells present during chronic infection have lost CD28 expression (17), and this skewing might contribute to a quantitatively inadequate response and ineffective control of viremia. Therefore, we evaluated if the loss of IL-2 production in HIV-specific CD28− CD8+ T cells represented a multitude of events acquired during infection or if restoring CD28 expression could reestablish the ability of these effector cells to produce sufficient IL-2 to overcome the dependence on CD4+ Th cells. CD28 was expressed in HIV-specific CD28− CD8+ CTL clones by retroviral transduction, and the ability to produce IL-2 was assessed. The parental clones did not produce IL-2 in response to Ag stimulation, whereas the CD28-transduced T cell clones acquired this capacity (Fig. 5 A). The IL-2 production was again abrogated by addition of CTLA-4–Ig (Fig. 5 A).
Figure 5.
IL-2 production and proliferation of CD28-transduced HIV-specific CD8+ T cell clone after Ag stimulation requires an intact CD28 costimulatory signal. (A) IL-2 production by parental and CD28-transduced HIV-specific CD8+ T cells. HLA A*0301-positive LCLs pulsed with HIVgag/20–28 were incubated with the parental (○ and □) or CD28-transduced (• and ▪) HIV-specific CD8+ T cell clones TL-52-H8 (○ and •) and SM-28A2 (□ and ▪). CTLA-4–Ig was added to indicated wells. The data shown is the mean of two samples for each clone. (B) Parental TL-52-H8 (white bar) and the CD28-transduced clone (black bar) were stimulated with HLA A*0301+ LCLs alone or pulsed with the HIVgag/20–28 peptide. Selected wells were supplemented with CTLA-4–Ig and/or IL-2. Proliferation was assessed by incorporation of [3H]-TdR. The data represents the mean [3H]-TdR incorporation from triplicate wells and is representative of three experiments. (C) The HIV-specific CD28− CD8+ T cell clone SM-28A2 was transduced with either the intact CD28 or a mutant CD28 gene, and CD28 surface expression was assessed. (D) IL-2 production was quantified after Ag stimulation of the parental, and CD28− and CD28 mutant-transduced SM-28A2. HLA A*0301+ APCs were pulsed with the HIVgag/20–28 peptide and incubated with either the parental (white bar), the CD28-transduced (black bar), or the CD28 mutant-transduced (gray bar) HIV-specific CD8+ T cell clone. Supernatants were harvested at 36 h, and IL-2 was measured by ELISA. The data represents the means of two experiments.
We next evaluated if IL-2 production by CD28-transduced HIV-specific CD8+ T cells was sufficient for autocrine proliferation after Ag stimulation. Stimulation with peptide-pulsed APCs induced a proliferative response in the CD28-transduced, but not the parental HIV-specific CTL clones, and addition of CTLA-4–Ig abrogated proliferation (Fig. 5 B). Supplementing IL-2 restored the proliferative response in the presence of CTLA-4–Ig, confirming adequate TCR triggering by the APCs and excluding nonspecific toxicity as the reason for CTLA-4–Ig blocking.
Introduction of a constitutive CD28 gene into CD28− CD8+ HIV-specific CTLs could potentially be explored as an adoptive therapy strategy to improve control of viremia in HIV-infected individuals. Therefore, we determined if expression of CD28 in previously CD28− HIV-specific CTLs interfered with the cytolytic function of transduced CTLs. Parental and transduced clones demonstrated equivalent lysis of targets expressing HIVgag and no nonspecific lysis of CMVpp65-expressing targets (unpublished data).
Enhanced IL-2 Production Is Dependent On Intracellular Signals Delivered by CD28.
CD28 can serve as an adhesion molecule stabilizing the immunological synapse (11), which might lead to a stronger TCR stimulus and enhanced cytokine production independent of a requirement for CD28-mediated signaling. The intracellular portion of CD28 contains four tyrosines, which are linked to activation of the phosphatidylinositol 3-kinase and microtubule-associated protein kinase pathways. Individually, each of the distal tyrosines impact the costimulatory signal for enhanced IL-2 production (19). Therefore, a mutated CD28 receptor was generated with a distal tyrosine changed to phenylalanine, a mutation that reduces the costimulatory signal for IL-2 production by ∼80%. Retroviral transduction with either intact or mutant CD28 led to similar levels of CD28 expression (Fig. 5 C). The parental and the transduced CTLs were stimulated with APCs pulsed with the HLA A*0301 HIVgag/20–28 peptide, and supernatant was harvested 36 h later. No IL-2 production could be detected from the parental clone, whereas IL-2 was readily detected from the CD28-transduced clone. IL-2 production was reduced by ∼80% in CD8+ T cells transduced with the CD28 mutant (Fig. 5 D) compared with the intact CD28 molecule. Thus, the capacity of CD28 to deliver a costimulatory signal was essential for efficiently reconstituting IL-2 production in Ag-specific CD8+ T cells.
