Abstract
Toll-like receptor (TLR)4 has recently been shown to reside in the Golgi apparatus of intestinal crypt epithelial m-ICcl2 cells, colocalizing with internalized lipopolysaccharide (LPS). Here we demonstrate that disruption of the integrity of the Golgi apparatus significantly reduced LPS-mediated nuclear factor κB activation. Also, the TLR4 adaptor protein MyD88 and the serine/threonine kinase IRAK-1 were rapidly recruited to the Golgi apparatus upon stimulation. LPS-mediated activation required lipid raft formation and intact clathrin-dependent internalization. In contrast to macrophages, prevention of ligand internalization by use of LPS-coated beads significantly impaired recognition by epithelial cells. The localization of TLR4 to the Golgi apparatus was abrogated by expression of a genetically modified form of the TLR4 binding chaperone gp96. Thus, our data provide evidence that in contrast to the situation in macrophages, LPS recognition in intestinal epithelial cells may occur in the Golgi apparatus and require LPS internalization.
Keywords: endotoxin, Golgi apparatus, innate immunity, mucosal immunology
Introduction
The innate immune system includes a family of transmembrane receptors that mediate recognition of conserved microbial structures to activate the host immune defenses (1). The best studied member of this family is Toll-like receptor (TLR)4. TLR4 acts as homodimer together with the accessory protein MD-2 and mediates recognition of LPS, a constituent of the outer cell membrane of all Gram-negative bacteria (2).
Although the expression of most TLRs seems to be largely restricted to cells of the myeloid lineage such as dendritic cells and monocytes/macrophages, some TLRs such as TLR4 have also been found in nonprofessional immune cells such as endothelial cells, fibroblasts, adipocytes, and epithelial cells. In addition, the cellular localization of TLRs seems to be restricted to specific subcellular compartments. TLR4 and TLR2 were demonstrated on the surface of macrophages and dendritic cells (3, 4). TLR2 is recruited to the phagosome upon interaction with its ligand yeast zymosan (3). In contrast, TLR9 resides in an intracellular compartment and requires internalization of its ligand, bacterial DNA, for recognition (5). Finally, expression of TLR5, the receptor for bacterial flagellin, might be restricted to the basolateral surface membrane of polarized intestinal epithelial cells and stimulation requires transepithelial transport (6). Thus, the involvement of cellular transport might represent a regulatory barrier to avoid unintended stimulation, for example, through flagellin produced by bacteria of the intestinal resident microflora (7).
The intestinal mucosal surfaces are continuously exposed to large amounts of bacterial products such as LPS. The fact that the intestinal epithelium seems to be unresponsive to intestinal luminal LPS was explained by low or absent expression of TLR4 and MD-2 as well as the coreceptor molecule CD14 in cells constituting the intestinal mucosal surface (8, 9). However, bacterial intestinal infection induces the rapid development of local inflammation and immune activation as, for example, seen during infection with the invasive pathogen Salmonella. This paradox might be explained by the surprising observation that m-ICcl2 cells derived from the crypt epithelium of the murine small intestine exhibit a highly LPS-susceptible phenotype and express CD14, TLR4, and MD-2 (10, 11). Interestingly, immunohistology for TLR4 revealed a predominant paranuclear localization of TLR4 identified as the Golgi apparatus. Furthermore, similar to other cell types, m-ICcl2 cells were shown to internalize LPS, which rapidly colocalized with intracellular TLR4 (11, 12). Because close interaction between LPS and TLR4 is required for recognition, LPS internalization might therefore facilitate physical proximity with its intracellular cognate receptor (13).
In this study we have evaluated the functional importance of intracellular TLR4 for LPS-mediated immune activation using the model of intestinal crypt epithelial m-ICcl2 cells. We demonstrate the requirement of internalization, cell traffic, and intact function of the Golgi apparatus for LPS-mediated stimulation and show that the initiation of the TLR4-mediated signal transduction cascade occurs at the site of the Golgi apparatus. Finally, we provide evidence that the endoplasmic reticulum (ER)-resident heat shock protein, glycoprotein 96 (gp96; also called grp94 for glucose-regulated protein 94), is critically involved in the subcellular localization of TLR4 in m-ICcl2 cells.
Materials and Methods
Antibodies and Reagents.
The goat anti–TLR4 antibody, the rabbit anti–MyD88 antibody, the mouse anti–IL-1R–associated kinase (IRAK)-1 antibody, as well as the rabbit anti–p65 antibody were purchased from Santa Cruz Biotechnology, Inc. The rabbit anti–gp96 antiserum was obtained from StressGen Biotechnologies and the rat monoclonal anti–gp96 antibody was obtained from Neomarkers. The rabbit anti–TLR4 antiserum was recently described (13). The rat MTS510 antibody was provided by K. Miyake (University of Tokyo, Tokyo, Japan). Recombinant gp96 was obtained from Immatics Biotechnologies. The nuclear factor (NF)-κB reporter construct pBIIX-luciferase carrying two copies (2× NF-κB) of the κB sequences from the Igκ enhancer was provided by S. Ghosh (Yale University Medical School, New Haven, CT) and the MyD88-GFP expression plasmid pef-EGFP-MyD88m was provided by H. Wagner (Technische Universität München, München, Germany) (14). The gp96tm expression plasmid was provided by Z. Li (University of Connecticut School of Medicine, Farmington, CT) (15). Escherichia coli K12 D31m4 (Re) LPS and lipid A were purchased from List Biological Laboratories. Control experiments with this LPS preparation did not exhibit any stimulating activity on peritoneal macrophages from TLR4-deficient mice at the concentrations used in this study. Also, repurification by phenol extraction did not result in loss of stimulatory activity. BODIPY TR C5 Ceramide was purchased from Molecular Probes and murine recombinant IL-1β and TNF-α as well as anti–macrophage inflammatory protein (MIP)-2 were obtained from Nordic BioSite. If not stated otherwise, all reagents were purchased from Sigma-Aldrich.
Cell Culture.
RAW 264.7 cells and m-ICcl2 cells were cultured as previously described (10, 11). Transfection was performed using the Lipofectamine 2000 transfection reagent from Invitrogen according to the manufacturer's instructions. Stabile cell lines expressing the NF-κB luciferase reporter construct and the modified gp96 (gp96tm) were engineered by antibiotic selection using the neomycin analogue G418 and Hygromycin B (Invitrogen). All plasmid DNA for transfection experiments was prepared using the endotoxin-free plasmid kit (QIAGEN).
Cell Stimulation Assays.
Cells were seeded on culture plates coated with 2 mg/ml rat tail collagen type 1 diluted 1:100 in ethanol/water (60:40 vol/vol) and incubated for 6 d with medium changes every second day (10, 11). LPS or lipid A was vortexed, sonicated for 15 min, and added to the cells at the appropriate concentration. For the determination of luciferase activity, cells were stimulated for 2 h, washed with cold PBS, and incubated for 10 min in lysis buffer (Promega). Luminescence was recorded after the addition of substrate (Promega) using a TD 20/20 Luminometer (Turner Designs Instruments). If not stated otherwise, m-ICcl2 cells were exposed to the various drugs to be tested for 30 min before LPS stimulation. Cell viability was monitored using the CytoTox 96 cytotoxicity assay (Promega). Covalently linked LPS-coated beads were prepared as follows. 1,4-bis (2:3-epoxypropoxy) butane cross-linked 4% beaded agarose was incubated in 0, 1, and 100 μg/ml LPS in 0.1 M carbamate buffer, pH 11.0, for 24 h. Subsequently, beads were blocked with ethanolamine and extensively washed with 0.1 M glycin and 0.5% Tween 20 to remove residual soluble LPS. Finally, LPS-coated beads were washed in PBS, pH 7.4, and added to confluent cells at a concentration of 3.3 mg beaded agarose per cm2. Cell culture supernatant was harvested 6 h after stimulation and stored at −20°C. The quantitative analysis of the MIP-2 was performed using an ELISA technique as recently described (16). To reverse the effect of methyl-β-cyclodextrin (MβCD), cells were washed and incubated for 1.5 h in medium containing 100 μM 5-cholesten-3β-ol (Sigma-Aldrich) before LPS stimulation. Potassium depletion was performed by incubation of polarized cells for 5 min at 37°C in hypotonic medium (DMEM/water, 1:1). Subsequently, cells were washed and incubated in medium (100 mM NaCl, 50 mM Hepes, pH 7.4, 1 mg/ml bovine serum albumin) in the absence or presence of potassium (10 mM KCl) and stimulated with 10 ng/ml LPS for 2 h. Potassium depletion itself did not lead to significant cell stimulation or reduction of cell viability.
