Abstract
Human membrane cofactor protein (CD46) protects host cells against complement attack and may function as a receptor for pathogenic Neisseriae. We assessed CD46 expression in the human cervical cell line ME-180 after exposure to Neisseria gonorrhoeae. Piliated but not nonpiliated gonococci adhered to cells and produced up to an 80% reduction in CD46 surface expression by 6 h that persisted for at least 24 h. This response required a minimum multiplicity of infection of 10 and was not prevented by antibodies to CD46. CD46 down-regulation was not attributable to intracellular retention or a global or specific shutdown of mRNA or protein synthesis. Substantial quantities of CD46 were found in the supernatants, indicating a specific shedding of this protein. Adherent gonococci lacking the pilus retraction protein PilT did not down-regulate CD46 but de-repression of pilT expression restored CD46 down-regulation. After experimental infection of human volunteers with a gonococcal variant incapable of inducing CD46 down-regulation, variants of this strain were reisolated that exhibited CD46 down-regulation. Pilus-mediated interactions of gonococci with human epithelial cells results in a pathogen-induced manipulation of the host cell environment in which a membrane protein is removed from epithelial cells by liberation into the surrounding milieu.
Keywords: Type IV pilus, PilT, pilus retraction, PilE, protein shedding
Introduction
The regulators of complement activation, a family of plasma and membrane proteins that protect the host from complement attack, are frequently targeted by pathogenic microorganisms (for review see reference 1). Membrane cofactor protein (CD46) is a member of this family that is expressed on nearly all human cells. CD46 regulates complement activation by serving as a cofactor for the plasma serine protease factor I to cleave and thereby inactivate C3b and C4b deposited on the same surface on which CD46 is expressed (for review see reference 2). CD46 has also been identified as a cellular receptor for several human pathogens, including some strains of the measles virus (MV; references 3, 4), Streptococcus pyogenes (5), human herpes virus 6 (6), and the pathogenic Neisseriae (7).
The role of CD46 in microbial pathogenesis has been most studied in the case of the MV. MV-induced signaling responses through CD46 include enhanced IL-12 secretion (8) and increased release of nitric oxide through modulation of IFN α/β synthesis (9, 10). In addition, regions of the cytoplasmic tail of CD46 are involved in the MV-induced processes of syncytia formation and down-regulation of CD46 (11–16). The inhibition of this interaction with CD46 peptides or mAbs specific to CD46 results in a loss of viral infectivity (17–20). However, usage of this receptor may come at a price, as the resulting loss of CD46 renders MV-infected cells more susceptible to complement attack (15, 21, 22).
Initial adherence of Neisseria gonorrhoeae to the human mucosal epithelium is thought to require CD46 and is facilitated by a multifunctional filamentous appendage known as the Type IV pilus (Tfp). In addition to host cell adhesion, expression of this organelle is associated with bacterial autoagglutination, competency for DNA transformation, and a mode of surface translocation known as twitching motility (for review see reference 23). These processes are reliant on coordinate expression and presentation of the multiple protein constituents of the pilus. The pilus components PilE and PilC, both of which exhibit variable expression, are two major adhesins in pilus-mediated gonococcal attachment to host cells. PilE, or pilin, is the major pilus subunit, and comprises the bulk of the pilus fiber. pilE exhibits frequent sequence variation which arises from RecA-dependent gene conversion between the pilE expression locus and one of several silent pilS loci (24–26). These pilin structural alterations modulate many aspects of pilus biology including antigenicity, pilus-mediated adhesion, and tissue tropism (27–29). Changes in PilE are thought to contribute to host receptor recognition either through direct, high-affinity interactions with host cell receptors or by acting as a “molecular scaffold” for other adhesion-promoting pilus constituents (for review see reference 23).
The pilus-associated protein PilC has also been identified as a major pilus-associated adhesin based on the failure of PilC null mutants to adhere to epithelial cells and inhibition of pilus-mediated gonococcal attachment to epithelial cells by purified PilC preparations (28, 30). This protein has been localized to the outer membrane as well as to the tip of the pilus fiber (30, 31) and is expressed from two loci (pilC1 and pilC2), both of which undergo high frequency on/off phase variation (32, 33). In addition to its apparent role in host cell receptor recognition, PilC modulates the dynamics of pilus fiber assembly and is essential for full competence for DNA transformation (32–35).
Evidence for the role of CD46 as a pilus receptor includes binding of purified pili to CD46 in an overlay assay, inhibition of gonococcal adherence by mAbs to CD46, and adherence of piliated (P+) N. gonorrhoeae to CD46 transfectants of otherwise nonpermissive cells (7). However, the finding of an inverse correlation between CD46 expression and adherence levels of P+ N. gonorrhoeae to various epithelial cell lines challenged the perception of CD46 as a classical receptor for N. gonorrhoeae (36). Other studies indicate a potential role for CD46 in N. gonorrhoeae pathogenesis, especially an involvement of CD46 in intracellular signaling associated with gonococcal attachment. These analyses include the induction of a CD46-dependent calcium flux upon incubation of ME-180 cells with purified N. gonorrhoeae pili (37) and phosphorylation of Tyr 354 in the cytoplasmic tail of CD46 by the src kinase c-Yes after adherence of P+ bacteria to A431 human endocervical cells (38). Additionally, Chinese hamster ovary cells expressing tail deletion constructs of CD46 did not support bacterial adhesion (39), indicating that the CD46 cytoplasmic tail plays a role in promoting adhesion.
Other responses of the host cell to pilus-mediated adherence of the pathogenic Neisseriae have been described previously, including cytotoxicity for human cells (40, 41), increased lysosome exocytosis (42), and cortical actin plaque formation (43). The latter process is enhanced by expression of the inner membrane protein PilT of N. gonorrhoeae, an ATPase thought to mediate retraction of the Tfp (44). PilT is dispensable for pilus biogenesis and pilus-mediated adhesion to cultured epithelial cells but is essential to DNA uptake and twitching motility (45). The role of PilT in pilus retraction and its contributions to cortical actin plaque formation have sparked speculation that the tensile forces exerted on the host cell membrane by retractile pili of adherent N. gonorrhoeae activate mechanosensory signaling pathways in the host cell to enhance gonococcal infectivity (43).
The putative role of CD46 as a cellular receptor for pilus-associated adhesins (28, 30) in conjunction with the dynamic nature of pilus-mediated gonococcal adherence to host epithelial cells (for review see reference 23) led us to explore the effects of pilus-mediated adherence on CD46 expression and function. Here, we describe the down-regulation of CD46 in cultured human epithelial cells after exposure to gonococci expressing retractile pili. The data are consistent with a cellular response to pilus-mediated gonococcal attachment that is reliant on PilT-dependent processes. In addition, after inoculation of male volunteers with a non–down-regulating variant of N. gonorrhoeae, gonococcal pilin variants of this strain were isolated that exhibited CD46 down-regulation. We hypothesize that the down-regulation of CD46 represents a pathogen-induced alteration in the host cell designed to promote gonococcal infectivity.
Materials and Methods
Cell Lines and Bacterial Strains.
The cell lines ME-180, SiHa, FaDu, HeLa, Hep-2, Chang, and T84 (HTB-33, HTB-35, HTB-43, CCL-2, CCL-23, CCL-20.3, and CCL-248, respectively) were obtained from American Type Culture Collection. ME-180 cervical epithelial cells were maintained in McCoy's 5A medium supplemented with 10% heat-inactivated FCS. Chang cells were maintained in Medium 199 supplemented with 10% FCS. Other cell lines were maintained in DMEM + 10% FCS.