Discussion
This paper describes a major functional consequence of the loss of CD28 expression after maturation of CD8+ T cells from naive cells to Ag-experienced memory/effector T cells. Only the CD28+ subset of CMVpp65-specific CD8+ T cells, which represent a minority of the memory T cells in most CMV seropositive individuals, was capable of producing IL-2 after Ag stimulation. Reconstitution of CD28 surface expression in primary CD28− CD8+ T cells and T cell clones by introduction of the gene under a constitutive promoter restored the capacity for IL-2 production after stimulation through the TCR and CD28.
Infection with herpes viruses results in virus persistence, requiring maintenance of a strong specific CD8+ T cell response to contain the virus and prevent progressive infection (20). The memory/effector CD8+ T cell population that provides protection is comprised of phenotypically diverse subsets of cells. We chose to study the consequences of CD28 expression and signaling in CMV-specific CD8+ T cells, since there is a major response to an epitope of CMVpp65 in HLA A*0201 individuals which permits direct isolation of Ag-specific CD8+ T cells with tetramers (3). In our study, CD8+ T cells from A*0201+ individuals specific for this CMVpp65/495–503 epitope represented on average 1.27% of the CD8bright T cell population, and in this CMV-reactive population the majority of CD8+ T cells were CD28− (on average 61%), consistent with previous reports (3).
CD28 expression is not invariant by CD8+ T cells. Naive CD8+ T cells express CD28 and can produce IL-2 after physiologic interaction with an APC. As we have shown, this ability to produce IL-2 remains intact for the subset of memory CD8+ T cells that retain CD28 expression. However, the CD28− CD8+ subset can only produce significant amounts of IL-2 if an extremely strong signal is delivered through cross-linking of the TCR with anti-CD3 antibodies or treatment with PMA and ionomycin (21). This response to such nonphysiologic stimuli implies that the inability of CD28− CD8+ T cells to produce IL-2 in response to Ag is not due to chromosomal inaccessibility of the IL-2 gene and that production of limited amounts of IL-2 might also occur in vivo under special stimulation conditions. There are a large number of costimulatory molecules, including CD2, CD27, 41BB, and LFA-1, that can deliver signals which enhance the strength of the TCR signal, but the most potent costimulatory signal for IL-2 production is delivered via CD28. Therefore, we questioned if restoring CD28 expression would be sufficient to reconstitute the ability of CD28− CD8+ T cells to produce IL-2 in response to Ag. Remarkably, the loss of expression of CD28 appeared to be the only critical blockade to IL-2 production by CD28− CD8+ T cells. Reconstitution of IL-2 production required CD28 to function as a signaling molecule and not just an adhesion molecule, since expression of CD28 with a point mutation of a tyrosine residue in the signaling domain of the cytoplasmic tail failed to restore the ability to produce IL-2.
Why should human Ag-specific CD8+ T cells specifically down-regulate the expression of CD28 and therefore lose the capacity to produce IL-2? One possibility is that this contributes to regulating the size of the Ag-specific CD8+ T cell response. The magnitude of the CD8+ response after acute infections can be enormous, representing ∼50% of all CD8+ T cells (1), and it is essential that these expanded populations be regulated and ultimately contract after Ag clearance to maintain lymphoid homeostasis. Phenotypic analysis of EBV-specific CD8+ T cells during the peak of a primary EBV response has shown that up to 90% have lost CD28 expression (1, 17). Since IL-2 is a potent growth and survival factor during the acute response (7), the loss of endogenous IL-2 production by CD28− CD8+ Ag-specific T cells should make these cells incapable of autonomously sustaining the response after target recognition. Thus, continued expansion of CD8+ T cells that have become CD28− during the acute response may be regulated by the requirement for sufficient Ag and inflammation to attract APCs and responding CD4+ Th cells that can provide paracrine IL-2. The loss of CD28 expression also results in decreased production of intracellular antiapoptotic proteins, potentially increasing susceptibility to death from cytokine withdrawal and thereby promoting contraction of the response.
