Abstract
There exists a bidirectional regulatory circuit between the nervous and immune systems. This regulation has been shown to be mediated in part through neuroendocrine hormones and cytokines. Both systems have receptors for both types of signal molecules. The nervous system has receptors for cytokines and it also synthesizes cytokines. The immune system synthesizes and responds to cytokines. So, it is not too farfetched to believe that neuroendocrine peptide hormones could bind to leukocytes and modulate immune functions. However, it is not widely known that the immune system also synthesizes functional, neuropeptide hormones. This will be discussed in this paper citing a plethora of evidence. The aim of this paper is to summarize this evidence by using three neuropeptides that are synthesized by leukocytes and modulate immune functions as examples; corticotropin (ACTH), endorphin (END), and corticotropin releasing factor (CRF). The production and action of these three neuropeptides in the immune system will be explained. Finally, the potential physiological role of leukocyte-derived ACTH, END, and CRF in inflammation as a localized hypothalamic-pituitary like axis is discussed.
1. Introduction
Much progress has been made in the field of psychoneuroimmunology (PNI) in the 28 years I have been involved in the field. Over the last year, I have had the honor of reading every abstract submitted for the annual meeting of the Psychoneuroimmunology Research Society. I also acted as guest editor for a series of reviews highlighting the developments of PNI to celebrate the 20th anniversary of Brain Behavior and Immunity. The molecular biology methodology combined with sophisticated behavioral approaches show PNI has come of age as a “mature science.” To see how much the field in general has progressed during the past 20 years, I encourage you to read the 2007 series “20 Years of Brain Behavior and Immunity.” In particular, PNI has made immense strides in understanding cytokines in the brain, sickness behavior, vagal afferent mediation of cytokine activity, sympathetic innervation and regulation of immune responses. Some of the early research topics have been slower to develop (or had fewer people interested in researching them). One such topic is the production of neuropeptides by leukocytes.
There are many reasons for the production of neuropeptides by the immune system being an under-developed topic. But, I believe the most prominent to be that the different specialties such as immunology and endocrinology do not know how to interpret or integrate such a finding in their systems. The difficulty immunologists have had in accepting PNI is well known and continues today. But the immunologists were also hesitant, at first to embrace cytokines since they were not antigen specific factors. We still do not know what the essential role of IL-1 is, much less how a neuropeptide fits into the regulatory scheme of the immune system. This has been controversial from the very beginning. The first RO1 grant application Ed Blalock and I submitted on the topic prompted a site visit by the NIH reviewers to see if the findings were real. Even though there are over a hundred publications on the topic, it is rare to find leukocyte production of neuropeptides mentioned in immunology or neuroscience text books.
Many questions arose with the first evidence of leukocyte hormone production. Why does this production occur? What is the spectrum of neuropeptides produced? Does it occur through the same mechanisms as in the prototype tissues? And finally, what is the role of leukocyte production of neuropeptides? These are some of the same questions my laboratory has been trying to address, particularly in regard to corticotropin (ACTH) and corticotropin releasing factor (CRF). In this article I will discuss the production of three neuropeptides that are synthesized by leukocytes; ACTH, endorphin (END), and CRF and speculate on how they may interact in a localized circuit to affect immune responses.
2. Pro-opiomelanocortin (POMC) production in the immune system
The finding that leukocytes produce neuropeptides came early in the development of the PNI field. When I joined Ed Blalock’s laboratory he was studying interferon’s (IFN) novel, endocrine-like effects (another fundamental family of cytokines that was slow to be accepted as an immunological regulatory factor). Blalock found that IFN-α exhibited adrenergic activity on mouse myocardial cells and would increase their beat frequency in addition to inducing an antiviral response in the cells (Blalock and Stanton, 1980). This was a specific, IFN-α receptor mediated effect because IFN is a species-specific cytokine and neither human IFN-α or murine IFN-γ had an effect on the murine myocardial cells. We started looking at other hormonal activities associated with IFNs (Smith and Blalock, 1981b). Mouse adrenal tumor cells (Y-1) and melanoma cells both responded to IFN-α. The Y-1 cells changed morphologically to a more rounded shape and the melanoma cells increased their pigmentation. ACTH causes similar changes in both cell types. One possible explanation other than an IFN receptor-mediated effect, was a structural homology (primary or tertiary) such that IFN could bind the ACTH receptor (melanocortin 2 receptor; MC2R). This was before the sequence of IFN-α was known, but was what our preliminary evidence suggested, a structural homology or common sequence between ACTH and IFN-α(Blalock and Smith, 1980). With further investigation we determined that the ACTH was co-expressed with the IFN in our system and present in the partially purified IFN preparation (Smith and Blalock, 1981a). Thus it was discovered that leukocytes could synthesize a neuropeptide.
Other hormones and neuropeptides have also been shown to be produced by, and have activity on, lymphocytes. Examples include CRF, END, enkephalins (ENK), growth hormone, prolactin, thyroid stimulating hormone (TSH), arginine vasopressin/oxytocin, vasoactive intestinal peptide, luteinizing hormone, substance P, and others (Kelley, Brief, Westly, Novakofski, Bechtel, Simon, and Walker, 1986;Davila, Brief, Simon, Hammer, Brinster, and Kelley, 1987;Blalock, Whitaker, Benveniste, and Bost, 1989;Hughes and Chin, 1994). Since leukocyte neuropeptide production occurs in many organisms, including less advanced ones such as invertebrates, this feature has been conserved through evolution. To be maintained it must serve a fundamental role (Stefano and Smith, 1995). These other neuropeptides and hormones likely have important roles in the systems. However, this paper will focus on ACTH, END, and CRF interactions in the immune system.
ACTH was the first and probably the best, characterized neuropeptide produced by leukocytes (Smith, 1994). ACTH was first observed in cell culture supernatant fluid from lymphocytes stimulated with Newcastle disease virus (NDV) to produce IFN (Smith et al., 1981a; Blalock et al., 1980). The IFN could be removed from the preparation by proteolytic digestion and acid treatment to which ACTH is stable. Purification and biochemical characterization proved the leukocyte product to be actual ACTH. The leukocyte-derived ACTH had the same electrophoretic mobility on polyacrylamide gels, same migration on reverse phase high performance liquid chromatography (HPLC), was recognized by anti-ACTH antiserum, and was co-produced with END (Smith, Galin, LeBoeuf, Coppenhaver, Harbour, and Blalock, 1990;Smith et al., 1981a). Leukocyte ACTH would induce mouse Y-1 adrenal cells to round up morphologically and to secret corticosteroids. The amino acid sequence of the purified, ACTH peptide and that of a cDNA from RT-PCR of the mRNA was identical with that from the pituitary (Smith et al., 1990; Smith et al., 1981a). Since ACTH and endorphin are cleavage products of POMC, we believe that the same POMC gene expressed in the pituitary gland is also expressed in leukocytes.
ACTH is produced by lymphocytes in response to various inducers in addition to NDV (Smith et al., 1981a; Westly, Kleiss, Kelley, Wong, and Yuen, 1986). Other inducers include bacterial lipopolysaccharide (LPS) (Harbour-McMenamin, Smith, and Blalock, 1985; Smith et al., 1981a), CRF (Smith, Morrill, Meyer III, and Blalock, 1986), and other viruses such as human immunodeficiency (HIV) (Smith, Hughes, Jr., Hashemi, and Stefano, 1992;Hashemi, Hughes, and Smith, 1998), and Epstein-Barr (EBV) viruses (Oates, Allaway, Armstrong, Boyajian, Kehrl, and Prabhakar, 1988).
The first studies to determine if leukocytes produced ACTH in vivo used a surgical approach to delete the primary source of the neuroendocrine hormones. Hypophysectomized mice were injected with NDV, and at multiple time points sacrificed, to measure serum corticosterone and then the splenocytes were stained by immunofluorescence for ACTH (Smith, Meyer, and Blalock, 1982). There was a time-dependent rise in serum corticosterone that correlated with ACTH production by the splenocytes. Interestingly, pretreatment of the mice with dexamethasone prevented the splenocytes’ ACTH production and serum corticosterone elevation. Thus leukocytes produce a biologically relevant amount of ACTH and corticosteroids are negative regulators of leukocyte ACTH similar to pituitary ACTH. Using an avian model of combined hypophysectomized and bursectomized animals, Bayle et al. showed B-lymphocytes produced ACTH in vivo in response to a bacterial antigen (Bayle, Guellati, Ibos, and Roux, 1991). Leukocyte ACTH production or activity has also been proved in human clinical studies (Meyer III, Smith, Richards, Cavallo, Morrill, and Blalock, 1987;DuPont, Somers, Van Steirteghem, Warson, and Vanhaelst, 1984;Fehm, Holl, Spath-Schwalbe, Born, and Voigt, 1988).
As I mentioned above there has been controversy about neuropeptide production by leukocytes since the beginning. A few labs have not been able to detect POMC expression by leukocytes (van Woudenberg, Metzelaar, van der Kleij, de Wied, Burbach, and Wiegant, 1993), but as described above many labs have replicated this finding. The major question and controversy has primarily been over the in vivo studies and whether sufficient ACTH is produced by the leukocytes to have a systemic or endocrine-like effect. For example, one study only detected approximately a two-fold increase in corticosterone in hypophysectomized mice after NDV injection which was not statistically significant (Dunn, Powell, Moreshead, Gaskin, and Hall, 1987) compared to the approximately five-fold increase we found (Smith et al., 1982). It is possible that differences in the experimental systems or animal handling could play a role in the discrepant results. But, the leukocyte contribution of ACTH is certainly much less than the pituitary’s. At the high end we estimated, that if 100% of the leukocytes produced ACTH in vivo at the levels seen under culture conditions, the contribution would be approximately 10% of that by the pituitary. Therefore the majority of leukocyte-derived ACTH or other neuropeptide’s activity is likely to be paracrine or autocrine and endocrine effects will be small and difficult to see except under pathologic situations (DuPont et al., 1984). Examples of paracrine- or autocrine -like effects will be described later in this paper.
