Abstract
Since the time of Aristotle it has been thought that memories can be divided into two basic types; conscious recollections and familiarity-based judgments. Neuropsychological studies have provided indirect support for this distinction by suggesting that different regions within the human medial temporal lobe (MTL) are involved in these two forms of memory, but none of these studies have demonstrated that these brain regions can be fully dissociated. In a group of nondemented elderly subjects, we found that performance on recall and recognition tests was predicted preferentially by hippocampal and entorhinal volumes, respectively. Structural equation modeling revealed a double dissociation, whereby age-related reductions in hippocampal volume resulted in decreases in recollection, but not familiarity, whereas entorhinal volume was preferentially related to familiarity. The results demonstrate that the forms of episodic memory supported by the human hippocampus and entorhinal cortex can be fully dissociated, and indicate that recollection and familiarity reflect neuroanatomically distinct memory processes.
Keywords: recollection, familiarity, hippocampus, entorhinal cortex
INTRODUCTION
The medial temporal lobe (MTL) plays a critical role in supporting long-term memory for prior episodes. Patients with damage to the MTL can be profoundly amnestic, exhibiting deficits in tests of long-term recall and recognition memory, while performing normally on tests of intelligence and perception (Scoville and Milner, 1957; Parkin and Leng, 1993). In addition, studies of amnesics, as well as lesion studies of rats and nonhuman primates, suggest that different regions within the MTL support different types of episodic memory. Namely, the hippocampus appears to be critical for the recollection of qualitative information about a study event, such as where or when the event occurred, whereas the surrounding MTL cortex supports the assessment of stimulus familiarity (e.g., Eichenbaum et al., 1994; Aggleton and Brown, 1999). Recent neuroimaging evidence suggests that these two regions may be doubly dissociated (e.g., Davachi et al., 2003; Henson et al., 2003; Ranganath et al., 2004; Daselaar et al., 2006), but the neuropsychological evidence linking recollection and familiarity to the hippocampus and surrounding cortex has been limited to demonstrations of a single type of dissociation. That is, studies have demonstrated that damage to the hippocampus leads to a deficit in recollection, but has little or no effect on familiarity (Vargha-Khadem et al., 1997; Yonelinas et al., 2002). To our knowledge, the complementary neuropsychological dissociation in which the MTL surrounding the hippocampus is found to be more important for familiarity than recollection has never been reported. Although single dissociations are informative, only a double dissociation would provide conclusive evidence for the functional separability of these different brain regions (Shallice, 1988).
Examining the effects of aging provides a unique opportunity to determine the contribution of different MTL regions to memory, because increased age is associated with significant reductions in recollection (Light et al., 2000). Moreover, significant individual differences in familiarity are observed in the aged population (Davidson and Glisky, 2002). In addition, both the hippocampus and the surrounding entorhinal cortex exhibit age-related decreases in volume (Killiany et al., 2002; Raz et al., 2005). However, the relation between these specific forms of memory and particular MTL regions has never been directly examined in aging. In the current study, we found that, in nondemented aged subjects, recall was correlated most strongly with hippocampal volume, whereas recognition was correlated most strongly with entorhinal volume. These effects were examined further using structural equation modeling (SEM) which revealed a double dissociation, whereby recollection (but not familiarity) was directly related to the volume of the hippocampus, whereas familiarity was most directly related to the volume of the entorhinal cortex.
