Abstract
Macrophage clearance is essential for the resolution of inflammation. Much is known about how monocytes enter the inflammatory site but little is known about how resultant macro-phages are cleared. We have previously demonstrated that macrophage clearance from resolving peritonitis occurs by emigration into draining lymphatics rather than local apoptosis. We now examine mechanisms for this process, in particular by evaluating the hypothesis that modulation of adhesion interactions between macrophages and cells lining the lymphatics regulates the rate of macrophage clearance. We demonstrate in vivo that macrophages adhere specifically to mesothelium overlying draining lymphatics and that their emigration rate is regulated by the state of macrophage activation. We observed that macrophage–mesothelial adhesion is Arg-Gly-Asp (RGD) sensitive and partially mediated by very late antigen (VLA)-4 and VLA-5 but not αv or β2 integrins. Moreover, macrophage clearance into lymphatics can be blocked in vivo by RGD peptides and VLA-4 and VLA-5 but not β2 blocking antibodies. This is the first evidence that macrophage emigration from the inflamed site is controlled and demonstrates that this is exerted through specific adhesion molecule regulation of macrophage–mesothelial interactions. It highlights the importance of adhesion molecules governing entry of cells into the lymphatic circulation, thus opening a new avenue for manipulating the resolution of inflammation.
Keywords: peritonitis, integrins, lymphatic system, cell movement, receptors-very late antigen
Introduction
The mechanisms regulating the resolution of inflammation have been studied in detail for the neutrophil (1). However, the role of the macrophage in this process has received little attention. Macrophage clearance in acute resolving peritonitis is known to occur by emigration to the draining lymphatics rather than by local apoptosis, but the mechanisms regulating this are unknown (2). Kinetic studies on macrophages within the peritoneum imply that resident and inflammatory macrophages have dramatically different half-lives, which suggests that macrophage clearance might be regulated, a concept that has not been previously investigated (2, 3). Macrophages are central to effective wound healing (4). However, their accumulation is also a hallmark of chronic inflammation where they can potentiate tissue damage (5). Moreover, the elimination of macrophages from sites of chronic inflammation can lead rapidly to resolution of inflammation (6). Hence, an understanding of the process of macrophage emigration from the inflamed site is essential as it may impact directly upon the speed of inflammatory resolution and tissue injury.
In peritonitis, monocyte influx occurs directly across the encompassing peritoneal mesothelial lining and is regulated by adhesion molecules expressed both on the vascular endothelium and mesothelial cells (7). Efflux is believed to occur through milky spots found mainly on the subdiaphragmatic surface and omentum (2). These provide small openings in the mesothelial barrier and are intimately associated with the draining lymphatics (8).
From these observations, we hypothesized that in the resolution of peritonitis, specific adhesion molecules regulate macrophage adhesion to the mesothelium overlying the draining peritoneal lymphatics before their emigration. In addition, this adherence and transmigration provides a regulatory mechanism governing the rate of macrophage clearance. We examined the extent to which murine macrophage clearance is regulated in vivo then, using a novel ex vivo system, Arg-Gly-Asp (RGD) peptides (9) and blocking antibodies, determined the adhesion molecules involved. The role of these molecules in regulating macrophage clearance was finally tested in our in vivo peritonitis model.
Materials and Methods
Reagents and Antibodies.
All reagents, unless stated otherwise, were obtained from Sigma-Aldrich, plasticware and antibodies were from BD Biosciences, and culture media were from Life Technologies. The following antibodies were used: CD11a (M17/4), CD11b (M1/70, 5C6), CD18 (GAME-46, 2E6; American Type Culture Collection), CD49d (PS/2, R1-2, 9C10), CD49e (5H10-27), CD51 (H9.2B8), and F4/80 (Serotec). FITC-conjugated CD11c (HL3) and biotinylated Iad (MHC class II) were provided by D. Katz, University College London, London, United Kingdom. Isotype controls were: IgG1 (LO-DNP-1; Serotec), IgG2a (LO-DNP-16; Serotec), IgG2b (LO-DNP-11; Serotec), hamster IgM and IgG, and R-phycoerythrin–conjugated F(ab')2 goat anti–rat second layer (Star 73; Serotec). Antibodies for adhesion and in vivo studies were azide free and low endotoxin.
In Vivo Studies of Regulation of Macrophage Clearance.
BALB/c (H-2d) and C3HF/KAM (H-2k) mice (22–28 g) were used for joint resident/inflammatory macrophage studies. All other experiments used ICR mice (Harlan).
Comparison of Resident and Inflammatory Macrophage Clearance.