Also, these in vivo studies were before the field was aware of other peripheral sources of the neuropeptides and the neuroendocrine actions of cytokines which could play a role in the response. For instance, proinflammatory cytokines such as IL-1 are potent activators of the HPA axis (Berkenbosch, van Oers, del Rey, Tilders, and Besedovsky, 1987). The dogma was that IL-1 induction of pituitary ACTH release was indirect, through IL-1 induction of CRF which then acted directly on the pituitary corticotrophs. But, Payne et al. showed that the effect of the CRF was to sensitize the corticotrophs which then responded directly to the IL-1 and released ACTH (Payne, Weigent, and Blalock, 1994). Therefore, seeing endocrine-like effects of leukocyte products may be complicated by the involvement of co-factors.
3. Regulation of POMC expression in leukocytes
Regulation of POMC expression in the pituitary occurs at both the level of transcription through splicing of the mRNA and through post translational processing of the POMC precursor to bioactive peptides. A natural question was whether similar processes occurred with leukocyte POMC expression. Westly et al. (Westly et al., 1986) were the first to show transcription of the POMC gene in leukocytes. Using dot blots they showed that RNA from NDV-infected splenocytes hybridized to a recombinant plasmid probe for POMC and migrated in a Northern analysis at the same size as a positive control from AtT-20 pituitary cells. Mouse splenic macrophages were also shown to produce immunoreactive (ir)-β-END and the POMC mRNA from mouse and rat spleen tissue was of a size similar to that found in the pituitary (Lolait, Clements, Markwick, Cheng, McNally, Smith, and Funder, 1986). Oates et al. (Oates et al., 1988) showed that another virus, EBV, increased POMC transcripts, but these were several hundred bases smaller than pituitary transcripts. Subsequent studies had mixed results as to whether they found full length or truncated transcripts (Smith et al., 1990;Buzzetti, McLoughlin, Lavender, Clark, and Rees, 1989;Galin, LeBoeuf, and Blalock, 1990;Mechanick, Levin, Roberts, and Autelitano, 1992;Stephanou, Sarlis, Knight, Chowdrey, and Lightman, 1992).
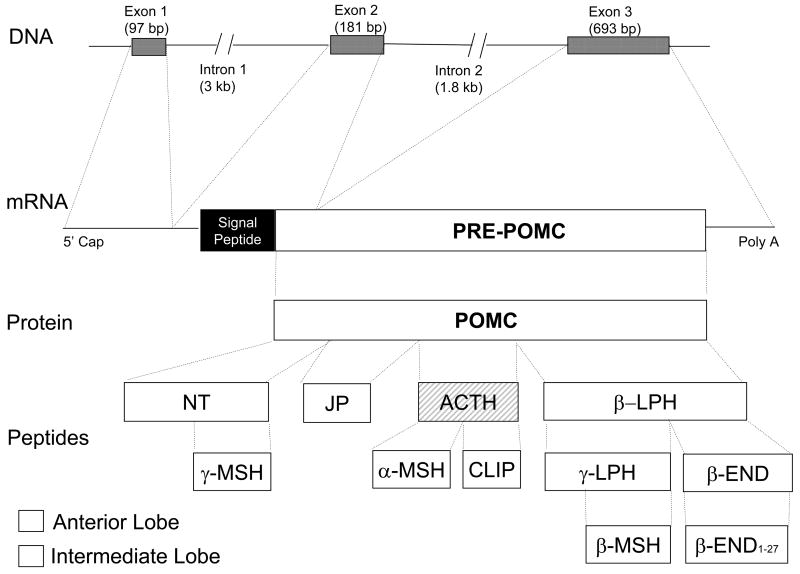
Since POMC mRNA is spliced, this raised questions about whether the signal sequence or one of the exons could be missing and what effect that would that have on expression, secretion, and function. Figure 1 shows that the POMC gene consists of three exons and two introns. Exon 1 contains non-translated sequences. Exon 2 codes for the signal peptide and the first amino acids of the N-terminal peptide. Exon 3 codes for most of the translated mRNA encoding ACTH and β-lipotropic hormone (LPH). The advent of RT-PCR and other molecular biological methodology has provided a means to answer these questions. Full length POMC mRNA is approximately 1.2 kb and various species of 0.8 and 1.5 kb have been reported in addition to the 1.2 kb species. Lyons and Blalock (Lyons and Blalock, 1997) used 5’ rapid amplification of cDNA ends (RACE) PCR methodology with primers designed to detect each exon. They sequenced the amplified products and found all 3 exons present in POMC mRNA from rat splenic mononuclear cells. They made the interesting observation that resting macrophages produced POMC, but lymphocytes needed to be stimulated. This might explain some of the discrepancies in earlier studies. Sitte et al.(Sitte, Busch, Mousa, Labuz, Rittner, Gore, Krause, Stein, and Schafer, 2007) used a semi-nested PCR methodology to analyze POMC mRNA in inflammation in vivo. They were able to confirm that all three exons were present in the POMC mRNA and further showed the signal sequence-encoding needed for regulated secretion of functionally active END. This seems to be proof that complete, functional POMC mRNA is transcribed by leukocytes. However, it is not present in all cells unless they are induced then alternatively spliced transcripts may also be produced.
Figure 1.
POMC gene structure and protein expression and processing. NT = N-terminal peptide, JP = joining peptide, LPH = lipotropic hormone
Another source of confusion regarding POMC production by leukocytes is the processing of the POMC precursor without which it has no inherent hormonal activity. POMC is processed in the anterior lobe of the pituitary gland into an N-terminal fragment, ACTH1-39 and β-LPH while the intermediate lobe produces α-melanocyte-stimulating hormone (MSH), γ-MSH, corticotropin-like intermediate lobe peptide (CLIP), γ-lipotropin (γ-LPH) and β-endorphin. The processing is highly regulated and is mediated by specific processing proteases (Loh, Parish, and Tuteja, 1985).
An interesting aspect of leukocyte POMC production is that while the same peptides are produced as in the pituitary, the patterns vary, and some are unique to leukocytes. Leukocytes stimulated with NDV or CRF produce ACTH1-39 and β-END (Smith et al., 1981a;Smith et al., 1986). Mitogen treatment increases the biosynthetic intermediate, non-glycosylated ACTH and full length POMC (Lyons et al., 1997). Lolait et al. found that splenic macrophages spontaneously produce POMC which was processed into ACTH and β-END (Lolait et al., 1986). In contrast, LPS treatment of B-lymphocytes induces a pattern unique to leukocytes. LPS stimulated leukocytes produce a truncated ACTH1-25 and endorphins of the same molecular weight of α- or β-END (Harbour-McMenamin et al., 1985; Harbour-McMenamin, 1986). As a consequence of this differential pattern of expression, leukocytes differ from any other extra-pituitary tissue which process POMC similar to that of the intermediate lobe of the pituitary.
Thus the type of POMC inducer may determine the cell type and processing pathway which ultimately leads to a certain set of peptides. Because the peptides have different activities, the effects vary with the particular pathways activated. As one might expect, cell type is also important to determine which peptides are produced. For example macrophages are able to produce α-MSH in response to tumor necrosis factor (Rajora, Ceriani, Catania, Star, Murphy, and Lipton, 1996) and also contain acetylating enzymes in addition to the proteolytic processing enzymes (Lolait et al., 1986). Mouse splenic macrophages can produce in addition to β-END1-31, lesser amounts of N-acetylated β-END1-16 (α-END),β-END1-17 (γ-END), β–END1-27, and β-END1-31 (Lolait et al., 1986). The different inducers, cell types, and processing pathways leading to a variety of peptides implies that a broad range of regulatory actions are possible from expression of the same POMC gene.
4. Leukocyte POMC inducers and producer cell types
Most, if not all leukocytes are able to synthesize POMC when properly stimulated. When 100% of the lymphocytes are infected with NDV as shown by immunofluorescence for NDV antigens, all of the cells also stained positive for ACTH and END (Smith et al., 1981a). The B-cell trophic virus EBV (Oates et al., 1988) and the T-cell trophic virus HIV (Hashemi et al., 1998) both induced POMC expression, further supporting evidence that most lymphocytes can produce ACTH.
Mitogens are another way to differentially activate specific populations of lymphocytes and will induce production of POMC products. Bacterial endotoxin (LPS) is a B-lymphocyte mitogen that induces high levels of ACTH and END (Smith et al., 1990;Harbour-McMenamin et al., 1985). Clarke et al. stimulated lymphocytes with the T-lymphocyte mitogen conconavalin A (ConA) for high levels of ACTH expression (Clarke, Gebhardt, and Blalock, 1993). Interestingly, Staphylococcus enterotoxin A (SEA) another T-lymphocyte mitogen and a super antigen, induces TSH production and not POMC (Smith, Phan, Kruger, Coppenhaver, and Blalock, 1983). Lyons and Blalock (Lyons and Blalock, 1995) made a thorough study of ACTH expression in rat leukocyte subpopulations. Using ConA or LPS as inducers, they found that B-, T-helper (CD4+), and cytotoxic T cells (CTL, CD8+) could all express ACTH but at different intensities. Peritoneal macrophages were positive without stimulation and this could be increased to 85% of the cells with activation. Therefore, dependent upon the stimulus, all lymphocytes and macrophages or a subpopulation of cells, will express POMC.
