Abstract
In contrast to protein antigens, processing of glycoproteins by dendritic cells (DCs) for presentation to T cells has not been well studied. We developed mouse T cell hybridomas to study processing and presentation of the tumor antigen MUC1 as a model glycoprotein. MUC1 is expressed on the surface as well as secreted by human adenocarcinomas. Circulating soluble MUC1 is available for uptake, processing, and presentation by DCs in vivo and better understanding of how that process functions in the case of glycosylated antigens may shed light on antitumor immune responses that could be initiated against this glycoprotein. We show that DCs endocytose MUC1 glycopeptides, transport them to acidic compartments, process them into smaller peptides, and present them on major histocompatability complex (MHC) class II molecules without removing the carbohydrates. Glycopeptides that are presented on DCs are recognized by T cells. This suggests that a much broader repertoire of T cells could be elicited against MUC1 and other glycoproteins than expected based only on their peptide sequences.
Keywords: antigen presenting cells, endocytosis, peptide epitopes, glycoepitopes, T cell hybridomas
Introduction
CD8+ and CD4+ T cells recognize protein antigens presented as peptides bound to MHC class I and II molecules (1, 2). Generation of class II–restricted peptide epitopes by APCs follows a multistep process (for a review, see reference 3). Upon entering the cell through endocytosis, the antigen is transported in endocytic vesicles to processing compartments where it undergoes proteolytic cleavages by specialized enzymes (4). The resulting peptides bind to MHC class II molecules residing in these compartments and are transported to the cell surface for presentation to CD4+ T cells (5). Various aspects of this pathway have been elucidated using as model antigens unglycosylated proteins that yield unglycosylated peptides for recognition by MHC class II–restricted T cells (6, 7). As a result, our understanding of processing of complex glycoproteins is much less advanced, even though many protein antigens that are encountered by the APC are glycosylated. Protein glycosylation is a posttranslational modification that can have great impact on immunogenic properties of many antigens (8, 9). Many viral envelope proteins are glycoproteins that use this biochemical characteristic to avoid immune detection (10, 11). In autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, changes in IgG glycoforms contribute to the immunopathology of these diseases (12). Allergens like the bee venom phospholipase A2 are glycoproteins and the carbohydrate structures are responsible for their immunogenic properties (13). In tumor cells, dramatic changes in protein glycosylation create tumor-specific glycoproteins that can be recognized by the immune system as tumor-specific antigens (14). Type II collagen (15, 16), bee venom allergen phospholipase A2 (13), and HIV envelope glycoprotein (17) are among the very few glycosylated antigens studied to date for their ability to generate class II–restricted glycoepitopes. The basic question that has not yet been answered is whether sugars are removed during antigen processing.
We have addressed the fate of sugars on glycoprotein antigens by studying the mucin-like glycoprotein MUC1. MUC1 is a human tumor antigen expressed in a variety of adenocarcinomas. MUC1 made by normal cells is highly glycosylated with branched O-linked oligosaccharides (18). By contrast, in tumor cells, MUC1 O-glycosylation is prematurely terminated, leading to the accumulation of short carbohydrate precursors such as monosaccharide Tn (GalNAcα1-O-S/T) or disaccharide T (Galβ1–3GalNAcα1-O-S/T), and their sialylated forms sTn and sT, respectively (for a review, see reference 19). These tumor-specific carbohydrates are O-linked to serines and threonines in the tandem repeat domain of the MUC1 molecule that consists of a variable number of 20 amino acid–long repeats. Each tandem repeat (HGVTSAPDTRPAPGSTAPPA) has five glycosylation sites, three threonines and two serines (for a review, see reference 20).
Our results obtained with MUC1-specific T hybridomas show that dendritic cells (DCs)* process and present MUC1 glycopeptides without removing the O-linked carbohydrates. Thus, in addition to peptide epitopes that are presented to CD4+ Th cells, there exist glycopeptide epitopes that are presented and could trigger a completely different repertoire of glycopeptide-specific T cells. In the case of MUC1, which has the same protein sequence in normal and tumor cells but different glycosylation profile, the peptide epitopes are shared between normal and tumor cells, but the glycopeptide epitopes are expected to be tumor specific. Thus, the knowledge that these epitopes are presented and can potentially be tumor-specific targets for glycopeptide-specific T cells may be critically important for tumor-specific immunotherapy.
Materials and Methods
Animals.
6–8-wk old C57BL/6 female mice used for immunizations were purchased from The Jackson Laboratory and housed at the University of Pittsburgh Cancer Institute (Pittsburgh, PA) Animal Facility. All experiments were approved by the IACUC of the University of Pittsburgh.
Chemical and Enzymatic Synthesis of MUC1 Peptides and Glycopeptides.
The 140mer and the 100mer peptides represent 7 and 5 repeats of a 20 amino acid sequence HGVTSAPDTRPAPGSTAPPA from the MUC1 tandem repeat region. These two peptides and the 13mer (HGVTSAPDTRPAP) and 9mer (HGVTSAPDT) were synthesized on a Chemtech 200 machine with N-(9-fluorenyl) methoxycarbonyl chemistry and purified by HPLC in the University of Pittsburgh Cancer Institute peptide synthesis facility.
Glycopeptides A1 to A7, and A13 were synthesized at the Institute of Organic Chemistry, University of Hamburg, and characterized at the Institute of Biochemistry II, University of Cologne, as described previously (21). Identical protocols were used for the synthesis of H1 and H2. Crude preparations of the glycopeptides were chromatographed on PLRP-S reversed-phase columns (250 × 4.6 mm, Polymer Laboratories) on a preparative scale and the eluted fractions analyzed for purity by MALDI mass spectrometry on a Bruker-Reflex III as described previously (22).
The GalNAc-100mer (Tn100mer) was prepared by enzymatic addition of GalNAc to a synthetic peptide substrate using recombinant UDP-GalNAc:polypeptide N-acetyl-galactosaminyltransferases rGalNAc-T1 and -T2 (provided by H. Clausen, School of Dentistry, University of Copenhagen, Denmark) under conditions described previously (22). The reaction mixtures were incubated at 37°C for up to 72 h with additions of cosubstrate and fresh enzyme at 24-h intervals. The reaction was monitored by matrix-assisted laser desorption/ionization (MALDI) mass spectrometric analysis. The final reaction product contained a total number of 15 GalNAc residues per 100mer peptide that were incorporated within threonine in VTSA region and adjoining serine and threonine within GSTA region, consistent with the site-specificity of the rGalNAc-Ts used (22).
The T100mer glycopeptide was synthesized from the Tn-100mer which was converted into the corresponding T-100mer by addition of galactose using a recombinantly expressed core1-Gal-transferase from Drosophila melanogaster (provided by T. Schwientek, Institute of Biochemistry, University of Cologne, Cologne, Germany). ∼2 mg of Tn-100mer was incubated in 1 ml of reaction buffer (100 mM MES, pH 6.5, 0.1% Triton X-100, 20 mM MnCl2, 20 mM DTT) containing 4 mM UDP-Gal and 0.5–1 mU of enzyme. Fresh cosubstrate and enzyme were added after 24 h and the reaction was stopped after 48 h. The products were separated by reversed phase chromatography on a PLRP-s column. According to mass spectrometry the major products contained 8–10 Gal residues per 100mer.
MCF-7 and T47D glycoforms were described previously (23). They were obtained by transfection of MCF-7 and T47D breast cancer cell lines with a vector encoding the fusion protein containing six tandem repeats of MUC1. The mammalian episomal expression vector pCEP-PU contains the signal peptide of the BM40 extracellular matrix protein, followed by a hexa-histidine sequence and a myc tag. Protein expression is driven by the cytomegalovirus promoter. The quality of the preparations was analyzed by SDS-PAGE followed by blotting onto nitrocellulose membranes and the fusion proteins were detected with an anti-myc mAb (Santa Cruz Biotechnology, Inc.).
The unmodified 19-mer (HGVTSAPDTRPAPGSTAPP) MUC1 peptide and the corresponding glycosylated analogs (W2-W7) were synthesized at The Wistar Institute by conventional solid-phase methods. The assembly was made on a Rainin PS3 automated synthesizer on TentaGel S-Ram-Fmoc resin with an initial load of 0.3 mmol/g (Advanced ChemTech). Standard Fmoc chemistry was used throughout (24) with four molar excess of the acylating amino acids and HATU (1-hydroxy-7-azabenzotriazole uronium salt) activation, recommended for the synthesis of complex peptides (25). FmocSer/Thr(maltoseGalNAc/maltohexoseGalNAc α1;O)-OPfp (26) glycoamino acids were incorporated in the same manner as the unmodified amino acids, except that they were coupled in 1.5 molar excess (to reduce glycoamino acid usage) in the presence of di-isopropyl-ethyl-amine (DIPEA).
