Abstract
Respiratory infections are the third leading cause of death worldwide. Illness is caused by pathogen replication and disruption of airway homeostasis by excessive expansion of cell numbers. One strategy to prevent lung immune–mediated damage involves reducing the cellular burden. To date, antiinflammatory strategies have affected both antigen-specific and naive immune repertoires. Here we report a novel form of immune intervention that specifically targets recently activated T cells alone. OX40 (CD134) is absent on naive T cells but up-regulated 1–2 d after antigen activation. OX40–immunoglobulin fusion proteins block the interaction of OX40 with its ligand on antigen-presenting cells and eliminate weight loss and cachexia without preventing virus clearance. Reduced proliferation and enhanced apoptosis of lung cells accompanied the improved clinical phenotype. Manipulation of this late costimulatory pathway has clear therapeutic potential for the treatment of dysregulated lung immune responses.
Keywords: costimulation, influenza, weight loss, inflammation
Introduction
According to the World Health Organization lower respiratory tract infections are the third most common cause of death (1). Influenza virus is a causative agent of both epidemic and pandemic respiratory viral illness with over 26,000 deaths attributed to the last outbreak in Britain alone (2). Though pivotal for viral clearance, over exuberant T cell responses result in airway occlusion (3, 4) and contribute significantly to pathology (5). Therefore, in the absence of universal vaccine candidates, inhibition of inflammatory T cells presents a novel strategy for therapeutic intervention in the treatment of influenza virus infection.
Several processes could be inhibited to thwart T cell proliferation including a reduction of antigen presentation by MHC, inhibition of costimulatory B7 molecules, or depletion of T cells themselves. Such strategies, however, affect both bystander and antigen-activated T cell repertoires. A more definitive approach would selectively inhibit only those T cells recently activated by antigen. OX40 (CD134) is an ideal candidate because unlike CD28, it is absent on naive T cells but up-regulated 1–2 d after antigen encounter (6). OX40 imparts a survival signal to the T cell preventing activation-induced cell death (7) but up-regulating anti-apoptosis gene expression (8) and cytokine production (9). Similarly, OX40 ligand (OX40L) is expressed only at low levels on resting APCs and is up-regulated after CD40–CD40L interactions (6, 10).
The critical role for OX40 is revealed in OX40-deficient mice where CD4+ T cell responses are abrogated during both Th1-driven responses to viral infection (11) and Th2-dominated allergic inflammation (12). Furthermore, inhibition of OX40 using soluble OX40 fusion proteins or antibody abrogates the detrimental effects of colitis in SCID mice (13), inflammatory bowel disease (14), and allergic encephalomyelitis (15).
Manipulation of this late costimulatory pathway has not been tested during virus-induced inflammation. We show that interruption of OX40/OX40L with an OX40–Ig fusion protein ameliorates influenza-driven T cell immunopathology and associated illness without preventing viral clearance. This beneficial outcome is directly linked to a reduced inflammatory infiltrate caused by an inhibition of proliferation and enhanced apoptosis. These findings show that OX40 costimulation plays a pivotal role in immunopathology to respiratory viral infection and highlights a novel therapeutic approach for the treatment of influenza-induced disease.
Materials and Methods
Mice and Virus.
8–12-wk-old female BALB/c (Harlan Olac Ltd.) were kept in pathogen-free conditions according to Home Office guidelines. Influenza A strain X31 (hemagglutinin [HA] titre 1024) was provided by A. Douglas (National Institute for Medical Research, London, United Kingdom).
Production of OX40 Fusion Proteins.
The generation of murine OX40–mIgG1 fusion protein (OX40–Ig) and the OX40–IgG4 fusion protein containing a mutation of Leu (235) to Glu in the Fc region of IgG4 has been previously described (15, 16). Constructs were deemed LPS-free by gas chromatography-mass spectrometry of fatty acid methyl esters. In brief, samples were derivatized using methanolic HCl and resulting fatty acid methyl esters dissolved in hexanes before column injection on a Stabilwax column (30 m × 0.25 mm internal diameter; Restek Corp.). Samples were analyzed using a temperature gradient of 150–250°C at a rate of 3°C/min. Characteristic retention times and spectra of known standards were used to identify any fatty acid methyl esters present in the samples.