METHODS
We examined recall and recognition memory in a group of nondemented elderly subjects. The participants (N = 157) were selected to be between the ages of 65 and 80, and to have Mini-Mental State Exam scores (Folstein et al., 1975) of 26 or greater, indicating that they were not demented. Each participant completed the Memory Assessment Scale (MAS; LaFosse et al., 1998) that included measures of recall and recognition. Participants were presented with 12 words to encode, followed by a free-recall test. The study-test cycle was repeated six times, or until all the words could be recalled. After a 3-min delay, subjects received a free-recall test followed by a cued-recall test. Following an additional 30-min delay, subjects were presented with a delayed free-recall and cued-recall test, and then a delayed recognition test that included a mixture of 12 studied items, 12 related lures, 12 semirelated lures, and 6 unrelated lures. Recall was measured as the proportion of recalled items in each recall test, and recognition was measured as the proportion of correctly recognized studied items, related lures, semirelated lures, and unrelated lures. The MAS battery was used because it provided multiple measures of recall and recognition that are standardized for aged participants, and because previous work has demonstrated that recall and recognition in this type of test can be used to separate the contribution of recollection and familiarity (e.g., Yonelinas et al., 2002). Neither the recall nor recognition test measures are expected to provide pure measures of recollection or familiarity. However, because recall requires retrieval of items from the study event, recall is expected to rely heavily on recollection. In contrast, because participants are presented with test items as retrieval cues in the recognition test, recognition benefits from familiarity as well as recollection (Mandler, 1980).
All participants were scanned on a 1.5 T magnet, and brain volumes were measured using standardized methods on volumetric T1-weighted images (Fig. 1A). Entorhinal cortex was manually traced in 63 subjects by an expert operator using methods previously published (Du et al., 2003). Automated hippocampal volumetry was carried out on all subjects using a commercially available high-dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO) (Csernansky et al., 2000). After manual identification of both global and local landmarks, a coarse transformation was computed using landmark matching. Automated hippocampal morphometry was then performed by a fluid image matching transformation (Haller et al., 1997). This software was validated by comparison with a manual method to trace the hippocampus (Hsu et al., 2002), using same day back-to-back MRI studies on 60 subjects. Correlation between automated and manual measurements of hippocampal volumes was 0.92. Automated measurements achieved an intraclass correlation coefficient across scans of 0.94, compared to 0.99 for manual measurements. Both entorhinal and hippocampal volumes were normalized to the subject's total intracranial volume. Memory and volumetric results are presented in Table 1, along with the correlations among each measure.
FIGURE 1.
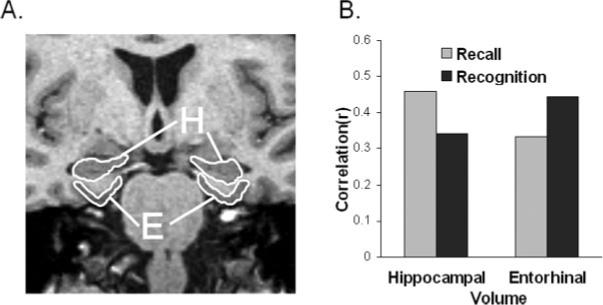
A: Hippocampal (H) and entorhinal (E) volumes were measured using a volumetric T1-wieghted image on a 1.5 T Siemans scanner. Brain volumes for each subject were normalized for brain size by dividing by total intracranial volume. B: Pearson correlations between medial temporal lobe volumes (i.e., hippocampal and entorhinal cortex) and mean recognition and recall scores. Recall was most strongly predicted by hippocampal volume, whereas recognition was most strongly predicted by entorhinal volume.
TABLE 1.