Intraperitoneal injection of 1 ml 4% thioglycollate (TG; Difco) induced a sterile peritonitis with leukocyte numbers (>93% macrophages) peaking on day 5 and declining to baseline by day 10. H-2d peritoneal phagocytes were labeled in vivo by 1 ml 0.5 μM red fluorescent marker PKH26-PCL intraperitoneally 5 d after TG2. This labeled >87% of phagocytes and was cleared rapidly so no label was acquired by fresh H-2k macrophages instilled 2 h later and was distinguished by flow cytometry using FITC-conjugated anti–H-2Dd IgM and FITC-conjugated anti–H-2Kk IgG2a with FITC-conjugated anti-CD15 IgM and FITC-conjugated anti-CD45RO IgG2a as controls (Dako). Resident phagocytes were labeled in vivo by 1 ml 0.5 μM green fluorescent dye PKH2-PCL intraperitoneally in the absence of TG.
Fluorescent resident and inflammatory macrophages were lavaged, mixed in a 1:1 ratio of red/green cells (confirmed by flow cytometry) and 2.5 × 106 or 1.25 × 107 cells instilled intraperitoneally into mice with no inflammation or 5 d after TG. 3 or 10 d later abdominal wall, spleen, liver, kidney, para-aortic and parathymic lymph nodes, and heart and lung were harvested for frozen sectioning. Macrophage totals in peritoneal lavage were determined from cell counts and differential counts from cytospins, counting >400 cells per slide. Resident and inflammatory macrophage numbers were determined by dual color flow cytometry from percent of green and red cells in gates generated on CD11b and F4/80+ cells. Flow cytometry demonstrated <2.5% of the PKH26-PCL–labeled population was CD11c+ whereas biotinylated staining showed <8% positive for MHC class II antigen. Therefore, few of the fluorescent cells migrating to the lymph nodes late in inflammation were of dendritic phenotype.
In Vivo Modulation of Inflammatory Macrophage Clearance.
1 ml of test agents (GRGDTP peptide [RGD], control RGES peptide [RGE], PBS, or antibodies) were injected intraperitoneally 2 and 26 h after fluorescently labeling day 5 macrophages. 24 h later cells were lavaged, macrophages counted, and adhesion molecule surface expression determined by indirect immunofluorescence. Mesothelial lining imprints of cecum, spleen, and liver were prepared by applying air-dried tissue onto 5% gelatin-coated slides and labeled macrophages on these imprints were quantified blindly by two observers. Parathymic lymph nodes were snap frozen and paired and serial 7-μm frozen sections were cut at 280 μm intervals spanning the whole node. Sections were scored blindly from 0 (no labeled cells) to 8 (maximum labeled cells) by two observers using predefined control slides. In all cases interobserver variability was <15% and the distribution of cells within nodes was unaffected by treatments.
To outline lymphatics, TG challenged mice were injected intraperitoneally with 1 ml India ink or PBS. 30 min later PKH26-PCL–labeled macrophages were instilled and 1 h later the diaphragm was dissected, washed, and the distribution of India ink and macrophages was determined.
Ex Vivo Macrophage–Mesothelial Adhesion.
Macrophages from mice 5 d after TG, were labeled in vitro using 5-chloromethylfluorescein diacetate (Cambridge Bioscience). Diaphragms from the same mice were washed, clamped in customized rings (5 mm internal diameter retaining 100 μl above and 75 μl below the surface), and orientated with peritoneal mesothelial surface uppermost. Rings were incubated with test medium for 30 min at 37°C while 1.5–3 × 104 labeled cells were preblocked by rotating with test medium for 30 min at 4°C before being added to the upper chamber in Dulbecco's phosphate-buffered saline. After 1 h, the rings were washed gently to remove nonadherent cells and adherent cells were counted. Mesothelial integrity was confirmed by en face silver staining of intercellular junctions as previously described (10). To identify lymphatics in ex vivo diaphragmatic tissue, 100 μl media containing 2 μl India ink was added to the upper chamber, incubated for 30 min, and washed before the addition of labeled cells.
Determination of Apoptosis.
To determine RGD-induced apoptosis, cells lavaged 5 d after TG were incubated in Iscove's modified DMEM supplemented with 10% FCS, l-glutamine, 100 U penicillin, and streptomycin in 8-well slides at 1.5 × 105 cells/well and up to 0.5 mg/ml RGD or RGE. Cells were examined for up to 48 h and apoptosis was quantified by nuclear morphology and staining with acridine orange (10 μg/ml).
Statistics.
