Abstract
We have established that the route of immunization with peptide-pulsed, activated DC leads to memory CD8+ T cells with distinct distributions in lymphoid tissues, which determines the ability to control tumors growing in different body sites. Both intravenous (i.v.) and subcutaneous (s.c.) immunization induced memory T cells in spleen and control of metastatic-like lung tumors. s.c. immunization also induced memory T cells in lymph nodes (LNs), imparting protection against subcutaneously growing tumors. In contrast, i.v. immunization-induced memory was restricted to spleen and failed to impart protective immunity against subcutaneously growing tumors. Memory cell distribution and tumor control were both linked to injection route–dependent localization of DCs in lymphoid compartments. Using peripheral LN–ablated mice, these LNs were shown to be essential for control of subcutaneously growing tumors but not lung metastases; in contrast, using immunized asplenic mice, we found that the spleen is necessary and sufficient for control of lung tumors, but unnecessary for control of subcutaneously growing tumors. These data demonstrate the existence of a previously undescribed population of splenic-resident memory CD8 T cells that are essential for the control of lung metastases. Thus, regional immunity based on memory T cell residence patterns is an important factor in DC-based tumor immunotherapy.
Keywords: active immunotherapy, regional immunity, vaccination, T cell memory, T cell homing
Introduction
DCs are potent antigen-presenting cells and are effective in inducing both antiviral (1) and antitumor immune responses in experimental animals (2) and humans (3). Immature DCs in peripheral tissues have high phagocytic and macropinocytotic activity, express a wide variety of receptors that augment antigen uptake, and have low surface expression of class I and II MHC and costimulatory molecules. Inflammatory stimuli induce DC maturation, characterized by cessation of phagocytosis and increased cell surface expression of class I and II MHC molecules and costimulatory molecules (4, 5). Maturing DCs down-regulate chemokine receptors associated with tissue retention and simultaneously up-regulate CCR7 and CD62L, allowing them to migrate through the lymphatics to adjacent lymphoid tissue, where they present a snapshot of recently acquired antigens to T cells (6, 7).
To interact with DCs, naive T cells enter LN and mucosal-associated lymphoid tissue based on expression of specific adhesion molecules and chemokine receptors (8). After the immune response has subsided, subpopulations of central memory cells have been defined that express adhesion molecules that enable homing to either peripheral or intestinal LN (9). However, regulation of T cell migration to spleen and other LN compartments is not understood. An important but unexplored issue is whether the localization of the primary immune response in different lymphoid tissues is reflected in the distribution of these central memory cells. Finally, it should also be noted that these homing pathways have been defined using CD4 T cells, and their relevance for CD8 T cells is relatively unexplored.
DCs treated exogenously to express appropriate antigens offer an important avenue for vaccination against tumors (10–14). Antigen has been introduced into immature DC by viral infection (15), RNA transfection (16), and incubation with apoptotic (17) or necrotic cells (18) or tumor lysates (19), whereas synthetic peptides are most effective when pulsed onto mature DCs (20). Such antigen-pulsed DCs distribute in different lymphoid compartments when injected into the body by different routes (11, 12, 21). However, although these different distributions might be expected to stimulate regionally localized T cell responses, as yet there is no evidence in support of this possibility.
Recently, we described a model for evaluating immune responses to a melanoma peptide antigen, tyrosinase369–377 (Tyr369), using mice that express a recombinant human HLA-A*0201 class I MHC molecule (2, 22). Expression of tyrosinase protein in these mice leads to substantial but incomplete tolerance to this epitope (22). However, the residual T cell population in tyrosinase-expressing animals could be expanded to control melanoma outgrowth by immunization with Tyr369-pulsed DC (2). Using this model in the present work, we have established that the route by which DCs are introduced leads to differences in their distribution in lymphoid tissues. In turn, this determines the location of the primary immune response, the distribution of memory cells, and the ability to control the outgrowth of tumors at different sites in the body.
Materials and Methods
Animals.
Transgenic mice on the C57BL/6 background expressing a chimeric MHC class I composed of the α1 and α2 domains of HLA-A*0201 and the α3 domain of H2-Dd (AAD) have been described previously (23). Mice with a radiation-induced excision of the tyrosinase (c) coding locus on chromosome 7 (c 38R145L/c 38R145L; reference 24) were crossed onto the C57BL/6-AAD background (albino AAD) as described previously (22). Asplenic C57BL/6 Hox11−/− mice (Tlx tmSjk; The Jackson Laboratory; reference 25) were crossed onto the C57BL/6-AAD background. All animals were maintained in pathogen-free facilities at the University of Virginia. All protocols were consistent with accepted National Institutes of Health guidelines for the care and use of laboratory animals and approved by the University of Virginia Institutional Animal Care and Use committee.
Peptides.
Synthetic peptides were made by standard Fmoc chemistry using a peptide synthesizer (model AMS422; Gilson Co. Inc.) and purified to >98% purity by reverse-phase HPLC on a C-8 column (Vydac). Purity and identity were confirmed using a triple quadrupole mass spectrometer (Finnigan).
Cell Lines.
B16-F1 (CRL-6323) was obtained from the American Type Culture Collection and maintained in RPMI 1640 (Mediatech) supplemented with 5% FBS (Sigma-Aldrich) and SexXtend (Irvine Scientific). B16-F1 was transfected with a plasmid containing the genes for AAD and G418 resistance under the control of the class I promoter, as described previously (2), and the resultant cell line is referred to as B16-AAD. B16-AAD cells were >98% AAD positive and expressed AAD stably for at least 6 wk in the absence of G418.
DC Culture.
DCs were generated by culturing mouse bone marrow cells in GM-CSF and IL-4 (BD Biosciences), as described previously (26), and activated by overnight culture with an equal number of irradiated (3,000 rads) NIH-3T3 cells transfected to express CD40L (gift of R. Lapoint, University of Montreal, Quebec, Canada). The resulting activated DCs were CD80hiCD86hi, and >85% expressed IL-12 by intracellular staining (unpublished data). DC expression of CD62L was assessed by flow cytometry using anti-CD62L mAb (BD Biosciences). CCR7 expression was determined by flow cytometry using ELC-19 fusion protein (gift of U. von Andrian, Harvard University, Cambridge, MA) and anti–human IgG mAb (Jackson ImmunoResearch Laboratories).
Immunization.
CD40L-activated DCs were pulsed with the indicated concentration of peptide for 4 h at 37°C in HBSS containing 5% FBS and 5 μg/ml human β2-microglobulin (Calbiochem). In all experiments, an altered ligand of Tyr369 (Tyr369Y, YMDGTMSQV) was used as the immunogen, as described previously (2). The HLA-A*0201–restricted epitope of the influenza M1 protein (GILGFVFTL) was used as an irrelevant control peptide. Cells were washed twice, resuspended in physiologic saline, and 100 μl of cell suspension injected by the s.c. route into the scapular fold or flank, as indicated, or i.v. into the dorsal tail vein. Unless indicated otherwise, mice received 105 DCs. For analysis of recall responses, DC-immunized mice were injected i.v. with 107 PFU of hTyrVac, a recombinant vaccinia virus encoding human tyrosinase.
Tumor Induction and Measurements.