Mouse Infections and Treatment.
On day 0 mice were anesthetized and intranasally infected with 50 HA units influenza virus. Mice were injected i.p. with 100 μg OX40–Ig fusion protein (Xenova Pharmaceuticals) or mouse IgG (Caltag) on alternate days. The weight of mice was monitored daily and illness severity was scored as follows: 0, healthy; 1, barely ruffled fur; 2, ruffled fur but active; 3, ruffled fur and inactive; 4, ruffled fur, inactive, and hunched; 5, dead. Mice were killed on various days after infection by injection of 3 mg pentobarbitone and exsanguinated via the femoral vessels. Some mice were challenged with 50 HA units influenza virus 3 wk later and killed on various days as indicated in the text.
Cell Recovery and Flow Cytometry.
Single cell suspensions from bronchoalveolar lavage fluid (BAL), lung tissue, and mediastinal LNs (MLNs) were stained for surface markers and intracellular cytokines and analyzed by flow cytometry as previously described (17). All antibodies were purchased from BD Biosciences except anti–OX40-FITC (Serotec). Influenza-specific CD8+ T cells were identified using an APC-labeled MHC class I tetramer (H-2d) loaded with the immunodominant peptide TYQRTRALV from the influenza nucleoprotein (National Institute of Allergy and Infectious Diseases Tetramer Facility). For apoptosis analysis, binding of PE-labeled annexin V was detected according to the manufacturer's instructions. All data were acquired on a FACSCalibur™ and 30,000 lymphocyte events were analyzed with CELLQuest™ Pro software (BD Biosciences).
5-bromo-2′-deoxyuridine (BrdU) Labeling and Detection.
Mice were treated with 0.8 mg/ml BrdU (Sigma-Aldrich) in their drinking water for 4 d. MLNs were taken and cells were stained with CyChrome anti-CD4 and anti–CD8-PE as previously described (17). After washing, cells were resuspended in 500 μl ice-cold 0.15 M NaCl. While mixing gently, 1,200 μl ice-cold 95% ethanol was added drop-wise. Cells were incubated at 4°C in the dark for 30 min before washing with cold PBS and fixing for 30 min at room temperature (RT) with 1% paraformaldehyde and 0.01% Tween-20. Cells were then centrifuged and incubated with 50 U DNase I (Sigma-Aldrich) in 4.2 mM MgCl2, 0.15 M NaCl, pH 5.0, for 10 min at RT. After centrifugation, cells were incubated in the dark at RT for 30 min with FITC anti-BrdU (BD Biosciences), washed in PBS/3%BSA/0.01% sodium azide, and analyzed on a FACSCalibur™. Lymphocytes positive for FITC anti-BrdU were detected with CELLQuest™ Software (BD Biosciences) and were identified to have undergone postthymic proliferation (18).
Lung Influenza Titer.
Clearance of influenza was assessed in lung homogenates 2, 4, 7, and 11 d after virus challenge. Homogenized cells were freeze thawed three times, centrifuged at 4,000 g, and supernatants were titrated in doubling dilutions on Madin Darby canine kidney cell monolayers in flat-bottomed 96-well plates. After incubation at RT for 3 h, samples were overlaid with methylcellulose and incubated for 72 h at 37°C. Cell monolayers were washed and incubated with anti-influenza antibody (Serotec), followed by anti–mouse–horseradish peroxidase (DakoCytomation) and infected cells were detected using 3-amino-9-ethylcarbazole (AEC; Sigma-Aldrich). Infectious units were enumerated by light microscopy and total plaque-forming units per lung were quantified (number of plaques × dilution factor × total volume of lung homogenate).
Statistics.
Statistical significance was evaluated using a two-tailed Student's t test assuming unequal variance with the Minitab software program. Where more than one comparison was made within an experiment, the Bonferroni correction was applied.