Data are expressed as mean ± SEM with comparison by two-tailed Mann-Whitney U test. Differences were considered significant if P < 0.05.
Results
Macrophage Emigration Is Regulated and Depends on Activation State.
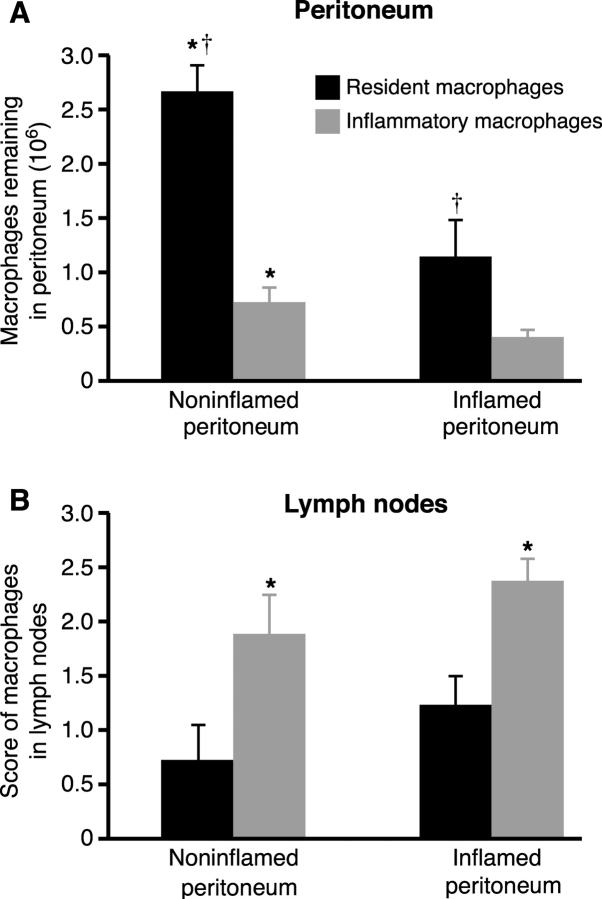
Clearance rates of noninflamed (resident) and inflammatory macrophages in the presence or absence of inflammation were compared by instilling equal numbers into the peritoneum in the presence/absence of resolving peritonitis and determining the number remaining after 3 (Fig. 1 A) and 10 d. Recovery of resident macrophages always exceeded that of inflammatory macrophages with over three and a half times more resident than inflammatory macrophages recovered from the noninflamed peritoneum (2.65 ± 0.25 × 106 vs. 0.72 ± 0.14 × 106, P < 0.005) and nearly three times more from the inflamed peritoneum (1.14 ± 0.3 × 106 vs. 0.4 ± 0.07 × 106, P < 0.05). Resolving peritonitis dramatically augmented the clearance of all macrophages with significantly more resident (P < 0.005) and inflammatory (P < 0.05) macrophages recovered from the noninflamed than the inflamed peritoneum after 3 d. Differences between resident and inflammatory macrophage recovery persisted when examined after 10 d (0.58 ± 0.2 × 106 vs. 0.03 ± 0.03 × 106, P < 0.005 from noninflamed and 0.26 ± 0.07 × 106 vs. 0.013 ± 0.007 × 106, P < 0.01 from inflamed peritoneum; n = 6/group). These differences were maintained when fivefold fewer cells were transferred (n = 3/group, unpublished data).
Figure 1.
Macrophage emigration is regulated. A total of 12.5 × 106 fluorescent green–labeled resident and red-labeled inflammatory macrophages were instilled into the peritoneal cavity of mice in the absence of peritonitis (noninflamed peritoneum) or mice with resolving peritonitis (inflamed peritoneum). (A) The number of resident (black bars) and inflammatory (gray bars) macrophages recovered from the peritoneal cavity after 3 d is shown. † significantly greater resident than inflammatory macrophage recovery (P < 0.05, n = 8/group). *, significantly greater recovery from noninflamed than inflamed peritoneum (P < 0.05). (B) Fluorescent-labeled macrophages in draining parathymic lymph nodes from the same mice. *, significantly more inflammatory than resident macrophages (P < 0.05, n = 8/group).
Fig. 1 B demonstrates that reduced peritoneal recovery of inflammatory macrophages was due to faster emigration into the draining lymphatics as there were always significantly more inflammatory than resident macrophages in the parathymic nodes. Analysis of the peritoneal lining of the abdominal wall, spleen, liver, kidney, and para-aortic lymph nodes for fluorescent cells verified that differences were not due to widespread nonspecific intra-abdominal adhesion. Consistent with reduced clearance rates in vivo, resident macrophage adhesion to noninflamed mesothelium was only 71 ± 4.1% (n = 4, P < 0.05) that of inflammatory macrophage adhesion to the inflamed mesothelium when examined ex vivo.