Teleologically, the differentiation of CD8+ T cells to a CD28− CD8+ population of memory T cells dependent on other cells to provide IL-2 may also be important for limiting autonomous outgrowth of autoreactive CD8+ T cells. Thus, even if naive autoreactive CD8+ T cells do get triggered, the persistence of the autoantigen may drive maturation to a CD28− CD8+ phenotype, and the response of such CD28− CD8+ T cells would not be maintained unless there were a concurrent loss of tolerance in the CD4+ Th cell compartment.
It has been proposed that memory CD8+ T cells can be divided into central and peripheral compartments based on the expression of CCR7, which can attract T cells to lymphoid tissue (22). In individuals with chronic CMV, EBV, or HIV infection, CCR7 expression is found on ∼10% of all CD8+ virus-specific T cells in the peripheral blood (17). The vast majority these CCR7+ CD8+ T cells also express CD28 (23). Thus, this population, which retains the ability to produce IL-2 and expand in response to Ag in the absence of a CD4+ Th cell response, might efficiently initiate responses in the LN upon reexposure to Ag and replenish effector CD8+ T cells to contain the pathogen. If the pathogen persists, a CD4+ Th cell response might be essential to provide the necessary factors for more extensive expansion of the CD8+ T cell response.
The observation that restoring CD28 expression corrects the defect in IL-2 production in CD28− CD8+ T cells has implications for the treatment of HIV with adoptively transferred virus-specific CD8+ CTL. In HIV-infected individuals, the majority of Ag-specific CD8+ T cells are CD28− (17) and therefore dependent on IL-2 from exogenous sources. Unfortunately, HIV-specific CD4+ Th cells are mostly eliminated early during the course of the disease (24), which limits the host's ability to generate and maintain an effective CD8+ T cell response to the virus. Adoptively transferred autologous HIV-specific CD8+ CTLs have been shown to have transient antiviral activity and home to sites of infection in vivo but exhibited limited in vivo persistence (18). This likely reflects in part the absence of IL-2 production, since in other settings the persistence of transferred Ag-specific CD8+ cells can be prolonged by either the presence of an endogenous Ag-specific CD4+ Th cell response (25), the coadministration of Ag-specific CD4+ Th cells (26), or the administration of IL-2 (27). As HIV-infected CD4+ Th cells and macrophages express CD80/86 (28, 29), reconstituting CD28 in HIV-specific CD28− CD8+ T cells before cell administration might promote in vivo proliferation and persistence of the transferred CD8+ CTLs after target recognition, which should improve antiviral activity. Additionally, transfer of CD28+ CD8+ CTLs may facilitate structured treatment interruptions in patients on antiretroviral therapy (30), since this might establish an effector response that is not dependent on induction of a CD4+ response to HIV to promote effective CD8+ control of the resulting viremia (10, 24).
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (to M.S. Topp), a Postdoctoral Fellowship from the Cancer Research Institute (to M.S. Topp), and by National Institutes of Health grants AI 41754, AI43650, CA18029, HL66947, and CA33084.
M.S. Topp and S.R. Riddell contributed equally to this work.