All bacteria were grown for 18–20 h on GC agar supplemented with Isovitalex (Becton Dickinson) at 37°C in a 5% CO2 atmosphere. Piliation state and Opa expression of each population was determined by observation of colony morphology using an inverted stereomicroscope with a diascopic base. Colonies of P+ bacteria were identified by their dense, domed colonies as described by Swanson et al. (46). N. gonorrhoeae strains used in this work are summarized in Table I. N401 and its derivatives lacking the pilus constituents PilE, PilT, PilV, and PilU have been described previously as indicated in Table I. Based on colony morphology, cultures were judged to be >95% homogeneous in their piliation state before use. Where indicated, de-repression of PilT expression was achieved using isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma-Aldrich).
Table I.
Strains and Variants of Neisseria gonorrhoeae Used in This Study
| Strain/variant | Parent strain | Piliationa | Relevant characteristics | Ref. |
|---|---|---|---|---|
| N401 (recA6(kan))b | N400 | + | wild-type pilus expression | 45 |
| MW24 (PilEind) | N401 | − | lacks major pilus subunit | 35 |
| GT104 (PilTind) | N401 | + | nonretractile pilic | 45 |
| GU5 (pilU::Tnerm5) | N400 | + | hyperautoagglutinative | 54 |
| GV2 (PilV::kan) | N400 | + | poorly adherent | 55 |
| P1 | FA1090 | + | inoculum for male volunteers | d |
| P3, P6, P7, P11, P17 | FA1090 | + | isolated from infected male volunteers | d |
As determined by colony morphology.
recA6 is an IPTG-inducible allele of recA. In the absence of induction, recA is not expressed, minimizing antigenic variation.
PilT expression can be de-repressed with IPTG, restoring pilus retraction.
Unpublished data.
Pilin variants selected by in vivo passage of N. gonorrhoeae strain FA1090 through human hosts were isolated from male subjects, who were infected experimentally with this strain, using the procedures described by Cohen et al. (47) and Jerse et al. (48). The inoculum was a P+ variant of strain FA1090 used in previous analyses (48, 49); the pilin expressed by this variant was arbitrarily designated P1. Gonococci cultured from urine or urethral swab specimens from infected subjects show shifts in the preponderance of variants expressing new pilin sequences (unpublished data). The colony variants designated P3, P6, P7, P11, and P17, each of which expresses pilin protein (PilE) with a different primary sequence, are representative of some of the pilin variants that predominated in experimentally infected subjects at different times after inoculation.
To minimize variation, in vivo-derived variants of strain FA1090 were cultured directly from frozen stock and P+ bacteria were selected based on colony morphology, recultured, and pooled. Pooled bacteria were used to prepare a frozen stock from which bacteria were cultured for each independent experiment without additional passage. Before each experiment, plates were examined to determine piliation state and the predominant colony morphology for each variant (>65%) was indicative of a P+ state.
In some experiments, bacteria were heat killed by incubation for 5 min at 95°C before addition to cultured cells or were treated with chloramphenicol at a concentration of 200 μg/ml 30 min before and during the incubation with cultured cells.
Immunostaining of Cell Surface Proteins for FACS® Analysis.
ME-180 cells were seeded in 6-well tissue culture–treated plates at 1.5 × 105 cells/well and incubated overnight. Gonococci, suspended in fresh, serum-free cell culture medium, were added to each well at the appropriate multiplicity of infection (MOI; based on OD600 where OD600 of 1.0 = 109 bacteria/ml). Cells were incubated at 37°C in a 5% CO2 atmosphere. At indicated time points, monolayers were washed three times with McCoy's 5A medium, and trypsin was added for 1 min at RT. The cells were scraped into 350 μl FACS® buffer (1% FCS in PBS) followed by a 5-min centrifugation. Pelleted cells were resuspended in FACS® buffer containing 25 μg/ml TRA-2-10, a mAb specific to the NH2-terminal complement control protein repeat of CD46 (50), followed by a 15–30 min incubation at 4°C. Next, the cells were washed twice in cold FACS® buffer and resuspended in a 1:100 dilution of FITC-conjugated goat anti–mouse antibody (Sigma-Aldrich). After 15–30 min incubation at 4°C, cells were washed twice and resuspended in FACS® buffer with 0.5% paraformaldehyde. Other primary antibodies similarly used in this work included anti–decay-accelerating factor (DAF; Accurate Chemical and Scientific Corporation), anti–E-cadherin (Becton-Dickinson), and anti-HLA (BD Biosciences).
Western Immunoblot and ELISA.
ME-180 cells were infected with N401 (P+), MW24 (nonpiliated [P−]), or medium alone as described for immunostaining. At each time point, cells were harvested, counted, and washed in PBS before resuspension in lysis buffer (1% Nonidet P-40 and 0.05% SDS in PBS) with Complete Protease Inhibitor Cocktail (Roche) at a concentration of 2 × 107 cells/ml (51). After a 15–20 min incubation on ice, the solubilized-cell preparations were centrifuged at 12,000 g for 10 min. The lysates were electrophoresed on 10% SDS-PAGE and transferred to a nitrocellulose membrane using an XCell II Blot Module (Novex) as per the manufacturer's recommendations. Membranes were incubated in Tris buffered saline with 0.05% Tween 20 containing 2% nonfat dry milk for 1 h at 37°C, washed, and immunoblotted using rabbit polyclonal anti-CD46 antiserum (1:7,000) followed by HRP-conjugated donkey anti–rabbit IgG (1:7,000). Blots were washed in Tris buffered saline with 0.05% Tween 20 before being developed with reagents (Supersignal West Pico Luminol; Pierce Chemical Co.). To verify equal loading, blots were stripped using the Western Blot recycling kit (ReBlot Plus; Chemicon International), probed with a goat anti–glyceraldehyde-3-phosphate dehydrogenase (1:1,000; Chemicon International) followed by an HRP-conjugated donkey anti–goat secondary antibody (1:15,000; Jackson ImmunoResearch Laboratories). CD46 in cell lysates was also quantified by ELISA as described by Liszewski et al. (51).
Preparation of Cell Culture Supernatants.
106 ME-180 cells were incubated with N401 (MOI = 1,000), MW4 (MOI = 1,000), or medium alone for 5.5 h. Supernatants (12 ml each) were collected and monolayers were washed once with 12 ml of medium. The wash was pooled with the supernatant and centrifuged at 30,000 g for 1 h at 4°C. These supernatants were transferred to a centrifugal filter device (Centricon-Plus-20; Millipore) and concentrated to 1.5 ml as per the manufacturer's instructions. Corresponding cell monolayers were lysed in 1.5 ml lysis buffer and both lysates and concentrated supernatants were analyzed by Western immunoblot and ELISA.
RT-PCR.
RT-PCR analysis was performed as described by Wang et al. (52).
Adherence Assays.
Adherence assays were performed as described previously (36). In brief, bacteria were fluorescently labeled by incubation with carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) for 20 min at RT, and labeling of each bacterial population was assessed by FACS® to ensure equivalent staining of all populations being tested. Bacteria were washed twice with PBS, resuspended to OD600 = 1.0, and added to 6-well plates containing 70–80% confluent monolayers of the cultured epithelial cell lines to achieve an MOI of 100. After a 4-h incubation at 37°C with 5% CO2, cells were washed, trypsinized for 1 min, and resuspended in 300 μl FACS® buffer. Flow cytometry was performed as described in the next paragraph. Adherence was also monitored using an inverted light microscope and fluorescence microscopy.
Flow Cytometry.