On completion of the assembly of the peptide chain, the Fmoc protection on the NH2-terminal amino acid was retained. Still polymer-bound, the azido group on the sugar ring was transformed into an acetamido group using thioacetic acid for 4 d with consecutive changes of the reagent (27). After reducing the azido group, the Fmoc protection on the NH2-terminal amino acid was removed using 20% piperidine in dimethyl formamide. The resulting glycopeptides, as well as the unmodified 19-mer peptide were cleaved from the solid support with trifluoroacetic acid in the presence of thioanisol (5%), and water (5%) as scavengers for 2 h. Deacetylation of the sugar hydroxyl group was accomplished by treatment with 0.1 M NaOH (28). Reaction time of 10 min was sufficient for the peptides containing trisaccharide, and 20 min was needed for those carrying heptasaccharide side-chains. After cleavage, the peptides were purified by RP-HPLC (preparative runs: isocratic elution of 5% solvent B for 5 min followed by a linear gradient from 5 to 65% solvent B for 300 min). The final products were characterized by RP-HPLC (during the analytical runs the same gradient was developed over a 45-min period) and MALDI-MS.
All peptides are listed in Table I.
Table I.
MUC1 Peptides and Glycopeptides
| Peptide | Sequence and glycosylation sitesa | Carbohydrate type and length |
|---|---|---|
| 100mer | (GVTSAPDTRPAPGSTAPPAH) × 5 | None |
| 19mer | HGVTSAPDTRPAPGSTAPP | None |
| 13mer | HGVTSAPDTRPAP | None |
| 9mer | HGVTSAPDT | None |
| Tn-100mer | (GVTSAPDTRPAPGSTAPPAH) × 5 | GalNAc(α1-O) |
| H1 | AHGVTSAPDTRPAPGSTAPPA | GalNAc(α1-O) |
| H2 | AHGVTSAPDTRPAPGSTAPPA | GalNAc(α1-O) |
| A13 | AHGVTSAPDTRPAPGSTAPPA | GalNAc(α1-O) |
| A1 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A2 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A3 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A4 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A5 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A6 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| A7 | AHGVTSAPDTRPAPGSTAPPA | Gal(β1-3)-GalNAc(α1-O) |
| W2 | HGVTSAPDTRPAPGSTAPP | Glc(α1-4)-Glc(β1-4)-GalNAc(α1-O) |
| W3 | HGVTSAPDTRPAPGSTAPP | Glc(α1-4)-Glc(β1-4)-GalNAc(α1-O) |
| W4 | HGVTSAPDTRPAPGSTAPP | Glc(α1-4)-Glc(β1-4)-GalNAc(α1-O) |
| W6 | HGVTSAPDTRPAPGSTAPP | [Glc(α1-4)-Glc]×3-GalNAc(α1-O) |
| W7 | HGVTSAPDTRPAPGSTAPP | [Glc(α1-4)-Glc]×3-GalNAc(α1-O) |
Glycosylated threonine (T) and serine (S) residues are marked in bold.
Generation of DCs.
DCs were generated as described previously (29), from bone marrow precursors (C57Bl/6 mice) in the presence of 10 ng/ml murine GM-CSF and 10 ng/ml IL-4 (gifts from Immunex). DCs were purified after 5 d in culture, using a Nycoprep gradient (Nycomed). This protocol produces phenotypically immature DCs (30).
Generation of T Cell Hybridomas.
For peptide-specific hybridomas, purified immature DCs were loaded overnight at 37°C, with 20 μg/ml of 140-mer MUC1 peptide. Additional soluble 140-mer MUC1 peptide was added to the preloaded DCs at a final concentration of 100 μg per mouse, right before vaccination. C5BL/6 mice were immunized with 5 × 105 DCs per mouse subcutaneously in the right hind flank and boosted twice at 3-wk intervals. 10 d after the last boost, inguinal lymph nodes and spleen lymphocytes were pooled and plated in 6-well plates (Becton Dickinson) at two million cells/ml in complete DMEM-10 medium (ICN) and restimulated with soluble 140-mer peptide (20 μg/ml). After 36 h of culture, the cells were fused in the presence of polyethylene glycol (Sigma-Aldrich) with the BW5147 TCR α−β− lymphoma cell line (American Type Culture Collection), according to published protocols (31, 32). Fused cells were monitored for 2–3 wk and cells showing growth were tested for CD3 and CD4 expression by FACS® (antibodies purchased from BD PharMingen). The CD3+ hybridomas were screened for antigen recognition using an ELISA-based IL-2 detection protocol. Antigen-specific hybridomas were subcloned by limiting dilution.
For glycopeptide-specific hybridomas, inguinal and mesenteric lymph nodes and spleens from C57Bl/6 mice were harvested and lymphocytes primed in vitro with DCs loaded overnight (as described above) with pooled A2-A7 (see Table I) MUC1 21mer glycopeptides (20 μg/ml each). Every 7 d, T cell cultures were restimulated with overnight-loaded DCs. 3 d after the third restimulation, viable T cells were fused with BW5147 lymphoma cells as described above. The same phenotypic (CD3, CD4 expression) and functional screening (IL-2 release in response to the antigen) was performed as with the peptide-specific T hybridomas.
Antigen Specificity and IL-2 Detection by ELISA.
DCs were loaded with antigen (20 μg/ml, unless otherwise indicated) for 16 h.
Equal amounts (in μg/ml) of all short and long peptides and glycopeptides were assayed rather than equimolar quantities because the 100mer comprises five tandem repeats/molecule, each repeat generating one epitope (up to five epitopes per molecule) upon processing by DCs.
Antigen-loaded and control, no antigen DCs were added at 104 cells per well (unless otherwise indicated) to Immulon 4 ELISA plates (Dynex Technologies Inc.), in triplicates. The T hybridoma cells were added at a density of 105 cells per 100 μl per well, for 24 h. 100 μl of the supernatant was tested for the presence of IL-2 by ELISA, using IL-2 detection kit (BD PharMingen). ELISA plates were read in an automatic ELISA reader, using MRX Revelation software (Dynex Technologies). Data was plotted using Cricket graph software.
Confocal Laser Scanning Microscopy.
MUC1 peptides and glycopeptides were directly conjugated to either Cy3 or Cy5 fluorescent dyes according to manufacturer's protocol (Amersham Pharmacia Biotech). Free dye was separated from the conjugated protein by mass chromatography and the final concentration of protein determined in collected fractions using a protein assay kit (Bio-Rad Laboratories).
Day 5 immature DCs were resuspended in polypropylene tubes at 5 × 104 cells per 250 μl of AIM V DC medium and exogenously pulsed with 20 μg/ml fluorescently labeled peptides for 2 h. For the last 30 min of incubation, DCs were pulsed with 5 μM of green fluorescent BODIPY FL Pepstatin A (Molecular Probes). After uptake and processing, DCs were briefly fixed in 2% paraformaldehyde, washed in serum-containing medium and indirectly stained for intracellular and/or cell surface MHC class II. Staining with primary anti–mouse IAb antibody (BD PharMingen) was followed by secondary Alexa 488, Alexa 546, or Cy5-labeled (Molecular Probes) antibodies. When intracellular staining was employed, the antibody was diluted in 0.1% Triton X-100.
The final concentration of antibodies used for staining was 5 μg/ml. After staining, DCs were mounted on glass slides and immediately analyzed by confocal laser microscopy at the University of Pittsburgh Center for Biological Imaging Facility, using a Leica TCS NT confocal LSM microscope. Images were collected using the ×100 objective, as serial sections. Individual sections are presented (of generally 4–8 cells collected). Cy5 is a far-red fluorescent dye to which we have assigned the color blue.
Antigen Processing Assay.
Day 5 immature DCs were either fixed in 1% paraformaldehyde for 10 min at room temperature or pretreated for 30 min at 37°C with either of the following inhibitors (all from Sigma-Aldrich): 2 mM sodium azide; 2 deoxyglucose (100 μM); or a protease inhibitors cocktail 25- or 100-fold diluted (concentration of some of the protease inhibitors in the stock solution are 100 μg/ml aprotinin; 500 μM leupeptin; 500 μM E-64; 50 mM AEBSF; and 100 mM EDTA). Cells were extensively washed upon treatment and then resuspended in polypropylene tubes (Becton Dickinson) in 200 μl final volume of AIM V serum-free medium. Viability of DCs was assessed before and after pretreatment by Trypan Blue (GIBCO BRL) exclusion method. Control DCs were treated with DMSO only (used as a vehicle for protein inhibitors; Sigma-Aldrich). Synthetic peptides were then added at 20 μg/ml and DCs incubated at 37°C (or on ice, in inhibition experiments) in a humidified CO2 incubator for 16 h (unless otherwise stated). DCs were plated in 96-well plates at a density of 104 cells per well and T hybridomas added at a density of 105 cells per well. Experiments were performed in triplicate and at least on two occasions.
Detection of Processed MUC1 Peptides and Glycopeptides on the Surface of DCs by FACS®.