Pearson Correlations Among Memory Measures and Brain Volumes, Along With Descriptive Statistics (Bottom of Table) for the Memory Scores and Brain Volumes
| IR1 | 1.000 | |||||||||||||||
| IR2 | 0.732 | 1.000 | ||||||||||||||
| IR3 | 0.624 | 0.748 | 1.000 | |||||||||||||
| IR4 | 0.582 | 0.715 | 0.811 | 1.000 | ||||||||||||
| IR5 | 0.569 | 0.719 | 0.807 | 0.817 | 1.000 | |||||||||||
| IR6 | 0.528 | 0.657 | 0.750 | 0.793 | 0.817 | 1.000 | ||||||||||
| SDR | 0.487 | 0.670 | 0.713 | 0.729 | 0.792 | 0.765 | 1.000 | |||||||||
| SDC | 0.475 | 0.686 | 0.715 | 0.732 | 0.763 | 0.768 | 0.877 | 1.000 | ||||||||
| R | 0.456 | 0.672 | 0.701 | 0.732 | 0.786 | 0.771 | 0.919 | 0.888 | 1.000 | |||||||
| C | 0.446 | 0.672 | 0.680 | 0.711 | 0.766 | 0.733 | 0.844 | 0.903 | 0.880 | 1.000 | ||||||
| RO | 0.269 | 0.436 | 0.447 | 0.447 | 0.442 | 0.433 | 0.421 | 0.508 | 0.480 | 0.485 | 1.000 | |||||
| RR | −0.314 | −0.416 | −0.414 | −0.431 | −0.489 | −0.536 | −0.625 | −0.562 | −0.605 | −0.559 | −0.183 | 1.000 | ||||
| RS | −0.250 | −0.313 | −0.314 | −0.359 | −0.329 | −0.402 | −0.490 | −0.490 | −0.518 | −0.476 | −0.129 | 0.705 | 1.000 | |||
| RN | −0.208 | −0.312 | −0.283 | −0.334 | −0.347 | −0.380 | −0.509 | −0.503 | −0.514 | −0.405 | −0.265 | 0.479 | 0.519 | 1.000 | ||
| Hippo | 0.344 | 0.405 | 0.393 | 0.343 | 0.401 | 0.357 | 0.445 | 0.394 | 0.441 | 0.400 | 0.220 | −0.405 | −0.265 | −0.205 | 1.000 | |
| Ento | 0.181 | 0.207 | 0.228 | 0.237 | 0.388 | 0.283 | 0.393 | 0.293 | 0.386 | 0.319 | 0.341 | −0.467 | −0.306 | −0.412 | 0.421 | 1.000 |
| IR1 | IR2 | IR3 | IR4 | IR5 | IR6 | SDR | SDC | R | C | RO | RR | RS | RN | Hippo | Ento | |
| Mean | 5.554 | 7.803 | 8.541 | 9.280 | 9.590 | 9.830 | 8.548 | 9.382 | 8.777 | 9.140 | 11.178 | 0.522 | 0.197 | 0.115 | 0.00322 | 0.00200 |
| SD | 1.827 | 2.271 | 2.208 | 2.192 | 2.100 | 2.184 | 3.274 | 2.294 | 3.187 | 2.656 | 1.504 | 1.179 | 0.746 | 0.438 | 0.00053 | 0.00049 |
| min | 2 | 2 | 2 | 4 | 4 | 4 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0.00167 | 0.00100 |
| max | 10 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 8 | 6 | 3 | 0.00455 | 0.00299 |
Brain volumes were measured in cubic millimeters and then normalized to total intracranial volume (volume/total intracranial volume). IR, immediate recall; SDR, short delay recall; SDC, short delay cued-recall; R, recall; C, cued-recall; RO, recognition old; RR, recognition related; RS, recognition semirelated; RN, recognition new; Hippo, hippocampus; Ento, entorhinal cortex; SD, standard deviation; min, minimum; max, maximum.
RESULTS
Figure 1B presents Pearson correlations between brain volumes and memory measures, and it indicates that recall was more strongly correlated with hippocampal volume, whereas recognition was more strongly correlated with entorhinal volume (the full correlation matrix is presented in Table 1). The recall measure included the mean of all of the free-recall and cued-recall scores from the MAS, with the exception of the free-recall scores from the 5th and 6th learning trials, which were not collected in several participants because they performed perfectly in the preceding trials. The recognition measure included all of the MAS recognition scores, including the proportion of correctly recognized old items, related lures, semirelated lures, and unrelated lures. To quantify the interaction observed in Figure 1B, we tested whether the pattern of correlations of the two brain volumes with the two memory measures differed significantly, or whether these patterns of correlation were similar. That is, we first tested a model that allowed the recall and recognition scores to correlate freely with hippocampal and entorhinal volumes. Then, we added the constraint that the difference between the recall and recognition correlations with hippocampal volume was equal to the difference between recall and recognition correlations with entorhinal volume. The constrained model provided a significantly poorer account than the full model, χ2(1) = 4.05, P < 0.05, indicating that the pattern of correlations of the recall and recognition scores with hippocampal and entorhinal volumes differed significantly.