Macrophage–Mesothelial Adhesion Localizes to Areas Overlying Draining Lymphatics.
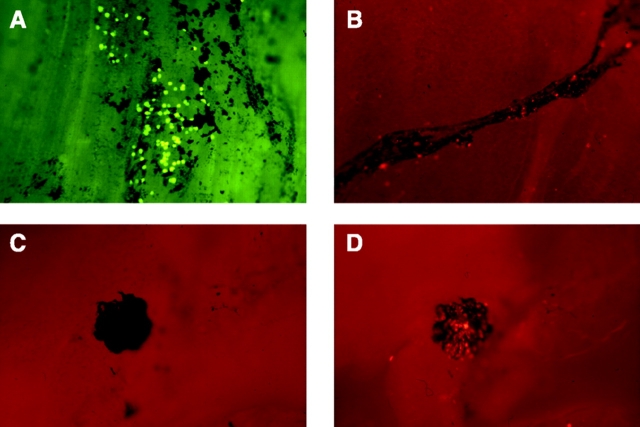
Macrophage–mesothelial adherence was patchy and markedly reduced over the mesothelium lining the tendinous compared with the muscular portion of the diaphragm, both when adhesion was examined in vivo or ex vivo. Adherence was localized to the India ink–positive areas suggesting focal adhesion to particular mesothelial cells or structures associated with the draining lymphatics. Macrophage binding to India ink–free areas was minimal (n = 3, Fig. 2 A). India ink outlined lymphatic vessels under the diaphragmatic mesothelial surface that contained emigrating macrophages by 1 h (Fig. 2 B). India ink stained multiple small patchy areas to which macrophages commonly adhered as well as classic milky spot structures to which macrophages always adhered strongly (Fig. 2, C and D). Macrophage adhesion was similarly patchy in the absence of India ink, which suggested this was not an artifact induced by the India ink particles.
Figure 2.
Macrophage–mesothelial adhesion is localized to regions overlying the draining lymphatics. (A) Ex vivo preparation of mesothelial surface of diaphragm with lymphatics localized by India ink. Adherence of inflammatory macrophages localizes to areas of mesothelium stained with India ink whereas virtually no macrophages adhere to India ink–free areas. Similar colocalization is seen when adhesion occurs in vivo. (B) Macrophages emigrating into draining lymphatics within 1 h of instillation. (C) India ink labeling of a milky spot and (D) the same section under fluorescent microscopy where fluorescent macrophages are seen to adhere (×80).
Very Late Antigen (VLA)-4 and VLA-5 Integrins Regulate Macrophage–Mesothelial Adhesion Ex Vivo.
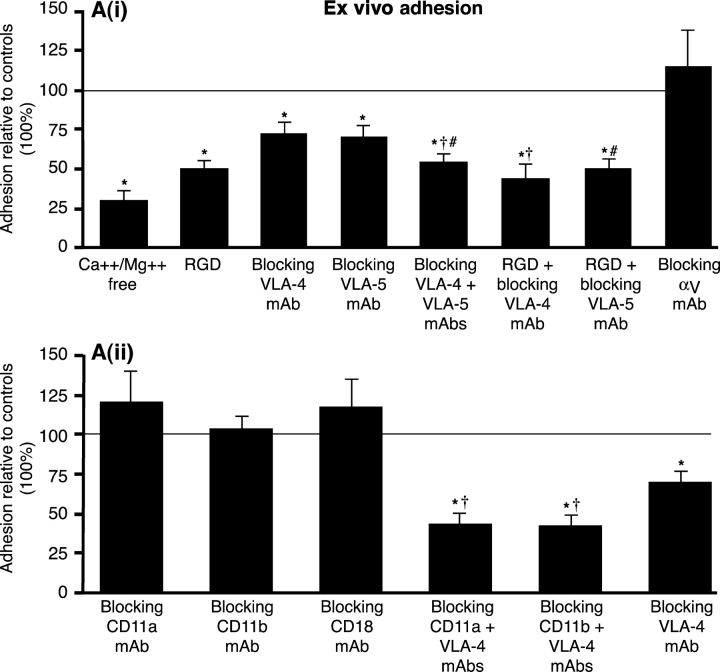
Fig. 3 A(i) demonstrates that inflammatory macrophage adhesion was reduced by 70.6 ± 6% in the absence of divalent cations (P = 0.0004), 50.5 ± 5.4% by RGD (P < 0.05), 29.2 ± 8% by blocking the α4 chain of VLA-4 (PS/2; P < 0.05), and confirmed by another combination of anti-α4 antibodies (R1-2 with 9C10, 20 μg/ml each), which decreased adhesion by 27.2 ± 6%. Blocking VLA-5 (5H10-27) significantly inhibited binding by 31.2 ± 11.8% (P < 0.05). In contrast, blocking αv, another RGD-inhibitable integrin, had no effect. The combination of blocking VLA-4 (PS/2) and VLA-5 (5H10-27) significantly increased inhibition of adhesion (46.8 ± 5.7%) over either PS/2 or 5H10-27 alone (P < 0.01). In addition, the combination of RGD with VLA-4 blocking (PS/2) appeared to increase inhibition over RGD alone, although this was not significant at the P < 0.05 level (56.9 ± 9 vs. 51.2 ± 6.2). Adhesion in the presence of RGD plus VLA-5 blocking (5H10-27) was not different to RGD alone (51.1 ± 6.8 vs. 49.8 ± 6).