One of the more interesting inducers of leukocyte POMC is CRF. To determine if leukocyte POMC production could be induced as it is in anterior pituitary cells, human peripheral blood mononuclear cells (PBMC) were treated with CRF and/or arginine vasopressin (AVP) (Smith et al., 1986). Immunofluorescence staining of the stimulated lymphocytes showed both ACTH and END immunoreactivity. CRF and AVP both individually induced POMC expression and in combination the effects were additive. Examining β-END induction by CRF and AVP, revealed that B-cells in a human PBMC culture were producing the β-END (Kavelaars, Ballieux, and Heijnen, 1989). Furthermore, the antibody to IL-1 blocked the induction suggesting it was an indirect mediator of the induction. ACTH production could be induced with exogenous IL-1 added to the PBMC cultures (Reder, 1992). Later Kavelaars et al. expanded this study to show that glucocorticoids inhibit CRF induction of IL-1 and subsequent β-END production (Kavelaars, Ballieux, and Heijnen, 1990b). Subcutaneous administration of CRF in rats induced IL-1 and then β-END secretion as well (Kavelaars, Berkenbosch, Croiset, and Ballieux, 1990). Kravchenco et al. in a study of CRF production by lymphocytes found that LPS and ConA treated human lymphocytes secreted ACTH and treatment with antiserum to CRF blocked ACTH production (Kravchenco and Furalev, 1994).
The intracellular signaling pathways by which CRF and AVP induce leukocyte POMC production were examined. Activation of protein kinase-C (PKC) or PKA pathways in T-cells or non-T-cells induces β-END secretion (Kavelaars, Ballieux, and Heijnen, 1991). Rapid induction appeared to be mediated via the PKC pathway while after 18h both kinases were involved. As expected, PKC activation was involved with ACTH secretion as well (Reder, 1992). CRF also activates the NF-κB signaling pathway and this may be responsible for induction of IL-1 (Smith, Hogan, and Hughes, Jr., 2001;Zhao and Karalis, 2002b;Smith, Dalmeida, and Hughes, 2004;Smith, Gregg, Hashemi, Schott, and Hughes, 2006). This subject will be discussed in the next section.
5. CRF production in the immune system
Ironically, CRF not only induces leukocytes to produce POMC, but is also produced by leukocytes. The accepted role of CRF is its release from the hypothalamus during stress and in circadian regulation for activation of the hypothalamic-pituitary-adrenal (HPA) axis but CRF is also associated with major depression and other behavioral disorders [for review see (Davila et al., 1987;Dautzenberg and Hauger, 2002)]. As mentioned previously modulation of immune responses can now be added to the list of CRF’s physiological roles. Some earlier reports found CRF immunoreactivity in spleen and thymus as well as other peripheral tissues, but whether the leukocytes were the source of the CRF was not determined. Stephanou et al. (Stephanou, Jessop, Knight, and Lightman, 1990) were the first to report that T and B lymphocytes plus neutrophils synthesized CRF mRNA. This was shown by using in situ hybridization and radioimmunoassay (RIA) of immunoreactive protein. Subsequently the CRF mRNA expression by leukocytes was confirmed using RT-PCR in lymphocytes and adherent cells from rat thymus and spleen (Aird, Clevenger, Prystowsky, and Redei, 1993).
T-lymphocytes were shown to contain CRF mRNA and their expression could be up regulated with phytohemagglutinin (PHA) and tumor promoting agent (TPA) (Ekman, Servenius, Castro, Lowry, Cederlund, Bergman, and Sjogren, 1993). Interestingly, stressors such as hypothermia, hyperosmolarity, and hypoxia could induce human lymphocytes and mouse splenocytes to secrete CRF (Kravchenco et al., 1994). LPS and ConA also stimulated CRF secretion by B and T-lymphocytes, respectively. In addition nordihydroguaiaretic acid, a lipoxygenase pathway inhibitor, was shown to be a potent inducer of splenic and thymic adherent cells in culture but IL-1 had no effect in this study (Aird et al., 1993). Hydrocortisone inhibits B- and T-lymphocyte CRF production (Kravchenco et al., 1994). Therefore, like POMC production by leukocytes, CRF is also under positive and negative regulation. Further, and also similar to POMC, different cell subpopulations produce CRF depending upon the inducer and this could be a factor in leukocyte CRF’s role in the immune system.
In contrast to what is known about POMC transcription, post translational cleavage, and the resulting products, little about CRF is known in this regard. CRF protein is secreted, because it has been isolated from thymus and spleen tissues as well as supernatant fluid from leukocyte cell cultures (Aird et al., 1993; Kravchenco et al., 1994). Most reports that have sized the leukocyte CRF show it to migrate at the same position or size as full length, active CRF1-41 (Redei, 1992;Ekman et al., 1993;Kravchenco et al., 1994). However, Stephanou et al. found the immunoreactive CRF to be different than CRF1-41 (Stephanou et al., 1990). The immunoreactive CRF was found to elute earlier on reverse phase HPLC, differ from the standard curve by RIA, and to be more labile when compared with bona fide CRF. While a thorough analysis of the mRNA transcripts has not been done, Aird et al. used a set of primers that included some of both exons of the gene and this suggested that the mRNA was expressed like the prototype in the hypothalamus (Aird et al., 1993).
In vivo, CRF was found synthesized outside of the hypothalamus in a number of tissues, especially by cells of the immune system (Jessop, Harbuz, Snelson, Dayan, and Lightman, 1997;Muglia, Jenkins, Gilbert, Copeland, and Majzoub, 1994;Aird et al., 1993;Schafer, Mousa, Zhang, Carter, and Stein, 1996;Audhya, Zwickler, Hutchinson, Brown, and Hollander, 1988;Stephanou et al., 1990;Bellinger, Felten, Lorton, and Brouxhon, 2001). Normal regulatory factors that are present during an immune response will induce leukocyte production of CRF. Bellinger et al. (Bellinger et al., 2001) found that i.p. injection of IL-2 stimulated the synthesis of CRF in nerves that innervate the F344 rat spleen and promoted the appearance of CRF-positive leukocytes in draining mesenteric lymph nodes. This is important because circulating levels of CRF are normally very low. These studies show that both CRF and receptor-bearing lymphocytes can be present in the same tissues to provide the proximity and necessary concentration for immune regulation. As an aside, lymphocytes and a T-lymphoma cell line (Jurkat) were shown to produce urocortin (UCN), a CRF-related peptide, (Bamberger, Wald, Bamberger, Ergun, Beil, and Schulte, 1998) which combined with the presence of CRF-R2 receptors in the periphery, including thymus and spleen, suggest that UCN may also play a role in immune system responses (Baigent and Lowry, 2000;Baigent, 2001).
6. ACTH and END effects on immune functions
POMC as a protein precursor is not known to modulate immune function directly. However ACTH and END, the peptides that result from post translational processing, have many activities in the immune system. To understand the immunomodulatory activity of ACTH it is necessary to consider not only direct effects on leukocytes, but also potential indirect actions. There are at least two ways ACTH acts indirectly. One is through induction of corticosteroids (Munck, Guyre, and Holbrook, 1984), and the other is through secondary transformation into MSH (Smith et al., 1992). Corticosteroid anti-inflammatory and immunosuppressive properties are well known and don’t need to be reviewed here. MSH is also a potent anti-inflammatory peptide (Lipton, ZHAO, Ichiyama, Barsh, and Catania, 1999). Although it will not be discussed further, MSH should be considered when ACTH activity requires more than a few hours of treatment to induce an effect. In that time ACTH may undergo proteolytic processing to MSH prior to the measured effect (Smith et al., 1992).
The first immunological effect directly attributable to ACTH was the inhibition of antibody production (Johnson, Smith, Torres, and Blalock, 1982). ACTH inhibited an in vitro antibody response against a T-lymphocyte dependent and a T-independent antigen. Interestingly, when two other labs examined different antigens, they found inhibition at high doses and enhancement at very low concentrations between 10−13 and 10−17M (Munn and Lum, 1989;Bost, Clarke, Xu, Kiyono, McGhee, and Pascual, 1990). The effects of ACTH on B-cells suggest possible mechanisms for these seemingly conflicting results. Alvarez-Mon et al. (Alvarez-Mon, Kehrl, and Fauci, 1985) found that ACTH enhances B-cell growth and differentiation when B-cell growth factor or IL-2 were present. Brooks (Brooks, 1990) demonstrated that ACTH functions as a late-acting B-cell growth factor and synergizes with IL-5, a B-cell growth factor. ACTH was reported to inhibit T-lymphocyte proliferation in response to a mitogen (Heijnen, Zijlstra, Kavelaars, Croiset, and Ballieux, 1987). Therefore depending upon the kinetics of the response and involvement of T-cells, ACTH treatment could have different effects on antibody production.
Because cytokines play a major role in regulating immune responses such as antibody production, it is of interest that the production of several cytokines is altered by ACTH. IFN-γ production by T-lymphocytes is inhibited (Johnson, Torres, Smith, Dion, and Blalock, 1984) and so is IFN-γ activation of tumoricidal activity of macrophages (Koff and Dunegan, 1985). In contrast ACTH induces tumor necrosis factor-α (TNF-α) production by adherent macrophages (Hughes and Smith, 1989).