Flow cytometry was performed using a FACSCalibur™ (Becton Dickinson) using forward and side scatter thresholds optimized for ME-180 cells. Fluorescence was acquired on a four-decade log scale wherein instrument settings were adjusted such that fluorescence of unstained cells fell within the first decade. Changes in fluorescence intensity resulting from binding of fluorescently labeled antibodies or bacteria to epithelial cells are expressed as mean fluorescence or geometric mean, respectively, that was calculated from histograms plotting fluorescence on an arbitrary linearized scale versus number of events. A minimum of 10,000 events were collected per sample and no gating was applied.
Immunofluorescent Microscopy.
ME-180 cells were cultured overnight in 6-well chambered slides and incubated with N. gonorrhoeae at an MOI of 100 for 4 h at 37°C in 5% CO2. After three to five washes (PBS + 1% FCS), gonococci adhering to the monolayer were stained with Syva Microtrak Reagent A (Trinity Biotech) or with a polyclonal antiserum against a major outer membrane protein of N. gonorrhoeae (1:100; Biodesign International) followed by a FITC-conjugated anti–rabbit IgG (Sigma-Aldrich). Next, cells were washed twice and fixed with PBS containing 2% paraformaldehyde and 0.5% glutaraldehyde. After the chambers were removed, coverslips were mounted using a 50% solution of glycerol in PBS. Cells were visualized at a magnification of 1,000 using a fluorescent microscope (model E400; Nikon) equipped with MagnaFire digital camera and imaging software.
Results
Adherence of P+ N. gonorrhoeae to ME-180 Cells Down-regulates CD46.
To determine if adherence of P+ gonococci to epithelial cells results in modulation of CD46 expression, ME-180 cells were incubated with N401 (P+), MW24 (P−), or medium alone for up to 24 h. FACS® and Western immunoblots demonstrated that incubation of ME-180 cells with P+ N. gonorrhoeae, but not P− N. gonorrhoeae, led to a reduction in CD46 expression (Fig. 1) . These results were confirmed by ELISA of CD46 in whole cell lysates (not depicted). Experiments wherein CD46 levels were examined by FACS® at 30-min intervals up to 8 h after addition of P+ N. gonorrhoeae established that the decrease in CD46 was detectable by 3.5 h and maximal by 6 h (unpublished data). A similar decline in CD46 expression to that seen with an MOI of 1,000 was induced by an MOI of 100, whereas an MOI of 10 induced a more modest decrease in CD46 and an MOI of 1 did not influence CD46 expression (Fig. 2) . In addition, incubation of ME-180 cells with heat-killed or chloramphenicol-treated N. gonorrhoeae did not lead to CD46 down-regulation (not depicted).
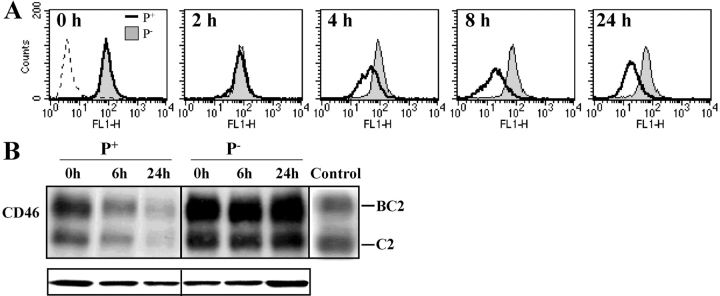
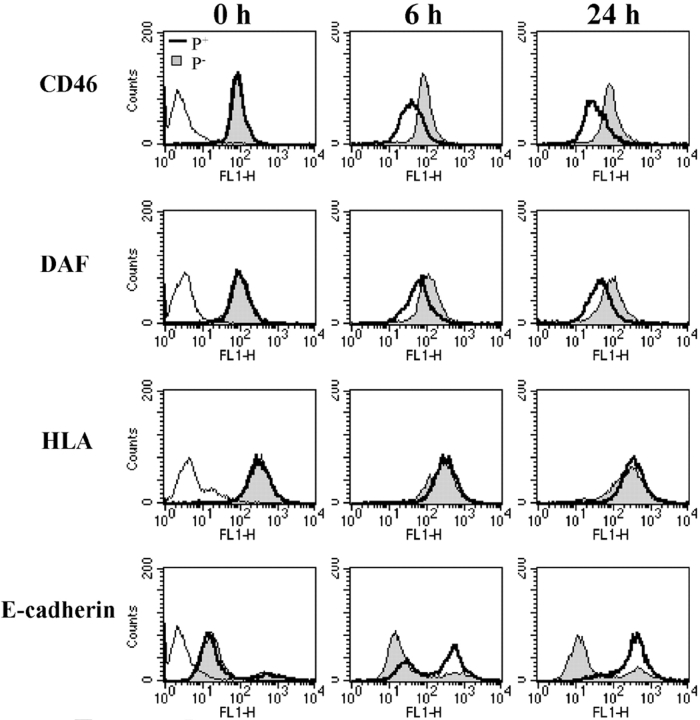
Figure 1.
Adherence of P+ N. gonorrhoeae to ME-180 cells leads to down-regulation of CD46. ME-180 cells were incubated with N401 (P+) N. gonorrhoeae (MOI = 1,000), MW24 (P−) N. gonorrhoeae (MOI = 1,000), or medium alone for up to 24 h. CD46 levels were assessed at time points indicated by FACS® (A) or Western immunoblot (B). The Western immunoblot shows the typical SDS-PAGE electrophoretic pattern of CD46 expressed by ME-180 cells, consisting predominantly of the more heavily O-glycosylated upper band isoforms (BC1 and BC2) and smaller amounts of the lower band isoforms (C1 and C2). (A and B) CD46 levels in uninfected cells are similar to P− infected controls and have been omitted for clarity. In A and in subsequent figures, controls wherein primary antibody was omitted are represented as a peak in the first decade of the four-decade log scale. (B) “Control” lane contains cell lysates of CHO cells transfected with BC2 or C2 isoforms of CD46.
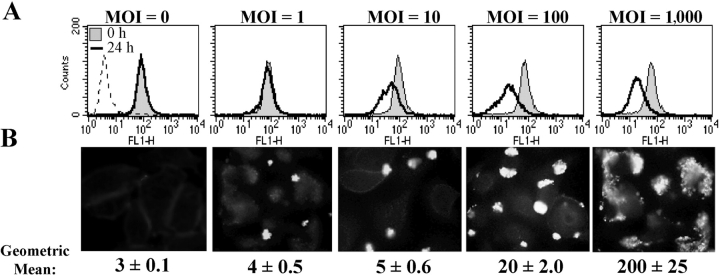
Figure 2.
CD46 down-regulation is dependent on bacterial adherence. (A) FACS® was used to evaluate CD46 expression on ME-180 cells that were incubated with N401 (P+) N. gonorrhoeae at the MOIs noted for 0 or 24 h. Bacterial adherence at each MOI was also assessed at 4 h after infection using immunofluorescent microscopy at a magnification of 1,000 and by FACS® (B). Images in B are representative of one of at least ten fields examined in each of three similar experiments. Values in B indicate mean ± SD of triplicate samples from one of three independent experiments.
Six additional human epithelial cell lines (T84, HeLa, Hep-2, FaDu, SiHa, and Chang) were investigated for CD46 down-regulation (unpublished data). At an MOI of 1,000, this phenomenon was observed in T84 and intermittently in HeLa, but the effect was not seen in the remaining four cell lines despite similar or greater levels of CD46 expression. Consistent with previous papers, gonococcal adherence to all non–down-regulating cell lines were significantly lower than those observed with ME180 and T84 (36, 39). Due to the high levels of bacterial adherence and CD46 down-regulation observed in ME-180, this cell line was subsequently used to further investigate the bacterial and host cell interactions involved in CD46 down-regulation.
