Abstract
A possible involvement of the mitogen-activated protein (MAP) kinase cascade in the inhibition of macrophage interleukin-12 (IL-12) production by Legionella pneumophila infection was examined. The results of MAP kinase inhibition by p42/44 and p38 MAP kinase inhibitors and of p42/44 MAP kinase activity assays indicate that L. pneumophila infection of macrophages causes a selective inhibition of lipopolysaccharide-induced IL-12 production by activating the p42/44 MAP kinase cascade. In addition, it was also revealed that the p38 MAP kinase may be important for the production of IL-12 but not for the inhibition caused by L. pneumophila infection.
Interleukin-12 (IL-12), a key cytokine produced by macrophages in regulation of the development of cell-mediated immunity (16, 30), is essential in the host defense against intracellular pathogens, such as Legionella pneumophila (3, 7, 15, 25). Some intracellular pathogens have been shown to suppress macrophage IL-12 production. For instance, the interaction of Leishmania spp. (2, 4, 11), measles virus (17), Histoplasma capsulatum (22), and human immunodeficiency virus (5, 6) with monocytes/macrophages results in a marked decrease in IL-12 production. We have shown that L. pneumophila also suppresses in vitro mouse peritoneal macrophage IL-12 production induced by bacterial lipopolysaccharide (LPS) at steady-state levels of message (24). However, the molecular mechanism of the suppression is not yet clear.
The signal transduction of bacterial LPS in monocytes/macrophages involves binding to cell surface CD14 associated with a transmembrane receptor(s), such as Toll-like receptors (29), and has been shown to include activation of some signal transduction molecules, such as the mitogen-activated protein (MAP) kinases, p38, p42/44 (ERK), and p54 (stress-activated protein kinase/JNK) (14, 19, 21). Although the relationship between the activation of these signaling molecules and induced cytokine expression is still unclear, this association has been increasingly recognized (11, 12). In the present study, therefore, we examined a possible involvement of the MAP kinase cascade in the regulation of macrophage IL-12 production by L. pneumophila infection. Since alveolar macrophages are the preferential site for growth of L. pneumophila during infection, our currently established in vitro alveolar macrophage infection model with L. pneumophila (23) was utilized in this study.
The MH-S murine alveolar macrophage cell line, purchased from the American Type Culture Collection (Manassas, Va.), was utilized in this study. The cells were cultured in 24-well tissue culture plates at a concentration of 5 × 105 cells/ml in RPMI 1640 medium containing 10% heat inactivated fetal calf serum (HyClone Laboratories, Logan, Utah) without antibiotics. L. pneumophila M124 was cultured on buffered charcoal yeast extract medium (Becton Dickinson, Sparks, Md.) for 3 days at 37°C (13). The bacterial suspensions were prepared in pyrogen-free saline, and the concentration of bacteria was determined by spectrophotometry. The MH-S cell monolayers were infected with L. pneumophila (infectivity ratio, 10 bacteria per cell) for 30 min, washed to remove nonphagocytosed bacteria, and incubated in RPMI 1640 medium containing 10% fetal calf serum with or without 1 μg of Escherichia coli LPS (Sigma Chemical, St. Louis, Mo.) per ml. In some experiments, the cell monolayers were pretreated with either PD98059 (p42/44 MAP kinase inhibitor), SB203580 (p38 MAP kinase inhibitor), or SB202474 (negative control compound) (Calbiochem, San Diego, Calif.) 2 h prior to infection. The amounts of IL-1α, IL-6, IL-10, and IL-12 p40/p70 in culture supernatants at 24 h after infection were determined by enzyme-linked immunosorbent assay (ELISA) (Pharmingen International, San Diego, Calif.). The ELISA for IL-12 p40/p70 utilized in this study measured the IL-12 p40-IL-12 p70 heterodimer. RNA isolation from macrophages and reverse transcription-PCR with primers for β2-microglobulin, IL-12 p35, and IL-12 p40 were performed as described previously (24). The p42/44 MAP kinase activity assay was conducted with a nonradioactive p44/42 MAP kinase assay kit (Cell Signaling Technology, Beverly, Mass.) in accordance with the manufacture's manual. In brief, the cell lysates (1 mg/ml) were incubated with immobilized phospho-p44/42 MAP kinase monoclonal antibody for immunoprecipitation. The immunoprecipitated pellets with fusion protein and ATP were then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before transfer to a nitrocellulose membrane. The blotted membranes were reacted with primary and horseradish peroxidase-conjugated secondary antibodies, followed by detection with chemiluminescent reagent. All experiments were repeated at least three times. Statistical analysis was performed with the paired Student's t test.
