Abstract
Purpose
To determine the incidence and natural history of cataracts in children with congenital toxoplasmosis.
Methods
Children referred to the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) between 1981 and 2005 were examined by ophthalmologists at predetermined times according to a specific protocol. The clinical course and treatment of patients who developed cataracts was reviewed.
Results
In the first year of life, 134 of 173 children examined were treated with pyrimethamine, sulfadiazine, and Leucovorin, while the remaining 39 were not treated. Cataracts occurred in 27 eyes of 20 patients (11.6%, 95% confidence interval [7.2%, 17.3%]). Fourteen cataracts were present at birth, and 13 developed postnatally. Locations of the cataracts included anterior polar (3 eyes), anterior subcapsular (6), nuclear (5), posterior subcapsular (7), and unknown (6). Thirteen cataracts were partial, 9 total, and 5 with unknown complexity. Twelve cataracts remained stable, 12 progressed, and progression was not known for 3. Five of 27 eyes had cataract surgery, with 2 of these developing glaucoma. Sixteen eyes of 11 patients had retinal detachment and cataract. All eyes with cataracts had additional ocular lesions.
Conclusions
In the NCCCTS cohort, 11.6% of patients were diagnosed with cataracts. There was considerable variability in the presentation, morphology, and progression of the cataracts. Associated intraocular pathology was an important cause of morbidity.
Keywords: Cataracts, Congenital Toxoplasmosis, Eyes
Introduction
Congenital toxoplasmosis causes substantial visual and neurologic morbidity.4–14 Even treatment initiated at birth may commence too late to prevent ocular damage.4,5,11,14 Cataracts in children with congenital toxoplasmosis have been described by others.14 We previously described our experience in the National Collaborative Chicago-Based Congenital Toxoplasmosis Study (NCCCTS) for the first 75 children, of whom 7 (9.3%) had cataracts.11 To better understand how cataracts manifest in children with congenital toxoplasmosis, we further studied the characteristics and natural history of cataracts in the updated NCCCTS cohort of 173 children followed prospectively and longitudinally from 1981 to 2005.4
Materials and Methods
Patient Treatments and Evaluations
This work was performed with Institutional Review Board approval granted from the University of Chicago. Informed consent was obtained for all participants in accordance with Health Insurance Portability and Accountability Act of 1996 guidelines.
One hundred seventy-three children with congenital toxoplasmosis were diagnosed serologically and referred to our study as previously described.4 One hundred twenty-four children were treated at diagnosis with 100 mg/kg of sulfadiazine taken daily in two divided doses for 12 months, and 1mg/kg of pyrimethamine taken once daily for either two months (Treatment 1) or six months (Treatment 2). Both Treatments 1 and 2 were followed by the same dosage of Leucovorin (folinic acid) as described and pyrimethamine administered on Mondays, Wednesdays, and Fridays for the remainder of a year.4 The children’s physicians managed treatment with the consultation of the study’s principal investigators. Ten children received treatment different than the previously described recommendation,4 with varying regimens including one or all of spiramycin, pyrimethamine, sulfonamides, and folinic acid, Thirty-nine children, referred to as “historical patients,” were not diagnosed with congenital toxoplasmosis until after their first year of life, and therefore they did not received treatment during this time.
To remove economic barriers that might affect the ability of families to participate in the study, all patients were provided complimentary accommodations and travel for one-day evaluations in Chicago. The children participating in these comprehensive evaluations were at or as close as possible to the ages of < 2.5 months, 1, 3.5, 5, 7.5, 10, 15, 20 years old. The evaluation included a review of medical history, and a general physical examination, ophthalmologic evaluation, psychological and developmental testing, a neurologic exam, audiology testing, and hematologic laboratory testing (i.e., complete blood count including differential, white blood cell, and platelet counts). A brain CT scan was obtained in the newborn period.
Ophthalmologic data were collected for each study participant and entered into a database. Twenty study patients who had cataracts were identified and characteristics of their cataracts were determined including morphology, complexity, associated conditions, and natural history (progression).