END peptides also have many effects on in vitro and in vivo immune responses (Smith, 2003). There are conflicting results concerning what responses are affected and in which direction. This is due in large part to the multiple endogenous and exogenous opioids that act with varying efficacy through multiple types of opiate receptors. Therefore any response is dependent upon the receptor subtype expressed on the target cell and the particular opioid ligand. Through the μ opiate receptor (MOR), α-, β-, and γ-END inhibit the in vitro antibody response to sheep red blood cells (Johnson et al., 1982). Typically β-END is the proteolytic product reported in leukocytes (Kavelaars et al., 1989;Lolait et al., 1986). β-END has been found to enhance natural cytotoxicity of lymphocytes and macrophages towards tumor cells (Mathews, Froelich, Sibbitt, Jr., and Bankhurst, 1983) and natural killer (NK) cells (Kay, Allen, and Morley, 1984). Another reason for the different responses seen with the endogenous opioids may be β-END’s affect on cAMP. β-END is a true modulator, elevating levels in cells with a low baseline and decreasing cAMP levels in cells with high levels of cAMP (Kavelaars, Ballieux, and Heijnen, 1990a). Since β-END binds with highest affinity to the MOR like morphine, the many studies of this drug of abuse and immune function are pertinent and suggest numerous immune functions could be affected by β-END (Carr, Rogers, and Weber, 1996).
7. CRF effects on immune functions
Our study was the first to show that CRF could directly affect lymphoid cells after finding that CRF induced lymphocytes to produce ACTH and END (Smith et al., 1986). Since then others have joined us in showing additional effects of CRF on more traditional functions of the immune system [see for review (Karalis, Muglia, Bae, Hilderbrand, and Majzoub, 1997;Jessop, Harbuz, and Lightman, 2001;Smith, 2002)]. Typically, studies have looked at CRF immune effects in two general ways. First systemically, where CRF was injected and subsequent immune responses such as natural killer (NK) cell activity were measured (Irwin, Vale, and Britton, 1987;Irwin, Hauger, Brown, and Britton, 1988;Irwin, Hauger, and Brown, 1992). The modulation was usually attributed to CRF activation of the HPA axis and the suppressed immune function due to corticosteroid production. The other approach was generally in vitro where CRF was applied directly to the leukocytes, isolated from other neuroendocrine influence.
CRF receptors were identified in lymphoid tissues and on dissociated leukocytes by several laboratories. Radio-labeled CRF binds to macrophage-rich areas of the spleen (Webster, Tracey, Jutila, Wolfe, Jr., and De Souza, 1990;Webster and De Souza, 1988) and isolated PBMC (Singh and Fudenberg, 1988). There are multiple species of CRF receptors (CRF-R) but CRF-R1 and CRF-R2, the major two, are both found on leukocytes (Smith et al., 2006). Monoclonal antibody staining methods and RT-PCR were utilized to show that the CRF-R1 is expressed in spleen, particularly by activated neutrophils (Radulovic, Dautzenberg, Sydow, Radulovic, and Spiess, 1999). Only a subset of non-stimulated lymphocytes express CRF receptors, but the percentage of immunoreactive cells can be increased with mitogen treatment (Smith et al., 2006). There is also preliminary evidence for CRF-R2 expression in both the spleen and thymus (Baigent, 2001).
Depending upon the immune measure, CRF was reported to inhibit some responses yet enhance others. The dichotomy may be due in part to the two different approaches, where other mediators such as corticosteroids are likely to be involved. In vitro, CRF will enhance B and T cell mitogenesis, IL-1, IL-2, and IL-6 induction, plus NK activity (Kavelaars et al., 1989;McGillis, Park, Rubin-Fletter, Turck, Dallman, and Payan, 1989;Singh, 1989;Singh and Leu, 1990;Leu and Singh, 1991;Leu and Singh, 1992). CRF inhibits phagocyte activation, total antibody production, and NK activity (Smith, Hughes, Cadet, and Stefano, 1992;Pawlikowski, Zelazowski, Dohler, and Stepien, 1988;Leu and Singh, 1993). Monocyte/macrophage activity is affected through both of the major CRF-R. CRF enhances monocyte adhesion and NO release through CRF-R1 and R2 (Wilbert-Lampen, Straube, Trapp, Deutschmann, Plasse, and Steinbeck, 2006), while UCNs will induce macrophage apoptosis and the expression of toll like receptor 4 (TLR4) through CRF-R2 (Tsatsanis, Androulidaki, Dermitzaki, Charalampopoulos, Spiess, Gravanis, and Margioris, 2005;Tsatsanis, Androulidaki, Alissafi, Charalampopoulos, Dermitzaki, Roger, Gravanis, and Margioris, 2006).
In contrast, in vivo CRF inhibits NK cell activity and antibody production (Irwin et al., 1987;Irwin et al., 1988). Otherwise, in vivo CRF is generally reported to be pro-inflammatory (Jessop et al., 1997;Schafer, Carter, and Stein, 1994). CRF deficient (CRF−/−) mice have been generated by a targeted mutation approach and have provided some insight into CRF effects on immune functions (Muglia, Jacobson, Dikkes, and Majzoub, 1995;Muglia, Jacobson, Weninger, Karalis, Jeong, and Majzoub, 2001). In CRF-deficient mice there is a near-normal glucocorticoid response to inflammation and systemic immune challenge (Muglia, Bethin, Jacobson, Vogt, and Majzoub, 2000). A carrageenin-induced inflammatory response in normal and adrenalectomized CRF−/− mice showed corticosterone levels in CRF−/− are 60% lower than in CRF+/+ mice (Karalis et al., 1997). But the corticosterone levels were 10-fold above their own baseline of non-inflamed CRF−/− mice, suggesting there was stimulation of the HPA axis in the absence of CRF. In the adrenalectomized CRF−/− mice implanted with a corticosterone pellet to produce glucocorticoid levels similar to CRF+/+ mice, the inflammatory response was much lower. This suggests that when glucocorticoid levels are similar, CRF is required for the induction of the inflammatory response (Karalis et al., 1997). A later study found that both CRF and epinephrine enhance the cellular infiltration in the carrageenin inflammation model (Karalis, Kontopoulos, Muglia, and Majzoub, 1999). This occurs in a mutually exclusive manner. Removing one or the other factor only partially reduces the effect and removal of both is necessary to reach control levels. The effect on cellularity may be maximal from either CRF or epinephrine and excess of either substance cannot elevate it further. In the CRF−/− mice, part of the enhanced inflammation was due to decreased glucocorticoids.
Clearly CRF has several effects on immune system function. However, the effects can be inhibitory or enhancing and the mechanism(s) for this dichotomy are unknown. The presence of multiple CRF-Rs and/or different cell types could explain some of these discrepancies. The assumption was made that the inhibitory activities, at least in vivo, are through glucocorticoid production and that the direct CRF effects are proinflammatory. CRF-Rs can also activate multiple intracellular signaling pathways. CRF elevates cAMP and PKA in lymphocytes like it does in pituitary corticotrophs (Dave, Eiden, and Eskay, 1985;Smith and Johnson, 1989;Singh and Leu, 1993). CRF also enhances the NF-κB intracellular signaling pathway in leukocytes (Smith et al., 2001;Zhao et al., 2002b;Smith et al., 2004;Smith et al., 2006). This second intracellular signaling pathway is one that would tend to enhance immune functions, while the G-protein linked elevation of cAMP is generally inhibitory. NF-κB’s immune activating activities are extensive and if induced by CRF would likely be important (Baeuerle and Henkel, 1994). Therefore the enhancing and inhibitory effects of CRF could also occur simultaneously at the level of the target cell. Our study was the first to report CRF induction of NF-κB in lymphocytes using gel shift and later gene reporter assays (Smith et al., 2001;Smith et al., 2004;Smith et al., 2006). Recent studies have shown that CRF also enhances NF-κB activity in thymocytes (Zhao and Karalis, 2002a). Zhao and Karalis found that CRF decreased IκBα an inhibitor of NF-κB, but dexamethasone, which also inhibits IκBα (Auphan, DiDonato, Rosette, Helmberg, and Karin, 1995), had no effect on the CRF enhancement. Interestingly, ACTH inhibits NF-κB by elevating IκBα (Smith et al., 2001;Smith et al., 2004). This effect could potentially serve as a negative regulator to counter excessive or prolonged CRF enhancement of NF-κB. CRF will still enhance T-cell mitogenesis in NF-κB deficient cells which is further evidence that CRF operates through multiple pathways (Smith et al., 2006). Thus there are multiple pathways to mediate CRF’s many effects on immune functions.
7. Leukocyte interaction utilizing hypothalamic-pituitary neuropeptides
Since CRF induces POMC in the HPA axis, it is natural to wonder if there is an HPA-like relationship operating in the periphery through leukocyte CRF and POMC. Recent studies suggest that there is indeed a localized regulatory circuit that resembles the hypothalamus-pituitary axis. In particular, this regulatory interaction can be demonstrated in vivo in association with inflammation (Schafer et al., 1994;Schafer et al., 1996;Jessop, Lightman, and Chowdrey, 1994;Jessop, Renshaw, Lightman, and Harbuz, 1995;Webster, Elenkov, and Chrousos, 1997;Schafer, Mousa, and Stein, 1997).