These differences in correlations were subtle in some cases, but all 10 of the recall measures we obtained correlated more highly with hippocampal than with entorhinal volumes, whereas all four of the recognition measures correlated more highly with the entorhinal than with the hippocampal volumes, indicating that the directional effect was quite reliable (Table 1). Note that, because recognition is an easier task than recall, and recognition scores were quite high, it may be a less sensitive measure than recall. The current results, however, indicate that the recall and recognition measures were related in opposite ways to the hippocampal and entorhinal volumes, producing a crossover interaction; thus, it was not the case that one of the measures was simply less sensitive than the other. Although single dissociations can be produced by differences in overall levels of performance, double or crossover dissociations like that seen in the current study cannot (Shallice, 1988). In addition, several aspects of the current results suggest that the conclusions were not biased by differences in overall level of performance. For example, in every measure of recall that we obtained (8 measures) and every measure of recognition (4 measures), the same dissociative pattern was observed. Performance varied considerably across these measures, suggesting that the level of performance was not a critical factor. In addition, to determine whether the high recognition scores were responsible for the dissociative pattern of results, we conducted a secondary analysis that included only the subjects with overall recognition scores in the lower half based on a medial split, thus removing any potential bias due to high recognition scores. The results indicated that, if anything, our initial analysis may have underestimated the degree to which the measures dissociated. That is, recognition was more highly correlated with entorhinal cortex (0.36) than with hippocampal volume (0.21), a difference of 0.16, which is larger than the difference seen in the initial overall analysis (i.e. a difference of 0.10). Conversely, delayed recall in the secondary analysis was more highly correlated with hippocampal cortex (0.35) than with entorhinal cortex (0.22), a difference of 0.13, which is larger than the difference seen in the initial overall analysis (i.e., a difference of 0.07). Thus, there is no evidence that the dissociative pattern of results we observed between the memory and brain measures was produced by differences in overall levels of performance.
This distinctive pattern of correlations is consistent with the claim that recall and recognition memory are differentially dependent on the hippocampus and entorhinal cortex. However, because recall and recognition tests do not provide pure measures of recollection and familiarity, the prior analyses do not indicate how these two regions are related to recollection and familiarity. In order to answer this question we used SEM (Joeskog, 1974). We began with an a priori model that has proven useful in previous studies of hippocampal function (Yonelinas et al., 2002; Quamme et al., 2004), and then tested each of the important assumptions of that model as well as various alternative accounts of the results. The model assumes that two latent variables explain the covariances among recall and recognition measures, and these latent variables correspond to the constructs of recollection and familiarity. In the model, hippocampal volume is related directly to recollection, and entorhinal volume is related directly to familiarity. For reasons argued earlier, it predicts that recollection relates to recall and recognition, whereas familiarity relates to recognition but not to recall. Furthermore, based on earlier work (Light et al., 2000; Yonelinas, 2002), the model assumes that age is negatively related with recollection, but is unrelated to familiarity, and that hippocampal volume mediates the relation between age and recollection.
The SEM, diagrammed in Figure 2, provided a statistically acceptable account of the observed covariances among age, the brain volume measures, and memory performance, χ2(22) = 31.71, P > 0.05, CFI = 0.985, RMSEA = 0.053 (CI = 0−0.091), and it was the best fitting model that could be identified in the current study. Most critically, it indicated that age-related decreases in hippocampal volume led selectively to lower levels of recollection, which resulted in decreases in recall and recognition performance. In contrast, reduced entorhinal volume was related uniquely to reductions in familiarity, which resulted in a selective decrease in recognition.
FIGURE 2.