We initially determined how in vitro L. pneumophila infection of cells could affect the IL-12 p40/p70 production in response to LPS, since it is known that p40 is inducible but p35 is constitutive (9). The MH-S macrophages were infected with L. pneumophila or stimulated with either LPS alone or LPS in combination with bacteria, and the production of IL-1α, IL-6, IL-10, and IL-12 p40/p70 in cell culture supernatants was determined. As shown in Table 1, L. pneumophila infection of cells induced the production of IL-1α, IL-6, and IL-10, but IL-12 p40/p70 was not significantly produced. Furthermore, infection of cells with L. pneumophila significantly down-regulated the LPS-induced production of IL-12 p40/p70. However, the infection did not alter the LPS-induced production of IL-1α, IL-6, and IL-10. To analyze whether L. pneumophila infection affects IL-12 production at the level of gene transcription, we also examined steady-state levels of IL-12 p35 and IL-12 p40 mRNA isolated from cells infected with L. pneumophila or stimulated with either LPS alone or LPS in combination with bacteria by reverse transcription-PCR. L. pneumophila infection resulted in suppression of mRNA accumulation for the IL-12 p40 gene in response to LPS stimulation at 24 h after infection but not IL-12 p35 (data not shown), similar to our previous study with mouse peritoneal macrophages (24). That is, the levels of IL-12 p35 mRNA were always high and minimally affected by the treatments. In contrast, IL-12 p40 mRNA was not detected in nontreated control cells but was obviously induced by LPS. In addition, the levels of IL-12 p40 mRNA in response to LPS stimulation were markedly suppressed by L. pneumophila infection.
TABLE 1.
Effect of L. pneumophila infection on levels of IL-1α, IL-6, IL-10, and IL-12 p40/p70 production induced by LPS
| Treatment | Levela of:
|
|||
|---|---|---|---|---|
| IL-1α (ng/ml) | IL-6 (ng/ml) | IL-10 (pg/ml) | IL-12 (ng/ml) | |
| Control | 0.13 ± 0.09 | 0.29 ± 0.12 | 24.9 ± 14.5 | 0.22 ± 0.12 |
| L. pneumophila | 1.82 ± 0.34 | 3.76 ± 0.62 | 242.7 ± 19.6 | 0.62 ± 0.26 |
| LPS | 2.45 ± 0.51 | 22.1 ± 2.54 | 572.6 ± 79.6 | 5.75 ± 0.84* |
| LPS + L. pneumophila | 2.51 ± 0.59 | 23.5 ± 2.18 | 563.9 ± 68.1 | 3.18 ± 0.55* |
The amount of cytokine in the culture supernatants obtained at 24 h after infection was measured by ELISA. Results are expressed as means ± standard deviations for three independent experiments. *, P < 0.05.
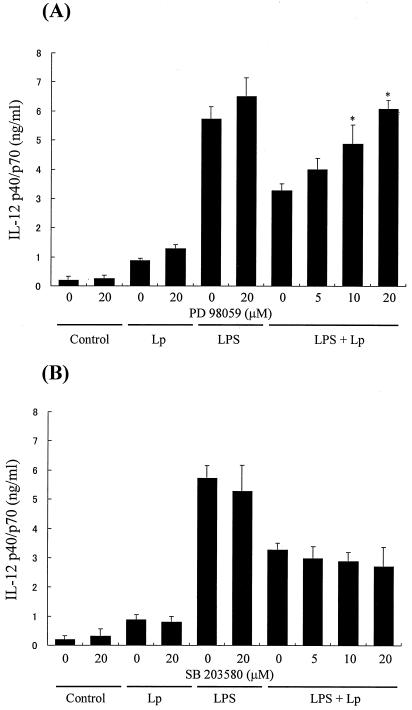
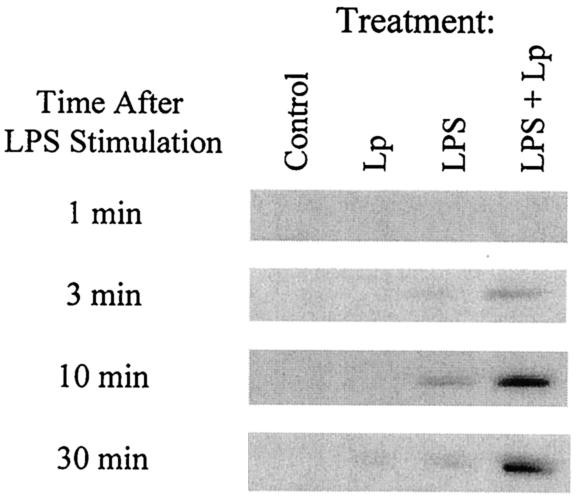
Since it is known that the activation of the MAP kinase cascade is involved in the regulation of cytokine expression (11, 12), treatment of macrophages with MAP kinase inhibitors may alter the IL-12 p40/p70 production if the MAP kinase cascade is involved in the inhibition of IL-12 production by L. pneumophila infection. As shown in Fig. 1A, treatment of cells with PD98059 (p42/44 MAP kinase inhibitor) markedly diminished the L. pneumophila infection-induced inhibition of IL-12 production in response to LPS in a dose-dependent manner. The PD98059 treatment also slightly up-regulated the production of IL-12 in response to both bacterial infection and LPS stimulation, but this was not significant. In contrast, SB203580 (a p38 MAP kinase inhibitor) did not alter the inhibition of IL-12 production by infection (Fig. 1B). The negative control compound SB202474 did not change production of IL-12 in any group (data not shown). From these results, it seemed likely that the activation of p42/44 MAP kinase could be involved in the suppression of LPS-induced IL-12 production by L. pneumophila infection. In order to investigate such a possibility, the effect of L. pneumophila infection on LPS-induced macrophage p42/44 MAP kinase activity was examined. As shown in Fig. 2, LPS stimulated a rapid increase in the levels of p42/44 MAP kinase activity, which peaked at 10 min after stimulation. Although bacterial infection alone weakly induced p42/44 MAP kinase activity only at 30 min after infection, L. pneumophila infection markedly enhanced LPS-induced p42/44 MAP kinase activity in macrophages. On the other hand, noninfected control macrophages did not show any detectable expression of p42/44 MAP kinase activity. Therefore, it is conceivable that there is a dose-response relationship where excessive levels of p42/44 MAP kinase activation might suppress IL-12 production, whereas lower levels may be insufficient to suppress production.