DCs were loaded as above, washed, fixed, and stained for 30 min at 4°C, in the dark. Staining was performed either directly using FITC-labeled Vicia villosa lectin (EY Laboratories) or indirectly with anti-MUC1 monoclonal antibodies VU-4-H5, VU-3-C6 (33) and BCP9 followed by ALEXA 488 anti–mouse secondary antibody (Molecular Probes). Surface staining of DCs with the fluorescent lectin was performed on DCs pulsed with the antigen either post- or prior fixation in 1% PFA for 10 min at room temperature. Fixed cells were extensively washed upon fixation. Stained cells were immediately analyzed with a Becton Dickinson FACScalibur™ and data analyzed with CELLQuest™ software.
Results
MUC1 Peptide Specificity and MHC Class II Restriction of the CD4+ T Hybridoma VF5.
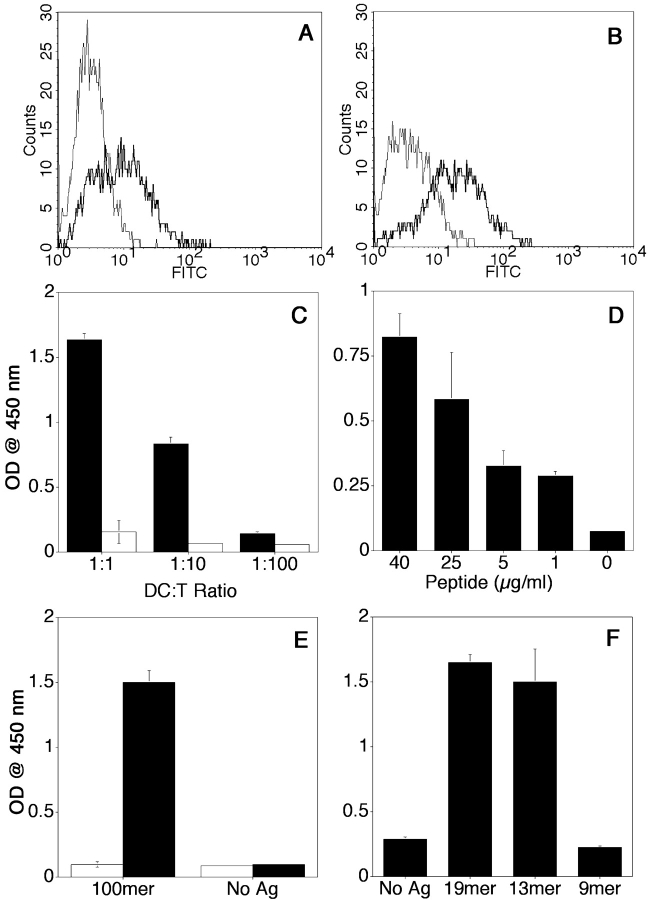
MUC1 peptide-specific T hybridomas were generated after an in vivo DCs based immunization protocol, and screened for specificity by coincubation with immature bone marrow–derived DCs exogenously loaded for 16 h with the synthetic MUC1 100mer peptide. The 100mer peptide (Table I) represents five 20 amino acids-long repeats from the MUC1 tandem repeat region. The supernatants of these cocultures were tested for the presence of IL-2 by ELISA and compared with supernatants from cocultures with control DCs that did not receive antigen. We identified one stable MUC1 peptide-specific T cell hybridoma (clone VF5). CD3 and CD4 expression by this T cell clone are shown in Fig. 1, A and B . This clone produced IL-2 in response to DCs loaded with MUC1 100mer peptide and not in response to control DCs. The response was dose-dependent and varied according to various T/DC ratios and various peptide concentrations (Fig. 1, C and D). Monoclonality was achieved through multiple subclonings by limiting dilution, and confirmed by PCR analysis of the TCR genes (unpublished data).
Figure 1.
Phenotypic and functional characterization of MUC1 peptide-specific T hybridoma VF5. Flow cytometric analysis was performed for CD3 (A) and CD4 (B) expression. Cells were incubated in the presence of either FITC-labeled isotype control antibody (thin line) or anti-CD3 or anti–CD4-FITC (thick line) antibodies. Antigen specificity was assessed in IL-2 detection assay, by ELISA (C and D). Day 5 bone marrow–derived DCs were loaded with 40 μg/ml 100mer MUC1 peptide and coincubated with VF5 T cells at various DC/T cell ratios (x axis), in triplicate, for 24 h (C). DCs were loaded for 16 h with various concentrations of 100mer peptide (in μg/ml, on the x axis) and added to VF5 T cells at a 1:10 ratio (D). VF5 cells were coincubated (1:10 DC/T cell ratio) with either syngenic DCs from C57Bl/6 (I-Ab) mice or with allogeneic DCs from B6.NOD mice (I-Ag7) mice, pulsed with 20 μg/ml antigen as described above (E). DCs were loaded with 20 μg/ml of 100mer, 19mer, 13mer, and 9mer MUC1 peptides, in triplicates, for 16 h and then added to VF5 cells at a 1:10 ratio (F). After 24 h of coculture, the supernatant was tested for IL-2 by ELISA (C–F). Results represent means of optical density units. Every assay had an IL-2 standard curve included. IL-2 production by T cells in the presence of antigen-loaded DCs was compared with IL-2 production in the presence of control DCs and statistical significance determined using Student's t test (P < 0.005).
MHC class II restriction of clone VF5 was confirmed using DCs generated from B6.NOD mice (H-2g7) mismatched in MHC class II with C57BL6 mice (I-Ab). Fig. 1 E shows that clone VF5 produces IL-2 in response to the 100mer peptide only when presented by the C57BL6 DC (I-Ab) and not by B6.NOD DC (I-Ag7). The B6.NOD DCs can otherwise function well as APC, demonstrated by their ability in the same experiment to stimulate a class I (H-2b) restricted T hybridoma specific for the 8mer (SIINFEKL) OVA peptide (unpublished data).
Long peptides, such as the MUC1 100mer, exogenously administered to DCs are expected to be enzymatically cleaved into 13–25 amino acid long epitopes that are then presented on MHC class II molecules on the cell surface (3). Consistent with that, IL-2 secretion by clone VF5 was also detected in response to a 19mer peptide HGVTSAPDTRPAPGSTAPP (Fig. 1 F). By testing even shorter peptides, we determined that the minimal epitope recognized by VF5 hybridoma resides within the 13mer HGVTSAPDTRPAP sequence. Further truncation to a 9mer peptide HGVTSAPDT abrogates the response presumably because this peptide is too short to bind to I-Ab.
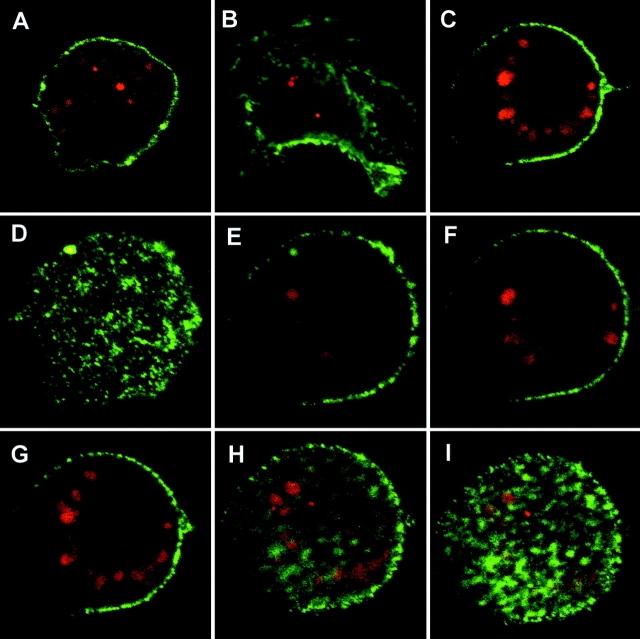
The Long and the Short MUC1 Peptides Are Internalized by DCs and Trafficked to Endocytic Vesicles.
We fluorescently labeled the 100mer, 19mer, and 13mer peptides with Cy3 (a red fluorescent dye) and by confocal scanning microscopy tracked their presence inside DCs after a 2-h loading period. DCs were stained for class II on the surface using a green fluorescent antibody. Fig. 2 demonstrates intense accumulation of the 100mer (Fig. 2 A), 19mer (Fig. 2 B), and 13mer (Fig. 2 C) peptides inside (rather than on the surface of) DCs. The discrete, vesicular staining of internalized fluorescent peptides suggests endosome-like structures. Further proof that these vesicles are intracellular was obtained by consecutive scanning sections through cells, and one such example is shown for the 13mer peptide in panels D–I.
Figure 2.