Antibodies to CD46 Do Not Block CD46 Down-regulation.
Because N. gonorrhoeae adherence and pilus-induced Ca2+ flux are inhibited by treatment with mAbs to CD46 (7, 37), we asked if N. gonorrhoeae–induced CD46 down-regulation could be similarly blocked. ME-180 cells were incubated with the CD46-specific mAbs GB24 and TRA-2–10 (50) for 30 min before addition of N401 (P+) N. gonorrhoeae, MW24 (P−) N. gonorrhoeae, or medium alone. Antibody cross-linking alone did not result in modulation of CD46 expression and these mAbs did not inhibit CD46 down-regulation resulting from adherence of P+ N. gonorrhoeae to ME-180 cells (Fig. 3 A). Furthermore, microscopic examination of N. gonorrhoeae adherence in the presence of mAbs to CD46 (Fig. 3 B) indicated similar levels of adherence in the presence of these and other mAbs to CD46, including 122-2, E4.3, and J4-48 (Research Diagnostics Inc.) as well as a rabbit polyclonal antiserum (not depicted). These results suggest that the down-regulation of CD46 may not require direct contact between pili and the ectodomain of CD46.
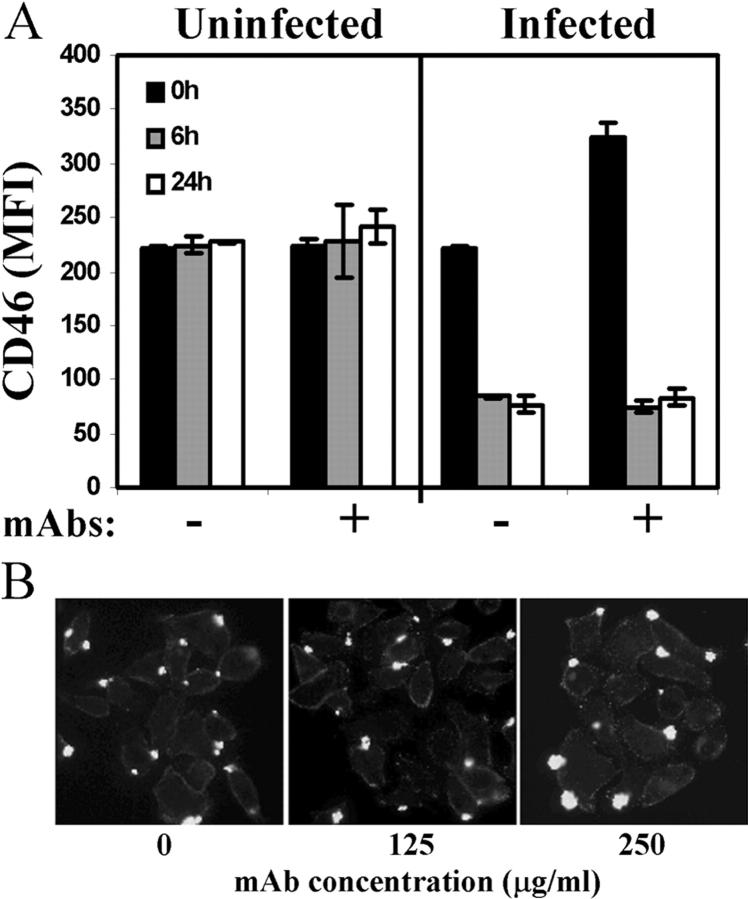
Figure 3.
Antibodies to CD46 do not inhibit CD46 down-regulation or adherence of P+ N. gonorrhoeae to CD46. (A) ME-180 cells were incubated for 30 min with the CD46-specific mAbs TRA-2-10 and GB24 (100 μg/ml each), which recognize epitopes in the NH2-terminal and COOH-terminal repeating modules of the ectodomain, respectively (50). P+ N. gonorrhoeae, P− N. gonorrhoeae, or medium alone was added. Next, cells were analyzed for CD46 expression by FACS® at time points indicated. Values represent mean ± SD of triplicate samples from one of three similar experiments. (B) ME-180 cells were incubated for 30 min with anti-CD46 mAbs GB24 and TRA-2-10 at the concentrations shown. Cells were incubated with N401 (P+) N. gonorrhoeae at an MOI of 100 for 4 h and washed; adherent bacteria were detected by immunofluorescent staining at a magnification of 400. Images are representative of at least 10 fields from one of three separate experiments.
Mechanism of CD46 Down-regulation.
Studies of CD46 down-regulation in response to MV infection have indicated that surface expression of CD46 declines but total cellular levels of CD46 remain constant, suggesting internalization without intracellular degradation (13). However, in the case of N. gonorrhoeae–induced CD46 down-regulation, a comparison of changes in CD46 expression on the cell surface (Fig. 1 A) and in the whole cell lysates (Fig. 1 B) established that CD46 is lost not only from the cell surface but also is not detectable intracellularly. However, Western immunoblot analysis of treated cell supernatants revealed substantial quantities of CD46 with the expected molecular weight in the supernatants of cells infected with P+ bacteria (Fig. 4) . Quantitation by ELISA of CD46 in cell lysates and the corresponding concentrated cell supernatants indicated that 25–50% of the CD46 lost from infected cells was recovered in the supernatants. In addition, RT-PCR analyses established that the loss of CD46 is not accompanied by decreased quantities of CD46 mRNA (not depicted).
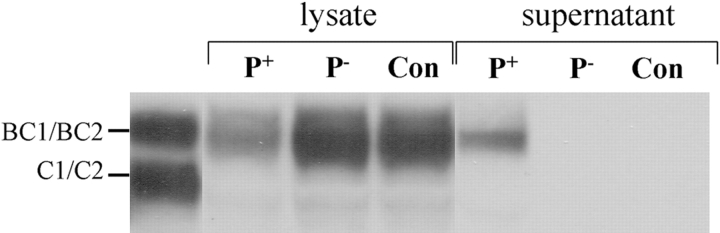
Figure 4.
CD46 is found in supernatants after down-regulation. Cell culture supernatants of ME-180 cells were harvested and concentrated eightfold (1.5 ml final volume) after infection with N401 (P+), MW4 (P−), or medium alone (Con). These supernatants and corresponding cell monolayers (solubilized in 1.5 ml lysis buffer) were analyzed for CD46 by Western immunoblot. Lane one contains solubilized preparation of Hep-2 epithelial cells. Results are representative of three independent experiments.
We also addressed the possibility that CD46 down-regulation was due to a more global loss of membrane constituents. Surface expression of DAF (CD55), HLA, and E-cadherin was assessed by FACS® under conditions that induced CD46 down-regulation (Fig. 5) . DAF, a complement regulatory protein functionally and structurally related to CD46 (for review see reference 53), showed modest down-regulation in response to P+ N. gonorrhoeae adherence. However, HLA expression remained constant whereas the cell adhesion molecule E-cadherin was up-regulated. These data indicate that the loss of CD46 is not a consequence of a generalized reduction in expression or surface presentation of membrane proteins. In addition, the coordinate down-regulation of CD46 and of the structurally and functionally related DAF protein may be related to a cooperative interaction between these proteins (54).
Figure 5.
Adherence of P+ N. gonorrhoeae also alters expression of DAF and E-cadherin but not HLA. ME-180 cells were incubated with N401 (P+) N. gonorrhoeae (MOI = 1,000), MW24 (P−) N. gonorrhoeae (MOI = 1,000), or medium alone. Surface expression of CD46, DAF, HLA, and E-cadherin were assessed by FACS®. Profiles for uninfected samples were identical to those for P− N. gonorrhoeae–infected samples and are not shown for clarity. Results are representative of three independent experiments.