In these studies, inflammation was induced in rats by injecting them with Freund's adjuvant, which results in unilateral hindpaw inflammation. Stein et al. showed opioids from immune cells bound receptors on sensory nerves, inhibiting inflammatory pain (Stein, Gramsch, Hassan, Przewlocki, Parsons, Peter, and Herz, 1990;Stein, Gramsch, and Herz, 1990). This group has thoroughly characterized the production and action. β-END is detected in the synovial fluid and leukocytes of inflamed joints (Stein et al., 1990;Stein, Hassan, Lehrberger, Giefing, and Yassouridis, 1993). Synthesis of β-END and met-ENK by B and T lymphocytes was demonstrated at the protein level and by expression of POMC and proenkephalin mRNA (Przewlocki, Hassan, Lason, Epplen, Herz, and Stein, 1992). Total body irradiation or immunosuppression reduced the antinociceptive effect in the inflamed paw and demonstrated that the opioids were synthesized de novo.
Many of the factors found to induce opioid production by leukocytes in vitro are present in inflamed tissues, particularly cytokines. CRF with or without co-production of IL-1 is also produced at the site and both factors appear to be opioid inducers (Schafer et al., 1994;Mousa, Schafer, Mitchell, Hassan, and Stein, 1996). Immunohistochemical methods show lymphoid cells and peripheral fibers are producing β-END, met-ENK, dynorphin, and other factors (Schafer et al., 1994;Hassan, Pzewlocki, Herz, and Stein, 1992). Migration of the opioid-containing leukocytes to the sites of inflammation seems to be mediated in part through co-expression with adhesion molecules (Mousa, Machelska, Schafer, and Stein, 2000). Furthermore, there are intriguing data to suggest that other neurally derived factors may down regulate the opioid response (Schafer, Zhou, and Stein, 1998). In a related animal model, adjuvant-induced arthritis (AA), spleen POMC mRNA and IL-1β are increased (Stephanou et al., 1992). Characterization of this model showed elevated ACTH, β-END, and CRF levels in the spleen and thymus of AA animals (Jessop et al., 1994;Jessop et al., 1995). These neuropeptides seemed to be under negative control by glucocorticoids as found with NDV as an inducer (Smith et al., 1982). In other experiments antisense oligonucleotides, which block CRF expression also blocked the splenocyte proliferation (Jessop et al., 1997).
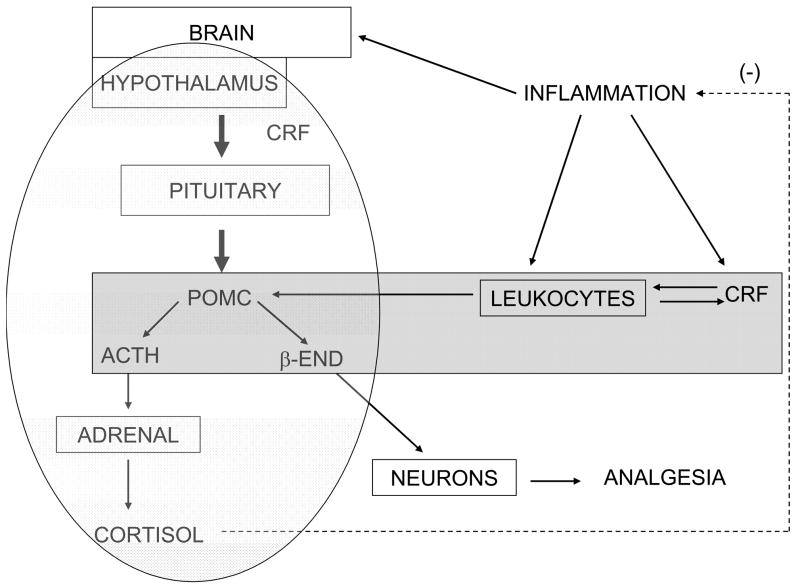
Measures of nociception have shown that the opioids reduce the pain associated with the inflammation (Rittner, Brack, Machelska, Mousa, Bauer, Schafer, and Stein, 2001). If opiate receptor antagonists such as naloxone are injected at the inflammatory site there is an increase in the pain level. Therefore, the CRF that is induced in leukocytes during inflammation induces β-END among other endogenous opioids as CRF does in the pituitary (figures 2 and 3). Furthermore this CRF and POMC induction also appears to be an inherent means for leukocytes to control the pain associated with inflammation. Further study could show whether the leukocyte-derived ACTH in inflamed tissue contributed to increased corticosteroid release from the adrenal to limit the inflammatory response.
Figure 2.
Diagram of interactions involving the HPA axis (light shading) and putative leukocyte hypothalamus-pituitary-like axis (darker shading) during inflammation. Solid arrows indicate induction and the dashed arrow denotes cortisol’s inhibition of inflammation. In this depiction a possible sequence of events is as follows. During an inflammatory response IL-1 and other factors are released at the site and attract more leukocytes, particularly neutrophils and macrophages to the site. CRF is produced by these cells and more would then be induced by the IL-1. The CRF would induce more IL-1 and POMC expression resulting in ACTH and β-END. The CRF would enhance NF-κB activity, proinflammatory cytokine production, and inflammation. The local ACTH would down regulate the NF-κB starting to check the inflammation. The β-END would induce analgesia in the local neurons. The proinflammatory cytokines activate the HPA axis resulting in corticosteroid release that would be the major factor to hold the inflammation in check. Possibly the leukocyte ACTH could contribute to stimulating the adrenal gland corticosteroid release.
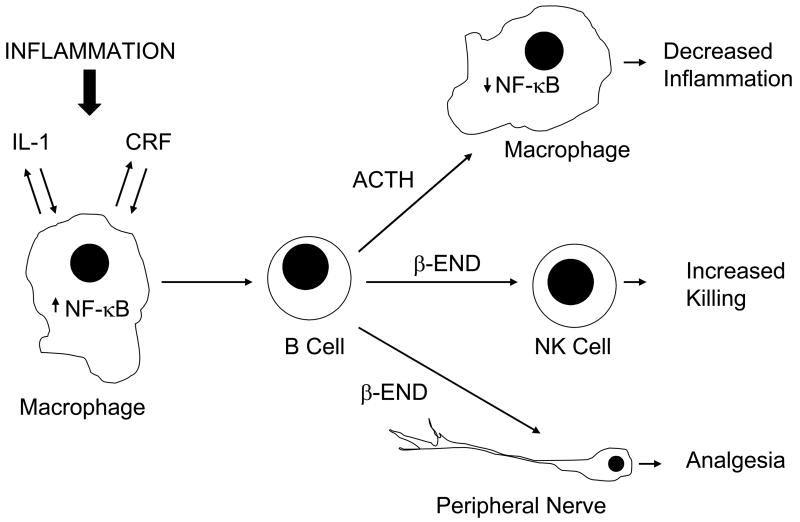
Figure 3.
A cellular level diagram of how CRF, ACTH, and β-END may interact in paracrine and autocrine manners during inflammation. The figure is conceived from one by Weigent and Blalock (Weigent and Blalock, 1997) and modified to incorporate the concepts concerning the role these neuropeptides may play in inflammation (Schafer et al., 1996;Karalis et al., 1991;Jessop et al., 2001).
8. Conclusions and future directions
Clearly, the evidence shows that leukocytes do synthesize neuropeptides. Furthermore they can be processed and secreted in biologically relevant amounts similar to their neuroendocrine prototypes. The in vivo studies of inflammation in particular show an important regulatory role for leukocyte neuropeptides. With the weight of evidence and potential clinical implications, leukocyte-derived neuropeptides can no longer be ignored.
Some of the difficulty in showing the biological relevance of leukocyte neuropeptides in vivo is because the studies are done in the presence of the endogenous neuropeptides. These are generally present in relatively high basal concentrations and secreted even more during the stress of handling. Ideally an animal model is needed in which the neuroendocrine contribution of the neuropeptide is absent, to show only what the leukocytes contribute. Kley Hughes and I have developed such a model for IL-10 to investigate the converse, the role IL-10 from the HPA axis plays in the absence of immune system-derived IL-10 (Koldzic-Zivanovic, Tu, Juelich, Rady, Tyring, Hudnall, Smith, and Hughes, 2006). In this model we create chimeric mice that are genetically deficient in IL-10 (IL-10−/−) except for the immune system which has been reconstituted by a bone marrow transplant with cells from IL-10+/+ congenic mice. Unfortunately, for these studies it was only possible to reconstitute the immune system rather than neuroendocrine tissues. Therefore the role of neuroendocrine IL-10 in this model is the inverse of what one would choose to measure. Those functions which disappear in the IL-10−/− compared to the IL-10+/+ reconstituted mice would be due to neuroendocrine-derived IL-10.
A related approach to study the role of leukocyte CRF was devised. In this model a CRF−/− mouse is irradiated to eliminate its endogenous immune system and the animal is reconstituted with bone marrow from a CRF+/+ congenic mouse. Thus leukocytes (CRF+/+) would be the sole source of CRF in an otherwise deficient mouse. Experiments to measure only the leukocyte’s CRF effect on inflammation could be done using the inflammation models of Karalis et al. (Karalis, Sano, Redwine, Listwak, Wilder, and Chrousos, 1991) or Jessop et al. (Jessop et al., 1997). It is our hope that by these means we will finally determine the role of at least one leukocyte-derived neuropeptide.