Intracellular localization of MUC1 synthetic peptides after endocytosis by DCs. DCs were exogenously loaded with Cy 3 (red)-labeled 100mer (A), 19mer (B), and 13mer (C) peptides for 2 h, washed, fixed, and counterstained green for cell surface IAb and analyzed with confocal laser microscopy. Panel C represents one section from a series of 16 0.5-μm thick sections taken through a cell pulsed with 13mer peptide, six of which are shown in panels D-I. All images were acquired at ×100 original magnification.
MUC1 Glycopeptides Are Not Deglycosylated during Processing by DCs.
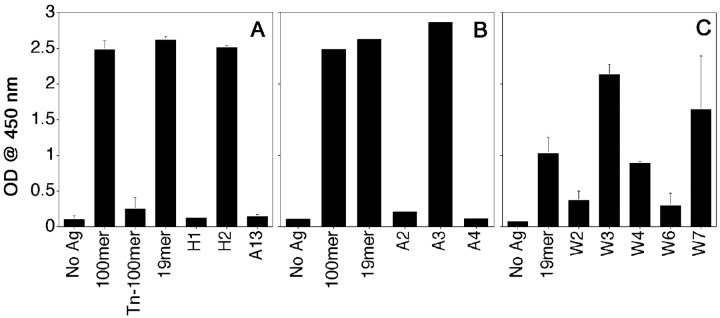
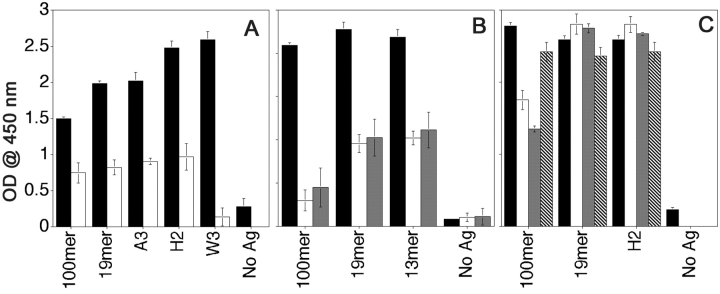
We synthesized a 100mer MUC1 peptide with a monosaccharide GalNAc linked to at least three out of five (two serines and three threonines) O-glycosylation sites within each tandem repeat (Tn-100mer, Table I). We also synthesized a series of 19mer and 21mer peptides with specific threonines and serines glycosylated with mono-, di-, tetra-, or heptasaccharides (shown in Table I). These glycopeptides were designed to represent different forms of MUC1 produced by tumor cells. We analyzed the ability of the peptide-specific hybridoma VF5 to recognize epitopes derived after processing of these glycopeptides by DC (Fig. 3) . Unglycosylated peptides, 100mer and 19mer served as positive controls. In Fig. 3 A, we see that compared with the DCs loaded with the unglycosylated 100mer, which was recognized very well, there was no recognition of DCs loaded with the glycosylated Tn-100mer. Yet Tn-100mer was also endocytosed (Fig. 3 D, blue) when fed to DCs simultaneously with the 100mer (red) and colocalized with it in endocytic compartments (Fig. 3 E, purple). It also colocalizes (yellow) with I-Ab (green) in intracellular MHC class II–rich compartments (Fig. 3 F). In Fig. 3 A we also see that the hybridoma was able to secrete IL-2 in response to an epitope derived from the monosaccharide-substituted glycopeptide H2, glycosylated on the third threonine (counting from the NH2 terminus of the peptide), but not in response to epitopes derived from glycopeptide H1, glycosylated on the first threonine, or glycopeptide A13, glycosylated on the second threonine. We concluded that the glycosylation of the first two threonines interfered with the recognition of the peptide epitope by the hybridoma, suggesting that the sugars were not removed during processing.
Figure 3.
Selective recognition by peptide-specific clone VF5 of DC loaded with different glycopeptides. DCs were loaded with indicated MUC1 peptides and glycopeptides at 20 μg/ml for 16 h, then coincubated with VF5 cells at 1:10 ratio, for 24 h. Culture supernatants were assayed for IL-2 presence by ELISA as described above. (A) DCs were loaded with 100mer and 21mer peptides modified with tumor-like monosaccharide Tn (GalNAc) antigen. (B) DCs were loaded with peptides modified with tumor-like disaccharide T (Gal-GalNAc) antigen. (C) DCs were loaded with trisaccharide glycopeptides (W2, W3, and W4) or heptasaccharide glycopeptides (W6 and W7). Precise sites of glycosylation of all glycopeptides can be found in Table I. Results are represented as means of optical density units. (D and E) Confocal micrographs of DCs (stained green for cell surface MHC class II) exogenously pulsed with Cy5-labeled (blue) Tn100mer antigen alone (D) or together with Cy3–100mer (red) MUC1 peptide (E) for 2 h. Colocalization of peptide and glycopeptide in vesicular compartments (purple) marked with arrows. (F) Colocalization (in yellow) of Tn-100mer peptide (red) with I-Ab (green) in DCs stained, after 2-h antigen pulse, for cell surface and intracellular MHC class II. Images are single scanned sections acquired at original magnification: ×100.
When DCs were fed 21mer peptides (Table I) bearing disaccharide Gal-GalNAc linked to individual serines and/or threonines (known as the tumor-specific T antigen), similar pattern of responses from the T hybridoma was seen (Fig. 3 B). Glycosylation on the second threonine (glycopeptide A2) and first serine (glycopeptide A4) interfered with the epitope recognition by the hybridoma, while the presence of the disaccharide at the COOH-terminal threonine did not (glycopeptide A3). Glycosylation of the serine in the COOH-terminal sequence GSTAPPA also did not interfere with the recognition by T cells (unpublished data), which may suggest that it is cleaved during processing.
Similar results were obtained from experiments with peptides bearing elongated sugars (trisaccharide-peptides W2, W3, and W4, or heptasaccharide-peptides W6 and W7, Table I). These glycopeptides were tested under the hypothesis that longer sugar chains might be handled differently by the DC antigen processing compartments. What we found was that regardless of the sugar chain length, the same pattern of epitope recognition was detected: sugars within the minimal HGVTSAPDTRPAP epitope interfered with the hybridoma recognition while sugars within the GSTAPPA COOH-terminal sequence did not (Fig. 3 C).
Recognition of MUC1 Peptides and Glycopeptides Requires Active Uptake and Transport to Acidic, Enzyme-rich Endocytic Compartments.
While complex protein antigens and long synthetic peptides are expected to require intracellular processing before their presentation on MHC class II, small peptides derived from some antigens (but not others) could directly bind to cell surface MHC class II molecules (34–36) and be presented equally well by fixed and nonfixed APCs (37, 38). We showed earlier in Fig. 2 that MUC1 peptides, regardless of their length, are endocytosed by DCs. In Fig. 4 A we confirm that metabolically active DCs are required for MUC1 processing and presentation to the VF5 hybridoma since DCs fixed before the addition of peptides are not able to elicit a response. Fixing cells after incubation with the peptides did not affect peptide presentation, suggesting that the ability of the DCs to successfully activate T hybridomas was preserved. Similarly, DCs pulsed on ice or pretreated with sodium azide/2 deoxyglucose before antigen pulse to disrupt active endocytosis (34), generated significantly lower IL-2 responses (Fig. 4 B) suggesting that endocytosis is necessary for VF5 epitope presentation.
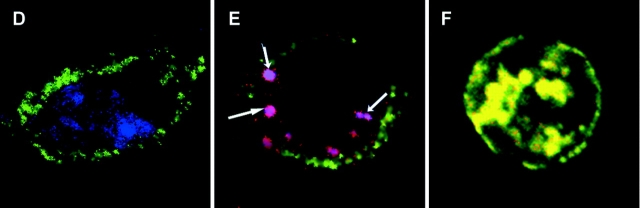
Figure 4.
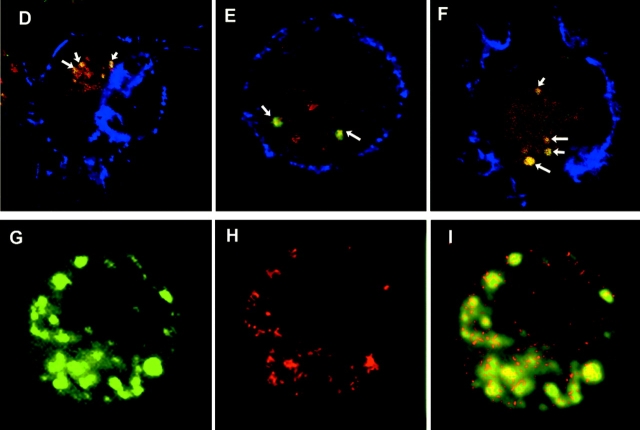
Inhibition of uptake and processing in endocytic compartments of both long and short MUC1 peptides and glycopeptides. DCs were either briefly fixed in 1% PFA (A, white bars), pretreated with 2 mM sodium azide/100 μM 2 deoxyglucose (B, white bars) or with a protease inhibitors cocktail (C) 100-fold (white bars) or 25-fold diluted (gray bars), 30 min before addition of 20 μg/ml MUC1 peptides and glycopeptides. No inhibitor was added to control DCs (black bars in A–C). In panel B, DCs were pulsed with antigen on ice (gray bars). In panel C, hatched bars represent DMSO control treated DCs. After 6 h of pulse, treated and untreated DCs were incubated with the VF5 cells and IL-2 was measured by ELISA. (D–F) Confocal micrographs of DCs exogenously pulsed with Cy3-labeled (red) 100mer (D), 19mer (E), and 13mer (F) MUC1 peptides for 2 h. During the last 30 min of the pulse period, DCs were also fed BODIPY FL Pepstatin A (green). Surface staining for MHC class II is shown in blue. Overlay sections show area of red-green colocalization (yellow) marked with arrows. (G–I). Confocal micrograph of DCs pulsed with Cy5-labeled 19mer peptide for 2 h and stained after the pulse for extracellular and intracellular I-Ab (green, G). Colocalization of antigen (red, H) and MHC class II in yellow (I).