Results
Characteristics of children in this study
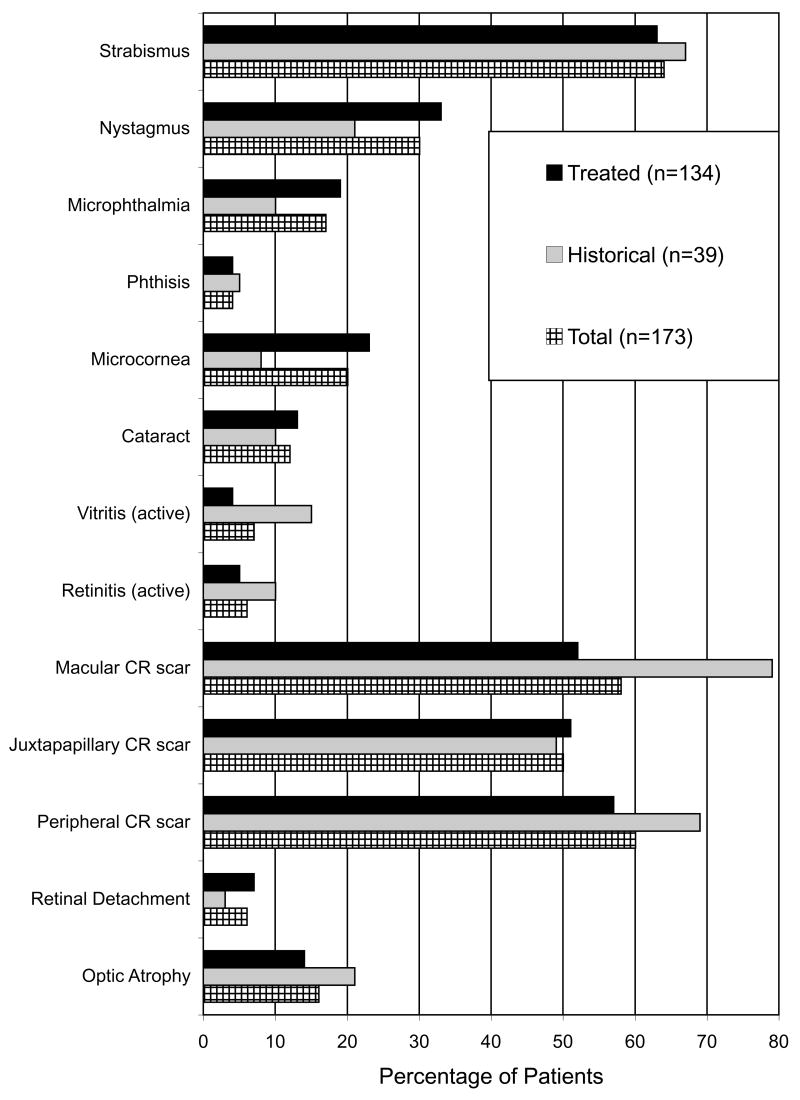
Children were referred from throughout the United States with predominance from the New York, Chicago, and Los Angeles metropolitan areas. Ophthalmologic manifestations in these children are summarized in Figure 1. Although chorioretinal scars were the most frequent finding, 20 (11.6%, 95% confidence interval [7.2%, 17.3%]) of 173 patients had cataracts.
Figure 1.
Summary of ophthalmologic manifestations in 173 patients with congenital toxoplasmosis. Chorioretinal scars were the most common finding with a high incidence of strabismus. CR = Chorioretinal.
Identification and Characterization of Cataracts
Twenty children (27 eyes) had cataracts. Seven were bilateral, thirteen were unilateral and all occurred in the presence of other ocular lesions. There were 16 children (21 eyes) with cataracts among the 134 children treated in their first year of life and 4 children (6 eyes) with cataracts among the 39 children diagnosed after their first year of life. All of the 16 children who were treated in the first year of life and developed cataracts had severe disease with neurologic signs at birth. It is unknown whether the four children diagnosed after their first year of life, rather than at birth, had severe neurologic disease at birth that might have been identified with brain computed tomography scan or detailed neurologic examinations.
Posterior subcapsular cataracts were noted most often (7); however, nuclear (5), anterior subcapsular (6), and anterior polar cataracts (3) also were observed frequently. The morphology of 6 cataracts was unknown. Other characterization of the cataracts includes complexity, time of development, and natural history. Thirteen were partial (48.1%), nine were total (33.3%), and five were unknown (18.5%) (Figure 2) Fourteen cataracts (51.9%) were detected at birth and thirteen (48.1%) were observed postnatally. Twelve were stable (44.4%), twelve progressive (44.4%), and the natural history was not known for three cataracts (11.1%).
Figure 2.