Results and Discussion
OX40–Ig Treatment Reduces Influenza-induced Weight Loss and Exuberant T Cell Inflammation During Infection.
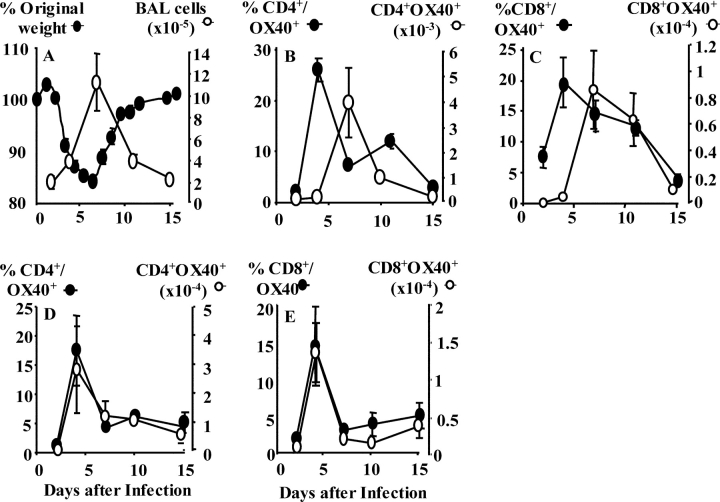
Intranasal influenza virus infection results in rapid accumulation of lymphocytes within the lung and airways (4). Maximal T cell expansion at 7 d coincides with peak weight loss (Fig. 1 A). The association between cellular infiltration and weight loss is characteristic of many lung viral infections (19, 20). The kinetics of OX40 expression by BAL CD4+ (Fig. 1 B) and CD8+ (Fig. 1 C) T cells followed a similar pattern in that the highest proportion was present 4 d after influenza infection. This profile was also evident in the lung (unpublished data). By calculating the total number of CD4+ OX40+ and CD8+ OX40+ T cells the highest numbers coincided with maximum weight loss at day 7. OX40 expression was also present in the lung-draining MLN. The proportion and total numbers of OX40-expressing CD4+ (Fig. 1 D) and CD8+ (Fig. 1 E) T cells peaked at 4 d.
Figure 1.
Influenza infection induces weight loss, pulmonary inflammation, and OX40 expression by T cells. (A) Mice were infected with influenza, BAL removed 2, 4, 7, 11, and 15 d after infection, and total viable cells were enumerated (right axis). Weight loss was monitored daily and expressed as percent of original body weight (left axis). Results represent mean values ± SEM from four mice per group. BAL (B and C) and MLNs (D and E) were removed from influenza-infected mice and cells were stained with OX40-FITC and APC-CD4 (B and D) or PercP-CD8 (C and E). Results represent the mean percent (left) and total number (right) of OX40+ T cells ± SEM from five individual mice and is representative of three separate experiments.