Subcutaneous tumors were established by injection of 4 × 105 B16-AAD in 200 μl of physiologic saline. Lung metastases were induced by tail vein injection of 4 × 105 B16-AAD in 200 μl of physiologic saline. Tumor cells were 100% viable by trypan blue exclusion (unpublished data). Mice were evaluated for subcutaneoulsy growing tumor by palpation, and 100% of B16-AAD–challenged naive tyrosinase+ animals developed palpable tumors by day 10. Tumor growth was measured at ∼72 h intervals using a vernier caliper, as described previously (2). Mice challenged with tumor i.v. were killed on day 21 after the injection, and surface lung metastatic lesions enumerated using a 40 magnification dissecting scope with the aid of an eyepiece grid. All analyses were performed in a blinded manner.
Tumor Resection and Evaluation of Infiltrating Lymphocytes.
Subcutaneous tumors were resected, digested in 0.5% collagenase D (Roche Diagnostics) for 45 min, and homogenized. The suspension was filtered through nylon, and then debris and dead cells were separated on a Ficoll-Paque gradient (Amersham Biosciences). Lymphocytes were separated on a Lympholyte-M gradient (Accurate Chemical). Antigen-specific CD8+ T cells were enumerated using HLA-A*0201–Tyr369Y tetramers (National Institute of Allergies and Infectious Diseases Tetramer Core Facility) at 1:100 dilution. Cells were costained with anti-CD8 and anti–TCR-β chain (BD Biosciences) at 1:1,000 dilution and analyzed by flow cytometry.
DC Tracking Studies.
To determine lymphoid distribution of DC after injection, CD40L-activated DCs were labeled with 5-carboxyfluorescein diacetate, succinimidyl ester) (CFSE; Molecular Probes) and injected (105 cells) s.c. or i.v. At various times after injection, lymphoid cells from spleen and LN were collected and stained with anti-CD11c (BD Biosciences). The number of infiltrating DC (CD11c+CFSEhi cells) was quantitated by flow cytometry.
LN Ablation.
Albino AAD mice devoid of peripheral LN were generated using soluble lymphotoxin β receptor Ig as described previously (27). Peripheral LN depletion was confirmed at the termination of each experiment. LN ablation was scored positive when axillary, brachial, inguinal, and popliteal LN could not be visually detected by inspection at 100× magnification after s.c. injection with Lymphazurin 1% tracking dye (Ben Venue Labs).
Ex Vivo Analysis of Antigen-specific T Cells.
CD8+ T cells were enriched from spleens and LN of naive and immunized mice using a StemSep column (StemCell Technologies, Inc.) according to the manufacturer's instructions. Preparations were consistently 85–95% CD8+ as assessed by flow cytometry. Enriched CD8+ T cells were directly assessed for cytokine production using target/stimulator cells that had been pulsed overnight with 100 μM of peptide. Peptide-pulsed stimulator cells were incubated with CD8+ T cells for 5 h at a ratio of 1:1 in medium supplemented with 50 U/ml IL-2 (Chiron Corp.) and 10 μg/ml Brefeldin A (Sigma-Aldrich). Stimulated cells were stained with PE-conjugated anti-CD8 (BD Biosciences), washed, fixed, and permeabilized in Cytofix/Cytoperm (BD Biosciences), further stained with FITC-conjugated anti-IFNγ (BD Biosciences) or isotype-matched controls, and analyzed by flow cytometry. Antigen-specific cells were also enumerated using HLA-A*0201-Tyr369Y tetramers.
Statistics.
Statistical comparisons were performed using Student's t test; survival was plotted using Kaplan-Meier curves and statistical relevance was determined using log-rank comparison. Unless noted, data are presented as means ± SD of pooled data from four to six independent experiments.
Online Supplemental Material.
Fig. S1 shows three independent tumor outgrowth experiments in which mice expressing a recombinant class I MHC molecule (AAD) were immunized once with 105 DCs and challenged 21 d later with 4 × 105 s.c. injected B16-AAD melanoma. DCs were unpulsed or pulsed with 1 μM of either Tyr369Y or influenza A M1 peptide. Data in each group are mean of five separate animals ± SD. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20021348/DC1.
Results
Intravenous Immunization with Peptide-pulsed DC Fails to Control Subcutaneous Melanoma Outgrowth.
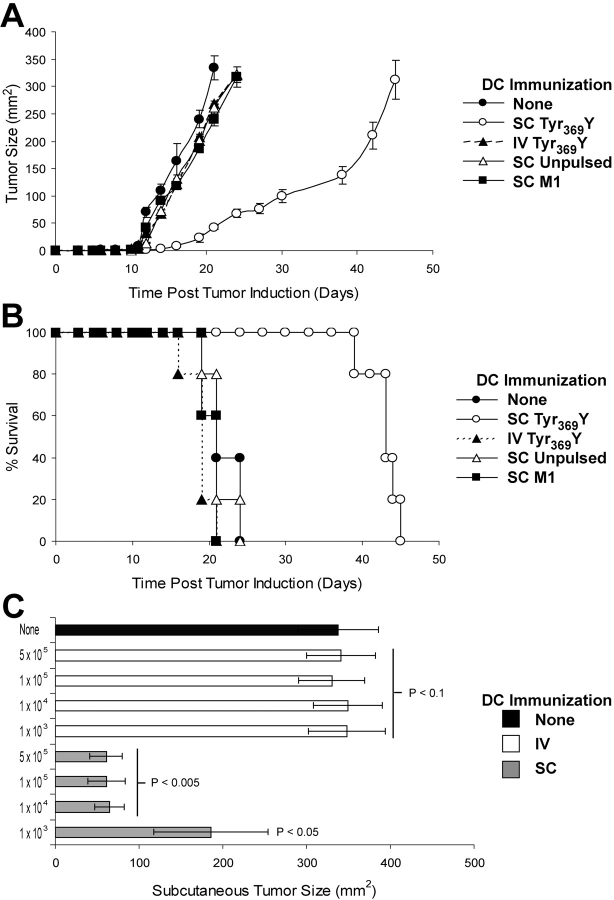
Mice expressing a recombinant class I MHC molecule (AAD) were immunized with CD40L-activated and tyrosinase peptide (Tyr369Y)–pulsed DCs by either the s.c. or i.v. route. 3 wk later, mice were challenged with a s.c. bolus of B16-AAD melanoma cells. In six experiments involving five animals in each group (representative experiments shown in Fig. 1 A and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20021348/DC1), measurable outgrowth was delayed by 8.7 ± 1.5 d in mice immunized s.c. with Tyr369Y-pulsed DCs, compared with unimmunized littermates (P < 0.005). Survival, defined as the time point when tumors achieved or exceeded 300 mm2, was also significantly (P < 0.001) enhanced by a mean of 22.3 ± 2.4 d in the same experiments (one representative experiment is shown in Fig. 1 B). Surprisingly, tumor outgrowth was not delayed in animals immunized i.v. with the same DCs and no survival advantage was conferred, although this immunization has been shown previously to induce a significant antigen-specific CD8+ T cell response in spleen (20). s.c. immunization with unpulsed DCs or DCs pulsed with influenza M1 peptide did not delay tumor outgrowth or enhance survival, establishing that a tumor antigen-specific immune response was required. In these experiments, 105 DCs were injected; however, no control of outgrowth of subcutaneously growing tumors was observed in mice immunized i.v. with numbers of Tyr369Y-pulsed DCs ranging between 103 and 5 × 105 per animal (Fig. 1 C). In contrast, as few as 104 DCs injected by the s.c. route were sufficient for maximal control (Fig. 1 C). These data demonstrate that the immunization with DCs via the i.v. route fails to induce an immune response capable of controlling subcutaneously growing melanomas.