Immunoblotting and Immunoprecipitation.
Cells were grown on collagen-coated 24-well plates for 6 d. The supernatant was removed and 200 μl lysis buffer (3:1 WB/SB vol/vol, SB: 250 mM Tris, pH 6.5, 8% SDS, 40% glycerol and WB: 50 mM Tris, pH 7.4, 120 mM NaCl) supplemented with proteinase inhibitor cocktail Complete Mini (Roche Diagnostics) was added. Cells were sonicated and 5 μg total protein per lane was separated on a 10% acrylamide gel (DC Protein Assay; Bio-Rad Laboratories). Proteins were blotted on nitrocellulose and stained for gp96 using the rat monoclonal anti–gp96 antibody (Neomarkers) and for actin using a rabbit polyclonal antibody (Sigma-Aldrich). For coimmunoprecipitation analysis, 0.5 × 107 cells were washed in ice-cold PBS, lysed in buffer I (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Nonidet P40, 0.25% sodium-deoxycholate, proteinase inhibitor cocktail), sonified, centrifuged, and precleared with 50 μl protein A agarose (Roche Diagnostics) for 3 h at 4°C. 5 μg polyclonal anti-TLR4 antiserum was added to the precleared supernatant for 1 h at 4°C followed by the addition of 50 μl protein A agarose and incubation overnight at 4°C. Bound protein was washed twice in lysis buffer followed by two washing steps in buffer II (similar to buffer I but 250 mM NaCl, 0.1% Nonidet P40, 0.025% sodium-deoxycholate) and III (similar to buffer I but 0.1% Nonidet P40, 0.025% sodium-deoxycholate). Agarose-bound protein was separated on a 7% SDS acrylamide gel. After transfer on nitrocellulose, filters were incubated with the rabbit anti–TLR4 antiserum (1:500), the rat anti–gp96 monoclonal antibody (1:200), or the rabbit anti–gp96 antiserum (1:1,000) for 2 h at room temperature (RT) followed by incubation with the peroxidase-conjugated secondary antibody. Staining was detected using the Renaissance chemiluminescence kit (NEN Life Science Products) in combination with Hyperfilm ECL (Amersham Biosciences).
Immunofluorescence Staining Procedure.
Immunostaining for TLR4 and the NF-κB subunit p65 was performed as recently described (16). For detection of MyD88 and IRAK-1, cells were fixed with 3.5% paraformaldehyde for 15 min at RT and blocked with 10% normal serum. The anti-MyD88 antiserum was preincubated in 25% normal mouse serum in 4% BSA/PBS for 1 h and added to the cells for 45 min at RT. The mouse monoclonal anti–IRAK-1 antibody was added to the cells in 0.2% saponin 4% BSA/PBS. Slides were washed and incubated for 45 min with the appropriate conjugated secondary antibody (Jackson ImmunoResearch Laboratories). For double immunostaining, cells were subsequently incubated with the other primary antibody for 45 min at RT followed by incubation with the appropriate secondary antibody (Jackson ImmunoResearch Laboratories). The Golgi apparatus was identified using BODIPY TR C5 Ceramide complexed to BSA according to the manufacturer's instructions. Counterstaining was performed using Hoechst 33258 (DAPI) nuclear stain purchased from Pierce Chemical Co. Slides were mounted in Vectastain (Vector Laboratories) and analyzed under a Nikon Eclipse E400 microscope (Global Medical Instrumentation) connected to a Hamamatsu C4742-98 digital camera (Hamamatsu). Staining for FACS® analysis was performed on ice using the rat monoclonal MTS510 antibody or an IgG2a isotype control (BD Biosciences). Analysis was conducted with a FACScan™ cytometer (Becton Dickinson). For confocal laser scanning microscopy, images were collected on an inverted Nikon Diaphot 200 microscope attached to a Multiprobe 2001 confocal laser scanning microscope (Molecular Dynamics) equipped with a krypton/argon laser. Stacks of images were collected through a ×100 objective (numerical aperture 1.4) using a z-step of 0.15 μm and analyzed using the Volume Workbench software (Molecular Dynamics) based on maximum intensity projections.
Statistical Analysis.
Results are given as the mean ± SD from at least three separate experiments. Statistical analyses were performed using the Student's t test. A P value <0.05 was considered significant.
Results
LPS-mediated TLR4 Activation Requires Intact Cell Traffic.
Initiation of cellular activation by intracellular TLR4 would require LPS internalization and transport to facilitate receptor–ligand interaction (13). LPS stimulation of m-ICcl2 cells was therefore analyzed under various conditions interfering with internalization and cell traffic. Due to disturbance of cytokine secretion under these conditions, stable transfected m-ICcl2 cells expressing an NF-κB luciferase reporter gene construct were used to analyze LPS-mediated cellular activation. Incubation of the cells with 10 ng/ml LPS for 2 h induced a rapid response to LPS, with 20-fold increase in luciferase activity (not depicted). The sensitivity of this reporter gene system was comparable to the induction of the chemokine MIP-2 in the cell culture supernatant after LPS stimulation (unpublished data and 11).
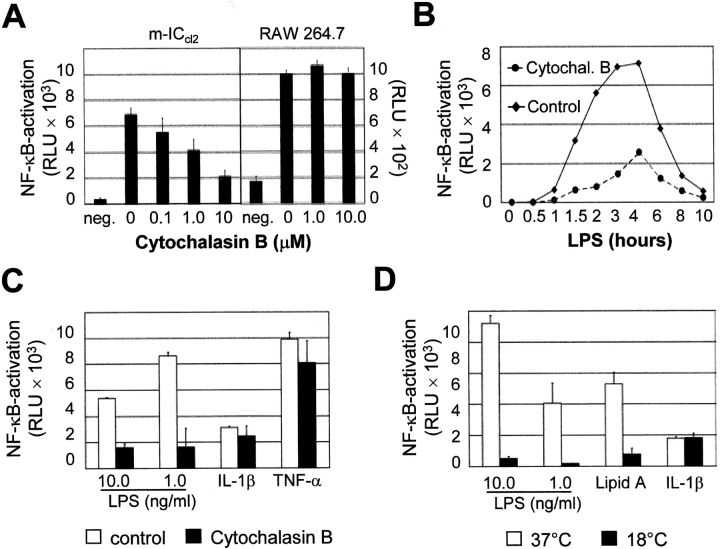
Preincubation of the cells with cytochalasin B, an inhibitor of actin polymerization, before LPS exposure led to a dose-dependent decrease in luciferase activity in m-ICcl2 cells, indicating the involvement of an actin-dependent process for LPS recognition. No significant reduction was observed in RAW 264.7 cells carrying the NF-κB luciferase reporter construct (Fig. 1 A). Similarly, a significant inhibitory effect on LPS-mediated stimulation of m-ICcl2 cells was found after preincubation with latrunculin A, another inhibitor of microfilament-mediated processes (unpublished data). The time course illustrates the profound inhibition of NF-κB activation after LPS stimulation in the presence of 10 μM cytochalasin B (Fig. 2 B). To assess the specificity of the cytochalasin-mediated inhibitory effect, m-ICcl2 cells preincubated with 10 μM cytochalasin B were stimulated with LPS, IL-1β, or TNF-α. Cytochalasin B inhibited the LPS-mediated cell activation whereas NF-κB stimulation through IL-1β or TNF-α was not significantly affected (Fig. 1 C). Also, microtubules are involved in cellular internalization and interaction of LPS with microtubule proteins has been reported (17, 18). However, preincubation with the microtubule inhibitor colchicine had no effect on LPS-mediated cell activation (unpublished data). LPS-mediated stimulation of m-ICcl2 cells was also analyzed at 18°C, a temperature known to block transport processes from endosomes to lysosomes and/or the Golgi complex (19, 20). Although a strong NF-κB activation after stimulation with LPS or lipid A at 37°C was noted, this response was almost abolished at 18°C. In contrast, cellular stimulation by IL-1β was maintained at lower temperatures and reached similar levels after 6 h at 18°C as compared with 2 h at 37°C (Fig. 1 D).