FIG. 1.
Effect of PD98059 (p42/44 MAP kinase inhibitor) (A) and SB203580 (p38 MAP kinase inhibitor) (B) on IL-12 p40/p70 production of MH-S alveolar macrophages. The cells were pretreated with or without a MAP kinase inhibitor 2 h prior to infection. After incubation, the cells were infected with L. pneumophila (Lp) or stimulated with either LPS (1 μg/ml) alone or LPS in combination with bacteria, and the amount of IL-12 p40/p70 in macrophage culture supernatants obtained at 24 h after infection was determined by ELISA. Results are expressed as means plus standard deviations for three independent experiments. *, P < 0.05 (significantly different from the non-inhibitor-treated control group).
FIG. 2.
Effect of L. pneumophila infection on LPS-induced p42/44 MAP kinase activity of MH-S alveolar macrophages. The cells were infected with L. pneumophila (Lp) for 30 min, washed to remove nonphagocytosed bacteria, and stimulated with or without LPS. The cell lysates were collected and the p42/44 MAP kinase activity was measured, as described in text. Representative results of three experiments are shown.
Since IL-10 is known to suppress IL-12 production in the presence of LPS (8, 28), it is possible that the up-regulation of IL-12 production by PD98058 is mediated by the down-regulation of macrophage IL-10 production by the MAP kinase inhibitor. Therefore, the effect of PD98059 on macrophage IL-10 production induced by LPS in combination with L. pneumophila infection was determined. The production of IL-10 at 24 h after incubation was not affected by PD98059, even at a concentration as high as 20 μM (data not shown).
Thus, the present study demonstrated that L. pneumophila suppressed in vitro macrophage IL-12 production in response to LPS at the level of message accumulation, because the suppression was associated with decreased mRNA levels for IL-12 p40. This is consistent with a prior report of IL-12 suppression by Leishmania (11) and a previous study using mouse peritoneal macrophages (24). The suppression of cytokines by L. pneumophila infection was selective for IL-12, since IL-1α, IL-6, and IL-10 induced by LPS were not altered by infection. Therefore, it is conceivable that the suppression of IL-12 by L. pneumophila infection is not the result of a generalized failure of macrophage function.
The results of MAP kinase inhibition by p42/44 MAP kinase and p38 MAP kinase inhibitors indicated that L. pneumophila infection of macrophages caused a selective inhibition of LPS-induced IL-12 by activating the p42/44 MAP kinase cascade. The p38 MAP kinase may not be involved in the IL-12 inhibition caused by L. pneumophila infection. The signaling pathways involved in monocyte/macrophage cytokine production are not yet well understood. LPS has previously been shown to activate some MAP kinase pathways in monocytes/macrophages (14, 19, 21). It has been revealed that there are parallel MAP kinase cascades that can be activated individually and simultaneously (18, 20, 27), suggesting independent signaling roles for these MAP kinase cascades. In fact, it is known that monocyte production of IL-1β, IL-10, and tumor necrosis factor alpha is regulated by the p38 and p42/44 MAP kinase pathways differentially (12). The present study showed that p42/44 MAP kinase plays an important role in the down-regulation of signals leading to the induction of IL-12. This negative regulatory role of p42/44 MAP kinase in IL-12 synthesis was supported by a p42/44 MAP kinase activity assay, because L. pneumophila infection markedly enhanced LPS-induced p42/44 MAP kinase activity in macrophages. These results are consistent with a prior report of IL-12 suppression by Leishmania lipophosphoglycans (11) regarding regulation of IL-12 through MAP kinase activation.