Also, we wanted to see if both, the long and the short peptides, once internalized, were subjected to proteolytic cleavage into smaller fragments. Our assumption was that this would be required for the long peptide, the MUC1 100mer, but that the shorter peptides may or may not be further trimmed. Intracellular processing compartments in DCs are rich in proteolytic enzymes. The most prominent enzymes are cysteine proteases (such as Cathepsins B, H, L, S, etc.), aspartic proteases (Cathepsins D and E), and asparaginyl endopeptidase. We used a cocktail of chemical reagents that could specifically inhibit serine, cysteine, aspartic, and metalloproteases. As seen in Fig. 4 C, we successfully inhibited processing of the 100mer peptide but not of the small peptides suggesting that even though they are longer than average class II binding peptides they may still bind. If cleavage of the COOH-terminal segment is blocked it is not expected to interfere with the MHC-peptide-TCR complex formation being outside the VF5 epitope.
To show that lack of inhibition seeing with the short peptides is not due to their exclusion from the antigen processing compartments, we used confocal microscopy to colocalize Cy3 labeled MUC1 peptides (red) with other molecules that reside in the endosomes. Green fluorescent pepstatin A is a marker for endocytic compartments (39). Pepstatin A is internalized through an endocytic pathway and transported to lysosomes where it binds cathepsin D, one of the major endopeptidases involved in protein degradation. We show that it colocalizes with the 100mer (Fig. 4 D), 19mer (Fig. 4 E), and 13mer (Fig. 4 F) peptides. Another marker of the intracellular processing compartments of the endocytic pathway is MHC class II. Trafficking of the 100mer peptide to MHC class II-rich compartments (MIIC) has been reported previously (40). Here we show that the 19mer peptide (red, Fig. 4 H) and intracellular I-Ab (green, Fig. 4 G), colocalize in the same vesicles (Fig. 4 I), confirming trafficking of the small MUC1 peptides to vesicular, enzyme-rich MIIC compartments.
MUC1-derived Glycoepitopes Are Presented on the DC Cell Surface.
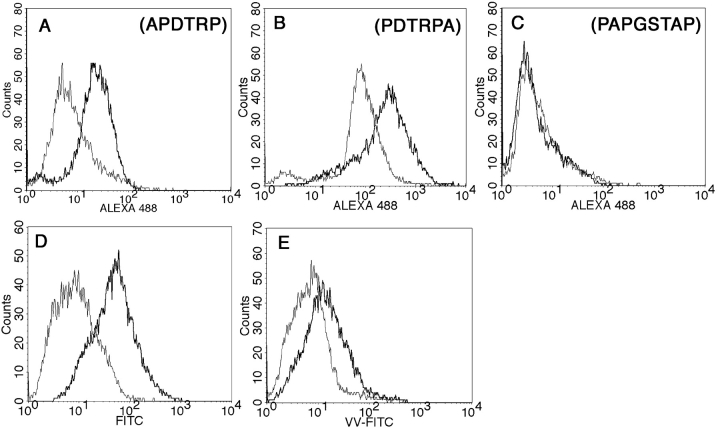
We interpreted the lack of reactivity of the VF5 hybridoma with some of the glycopeptides to be due to the persistence of sugars in the epitope after processing. However, an alternative possibility is that glycosylation could interfere with the processing or binding of glycopeptides to MHC class II molecules and therefore they may not be presented. To rule out this latter possibility we confirmed the presence on the DC cell surface of processed MUC1 glycoepitopes derived from the longest glycopeptide, the Tn-100mer. We stained DCs loaded with the Tn-100mer with anti-MUC1 antibodies that specifically recognize defined epitopes within a MUC1 tandem repeat. mAbs that recognize peptides bound to MHC class II molecules are rare (41). Several anti-MUC1 antibodies have been reported to recognize both the native MUC1 as well as MUC1 peptides in MHC (42). DCs pulsed with Tn-100mer were washed, fixed, and then stained with anti-MUC1 mAbs VU-4-H5 (Fig. 5 A), VU-3-C6 (Fig. 5 B), and BCP9 (Fig. 5 C). VU-4-H5 and VU-3-C6 recognize overlapping epitopes (33) within the DTRPAP sequence that are in the epitope recognized by the VF5 hybridoma. Glycosylation of these epitopes does not interfere with their recognition by the antibodies. The epitope recognized by antibody BCP9 (PAPGSTAP) lies in the COOH-terminal region of the MUC1 tandem repeat (43). This is also the region where glycosylation of serine and threonine residues does not interfere with the recognition of the VF5 epitope, suggesting that it is cleaved during glycopeptide processing. VU-3C6 and VU-4H5 react with their epitopes while BCP9 does not. If the Tn-100mer was merely bound on the surface of the DCs, all three antibodies would show positive staining. The lack of staining with BCP9 indicates that during processing of the Tn-100mer its epitope is destroyed while the epitopes recognized by the other two antibodies are preserved. Thus, even though the peptide specific clone VF5 does not “see” these epitopes, they are nevertheless processed and presented on DCs.
Figure 5.
Detection of processed MUC1 glycoepitopes on DC surface by antibody and lectin staining. DCs were pulsed for 16 h with 20 μg/ml Tn-100mer MUC1 peptide and stained with anti-MUC1 antibodies VU-4-H5 (A), VU-3-C6 (B) and BCP9 (C). Epitopes recognized by each antibody are shown. Goat anti–mouse ALEXA 488 was used as a fluorescent secondary antibody. (D and E) Staining with FITC-labeled Vicia villosa lectin highly specific for Tn antigen. Thin line histograms represent staining of control DCs, thick line represents staining of Tn-100mer-loaded DCs (D). DCs were loaded with Tn-100mer either prior (thick line) or after (thin line) fixation in 1% PFA for 10 min at room temperature (E).
To confirm that monosaccharide residues are still attached to the processed MUC1 peptide epitopes presented on the cell surface, we stained the same Tn-100mer-loaded DCs with FITC-labeled lectin Vicia villosa that specifically binds GalNAc (Tn antigen). Histograms in Fig. 5 D show positive staining of the Tn-100mer-loaded DCs compared with control DCs. To rule out staining of unprocessed Tn-100mer nonspecifically bound to the DC surface, we used as an additional control Tn-100mer-loaded fixed DCs unable to endocytose antigens (Fig. 5 E).
Processed Glycopeptides Are Recognized by a Glycopeptide-specific T Hybridoma.