PilT Is Necessary for Induction of CD46 Down-regulation.
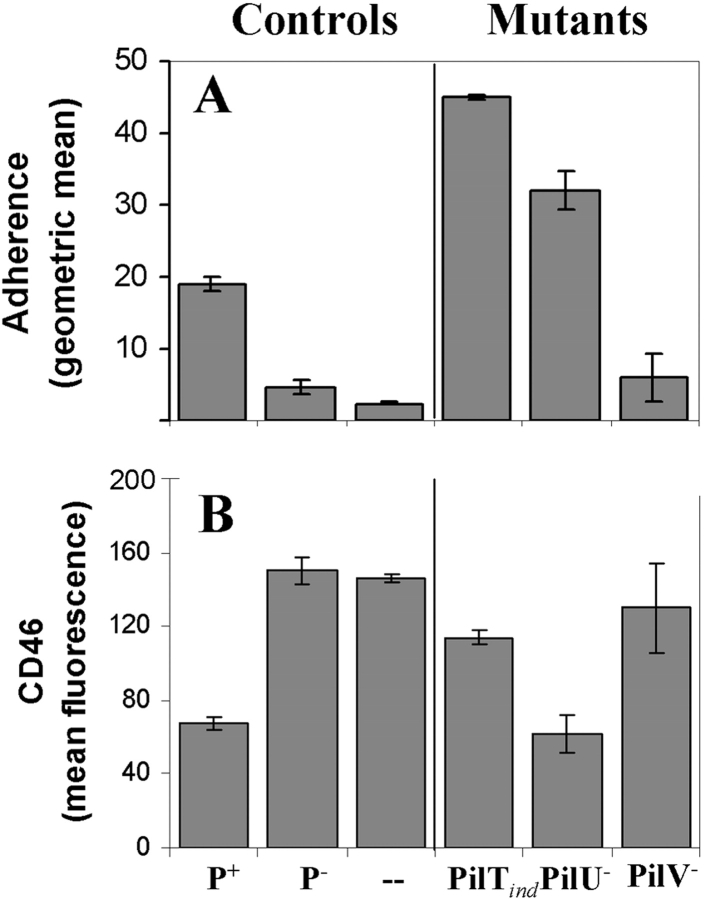
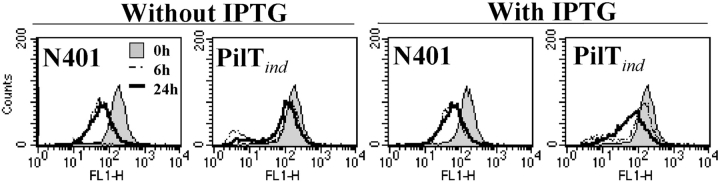
Given the dependence of CD46 down-regulation on pilus-mediated adherence, we assessed adherence levels and CD46 regulatory capabilities of mutants (Table I) lacking specific components of this organelle. The PilTind mutant (which does not express the pilus retraction protein PilT in the absence of the inducer IPTG) was hyperadherent in comparison to N401 (Fig. 6 A) and did not result in CD46 down-regulation (Fig. 6 B). De-repression of PilT restored the capacity to down-regulate CD46 in ME-180 cells (Fig. 7) , indicating that PilT was essential to the induction of CD46 down-regulation. Down-regulation of CD46 after induction of PilT expression in the PilTind mutant occurred several hours later than that seen with N401, probably reflecting either delayed or reduced expression of PilT in this mutant.
Figure 6.
CD46 down-regulation by pilus mutants of strain N401. N401 (P+) and its mutants (Table I), MW4 (P−), GT104 (PilTind), GV2 (PilV−), or GU5 (PilU−), were analyzed for adherence (A) and CD46 down-regulation (B). Adherence was assessed by FACS® at 4 h after infection (MOI = 100) whereas CD46 levels were analyzed by FACS® after incubation of bacteria with ME-180 cells for 6 and 24 h (MOI = 100). Results at 6 h (not depicted) were similar to those at 24 h. Data are expressed as mean ± SD of triplicate samples from one of three independent experiments.
Figure 7.
PilT is necessary to induce CD46 down-regulation. ME-180 cells were incubated with the PilTind mutant in the presence or absence of IPTG (for de-repression of PilT expression). CD46 expression was examined by FACS® at 0, 6, and 24 h after infection. Results are representative of three similar experiments.
Interruption of pilT expression is known to also eliminate expression of the downstream gene pilU, which encodes a protein that contributes to pilus-mediated adherence and autoaggregation (55). Therefore, it was necessary to evaluate CD46 down-regulation by PilU− N. gonorrhoeae to ensure that the loss of CD46 down-regulation in the PilTind mutant was not due to disruption of PilU expression. The PilU− mutant, which expresses retractile pili (55), was also hyperadherent (Fig. 6 A) and induced CD46 down-regulation similar to that seen with the control strain N401 (Fig. 6 B), establishing that disruption of pilU gene expression does not account for loss of CD46 down-regulation observed for PilT− N. gonorrhoeae.
Together, these results imply a correlation between adherence, retractile Tfp, and CD46 down-regulation. Given the role of the pilus-associated protein and putative adhesin PilC in promoting adherence (28, 30), we sought to examine a potential role for PilC in induction of CD46 down-regulation. However, it was not possible to test mutants expressing retractile Tfp in the absence of PilC as the requirement for PilC in pilus biogenesis can only be suppressed in a PilT null background (45). Alternatively, we assessed a PilV null mutant that retains expression of retractile Tfp but adheres inefficiently due to a defect in presentation or exposure of PilC (56). This mutant did not induce CD46 down-regulation (Fig. 6 B). Based on these data, we conclude that CD46 down-regulation is reliant on expression of PilT and proper presentation of PilC.
CD46 Down-regulation by In Vivo–derived Variants of N. gonorrhoeae Strain FA1090.