Figure 1.
s.c., but not i.v., immunization with peptide-pulsed DCs controls s.c. melanoma outgrowth. Mice were immunized once with unpulsed or 1 μM peptide-pulsed DCs, and B16-AAD melanoma was injected s.c. 21 d later. (A) Kinetics of tumor outgrowth after i.v. or s.c. immunization with 105 DCs pulsed with either Tyr369Y or a peptide corresponding to residues 57–66 of the influenza A M1 protein. Data are the mean of five separate animals ± SD; representative data from one of six similar experiments are shown. Additional representative experiments are shown in Fig. S1. (B) Kaplan-Meier survival curves after B16-AAD challenge in mice immunized i.v. or s.c. with 105 DCs pulsed with Tyr369Y- or M1 flu peptide-pulsed DCs, scored as days from challenge until tumor diameter exceeds 300 mm2. Survival was significantly increased in s.c. immunized animals compared with untreated (P < 0.001). Data are for groups of five animals; representative data from one of six similar experiments are shown. (C) Day 21 s.c. tumor size in mice immunized i.v. or s.c. with 103 to 5 × 105 DCs pulsed with Tyr369Y (1 μM). P-values indicate statistical significance compared with unimmunized control (black bar) by two-sample Student's t test.
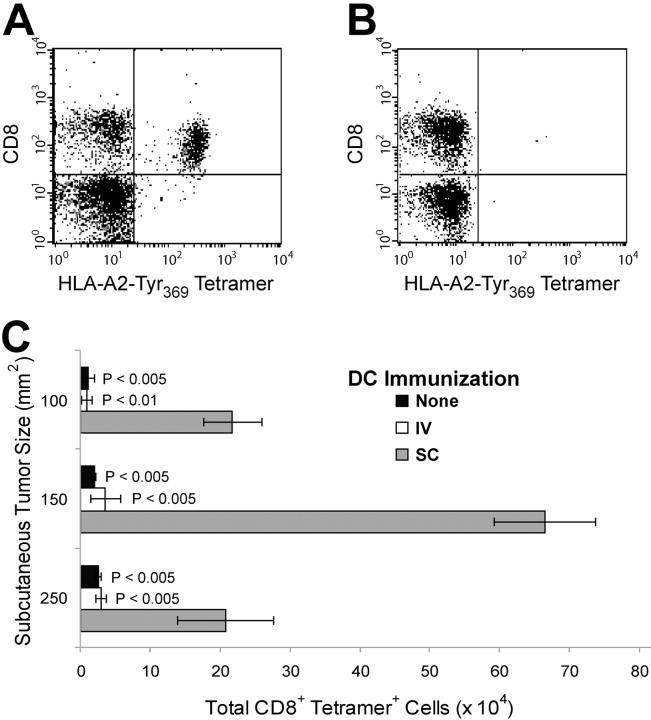
To further investigate the failure of i.v. immunization with DCs to control subcutaneously growing tumors, we used HLA-A*0201-Tyr369Y tetramers to enumerate Tyr369Y-specific CD8+ T cells infiltrating tumors that had achieved comparable sizes in s.c. or i.v. immunized mice. A substantial population of Tyr369Y-specific CD8+ cells was recovered from 150 mm2 tumors growing in s.c. immunized mice (Fig. 2 A). In contrast, there was no significant infiltration of antigen-specific T cells in tumors of equivalent size from i.v. immunized animals (Fig. 2 B). Similar data were obtained when tumors of different sizes, obtained at different points during their outgrowth, were compared (Fig. 2 C). Although tumors from i.v. immunized animals achieved a given size at an earlier time relative to those from s.c. immunized mice, infiltration of 100-mm2 tumors obtained from s.c. immunized mice on day 15 was still substantially greater than that of 150-mm2 tumors from i.v. immunized mice obtained on the same day. These data suggest that either the memory T cells primed by i.v. immunization are not reactivated by a subcutaneously growing tumor, or that they are activated but do not infiltrate the tumor.
Figure 2.
Antigen-specific CTL infiltrate subcutaneously growing tumors only after s.c. delivery of immunizing DCs. Melanomas from naive or DC-immunized tumor-challenged mice were analyzed for infiltrating Tyr369Y-specific CD8+ lymphocytes using HLA-A*0201+ Tyr369Y tetramers. (A) Infiltration of 150 mm2 subcutaneously growing B16-AAD tumor from s.c. immunized mouse. (B) Infiltration of 150 mm2 s.c. growing B16-AAD tumor from i.v. immunized mouse. (C) Infiltration of CD8+ tetramer+ cells into tumors of 100, 150, or 250 mm2. P-values indicate statistical significance compared with s.c. immunized animals (gray bar), in the same time point grouping, by two-sample Student's t test.
I. v. Immunization with Peptide-pulsed DCs Enables Control of Metastatic-like Lung Melanomas.
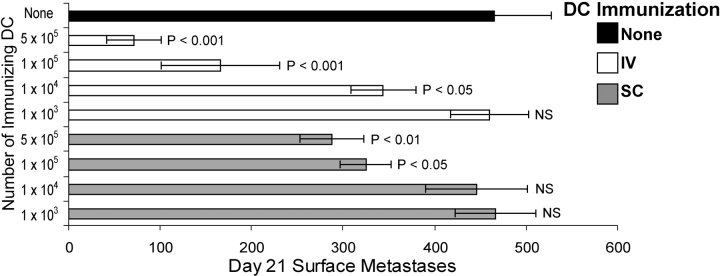
We showed previously that i.v. immunization with Tyr369Y-pulsed DCs induced an antigen-specific CD8+ response in spleen (26). To determine whether these T cells were functional in controlling tumors growing in other body sites, we evaluated the ability of mice immunized with DCs via the i.v. route to control metastatic-like lung tumors (Fig. 3) . i.v. immunization with 105 Tyr369Y-pulsed DCs resulted in a 70 ± 9.3% reduction in the number of lung lesions 21 d after challenge, as compared with the number in untreated control mice. s.c. immunization with the same number of DCs also led to a significant decrease in the number of lungs lesions (29 ± 9.1%). In contrast to results with subcutaneously growing tumors shown in Fig. 1 C, control of lung tumor metastases correlated (logarithmic r 2 = 0.993) with the number of DCs used for immunization over the entire range of 103 to 5 × 105 per animal, regardless of delivery route. The ability of T cells induced by i.v. immunization to control lung metastatic lesions demonstrates they were tumor-reactive and not functionally compromised, and suggests that their failure to control subcutaneously growing B16-AAD was due either to an inability of memory T cells to be reactivated by subcutaneously growing tumors, or to an inability of reactivated effector cells to localize subcutaneously. Because DCs from subcutaneously growing tumors have been shown to migrate to regional draining LN (28), we favored the first interpretation and hypothesized that DCs introduced via different routes would migrate to distinct lymphoid compartments and only stimulate the production of memory T cells at those sites.
Figure 3.
Metastatic-like lung lesions are controlled by i.v. immunization and partially controlled by s.c. immunization with peptide-pulsed DCs. Mice were immunized with the indicated number of 1 μM Tyr369Y-pulsed DCs, delivered either s.c. or i.v., and B16-AAD melanoma was injected i.v. 21 d later. Surface lesions were enumerated 21 d after tumor inoculation. P-values indicate statistical significance compared with unimmunized control animals (black bar) by two-sample Student's t test.