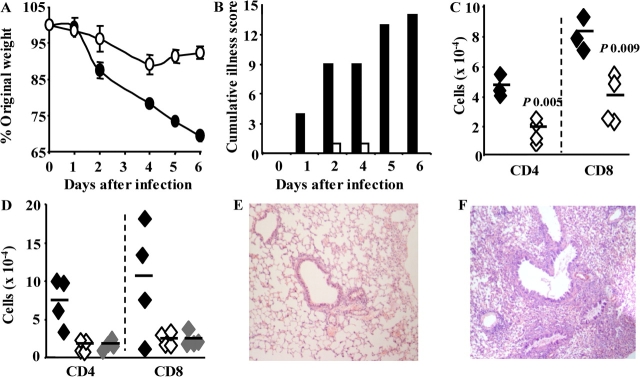
Because excess T cell infiltration into the virally infected lung compromises lung function and compliance, we next examined the effect of reducing T cell costimulation via OX40. Control-treated mice lost >25% of their initial body weight, whereas those treated with OX40–Ig did not (Fig. 2 A). Visual inspection revealed that control-treated, influenza-infected mice, especially at day 6, appeared hunched, immobile, and severely cachexic, whereas OX40–Ig-treated mice were consistently indistinguishable from uninfected controls (Fig. 2 B). The striking elimination of weight loss and illness severity was accompanied by a reduction in pulmonary inflammation including neutrophils (unpublished data) and CD4+ and CD8+ T cells (Fig. 2 C). The total number of OX40-expressing T cells was also reduced in line with the general reduction in inflammation (unpublished data). However, the percent of T cells expressing OX40 was unaffected. This was not surprising because OX40 expression is induced by CD28 and TCR signals and does not rely on interaction with OX40L. OX40L expression has been observed on some T cells cultured in vitro (21). Although reduced T cell numbers in our study may reflect depletion after OX40–Ig binding, we do not believe this to be the case because OX40–Ig did not enhance T cell death in vitro (unpublished data) and an alternative OX40–Ig fusion that does not fix complement or promote ADCC produced an identical effect (Fig. 2 D). The benefit of manipulating OX40 costimulation is clearly evident in hematoxylin and eosin–stained sections of lung tissue. Cellular infiltration around the airways and blood vessels was markedly reduced in OX40–Ig-treated mice compared with control IgG–treated mice (Fig. 2, E and F, respectively).
Figure 2.
OX40–Ig treatment prevents illness and T cell inflammation during influenza infection. (A) BALB/c mice (n = 4) were infected with influenza, treated with mouse IgG or OX40–Ig on alternate days, and weight loss was monitored and expressed as percent of original weight (P < 0.05 days 2–6). Illness was scored from 1–5 based on the degree of immobility, cachexia, and ruffled fur. The cumulative illness score was calculated by treatment group (B). (C) Total numbers of BAL CD4+ and CD8+ T cells 6 d after infection were determined by multiplying the percent of positive cells by flow cytometry with the total viable cells recovered from the BAL of IgG or OX40–Ig-treated mice. (D) In an identical experiment, BAL CD4+ and CD8+ T cell numbers were determined in mice treated with IgG, OX40–Ig, and OX40–mutIgG (gray symbols). Open symbols, OX40–Ig-treated mice; closed symbols, control IgG–treated mice. E and F show representative hematoxylin and eosin–stained lung sections 7 d after influenza infection in OX40–Ig- (E) or IgG- (F) treated mice. All experiments were repeated two to three times with five mice per group.
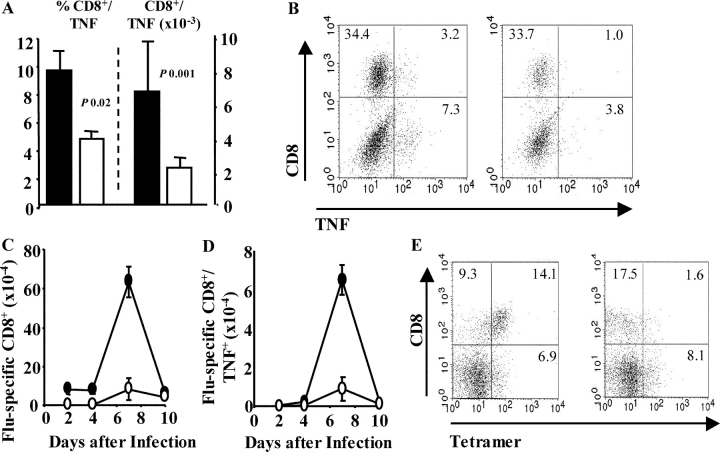
Using flow cytometry and intracellular cytokine staining, the number and proportion of CD8+ T cells producing TNF were found to be reduced in OX40–Ig-treated mice (Fig. 3, A and B) . Tetramer-binding CD8+ T cells (Fig. 3, C and E) and their production of TNF (Fig. 3 D) were severely reduced by OX40–Ig treatment. Although TNF is greatly increased systemically in some viral infections, we did not observe elevated serum TNF by ELISA or cytometric bead array after influenza virus infection (unpublished data). Anti-TNF antibodies reduce weight loss and cell recruitment during influenza infection (5). Precisely how this works, however, is unclear. Influenza virus replication is restricted to the respiratory epithelium. In this situation we believe that TNF indirectly causes weight loss by enhancing cell recruitment and proliferation leading to airway occlusion. These results imply that OX40 signaling plays a critical role during T cell–mediated immunopathology elicited by influenza infection.