Figure 1.
Requirement of intact cell traffic for LPS-mediated cell activation. (A) Effect of cytochalasin B on LPS-induced cellular stimulation of m-ICcl2 cells and RAW 264.7 cells carrying an NF-κB luciferase reporter construct. Data are presented as relative light units (RLU). (B) Time course of luciferase production after LPS stimulation (10 ng/ml) in the presence or absence of cytochalasin B. (C) Comparison of the inhibitory effect of 10 μM cytochalasin B on cellular stimulation mediated by LPS, lipid A, IL-1β, or TNF-α. (D) Effect of the incubation temperature on LPS-mediated NF-κB stimulation. Stimulation was performed with 10.0 or 1.0 ng/ml LPS, 10 ng/ml lipid A, or 100 ng/ml IL-1β at 37 or 18°C for 2 or 6 h, respectively. (E) Comparison of MIP-2 secretion by macrophage-like RAW 264.7 cells and intestinal epithelial m-ICcl2 cells in response to soluble LPS (sLPS) versus LPS covalently linked to agarose beads. The data are presented as percent of MIP-2 secretion obtained after exposure to 1.0 ng/ml sLPS. Agarose beads were coated in the absence or presence of 1.0 or 100.0 μg/ml LPS. (F) The micrograph illustrates the situation of RAW 264.7 and m-ICcl2 cells exposed to agarose beads. ×200. (G) FACS® analysis for TLR4 on primary macrophages and m-ICcl2 cells using the rat monoclonal anti–TLR4/MD-2 antibody MTS510. Dotted line, isotype control.
Figure 2.
LPS-mediated NF-κB activation requires the formation of lipid rafts and intact clathrin-dependent internalization. (A and B) Dose-dependent effect of filipin (A) or MβCD (B) pretreatment on LPS-induced NF-κB activation. Note that subsequent replenishment of plasma membrane cholesterol completely reversed the inhibitory effect of MβCD. (D and E) Effect of the inhibition of the clathrin-dependent internalization by (C) monodansylcadaverin (MDC) and (D) chlorpromazine (CPZ) on LPS-induced NF-κB activation. (E) Effect of potassium depletion on LPS-mediated cell activation. Stimulation with IL-1β was added as a control. Data are presented as relative light units (RLU).
Macrophage-like RAW 264.7 cells and primary macrophages show marked surface expression of TLR4. In contrast, expression in m-ICcl2 cells was predominantly localized to the Golgi apparatus with little surface staining detected (Fig. 1 G; references 4 and 11). To test whether LPS internalization is required for activation of m-ICcl2 cells as opposed to direct surface membrane detection of LPS by RAW 264.7 cells, both cell types were analyzed in respect to their responsiveness to soluble LPS versus LPS covalently linked to agarose beads. The addition of soluble LPS resulted in significant cellular activation of both cell types, whereas only RAW 264.7 cells but not m-ICcl2 cells exhibited significant MIP-2 secretion upon incubation with beads coated at 1 μg/ml LPS (Fig. 2, E and F). Also, exposure of cells with beads coated at 100 μg/ml LPS resulted in a significantly higher response of macrophage-like RAW 264.7 cells as compared with intestinal epithelial m-ICcl2 cells (P < 0.001). The discrete increase in MIP-2 secretion observed in m-ICcl2 cells incubated with beads coated at high LPS concentrations might reflect stimulation by noncovalently linked LPS molecules released during the incubation period. Altogether, these data suggest an active role of internalization and cell traffic in the process of LPS-mediated activation of m-ICcl2 cells.
LPS-mediated Cellular Activation Requires the Presence of Lipid Rafts and Clathrin-dependent Internalization.
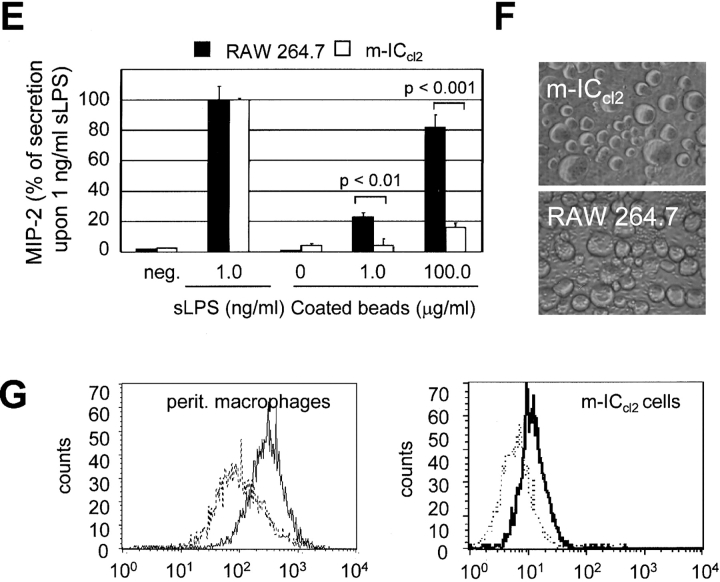
The presence of specialized detergent-insoluble plasma membrane microdomains, highly enriched in cholesterol and sphingolipids, has recently been implicated in the process of LPS recognition (21). The participation of these so-called lipid rafts in LPS-mediated cellular stimulation was analyzed using m-ICcl2 cells treated with MβCD and filipin. Both agents bind cholesterol and thereby impede the formation of lipid rafts. Pretreatment of m-ICcl2 cells with filipin or MβCD significantly reduced LPS-mediated NF-κB activation in a dose-dependent manner (Fig. 2, A and B). Whereas filipin forms a stable complex together with cholesterol, MβCD actively removes the lipid from the plasma membrane. Accordingly, the inhibitory effect of MβCD was reversed by the addition of cholesterol before LPS stimulation (Fig. 2 B). Thus, in accordance with a recently published report (21) using transfected Chinese hamster ovary cells, LPS-mediated cellular activation of epithelial m-ICcl2 cells requires the presence of intact lipid rafts.
Lipid rafts are involved in the recruitment of molecule complexes and the initiation of cell signaling and have been further implicated in the formation of endocytic vesicles. Both clathrin- as well as caveolin-dependent internalization requires the presence of lipid rafts on the plasma membrane (22). The fact that LPS-induced NF-κB activation was resistant to microtubule disruption provided evidence for an involvement of clathrin-coated pits because microtubuli have been shown to contribute to caveolae-mediated internalization (17). Indeed, inhibition of clathrin-mediated internalization by preincubation with monodansylcadaverin or chlorpromazine abolished the LPS-induced NF-κB activation (Fig. 2, C and D). Monodansylcadaverin acts as an inhibitor of the membrane-bound enzyme transglutaminase that actively participates in internalization via the clathrin-dependent pathway. The cationic amphiphilic drug chlorpromazine causes redistribution of the clathrin pit component AP-2 from the plasma membrane to endosomes. Also, potassium depletion significantly reduced LPS-mediated cell activation (Fig. 2 E). Cellular potassium depletion blocks the formation of clathrin-coated pits as previously demonstrated by electron microscopy. These findings demonstrate that LPS-mediated NF-κB activation in m-ICcl2 cells requires the formation of coated pits, suggesting a clathrin-dependent pathway of LPS internalization.