Tumor Control Correlates with Distribution of DCs in Secondary Lymphoid Tissues after s.c. and i.v. Immunization.
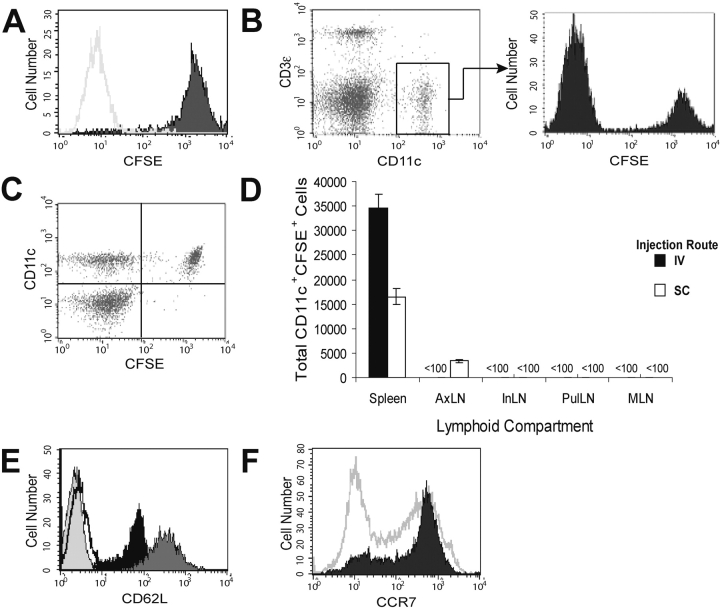
To determine whether the immunization route dictates the lymphoid compartment distribution of DCs, 105 CD40L-activated DCs were labeled with CFSE, injected either s.c. or i.v., and their location assessed at various times by gating on both CFSEhi and CD11c+ events in cell suspensions isolated from LN and spleen. The level of CFSE fluorescence was equivalent in CD11c+ cells before transfer (Fig. 4 A) and after recovery from spleen (Fig. 4 B) and LN (not depicted). Furthermore, all recovered CFSEhi cells were also CD11c+ (Fig. 4 C), establishing that the cells identified were unlikely to be endogenous DC or macrophages that acquired CFSE from the adoptively transferred population. After injection via the s.c. route, DCs were detected in the axillary LN (AxLN) draining the injection site and in spleen (Fig. 4 D). However, no DCs were observed in inguinal LN (InLN) and popliteal LN, both of which drain skin but are outside the drainage of the injection site (Fig. 4 D and not depicted). In addition, DCs were not observed in either mesenteric LN (MLN) or pulmonary LN (Fig. 4 D, PulLN) at any time up to 72 h after the injection (Table I). The failure to find these DCs in LN other than those draining the injection site was surprising because they expressed both CD62L (Fig. 4 E) and CCR7 (Fig. 4 F). However, the level of CD62L was only 20% as much as that expressed on splenic T cells. Similar low levels on B and T lymphocytes have been associated with a failure to migrate into peripheral LN (29). Interestingly, the number of s.c. injected DCs in the AxLN was substantially smaller (∼3,000 cells in several experiments) than in the spleen (∼16,000), and whereas the number of DCs in AxLN remained constant when up to 5 × 105 DC were injected, the number of DCs in spleen directly correlated with the number injected (unpublished data). These data suggest that the number of DCs injected was significantly greater than the holding capacity of the draining LN, and that the excess DCs migrated via the blood, but not lymphatics, to the spleen.
Figure 4.
Phenotype and distribution of CD40L-activated DCs after s.c. and i.v. injection. CD40L-activated DCs were labeled with CFSE, adoptively transferred, and recovered from the indicated lymphoid organs at the indicated times. (A) Fluorescence of CFSE-stained (shaded histogram) and unstained (white histogram) CD40L-activated DCs before injection. (B) CD11c staining and CFSE staining of gated CD11c+ cells recovered from spleen 24 h after injection. (C) CFSE and CD11c staining of total spleen cells, excluding red blood cells and debris, recovered 24 h after injection. (D) Total CD11c+CFSE+ cells in various lymphoid compartments 4 h after injection of 105 CFSE-labeled CD40L-activated DCs either s.c. in the scapular region or i.v. (E) CD62L expression on CD40L-activated DC (black histogram), splenic CD8+ T cells (dark gray histogram), unstained DCs (light gray histogram), and isotype-control stained DCs (white histogram). (F) CCR7 expression on CD40L-activated DCs (shaded histogram) and splenocytes (white histogram).
Table I.
Quantitation of CFSE+ CD11c+ DC in LN and Spleen after Injection of 105 Cells
| Time after injection |
i.v.-injected DCa
|
s.c.-injected DCa
|
||
|---|---|---|---|---|
| Spleen | Axillary LN | Spleen | Axillary LN | |
| h | ||||
| 4 | 26.2 ± 1.6 | <0.1 | 15.9 ± 1.2 | 3.0 ± 0.2 |
| 24 | 9.4 ± 1.4b | <0.1 | 7.4 ± 0.5c | 0.76 ± 0.02b |
| 72 | 2.8 ± 0.3b | <0.1 | 2.3 ± 0.5b | 0.26 ± 0.09b |
Data represent mean ± SD for three mice.
Percentage of injected cells.
P < 0.005 compared with 4-hr time point for the same injection route and lymphoid organ.
P < 0.05 compared with 4-hr time point for the same injection route and lymphoid organ.
As expected, DCs injected via the i.v. route were found in large numbers in the spleen (approximately twice as many as had been observed after s.c. injection). However, i.v. injected DCs were never detected in any LN compartment examined (Fig. 4 D), including skin-draining LN, MLN, and PulLN, even though these cells express both CD62L and CCR7. Nonetheless, these data demonstrate that CD40L-activated DCs injected via the i.v. route fail to access LN compartments, but show enhanced representation in the spleen.
DC Distribution in Secondary Lymphoid Tissue Determines the Sites of Antigen-specific T Cell Expansion.