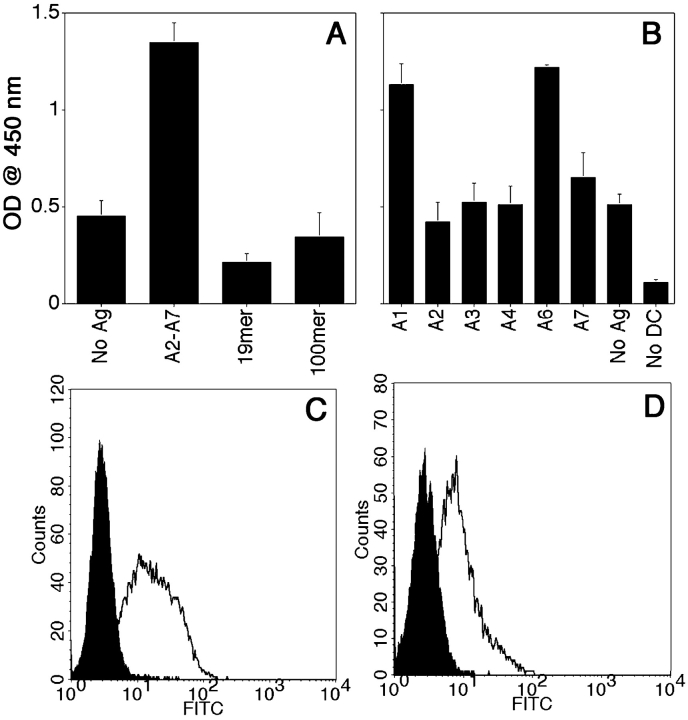
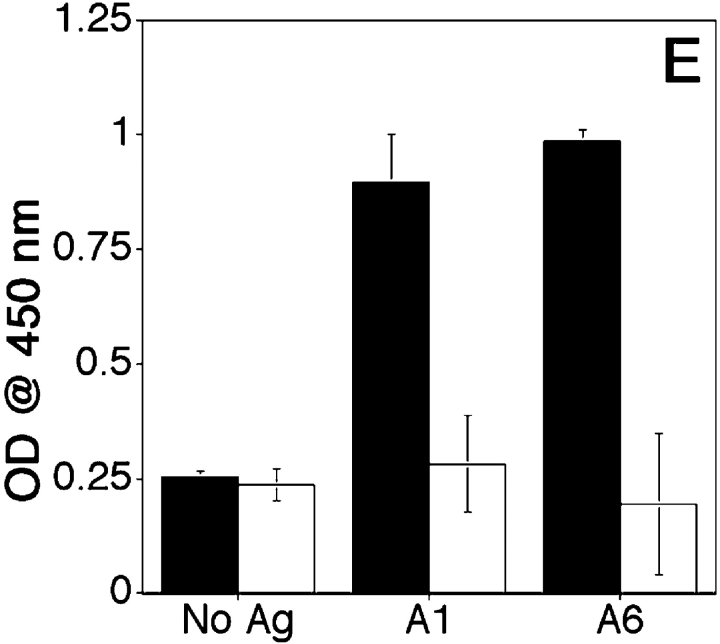
Presentation of glycosylated MUC1 epitopes by DCs is most conclusively demonstrated by their recognition by T cells. We generated T cell hybridomas by in vitro priming and restimulation of T lymphocytes with DCs loaded with pooled disaccharide-substituted glycopeptides. We isolated one hybridoma, VF9, that showed specificity for DCs loaded with the pooled priming glycopeptides A2-A7 and did not react with DCs loaded with unglycosylated 19mer and 13mer (Fig. 6 A). The response of VF9 hybridoma to DCs loaded with the individual glycopeptides (Fig. 6 B) shows specificity for two glycopeptides (A1 and A6), which have in common a disaccharide (T antigen) attached to the first threonine (T1). This hybridoma does not recognize the monosaccharide-substituted H1 glycopeptide or the corresponding unglycosylated peptide (unpublished data), suggesting that presence of the disaccharide is required. VF9 is CD3+ (Fig. 6 C), CD4+ (Fig. 6 D) and I-Ab–restricted T hybridoma (Fig. 6 E).
Figure 6.
Characterization of VF9, a glycopeptide-specific T hybridoma. DCs were loaded for 16 h with either pooled A2-A7 glycopeptides (20 μg/ml each) (A), or individual glycopeptides (B). DCs were then coincubated with VF9 cells for 24 h and supernatants tested for IL-2 presence by ELISA, as described above. (C and D) Flow cytometric analysis was performed for CD3 (C) and CD4 (D) expression. Cells were incubated in the presence of either FITC-labeled isotype control antibody (filled histograms) or anti-CD3 or anti-CD4-FITC antibodies. (E) VF9 cells were coincubated (1:10 DC/T cell ratio) with either syngeneic DCs from C57Bl/6 (I-Ab) mice or with allogeneic DCs from B6.NOD mice (I-Ag7) mice, pulsed with 20 μg/ml antigen, and IL-2 presence assayed by ELISA, as described above.
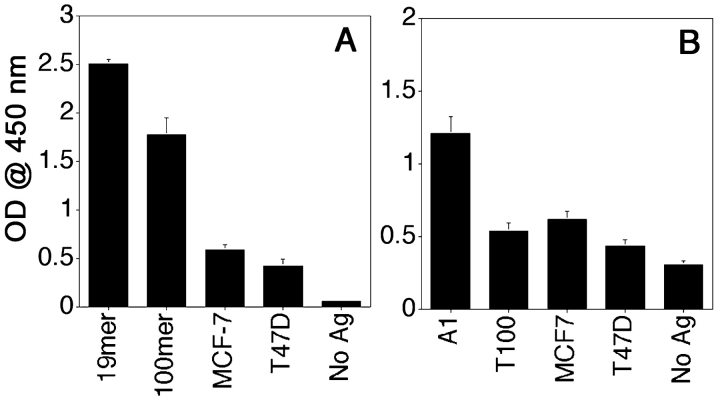
Naturally Glycosylated Tumor MUC1 Is Processed by DCs to Yield Peptide and Glycopeptide Epitopes Recognized by VF5 and VF9.
Having one peptide-specific hybridoma that recognizes a nonglycosylated epitope and a glycopeptide-specific hybridoma that recognizes a tumor-specific glycopeptide bearing a Tn antigen, we could begin to explore processing of MUC1 glycosylated in vivo by tumor cells (Fig. 7) . A gene construct encoding a six-tandem repeat MUC1 fragment was expressed in MCF-7 and T47D breast cancer cell lines. Complete O-glycan profiles of the recombinant proteins purified from these cell lines have been reported to represent the authentic glycosylation pattern seen on MUC1 made by breast tumor cells (23). While both cell lines produced underglycosylated tumor MUC1 distinct from normal MUC1, the average density of glycans per repeat was estimated to be 3 for MCF7 and 4.8 for T47D. We used these naturally glycosylated tumor MUC1 preparations to investigate if their processing by DCs would yield the peptide epitope recognized by the VF5 hybridoma, or the glycopeptide epitope recognized by the VF9. The response of the VF5 hybridoma to DCs loaded with MCF7-derived MUC1 was higher than to DCs loaded with T47D-derived MUC1 (Fig. 7 A) consistent with the higher probability of generating a nonglycosylated peptide epitope from the MCF-7 glycoform with fewer residues glycosylated.
Figure 7.
VF5 and VF9 responses to tumor MUC1 glycoforms. DCs were loaded for 16 h with antigen and coincubated with peptide-specific VF5 (A) or VF9 hybridomas (B) for 24 h and supernatants tested for IL-2 presence by ELISA, as described above.
These tumor forms of MUC1 also yielded VF9-specific epitopes (Fig. 7 B), according to the expected density of T antigens present in the respective preparations; MUC1 glycosylated by MCF-7 cells has a 12.9% incidence of T antigen as opposed to 6.9% on MUC1 produced by T47D breast cancer cells (23). This difference is reflected in the slightly higher amount of IL-2 released by VF9 hybridoma in response to MCF-7–derived MUC1 compared with T47D-derived MUC1. The efficiency of processing of these natural glycoforms into VF9 epitopes is similar to the efficiency of generation of this epitope from a synthetic T-100mer MUC1 that has every Thr within the VTSA sequence glycosylated with the disaccharide T antigen.
Discussion
In attempting to understand immune responses to various self- or foreign antigens, a lot of attention has been paid to how they are processed by APCs and what sort of epitopes are presented to T cells. Most of these studies to date have focused on the protein sequence of specific antigens and the peptide epitopes recognized by MHC class I– or class II–restricted T cells. In many instances, though, the native molecular forms available to DCs for uptake, processing, and presentation are glycosylated or otherwise posttranslationally modified. Recent findings that MHC class II and I molecules can bind and present glycoepitopes to CD4+ (13, 15, 16) and CD8+ T cells (44–48), predicts that the immune repertoire will be broader than initially thought. In fact, studies on autoimmune response have provided useful insights on the repertoire of posttranslationally modified immune epitopes.
According to them, antibodies elicited by modified self-proteins tend to be promiscuous in their ability to bind either the modified or the unmodified form of protein most probably due to the contribution of conserved amino acids flanking the modified residue. By contrast, T cell responses are specific for the modified self-antigen with little or no cross-reactivity with the unmodified self-antigen, as seen in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis, collagen-induced arthritis, celiac disease, etc. (15, 49–52). For example, the acetylated NH2-terminal peptide of myelin basic protein (MBP-Ac1–11) is required for generating EAE, a murine model of human multiple sclerosis. Nonacetylated peptide fails to stimulate T cells and will not elicit EAE (53). In a similar manner, systemic lupus erythematosus can be elicited in a murine model, with an isoaspartyl-modified form of self-Ag (54). Recently, it has been shown that in DR4-transgenic mice expressing human type II collagen, the rheumatoid arthritis glycoprotein antigen, T cell responses to a “naked” peptide epitope are strongly tolerized or even deleted, whereas T cells specific for the corresponding glycosylated peptides persist (55). While these observations have been made regarding the T cell specificity, very little is known about the antigen processing pathways that give rise to these epitopes.
We were particularly interested in what happens to complex oligosaccharides when glycoproteins are taken up, processed, and presented to T cells by DCs. We have addressed processing and presentation of glycoprotein antigens using as a model the tumor antigen MUC1 glycopeptides. As is the case with most antigens studied to date, processing and presentation by DCs of unglycosylated MUC1 peptides and their ability to elicit helper responses has received considerable attention, while nothing is known about how DCs handle glycosylated, synthetic or native forms of MUC1 and what is the nature of the epitopes presented to T cells.