Figure 3.
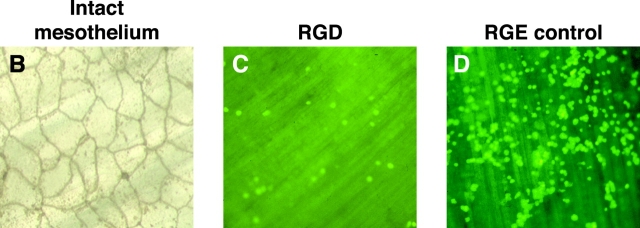
β1 integrins mediate macrophage–mesothelial adhesion. (A) (i) Macrophage–mesothelial adhesion ex vivo is significantly reduced (P < 0.05) by the following: Ca2+/Mg2+-free media (control: Ca2+/Mg2+-containing media, n = 5), 0.5 mg/ml RGD in standard media (control: RGE, n = 5), blocking: VLA-4 (PS/2 10 μg/ml, n = 6), VLA-5 (5H10–27 10 μg/ml, n = 9), VLA-4 + VLA-5 (n = 7), VLA-4 + RGD (n = 9), and VLA-5 + RGD (n = 8), but not by blocking αv (H9.2B8, 10 μg/ml, n = 6). Controls (isotype monoclonals, F4/80 and/or RGE) represent 100% adhesion. Adhesion significantly reduced compared to the following: controls (*), blocking VLA-4 (†), and blocking VLA-5 (#). (ii) Blocking β2 integrins: CD11a (M17/4, n = 8), CD11b (M1/70, 5C6 both n = 6), and CD18 (GAME-46, 2E6 both n = 9) do not inhibit adhesion, however blocking both β1 and β2 integrins, VLA-4 + CD11a (n = 6) and VLA-4 + CD11b (M1/70, n = 6), inhibits adhesion significantly more than blocking β1 integrins alone (PS/2, n = 6). Adhesion was significantly reduced (P < 0.05) compared to the following: controls (*) and blocking VLA-4 (†). (B) En face silver staining demonstrating the integrity of mesothelial surface after 6 h is shown (×300). (C and D) Adhesion of fluorescent-labeled macrophages to mesothelium ex vivo in the presence of RGD or RGE, which shows the ease of visual quantification of assay (×100).
Importantly, Fig. 3 A(ii) shows that blocking β2 integrin function with CD11a (M17/4), CD11b (5C6, M1/70), and CD18 (2E6, GAME-46) blocking antibodies had no effect on adhesion. However, the combination of VLA-4 (PS/2) with either CD11a or CD11b blocking antibodies resulted in significantly greater inhibition than with PS/2 alone (57.7 ± 8.2 and 58.5 ± 7.9 vs. 30.9 ± 7.5, respectively; P < 0.05). Adhesion was compared with equivalent concentrations of isotype control antibody, protein alone, and with F4/80 (used to determine whether there were any Fc-mediated effects). There was no difference between these controls. Silver nitrate staining confirmed mesothelial surface integrity (Fig. 3 B) and adhesion was easily quantifiable by fluorescent microscopy (Fig. 3, C and D). Macrophage adhesion to the mesothelium lining the abdominal wall was reduced by >50% compared with the subdiaphragmatic mesothelium, confirming the specificity of this region for emigration (P < 0.05, n = 3). The difference in adhesion induced by RGD peptide was not due to any proapoptotic effect of RGD on macrophages altering their ability to adhere, as there was no difference in the number of apoptotic cells when peritoneal lavage cells (>90% macrophages) were exposed to 0.5mg/ml RGD or controls for up to 48 h in vitro.