Acknowledgments
I would like to thank my many colleagues I have worked with and friends made throughout the last 28 years, who helped make my research possible and enjoyable. In particular, I want to acknowledge Drs. J. Edwin Blalock and Thomas K. Hughes with whom I started in PNI research and who continue to provide wonderful advice and friendship. I also want to acknowledge the instrumental contributions of Dr. Jeannine Majde-Cottrell and the Office of Naval Research, the National Institutes of Health, and the John Sealy Memorial Endowment Fund at UTMB that supported my research. Finally, I wish to thank Layne Dearman for her excellent editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aird F, Clevenger CV, Prystowsky MB, Redei E. Corticotropin-releasing factor mRNA in rat thymus and spleen. Proc Natl Acad Sci U S A. 1993;90:7104–7108. doi: 10.1073/pnas.90.15.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Mon M, Kehrl JH, Fauci AS. A potential role for adrenocorticotropin in regulating human B lymphocyte functions. J Immunol. 1985;135:3823–3826. [PubMed] [Google Scholar]
- Audhya T, Zwickler D, Hutchinson B, Brown C, Hollander CS. Stress-induced changes in corticotropin-releasing factor (CRF) in immune tissues and hypothalamus: studies toward defining a role for CRF in neuroimmunomodulation. Transactions of the Association of American Physicians. 1988;101:62–69. [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of its synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–820. doi: 10.1016/s0196-9781(01)00395-3. [DOI] [PubMed] [Google Scholar]
- Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Wald M, Bamberger AM, Ergun S, Beil FU, Schulte HM. Human Lymphocytes Produce Urocortin, But Not Corticotropin-Releasing Hormone. J Clin Endocrinol Metab. 1998;83:708–711. doi: 10.1210/jcem.83.2.4693. [DOI] [PubMed] [Google Scholar]
- Bayle JD, Guellati M, Ibos F, Roux J. Brucella abortus antigen stimulates the pituitary-adrenal axis through the extrapituitary B-lymphoid system. Progress in Neuroendocrinimmunology. 1991;4:99–105. [Google Scholar]
- Bellinger D, Felten DL, Lorton D, Brouxhon SM. Effects of interleukin-2 on the expression of corticotropin-releasing hormone in nerves and lymphoid cells in secondary lymphoid organs from the Fischer 344 rat. J Neuroimmunol. 2001;119:37–50. doi: 10.1016/s0165-5728(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders FX, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Blalock JE, Smith EM. Human leukocyte interferon: Structural and biological relatedness to adrenocorticotropic hormone and endorphins. Proc Natl Acad Sci U S A. 1980;77:5972–5974. doi: 10.1073/pnas.77.10.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock JE, Stanton JD. Common pathways of interferon and hormonal action. Nature. 1980;283:406–408. doi: 10.1038/283406a0. [DOI] [PubMed] [Google Scholar]
- Blalock JE, Whitaker JN, Benveniste EN, Bost KL. Use of peptides encoded by complementary RNA for generating anti-idiotypic antibodies of predefined specificity. Methods in Enzymology. 1989;178:63–74. doi: 10.1016/0076-6879(89)78006-x. [DOI] [PubMed] [Google Scholar]
- Bost KL, Clarke BL, Xu JC, Kiyono H, McGhee JR, Pascual D. Modulation of IgM secretion and H chain mRNA expression in CH12.LX.C4.5F5 B cells by adrenocorticotropic hormone. J Immunol. 1990;145:4326–4331. [PubMed] [Google Scholar]
- Brooks KS. Adrenocorticotropin (ACTH) functions as a late-acting B cell growth factor and synergizes with interleukin 5. Journal of Molecular and Cellular Immunology. 1990;4:327–337. [PubMed] [Google Scholar]
- Buzzetti R, McLoughlin L, Lavender PM, Clark AJ, Rees LH. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignancies. J Clin Invest. 1989;83:733–737. doi: 10.1172/JCI113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Rogers TJ, Weber RJ. The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med. 1996;213:248–257. doi: 10.3181/00379727-213-44056. [DOI] [PubMed] [Google Scholar]
- Clarke BL, Gebhardt BM, Blalock JE. Mitogen-stimulated lymphocytes release biologically active corticotropin. Endocrinology. 1993;132:983–988. doi: 10.1210/endo.132.3.8382604. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger R. The CRF peptide family and their receptors: Yet more partners discovered. Trends in Pharmacological Sciences. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dave J, Eiden L, Eskay R. Corticotropin-releasing factor binding to peripheral tissue and activation of the adenylate cyclase-adenosine 3′,5′-monophosphate system. Endocrinology. 1985;116:2152–2159. doi: 10.1210/endo-116-6-2152. [DOI] [PubMed] [Google Scholar]
- Davila DR, Brief S, Simon J, Hammer RE, Brinster RL, Kelley KW. Role of growth hormone in regulating T-dependent immune events in aged, nude, and transgenic rodents. J Neurosci Res. 1987;18:108–116. doi: 10.1002/jnr.490180118. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Powell ML, Moreshead WV, Gaskin JM, Hall NR. Effects of Newcastle disease virus administration to mice on the metabolism of cerebral biogenic amines, plasma corticosterone, and lymphocyte proliferation. Brain, Behavior, and Immunity. 1987;1:216–230. doi: 10.1016/0889-1591(87)90024-9. [DOI] [PubMed] [Google Scholar]
- DuPont AG, Somers G, Van Steirteghem AC, Warson F, Vanhaelst L. Ectopic adrenocorticotropin production: Disappearance after removal of inflammatory tissue. J Clin Endocrinol Metab. 1984;58:654–658. doi: 10.1210/jcem-58-4-654. [DOI] [PubMed] [Google Scholar]
- Ekman R, Servenius B, Castro MG, Lowry PJ, Cederlund AS, Bergman O, Sjogren HO. Biosynthesis of corticotropin-releasing hormone in human T-lymphocytes. J Neuroimmunol. 1993;44:7–13. doi: 10.1016/0165-5728(93)90262-w. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Holl R, Spath-Schwalbe E, Born J, Voigt KH. Ability of corticotropin releasing hormone to stimulate cortisol secretion independent from pituitary adrenocorticotropin. Life Sci. 1988;42:679–686. doi: 10.1016/0024-3205(88)90459-6. [DOI] [PubMed] [Google Scholar]
- Galin FS, LeBoeuf RD, Blalock JE. A lymphocyte mRNA encodes the adrenocorticotropin/β-lipotropin region of the pro-opiomelanocortin gene. Progress in Neuroendocrinimmunology. 1990;3:205–208. [Google Scholar]
- Harbour-McMenamin D. B lymphocyte enriched populations produce immunoreactive endorphin that binds to delta opiate receptors and may play a role in endotoxic shock. ICSU Short Report 4. 1986:378–379. Ref Type: Journal (Full) [Google Scholar]
- Harbour-McMenamin D, Smith EM, Blalock JE. Bacterial lipopolysaccharide induction of leukocyte-derived corticotropin and endorphins. Infect Immun. 1985;48:813–817. doi: 10.1128/iai.48.3.813-817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi FB, Hughes TK, Smith EM. Human immunodeficiency virus induction of corticotropin in lymphoid cells. J Clin Endocrinol Metab. 1998;83:4373–4381. doi: 10.1210/jcem.83.12.5323. [DOI] [PubMed] [Google Scholar]
- Hassan AH, Pzewlocki R, Herz A, Stein C. Dynorphin, a preferential ligand for kappa-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neurosci Lett. 1992;140:85–88. doi: 10.1016/0304-3940(92)90688-4. [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Zijlstra J, Kavelaars A, Croiset G, Ballieux RE. Modulation of the immune response by POMC-derived peptides. I. Influence on proliferation of human lymphocytes. Brain Behav Immun. 1987;1:284–291. doi: 10.1016/0889-1591(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Hughes TK, Chin R. Interactions of neuropeptides and lymphokines. In: Scharrer B, Smith EM, Stefano GB, editors. Neuropeptides and Immunoregulation. Springer-Verlag; Berlin: 1994. pp. 100–115. [Google Scholar]
- Hughes TK, Smith EM. Corticotropin (ACTH) induction of tumor necrosis factor alpha by monocytes. J Biol Regul Homeost Agents. 1989;3:163–166. [PubMed] [Google Scholar]
- Irwin M, Hauger R, Brown M. Central corticotropin-releasing hormone activates the sympathetic nervous system and reduces immune function: increased responsivity of the aged rat. Endocrinology. 1992;131:1047–1053. doi: 10.1210/endo.131.3.1505449. [DOI] [PubMed] [Google Scholar]
- Irwin M, Hauger R, Brown M, Britton K. CRF activates autonomic nervous system and reduces natural killer cytotoxicity. American Journal of Physiology -Regulatory Integrative & Comparative Physiology. 1988:R744–R747. doi: 10.1152/ajpregu.1988.255.5.R744. [DOI] [PubMed] [Google Scholar]
- Irwin M, Vale W, Britton K. Central corticotrtopin-releasing factor suppresses natural killer cytotoxicity. Brain Behav Immun. 1987;1:81–87. doi: 10.1016/0889-1591(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Harbuz MS, Snelson CL, Dayan CM, Lightman SL. An antisense oligodeoxynucleotide complementary to corticotropin- releasing hormone mRNA inhibits rat splenocyte proliferation in vitro. J Neuroimmunol. 1997;75:135–140. doi: 10.1016/s0165-5728(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Lightman SL, Chowdrey HS. Effects of a chronic inflammatory stress on levels of pro-opiomelanocortin-derived peptides in the rat spleen and thymus. J Neuroimmunol. 1994;49:197–203. doi: 10.1016/0165-5728(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Renshaw D, Lightman SL, Harbuz MS. Changes in ACTH and beta-endorphin immunoreactivity in immune tissues during a chronic inflammatory stress are not correlated with changes in corticotropin-releasing hormone and arginine vasopressin. J Neuroimmunol. 1995;60:29–35. doi: 10.1016/0165-5728(95)00049-8. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Harbuz MS, Lightman SL. CRH in chronic inflammatory stress. Peptides. 2001;22:803–807. doi: 10.1016/s0196-9781(01)00394-1. [DOI] [PubMed] [Google Scholar]