References
- 1.Callan, M.F., L. Tan, N. Annels, G.S. Ogg, J.D. Wilson, C.A. O'Callaghan, N. Steven, A.J. McMichael, and A.B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B.C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of alpha beta TCR+ T cells. Nat. Immunol. 1:107–111. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie, G.M., M.R. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J.I. Bell, and P.A. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74:8140–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matloubian, M., R.J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen, E.M., E.E. Lemmens, T. Wolfe, U. Christen, M.G. von Herrath, and S.P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger, S.P., R.E. Toes, E.I. van der Voort, R. Offringa, and C.J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. [DOI] [PubMed] [Google Scholar]
- 7.Vella, A.T., S. Dow, T.A. Potter, J. Kappler, and P. Marrack. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 95:3810–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMichael, A.J., and S.L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature. 410:980–987. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D.C., J.M. Brenchley, M.R. Betts, D.R. Ambrozak, B.J. Hill, Y. Okamoto, J.P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 417:95–98. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg, E.S., J.M. Billingsley, A.M. Caliendo, S.L. Boswell, P.E. Sax, S.A. Kalams, and B.D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 278:1447–1450. [DOI] [PubMed] [Google Scholar]
- 11.Life, P., J.P. Aubry, S. Estoppey, V. Schnuriger, and J.Y. Bonnefoy. 1995. CD28 functions as an adhesion molecule and is involved in the regulation of human IgE synthesis. Eur. J. Immunol. 25:333–339. [DOI] [PubMed] [Google Scholar]
- 12.Boise, L.H., A.J. Minn, P.J. Noel, C.H. June, M.A. Accavitti, T. Lindsten, and C.B. Thompson. 1995. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 3:87–98. [DOI] [PubMed] [Google Scholar]
- 13.Ragheb, J.A., M. Deen, and R.H. Schwartz. 1999. CD28-mediated regulation of mRNA stability requires sequences within the coding region of the IL-2 mRNA. J. Immunol. 163:120–129. [PubMed] [Google Scholar]
- 14.Lenschow, D.J., T.L. Walunas, and J.A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233–258. [DOI] [PubMed] [Google Scholar]
- 15.Azuma, M., J.H. Phillips, and L.L. Lanier. 1993. CD28− T lymphocytes. Antigenic and functional properties. J. Immunol. 150:1147–1159. [PubMed] [Google Scholar]
- 16.Posnett, D.N., J.W. Edinger, J.S. Manavalan, C. Irwin, and G. Marodon. 1999. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+CD28− cytotoxic effector clones. Int. Immunol. 11:229–241. [DOI] [PubMed] [Google Scholar]
- 17.Appay, V., P.R. Dunbar, M. Callan, P. Klenerman, G.M. Gillespie, L. Papagno, G.S. Ogg, A. King, F. Lechner, C.A. Spina, et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385. [DOI] [PubMed] [Google Scholar]
- 18.Brodie, S.J., D.A. Lewinsohn, B.K. Patterson, D. Jiyamapa, J. Krieger, L. Corey, P.D. Greenberg, and S.R. Riddell. 1999. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat. Med. 5:34–41. [DOI] [PubMed] [Google Scholar]
- 19.Teng, J.M., P.D. King, A. Sadra, X. Liu, A. Han, A. Selvakumar, A. August, and B. Dupont. 1996. Phosphorylation of each of the distal three tyrosines of the CD28 cytoplasmic tail is required for CD28-induced T cell IL-2 secretion. Tissue Antigens. 48:255–264. [DOI] [PubMed] [Google Scholar]
- 20.Li, C.R., P.D. Greenberg, M.J. Gilbert, J.M. Goodrich, and S.R. Riddell. 1994. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 83:1971–1979. [PubMed] [Google Scholar]
- 21.Hamann, D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 23.Hislop, A.D., N.H. Gudgeon, M.F. Callan, C. Fazou, H. Hasegawa, M. Salmon, and A.B. Rickinson. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167:2019–2029. [DOI] [PubMed] [Google Scholar]
- 24.Kalams, S.A., and B.D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter, E.A., P.D. Greenberg, M.J. Gilbert, R.J. Finch, K.S. Watanabe, E.D. Thomas, and S.R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038–1044. [DOI] [PubMed] [Google Scholar]
- 26.Heslop, H.E., C.Y. Ng, C. Li, C.A. Smith, S.K. Loftin, R.A. Krance, M.K. Brenner, and C.M. Rooney. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2:551–555. [DOI] [PubMed] [Google Scholar]
- 27.Yee, C., J.A. Thompson, P. Roche, D.R. Byrd, P.P. Lee, M. Piepkorn, K. Kenyon, M.M. Davis, S.R. Riddell, and P.D. Greenberg. 2000. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell–mediated vitiligo. J. Exp. Med. 192:1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolthers, K.C., S.A. Otto, S.M. Lens, D.N. Kolbach, R.A. van Lier, F. Miedema, and L. Meyaard. 1996. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4+ T cells. Eur. J. Immunol. 26:1700–1706. [DOI] [PubMed] [Google Scholar]
- 29.Low, P., C. Weber, E. Harrer, P. Rohwer, J.R. Kalden, and T. Harrer. 1997. CD80 expression on monocytes in HIV-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:264–268. [DOI] [PubMed] [Google Scholar]
- 30.Altfeld, M., and B.D. Walker. 2001. Less is more? STI in acute and chronic HIV-1 infection. Nat. Med. 7:881–884. [DOI] [PubMed] [Google Scholar]