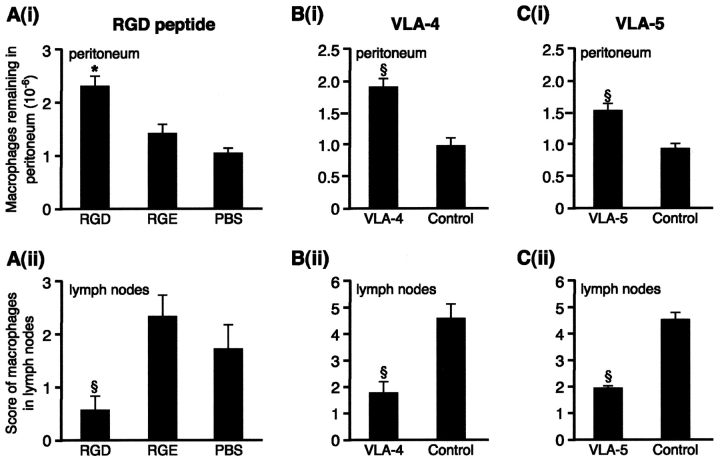
Macrophage Emigration In Vivo Is Regulated by VLA-4 and VLA-5 Integrins.
Inflammatory macrophages express significant levels of CD11b, CD49d, and CD49e (5.1 ± 0.85, 2.23 ± 0.31, and 2.27 ± 0.48-fold greater than isotype controls, respectively; all P < 0.05, n = 8 mice). Repeated intraperitoneal injection of RGD significantly delayed macrophage clearance in vivo as evidenced by increased numbers of fluorescent macrophages remaining in the peritoneum compared with RGE and medium controls (P < 0.05, Fig. 4) . In addition, RGD significantly reduced the number of labeled macrophages in the parathymic nodes in the RGD-treated mice compared with either control (P < 0.01). Controls were similar for both peritoneal recovery and lymph node scores. There were no differences between the groups in the number of fluorescent macrophages adherent to bowel, spleen, or liver and the mesothelial surfaces of these organs remained intact. Corroborating the ex vivo data, macrophage emigration into the draining lymphatics was also significantly inhibited by blocking VLA-4 (PS/2) and VLA-5 (5H10-27), which increased the number of macrophages remaining in the peritoneum (P < 0.01 for blocking both VLA-4 and VLA-5) and reduced emigration into the lymphatics (P < 0.01 for blocking both VLA-4 and VLA-5). They did not alter the distribution of macrophages on the mesothelial surface of spleen, bowel, or liver. Blocking CD11b had no effect on macrophage clearance in vivo, there being no significant difference in macrophage recovery from the peritoneum for M1/70, isotype, or F4/80 controls (0.89 ± 0.2 vs. 1.18 ± 0.19 and 0.96 ± 0.09 × 106, respectively; n = 12). Likewise, lymphatic emigration was equally unaffected by blocking CD11b. Antibody was used at 100 μg for each injection and increasing to 225 μg had no additional effect (n = 5, unpublished data).
Figure 4.
VLA-4 and VLA-5 regulate macrophage clearance from resolving peritonitis. (A) (i) After fluorescently labeling macrophages in vivo, 0.4 mg RGD or controls, RGE (0.4 mg) or PBS, were injected 2 and 26 h later and macrophage recovery from peritoneum was determined 24 h later. This was significantly greater for RGD than controls. *, P < 0.05. n = 13 RGD, 8 RGE, and 10 PBS. Controls were not different. (ii) RGD significantly reduced the number of macrophages reaching draining lymph nodes compared with RGE or PBS (§, P < 0.01). (B) (i) Blocking VLA-4 (PS/2) led to significantly more macrophages remaining in the peritoneum (§, P < 0.01; n = 10 VLA-4; 9 controls) and (ii) significantly less in the lymph nodes (§, P < 0.01) than isotype control. (C) (i) Blocking VLA-5 (5H10–27) led to significantly more macro-phages remaining in the peritoneum (§, P < 0.01; n = 10 VLA-5; 10 controls) and (ii) significantly less in the lymph nodes (§, P < 0.01) than isotype control.
Discussion
This study demonstrates for the first time that macrophage clearance from the inflamed peritoneum can be regulated, a finding that may have significant implications for our understanding of mechanisms leading to mononuclear persistence in chronic inflammation. Macrophages are capable of undergoing apoptosis, however they are generally resistant to this and during acute resolving peritoneal inflammation are cleared by emigration, not apoptosis (2, 11). Hence, mechanisms regulating emigration may have powerful effects on inflammation.