Figure 3.
OX40–Ig reduces TNF and tetramer binding of CD8+ T cells. (A) Influenza-infected mice were treated with IgG or OX40–Ig and lung cells were stained for CD8 and intracellular TNF. Total numbers were calculated by multiplying total viable cells by the percent of CD8+/TNF+ cells. Mean percent (left) and total numbers (right) ± SEM from five mice per group are shown. Representative plots of lung CD8 versus TNF in lymphocytes 7 d after infection in IgG- (left) or OX40–Ig- (right) treated mice (B). (C and E) Lung cells were removed from IgG- or OX40–Ig-treated, influenza-infected mice 0, 2, 5, 7, and 10 d after infection and stained with anti–CD8-PercP, tetramer-APC, and anti–TNF-FITC. Total numbers were calculated by total viable cells × percent CD8+/Tetramer+ (C) or CD8+/Tetramer+/TNF+ (D). Results are mean values ± SEM of five mice per group. Representative plots of CD8 versus tetramer are shown in E in IgG- (left) or OX40–Ig- (right) treated mice 7 d after infection. All results are representative of at least two independent experiments containing five mice per group. Open symbols, OX40–Ig-treated mice; closed symbols, IgG-treated mice.
Delayed OX40–Ig Treatment Inhibits Weight Loss in Influenza-infected Mice with Established Illness and Does Not Affect Viral Clearance or Recall Responses.
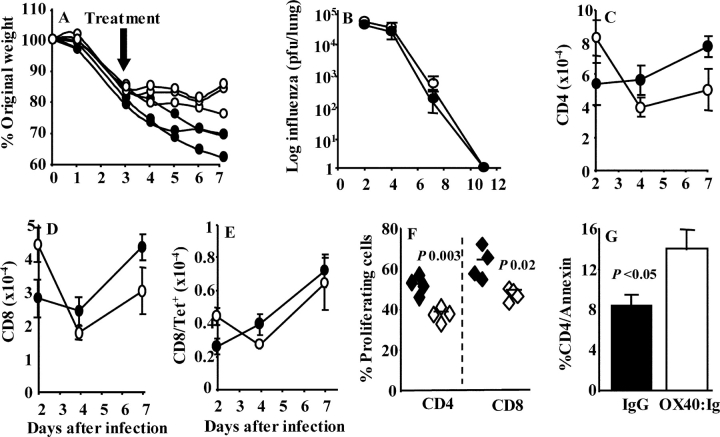
Next, we delayed OX40–Ig treatment during influenza virus infection until the mice had lost 20% of their original weight. Such delayed treatment still reversed the decline of weight compared with controls (Fig. 4 A; P < 0.05 days 5–7) and mice appeared perfectly healthy. Plaque assays performed on lung homogenates in a time course study did not reveal any delay in influenza virus clearance when OX40–Ig was administered on day 0 (Fig. 4 B) or 3 d after infection (unpublished data).
Figure 4.
OX40–Ig reduces established illness and T cell proliferation, increases apoptosis, but does not inhibit viral clearance or recall responses. (A) Mice (n = 3) were infected with influenza virus on day 0 and treated with IgG or OX40–Ig on days 3, 4, and 5 after infection. Weight loss is expressed as percent of original weight. (B) Mice (n = 4) were infected with influenza and treated with either IgG or OX40–Ig. On days 2, 5, 7, and 11 after infection, lung tissue was homogenized and viral titre was quantified by plaque assay. (C–E) Mice (n = 4) were infected with influenza and treated with IgG or OX40–Ig as in B. 3 wk later mice were challenged with influenza and on days 2, 5, and 7 after infection, CD4+ (C), CD8+ (D), and CD8+ tetramer+ (E) T cells were enumerated in lung tissue. (F and G) Proliferating MLN CD4 and CD8 T cells (F, by BrdU incorporation) and apoptotic annexin V–stained lung cells (G) were assessed in control- or OX40–Ig-treated mice 4 and 7 d, respectively, after influenza infection. Closed symbols, IgG control; open symbols, OX40–Ig. Mean values ± SEM are shown and are representative of two separate experiments.