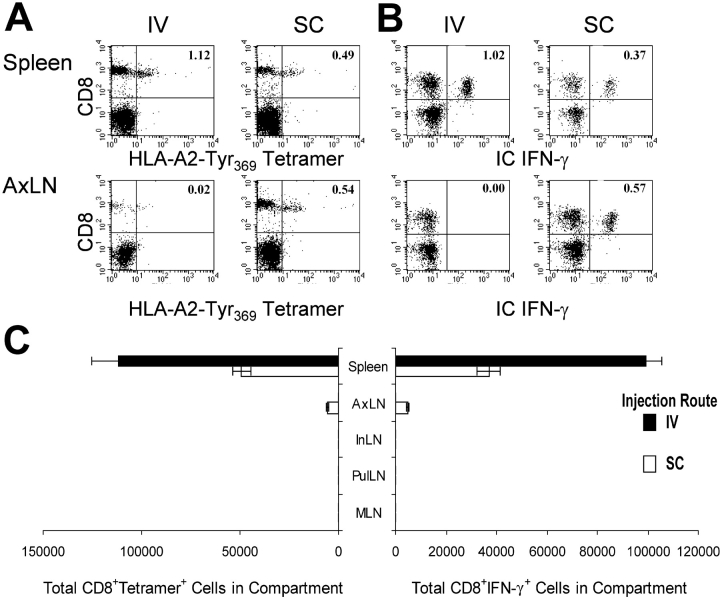
The aforementioned results established a direct correlation between the presence of immunizing DCs in LN and protection against melanoma growing in a s.c. site, and between the number of immunizing DCs present in spleen and the extent to which melanoma growing in the lungs was controlled. This suggested the hypothesis that the presence of DCs defines the secondary lymphoid compartments in which antigen-specific T cells are activated or expanded. To evaluate this possibility, we determined the peak magnitude of the primary antigen-specific T cell responses in different lymphoid compartments 7 d after s.c. or i.v. immunization with peptide-pulsed DCs. Tyr369Y-specific CD8+ cells were quantified in different LN subsets and in spleen by surface staining with HLA-A2-Tyr369Y tetramer (Fig. 5 A) and by measuring antigen-induced intracellular IFN-γ accumulation (Fig. 5 B). After s.c. immunization, comparable numbers of antigen-specific CD8+ T cells were detected in the draining AxLN and the spleen (Fig. 5 C). However, no significant response was detected in distal skin-draining InLN, nor in MLN or PulLN. In contrast, i.v. immunization with DCs induced approximately twice as many antigen-specific CD8+ T cells in the spleen as did s.c. immunization (Fig. 5 C). However, i.v. immunization failed to induce any significant antigen-specific primary responses in any LN examined. Thus, the size and location of primary CD8+ T cell responses on day 7 after immunization correlated directly with the distribution of DCs observed shortly after injection, as well as with the ability to control subsequent tumor outgrowth 2–3 wk later.
Figure 5.
Immunization route influences the location of DC-induced antigen-specific T cell expansion. Mice were immunized with 1 μM 105 Tyr369Y-pulsed DCs, delivered either s.c. or i.v. 7 d later, spleen and LNs were evaluated for total Tyr369Y-specific CD8+ T cells using either (A) HLA-A*0201–Tyr369Y tetramers or (B) intracellular production of IFN-γ by CD8+ T cells after stimulation with peptide-pulsed targets. Numbers in dotplots represent the percentage of CD8+ tetramer+ events (A) or CD8+ IFN-γ+ events (B) in the total lymphocyte population of the indicated lymphoid compartment. (C) Total tetramer+ and IFN-γ+ CD8+ cells are shown in each compartment.
DC Immunization by Different Routes Induces Distinct Populations of Memory T Cells That Reside in Either LN or Spleen.
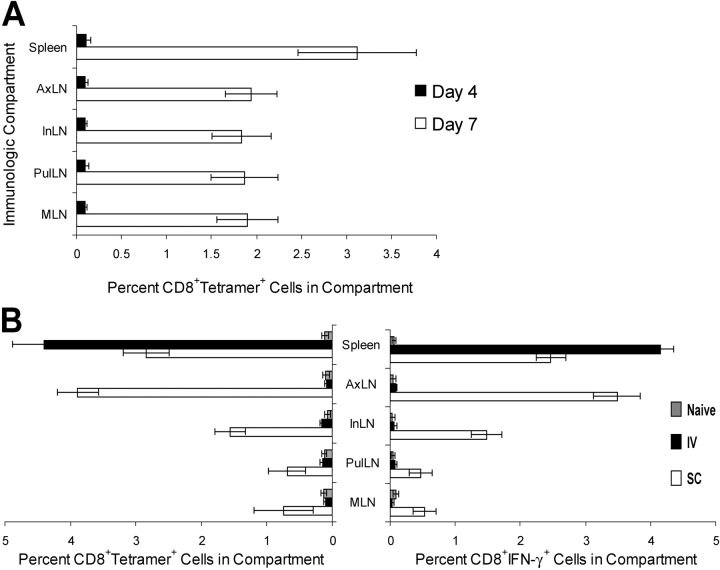
Despite the localization of primary T cell responses shown in the previous section, control of tumor outgrowth in the protective immunization model used here depends on the distribution and/or reactivation of memory cells. Attempts to quantify the apparently small number of AAD+ Tyr369-specific memory cells in LN and spleen from mice bearing subcutaneouay growing B16-AAD tumors using MHC tetramers or antigen-induced IFN-γ accumulation were unsuccessful (unpublished data). Consequently, we determined the location of memory T cells induced by DC immunization by examining recall responses to recombinant vaccinia virus encoding human tyrosinase (hTyrVac). In contrast to DCs, hTyrVac induced equivalent antigen-specific primary responses in spleen and multiple LN after i.v. injection (Fig. 6 A). Although primary responses to hTyrVac in naive animals were maximal at day 7, they remained minimal on day 4. Thus, by evaluating AAD+Tyr369Y-specific T cells on day 4 after hTyrVac challenge in animals that had been immunized previously with Tyr369Y-pulsed DCs, we visualized only the response of the memory cells.
Figure 6.
Recall responses are enriched in the compartments where primary responses are observed. (A) Antigen-specific hTyrVac-induced primary responses at days 4 and 7, measured as intracellular accumulation of IFN-γ by CD8+ T cells incubated with peptide-pulsed targets. (B) Antigen-specific hTyrVac-induced recall responses in mice immunized previously (21 d) with 1 μM 105 Tyr369Y-pulsed DCs. Tyr369Y-specific responses were measured by costaining with anti-CD8 and HLA-A*0201–Tyr369Y tetramers, and by assessing intracellular accumulation of IFN-γ by CD8+ T cells incubated with peptide-pulsed targets.
In animals immunized with DCs via the s.c. route, recall responses were robust in draining AxLN and spleen, in keeping with the location of the peak primary T cell response (Fig. 6 B). Significant recall responses were also observed in peripheral LN residing in draining beds not targeted by the scapular-region s.c. immunization (InLN and popliteal LN), and in which no DC infiltration or primary T cell response had been seen (Fig. 6 B and not depicted). However, these responses were invariably lower than those observed in AxLN. Unexpectedly, recall responses were also noted in MLN and PulLN, although again these responses were of lower magnitude than in InLN and popliteal LN. In animals immunized with DCs via the i.v. route, memory responses were observed exclusively in spleen, demonstrating that memory T cells resulting from a primary response confined to the spleen do not migrate to major LN compartment subsets. Based on this result, the CD8+ memory T cells observed in peripheral and mucosal LN in distinct drainages after a primary response in the AxLN most likely arise from cells that leave the AxLN and circulate.
LN Memory T Cells Are Necessary and Sufficient for Control of Subcutaneously Growing Tumors, Whereas Spleen-resident Memory T Cells Are Necessary and Sufficient for Control of Lung Metastatic-like Lesions.