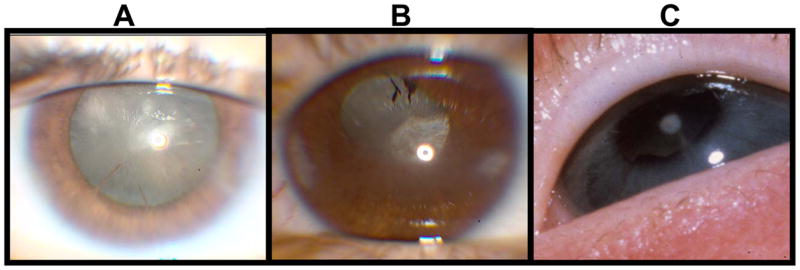
The left photograph is a total cataract. The middle photograph is a cataract that clinically was of unknown type. The type of cataract was unknown at the time of the examination. Later the eye was enucleated and pathology revealed an anterior subcapsular plaque and marked cataractous changes. The right photograph is a partial cataract (anterior polar).
Five of 27 cataractous eyes had surgery (18.5%). One or a combination of the following surgeries were performed: extra capsular cataract extraction, pars plana vitrectomy, pars plana lensectomy, membrane peeling, pulpilloplasty, peripheral iridectomy. Two of 27 eyes with cataracts also had glaucoma (7.4%) and sixteen of 27 eyes with cataracts also had retinal detachment (59.3%). Two patients had persistent fetal vasculature (persistent hyaloid artery and persistent hyperplasia of the primary vitreous). One eye of 27 (3.7%) was enucleated. Additional features of the demographics, patient characteristics, cataract morphology, and a representative case are illustrated in the e-supplement, available at jaapos.org.
Discussion
The pathogenesis of cataracts in congenital toxoplasmosis is not known. The retina and choroid are generally affected first; then iridocyclitis and cataracts can develop, as secondary complications of retinochoroiditis. In sequential clinical examinations carried out on the eyes of mice that had been infected in utero with Toxoplasma gondii, 3 patterns of clinical disease were seen. These patterns included crystalliform cataracts, acute uveitis that progressed into a chronic inflammatory disease with secondary opaque cataracts, and multiple discrete foci of deep retinal disturbances. Immunocytochemical staining for Toxoplasma antigen revealed only intraretinal Toxoplasma cysts, but no free organisms or extracystic antigen were demonstrated. Selective photoreceptor destruction was the most prominent histopathological feature.15 In one study of a murine model, DNA deposition led to cataract formation.16 It may be that both parasite replication and inflammatory cells arriving via the hyaloid artery during lens development cause such DNA deposition in the lens in congenital toxoplasmosis.
The incidence of cataracts was 11.6% in our NCCCTS cohort, most commonly occurring in those with the most severe disease. These cataracts affected any part of the lens and they varied in complexity from partial to total. In 11 children (16 eyes) in our study, cataracts were associated with such severe intraocular pathology (e.g. retinal detachment) that intervention was not performed. Some of these patients eventually had no light perception and phthisis. It is important to evaluate the retina of children with congenital toxoplasmosis and cataracts.
The child discussed in the Case 1 in the online supplement was treated for toxoplasmosis prior to, at the time of, and after his cataract surgery. It is important to administer anti-parasitic medication during surgery because reactivations of chorioretinitis have accompanied surgery for cataracts in patients with congenital toxoplasmosis.17
Intraocular pressure measurements may be overlooked when treating young children with active disease. Rosenbaum and colleagues 18 suggested that as many as 38% of older children and adults who have active lesions consistent with toxoplasmic chorioretinitis have elevated intraocular pressure. We have cared for a patient with eye pain during active toxoplasmic chorioretinitis in whom intraocular pressure was elevated (unpublished observation, February 24, 2006).
In conclusion, patients with congenital toxoplasmosis are at high risk for cataracts and other intraocular pathology and may benefit from cataract removal.