LPS-induced TLR4 Signaling Is Initiated at the Site of the Golgi Apparatus.
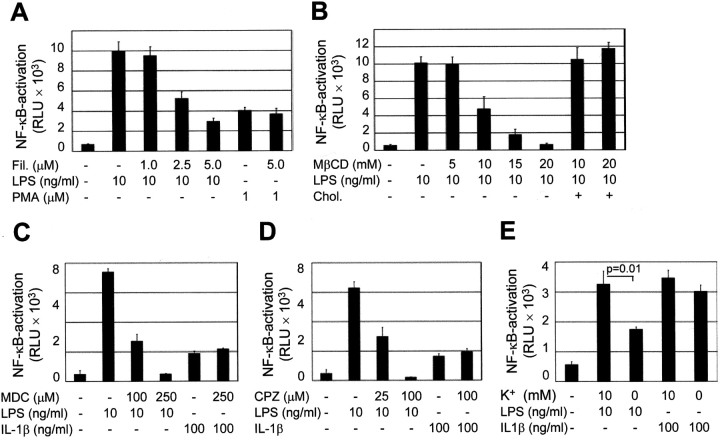
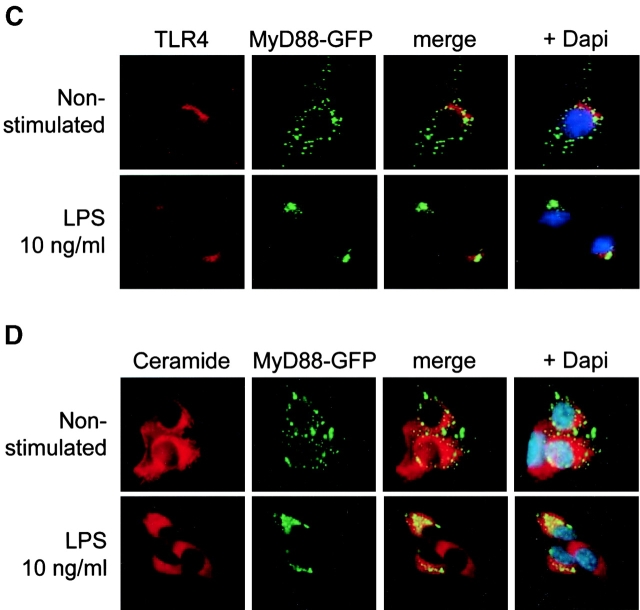
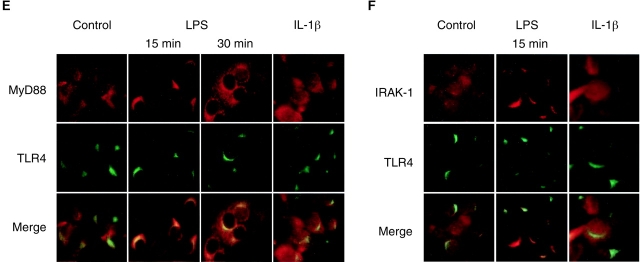
Activation of TLR4 leads to recruitment of the adaptor protein MyD88 and the serine/threonine kinase IRAK-1, facilitating downstream signaling. Therefore, we analyzed the cellular localization of MyD88 and IRAK-1 in the presence or absence of LPS to determine the localization of the initiation of the TLR4-mediated signaling cascade. In a first attempt, we used an MyD88-GFP fusion construct recently described (14). Fluorescence microscopy revealed a diffuse cytoplasmic staining of MyD88-GFP in untreated cells. Subsequent stimulation with 10 ng/ml LPS induced rapid redistribution of MyD88-GFP and paranuclear condensation after 15 min exposure (Fig. 3 A). This paranuclear redistribution of MyD88-GFP was observed after stimulation with LPS but not with IL-1β (Fig. 3 B). It colocalized with intracellular TLR4 staining as well as the cellular distribution of the Golgi marker C5 ceramide, indicating that signaling occurred from this TLR4+ compartment (Fig. 3, C and D). In contrast, LPS stimulation of murine macrophage-like RAW 264.7 cells expressing MyD88-GFP induced recruitment to the cell membrane as recently described (unpublished data and 14). In addition, endogenous MyD88 was detected in m-ICcl2 cells using immunohistochemistry using an MyD88-specific antiserum. Similar to the MyD88-GFP fusion protein, endogenous MyD88 concentrated at a paranuclear localization 15 min after LPS stimulation at a concentration of 10 ng/ml and colocalized with TLR4 (Fig. 3 E). Also, IRAK-1 was redistributed toward the TLR4+ subcellular compartment 15 min after exposure to LPS (Fig. 3 F). These data demonstrate assembly of the TLR4-mediated cytoplasmic signaling cascade at the site of the Golgi apparatus in intestinal crypt epithelial m-ICcl2 cells and suggest an active role of Golgi-resident TLR4 in the process of LPS-mediated cell activation.
Figure 3.
Recruitment of the TLR4 adaptor protein MyD88 and IRAK-1 to the Golgi apparatus upon LPS stimulation. (A) m-ICcl2 cells transfected with an MyD88-GFP fusion protein were left untreated or stimulated with 10 ng/ml LPS for the indicated time period. (B) m-ICcl2 cells transfected with an MyD88-GFP fusion protein were left untreated or stimulated with 10 ng/ml LPS or 100 ng/ml IL-1β. The cellular activation is illustrated by the nuclear translocation of the NF-κB subunit p65. (C) m-ICcl2 cells carrying the MyD88-GFP expression plasmid were left untreated or stimulated with LPS, fixed, and immunostained for TLR4 (red). (D) m-ICcl2 cells carrying the MyD88-GFP expression plasmid were incubated with 5 μM TR C5 ceramide to identify the Golgi apparatus. Subsequent stimulation with 10 ng/ml LPS for 15 min illustrates recruitment of the TLR4 adaptor protein MyD88 to the Golgi apparatus. (E) Double immunostaining of m-ICcl2 cells for MyD88 (red) and TLR4 (green) after stimulation with 10 ng/ml LPS or 100 ng/ml IL-1β for the indicated time. Note the transient change of the TLR4+ Golgi apparatus from green to yellow resulting from an overlay of the FITC-labeled TLR4 and the TxR-labeled MyD88 (merge images), indicating colocalization. (F) Double immunostaining of m-ICcl2 cells for IRAK-1 (red) and TLR4 (green) in unstimulated and LPS- or IL-1β–stimulated cells for 15 min. ×1,000.
TLR4 Signaling Requires Functional Compartmental Integrity of the Golgi Apparatus.