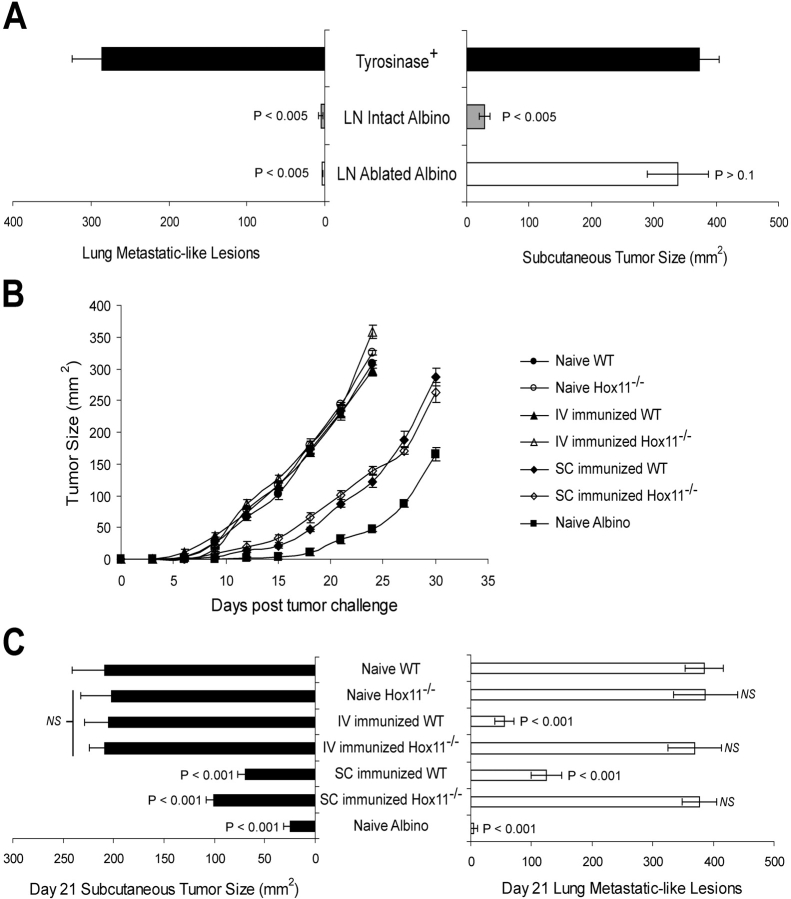
The results in the previous section suggested that Tyr369Y-specific memory cells in skin-draining LN and spleen enable, respectively, the control of tumors growing either subcutaneously or in the lungs. To directly define the requirement for peripheral LN in controlling tumor outgrowth, we used mice whose peripheral LN had been ablated by perinatal administration of lymphotoxin β receptor immunoglobulin Ig fusion protein (27). Both mucosal LN and the splenic compartments in these mice are normal (27 and unpublished data). These experiments were performed in AAD+ albino mice instead of AAD+ tyrosinase+ mice because their lack of tolerance to tyrosinase (22) enables AAD+ albino mice to control B16-AAD outgrowth without prior immunization (2). Peripheral LN–ablated AAD+ albino mice and intact mice controlled B16-AAD lung metastatic lesions comparably, and substantially better than did intact AAD+ tyrosinase+ mice (Fig. 7 A). However, the ability of peripheral LN–ablated AAD+ albino mice to control subcutaneously growing B16-AAD was substantially diminished compared with that of intact mice, and was only marginally better than that of AAD+ tyrosinase+ mice (Fig. 7 B). Thus, the absence of peripheral LN resulted in a selective failure to control the outgrowth of subcutaneously growing tumors.
Figure 7.
Peripheral LNs and spleen, respectively, are necessary and sufficient to control the outgrowth of s.c. and lung metastatic-like tumors. (A) Tumor outgrowth at day 21 after B16-AAD challenge of intact tyrosinase+ and albino mice and LN-ablated albino mice. P-values indicate statistical significance compared with intact tyrosinase+ animals (black bar) by two-sample Student's t test. (B) Outgrowth of s.c. melanoma in AAD+ normal or asplenic (Hox11−/−) mice immunized previously with peptide-pulsed DCs, either i.v. or s.c. Data are mean ± SD for five animals. (C) Day 21 outgrowth of subcutaneously growing melanomas and lung metastatic-like lesions in immunized and naive AAD+ and AAD+Hox11−/− mice. Data are mean ± SD for 10 pooled animals from two independent experiments.
To directly define the requirement for spleen-resident memory T cells in controlling tumor outgrowth, we used asplenic AAD+Hox11−/− mice (25). These mice were tyrosinase+ and, therefore, partially tolerant, so we evaluated tumor outgrowth in both naive and immunized animals. After s.c. immunization, AAD+Hox11−/− mice and AAD+ mice with normal spleens both controlled subcutaneously growing B16-AAD to a similar extent (Fig. 7, B and C). Together with the LN ablation results, this lack of dependence on the spleen further emphasizes the importance of LN-resident immunity in controlling the outgrowth of subcutaneously growing tumors. In contrast, regardless of the route of immunization, AAD+Hox11−/− mice were incapable of controlling B16-AAD outgrowth in lung (Fig. 7 C). Thus, the absence of spleen-resident T cells resulted in a selective failure to control the outgrowth of tumors in lung, establishing both the necessity and sufficiency of the spleen to mediate immunity against tumors growing in the lungs.
Discussion
In the present paper, we have established that the location of primary T cell activation, the distribution of memory T cells, and control of localized tumor outgrowth are determined by the route of immunization with tumor antigen-pulsed DCs. After s.c. injection, a small number of DCs apparently saturate draining LN and the remainder enter the spleen, but not noncontiguous LN beds. Subsequent antigen-specific primary CD8+ T cell responses are limited to the lymphoid compartments in which DCs localize, whereas memory cells redistribute to LN outside the draining node bed of the immunization site. In contrast, i.v. injected DCs enter only spleen, leading to primary and memory CD8+ T cells that are present in the spleen, but absent from major LN compartments. This demonstrates that memory CD8+ T cell distributions, which presumably reflect their homing characteristics, are defined by the compartment (LN or spleen) in which the initial APC–T cell interactions occur. In keeping with this, we also showed that peripheral LNs are necessary and sufficient for control of subcutaneously growing tumors. Conversely, spleen-resident memory T cell immunity is required for control of tumor growing as metastatic-like lung lesions, but spleen is not necessary for the induction or maintenance of effective antitumor T cells in peripheral LN. Collectively, these data demonstrate a correlation between the presence of antigen-specific memory T cells in lymphoid compartments that are directly accessible to tumor cells or to DC-presenting tumor antigens, and the capacity to control tumor outgrowth. These results illustrate fundamental processes of T cell localization that are important for immunologic control of tumors growing in different sites in the body, and also demonstrate the critical nature of the immunization route when DCs are used as immunotherapeutic adjuvants.