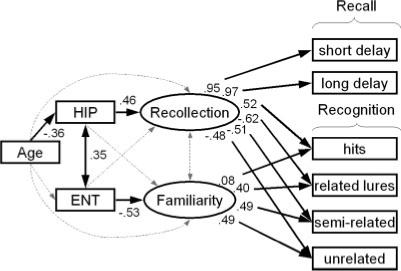
Modeling the effects of aging on recollection and familiarity. The ellipses and rectangles represent latent and manifest variables, respectively. Solid arrows reflect significant standardized regression coefficients and dotted arrows reflect nonsignificant coefficients. The model assumes that recognition relies on recollection and familiarity, whereas recall relies solely on recollection. Recall scores represent proportion of correctly recalled items in short-delay free recall and long-delay free recall. Recognition scores represent the proportion of recognized studied items (hits), correct rejections for related lures, semirelated lures, and unrelated lures. Hippocampal volume (HIP) was allowed to influence Recollection, entorhinal volume (ENT) was allowed to influence familiarity, and aging was allowed to influence HIP.
The only expected model loading that was not statistically significant was that between familiarity and the recognition hit rate. On a priori grounds that connection was included in all the models, but excluding it did not alter any of the conclusions. The lack of a strong relationship between familiarity and recognition hit rate may reflect the fact that recognition memory was quite good. That is, because familiarity is expected to contribute to the hit rate only when recollection fails (Mandler, 1980; Jacoby, 1991), familiarity should load most heavily on new item scores. In fact, a subsequent analysis of demented individuals who were expected to exhibit pronounced deficits in recollection indicated that the loading between familiarity and hit rate was significant in this group.
To verify the conclusions of the model analysis, we tested various alternative hypotheses. First, allowing hippocampal volume to influence familiarity directly in addition to recollection did not improve model fit, χ2(1) = 1.19, ns, indicating that differences in hippocampal volume influenced recollection, but not familiarity. Conversely, allowing entorhinal cortex volume to influence recollection directly did not improve model fit, χ2(1) = 3.29, ns, indicating that entorhinal cortex had selective effects on familiarity. Note, however, that this latter difference was close to significant (i.e., P < 0.07), suggesting that the entorhinal cortex may play a secondary role in recollection as well as familiarity.
The dissociative effects of hippocampal and entorhinal cortex on recollection and familiarity are illustrated in Figure 3, which shows that in a model in which both brain volumes were allowed to influence both processes, hippocampal volume influenced recollection more than familiarity, whereas entorhinal volume influenced familiarity more than recollection.
FIGURE 3.
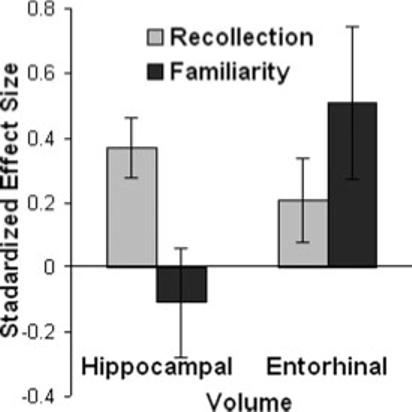
The effects of increasing hippocampal and entorhinal cortex by one standard deviation on recollection and familiarity. Changes in hippocampal volume had larger effects on recollection than familiarity, whereas changes in entorhinal volume had larger effects on familiarity than recollection.
Several alternative hypotheses regarding the effects of aging on memory were also tested. For example, allowing age to influence entorhinal cortex volume as well as hippocampal cortex volume did not significantly improve the fit of the model, χ2(1) = 1.50, ns, indicating that aging had relatively selective effects on the hippocampus, and not on entorhinal cortex. In addition, allowing effects of age directly on recollection and familiarity did not improve the fit of the model, χ2(2) = 1.11, ns, indicating that hippocampal volume effectively mediated all age-related changes in recall and recognition.