We addressed important aspects of MUC1 processing by DCs and assessed the impact of glycosylation on its presentation to T cells using MHC class II–restricted T cell hybridomas. Using peptide-specific clone VF5 we found that DCs did not remove carbohydrates from glycopeptides that were otherwise efficiently internalized and transported to enzyme- and MHC class II–rich processing compartments. It has been previously suggested that, if not removed, sugars might prevent peptide binding to MHC class II or they might interfere with TCR binding to the peptide–MHC complex. Our results support the latter. Glycoepitopes were generated even from the MUC1 Tn-100mer with at least 15 monosaccharides attached to the peptide backbone, however sugars attached to them prevented recognition by VF5. To further support these observations we studied the glycopeptide-specific VF9 hybridoma that requires the presence of the disaccharide for the recognition of its epitope and is not cross-reactive to the “naked” or monosaccharide-substituted corresponding peptide.
Many of the in vitro glycosylated synthetic glycopeptides used here display O-linked glycans like the Tn and T antigens, found on cancer-associated MUC1. We have used them as experimental tools to determine that saccharides are not removed by DCs after endocytosis and that resulting MUC glycoepitopes are presented at the cell surface generating glycopeptide-specific T cell responses. Yet the in vivo glycosylation of MUC1 occurring in tumor cells is an intricate biosynthetic process generating complex glycosylation patterns that differ from normal cells and may vary from one tumor cell type to another (23). MCF-7 and T47D are two different breast cancer lines, which we used as a source of secreted, in vivo glycosylated MUC1 glycoproteins. MUC1 derived from MCF-7 exhibits a substantially lower glycosylation density compared with T47D, and consequently a higher yield of “naked” MUC1 epitopes recognized by VF5. The relative incidence of T tumor antigen among other sugars on MCF-7 MUC1 is also higher than on T47D-derived MUC1, which resulted in a more efficient generation of glycoepitopes recognized by VF9.
In addition to class II–restricted epitopes, DCs that actively acquire exogenous antigens can generate MHC class I–restricted peptides via both TAP-dependent and TAP-independent pathway (56–59). Very little is currently known about class I–restricted glycopeptides and it will be equally important to determine if class I–restricted MUC1 glycoepitope-specific CD8+ T cells can be generated. While adenocarcinomas express very low levels of MHC class II molecules and CD4+ T cells are not expected to react with tumor cells, CD8+ T cells are expected to have a direct antitumor effect. Understanding the intracellular fate of glycosylated antigens taken up by DCs, and especially those whose glycosylation is different between normal and tumor cells, could have great impact on the design of cancer vaccines.
Acknowledgments
We thank Dr. Russell Salter for his advice, Sean Alber for assistance with microscopy, and Edyta Zielinska and Ana Ivic for help with functional screening of T hybridomas.
A. Vlad was supported by dissertation research grant (DISS2000495) from The Susan G. Komen Breast Cancer Foundation and Bob and Colleen Woeber Breast Cancer Research Fund. The work was supported by grants PO1CA73743 and RO1CA56103 to O.J. Finn, RO1CA84106, Deutsche Forschungsgemeinschaft (Ha2092/4-4 and Ha2092/8-1) and by grants of the Köln-Fortune Programme to F.-G. Hanisch, and a National Institutes of Health grant GM45011 to L. Otvos.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; MIIC, MHC class II-rich compartment.
References
- 1.Lehner, P.J., and P. Cresswell. 1996. Processing and delivery of peptides presented by MHC class I molecules. Curr. Opin. Immunol. 8:59–67. [DOI] [PubMed] [Google Scholar]
- 2.Germain, R.N., F. Castellino, R. Han, C. Reis e Sousa, P. Romagnoli, S. Sadegh-Nasseri, and G.M. Zhong. 1996. Processing and presentation of endocytically acquired protein antigens by MHC class II and class I molecules. Immunol. Rev. 151:5–30. [DOI] [PubMed] [Google Scholar]
- 3.Pieters, J. 2000. MHC class II-restricted antigen processing and presentation. Adv. Immunol. 75:159–208. [DOI] [PubMed] [Google Scholar]
- 4.Watts, C. 2001. Antigen processing in the endocytic compartment. Curr. Opin. Immunol. 13:26–31. [DOI] [PubMed] [Google Scholar]
- 5.Harding, C.V. 1996. Class II antigen processing: analysis of compartments and functions. Crit. Rev. Immunol. 16:13–29. [DOI] [PubMed] [Google Scholar]
- 6.Pierre, P., S.J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R.M. Steinman, and I. Mellman. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 388:787–792. [DOI] [PubMed] [Google Scholar]
- 7.Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R.N. Germain, I. Mellman, and R.M. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockhausen, I., J. Schutzbach, and W. Kuhns. 1998. Glycoproteins and their relationship to human disease. Acta Anat. (Basel). 161:36–78. [DOI] [PubMed] [Google Scholar]
- 9.Finn, O.J. 2000. Glycoprotein antigens. In Principles and Practice of the Biological Therapy of Cancer. S. Rosenberg, editor. Lippincott, Philadelphia. 549–556.
- 10.Olofsson, S. 1992. Carbohydrates in herpesvirus infections. APMIS Suppl. 27:84–95. [PubMed] [Google Scholar]
- 11.Freed, E.O., and M.A. Martin. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J. Biol. Chem. 270:23883–23886. [DOI] [PubMed] [Google Scholar]
- 12.Rademacher, T.W., R.H. Jones, and P.J. Williams. 1995. Significance and molecular basis for IgG glycosylation changes in rheumatoid arthritis. Adv. Exp. Med. Biol. 376:193–204. [DOI] [PubMed] [Google Scholar]
- 13.Dudler, T., F. Altmann, J.M. Carballido, and K. Blaser. 1995. Carbohydrate-dependent, HLA class II-restricted, human T cell response to the bee venom allergen phospholipase A2 in allergic patients. Eur. J. Immunol. 25:538–542. [DOI] [PubMed] [Google Scholar]
- 14.Kim, Y.J., and A. Varki. 1997. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 14:569–576. [DOI] [PubMed] [Google Scholar]
- 15.Corthay, A., J. Backlund, J. Broddefalk, E. Michaelsson, T.J. Goldschmidt, J. Kihlberg, and R. Holmdahl. 1998. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur. J. Immunol. 28:2580–2590. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsson, E., J. Broddefalk, A. Engstrom, J. Kihlberg, and R. Holmdahl. 1996. Antigen processing and presentation of a naturally glycosylated protein elicits major histocompatibility complex class II-restricted, carbohydrate-specific T cells. Eur. J. Immunol. 26:1906–1910. [DOI] [PubMed] [Google Scholar]
- 17.Surman, S., T.D. Lockey, K.S. Slobod, B. Jones, J.M. Riberdy, S.W. White, P.C. Doherty, and J.L. Hurwitz. 2001. Localization of CD4+ T cell epitope hotspots to exposed strands of HIV envelope glycoprotein suggests structural influences on antigen processing. Proc. Natl. Acad. Sci. USA. 98:4587–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gendler, S.J., and A.P. Spicer. 1995. Epithelial mucin genes. Annu. Rev. Physiol. 57:607–634. [DOI] [PubMed] [Google Scholar]
- 19.Baldus, S.E., and F.G. Hanisch. 2000. Biochemistry and pathological importance of mucin-associated antigens in gastrointestinal neoplasia. Adv. Cancer Res. 79:201–248. [DOI] [PubMed] [Google Scholar]
- 20.Ciborowski, P., E.M. Hiltbold, S. Barrat-Boyes, and O.J. Finn. 1996. MUC1 mucin as a tumor antigen in breast cancer. In Breast Cancer: Molecular Genetics, Pathogenesis and Therapeutics. A.M. Bowcock, editor. Humana Press Inc., Totowa, NJ. 453–468.