Some previous work has examined, in a more preliminary way, the impact of DC immunization via different routes on either immune responses or antitumor efficacy. Lambert et al. (19) compared i.v., s.c., and intranodal immunization and concluded that intranodal immunization was superior for the induction of antitumor immunity. However, only lung metastases were examined in this paper, and consequently there was no evaluation of differences in immunity to tumors growing in different sites. There was also no examination of DC migration patterns or the location of memory responses. This work did show that tumor control was comparable after immunization via s.c. and i.v. routes, whereas in our work, the s.c. route was somewhat less effective. However, paradoxically, this work also showed that the number of antigen-specific T cells recovered from spleen was lower after s.c. than i.v. immunization. Our results demonstrate a direct relationship between the number of DCs infiltrating the spleen (depending, in turn, on immunization route), the size of the primary and memory responses, and control of lung tumor outgrowth. In a similar vein, Eggert et al. (11) and Okada et al. (12) compared s.c. and i.v. immunization with DCs, but only evaluated subcutaneously implanted tumors. As we have demonstrated, both of these groups showed that i.v. immunization failed to control outgrowth of tumor in s.c. compartments. However, neither of these papers examined control of tumors growing in different sites nor lymphoid distribution of antigen-specific T cells. Instead, both groups reported that i.v. immunization failed to induce any immune response in the spleen at all. This was despite the demonstration by Eggert et al. (11) of a significant number of DCs in this organ. Thus, we have reached an entirely different conclusion from these previous works. We find that i.v. injection of DCs is not a deficient route of immunization, but rather that it induces large responses in the spleen, and that these responses are effective in controlling lung metastases. Finally, Fong et al. (13) reported that intradermal and intralymphatic injection of peptide-pulsed PBMC-derived DCs led to antigen-specific immune responses in melanoma patients, whereas i.v. immunization did not. However, this work did not evaluate DC migration, or the existence and location of Ag-specific T cells in any organs except blood, and there was no examination of the ability of these immunizations to control tumors. In contrast to our work and the papers cited herein, Zitvogel et al. (30) has demonstrated that i.v. immunization with DCs results in effective control of s.c. or intradermal tumors (30). However, these analyses used repetitive immunization with large numbers of relatively immature DCs over a short time interval. DC migration was not evaluated, nor was there any evaluation of primary and memory T cell responses in skin-draining LNs. Neither of these analyses used B16, which may be relevant to the ability of tumors to initiate an immune response in local versus distal lymphoid compartments. Collectively, no previous work has suggested the existence of regionally distinct immune responses, nor examined control of tumors growing in different sites in the body. The present paper is the first comprehensive evaluation of the impact of DC immunization route on localization of primary and memory T cell responses and subsequent control of tumor outgrowth, and the first to demonstrate differences in the effectiveness of local immunity against tumors growing in different sites.
A central observation in the present work is that i.v. injection of DCs induces primary activated and memory T cells that are localized in spleen, but absent from major LN subsets. This contrasts strongly with the systemic immunity in both spleen and several different noncontiguous LNs induced by injection of vaccinia virus via the same route. Although it is formally possible that virus introduced i.v. could be cleared in the spleen but lead to the activation of DCs or T cells that circulate systemically, we favor the hypothesis that the virus itself disseminates widely throughout the body, activating discreet populations of primary and memory T cells in different lymphoid compartments. Previous work has established that memory T cells differentially express CD62L and α4β7 integrin, and that these molecules enable extravasation into peripheral LNs or into intestinal LNs and lymphoid tissue, respectively (31). However, the factors that might control any selective extravasation process into the white pulp of the spleen (as well as PulLN) remain undefined (32). Our results also demonstrated that s.c. injection of DCs led specifically to primary T cell responses in draining LN, and memory responses in both noncontiguous peripheral LN and mucosal LN. These results suggest that DCs in peripheral LNs stimulate the development of CD8+ memory T cells that circulate indiscriminately among different LN compartments. Based on previous work (31), we had expected that CD8+ central memory cells induced in the AxLN by s.c. injected DCs would have accessed only cutaneous-draining LNs. It is unclear whether our results reflect a difference in the homing of memory CD8+ cells induced by our immunization protocol, or whether small populations of memory cells arise from cells that rapidly migrate into these LN before expression of tissue-specific receptors. Nonetheless, our data demonstrate that memory cell localization is defined by the immunologic compartment in which the initial APC–T cell interaction occurs and point to the existence of a distinct population of memory CD8+ T cells that are selectively resident in the spleen. Further analysis of the homing characteristics and receptors expressed on these T cells is clearly warranted.
Recent work has highlighted the existence of effector memory T cells that persist in an activated state in nonlymphoid tissue, in addition to the central memory T cells that remain in lymphoid organs after the immune response has subsided (33, 34). Our model system does not provide sufficient sensitivity to directly examine the existence and location of effector memory T cells after immunization with DCs via different routes. Thus, it is possible that i.v. immunization with peptide-pulsed DCs induces a population of lung-resident effector memory cells that participate in the control of subsequent tumor outgrowth. Nonetheless, our results establish that control of lung metastatic-like lesions is directly correlated with the number of antigen-specific recall T cells in the spleen, which are presumably differentiated from local central memory cells, and absence of spleen precludes protective immunization using peptide-pulsed DC. In addition, control is apparently not enhanced by the presence of memory cells in PulLNs. Similarly, the essential role of peripheral LNs in the control of s.c. tumor in unimmunized albino mice cannot be attributed to effector memory cells, although this may differ in immunized tyrosinase+ mice. Thus, the role of effector memory in controlling tumor outgrowth, while not formally excluded, seems to have limited significance in this model. At the least, the distribution of central memory cells in peripheral LNs and spleen may serve as a proxy for the distribution and magnitude of effector memory cells.
These considerations highlight an additional uncertainty in understanding why memory T cells induced by i.v. immunization fail to control the outgrowth of subcutaneous tumors. It is possible that T cells activated in spleen are incapable of infiltrating cutaneous sites, a limitation that would presumably apply to both effector and effector memory T cells. Alternatively, antigen from subcutaneously growing tumors may not reach the spleen, such that activation of splenic T cells fails to occur. Although we have shown that excess s.c. injected DCs migrate to the spleen, the number that infiltrate tumors and subsequently migrate to regional LNs is almost certainly lower than the number we inject. Indeed, it has been demonstrated that antigens captured in the skin are presented by DCs exclusively in the draining LNs, and skin-derived antigens are found in spleen only in the absence of LNs (35). Tumor-induced DC dysfunction may further limit the number of activated tumor antigen-bearing DCs that exit the tumors, as many tumors release immunosuppressive factors and chemokines that interfere with DC activation and maturation (36). Further work is required to distinguish between these two possibilities.
Our work demonstrates that CD8+ T cell memory induced in a peripheral LN drainage bed leads to the seeding of memory cells in distinct LN beds, even though these compartments were devoid of injected DCs or Ag-specific CD8+ primary immune responders. Our data show that primary responses in LN occur only in the draining node, and, therefore, involve only a small number of both DCs and responding T cells. However, from the point of view of immunotherapy, distribution of T cell memory throughout many LN compartments may improve the likelihood of responding to newly arising cutaneous metastases at distant sites. On the other hand, because control of lung tumor outgrowth is unaffected by the presence or absence of peripheral LN in albino mice, these data suggest that treatment of tumors in highly vascularized organs may be best achieved by immunizations that maximize the splenic T cell response.
In summary, our results provide strong evidence for nonoverlapping populations of LN- and spleen-resident CD8+ memory T cells that are differentially involved in controlling tumors that grow in different body sites. Furthermore, we demonstrated that these populations are differentially induced depending on the route of immunization with peptide-pulsed DCs. These data have significant clinical implications, providing a basis for improvements in tumor immunotherapy as well as an understanding of T cell homing and regional immunity in general.
Acknowledgments
We thank Drs. C.L. Slingluff, Jr. and K. Tung for suggestions and insightful discussions.
These studies were supported by U.S. Public Health Service grants CA78400 (to V.H. Engelhard), HD37104, and DK58891 (both to Y.-X. Fu). S.L. Sheasley was supported by grant GM07627, and D.W. Mullins was a fellow of the American Cancer Society.
The online version of this article includes supplemental material.
Abbreviations used in this paper: AxLN, axillary LN; CFSE, 5-carboxyfluorescein diacetate, succinimidyl ester; hTyrVac, recombinant vaccinia virus encoding human tyrosinase; InLN, inguinal LN; MLN, mesenteric LN; PulLN, pulmonary LN.