- Johnson HM, Smith EM, Torres BA, Blalock JE. Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci U S A. 1982;79:4171–4174. doi: 10.1073/pnas.79.13.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM, Torres BA, Smith EM, Dion LD, Blalock JE. Regulation of lymphokine (gamma-interferon) production by corticotropin. J Immunol. 1984;132:246–250. [PubMed] [Google Scholar]
- Karalis KP, Kontopoulos E, Muglia LJ, Majzoub JA. Corticotropin-releasing hormone deficiency unmasks the proinflammatory effect of epinephrine. Proc Natl Acad Sci U S A. 1999;96:7093–7097. doi: 10.1073/pnas.96.12.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis KP, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- Karalis KP, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Ballieux RE, Heijnen CJ. Differential effects of beta-endorphin on cAMP levels in human peripheral blood mononuclear cells. Brain Behav Immun. 1990a;4:171–179. doi: 10.1016/0889-1591(90)90020-q. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Ballieux RE, Heijnen CJ. Two different signalling pathways for the induction of immunoreactive beta-endorphin secretion by human peripheral blood mononuclear cells. Endocrinology. 1991;128:765–770. doi: 10.1210/endo-128-2-765. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Ballieux RE, Heijnen CJ. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive beta- endorphin by human peripheral blood mononuclear cells. J Immunol. 1989;142:2338–2342. [PubMed] [Google Scholar]
- Kavelaars A, Ballieux RE, Heijnen CJ. Beta-endorphin secretion by human peripheral blood mononuclear cells: Regulation by glucocorticoids. Life Sci. 1990b;46:1233–1240. doi: 10.1016/0024-3205(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Berkenbosch F, Croiset G, Ballieux RE. Induction of beta-endorphin secretion by lymphocytes after subcutaneous administration of corticotropin-releasing factor. Endocrinology. 1990;126:759–764. doi: 10.1210/endo-126-2-759. [DOI] [PubMed] [Google Scholar]
- Kay N, Allen J, Morley JE. Endorphins stimulate normal human peripheral blood lymphocyte natural killer activity. Life Sci. 1984;35:53–59. doi: 10.1016/0024-3205(84)90151-6. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, Walker EB. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986;83:5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Dunegan MA. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J Immunol. 1985;135:350–354. [PubMed] [Google Scholar]
- Koldzic-Zivanovic N, Tu H, Juelich TL, Rady PL, Tyring SK, Hudnall SD, Smith EM, Hughes TK. Regulation of adrenal glucocorticoid synthesis by interleukin-10: A preponderance of IL-10 receptor in the adrenal zona fasciculata. Brain, Behavior, and Immunity. 2006;20:460–468. doi: 10.1016/j.bbi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kravchenco I, Furalev V. Secretion of immunoreactive corticotropin releasing factor and adrenocorticotropic hormone by T- and B- lymphocytes in response to cellular stress factors. Biochemical and Biophysical Research Communications. 1994;204:828–834. doi: 10.1006/bbrc.1994.2534. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Singh V. Modulation of natural killer cell-mediated lysis by corticotropin-releasing neurohormone. J Neuroimmunol. 1991;33:253–260. doi: 10.1016/0165-5728(91)90113-l. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Singh V. Stimulation of interleukin-6 production by corticotropin-releasing factor. Cell Immunol. 1992;143:220–227. doi: 10.1016/0008-8749(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Singh V. Suppression of in vitro antibody production by corticotropin-releasing factor neurohormone. J Neuroimmunol. 1993;45:23–29. doi: 10.1016/0165-5728(93)90159-v. [DOI] [PubMed] [Google Scholar]
- Lipton JM, ZHAO H, Ichiyama T, Barsh GS, Catania A. Mechanisms of Antiinflammatory Action of alpha-MSH Peptides: In Vivo and in Vitro Evidence. Ann N Y Acad Sci. 1999;885:173–182. doi: 10.1111/j.1749-6632.1999.tb08674.x. [DOI] [PubMed] [Google Scholar]
- Loh YP, Parish D, Tuteja R. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem. 1985;260:7194–7205. [PubMed] [Google Scholar]
- Lolait SJ, Clements JA, Markwick AJ, Cheng C, McNally M, Smith AI, Funder JW. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986;77:1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons PD, Blalock JE. The kinetics of ACTH expression in rat leukocyte subpopulations. J Neuroimmunol. 1995;63:103–112. doi: 10.1016/0165-5728(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Lyons PD, Blalock JE. Pro-opiomelanocortin gene expression and protein processing in rat mononuclear leukocytes. J Neuroimmunol. 1997;78:47–56. doi: 10.1016/s0165-5728(97)00081-7. [DOI] [PubMed] [Google Scholar]
- Mathews PM, Froelich CJ, Sibbitt WL, Jr, Bankhurst AD. Enhancement of natural cytotoxicity by beta-endorphin. J Immunol. 1983;130:1658–1662. [PubMed] [Google Scholar]
- McGillis JP, Park A, Rubin-Fletter P, Turck C, Dallman MF, Payan DG. Stimulation of rat B-lymphocyte proliferation by corticotropin-releasing factor. J Neurosci Res. 1989;23:346–352. doi: 10.1002/jnr.490230316. [DOI] [PubMed] [Google Scholar]
- Mechanick JI, Levin N, Roberts JL, Autelitano DJ. Proopiomelanocortin gene expression in a distinct population of rat spleen and lung leukocytes. Endocrinology. 1992;131:518–525. doi: 10.1210/endo.131.1.1612033. [DOI] [PubMed] [Google Scholar]
- Meyer WJ, III, Smith EM, Richards GE, Cavallo A, Morrill AC, Blalock JE. In vivo immunoreactive adrenocorticotropin (ACTH) production by human mononuclear lymphocytes from normal and ACTH-deficient individuals. J Clin Endocrinol Metab. 1987;64:98–105. doi: 10.1210/jcem-64-1-98. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Machelska H, Schafer M, Stein C. Co-expression of beta-endorphin with adhesion molecules in a model of inflammatory pain. J Neuroimmunol. 2000;108:160–170. doi: 10.1016/s0165-5728(00)00284-8. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Schafer M, Mitchell WM, Hassan AH, Stein C. Local upregulation of corticotropin-releasing hormone and interleukin-1 receptors in rats with painful hindlimb inflammation. European Journal of Pharmacology. 1996;311:221–231. doi: 10.1016/0014-2999(96)00440-2. [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Bethin K, Jacobson L, Vogt SK, Majzoub JA. Pituitary-adrenal axis regulation in CRH-deficient mice. Endocrine Research. 2000;26:1057–1066. doi: 10.3109/07435800009048638. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Jacobson L, Weninger SC, Karalis KP, Jeong K, Majzoub JA. The physiology of corticotropin-releasing hormone deficiency in mice. Peptides. 2001;22:725–731. doi: 10.1016/s0196-9781(01)00385-0. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J Clin Invest. 1994;93:2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Munn NA, Lum LG. Immunoregulatory effects of alpha-endorphin, beta-endorphin, methionine-enkephalin, and adrenocorticotropic hormone on anti-tetanus toxoid antibody synthesis by human lymphocytes. Clinical Immunology & Immunopathology. 1989;52:376–385. doi: 10.1016/0090-1229(89)90152-9. [DOI] [PubMed] [Google Scholar]
- Oates EL, Allaway GP, Armstrong GR, Boyajian RA, Kehrl JH, Prabhakar BS. Human lymphocytes produce pro-opiomelanocortin gene-related transcripts. Effects of lymphotropic viruses. J Biol Chem. 1988;263:10041–10044. [PubMed] [Google Scholar]
- Pawlikowski M, Zelazowski P, Dohler K, Stepien H. Effects of two neuropeptides, somatoliberin (GRF) and corticoliberin (CRF), on human lymphocyte natural killer activity. Brain Behav Immun. 1988;2:50–56. doi: 10.1016/0889-1591(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Payne LC, Weigent DA, Blalock JE. Induction of pituitary sensitivity to interleukin-1: A new function for corticotropin-releasing hormone. Biochemical and Biophysical Research Communications. 1994;198:480–484. doi: 10.1006/bbrc.1994.1070. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Hassan AH, Lason W, Epplen C, Herz AX, Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- Radulovic M, Dautzenberg FM, Sydow S, Radulovic J, Spiess J. Corticotropin-releasing factor receptor 1 in mouse spleen: Expression after immune stimulation and identification of receptor-bearing cells. J Immunol. 1999;162:3013–3021. [PubMed] [Google Scholar]
- Rajora N, Ceriani G, Catania A, Star RA, Murphy M, Lipton JM. alpha-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. 1996;59:248–253. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- Redei E. Immuno-reactive and bioactive corticotropin-releasing factor in rat thymus. Neuroendocrinology. 1992;55:115–118. doi: 10.1159/000126104. [DOI] [PubMed] [Google Scholar]
- Reder AT. Regulation of production of adrenocotricotropin-like proteins in human mononuclear cells. Immunology. 1992;77:436–442. [PMC free article] [PubMed] [Google Scholar]