The rather surprising lack of an effect of OX40–Ig on virus clearance might be partially explained by stimulation of B cells. OX40 interaction with OX40L imparts a bidirectional signal to the T cell and the APC (22). Although soluble OX40 fusion proteins reduce the signal to the T cell, signaling to the APC remains intact and may even be enhanced. OX40–Ig treatment may therefore induce antiviral antibodies and elicit compensatory effects. However, analysis of influenza-specific antibody 4, 7, 11, 15, and 19 d after infection showed no differences in nasal IgA responses or serum IgG1 and IgG2a (unpublished data).
The ability of mice to respond to reinfection was not affected by OX40–Ig treatment despite the significant reduction in T cell immunity during the first infection. A time course analysis after rechallenge did not reveal any statistical differences between the numbers of CD4+ (Fig. 4 C) or CD8+ (Fig. 4 D) T cells and those binding MHC class I tetramer (Fig. 4 E). In both OX40–Ig-treated and control mice influenza virus was cleared 5 d after rechallenge. On day 2, virus titres were similar (1,080 ± 288 for control and 1,546 ± 246 plaque-forming units/lung in the OX40–Ig-treated group). The lack of an effect on recall responses is surprising and may suggest that sufficient immune memory was generated during the first infection. Influenza virus promotes a very large inflammatory response. It is likely that not all of the inflammatory cells are required to contain infection. The reduced inflammatory infiltrate may therefore still contain enough cells to seed the memory pool. No studies to date have addressed the number of antigen-specific cells required to eliminate a viral infection. In this study we show that a threefold reduction in T cells remains efficient and does not cause disease. Alternatively, efficient rechallenge responses may reflect recruitment of naive cells or the fact that OX40–Ig treatment was only administered to day 10 during the first infection. After day 10 some memory T cell responses may have been generated.
Impaired T Cell Responses in OX40–Ig-treated Mice Is a Consequence of Reduced Proliferation and Enhanced Apoptosis.
The reduction of T cells by OX40–Ig could represent reduced proliferation or increased apoptosis. Rather than artificially examining proliferation in vitro, we gave mice BrdU in their drinking water during infection. BrdU is incorporated into proliferating cells and can be detected by flow cytometry using an anti-BrdU antibody. Analysis of MLN cells 4 d after infection revealed that OX40–Ig treatment had significantly reduced both CD4+ and CD8+ T cell proliferation (Fig. 4 F). This effect was only observed at the peak of proliferation and not during the later stages of infection (unpublished data).
OX40 binding to its ligand induces the anti-apoptotic proteins Bcl-2 and Bcl-xL (8) and prevents activation-induced cell death (23). Reduced T cell numbers in OX40–Ig-treated mice may therefore result from increased cell death. The frequency of apoptotic CD4+ and CD8+ T cells was identified by annexin V staining and flow cytometry. Administration of OX40–Ig significantly enhanced the proportion of apoptotic CD4+ (Fig. 4 G) but not CD8+ T cells (unpublished data) in the airways during the later stages of infection (days 7 and 10), implying that OX40 costimulation is required to prolong pulmonary T cell survival.
The reduction in T cell infiltrate is similar to that observed in influenza virus–infected OX40−/− mice (11). Using such mice it is difficult to ascertain whether the improved outcome is due to reduced T cell function or an alteration of APC activation through OX40L. Our studies compliment and extend this in several important ways: (a) we show that both CD4+ and CD8+ T cells are affected, (b) that inflammatory cytokines are reduced, and (c) that proliferation is reduced and cell death increased.