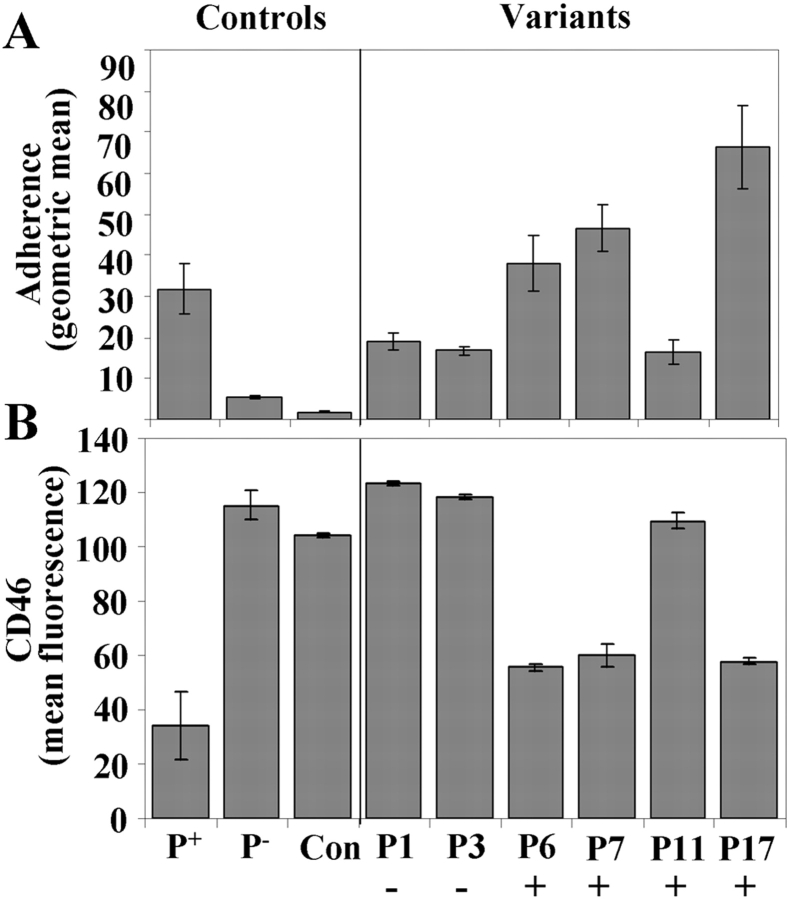
To determine if induction of CD46 down-regulation was induced by N. gonorrhoeae strains other than N401, the in vivo–derived variants of N. gonorrhoeae strain FA1090 (Table I) were tested for their ability to adhere to and induce CD46 down-regulation in ME-180 cells (Fig. 8) . Variants P3, P6, P7, P11, and P17 were isolated from experimentally infected male volunteers at various times after urethral inoculation with the variant designated P1 and are representative of variants arising in vivo expressing new pilin sequences (unpublished data). Microscopic examination and FACS®-based adherence assays revealed low levels of adherence for variants P1, P3, and P11 and high levels of adherence for variants P6, P7, and P17 when compared with adherence of strain N401 (Fig. 8 A). Incubation of ME-180 cells with those variants that adhered well to ME-180 resulted in CD46 down-regulation similar to that induced by N401, whereas the three remaining variants (all of which showed lower levels of adherence) did not induce CD46 down-regulation (Fig. 8 B). These results are consistent with the requirement for bacterial adherence for CD46 down-regulation and indicate that this response is not induced solely by N401 and its derivatives. Although not all of the in vivo–derived variants induced CD46 down-regulation, down-regulation occurred with three of the five variants that were reisolated from human volunteers after experimental inoculation with the non–down-regulating variant P1. In addition, one of the non–down-regulating variants (P3) was only isolated early in the experimental infection. Attachment of three of the four variants that persisted late in infection (P6, P7, P11, and P17), led to CD46 down-regulation. Together, these results suggest that those variants favored by in vivo selective pressures in the human male urethra are capable of CD46 down-regulation.
Figure 8.
Adherence levels and CD46 down-regulation by clinically relevant variants of N. gonorrhoeae strain FA1090. ME-180 cells were incubated with six variants of N. gonorrhoeae strain FA1090, strains N401 (P+) or MW24 (P−), or medium alone (Con). Variants P3, P6, P7, P11, and P17 (Table I) were isolated from male volunteers after inoculation with the variant designated P1. FACS® analysis of adherence (4 h after infection; MOI = 100) (A) and CD46 expression (6 h after infection; MOI = 1,000) (B). CD46 levels after a 24-h incubation were similar to those after a 6-h incubation (not depicted). Results are expressed as mean ± SD of triplicate samples from one of three independent experiments. Those variants that were detectable at the time urethritis developed (day 3 or 4) are indicated with a positive sign whereas the inoculating strain (P1) and the variant (P3) that were detected early in infection (day 1) but not at the time of urethritis are indicated with a minus sign. Characterization of Opa expression of these variants revealed no correlation between expression of a specific Opa protein and the ability of a variant to induce CD46 down-regulation (not depicted), indicating that the observed differences in adherence and CD46 down-regulation are most likely attributable to variations in their pilin sequences.
Discussion
We describe the down-regulation of CD46 after pilus-mediated adherence of N. gonorrhoeae to ME-180 cells. This effect was reliant on bacterial adherence, requiring an MOI of 10 or greater, was not inhibited by mAbs to CD46, and was accompanied by the accumulation of CD46 in the supernatant. The down-regulatory response was induced by the laboratory strain N401 as well as by P+, in vivo–derived variants of strain FA1090 and was inhibited by null mutations in the twitching motility protein PilT.
Although the mechanism underlying this reduction in CD46 expression remains under investigation, we have addressed several processes that could account for this down-regulation. In ME-180 cells challenged with P+ gonococci, both surface presentation and total cellular content of CD46 were reduced with similar kinetics, indicating that CD46 is not internalized and maintained as such within the cell. In addition, we conclude that after incubation with P+ gonococci, CD46 down-regulation was not due to a global reduction in expression or surface presentation of membrane proteins as was illustrated by the unchanged expression of HLA and up-regulation of E-cadherin. In addition, RT-PCR demonstrated that CD46 down-regulation does not coincide with a specific decrease in CD46 mRNA. However, substantial quantities of CD46 (at least 25–50% of that lost from the surface) were found in the media of infected cells. The molecular weight of CD46 in the supernatant is not detectably altered, arguing against a proteolytic cleavage event. Therefore, we currently favor and are pursuing the hypothesis that CD46 is lost from the cell surface into the supernatant via a shedding mechanism.
The down-regulation of CD46 resulting from pilus-mediated adhesion requires expression of the twitching motility protein PilT and is manifest 3.5–6 h after addition of bacteria. Certain other processes related to pilus-mediated adherence of N. gonorrhoeae show similar delayed kinetics and dependence on expression of PilT. For example, the PilT-dependent formation of cortical actin plaques begins within minutes of bacterial adhesion but is not maximal until 4–6 h after infection (43, 57). In addition, the progression from pilus-mediated adherence to intimate association by Neisseria meningitidis (58), which occurs in conjunction with a reduction in piliation and by tight juxtaposition of the bacterial and host cell membranes (for review see reference 23), is also PilT dependent and occurs between 4 and 9 h after infection. Due to variation in cell type and bacterial strain used for these studies, conclusions can only be cautiously drawn on the interrelatedness of CD46 down-regulation, cortical actin plaque formation, progression to intimate association, and other cellular responses for attachment of N. gonorrhoeae. Nevertheless, an attractive explanation for the role of PilT in inducing all three responses could involve tensile forces exerted by retractile pili inducing mechanosensory signaling responses in mammalian cells, as proposed by Merz and So (23).
Although MV and N. gonorrhoeae infection induce a similar modulation of CD46 expression, there were some notable differences regarding the microbial constituents necessary to induce this response. Modulation of CD46 expression by MV requires a direct, protein–protein interaction between the NH2-terminal region of CD46 and the MV hemagglutinin (12, 59). Inhibition of this interaction with mAbs to either protein prevents MV adherence as well as the induction of CD46 down-regulation (17, 18, 15, 19, 20). In contrast, our data from three lines of experimentation suggest that direct contact between CD46 and N. gonorrhoeae pili may not be necessary in the induction of N. gonorrhoeae–induced CD46 down-regulation or in adhesion of N. gonorrhoeae to host cells. We first demonstrated that preincubation of ME-180 cells with multiple mAbs to CD46, including those that are known to inhibit MV adherence (e.g., TRA-2-10; references 16–20) as well as those that have been described to inhibit N. gonorrhoeae attachment (e.g., GB24; reference 7), did not result in inhibition of N. gonorrhoeae adherence or the resulting down-regulation of CD46 (Fig. 3). Second, studies using ELISA and an overlay assay showed no direct interaction between purified pili and CD46 (unpublished data). Finally, upon examination of gonococcal adhesion to multiple cell lines, our data support the previously observed inverse correlation between CD46 expression and bacterial adhesion (36). The cell lines that supported high levels of N. gonorrhoeae adhesion (despite comparatively low levels of CD46 expression) also exhibited N. gonorrhoeae–induced down-regulation of CD46. As these data collectively suggest that N. gonorrhoeae adherence does not require a direct interaction between the N. gonorrhoeae pilus and CD46, we speculate that N. gonorrhoeae adherence and the induction of CD46 down-regulation may be reliant on interactions of bacterial adhesins with other as yet unidentified cell surface molecules. These molecules may not be present in cell lines that display neither high levels of gonococcal adhesion nor N. gonorrhoeae–induced CD46 down-regulation.