References
- 1.Bhardwaj, N., A. Bender, N. Gonzalez, L.K. Bui, M.C. Garrett, and R.M. Steinman. 1995. Stimulation of human anti-viral CD8+ cytolytic T lymphocytes by dendritic cells. Adv. Exp. Med. Biol. 378:375–379. [DOI] [PubMed] [Google Scholar]
- 2.Mullins, D.W., T.N. Bullock, T.A. Colella, V.V. Robila, and V.H. Engelhard. 2001. Immune responses to the HLA-A*0201-restricted epitopes of tyrosinase and glycoprotein 100 enable control of melanoma outgrowth in HLA-A*0201-transgenic mice. J. Immunol. 167:4853–4860. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, R.M., and M. Dhodapkar. 2001. Active immunization against cancer with dendritic cells: the near future. Int. J. Cancer. 94:459–473. [DOI] [PubMed] [Google Scholar]
- 4.Steinman, R.M., K. Inaba, S. Turley, P. Pierre, and I. Mellman. 1999. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum. Immunol. 60:562–567. [DOI] [PubMed] [Google Scholar]
- 5.Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- 6.Cyster, J.G. 1999. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J. Exp. Med. 189:447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto, F., and A. Lanzavecchia. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177:134–140. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, J.J., and E.C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336–341. [DOI] [PubMed] [Google Scholar]
- 9.Rott, L.S., M.J. Briskin, D.P. Andrew, E.L. Berg, and E.C. Butcher. 1996. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with β7 integrins and memory differentiation. J. Immunol. 156:3727–3736. [PubMed] [Google Scholar]
- 10.Gunzer, M., and S. Grabbe. 2001. Dendritic cells in cancer immunotherapy. Crit. Rev. Immunol. 21:133–145. [PubMed] [Google Scholar]
- 11.Eggert, A.A., M.W. Schreurs, O.C. Boerman, W.J. Oyen, A.J. de Boer, C.J. Punt, C.G. Figdor, and G.J. Adema. 1999. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 59:3340–3345. [PubMed] [Google Scholar]
- 12.Okada, N., M. Tsujino, Y. Hagiwara, A. Tada, Y. Tamura, K. Mori, T. Saito, S. Nakagawa, T. Mayumi, T. Fujita, et al. 2001. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br. J. Cancer. 84:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong, L., D. Brockstedt, C. Benike, L. Wu, and E.G. Engleman. 2001. Dendritic cells injected via different routes induce immunity in cancer patients. J. Immunol. 166:4254–4259. [DOI] [PubMed] [Google Scholar]
- 14.Serody, J.S., E.J. Collins, R.M. Tisch, J.J. Kuhns, and J.A. Frelinger. 2000. T cell activity after dendritic cell vaccination is dependent on both the type of antigen and the mode of delivery. J. Immunol. 164:4961–4967. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, J.M., Q. Yu, S.T. Piraino, S.E. Pennington, S. Shankara, L.A. Woodworth, and B.L. Roberts. 1999. Induction of anti-tumor immunity using dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J. Immunol. 163:699–707. [PubMed] [Google Scholar]
- 16.Boczkowski, D., S.K. Nair, J.H. Nam, H.K. Lyerly, and E. Gilboa. 2000. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 60:1028–1034. [PubMed] [Google Scholar]
- 17.Russo, V., S. Tanzarella, P. Dalerba, D. Rigatti, P. Rovere, A. Villa, C. Bordignon, and C. Traversari. 2000. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc. Natl. Acad. Sci. USA. 97:2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotera, Y., K. Shimizu, and J.J. Mule. 2001. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 61:8105–8109. [PubMed] [Google Scholar]
- 19.Lambert, L.A., G.R. Gibson, M. Maloney, B. Durell, R.J. Noelle, and R.J. Barth, Jr. 2001. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 61:641–646. [PubMed] [Google Scholar]
- 20.Bullock, T.N., D.W. Mullins, T.A. Colella, and V.H. Engelhard. 2001. Manipulation of avidity to improve effectiveness of adoptively transferred CD8+ T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J. Immunol. 167:5824–5831. [DOI] [PubMed] [Google Scholar]
- 21.Lappin, M.B., J.M. Weiss, V. Delattre, B. Mai, H. Dittmar, C. Maier, K. Manke, S. Grabbe, S. Martin, and J.C. Simon. 1999. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunol. 98:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colella, T.A., T.N.J. Bullock, L.B. Russell, D.W. Mullins, W. Overwijk, C.J. Luckey, R.A. Pierce, N.P. Restifo, and V.H. Engelhard. 2000. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J. Exp. Med. 191:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newberg, M.H., J.P. Ridge, D.R. Vining, R.D. Salter, and V.H. Engelhard. 1992. Species specificity in the interaction of CD8 with the α3 domain of MHC class I molecules. J. Immunol. 149:136–142. [PubMed] [Google Scholar]
- 24.Rinchik, E.M., J.P. Stoye, W.N. Frankel, J. Coffin, B.S. Kwon, and L.B. Russell. 1993. Molecular analysis of viable spontaneous and radiation-induced albino (c)-locus mutations in the mouse. Mut. Res. 286:199–207. [DOI] [PubMed] [Google Scholar]
- 25.Roberts, C.W., J.R. Shutter, and S.J. Korsmeyer. 1994. Hox11 controls the genesis of the spleen. Nature. 368:747–749. [DOI] [PubMed] [Google Scholar]
- 26.Bullock, T.N.J., T.A. Colella, and V.H. Engelhard. 2000. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 164:2354–2361. [DOI] [PubMed] [Google Scholar]
- 27.Rennert, P.D., P.S. Hochman, R.A. Flavell, D.D. Chaplin, S. Jayaraman, J.L. Browning, and Y.X. Fu. 2001. Essential role of lymph nodes in contact hypersensitivity revealed in lymphotoxin-α–deficient mice. J. Exp. Med. 193:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzo, A.L., R.A. Lake, D. Lo, L. Sherman, A. McWilliam, D. Nelson, B.W. Robinson, and B. Scott. 1999. Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 162:5838–5845. [PubMed] [Google Scholar]
- 29.Tang, M.L., D.A. Steeber, X.Q. Zhang, and T.F. Tedder. 1998. Intrinsic differences in L-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J. Immunol. 160:5113–5121. [PubMed] [Google Scholar]
- 30.Zitvogel, L., J.I. Mayordomo, T. Tjandrawan, A.B. DeLeo, M.R. Clarke, M.T. Lotze, and W.J. Storkus. 1996. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1–associated cytokines. J. Exp. Med. 183:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butcher, E.C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209–253. [DOI] [PubMed] [Google Scholar]
- 32.Nolte, M.A., A. Hamann, G. Kraal, and R.E. Mebius. 2002. The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunol. 106:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 34.Weninger, W., M.A. Crowley, N. Manjunath, and U.H. von Andrian. 2001. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194:953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemmi, H., M. Yoshino, H. Yamazaki, M. Naito, T. Iyoda, Y. Omatsu, S. Shimoyama, J.J. Letterio, T. Nakabayashi, H. Tagaya, et al. 2001. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 13:695–704. [DOI] [PubMed] [Google Scholar]
- 36.Gunzer, M., S. Janich, G. Varga, and S. Grabbe. 2001. Dendritic cells and tumor immunity. Semin. Immunol. 13:291–302. [DOI] [PubMed] [Google Scholar]