The reduction of both T cell numbers and proinflammatory cytokine secretion after OX40–Ig treatment raises the question of why lung viral replication is still effectively controlled? There are three plausible explanations: (a) innate immune mechanisms such as NK cells, which were not affected by OX40–Ig treatment (unpublished data), may mediate viral clearance. However, the role of NK cells in immunity to influenza virus infection is unclear. Increased susceptibility to influenza virus in the senescence-accelerated mouse is associated with impaired activity of NK cells, but CD8+ T cells are also defective in this model (24). (b) Influenza virus is highly inflammatory and a moderate reduction in the vigor of the inflammatory response might be beneficial without affecting virus clearance. This hypothesis is consistent with the efficient control of other viruses in OX40 knockout mice (25, 11). (c) Because OX40 is expressed 1–2 d after antigen activation, influenza virus infection might be controlled by earlier T cell–APC interactions.
The delayed expression of OX40 makes it an ideal target during immunopathological conditions because it enables initial T cell priming and containment of viral replication. In contrast, blockade of CD28, which is constitutively expressed by T cells, significantly delays influenza clearance (26). The lung microenvironment requires careful immune homeostasis. Excessive inflammation compromises gaseous exchange by occluding the airways and inducing mucus production. This is more apparent in the lungs than other mucosal sites. The gut, for example, can accommodate relatively large cell numbers while retaining its primary function. Evidence from murine models suggests that a reduction, but not elimination, of the lung inflammatory infiltrate is beneficial. Depletion of inflammatory cytokines (5), CD4+ or CD8+ T cells, or T cell subsets (27) all reduce weight loss without preventing virus clearance. Collectively, this implies that the problem is caused by the number of T cells present and not their phenotype. Manipulation of OX40 therefore presents a novel therapeutic strategy for treatment of immunopathological conditions by reducing the number of activated T cells while leaving the naive repertoire intact.
Acknowledgments
The authors would like to thank Paul Hitchen (Imperial College London) for help with determining the LPS content of OX40L–Ig fusion proteins, Lorraine Lawrence (Department Leukocyte Biology, Imperial College) for processing of samples for analysis of BrdU, and Professor Ita Askonas for critical review of the manuscript.
This work was supported by The Medical Research Council and The National Asthma Campaign.
Abbreviations used in this paper: BAL, bronchoalveolar lavage fluid; BrdU, 5-bromo-2′-deoxyuridine; HA, hemagglutinin; MLN, mediastinal LN; OX40L, OX40 ligand; RT, room temperature.
References
- 1.Murray, C.J., and A.D. Lopez. 1997. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 349:1436–1442. [DOI] [PubMed] [Google Scholar]
- 2.Curwen, M., K. Dunnell, and J. Ashley. 1990. Hidden influenza deaths. Br. Med. J. 300:896. [Google Scholar]
- 3.Doherty, P.C., D.J. Topham, R.A. Tripp, R.D. Cardin, J.W. Brooks, and P.G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105–117. [DOI] [PubMed] [Google Scholar]
- 4.Enelow, R.I., A.Z. Mohammed, M.H. Stoler, A.N. Liu, J.S. Young, Y.H. Lou, and T.J. Braciale. 1998. Structural and functional consequences of alveolar cell recognition by CD8(+) T lymphocytes in experimental lung disease. J. Clin. Invest. 102:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussell, T., A. Pennycook, and P.J.M. Openshaw. 2001. Inhibition of tumour necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566–2573. [DOI] [PubMed] [Google Scholar]
- 6.Gramaglia, I., A.D. Weinberg, M. Lemon, and M. Croft. 1998. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161:6510–6517. [PubMed] [Google Scholar]
- 7.Maxwell, J.R., A. Weinberg, R.A. Prell, and A.T. Vella. 2000. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J. Immunol. 164:107–112. [DOI] [PubMed] [Google Scholar]