The role of PilT in pilus retraction coupled with the necessity for PilT expression in the induction of CD46 down-regulation suggests that pilus retraction contributes to the induction of CD46 down-regulation. If an active bacterial response such as pilus retraction is necessary for CD46 down-regulation, activation of a cellular receptor through static binding of a bacterial adhesin could be an insufficient stimulus. Supporting evidence for this line of reasoning comes from the inability of the PilV− mutant to elicit this response. Loss-of-function mutants in the minor pilin subunit PilV retain retractile pili yet display reduced-affinity interactions with ME-180 cells, a property attributed to a concomitant defect in presentation of the adhesin PilC (56). The absence of CD46 down-regulation despite normal retractile capability of the PilV− pilus, coupled with the necessity of PilT in induction of this response, implies a simultaneous requirement for high-affinity adhesion and pilus retraction in the induction of CD46 down-regulation. This implies that, in contrast to the interaction between MV hemagglutinin and CD46, a static interaction between gonococcal adhesins and host cell surface receptors is insufficient to induce CD46 down-regulation.
Several pilus-associated properties influencing Neisseria adherence to human epithelial cells are affected by sequence variation in pilE arising from natural antigenic variation (for review see references 60, 61). Examination of pilE sequences after passage of N. gonorrhoeae strain FA1090 through the human host indicates rapid shifts in predominant pilin sequence (not observed upon in vitro passage), suggesting strong selective pressures for specific attributes of the gonococcal pilin (49). In this paper, induction of CD46 down-regulation by variants of strain FA1090 that had been reisolated from human hosts after inoculation with a non–down-regulating strain (variant P1) point to selective pressures for gonococci capable of modulating CD46 expression. Based on the dependence of CD46 down-regulation on pilus expression (as per experiments herein with pilus mutants of strain N401) combined with analyses of the effects of antigenic variation in PilE on in vivo selection and adherence to epithelial cells (27–29), alterations in the pilE sequence likely account for differences in CD46 modulatory capabilities of the in vivo–derived variants. Future papers will assess these variations in PilE as well as differences in PilT and PilC among the six FA1090 variants used in this work.
Although it remains to be determined if the loss of CD46 aids or hinders gonococcal pathogenesis, we propose that this process favors bacterial virulence and requires cross-talk between the pilus and CD46. The mechanism by which CD46 is shed from the cell membrane will be one focus of our investigations, as delineating this process may be fundamental to increasing our understanding of this interaction between a major human pathogen and CD46 of host cells.
Acknowledgments
The authors thank M.K. Liszewski for helpful input and M. Bogacki for editorial support.
This work was supported in part by funding from the National Institutes of Health (RO1 AI37618 to J.P. Atkinson and RO1 AI3496 to J. Cannon).
Abbreviations used in this paper: DAF, decay-accelerating factor; IPTG, isopropyl-β-d-thiogalactopyranoside; MOI, multiplicity of infection; MV, measles virus; P+, piliated; P−, nonpiliated; Tfp, Type IV pilus.
References
- 1.Lindahl, G., U. Sjobring, and E. Johnsson. 2000. Human complement regulators: a major target for pathogenic microorganisms. Curr. Opin. Immunol. 12:44–51. [DOI] [PubMed] [Google Scholar]
- 2.Liszewski, M.K., T.W. Post, and J.P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431–455. [DOI] [PubMed] [Google Scholar]
- 3.Naniche, D., G. Varior-Krishnan, F. Cervoni, T.F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorig, R.E., A. Marcil, A. Chopra, and C.D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 75:295–305. [DOI] [PubMed] [Google Scholar]
- 5.Okada, N., M.K. Liszewski, J.P. Atkinson, and M. Caparon. 1995. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc. Natl. Acad. Sci. USA. 92:2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro, F., P.E. Kennedy, G. Locatelli, M.S. Malnati, E.A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell. 99:817–827. [DOI] [PubMed] [Google Scholar]
- 7.Kallstrom, H., M.K. Liszewski, J.P. Atkinson, and A.B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639–647. [DOI] [PubMed] [Google Scholar]
- 8.Karp, C.L., M. Wysocka, L.M. Wahl, J.M. Ahearn, P.J. Cuomo, B. Sherry, G. Trinchieri, and D.E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 273:228–231. (published erratum appears in Science. 1997. 275:1053) [DOI] [PubMed] [Google Scholar]
- 9.Hirano, A., Z. Yang, Y. Katayama, J. Korte-Sarfaty, and T.C. Wong. 1999. Human CD46 enhances nitric oxide production in mouse macrophages in response to measles virus infection in the presence of gamma interferon: dependence on the CD46 cytoplasmic domains. J. Virol. 73:4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama, Y., A. Hirano, and T.C. Wong. 2000. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating production of alpha/beta interferon. J. Virol. 74:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yant, S., A. Hirano, and T.C. Wong. 1997. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J. Virol. 71:766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krantic, S., C. Gimenez, and C. Rabourdin-Combe. 1995. Cell-to-cell contact via measles virus haemagglutinin-CD46 interaction triggers CD46 downregulation. J. Gen. Virol. 76:2793–2800. [DOI] [PubMed] [Google Scholar]
- 13.Naniche, D., T.F. Wild, C. Rabourdin-Combe, and D. Gerlier. 1993. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J. Gen. Virol. 74:1073–1079. [DOI] [PubMed] [Google Scholar]
- 14.Schneider-Schaulies, J., J.J. Schnorr, U. Brinckmann, L.M. Dunster, K. Baczko, U.G. Liebert, S. Schneider-Schaulies, and V. ter Meulen. 1995. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc. Natl. Acad. Sci. USA. 92:3943–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider-Schaulies, J., L.M. Dunster, F. Kobune, B. Rima, and V. ter Meulen. 1995. Differential downregulation of CD46 by measles virus strains. J. Virol. 69:7257–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider-Schaulies, J., J.J. Schnorr, J. Schlender, L.M. Dunster, S. Schneider-Schaulies, and V. ter Meulen. 1996. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J. Virol. 70:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manchester, M., A. Valsamakis, R. Kaufman, M.K. Liszewski, J. Alvarez, J.P. Atkinson, D.M. Lublin, and M.B. Oldstone. 1995. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46). Proc. Natl. Acad. Sci. USA. 92:2303–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seya, T., M. Kurita, T. Hara, K. Iwata, T. Semba, M. Hatanaka, M. Matsumoto, Y. Yanagi, S. Ueda, and S. Nagasawa. 1995. Blocking measles virus infection with a recombinant soluble form of, or monoclonal antibodies against, membrane cofactor protein of complement (CD46). Immunology. 84:619–625. [PMC free article] [PubMed] [Google Scholar]
- 19.Devaux, P., and D. Gerlier. 1997. Antibody cross-reactivity with CD46 and lack of cell surface expression suggest that moesin might not mediate measles virus binding. J. Virol. 71:1679–1682. (published erratum appears in J. Virol. 1997. 71:4186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christiansen, D., P. Devaux, B. Reveil, A. Evlashev, B. Horvat, J. Lamy, C. Rabourdin-Combe, J.H. Cohen, and D. Gerlier. 2000. Octamerization enables soluble CD46 receptor to neutralize measles virus in vitro and in vivo. J. Virol. 74:4672–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tishon, A., M. Manchester, F. Scheiflinger, and M.B. Oldstone. 1996. A model of measles virus-induced immunosuppression: enhanced susceptibility of neonatal human PBLs. Nat. Med. 2:1250–1254. [DOI] [PubMed] [Google Scholar]