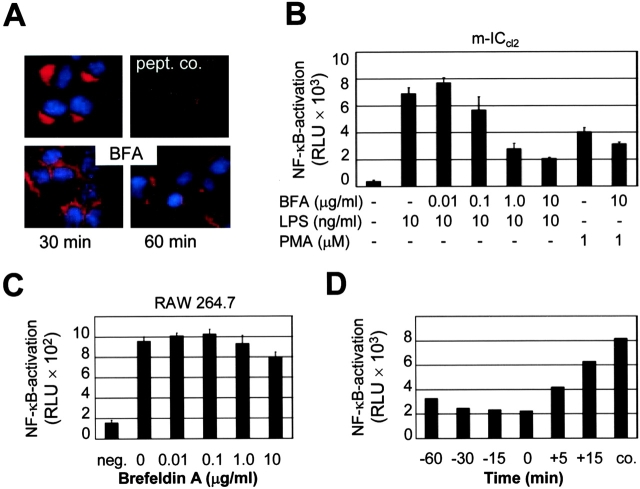
To analyze the functional importance of an intact Golgi apparatus for LPS recognition we measured LPS-induced cellular activation in the presence or absence of brefeldin A. Brefeldin A inhibits the small GTPase Arf leading to redistribution of proteins from the Golgi complex back to the ER and disruption of anterograde traffic (23). This effect is illustrated by disturbance of the defined paranuclear TLR4 staining of m-ICcl2 cells after exposure to brefeldin A (Fig. 4 A). Disturbance of the integrity of the Golgi compartment by preincubation with brefeldin A reduced LPS-mediated NF-κB activation in a dose-dependent manner but did not cause a similar reduction of cellular activation with phorbol ester (Fig. 4 B, PMA). This inhibitory effect was restricted to epithelial m-ICcl2 cells. Macrophage-like RAW 264.7 cells exhibited no significant reduction of LPS-mediated NF-κB activation in the presence of brefeldin A (Fig. 4 C). Interestingly, the inhibitory effect of brefeldin A was significantly reduced when the drug was added after exposure to LPS, suggesting that brefeldin A blocks the transfer of LPS to the TLR4+ Golgi apparatus but does not impair the subsequent recognition and signaling process (Fig. 4 D). Thus, the requirement of an intact Golgi apparatus for LPS-mediated cell activation further argues for an active role of TLR4 located in this subcellular compartment in the process of cellular LPS recognition.
Figure 4.
Requirement of the integrity of the Golgi apparatus for LPS-mediated m-ICcl2 cell activation. (A) Immunostaining of m-ICcl2 cells for TLR4 left untreated or incubated with 0.5 μg/ml brefeldin A (BFA) for 30 and 60 min. (B) Effect of brefeldin A on LPS-induced cellular stimulation. Stably transfected m-ICcl2 cells expressing a NF-κB luciferase reporter construct were preincubated with the indicated concentration of brefeldin A for 30 min and subsequently stimulated with LPS or PMA for 2 h. (C) Effect of brefeldin A on LPS-mediated stimulation of RAW 264.7 cells expressing a NF-κB luciferase reporter construct. (D) Kinetics of the inhibitory activity of brefeldin A added before (−) or after (+) the addition of LPS. Data are presented as relative light units (RLU).
The Heat Shock Protein gp96 Interacts with TLR4 in m-ICcl2 Cells.
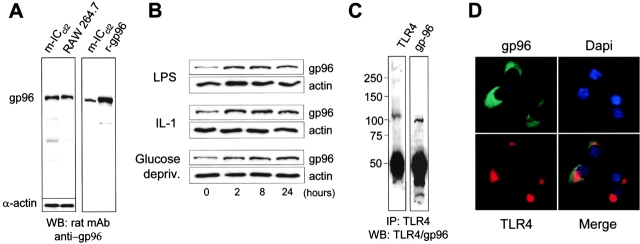
A recent report has described the importance of the heat shock protein gp96 for TLR-mediated recognition in a pre–B cell line, demonstrating direct interaction by coimmunoprecipitation of TLR1, TLR2, and TLR4 with gp96 (24). Moreover, gp96-deficient cells obtained by chemical mutagenesis revealed selective hyporesponsiveness to TLR ligands such as LPS or peptidoglycan, but not IL-1 or PMA. These results led us to analyze the expression of gp96 in our epithelial m-ICcl2 cell system. Immunoblotting revealed expression of gp96 in m-ICcl2 cells, quantitatively similar to the macrophage cell line RAW 264.7 (Fig. 5 A). Expression of gp96 in m-ICcl2 cells increased upon exposure to IL-1 or LPS as well as glucose deprivation as previously reported (Fig. 5 B). Similar to the situation in the pre–B cell line, gp96 also coimmunoprecipitated with TLR4 in m-ICcl2 cells (Fig. 5 C). Consistent with the carboxy-terminal KDEL sequence motif of gp96, which restricts the localization of gp96 to the cis-Golgi/ER, double immunostaining of gp96 using a polyclonal rabbit antiserum or a monoclonal rat antibody and TLR4 revealed only a partial colocalization (Fig. 5 D).
Figure 5.
The heat shock protein gp96 interacts with TLR4 in m-ICcl2 cells. (A) Immunoblot of whole m-ICcl2 and RAW 264.7 cell lysate (5 μg) or 200 ng recombinant gp96 (r-gp96) using a monoclonal rat anti–gp96 antibody. (B) Immunoblot of m-ICcl2 cell lysate for gp96 after incubation with 10 ng/ml LPS, 100 ng/ml IL-1β or glucose deprivation for the indicated times. Staining for actin is shown to demonstrate that equal amounts of protein were loaded on the gel. (C) Coimmunoprecipitation of m-ICcl2 cells with an anti-TLR4 antiserum. The presence of gp96 in the precipitate was detected using the monoclonal rat anti–gp96 antibody. (D) Double immunofluorescence for TLR4 and gp96 using a TxR-labeled (TLR4) and FITC-labeled (gp96) secondary antibody. ×1,000.
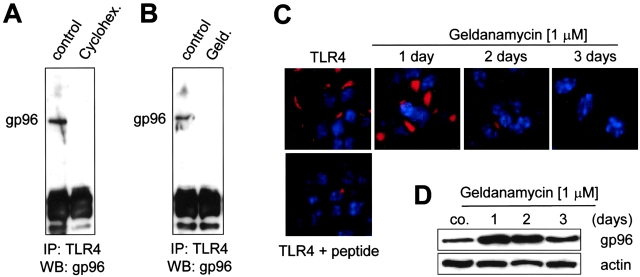
To further analyze the character of the TLR4–gp96 interaction, m-ICcl2 cells were incubated in the presence of the protein synthesis inhibitor cycloheximide or the benzoquinone ansamycin geldanamycin, shown to dissociate complexes between gp96 and one of its ligands, p185erbB2 (25). Coimmunoprecipitation experiments revealed dissociation of the TLR4–gp96 complex after incubation of the cells with 50 μg/ml cycloheximide or 1 μM geldanamycin for 6 h (Fig. 6, A and B) . Moreover, exposure of m-ICcl2 cells to geldanamycin for 2–3 d significantly reduced the immunostaining for TLR4 (Fig. 6 C). This was not due to diminished presence of gp96 itself because gp96 expression was rather enhanced by exposure to geldanamycin (Fig. 6 D). These results suggest that gp96 directly interacts with TLR4 and plays a role in the stability of the final TLR4 complex in m-ICcl2 cells. However, gp96 may not be part of the final LPS recognition complex.
Figure 6.
The transient interaction of TLR4 and gp96 is required for formation of stable TLR4 receptor. (A) m-ICcl2 cells were incubated in the absence (control) or presence of 50 μg/ml cycloheximide (Cyclohex.) for 6 h and coimmunoprecipitated using an anti-TLR4 antiserum. The presence of gp96 in the precipitate was detected using the rabbit anti–gp96 antiserum. (B) Coimmunoprecipitation of m-ICcl2 cells incubated with 1 μM geldanamycin (Geld.) for 6 h. (C) Immunostaining for TLR4 in m-ICcl2 cells that were left untreated or incubated in the presence of 1 μM geldanamycin for 1, 2, or 3 d. (D) Immunoblot for gp96 using 5 μg m-ICcl2 cell lysate incubated in the absence or presence of 1 μM geldanamycin for 1, 2, or 3 d. Immunostaining for actin is shown to demonstrate the amount of protein loaded on the gel.
Altered Localization of gp96 Leads to Cellular Redistribution of TLR4 and Golgi Apparatus–independent LPS Signaling.