- 8.Rogers, P.R., J. Song, I. Gramaglia, N. Killeen, and M. Croft. 2001. TI-OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 15:445–455. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, S., K.M. Toellner, C. Raykundalia, M. Goodall, and P. Lane. 1998. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 188:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuber, E., M. Neurath, D. Calderhead, H.P. Fell, and W. Strober. 1995. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 2:507–521. [DOI] [PubMed] [Google Scholar]
- 11.Kopf, M., C. Ruedl, N. Schmitz, A. Gallimore, K. Lefrang, B. Ecabert, B. Odermatt, and M.F. Bachmann. 1999. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 11:699–708. [DOI] [PubMed] [Google Scholar]
- 12.Jember, A.G., R. Zuberi, F.T. Liu, and M. Croft. 2001. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J. Exp. Med. 193:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malmstrom, V., D. Shipton, B. Singh, A. Al-Shamkhani, M.J. Puklavec, A.N. Barclay, and F. Powrie. 2001. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J. Immunol. 166:6972–6981. [DOI] [PubMed] [Google Scholar]
- 14.Higgins, L.M., S.A. McDonald, N. Whittle, N. Crockett, J.G. Shields, and T.T. Macdonald. 1999. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 162:486–493. [PubMed] [Google Scholar]
- 15.Weinberg, A.D., K.W. Wegmann, C. Funatake, and R.H. Whitham. 1999. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 162:1818–1826. [PubMed] [Google Scholar]
- 16.Taylor, L., M. Bachler, I. Duncan, S. Keen, R. Fallon, C. Mair, T.T. McDonald, and H. Schwartz. 2002. In vitro and in vivo activities of OX40 (CD134)-IgG fusion protein isoforms with different levels of immune-effector functions. J. Leukoc. Biol. 72:522–529. [PubMed] [Google Scholar]
- 17.Hussell, T., L.C. Spender, A. Georgiou, A. O'Garra, and P.J.M. Openshaw. 1996. Th1 and Th2 cytokine induction in pulmonary T-cells during infection with respiratory syncytial virus. J. Gen. Virol. 77:2447–2455. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Q., N. Strong, and N. Killeen. 2001. Homeostatic competition among T cells revealed by conditional inactivation of the mouse Cd4 gene. J. Exp. Med. 194:1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alwan, W.H., F.M. Record, and P.J.M. Openshaw. 1992. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus: comparison with the effects of CD8+ cells. Clin. Exp. Immunol. 88:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson, P.G., S. Hawke, and C.R. Bangham. 1996. Protection against lethal influenza virus encephalitis by intranasally primed CD8+ memory T cells. J. Immunol. 157:3065–3073. [PubMed] [Google Scholar]
- 21.Baum, P.R., R.B. Gayle, III, F. Ramsdell, S. Srinivasan, R.A. Sornensen, M.L. Watson, M.F. Seldin, E. Baker, G.R. Sutherland, and K.N. Clifford. 1994. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 13:3992–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuber, E., and W. Strober. 1996. The T cell–B cell interaction via OX40-OX40L is necessary for the T cell–dependent humoral immune response. J. Exp. Med. 183:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg, A.D., A.T. Vella, and M. Croft. 1998. OX-40: life beyond the effector T cell stage. Semin. Immunol. 10:471–480. [DOI] [PubMed] [Google Scholar]
- 24.Dong, L., I. Mori, M.J. Hossain, and Y. Kimura. 2000. The senescence-accelerated mouse shows aging-related defects in cellular but not humoral immunity against influenza virus infection. J. Infect. Dis. 182:391–396. [DOI] [PubMed] [Google Scholar]
- 25.Pippig, S.D., C. Pena-Rossi, J. Long, W.R. Godfrey, D.J. Fowell, S.L. Reiner, M.L. Birkeland, R.M. Locksley, A.N. Barclay, and N. Killeen. 1999. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40). J. Immunol. 163:6520–6529. [PubMed] [Google Scholar]
- 26.Lumsden, J.M., J.M. Roberts, N.L. Harris, R.J. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79–85. [DOI] [PubMed] [Google Scholar]
- 27.Walzl, G., S. Matthews, S. Kendall, J.C. Gutierrez-Ramos, A.J. Coyle, P.J. Openshaw, and T. Hussell. 2001. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J. Exp. Med. 193:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]