- 21.Karsten, U., C. Diotel, G. Klich, H. Paulsen, S. Goletz, S. Muller, and F.G. Hanisch. 1998. Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation. Cancer Res. 58:2541–2549. [PubMed] [Google Scholar]
- 22.Hanisch, F.G., S. Muller, H. Hassan, H. Clausen, N. Zachara, A.A. Gooley, H. Paulsen, K. Alving, and J. Peter-Katalinic. 1999. Dynamic epigenetic regulation of initial O-glycosylation by UDP-N-acetylgalactosamine:peptide N-acetylgalactosaminyltransferases. Site-specific glycosylation of MUC1 repeat peptide influences the substrate qualities at adjacent or distant Ser/Thr positions. J. Biol. Chem. 274:9946–9954. [DOI] [PubMed] [Google Scholar]
- 23.Muller, S., and F.G. Hanisch. 2002. Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin. High density and prevalent core 2-based glycosylation. J. Biol. Chem. 277:26103–26112. [DOI] [PubMed] [Google Scholar]
- 24.Fields, G.B., and R.L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161–214. [DOI] [PubMed] [Google Scholar]
- 25.Angell, Y.M., C. Garcia-Echeverria, and D.H. Rich. 1994. Comparative studies of the coupling of N-methylated sterically hindered amino acids during solid-phase peptide synthesis. Tetrahedron Lett. 35:5891–5894. [Google Scholar]
- 26.Cudic, M., H.C. Ertl, and L. Otvos, Jr. 2002. Synthesis, conformation and T-helper cell stimulation of an O-linked glycopeptide epitope containing extended carbohydrate side-chains. Bioorg. Med. Chem. 10:3859–3870. [DOI] [PubMed] [Google Scholar]
- 27.Meldal, M., T. Bielfeldt, S. Peters, K.J. Jensen, H. Paulsen, and K. Bock. 1994. Susceptibility of glycans to beta-elimination in Fmoc-based O-glycopeptide synthesis. Int. J. Pept. Protein Res. 43:529–536. [DOI] [PubMed] [Google Scholar]
- 28.Otvos, L., Jr., L. Urge, Z.Q. Xiang, G.R. Krivulka, L. Nagy, G.I. Szendrei, and H.C. Ertl. 1994. Glycosylation of synthetic T helper cell epitopic peptides influences their antigenic potency and conformation in a sugar location-specific manner. Biochim. Biophys. Acta. 1224:68–76. [DOI] [PubMed] [Google Scholar]
- 29.Mayordomo, J.I., T. Zorina, W.J. Storkus, L. Zitvogel, C. Celluzzi, L.D. Falo, C.J. Melief, S.T. Ildstad, W.M. Kast, A.B. Deleo, et al. 1995. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1:1297–1302. [DOI] [PubMed] [Google Scholar]
- 30.Soares, M.M., V. Mehta, and O.J. Finn. 2001. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J. Immunol. 166:6555–6563. [DOI] [PubMed] [Google Scholar]
- 31.White, J., M. Blackman, J. Bill, J. Kappler, P. Marrack, D.P. Gold, and W. Born. 1989. Two better cell lines for making hybridomas expressing specific T cell receptors. J. Immunol. 143:1822–1825. [PubMed] [Google Scholar]
- 32.Born, W., J. White, R. O'Brien, and R. Kubo. 1988. Development of T cell receptor expression: studies using T cell hybridomas. Immunol. Res. 7:279–291. [DOI] [PubMed] [Google Scholar]
- 33.Price, M.R., P.D. Rye, E. Petrakou, A. Murray, K. Brady, S. Imai, S. Haga, Y. Kiyozuka, D. Schol, M.F. Meulenbroek, et al. 1998. Summary report on the ISOBM TD-4 workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. Tumour Biol. 19(Suppl 1):1–20. [DOI] [PubMed] [Google Scholar]
- 34.Pathak, S.S., and J.S. Blum. 2000. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic. 1:561–569. [DOI] [PubMed] [Google Scholar]
- 35.Sette, A., L. Adorini, S.M. Colon, S. Buus, and H.M. Grey. 1989. Capacity of intact proteins to bind to MHC class II molecules. J. Immunol. 143:1265–1267. [PubMed] [Google Scholar]
- 36.Monji, T., and D. Pious. 1997. Exogenously provided peptides fail to complex with intracellular class II molecules for presentation by antigen-presenting cells. J. Immunol. 158:3155–3164. [PubMed] [Google Scholar]
- 37.Shimonkevitz, R., S. Colon, J.W. Kappler, P. Marrack, and H.M. Grey. 1984. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J. Immunol. 133:2067–2074. [PubMed] [Google Scholar]
- 38.Ceppellini, R., G. Frumento, G.B. Ferrara, R. Tosi, A. Chersi, and B. Pernis. 1989. Binding of labelled influenza matrix peptide to HLA DR in living B lymphoid cells. Nature. 339:392–394. [DOI] [PubMed] [Google Scholar]
- 39.Prigozy, T.I., O. Naidenko, P. Qasba, D. Elewaut, L. Brossay, A. Khurana, T. Natori, Y. Koezuka, A. Kulkarni, and M. Kronenberg. 2001. Glycolipid antigen processing for presentation by CD1d molecules. Science. 291:664–667. [DOI] [PubMed] [Google Scholar]
- 40.Hiltbold, E.M., A.M. Vlad, P. Ciborowski, S.C. Watkins, and O.J. Finn. 2000. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J. Immunol. 165:3730–3741. [DOI] [PubMed] [Google Scholar]
- 41.Dadaglio, G., C.A. Nelson, M.B. Deck, S.J. Petzold, and E.R. Unanue. 1997. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 6:727–738. [DOI] [PubMed] [Google Scholar]
- 42.Apostolopoulos, V., G. Chelvanayagam, P.X. Xing, and I.F. McKenzie. 1998. Anti-MUC1 antibodies react directly with MUC1 peptides presented by class I H2 and HLA molecules. J. Immunol. 161:767–775. [PubMed] [Google Scholar]
- 43.Xing, P.X., J. Prenzoska, K. Quelch, and I.F. McKenzie. 1992. Second generation anti-MUC1 peptide monoclonal antibodies. Cancer Res. 52:2310–2317. [PubMed] [Google Scholar]
- 44.Zhao, X.J., and N.K. Cheung. 1995. GD2 oligosaccharide: target for cytotoxic T lymphocytes. J. Exp. Med. 182:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haurum, J.S., G. Arsequell, A.C. Lellouch, S.Y. Wong, R.A. Dwek, A.J. McMichael, and T. Elliott. 1994. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J. Exp. Med. 180:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deck, B., M. Elofsson, J. Kihlberg, and E.R. Unanue. 1995. Specificity of glycopeptide-specific T cells. J. Immunol. 155:1074–1078. [PubMed] [Google Scholar]
- 47.Glithero, A., J. Tormo, J.S. Haurum, G. Arsequell, G. Valencia, J. Edwards, S. Springer, A. Townsend, Y.L. Pao, M. Wormald, et al. 1999. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity. 10:63–74. [DOI] [PubMed] [Google Scholar]
- 48.Speir, J.A., U.M. Abdel-Motal, M. Jondal, and I.A. Wilson. 1999. Crystal structure of an MHC class I presented glycopeptide that generates carbohydrate-specific CTL. Immunity. 10:51–61. [DOI] [PubMed] [Google Scholar]
- 49.van Stipdonk, M.J., A.A. Willems, S. Amor, C. Persoon-Deen, P.J. Travers, C.J. Boog, and J.M. van Noort. 1998. T cells discriminate between differentially phosphorylated forms of αB-crystallin, a major central nervous system myelin antigen. Int. Immunol. 10:943–950. [DOI] [PubMed] [Google Scholar]
- 50.Cao, L., D. Sun, and J.N. Whitaker. 1998. Citrullinated myelin basic protein induces experimental autoimmune encephalomyelitis in Lewis rats through a diverse T cell repertoire. J. Neuroimmunol. 88:21–29. [DOI] [PubMed] [Google Scholar]
- 51.Arentz-Hansen, H., R. Korner, O. Molberg, H. Quarsten, W. Vader, Y.M. Kooy, K.E. Lundin, F. Koning, P. Roepstorff, L.M. Sollid, and S.N. McAdam. 2000. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sollid, L.M. 2000. Molecular basis of celiac disease. Annu. Rev. Immunol. 18:53–81. [DOI] [PubMed] [Google Scholar]
- 53.Zamvil, S.S., D.J. Mitchell, A.C. Moore, K. Kitamura, L. Steinman, and J.B. Rothbard. 1986. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 324:258–260. [DOI] [PubMed] [Google Scholar]
- 54.Mamula, M.J., R.J. Gee, J.I. Elliott, A. Sette, S. Southwood, P.J. Jones, and P.R. Blier. 1999. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 274:22321–22327. [DOI] [PubMed] [Google Scholar]
- 55.Backlund, J., S. Carlsen, T. Hoger, B. Holm, L. Fugger, J. Kihlberg, H. Burkhardt, and R. Holmdahl. 2002. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263-270) in humanized transgenic mice and in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 99:9960–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, B., C.C. Norbury, R. Greenwood, J.R. Bennink, J.W. Yewdell, and J.A. Frelinger. 2001. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J. Immunol. 167:1283–1289. [DOI] [PubMed] [Google Scholar]
- 57.Yewdell, J.W., C.C. Norbury, and J.R. Bennink. 1999. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73:1–77. [DOI] [PubMed] [Google Scholar]
- 58.Norbury, C.C., B.J. Chambers, A.R. Prescott, H.G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280–288. [DOI] [PubMed] [Google Scholar]
- 59.Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. [DOI] [PubMed] [Google Scholar]