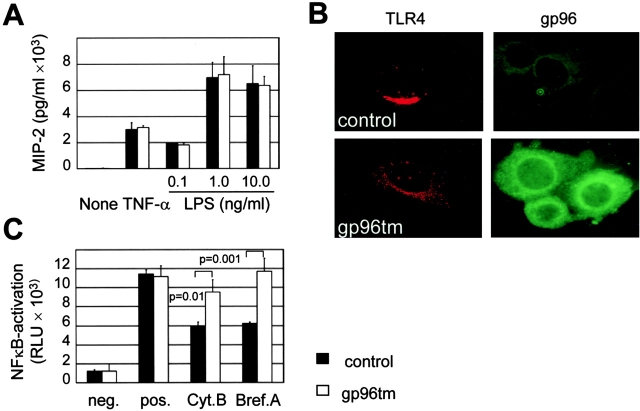
To analyze the role of gp96 during TLR4-mediated LPS recognition, m-ICcl2 cells were transfected with a previously described vector, expressing a genetically modified form of gp96 lacking the COOH-terminal KDEL sequence motif but instead harboring the transmembrane domain of the platelet-derived growth factor. Expression of this modified gp96 (called gp96tm) was demonstrated to result in localization of gp96 to the cell surface membrane (15). Therefore, LPS sensitivity, TLR4 localization, and susceptibility to cytochalasin B or brefeldin A as inhibitors of LPS-induced cell activation were analyzed in stably transfected m-ICcl2 cells expressing gp96tm or carrying the empty expression vector. Expression of gp96tm did not result in significant changes of MIP-2 secretion upon stimulation with LPS or TNF-α (Fig. 7 A). However, the cellular distribution of TLR4 was altered from the restricted paranuclear localization of TLR4 in wild-type cells to a cytoplasmic staining pattern in cells expressing gp96tm. This finding was consistent with a marked redistribution of gp96tm (Fig. 7 B). Moreover, LPS-mediated NF-κB activation in cells carrying gp96tm was significantly less affected by preincubation with brefeldin A or cytochalasin B as compared with wild-type cells (Fig. 7 C). Thus, gp96 seems to play an important role during the process of subcellular localization of the LPS recognition complex. These data further confirm the functional importance of an intact Golgi apparatus and cell traffic for LPS-mediated TLR4 activation in wild-type m-ICcl2 cells.
Figure 7.
Altered localization of gp96 leads to redistribution of TLR4. Stably transfected m-ICcl2 cells expressing a modified form of gp96 lacking the carboxy-terminal KDEL signal and supplemented with a transmembrane region (gp96tm) were compared with cells expressing an empty control vector. (A) Comparison of wild-type and gp96tm-expressing cells for MIP-2 production after stimulation with 50 ng/ml TNF-α or LPS at the indicated concentrations. (B) Immunostaining for TLR4 and gp96 in wild-type and gp96tm-expressing cells. The cellular localization of TLR4 was analyzed using confocal laser scanning microscopy. The picture represents an overlay of 15 confocal images spanning a 2-μm thick section through the nucleus of a representative cell. Staining for gp96 was performed on living nonfixed cells to illustrate the surface membrane localization of gp96. (C) Comparison of the inhibitory effect of 10 μM cytochalasin B and 5 μg/ml brefeldin A pretreatment on LPS-mediated cellular stimulation in wild-type and gp96tm-expressing cells. Data are presented as relative light units (RLU).
Discussion
Intestinal epithelial m-ICcl2 cells derived from enteric crypts have recently been shown to exhibit high expression of TLR4 predominantly localized to the Golgi apparatus. LPS added to the cell supernatant is rapidly internalized and colocalizes with intracellular TLR4 within minutes after exposure (11). Therefore, we speculated that the LPS internalization and directed transport to a TLR4+ subcellular compartment may facilitate receptor–ligand interaction and activation of intracellular TLR4. The requirement of LPS internalization for cellular stimulation remains controversial because internalization-independent LPS-mediated cellular stimulation has also been described (26–30). Interestingly, these studies have entirely analyzed macrophage/monocyte-like cells or used cell systems with overexpression of the LPS receptor molecule TLR4. In both instances, significant membrane surface expression of TLR4 has been reported, which was recently demonstrated to recognize LPS and mediate cellular activation in the absence of intact Golgi function (4, 26). Here we provide evidence that the controversial findings on the requirement of cellular trafficking for LPS-mediated cellular activation can be attributed to the different models used, namely the different cellular localization of the LPS recognition complex in different cell types or under different experimental conditions.
Internalization of LPS has been demonstrated in a number of studies (19, 31–34). However, the underlying mechanisms and the physiological importance remained largely undefined. It seems that internalization of LPS can occur through several different pathways, dependent on the aggregative state and structure of LPS and the functional integrity of the TLR4 receptor (35–37). Although large aggregates of LPS are rapidly internalized and directed to the lysosomal compartment to undergo deacylation, monomeric LPS is taken up more slowly and transported to the Golgi apparatus (12, 38, 39). Moreover, LPS internalization was recently described to require the presence of CD14 but to be independent on the presence of TLR4 on the plasma membrane (26, 27, 38). This situation is found in m-ICcl2 cells, which have previously been shown to express functional CD14 (11). The strong inhibitory effect of MβCD and filipin suggests an important role of lipid rafts for LPS uptake and cellular activation. Interestingly, lipid rafts have not only been associated with cellular uptake pathways such as clathrin- or caveolin-dependent internalization, but also with intercompartimental trafficking such as transport between endosomes and the Golgi apparatus (22). Moreover, it has been suggested that actin is directly involved in the intracellular movement of transport vesicles containing lipid rafts (40). The susceptibility of LPS stimulation to monodansylcadaverin, chlorpromazine, and potassium depletion also suggests an important role for a clathrin-dependent internalization pathway. This is in accordance with previously published work that used electron microscopy and described CD14-dependent LPS internalization via clathrin-coated pits (41).
Recent studies have shown that internalized LPS traffics to a cellular compartment, identified as the Golgi apparatus (11, 26, 31). Transport to the Golgi complex is limited to monomeric LPS, the form that induces cellular activation (31, 32). However, the importance of this process has not been fully elucidated. This represents an interesting issue especially because the transport of LPS to the Golgi apparatus is clearly different from detoxification of LPS molecules by deacylation, which occurs via the lysosomal pathway (39). Previous work by Visintin et al. (42) describing assembly of TLR4 with MD-2 in the ER-cis Golgi network suggested that a functional LPS recognition complex might be present in the Golgi apparatus. Using the Golgi disturbing agent brefeldin A, we demonstrate the requirement of intact Golgi function for LPS-mediated cellular activation of intestinal crypt epithelial m-ICcl2 cells. A second line of evidence suggesting an active role of TLR4 situated in the Golgi in the process of LPS-mediated cellular activation results from the redistribution of the TLR4 adaptor protein MyD88 and the serine/threonine kinase IRAK-1 to the TLR4+ Golgi apparatus of m-ICcl2 cells upon LPS exposure. The fact that TLR4 situated in the Golgi apparatus is able to recognize LPS and signal cellular activation in m-ICcl2 cells is in contrast to the recent work of Latz et al. (26), who described signaling of TLR4 exclusively from the cell surface of human embryonic kidney cells transfected with CD14, TLR4, and MD-2. Also, TLR4-mediated cellular activation was independent of brefeldin A. However, overexpression of TLR4 in this study resulted in pronounced surface expression of TLR4 facilitating CD14-independent stimulation with LPS. Thus, the different susceptibility to brefeldin A might be attributed to the different cellular distribution of TLR4. Moreover, signaling via the cell surface–resident TLR4 might have accounted for the major part of activated TLR4 impairing the visualization of intracellularly situated signaling TLR4 molecules. Alternatively, recognition of LPS on the cell surface might down-regulate subsequent activation of intracellular TLR4.