Here, we demonstrate a dramatic difference in clearance between activated and nonactivated macrophages. Nearly seven times more nonactivated macrophages remained in the noninflamed peritoneum after 3 d compared with activated macrophages in the inflamed peritoneum. These observations, along with the demonstration that adhesion localizes to the mesothelium overlying lymphatics, suggested specific adhesion molecule regulation that could be functionally up-regulated by inflammation. Adhesion was divalent cation and largely RGD sensitive and partially inhibited by blocking VLA-4 and VLA-5 but not by blocking CD11a, CD11b, or CD18, which suggests a predominant role for β1 integrins. Ex vivo results were validated by in vivo studies in which RGD and blocking VLA-4 and VLA-5 significantly delayed macrophage clearance, whereas blocking CD11b was ineffective. Alterations in peritoneal macrophage recovery was always mirrored by alterations in recovery from draining lymph nodes. Controls confirmed that differences in recovery were not due to nonspecific widespread adhesion. These data thus confirm a role for VLA-4 and VLA-5 in regulating inflammatory macrophage clearance from the inflamed peritoneum. They do not exclude the possibility that resident cells may depend on distinct molecular mechanisms for clearance.
Mesothelial cells express intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 and associated fibronectin strands can be demonstrated with inflammation, hence the ligands for VLA-4 and VLA-5 are present (12). VLA-5 and αv binding is RGD inhibitable whereas VLA-4 binding is normally through LDV-inhibitable not RGD-inhibitable mechanisms (9, 13). The increased inhibition from the combination of VLA-4 and VLA-5 blocking suggests that these two integrins are acting independently. When combined with RGD, VLA-4 further decreased adhesion but not significantly, and VLA-5 had no added effect over that of RGD. Whether vascular cell adhesion molecule 1 or fibronectin are the ligands responsible for VLA-4–mediated binding is currently unknown. Both VLA-4 and VLA-5 are expressed on monocytes and implicated in leukocyte influx into inflamed sites, in particular for migration through connective tissue (14). Given mesothelial intercellular adhesion molecule 1 expression, the lack of effect from blocking β2 integrins was surprising, however monocyte–fibronectin binding has been shown to be VLA-5 rather than β2 integrin dependent (15). In addition, the combination of β1 and β2 blocking had significantly greater inhibitory effect than VLA-4 alone, suggesting β2 integrins are functional but that β1 function is predominant.
VLA-4 inhibitors can attenuate inflammation, preventing monocyte and lymphocyte influx and thus potentially preventing the detrimental effects of excessive leukocyte accumulation (16, 17). However, we demonstrate a cautionary note about using such inhibitors in established inflammation as they can also prevent efflux. If the signal for leukocyte influx is waning, the balance of inflammatory cells at the inflamed site depends more on clearance. Hence the stage and nature of the inflammatory response need to be considered before modulating the function of these adhesion molecules.
Subdiaphragmatic macrophage–mesothelial adhesion, in close relationship with the lymphatics, has an anatomical explanation. Lymphatic drainage of the peritoneal cavity arises from this surface and the omentum where milky spots are found (8, 18). Electron microscopy demonstrates that these consist of dome-shaped mesothelial cells surrounding narrow pores overlying lymphatic vessels. This may have relevance for malignant cells that also adhere preferentially to milky spots, however the role of adhesion molecules in lymphatic metastases has received surprisingly little attention (19).
In summary, we propose that macrophages bind the mesothelium overlying draining lymphatics by integrin-mediated mechanisms involving VLA-4 and VLA-5, and regulation of these interactions affects macrophage emigration into the lymphatic circulation. As other coelomic cavities are mesothelial lined, these mechanisms might be important after pleural and pericardial inflammation. Moreover, as macrophage emigration to the lymphatics also occurs with inflammation in other organs such as the kidney and lung, an understanding of their clearance mechanisms is likely to have widespread significance (20, 21). Rapid and effective macrophage clearance may dictate the time course of inflammation and be a pivotal event determining the development of chronic inflammation. This work thus describes a newly recognized process that offers potential novel therapeutic approaches to promote the effective resolution of inflammation.
Acknowledgments
Medical Research Council UK funded G. Bellingan, P. Xu, and H. Cooksley.
References
- 1.Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5–S11. [DOI] [PubMed] [Google Scholar]
- 2.Bellingan, G.J., H. Caldwell, S.E. Howie, I. Dransfield, and C. Haslett. 1996. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157:2577–2585. [PubMed] [Google Scholar]
- 3.Van Furth, R. 1992. Development and distribution of mononuclear phagocytes. Inflammation: Basic Principles and Clinical Correlates. Second Edition. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 325–341.