The cytokine IL-10 has been shown to exhibit important deactivating effects on macrophages in murine models of legionella (26), leishmania (1), and mycobacterium (10) infection. Moreover, it is known that IL-10 is secreted by L. pneumophila-infected monocytes and macrophages, enhances bacterial growth, reverses the protective effect of gamma interferon, and blocks the secretion of tumor necrosis factor alpha by infected cells (26). However, modulation of IL-10 may not be directly involved in the suppression of IL-12 by L. pneumophila infection, which would explain why bacterial infection did not enhance the IL-10 production induced by LPS but did enhance suppression of IL-12 production, and the up-regulation of IL-12 production by p42/44 MAP kinase inhibitor was not associated with changes in IL-10 production.
Since IL-12 plays a key role in the development of T helper 1 (Th1) responses, which play an essential role in the development of cell-mediated immunity to pathogens (16), leading to production of gamma interferon (30), which can activate macrophages and monocytes to inhibit L. pneumophila growth (3, 25), the inhibition of IL-12 production by L. pneumophila infection may be exploited as a mechanism to evade the generation of Th1 immune responses and promote the bacterial survival in macrophages.
Acknowledgments
This work was supported by grant AI45169 from the National Institute of Allergy and Infectious Diseases and the American Lung Association of Florida.
Editor: J. M. Mansfield
REFERENCES
- 1.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj, N., T. W. Nash, and M. A. Horwitz. 1986. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 137:2662-2669. [PubMed] [Google Scholar]
- 4.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chehimi, J., S. E. Starr, I. Frank, A. D'Andrea, X. Ma, R. R. MacGregor, J. Sennelier, and G. Trinchieri. 1994. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 179:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chougnet, C., T. A. Wynn, M. Clerici, A. L. Landay, H. A. Kessler, J. Rusnak, G. P. Melcher, A. Sher, and G. M. Shearer. 1996. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J. Infect. Dis. 174:46-53. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 8.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Andrea, A., M. Rengaraju, N. M. Valiante, J. Chehimi, M. Kubin, M. Aste, S. H. Chan, M. Kobayashi, D. Young, E. Nickbarg, et al. 1992. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 176:1387-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis, M., and E. Ghadirian. 1993. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151:5425-5430. [PubMed] [Google Scholar]
- 11.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 12.Foey, A. D., S. L. Parry, L. M. Williams, M. Feldmann, B. M. Foxwell, and F. M. Brennan. 1998. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160:920-928. [PubMed] [Google Scholar]
- 13.Friedman, H., R. Widen, T. Klein, L. Searls, and K. Cabrian. 1984. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect. Immun. 43:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 17.Karp, C. L., M. Wysocka, X. Ma, M. Marovich, R. E. Factor, T. Nutman, M. Armant, L. Wahl, P. Cuomo, and G. Trinchieri. 1998. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur. J. Immunol. 28:3128-3136. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 21.Liu, M. K., P. Herrera-Velit, R. W. Brownsey, and N. E. Reiner. 1994. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J. Immunol. 153:2642-2652. [PubMed] [Google Scholar]
- 22.Marth, T., and B. L. Kelsall. 1997. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga, K., T. W. Klein, H. Friedman, and Y. Yamamoto. 2001. Alveolar macrophage cell line MH-S is valuable as an in vitro model for Legionella pneumophila infection. Am. J. Respir. Cell Mol. Biol. 24:326-331. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga, K., T. W. Klein, C. Newton, H. Friedman, and Y. Yamamoto. 2001. Legionella pneumophila suppresses interleukin-12 production by macrophages. Infect. Immun. 69:1929-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1988. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 140:3978-3981. [PubMed] [Google Scholar]
- 26.Park, D. R., and S. J. Skerrett. 1996. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J. Immunol. 157:2528-2538. [PubMed] [Google Scholar]
- 27.Saklatvala, J., W. Davis, and F. Guesdon. 1996. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos. Trans. R. Soc. Lond. B 351:151-157. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka, H., S. Maruo, N. Yamamoto, M. Wysocka, S. Ono, M. Kobayashi, H. Yagita, K. Okumura, T. Hamaoka, G. Trinchieri, and H. Fujiwara. 1997. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J. Leukoc. Biol. 61:80-87. [DOI] [PubMed] [Google Scholar]
- 29.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri, G. 1993. Interleukin-12 and its role in the generation of TH1 cells. Immunol. Today 14:335-338. [DOI] [PubMed] [Google Scholar]