- 22.Schnorr, J.J., L.M. Dunster, R. Nanan, J. Schneider-Schaulies, S. Schneider-Schaulies, and V. ter Meulen. 1995. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur. J. Immunol. 25:976–984. [DOI] [PubMed] [Google Scholar]
- 23.Merz, A.J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423–457. [DOI] [PubMed] [Google Scholar]
- 24.Koomey, M., E.C. Gotschlich, K. Robbins, S. Bergstrom, and J. Swanson. 1987. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 117:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehr, I.J., and H.S. Seifert. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Mol. Microbiol. 30:697–710. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Q.Y., D. DeRyckere, P. Lauer, and J.M. Koomey. 1992. Gene conversion in Neisseria gonorrhoeae: evidence for its role in pilus antigenic variation. Proc. Natl. Acad. Sci. USA. 89:5366–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virji, M., and J.E. Heckels. 1984. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J. Gen. Microbiol. 130:1089–1095. [DOI] [PubMed] [Google Scholar]
- 28.Rudel, T., J.P. van Putten, C.P. Gibbs, R. Haas, and T.F. Meyer. 1992. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol. Microbiol. 6:3439–3450. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson, A.B., D. Ilver, P. Falk, J. Pepose, and S. Normark. 1994. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 13:403–416. [DOI] [PubMed] [Google Scholar]
- 30.Rudel, T., I. Scheurerpflug, and T.F. Meyer. 1995. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 373:357–359. [DOI] [PubMed] [Google Scholar]
- 31.Rahman, M., H. Kallstrom, S. Normark, and A.B. Jonsson. 1997. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol. Microbiol. 25:11–25. [DOI] [PubMed] [Google Scholar]
- 32.Jonsson, A.B., G. Nyberg, and S. Normark. 1991. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 10:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonsson, A.B., J. Pfeifer, and S. Normark. 1992. Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of pili. Proc. Natl. Acad. Sci. USA. 89:3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudel, T., D. Facius, R. Barten, I. Scheuerpflug, E. Nonnenmacher, and T.F. Meyer. 1995. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA. 92:7986–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfgang, M., J.P. van Putten, S.F. Hayes, D. Dorward, and M. Koomey. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobiason, D.M., and H.S. Seifert. 2001. Inverse relationship between pilus-mediated gonococcal adherence and surface expression of the pilus receptor, CD46. Microbiol. 147:2333–2340. [DOI] [PubMed] [Google Scholar]
- 37.Kallstrom, H., M.S. Islam, P.O. Berggren, and A.B. Jonsson. 1998. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 273:21777–21782. [DOI] [PubMed] [Google Scholar]
- 38.Lee, S.W., R.A. Bonnah, D.L. Higashi, J.P. Atkinson, S.L. Milgram, and M. So. 2002. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. J. Cell Biol. 156:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallstrom, H., D. Blackmer Gill, B. Albiger, M.K. Liszewski, J.P. Atkinson, and A.B. Jonsson. 2001. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell. Microbiol. 3:133–143. [DOI] [PubMed] [Google Scholar]
- 40.Dunn, K.L., M. Virji, and E.R. Moxon. 1995. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb. Pathog. 18:81–96. [DOI] [PubMed] [Google Scholar]
- 41.McGee, Z.A., A.P. Johnson, and D. Taylor-Robinson. 1981. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 143:413–422. [DOI] [PubMed] [Google Scholar]
- 42.Ayala, P., L. Lin, S. Hopper, M. Fukuda, and M. So. 1998. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect. Immun. 66:5001–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merz, A.J., C.A. Enns, and M. So. 1999. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32:1316–1332. [DOI] [PubMed] [Google Scholar]
- 44.Merz, A.J., M. So, and M.P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature. 407:98–102. [DOI] [PubMed] [Google Scholar]
- 45.Wolfgang, M., P. Lauer, H.S. Park, L. Brossay, J. Hebert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330. [DOI] [PubMed] [Google Scholar]
- 46.Swanson, J., S.J. Kraus, and E.C. Gotschlich. 1971. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J. Exp. Med. 134:886–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen, M.S., J.G. Cannon, A.E. Jerse, L.M. Charniga, S.F. Isbey, and L.G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532–537. [DOI] [PubMed] [Google Scholar]
- 48.Jerse, A.E., M.S. Cohen, P.M. Drown, L.G. Whicker, S.F. Isbey, H.S. Seifert, and J.G. Cannon. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamrick, T.S., J.A. Dempsey, M.S. Cohen, and J.G. Cannon. 2001. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiol. 147:839–849. [DOI] [PubMed] [Google Scholar]
- 50.Liszewski, M.K., M. Leung, W. Cui, V. Bala Subramanian, J. Parkinson, P.N. Barlow, M. Manchester, and J.P. Atkinson. 2000. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46). J. Biol. Chem. 275:37692–37701. [DOI] [PubMed] [Google Scholar]
- 51.Liszewski, M.K., and J.P. Atkinson. 1996. Membrane cofactor protein (MCP; CD46). Isoforms differ in protection against the classical pathway of complement. J. Immunol. 156:4415–4421. [PubMed] [Google Scholar]
- 52.Wang, G., M.K. Liszewski, A.C. Chan, and J.P. Atkinson. 2000. Membrane cofactor protein (MCP; CD46) isoform-specific tyrosine phosphorylation. J. Immunol. 164:1839–1846. [DOI] [PubMed] [Google Scholar]
- 53.Liszewsi, M.K., and J.P. Atkinson. 1998. Membrane cofactor protein (CD46) and decay-accelerating factor (CD55). The Complement System. K. Rother, G.O. Till, and G.M. Hansch, editors. Springer Velag Berlin, Heidelberg. 146–162.
- 54.Brodbeck, W.G., C. Mold, J.P. Atkinson, and M.E. Medof. 2000. Cooperation between decay-accelerating factor and membrane cofactor protein in protecting cells from autologous complement attack. J. Immunol. 165:3999–4006. [DOI] [PubMed] [Google Scholar]
- 55.Park, H.S., M. Wolfgang, and M. Koomey. 2002. Modification of type IV pilus-associated epithelial cell adherence and multicellular behavior by the PilU protein of Neisseria gonorrhoeae. Infect. Immun. 70:3891–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winther-Larsen, H.C., F.T. Hegge, M. Wolfgang, S.F. Hayes, J.P. van Putten, and M. Koomey. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. USA. 98:15276–15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merz, A.J., and M. So. 1997. Attachment of piliated, Opa- and Opc-gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect. Immun. 65:4341–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pujol, C., E. Eugene, M. Marceau, and X. Nassif. 1999. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc. Natl. Acad. Sci. USA. 96:4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galbraith, S.E., A. Tiwari, M.D. Baron, B.T. Lund, T. Barrett, and S.L. Cosby. 1998. Morbillivirus downregulation of CD46. J. Virol. 72:10292–10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seifert, H.S. 1996. Questions about gonococcal pilus phase and antigenic variation. Mol. Microbiol. 21:433–440. [DOI] [PubMed] [Google Scholar]
- 61.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32:1124–1132. [DOI] [PubMed] [Google Scholar]