- 4.Leibovich, S.J., and R. Ross. 1975. The role of the macrophage in wound repair. A study with hydrocortisone and anti-macrophage serum. Am. J. Pathol. 78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Adams, D.O., and T.A. Hamilton. 1992. Macrophages as destructive cells in host defence. Inflammation: Basic Principles and Clinical Correlates. Second Edition. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 637–643.
- 6.Thepen, T., A.J. van Vuuren, R.C. Kieken, C.A. Damen, W.C. Vooijs, and J.G. van De Winkel. 2000. Resolution of cutaneous inflammation after local elimination of macrophages. Nat. Biotechnol. 18:48–51. [DOI] [PubMed] [Google Scholar]
- 7.Jonjic, N., G. Peri, S. Bernasconi, F.L. Sciacca, F. Colotta, P. Pelicci, L. Lanfrancone, and A. Mantovani. 1992. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J. Exp. Med. 176:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beelen, R.H., D.M. Fluitsma, and C.M. Hoefsmit. 1980. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum. J. Retic. Endothel. Soc. 28:585–599. [PubMed] [Google Scholar]
- 9.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697–715. [DOI] [PubMed] [Google Scholar]
- 10.Ryan, G.B., J. Grobety, and G. Majno. 1973. Mesothelial injury and recovery. Am. J. Pathol. 71:93–112. [PMC free article] [PubMed] [Google Scholar]
- 11.Navarre, W.W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2:265–273. [DOI] [PubMed] [Google Scholar]
- 12.Liang, Y., K. Jyoukura, N. Ogiwara, and K. Sasaki. 2001. Expression of adhesion molecules and fibronectin of activated peritoneal surface with lipopolysaccharide (LPS) analysed with immuno SEM. Ann. Anat. 183:353–356. [DOI] [PubMed] [Google Scholar]
- 13.Lobb, R.R., and M.E. Hemler. 1994. The pathophysiologic role of alpha 4 integrins in vivo. J. Clin. Invest. 94:1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang, X.Z., B.J. Lang, and A.C. Issekutz. 1998. Adhesion molecule mechanisms mediating monocyte migration through synovial fibroblast and endothelium barriers: role for CD11/CD18, very late antigen-4 (CD49d/CD29), very late antigen-5 (CD49e/CD29), and vascular cell adhesion molecule-1 (CD106). J. Immunol. 160:467–474. [PubMed] [Google Scholar]
- 15.Werr, J., X. Xie, P. Hedqvist, E. Ruoslahti, and L. Lindbom. 1998. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J. Exp. Med. 187:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen, A.R., J. McHale, J. Smith, H.T. Cook, A. Karkar, D.O. Haskard, R.R. Lobb, and C.D. Pusey. 1999. Endothelial expression of VCAM-1 in experimental crescentic nephritis and effect of antibodies to very late antigen-4 or VCAM-1 on glomerular injury. J. Immunol. 162:5519–5527. [PubMed] [Google Scholar]
- 17.Abraham, W.M., A. Gill, A. Ahmed, M.W. Sielczak, I.T. Lauredo, Y. Botinnikova, K.C. Lin, B. Pepinsky, D.R. Leone, R.R. Lobb, et al. 2000. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am. J. Respir. Crit. Care Med. 162:603–611. [DOI] [PubMed] [Google Scholar]
- 18.Leak, L.V. 1983. Interaction of mesothelium to intraperitoneal stimulation. I. Aggregation of peritoneal cells. Lab. Invest. 48:479–491. [PubMed] [Google Scholar]
- 19.Tsujimoto, H., A. Hagiwara, M. Shimotsuma, C. Sakakura, K. Osaki, S. Sasaki, T. Ohyama, M. Ohgaki, T. Imanishi, J. Yamazaki, et al. 1996. Role of milky spots as selective implantation sites for malignant cells in peritoneal dissemination in mice. J. Cancer Res. Clin. Oncol. 122:590–595. [DOI] [PubMed] [Google Scholar]
- 20.Lan, H.Y., D.J. Nikolic-Paterson, and R.C. Atkins. 1993. Trafficking of inflammatory macrophages from the kidney to draining lymph nodes during experimental glomerulonephritis. Clin. Exp. Immunol. 92:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen, A.G., B.A. Muggenburg, M.B. Snipes, and D.E. Bice. 1985. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 230:1277–1280. [DOI] [PubMed] [Google Scholar]