- Rittner HLM, Brack AMD, Machelska HP, Mousa SAP, Bauer MP, Schafer MMD, Stein CMD. Opioid Peptide-expressing Leukocytes: Identification, Recruitment, and Simultaneously Increasing Inhibition of Inflammatory Pain. [Article] Anesthesiology. 2001;95:500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- Schafer M, Carter L, Stein C. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci U S A. 1994;91:4219–4223. doi: 10.1073/pnas.91.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Mousa SA, Stein C. Corticotropin-releasing factor in antinociception and inflammation. European Journal of Pharmacology. 1997;323:1–10. doi: 10.1016/s0014-2999(97)00057-5. [DOI] [PubMed] [Google Scholar]
- Schafer M, Mousa SA, Zhang Q, Carter L, Stein C. Expression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc Natl Acad Sci U S A. 1996;93:6096–6100. doi: 10.1073/pnas.93.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Zhou L, Stein C. Cholecystokinin inhibits peripheral opioid analgesia in inflamed tissue. Neuroscience. 1998;82:603–611. doi: 10.1016/s0306-4522(97)00304-7. [DOI] [PubMed] [Google Scholar]
- Singh V. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol. 1989;23:257–262. doi: 10.1016/0165-5728(89)90058-1. [DOI] [PubMed] [Google Scholar]
- Singh V, Fudenberg HH. Binding of [125I]corticotropin releasing factor to blood immunocytes and its reduction in Alzheimer's disease. Immunol Lett. 1988;18:5–8. doi: 10.1016/0165-2478(88)90061-2. [DOI] [PubMed] [Google Scholar]
- Singh V, Leu SJ. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett. 1990;120:151–154. doi: 10.1016/0304-3940(90)90025-5. [DOI] [PubMed] [Google Scholar]
- Singh V, Leu SJ. Corticotropin-releasing factor-induced production of cyclic AMP by human peripheral blood immunocytes. Immunol Lett. 1993;35:239–245. doi: 10.1016/0165-2478(93)90189-9. [DOI] [PubMed] [Google Scholar]
- Sitte N, Busch M, Mousa SA, Labuz D, Rittner H, Gore C, Krause H, Stein C, Schafer M. Lymphocytes upregulate signal sequence-encoding proopiomelanocortin mRNA and beta-endorphin during painful inflammation in vivo. J Neuroimmunol. 2007;183:133–145. doi: 10.1016/j.jneuroim.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Smith EM. Corticotropin and immunoregulation. In: Scharrer B, Smith EM, Stefano GB, editors. Neuropeptides and immunoregulation. Springer-Verlag; Berlin: 1994. pp. 28–45. [Google Scholar]
- Smith EM. Opioid peptides in immune cells. In: Malchelska H, Stein C, editors. Immune Mechanisms of Analgesis. R. G. Landes Co; Berlin: 2002. pp. 51–68. [Google Scholar]
- Smith EM. Opioid peptides in immune cells. Advances in Experimental Medicine & Biology. 2003;521:51–68. [PubMed] [Google Scholar]
- Smith EM, Blalock JE. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981a;78:7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Blalock JE. The hormonal nature of the interferon system. Tex Rep Biol Med. 1981b;41:350–358. [PubMed] [Google Scholar]
- Smith EM, Galin FS, LeBoeuf RD, Coppenhaver DH, Harbour DV, Blalock JE. Nucleotide and amino acid sequence of lymphocyte-derived corticotropin: endotoxin induction of a truncated peptide. Proc Natl Acad Sci U S A. 1990;87:1057–1060. doi: 10.1073/pnas.87.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Hogan D, Hughes TK., Jr Nuclear factor-kappa B is a pivotal regulatory factor for neuro-immune interactions. Brain Behav Immun. 2001;15:184. [Google Scholar]
- Smith EM, Hughes TK, Cadet P, Stefano GB. Corticotropin-releasing factor-induced immunosuppression in human and invertebrate immunocytes. Cell Mol Neurobiol. 1992;12:473–481. doi: 10.1007/BF00711548. [DOI] [PubMed] [Google Scholar]
- Smith EM, Hughes TK, Jr, Hashemi F, Stefano GB. Immunosuppressive effects of corticotropin and melanotropin and their possible significance in human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1992;89:782–786. doi: 10.1073/pnas.89.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Johnson EW. A molecular basis for bidirectional communication between the neuroendocrine and immune systems. In: Hadden JW, Spreafico F, Yamamura Y, Austen KF, Dukor P, Masek K, editors. Advances in Immunopharmacology 4. Pergamon Press; Oxford: 1989. pp. 47–54. [Google Scholar]
- Smith EM, Meyer WJ, Blalock JE. Virus-induced corticosterone in hypophysectomized mice: a possible lymphoid adrenal axis. Science. 1982;218:1311–1312. doi: 10.1126/science.6183748. [DOI] [PubMed] [Google Scholar]
- Smith EM, Morrill AC, Meyer WJ, III, Blalock JE. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature. 1986;321:881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci U S A. 1983;80:6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Gregg M, Hashemi F, Schott L, Hughes T. Corticotropin Releasing Factor (CRF) Activation of NF-kB-Directed Transcription in Leukocytes. Cellular and Molecular Neurobiology. 2006:1–16. doi: 10.1007/s10571-006-9040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Dalmeida W, Hughes TK. Signaling pathway-related gene expression in neuroimmunoregulation. J Neuroimmunol. 2004;147:138–140. doi: 10.1016/j.jneuroim.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Smith EM. Adrenocorticotropin--a central trigger in immune responsiveness: Tonal inhibition of immune activation. Med Hypotheses. 1995;46:471–478. doi: 10.1016/s0306-9877(96)90028-6. [DOI] [PubMed] [Google Scholar]
- Stein C, Gramsch C, Hassan AH, Przewlocki R, Parsons CG, Peter K, Herz A. Local opioid receptors mediating antinociception in inflammation: Endogenous ligands. In: Quirion R, Jhamandas K, Gianoulakis C, editors. The International Nsarcotics Research Conference (INRC) '89. Alan R. Liss, Inc; New York: 1990. pp. 425–427. [PubMed] [Google Scholar]
- Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation: Local opioid receptors and beta-endorphin. J Neurosci. 1990;10:1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Hassan AH, Lehrberger K, Giefing J, Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342:321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Jessop DS, Knight RA, Lightman SL. Corticotrophin-releasing factor-like immunoreactivity and mRNA in human leukocytes. Brain Behav Immun. 1990;4:67–73. doi: 10.1016/0889-1591(90)90007-d. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Sarlis NJ, Knight RA, Chowdrey HS, Lightman SL. Response of pituitary and spleen pro-opiomelanocortin mRNA, and spleen and thymus interleukin-1 beta mRNA to adjuvant arthritis in the rat. J Neuroimmunol. 1992;37:59–63. doi: 10.1016/0165-5728(92)90155-e. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Alissafi T, Charalampopoulos I, Dermitzaki E, Roger T, Gravanis A, Margioris AN. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. Journal of Immunology. 2006;176(3):1869–77. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 2005;579:4259–4264. doi: 10.1016/j.febslet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- van Woudenberg AD, Metzelaar MJ, van der Kleij AA, de Wied D, Burbach JP, Wiegant VM. Analysis of proopiomelanocortin (POMC) messenger ribonucleic acid and POMC-derived peptides in human peripheral blood mononuclear cells: no evidence for a lymphocyte-derived POMC system. Endocrinology. 1993;133:1922–1933. doi: 10.1210/endo.133.5.8404638. [DOI] [PubMed] [Google Scholar]
- Webster E, De Souza EB. Corticotropin-releasing factor receptors in mouse spleen: Identification, autoradiographic localization, and regulation by divalent cations and guanine nucleotides. Endocrinology. 1988;122:609–617. doi: 10.1210/endo-122-2-609. [DOI] [PubMed] [Google Scholar]
- Webster E, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone acts on immune cells to elicit pro-inflammatory responses. Molecular Psychiatry. 1997;2:345–346. doi: 10.1038/sj.mp.4000314. [DOI] [PubMed] [Google Scholar]
- Webster E, Tracey D, Jutila MA, Wolfe SA, Jr, De Souza EB. Corticotropin-releasing factor receptors in mouse spleen: Identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990;127:440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- Weigent DA, Blalock JE. Production of peptide hormones and neurotransmitters by the immune system. Chemical Immunology. 1997;69:1–30. doi: 10.1159/000058652. [DOI] [PubMed] [Google Scholar]
- Westly HJ, Kleiss AJ, Kelley KW, Wong PK, Yuen PH. Newcastle disease virus-infected splenocytes express the proopiomelanocortin gene. J Exp Med. 1986;163:1589–1594. doi: 10.1084/jem.163.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbert-Lampen U, Straube F, Trapp A, Deutschmann A, Plasse A, Steinbeck G. Effects of corticotropin-releasing hormone (CRH) on monocyte function, mediated by CRH-receptor subtype R1 and R2: a potential link between mood disorders and endothelial dysfunction? Journal of Cardiovascular Pharmacology. 2006;47(1):110–6. doi: 10.1097/01.fjc.0000196240.58641.d3. [DOI] [PubMed] [Google Scholar]
- Zhao J, Karalis KP. Regulation of nuclear factor-κB by corticotropin-releasing hormone in mouse thymocytes. Molecular Endocrinology. 2002a;16:2561–2570. doi: 10.1210/me.2001-0334. [DOI] [PubMed] [Google Scholar]
- Zhao J, Karalis KP. Regulation of Nuclear Factor-{kappa}B by Corticotropin-Releasing Hormone in Mouse Thymocytes. Mol Endocrinol. 2002b;16:2561–2570. doi: 10.1210/me.2001-0334. [DOI] [PubMed] [Google Scholar]