To determine whether the effects of hippocampal and entorhinal volumes contribute to recollection and familiarity “above and beyond” any direct effects of age on these memory processes, we tested a model in which age was allowed to freely influence both recollection and familiarity, but there were no direct effects of the brain measures on recollection or familiarity. Then, we allowed hippocampal volume to directly influence recollection to determine whether the hippocampal volume had an effect on recollection that could be observed in addition to the effects of age alone. This difference was highly significant [χ2(1) = 35.26, P < 0.01]. Similarly, we also assessed whether allowed entorhinal cortex to influence familiarity led to an improvement in model fit in addition to any direct effect of age on familiarity, and this effect was also significant [χ2(1) = 8.75, P < 0.01]. So, the effects of hippocampal and entorhinal volumes on recollection and familiarity were significant even when we controlled for the effects of age.
Several additional theoretical assumptions were also tested. For example, allowing recollection to correlate with familiarity did not increase the fit of the model, χ2(1) = 1.19, ns, indicating that the two memory processes were acting independently. In addition, to test the possibility that the MTL reflects a unified memory system, we assessed a model in which both the hippocampus and the entorhinal cortex influenced a single latent memory factor that contributed to both recall and recognition, and found that it provided an unacceptable account for the data, χ2(26) = 73.45, P < 0.00001, CFI = 0.928, RMSEA = 0.108. Moreover, a model in which one memory factor contributed to recall and recognition whereas another contributed to recall alone (i.e., a generate-recognize model) was also found to provide an unacceptable account of the data, χ2(24) = 62.98, P < 0.0001, CFI = 0.941, RMSEA = 0.102.
DISCUSSION
The current results support three major conclusions. First, they demonstrate that the contribution of the human hippocampus and entorhinal cortex to recollection and familiarity can be doubly dissociated. Given the close proximity and high interconnectivity between these regions (Lavenex and Amaral, 2000), this functional separability is remarkable. The double dissociation cannot be accounted for by assuming that the hippocampus is generally more important for episodic memory than the entorhinal cortex, nor by assuming that recall performance is simply more sensitive than is recognition. The results thus provide the first unequivocal neuropsychological support from humans, showing that recollection is supported by the hippocampus whereas familiarity is supported by the surrounding medial temporal cortex.
The dissociation is consistent with dissociations observed in neuroimaging studies showing that hippocampal activity corresponds to subjective reports of “remembering” and accurate recollection of episodic details, whereas activity in the surrounding anterior temporal cortex corresponds to familiarity (Davachi et al., 2003; Henson et al., 2003; Ranganath et al., 2004). Moreover, the current results are consistent with a recent functional imaging study of aging that indicated that hippocampal activity related to recollection was reduced in normal aged subjects, whereas activity in the rhinal cortex was increased in the aged subjects (Daselaar et al., 2006).
The results are also consistent with previous animal and human studies showing that selective hippocampal lesions can disrupt recollection while leaving familiarity less affected (e.g., Aggleton and Brown, 1999; Eichembaum et al., 1994). We also note that a recent study reported that Parkinson's patients exhibited a selective deficit in familiarity, which was similar to the effects of entorhinal cortex that we observed (Davidson et al., 2006). Brain measures were not examined in that study, but it is possible that those patients exhibited reductions in entorhinal cortex.
Second, the results indicate that age-related hippocampal atrophy is associated with a reduction in recollection, but not familiarity. Although previous studies have established that aging reduces recollection to a greater extent than familiarity (Light et al., 2000; Howard et al., 2006), the neural basis for this deficit was unknown, with some evidence implicating the involvement of the prefrontal cortex and other evidence implicating the MTLs (e.g., Cabeza, 2002; Hedden and Gabrieli, 2004). The current results demonstrate that age-related declines in recollection can be explained by age-related hippocampal changes (i.e., variation in hippocampal volume accounted for virtually all of the age-related variation observed in the memory measures). Nonetheless, the current results should not be interpreted as ruling out a role for the prefrontal cortex in age-related recollection declines, because we examined memory only across a restricted age range (i.e., 65−80 years of age) and did not examine prefrontal cortex. Given that frontal lobe changes can be observed as early as middle age (Raz et al., 2005), it is possible that frontal lobe changes could also influence recollection across the lifespan. Future studies examining recollection and familiarity across a wider range of ages will be critical in addressing this issue.