A growing number of studies suggest that the process of LPS recognition involves considerably more proteins than previously discussed (21, 43). For example, the ER resident heat shock protein gp96 was recently shown to interact with TLRs. A pre–B cell line deficient in gp96 was demonstrated to lack surface expression of TLRs and exhibit hyporesponsiveness to TLR ligands but not IL-1 or PMA (24). Gp96 is a close homologue of the abundant cytoplasmic heat shock protein 90 and both gp96 and heat shock protein 90 exert chaperone function and have been identified in association with folding intermediates of a number of oligomeric proteins (44). However, gp96 is a surprisingly versatile molecule with multiple interesting features. Extracellular gp96 exerts high immunostimulatory potency and induces cytokine secretion and up-regulation of costimulatory molecules (45). It further binds and directs exogenous peptides into the MHC class I presentation pathway of antigen-presenting cells via the α2-macroglobulin receptor (CD91; reference 46). Interestingly, a direct connection between the proinflammatory action of extracellular heat shock proteins and cellular activation through members of the TLR family has been proposed (47).
Gp96 contains a carboxy-terminal retention/retrieval KDEL motif, which restricts its presence to the ER. KDEL-bearing molecules that gain access to the more distal Golgi apparatus as far as the trans Golgi network undergo retrograde transport to the ER mediated by Erd2p, the KDEL receptor (48). Similar to the study by Randow and Seed (24), a direct molecular interaction between gp96 and TLR4 occurs in m-ICcl2 cells. This was not necessarily expected because this interaction has so far only been documented in B cells, which express the TLR variant RP105. RP105 seems to cooperate with TLR4 to mediate LPS recognition, a situation exclusively found in B cells. Protein synthesis inhibition or disturbance of the gp96–ligand interaction markedly reduced the interaction between gp96 and TLR4. Thus, the interaction of TLR4 with gp96 seems to be limited to the early assembly of the LPS recognition complex, rather than to contribute to the formation of the final TLR4 receptor complex. Therefore, gp96 might be involved in the process of TLR4 assembly with the accessory protein MD2, a process that has been described to occur in the ER/cis Golgi compartment (43). In accordance with the literature, the lack of free gp96 may lead to intracellular accumulation of nonfunctional TLR4 and protein degradation (24). In the presence of gp96, the transport of gp96–TLR4-dimer–MD-2 complexes from the ER toward the Golgi apparatus may facilitate maturation of the N-linked glycosylation of both TLR4 and MD-2, shown to be required for intact signaling (49, 50). Thus, in accordance with the present findings, the mature LPS recognition complex might be present in the Golgi apparatus, able to signal cellular activation.
Deletion of the KDEL sequence motif and addition of a transmembrane domain to the native gp96 molecule has recently been shown to induce altered cellular localization of gp96 in transfected cells (15). Here we demonstrate that the lack of the restriction in the localization of gp96 to the ER/cis-Golgi also leads to redistribution of TLR4 within m-ICcl2 cells. Interestingly, altered localization of TLR4 was accompanied by a reduced dependency of LPS-mediated cellular activation on intact cellular traffic and integrity of the Golgi complex. These results confirm our previous findings that LPS-mediated TLR4 recognition in wild-type m-ICcl2 cells is mainly restricted to the Golgi compartment and requires LPS internalization for cellular activation. The presented data further strengthen the hypothesis that gp96 plays an active role in the assembly and positioning of the LPS recognition complex in m-ICcl2 cells. However, we cannot exclude the possibility that overexpression of the modified form of this versatile molecule itself directly or indirectly may cause TLR4 redistribution. We hope that this system might allow us to further investigate the role of intracellular LPS recognition by TLR4 in intestinal epithelial m-ICcl2 cells and unveil the mechanisms responsible for the cellular distribution of the TLR4 molecule in our model of intestinal crypt epithelial m-ICcl2 cells.
In conclusion, we show that LPS-induced TLR4 signaling in intestinal epithelial m-ICcl2 cells occurs at the site of the Golgi apparatus. LPS-mediated cellular activation requires ligand internalization that occurs via a lipid raft-dependent formation of clathrin-coated pits and intracellular transport to the Golgi compartment. The restricted localization of TLR4 to the Golgi apparatus of intestinal epithelial m-ICcl2 cells might be attributed to its chaperone gp96, facilitating assembly and transport from the ER to the cis-Golgi complex. This is in contrast to the situation in macrophages, where surface expression of TLR4 seems to allow recognition of LPS without prior internalization. LPS internalization may represent a regulatory step in the process of LPS-mediated activation of intestinal epithelial cells to avoid inadequate proinflammatory stimulation by the normal microflora. Thus, we propose a new concept of the process of LPS recognition in intestinal epithelial cells that may help to understand the existence of an effective enteric innate immune recognition system in the presence of an abundant intestinal bacterial microflora.
Acknowledgments
We are indebted to those who provided expression constructs and reagents.
The authors are supported by a grant from the Karolinska Institutet (to M.W. Hornef), the Swedish Medical Research Council (to S. Normark and M.W. Hornef), the Swedish Cancer Foundation (to S. Normark), and the Association Vaincre la Mucoviscidose (to A. Vandewalle).
Abbreviations used in this paper: ER, endoplasmic reticulum; gp96, glycoprotein 96; IRAK, IL-1R–associated kinase; MβCD, methyl-β-cyclodextrin; MIP, macrophage inflammatory protein; NF, nuclear factor; RT, room temperature; TLR, toll-like receptor.
References
- 1.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. [DOI] [PubMed] [Google Scholar]
- 2.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 3.Underhill, D.M., A. Ozinsky, A.M. Hajjar, A. Stevens, C.B. Wilson, M. Bassetti, and A. Aderem. 1999. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 401:811–815. [DOI] [PubMed] [Google Scholar]
- 4.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471–3475. [DOI] [PubMed] [Google Scholar]
- 5.Hacker, H., H. Mischak, T. Miethke, S. Liptay, R. Schmid, T. Sparwasser, K. Heeg, G.B. Lipford, and H. Wagner. 1998. CpG-DNA specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 17:6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gewirtz, A.T., T.A. Navas, S. Lyons, P.J. Godowski, and J.L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882–1885. [DOI] [PubMed] [Google Scholar]
- 7.Gewirtz, A.T., P.O. Simon, Jr., C.K. Schmitt, L.J. Taylor, C.H. Hagedorn, A.D. O'Brien, A.S. Neish, and L.J. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Invest. 107:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abreu, M.T., P. Vora, E. Faure, L.S. Thomas, E.T. Arnold, and M. Arditi. 2001. Decreased expression of toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccaride. J. Immunol. 167:1609–1616. [DOI] [PubMed] [Google Scholar]
- 9.Smith, P.D., L.E. Smythies, M. Mosteller-Barnum, D.A. Sibley, M.W. Russell, M. Merger, M.T. Sellers, J.M. Orenstein, T. Shimada, M.F. Graham, et al. 2001. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS and IgA-mediated activities. J. Immunol. 167:2651–2656. [DOI] [PubMed] [Google Scholar]
- 10.Bens, M., A. Bogdanova, F. Cluzeaud, L. Miqueroi, S. Kerneis, J.P. Kraehenbuhl, A. Kahn, E. Pringault, and A. Vandewalle. 1996. Transimmortalized mouse intestinal cells (m-ICcl2) that maintain a crypt phenotype. Am. J. Physiol. 270:C1666–C1674. [DOI] [PubMed] [Google Scholar]