Supplementary Material
Acknowledgments
This work was supported by Research to Prevent Blindness and the National Insitutes of Health RO1 AI27530. The Hyatt Hotel Foundation provided complementary lodging. We thank the families and physicians who permitted us to follow these children together with them. We also want to gratefully acknowledge airlines for providing complementary transportation during patient trips to Chicago. Other members of the Toxoplasmosis Study Group include Barbara Danis, Mark Stein, Linda Pfiffner, Jeanne Perkins, Sanford Meyers, Michael Kipp, Balaji Gupta, Ahmed Abdelsalam, Huiyuan Zhang, John Marcinak, Saeid Mojatahedi, Dianna Bardo, Marisha Humphries, Douglas Mack, Michael J. Kirisits, Diana Chamot, Ernest Mui, Ronald Thisted, and Adrian Esquivel (University of Chicago); Lazlo Stein (Northwestern University, Chicago; deceased); Andrew Suth (Argosy University, Chicago); Audrey Cameron (Mount Sinai Hospital, Chicago); Marie Weissbourd (Northwestern University Hospitial, Chicago); James McAuley (Rush University Medical Center, Chicago); Joyce Hopkins (Illinois Institute of Technology, Chicago); Dushyant Patel (Michael Reese Medical Center, Chicago); and Dave McLone (Children’s Memorial Hospital, Chicago)
This work was supported by RO1 AI27530 and the Research to Prevent Blindness Foundation.
Footnotes
This work was not presented at the AAPOS annual meeting or any other national meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor D. Congenital cataracts: changing perspective. In: Cotlier E, editor. Congenital Cataracts. Austin, TX: Landes Company; 1994. pp. 3–9. [Google Scholar]
- 2.James LM, McClearon AB, Waters GD. Congenital malformation surveillance data for birth defects prevention: Metropolitan Atlanta Congenital Defects Program (MADCP) 1968–1991 and birth defects monitoring program (BDMP) 1970–1991. Tetralogy. 1993;48:545. [Google Scholar]
- 3.Stewart-Bron SL, Haslum MN. Partial sight and blindness in children of the 1970 birth cohort at 10 years of age. J Epidemiol Community Health. 1988;42:17. doi: 10.1136/jech.42.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: The National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 5.McAuley J, Boyer K, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis. The Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 6.Roizen N, Kasza K, Mets M, et al. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis. Pediatrics. 2006 Aug;118(2):e379–90. doi: 10.1542/peds.2005-1530. Epub 2006 Jul 24. [DOI] [PubMed] [Google Scholar]
- 7.Eichenwald HF. A study of congenital toxoplasmosis, with particular emphasis on clinical manifestations, sequelae, and therapy. In: Siim JC, editor. Human Toxoplasmosis. Copenhagen, Demark: Munksgaard; 1960. pp. 41–9. [Google Scholar]
- 8.Wilson CB, Remington JS, Stagno S, Reynolds DW. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980;66:767–74. [PubMed] [Google Scholar]
- 9.Koppe JG, Loewer-Sieger DH, DeRoever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–6. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 10.Saxon SA, Knight W, Reynolds DW, et al. Intellectual deficits in children born with subclinical congenital toxoplasmosis: A preliminary report. J Peds. 1973;82:792. doi: 10.1016/s0022-3476(73)80068-x. [DOI] [PubMed] [Google Scholar]
- 11.Mets M, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–24. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 12.Roizen N, Swisher C, Stein M, et al. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics. 1995;95:11–20. [PubMed] [Google Scholar]
- 13.Brezin AP, Thulliez P, Couvreur J, Nobre R, McLeod R. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol. 2003;135:779–84. doi: 10.1016/s0002-9394(02)01704-x. [DOI] [PubMed] [Google Scholar]
- 14.Vutova K, Peicheva Z, Popova A, Markova V, Mincheva N, Todorov T. Congenital toxoplasmosis: eye manifestations in infants and children. Ann Trop Paediatr. 2002;22:213–8. doi: 10.1179/027249302125001507. [DOI] [PubMed] [Google Scholar]
- 15.Dutton GN. Graefes Clinicopathological features of a congenital murine model of ocular toxoplasmosis. Arch Clin Exp Ophthal. 1986;224:256–64. doi: 10.1007/BF02143066. [DOI] [PubMed] [Google Scholar]
- 16.Nishimoto S, Kawane K, Watanabe-Fukunaga R, et al. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–4. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- 17.Bosch-Driessen LH, Plaisier MB, Stilma JS, Van der Lelij A, Rothova A. Reactivations of ocular toxoplasmosis after cataract extraction. Ophthalmology. 2002;109:41–5. doi: 10.1016/s0161-6420(01)00845-4. [DOI] [PubMed] [Google Scholar]
- 18.Westfall AC, Lauer AK, Suhler EB, Rosenbaum JT. Toxoplasmosis retinochoroiditis and elevated intraocular pressure: a retrospective study. J Glaucoma. 2005;14:3–10. doi: 10.1097/01.ijg.0000146373.51495.c1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.