Third, the results provide evidence that individual difference in entorhinal cortex volumes in the aged were directly related to familiarity. These results are consistent with animal lesion results such as those indicating the entorhinal lesions can disrupt familiarity-based recognition (Eichenbaum et al., 1994; Aggleton and Brown, 1999), and with human neuroimaging results indicating that regions in the anterior MTL are related to familiarity (Davachi et al., 2003; Henson et al., 2003; Ranganath et al., 2004). The current results are also consistent with previous studies finding that aging is not correlated with entorhinal cortex volume (Rodrigue and Raz, 2004) or with familiarity (Light et al., 2000). The current results, however, do not indicate why entorhinal cortex volume was related to familiarity. One possibility is that this relation arose because some of the aged subjects may have had mild cognitive impairments and had preclinical Alzheimer's disease, which has been related to entorhinal atrophy (Killiany et al., 2002) and decreases in familiarity (Dalla Barba, 1997). Although this account leaves unexplained why entorhinal volumes were not related to age, previous work has suggested that changes in entorhinal volume may be related to episodic memory even when age is controlled (Rodrigue and Raz, 2004). Another possibility is that the individual differences we found reflect normal variation in entorhinal cortex volumes, and thus might emerge in a young sample as well.
The current results raise several additional questions about the contribution of different MTL structures to recognition that will be important to address in future studies. For example, how do other MTL regions such as the perirhinal or parahippocampal cortex contribute to recollection and familiarity? Imaging studies have suggested that the perirhinal cortex may be involved in familiarity, whereas the parahippocampal cortex may be important for recollection (Eichenbaum et al., 2007). Although age-related reductions in the temporal lobe volume in healthy aging are limited primarily to the hippocampus (Raz et al., 2005), other interconnected regions likely play a critical role in computing the familiarity signal that supports recognition memory. In addition, can familiarity be selectively disrupted by lesions to the entorhinal cortex? The current study showed that changes in entorhinal volume were related to familiarity, but had a nonsignificant effect on recollection. This latter effect, although nonsignificant, suggests that entorhinal cortex may play a limited role in recollection as well. One possibility is that as damage to the entorhinal cortex becomes more severe, as might occur in lesion patients, the hippocampus might become effectively isolated resulting in deficits in familiarity and recollection.
In sum, the current results provide direct support for models that assume that the hippocampus and the surrounding MTL regions make distinct and independent contributions to recognition memory. Age-related changes in the hippocampus influence recollection more than familiarity, whereas variations in entorhinal cortex influence familiarity more so than recollection.
REFERENCES
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Exploring the recognition memory deficit in Parkinson's disease: estimates of recollection versus familiarity. Brain. 2006;129:1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, et al. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampal system: Dissociating its functional components and recombining them in the service of declarative memory. Behav Brain Sci. 1994;17:449–776. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- Joreskog KG. In: Contemporary Developments in Mathematical Psychology: Measurement, Psychophysics and Neural Information Processing. Krantz DH, et al., editors. W.H. Freeman; New York: 1974. pp. 1–56. [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- LaFosse JM, Scarborough SL, Reed BR. Utility of a new verbal recognition memory task for the assessment of dementia. J Int Neuropsychol Soc. 1998;4:31. [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, La Voie DJ, Healy MR. Dual-process theories of memory in old age. In: Perfect TJ, Maylor EA, editors. Models of Cognitive Aging. Oxford University Press; New York: 2000. pp. 238–300. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psych Rev. 1980;87:252–271. [Google Scholar]
- Parkin AJ, Leng RC. Neuropsychology of the Amnesic Syndrome. Erlbaum; Hillsdale, New Jersey: 1993. [Google Scholar]
- Quamme JR, Yonelinas AP, Widaman KF, Kroll NE, Sauve MJ. Recall and recognition in mild hypoxia: Using covariance structural modeling to test competing theories of explicit memory. Neuropsychologia. 2004;42:672–691. doi: 10.1016/j.neuropsychologia.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. From Neuropsychology to Mental Structure. Cambridge University Press; Cambridge: 1988. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]