- 11.Hornef, M.W., T. Frisan, A. Vandewalle, S. Normark, and A. Richter-Dahlfors. 2002. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thieblemont, N., and S.D. Wright. 1999. Transport of bacterial lipopolysaccaride to the Golgi apparatus. J. Exp. Med. 190:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. USA. 97:2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad-Nejad, P., H. Häcker, M. Rutz, S. Bauer, R.M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharide activate toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958–1968. [DOI] [PubMed] [Google Scholar]
- 15.Zheng, H., J. Dai, D. Stoilova, and Z. Li. 2001. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J. Immunol. 167:6731–6735. [DOI] [PubMed] [Google Scholar]
- 16.Blaeke, A., Y. Delneste, N. Herbault, P. Jeannin, J.-Y. Bonnefoy, A. Beck, and J.-P. Aubry. 2002. Measurement of nuclear factor-kappa B translocation on lipopolysaccharide-activated human dendritic cells by confocal microscopy and flow cytometry. Cytometry. 48:71–79. [DOI] [PubMed] [Google Scholar]
- 17.Mundy, D.I., T. Machleidt, Y.S. Ying, R.G. Anderson, and G.S. Bloom. 2002. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 115:4327–4339. [DOI] [PubMed] [Google Scholar]
- 18.Ding, A.H., E. Sanchez, M. Tancinco, and C.F. Nathan. 1992. Interactions of bacterial lipopolysaccharide with microtubule proteins. J. Immunol. 148:2853–2858. [PubMed] [Google Scholar]
- 19.Detmers, P.A., N. Thieblemont, T. Vasselon, R. Pironkova, D.S. Miller, and S.D. Wright. 1996. Potential role of membrane internalization and vesicle fusion in adhesion of neutrophils in response to lipopolysaccharide and TNF-α. J. Immunol. 157:5589–5596. [PubMed] [Google Scholar]
- 20.Sandvig, K., A. Sundan, and S. Olsnes. 1984. Evidence that diphteria toxin and modeccin enter the cytosol from different vesicular compartments. J. Cell Biol. 98:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triantafilou, M., K. Miyake, D.T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603–2611. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, B.J., A.K. Kenworthy, R.S. Polishchuk, R. Lodge, T.H. Roberts, K. Hirschberg, R.D. Phair, and J. Lippincott-Schwarz. 2001. Rapid recycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward, T.H., R.S. Polishchuk, S. Caplan, K. Hirschberg, and J. Lippinkott-Schwartz. 2001. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randow, F., and B. Seed. 2001. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3:891–896. [DOI] [PubMed] [Google Scholar]
- 25.Chavany, C., E. Mimnaugh, P. Miller, R. Bitton, P. Nguyen, J. Trepel, L. Whitesell, R. Schnur, J.D. Moyer, and L. Neckers. 1996. p185(erbB2) binds to GRP94 in vivo-dissociation of the p185(erbB2)/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185(erbB2). J. Biol. Chem. 271:4974–4977. [DOI] [PubMed] [Google Scholar]
- 26.Latz, E., A. Visintin, E. Lien, K. Fitzgerald, B.G. Monks, E. Kurt-Jones, D.T. Golenbock, and T. Espevik. 2002. LPS rapidly traffics to and from the Golgi apparatus with the TLR4/MD-2/CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834–47843. [DOI] [PubMed] [Google Scholar]
- 27.Poussin, C., M. Foti, J.-L. Carpentier, and J. Pugin. 1998. CD14-dependent endotoxin internalization via a macropinocytotic pathway. J. Biol. Chem. 273:20285–20291. [DOI] [PubMed] [Google Scholar]
- 28.Hampton, R.Y., D.T. Golenbock, M. Penman, M. Krieger, and C.R. Raetz. 1991. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 352:343–344. [DOI] [PubMed] [Google Scholar]
- 29.Kitchens, R.L., R.J. Ulevitch, and R.S. Munford. 1992. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 176:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gegner, J., R.J. Ulevitch, and P.S. Tobias. 1995. Lipopolysaccharide (LPS) signal transduction and clearance. J. Biol. Chem. 270:5320–5325. [DOI] [PubMed] [Google Scholar]
- 31.Thieblemont, N., and S.D. Wright. 1999. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowan, D.B., S. Noria, C. Stamm, L.M. Garcia, D.N. Poutias, P.J. del Nido, and F.X. McGowan, Jr. 2001. Lipopolysaccharide internalization activates endotoxin-dependent signal transduction in cardiomyocytes. Circ. Res. 88:491–498. [DOI] [PubMed] [Google Scholar]
- 33.Beatty, W.L., S. Meresse, P. Gounon, J. Davoust, J. Mounier, P.J. Sansonetti, and J.P. Gorvel. 1999. Trafficking of Shigella lipopolysaccharide in polarized intestinal epithelial cells. J. Cell Biol. 145:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtman, S.N., J. Wang, C. Zhang, and J.J. Lemasters. 1996. Endocytosis and Ca2+ are required for endotoxin-stimulated TNF-α release by Kupffer cells. Am. J. Physiol. 271:G920–G928. [DOI] [PubMed] [Google Scholar]
- 35.Thieblemont, N., R. Thieringer, and S.D. Wright. 1998. Innate immune recognition of bacterial lipopolysaccharide: dependence on interaction with membrane lipids and endocytic movement. Immunity. 8:771–777. [DOI] [PubMed] [Google Scholar]
- 36.Thieblemont, N., and S.D. Wright. 1997. Mice genetically hyporesponsive to lipopolysaccharide (LPS) exhibit a defect in endocytic uptake of LPS and ceramide. J. Exp. Med. 185:2095–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchens, R.L., and R.S. Munford. 1998. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J. Immunol. 160:1920–1928. [PubMed] [Google Scholar]
- 38.Vasselon, T., E. Hailman, R. Thieringer, and P.A. Detmers. 1999. Internalization of monomeric lipopolysaccharide occurs after transfer out of cell surface CD14. J. Exp. Med. 190:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luchi, M., and R.S. Munford. 1993. Binding, internalization, and deacylation of bacterial lipopolysaccharide by human neutrophils. J. Immunol. 151:959–969. [PubMed] [Google Scholar]
- 40.Rozelle, A.L., L.M. Machesky, M. Yamamoto, M.H. Driessens, R.H. Insall, M.G. Roth, K. Luby-Phelps, G. Marriott, A. Hall, and H.L. Yin. 2000. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10:311–320. [DOI] [PubMed] [Google Scholar]
- 41.Kitchens, R.L., P. Wang, and R.S. Munford. 1998. Bacterial lipopolysaccharide can enter monocytes via two CD-14-dependent pathways. J. Immunol. 161:5534–5545. [PubMed] [Google Scholar]
- 42.Visintin, A., A. Mazzoni, J.A. Spitzer, and D.M. Segal. 2001. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to toll-like receptor 4. Proc. Natl. Acad. Sci. USA. 98:12156–12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Triantafilou, K., M. Triantafilou, and R.A. Dederick. 2001. A CD14-indendent LPS receptor cluster. Nat. Immunol. 2:338–345. [DOI] [PubMed] [Google Scholar]
- 44.Nicchitta, C.V. 1998. Biochemical, cell biological and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/gp96. Curr. Opin. Immunol. 10:103–109. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava, P.K. 1991. Protein tumor antigens. Curr. Opin. Immunol. 3:654–658. [DOI] [PubMed] [Google Scholar]
- 46.Berwin, B., M.F. Rosser, K.G. Brinker, and C.V. Nicchitta. 2002. Transfer of GRP94(Go96)-associated peptides onto endosomal MHC class I molecules. Traffic. 3:358–366. [DOI] [PubMed] [Google Scholar]
- 47.Vabulas, R.M., S. Braedel, N. Hilf, H. Singh-Jasaju, S. Herter, P. Ahmad-Nejad, C.J. Kirschning, C. da Costa, H.G. Rammensee, H. Wagner, et al. 2000. The ER-resident heat shock protein Gp96 activates dendritic cells via the TLR2/4 pathway. J. Biol. Chem. 277:20847–20853. [DOI] [PubMed] [Google Scholar]
- 48.Miesenböck, G., and J.E. Rothman. 1995. The capacity to retrieve escaped ER proteins extends to the trans-most cisternae of the golgi stack. J. Biol. Chem. 129:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Da Silva Correia, J., and R.J. Ulevitch. 2001. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 227:1845–1854. [DOI] [PubMed] [Google Scholar]
- 50.Mullen, G.E., M.N. Kennedy, A. Visintin, A. Mazzoni, C.A. Leifer, D.R. Davies, and D.M. Segal. 2003. The role of disulfide bonds in the assembly and function of MD-2. Proc. Natl. Acad. Sci. USA. 100:3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]